Abstract
OBJECTIVES: The primary objective of this study was to evaluate whether empirical enoxaparin doses according to Chest guidelines resulted in therapeutic antifactor Xa concentrations in pediatric patients. Secondary objectives were to determine the median enoxaparin dose that resulted in therapeutic anticoagulation, the median time to therapeutic concentrations, and the percentage of patients who experienced major bleeding.
METHODS: Patients in a tertiary medical center who were <18 years of age and received treatment doses of enoxaparin between July 2007 and June 2010 were included. Patients with <2 antifactor Xa concentrations or with only supratherapeutic concentrations and doses that were higher than recommended by the guidelines were excluded. Subgroup analysis was conducted by dividing children into 4 age groups: <2 months of age, 2 months to <1 year of age, 1 year to <3 years of age, and 3 to 17 years of age.
RESULTS: Thirty-two patients were included in the study. Thirty-seven percent of the patients achieved a therapeutic drug level with empirical dosing. The therapeutic dose ranged from 1 to 1.9 mg/kg in patients <1 year old, and 0.6 to 1.5 mg/kg in those =1 year of age. Comparison of the median therapeutic doses for patients 2 months to <1 year to that for patients =1 year old using the Mann-Whitney U test showed the median doses to be significantly difierent between the 2 groups (p=0.01). The antifactor Xa level became therapeutic on day 5 (median). There were no major bleeding events.
CONCLUSION: Less than 40% of patients were therapeutic with empirical dosing, which supports findings from other studies that suggest a need for modification of empirical treatment dosing of enoxaparin in children.
INDEX TERMS: blood coagulation factor inhibitors, child, enoxaparin, infant, low molecular weight heparin
INTRODUCTION
In recent years there has been an increase in the use of low-molecular-weight heparin (LMWH), enoxaparin, to treat pediatric thrombotic conditions. Some of the advantages of LMWH over un-fractionated heparin (UH) are decreased binding of nonspecific proteins, 100% renal elimination, and a more predictable dose response, thus requiring less monitoring than UH.1 Children have a larger volume of distribution and more rapid clearance of enoxaparin than adults.2 The current Chest guidelines, Antithrombotic Therapy in Children, recommend an initial enoxaparin dose of 1.5 mg/kg in children less than 2 months of age and 1 mg/kg in children 2 months and older. Monitoring of the antifactor Xa peak should be performed 4 to 6 hours after administration of the drug, with a goal level of 0.5 to 1 units/mL obtained on 2 consecutive days.3 Some published studies have found antifactor Xa concentrations to be subtherapeutic with these dosage recommendations.4–7
The purpose of this study was to determine whether the doses of enoxaparin as recommended in the 2001 Chest guidelines3 resulted in therapeutic antifactor Xa concentrations in pediatric patients in a tertiary pediatric teaching hospital. We hypothesized that the doses as recommended in the guidelines would result in therapeutic antifactor Xa concentrations in our population. Secondary objectives were to determine the daily enoxaparin dose necessary to achieve concentrations within the desired range when concentrations were not therapeutic with Chest guideline dosing, the number of days required to obtain a therapeutic antifactor Xa level after initiation of enoxaparin therapy, and the occurrence of bleeding events in patients receiving enoxaparin treatment.
MATERIALS AND METHODS
Patient Population
The Institutional Review Board approved this single-center study, and consent was waived due to the retrospective nature of the study. Individuals <18 years of age who received enoxaparin while hospitalized between July 1, 2007, and June 30, 2010, were identified using the hospital database. Patients were included in the study if they received treatment doses of enoxaparin. Patients were excluded if they did not have at least 2 antifactor Xa concentrations reported or if all concentrations were supratherapeutic with doses that were consistently greater than that recommended by the guidelines. The assay used to determine anti-Xa concentrations for LMWH was the STA-Rotachrom heparin assay kit (Stago, Toronto, Ontario, Canada).
Data Collection
A retrospective chart review was performed. Demographic information collected included patients' age, sex, height, and weight. Data recorded were indication for enoxaparin, most recent serum creatinine level prior to treatment initiation, time of drug administration, dose of enoxaparin (total dose and milligram of drug per kilogram of body weight; mg/kg), and anti-factor Xa concentrations. An antifactor Xa level was considered therapeutic at 0.5 to 1 units/mL, drawn 4 to 6 hours after dose administration. If more than 1 dose resulted in a therapeutic antifactor Xa level, the lowest dose was recorded. Patients were evaluated for the occurrence of bleeding. A major bleeding event was defined as bleeding into a major organ or cavity, consistent with the study by Baumann et al.5
Data Analysis
Data were analyzed using descriptive statistics, evaluating the population as a whole and dividing subjects into 4 age groups: <2 months, 2 months to <1 year, 1 year to <3 years, and 3 through 17 years of age. The Mann-Whitney U test was used to compare the median enoxaparin dose at which therapeutic anticoagulation was achieved, with a p value of <0.05 considered statistically significant.
