Abstract
While CAR therapy has begun to demonstrate efficacy, cell-engineering techniques that result in permanent genomic modification carry several safety concerns. CAR expression driven by RNA creates a platform for delivery of highly-active cell therapy while avoiding long-term CAR-driven toxicity. Using models of pediatric neuroblastoma, we have found that RNA CAR T cell activity is limited by ineffective tumor infiltration.
Keywords: chimeric antigen receptors, GD2, pediatrics, RNA, solid tumors
Abbreviations: Lenti CAR, lentivirally-modified chimeric antigen receptor T cell; RNA CAR, RNA-modified chimeric antigen receptor T cell
Chimeric antigen receptor (CAR) therapy has begun to demonstrate efficacy in clinical trials targeting hematopoietic malignancies.1 Experience with solid tumors has been much more limited, in large part due to difficulty in target antigen selection, as many solid tumor antigens are not unique to the tumor and are expressed by indispensable host tissues. Early trials of CARs for solid tumors yielded several adverse events and a call for safer CARs moving forward.2 As opposed to engineering with viral vectors, expression driven by RNA electroporation allows for a transiently expressed CAR molecule on the surface of delivered T cells. Previously published work has demonstrated that RNA CAR cells can mediate antitumor responses in xenograft models of both localized and intraperitoneal mesothelioma.3 In this study, six intraperitoneal injections did not result in disease control, unlike our experience with ALL in which three infusions of RNA CAR T cells resulted in disease cure.4
Previous trials of CAR T cells in neuroblastoma have demonstrated some efficacy,5 and recent advances in cell production have led to improved success of CAR therapy.6 To evaluate the activity of our CAR T cells against pediatric solid tumors, we investigated the efficacy of both permanently modified (lenti CARs) and transiently modified (RNA CARs) CAR T cells targeting the antigen GD2 in xenograft models of neuroblastoma.
To confirm antigen-driven cytotoxicity, we injected RNA CARs intratumorally into flank neuroblastoma and observed rapid tumor cell death, long-term disease stabilization and enhanced animal survival. We next developed a model of disseminated neuroblastoma using luciferase-expressing tumor cells injected intravenously. Delivery of a single infusion of lenti CARs resulted in disease control in all animals, with no disease detectable in > 60%. While all animals treated with GD2 lenti CARs had significantly enhanced survival as compared to those animals treated with control lenti CARs, all succumbed to xenogeneic graft-vs.-host disease, highlighting the robust proliferative potential of these permanently modified CAR T cells in vivo after encountering their target antigen.
Our previous experience with ALL indicated that a single infusion of RNA CARs was unlikely to result in a significant disease response, and thus we investigated the efficacy of our GD2 RNA CARs in our disseminated model of neuroblastoma with three distinct cell infusions. While these cells demonstrate some antitumor activity and were able to both slow disease progression and enhance animal survival, they were unable to result in disease eradication, and all animals died of disseminated cancer. To assess if this was simply an unmet dosing threshold, we increased the number of infusions to 9, for a total of 108 RNA CARs over the course of 8 weeks, and still did not observe disease eradication. These findings were consistent with those reported in the intraperitoneal mesothelioma model, expanded to a disseminated disease model treated with systemic cell delivery.
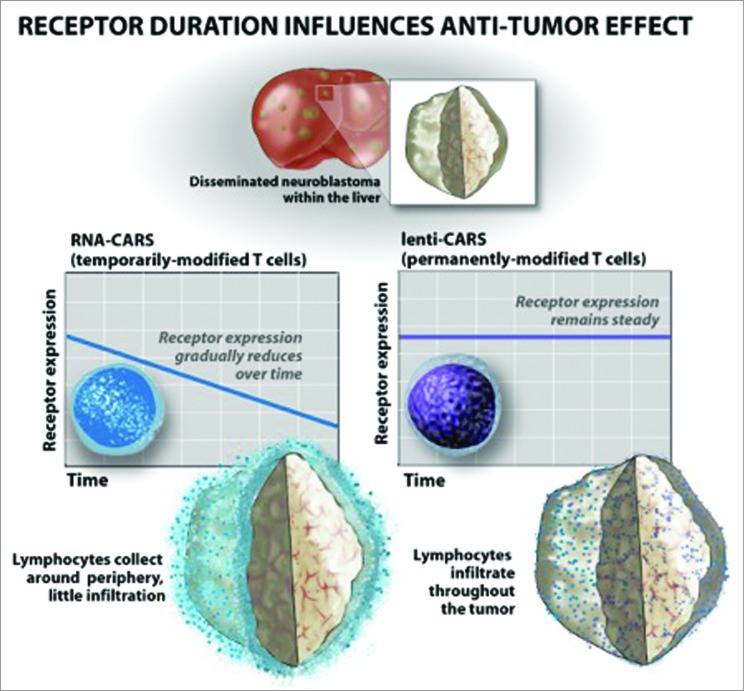
To investigate the interplay between our delivered CAR T cells and the tumor microenvironment, we performed serial immunohistological studies. Examination of excised animal livers, sites of heavy disease burden, demonstrated robust tumor infiltration by lenti CARs within 3 d of infusion. Conversely, RNA CARs are unable to penetrate tumors to any significant degree in the days following cell infusion. Interestingly, serial infusions simply result in ongoing accumulation of RNA CARs at the periphery of the tumor (Fig. 1). This observation highlights the fact that RNA CARs are able to home to tumor sites, suggesting that CAR expression is not immediately lost upon injection, but are unable to penetrate or proliferate in a significant way.
