Abstract
Hypoxia influences immune checkpoint receptors and their respective ligands. In support, we recently demonstrated that hypoxia selectively upregulates programmed cell death ligand 1 (PD-L1) on myeloid-derived suppressor cells (MDSCs) via hypoxia inducible factor 1 α (HIF-1α) binding to a hypoxia-response element (HRE) in the PD-L1 proximal promoter. Furthermore, blockade of PD-L1 under hypoxic conditions enhanced MDSC-mediated T-cell activation by attenuating MDSC secretion of IL-6 and IL-10.
Keywords: HIF-1α, hypoxia, IL-10, immune suppression, MDSC, PD-L1
Introduction
Tremendous progress has been made in the field of tumor immunology in the past decade and cancer immunotherapy is now a promise turned into reality. The recent approval by the FDA of cancer vaccines and monoclonal antibodies that block immune negative regulators has further ignited the hope of finally winning the fight against cancer. A large body of preclinical and clinical data indicates that antibody blockade of immune checkpoints can significantly enhance antitumor immunity. Recently, antibody-mediated blockade of programmed cell death 1 (PDCD1, better known as PD-1) and its ligand, PD-L1 has been shown to induce durable tumor regression in advanced cancers.1 Hypoxia is a common feature of all solid tumors and plays a central role in tumor progression and resistance to therapy.2 We have provided evidence that tumor hypoxic stress fosters the acquisition of immune resistance.3,4 In a recent study, we demonstrated that such stress is central in the regulation of immunosuppression.5 Hypoxic zones in tumors attract immunosuppressive cells such as myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs) and regulatory T cells (T regs).2 MDSCs are a heterogeneous group of relatively immature myeloid cells and several studies have described in detail mechanisms by which MDSCs mediate immune suppression. Although, hypoxia has been shown to regulate the function and differentiation of MDSCs,6 several major questions remain unanswered. The influence of hypoxia on the regulation of immune checkpoint receptors, including PD-1 and cytotoxic T lymphocyte associated protein 4 (CTLA-4), as well as their respective ligands (PD-L1, PD-L2, CD80 and CD86), on MDSCs remains largely unexplored. Furthermore, the potential contribution of these immune checkpoint receptors and their respective ligands on MDSC function under the influence of hypoxia remains unknown. (Fig. 1)
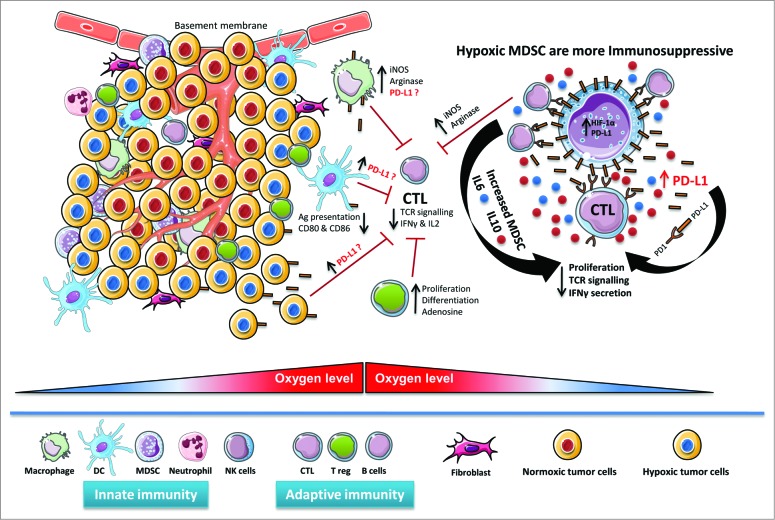
Figure 1.
Hypoxic regulation of PD-L1 expression in the tumor microenvironment. Hypoxic zones in tumors attract diverse immunosuppressive myeloid cells such as myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs), as well as lymphoid cells, such as regulatory T cells (Tregs) and cytotoxic T lymphocytes (CTLs). By virtue of hypoxia-inducible factor 1α (HIF-1α), hypoxia upregulates programmed cell death ligand 1 (PD-L1) expression on MDSCs, macrophages, antigen (Ag)-presenting dendritic cells and malignant cells in the tumor microenvironment. The potential contribution of increased PD-L1 on the surface of MDSCs, macrophages, dendritic cells and cancer cells in regulating immunosuppression within the hypoxic tumor microenvironment remains largely unknown. Hypoxic MDSC's are more immunosuppressive due to enhanced arginase and nitric oxide (iNOS) production. Hypoxia significantly increased IL-6 and IL-10 secretion from MDSCs, thereby increasing MDSC-mediated immunosuppression, decreasing T-cell function, and promoting tumor progression.
Hypoxic Regulation of Immune Checkpoint Receptors and Their Respective Ligands
Using several mouse tumor models, we first compared the expression level of immune checkpoint receptors and their respective ligands in splenic MDSCs relative to those of tumor-infiltrating MDSCs from tumor bearing mice. We found a relatively higher expression of PD-L1 on tumor-infiltrating MDSCs as compared to the levels on splenic MDSCs.7
Very interestingly, hypoxia dramatically and significantly increased the percentage of PD-L1 positive MDSCs isolated from spleen among different tumor-bearing mice. Similarly, hypoxic conditioning of live, tumor-bearing mice significantly upregulates PD-L1 expression on splenic MDSCs irrespective of tumor types. We further provided evidence that hypoxia inducible factor 1 α (HIF-1α) is a major regulator of PD-L1 mRNA and protein expression, and that HIF-1α regulates the expression of PD-L1 by binding directly to a hypoxia response element (HRE), namely HRE-4, in the PD-L1 proximal promoter.