RESULTS
A total of 108 hospitalized patients <18 years of age received enoxaparin from July 2007 through June 2010. Fifty patients were excluded because they received prophylactic doses of enoxaparin, 1 was excluded because enoxaparin doses were greater than guideline doses and all antifactor Xa concentrations were supratherapeutic, and 25 patients were excluded who did not have at least 2 antifactor Xa concentrations. Thirty-two patients were included in the study. The majority of patients were male (63%), and they ranged from 25-days-old to 17.5-years-old. Sixty-nine percent of the patients received the drug for deep vein thrombosis (DVT), 3% for pulmonary embolus (PE), 3% for a combination of DVT and PE, and 25% for other indications. Serum creatinine data were within normal limits for all patients in the study.
Twelve patients (37%) had antifactor Xa concentrations within the therapeutic range with empirical dosing, as recommended by the Chest guidelines, whereas 20 patients (63%) did not. Fifteen patients (47%) had a therapeutic antifactor Xa level at an adjusted dose. Five patients (16%) failed to obtain a therapeutic level prior to discharge, preventing determination of an enoxaparin dose that would result in a therapeutic antifactor Xa level.
The dosing results in the 4 defined age groups are shown in the Table. In the patients <2-months-old, 2-months to <1-year-old, 1 to < 3-years-old, and 3 to 17-years-old, 60%, 20%, 50%, and 38.5%, respectively, obtained therapeutic concentrations with doses according to the guidelines. Therapeutic antifactor Xa concentrations were achieved at an adjusted dose in 40% of patients <2 months, in 70% of patients 2 months to <1-year-old, in 25% of patients 1 year to <3- years-old, and in 38.5% of patients 3 to 17-years-old.
Table.
Dosage Results by Prespecified Age Groups
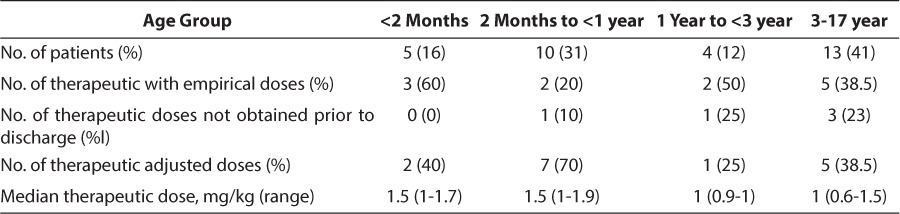
Twenty-seven patients (84%) had at least 1 antifactor Xa concentration between 0.5 and 1 unit/mL. The median dose to achieve a therapeutic antifactor Xa concentration was 1.5 mg/kg in patients <1 year of age and 1 mg/kg in patients 1 to 17-years-old. Because 1.5 mg/kg is an empirical guideline dose for infants <2-months-old, the Mann-Whitney U test was used to compare the median dose of patients 2 months to <1 year old (n=9) to those ≥1 year (n=13), and the difference between the 2 groups was found to be significant, with a p value of 0.01.
In patients with a therapeutic antifactor Xa concentration, the median day on which the concentration became therapeutic was day 5, with a range of day 1 to 37 of therapy. None of the patients experienced a major bleeding event. Only 1 patient experienced an episode of epistaxis, and enoxaparin was discontinued.
DISCUSSION
The overall results of this study found 37% of the patients to be therapeutic with empirical dosing of enoxaparin as recommended in the Chest guidelines, whereas 63% were not. When patients were divided into the 4 age groups, a higher percentage of those <2 months and 1 to <3 years of age obtained therapeutic concentrations at the dose recommended by the guidelines than in the other 2 age groups. This variability may be due in part to differences in the number of subjects in the groups, because the groups containing patients <2-months-old and 1 year to <3-years-old had notably fewer patients than the other 2 groups.