Figure 1.
RNA CARs are unable to penetrate tumor sites, while lenti CARs infiltrate and mediate anti-tumor responses.
While previous studies have demonstrated efficacy of CAR T cells targeting localized solid tumors,7 here we demonstrate long-term control of disseminated disease using systemically delivered therapy. Limited success of RNA CARs has been previously demonstrated, but the etiology remained unknown. The observed difference in efficacy of lenti vs. RNA CARs correlates with a significant disparity in tumor penetration, suggesting that one of the primary, and perhaps the most essential barriers to efficacy of transiently expressed CAR T cells is tumor penetration. We posit that CAR expression kinetics after antigen engagement in vivo play a key role in the differences observed. Enhancing timely delivery of these cells to tumor sites may enhance the efficacy of this therapy, as may prolongation of RNA construct expression.
Pediatric cancer represents a disease group in which safety of experimental therapies is of utmost concern, particularly when considering a therapy that has the potential to remain active within the patient for years. Experience with antibody therapy targeting GD2 has demonstrated significant antitumor activity, but also significant on-target off-tumor side effects in the form of neuropathic pain. While side effects from antibody therapy are not always observed when using cell-based therapies,8 this is a risk few would be willing to take when considering the potential long-term consequences. Thus, in this burgeoning field, we envision a dual role for RNA CARs. First, these cells, engineered with a guaranteed ‘off’ switch, could serve as the backbone for phase I studies of novel CARs, assessing safety and toxicity driven by on-target off-tumor side effects that cannot be modeled in animals. Second, these cells may serve as antitumor therapy in their own right, working differently, and with distinct treatment goals, than their lentiviral counterparts. Moreover, if the barrier of tumor penetration is overcome the efficacy of this therapy may approach that observed with permanently modified CAR cells, creating a platform for safer CAR therapies in the future.
Disclosure of Potential Conflicts of Interest
SAG received a commercial research grant from and is a consultant/advisory board member for Novartis. NS and DMB have no potential conflicts of interest to disclose.
References
- 1. Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. Annu Rev Med 2014; 65:333-47; PMID:; http://dx.doi.org/ 10.1146/annurev-med-060512-150254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heslop HE. Safer CARS. Mol Ther 2010; 18:661-2; PMID: 20357776; DOI: 10.1038/mt.2010.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL, Chew A, Carroll RG, Scholler J, Levine BL, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res 2010; 70:9053-61; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barrett DM, Zhao Y, Liu X, Jiang S, Carpenito C, Kalos M, Carroll RG, June CH, Grupp SA. Treatment of advanced leukemia in mice with mRNA engineered T cells. Hum Gene Ther 2011; 22:1575-86; PMID:; http://dx.doi.org/ 10.1089/hum.2011.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 2011; 118:6050-6; PMID:; http://dx.doi.org/ 10.1182/blood-2011-05-354449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barrett DM, Singh N, Liu X, Jiang S, June CH, Grupp SA, Zhao Y. Relation of clinical culture method to T-cell memory status and efficacy in xenograft models of adoptive immunotherapy. Cytotherapy 2014; 16:619-30; PMID:; http://dx.doi.org/ 10.1016/j.jcyt.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, Varela-Rohena A, Haines KM, Heitjan DF, Albelda SM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A 2009; 106:3360-5; PMID:; http://dx.doi.org/ 10.1073/pnas.0813101106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, Chew A, Zhao Y, Levine BL, Albelda SM, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res 2014; 2:112-20; PMID:; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0170 [DOI] [PMC free article] [PubMed] [Google Scholar]