We next investigated the functional consequences of hypoxia-induced upregulation of PD-L1 in MDSC-mediated T-cell suppression. The immunosuppressive activities of MDSCs enhanced in response to hypoxia were abrogated following PD-L1 blockade. Hypoxia significantly increased the secretion of the cytokines IL-6 and IL-10, as well as transforming growth factor β1 (TGFβ1) from MDSCs. More importantly, hypoxia upregulated IL-6 and IL-10 levels in MDSCs was significantly attenuated after PD-L1 blockade.7
Our data seem to be a more general mechanism, as blockade of tumor microenvironment-induced PD-L1 on myeloid dendritic cells has been shown to decrease myeloid dendritic cell-mediated immunosuppression, improve T-cell function, and decrease tumor progression.8
Potential Clinical Applicability of Immunotherapy
A myriad of distinct cell types comprise the tumor microenvironment. These include but are not limited to cancer cells, myeloid cells, lymphoid cells, endothelial cells and fibroblasts. With this in mind, we also examined whether hypoxia regulated PD-L1 is applicable to other cells in the tumor microenvironment. To this end, we used a panel of different mouse and human tumor cell lines expressing different levels of PD-L1 under normoxia. Interestingly, hypoxia significantly increased the expression of surface PD-L1 on these cells. Moreover, we also observed a slight but significant increase in PD-L1 expression on the surface of macrophages and dendritic cells isolated from naive mice splenocytes cultured under hypoxic conditions. Thus, hypoxia recapitulated the effect of the tumor microenvironment with regard to the expression of PD-L1 on MDSCs, macrophages, dendritic cells and tumor cells.7,9,10 Future experiments will attempt to further dissect to what degree hypoxia increased PD-L1 expression on MDSCs, macrophages, dendritic cells and tumor cells contributes to immunosuppression within the hypoxic tumor microenvironment.
Clearly, given the central role of hypoxia in the regulation of malignant disease progression and immune evasion, it is conceivable that its targeting might be considered as a potential strategy in the development of new combinatorial cancer therapies. In this regard it is tempting to speculate that targeting hypoxia will decrease immunosuppressive cell numbers, inhibit their function and eventually favor the in situ antitumor immune response. Therefore, novel immunotherapeutic approaches targeting tumor hypoxia via HIF-1α inhibitors in conjunction with PD-L1 blockade may boost the immune system in cancer patients. Exploiting hypoxia-associated immune escape routes holds great promise for overcoming diminished antitumor cytotoxic responses to restore the natural efficacy of immunity in cancer patients.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252-64; PMID:; http://dx.doi.org/ 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Noman MZ, Benlalam H, Hasmim M, Chouaib S. Cytotoxic T cells—stroma interactions. Bull Cancer 2011; 98:E19-24; PMID:; http://dx.doi.org/ 10.1684/bdc.2010.1295 [DOI] [PubMed] [Google Scholar]
- 3. Noman MZ, Buart S, Van Pelt J, Richon C, Hasmim M, Leleu N, Suchorska WM, Jalil A, Lecluse Y, El Hage F, et al. The cooperative induction of hypoxia-inducible factor-1 alpha and STAT3 during hypoxia induced an impairment of tumor susceptibility to CTL-mediated cell lysis. J Immunol 2009; 182:3510-21; PMID:; http://dx.doi.org/ 10.4049/jimmunol.0800854 [DOI] [PubMed] [Google Scholar]
- 4. Noman MZ, Janji B, Berchem G, Mami-Chouaib F, Chouaib S. Hypoxia-induced autophagy: a new player in cancer immunotherapy? Autophagy 2012; 8:704-6; PMID:; http://dx.doi.org/ 10.4161/auto.19572 [DOI] [PubMed] [Google Scholar]
- 5. Hasmim M, Noman MZ, Messai Y, Bordereaux D, Gros G, Baud V, Chouaib S. Cutting edge: Hypoxia-induced Nanog favors the intratumoral infiltration of regulatory T cells and macrophages via direct regulation of TGF-beta1. J Immunol 2013; 191:5802-6; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1302140 [DOI] [PubMed] [Google Scholar]
- 6. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12:253-68; PMID:; http://dx.doi.org/ 10.1038/nri3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 2014; 211:781-90; PMID:; http://dx.doi.org/ 10.1084/jem.20131916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 2003; 9:562-7; PMID:; http://dx.doi.org/ 10.1038/nm863 [DOI] [PubMed] [Google Scholar]
- 9. Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res 2014; 74:665-74; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-0992 [DOI] [PubMed] [Google Scholar]
- 10. Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, Johnson RS, Goldrath AW. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat Immunol 2013; 14:1173-82; PMID:; http://dx.doi.org/ 10.1038/ni.2714 [DOI] [PMC free article] [PubMed] [Google Scholar]