When broken down into the 4 subgroups, the median dose necessary for concentrations to be therapeutic in 3 of the groups, <2 months, 1 year to <3 years, and 3 through 17 years, was consistent with the Chest guidelines. In contrast, the patients in the group of 2-month-old to <1-year-old required a median dose of 1.5 mg/kg, which is higher than the currently recommended dose. Comparison of the median therapeutic dose of patients 2 months to 1 year and those 1 year and older indicated that the difference was significant between the 2 groups, supporting data from other studies that have shown there to be a need for higher dosing of enoxaparin in infants than in children and adolescents.4,5,7 In retrospective, single-center chart reviews, Fung, et al4 found patients <1 month, 1 month to <1 year, 1 to <6 years, and ≥6 years required average doses of 1.8, 1.64, 1.45, and 1.05 mg/kg, respectively, and Bauman et al5 determined that patients 3 to 12 months, 1 to 5 years, and 6 to 18 years of age needed doses of 1.48, 1.23, and 1.13 mg/kg, respectively. Ignjatovic et al7 reported median therapeutic enoxaparin doses in patients <2 months old, 2 months to 1 year, 1 to 5 years, 6 to 10 years, and 11 to 16 years of age of 1.50, 1.34, 1.16, 0.99, and 1.00 mg/kg, respectively. Comparing current study results to those of these other studies, some of the discrepancies may be due to the small patient sample size in our study, reporting of means versus medians, and differences in the ages of the subgroups analyzed.
There was significant variability in the times between initiation of treatment and patients' first therapeutic antifactor Xa concentration assay. Given that 5 of the 32 patients did not have a therapeutic antifactor Xa concentration assayed during their hospitalization, more routine monitoring of concentrations when therapy is initiated could potentially improve the rate at which therapeutic concentrations are reached.
The results of this study are limited by the small population of patients who met all the inclusion criteria, the 5 patients in whom the therapeutic enoxaparin dose was unable to be determined, and the potential for bias as a result of the large number of patients excluded. Some factors that could have contributed to variability in the antifactor Xa concentration results were differences in the amount of time between the blood draw and sample testing, performance of assay evaluation at 2 different laboratories during the study time frame, and differences in the blood sampling methods. The retrospective design and reliance on previously charted electronic data are additional limitations. Because of the unequal and small number of patients in the 4 different age groups, statistical comparison of the median therapeutic doses of the 4 groups of patients was not possible. An additional limitation was that we did not evaluate whether there was actual resolution of the thrombi.
CONCLUSIONS
In conclusion, this small study supports others that suggest a need for modification of empirical treatment dosing of enoxaparin in children. To determine specific dose recommendations, additional studies are needed to evaluate patient response and outcomes when alternative dosage regimens are administered.
ACKNOWLEDGMENTS
This work was presented orally at the Pediatric Pharmacy Advocacy Group Annual Meeting, Memphis, Tennessee, March 18, 2011; as a poster at the University of Maryland School of Pharmacy Research Day, Baltimore, Maryland, March 31, 2011; and orally at the Maryland Society of Health System Pharmacy Resident Research Night, Baltimore, Maryland, May 24, 2011.
ABBREVIATIONS
- DVT
deep vein thrombosis
- LMWH
low-molecular weight heparin
- PE
pulmonary embolus
- UH
unfractionated heparin
Footnotes
Disclosures The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Hirsh J, Warkentin TE, Shaughnessy SG et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(suppl 64S):64S–94S. doi: 10.1378/chest.119.1_suppl.64s. [DOI] [PubMed] [Google Scholar]
- 2.Ho SH, Wu JK, Hamilton DP et al. An assessment of published pediatric dosage guidelines for enoxaparin: a retrospective review. J Pediatr Hematol Oncol. 2004;26(9):561–566. doi: 10.1097/01.mph.0000139453.22338.d9. [DOI] [PubMed] [Google Scholar]
- 3.Monagle P, Michelson AD, Bovill E, Andrew M. Antithrombotic therapy in children. Chest. 2001;119(suppl 1):344S–370S. doi: 10.1378/chest.119.1_suppl.344s. [DOI] [PubMed] [Google Scholar]
- 4.Fung LS, Klockau C. Effects of age and weight-based dosing of enoxaparin on anti-factor Xa concentrations in pediatric patients. J Pediatr Pharmacol Ther. 2010;15(2):119–125. [PMC free article] [PubMed] [Google Scholar]
- 5.Bauman ME, Belletrutti MJ, Bajzar L et al. Evaluation of enoxaparin dosing requirements in infants and children: better dosing to achieve therapeutic levels. Thromb Haemost. 2009;101(1):86–92. [PubMed] [Google Scholar]
- 6.Malowany JI, Monagle P, Knoppert DC et al. Enoxaparin for neonatal thrombosis: a call for a higher dose for neonates. Thromb Res. 2008;122(67):826–830. doi: 10.1016/j.thromres.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Ignjatovic V, Najid S, Newall F et al. Dosing and monitoring of enoxaparin (low molecular weight heparin) therapy in children. Br J Haematol. 2010;149(5):734–738. doi: 10.1111/j.1365-2141.2010.08163.x. [DOI] [PubMed] [Google Scholar]


