Abstract
OBJECTIVES: Delay of antimicrobial administration in adult patients with severe sepsis and septic shock has been associated with a decrease in survival to hospital discharge. The primary objective of this investigation was to determine the time to first antimicrobial administration after the onset of sepsis in critically ill children. Secondary objectives included appropriateness of empiric antimicrobials and microbiological testing, fluid resuscitation during the first 24 hours after onset of sepsis, intensive care unit and hospital length of stay, and mortality.
METHODS: Retrospective, chart review of all subjects less than or equal to 18 years of age admitted to the pediatric intensive care unit (PICU) with a diagnosis of sepsis between January 1, 2011, and December 31, 2012.
RESULTS: A total of 72 subjects met the inclusion criteria during the study period. Median time to first antimicrobial administration by a nurse after the onset of sepsis was 2.7 (0.5–5.1) hours. Cultures were drawn prior to administration of antimicrobials in 91.7% of subjects and were repeated within 48 hours in 72.2% of subjects. Empiric antimicrobial regimens were appropriate in 91.7% of cases. The most common empiric antimicrobial regimens included piperacillin/tazobactam plus vancomycin in 19 subjects (26.4%) and ceftriaxone plus vancomycin in 15 subjects (20.8%). Median PICU length of stay was 129 (64.6–370.9) hours, approximately 5 days, and median hospital length of stay was 289 (162.5–597.1) hours, approximately 12 days. There were 4 deaths during the study period.
CONCLUSIONS: Time to first antimicrobial administration after onset of sepsis was not optimal and exceeded the recommendations set forth in international guidelines. At our institution, the process for treating pediatric patients with severe sepsis and septic shock should be modified to increase compliance with national guidelines.
INDEX TERMS: anti-infective agents, pediatrics, sepsis, septic shock
INTRODUCTION
Severe sepsis and septic shock are significant health problems in children. Over 20,000 children will develop septic shock annually, with a hospital mortality rate of 10.3%.1 Prompt recognition and treatment of severe sepsis and septic shock is vital to enhancing the probability of survival.
The 2012 Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock outline a series of goal-directed therapeutic end points that should be achieved within a specific time from the identification of severe sepsis or septic shock.2 One of the key recommendations of these guidelines is to administer effective intravenous antimicrobial agents within 1 hour of recognition of septic shock and severe sepsis without septic shock.2 Many of these end points are consistent with the 2007 Clinical Practice Parameters for Pediatric and Neonatal Septic Shock, which emphasize stepwise, goal-directed management of sepsis.3 Early, goal-directed therapy provides significant benefits to patient outcomes, including mortality, when utilized in the treatment of adult patients with severe sepsis.4,5 In pediatric patients, adherence to sepsis guidelines reduced hospital length of stay by 57%.6 Previous studies have identified negative consequences of delays in treatment, including an increase in mortality in adult patients with sepsis. The delay of antimicrobial administration, particularly appropriate antibiotics, in severe sepsis and septic shock has been associated with a decrease in survival to hospital discharge.5,7
A previous study at our institution has identified shortcomings in the treatment of pediatric septic shock, including a delay in antimicrobial administration of over 7.5 hours in patients requiring vasopressor therapy.8 Our institution is located within a larger academic medical center in a major city and contains 32 general inpatients, 24 pediatric intensive care units (PICUs), 30 neonatal intensive care units (NICUs), and 10 NICU step-down beds. The treatment of severe sepsis and septic shock in pediatric patients is currently directed by an individual provider's orders. While computerized physician order entry (CPOE) exists, no structured care plan or order set is available for the management of pediatric sepsis.
The primary objective of this investigation was to determine the time to first antimicrobial administration after the onset of sepsis in critically ill children admitted to the PICU. Secondary objectives included the appropriateness of empiric antimicrobials and microbiological testing, time to appropriate antimicrobial administration after the onset of sepsis, time to administration of first and appropriate antimicrobials after initial triage, fluid resuscitation during the first 24 hours after onset of sepsis, and intensive care unit and hospital length of stay.
MATERIALS AND METHODS
This was a retrospective, chart review that was approved by the University of Maryland, Baltimore Institutional Review Board with informed consent waived. Eligible subjects included all of those less than or equal to 18 years of age admitted to the PICU with a diagnosis of sepsis between January 1, 2011, and December 31, 2012. Subjects were selected during the study period by identifying those with admission and/or discharge International Classification of Diseases, Ninth Revision (ICD-9) codes for septicemia (038.0–038.9), severe sepsis (995.92), or septic shock (785.52).
Data were extracted from the electronic medical record, which included electronic medication administration record, daily flow sheets, and emergency department (ED) triage documents. Demographic data collected for each subject included age (years), sex, past medical history, and date and time of admission and discharge from hospital and PICU. For the purposes of this study, the time of onset of sepsis for each subject was defined as the time at which the first fluid bolus was ordered upon admission. Time of triage was defined as the date and time of the first recorded vital signs on the admission flow sheet. For initial fluid orders, data recorded included the name of fluid ordered, quantity, date and time of physician order, date and time of pharmacist verification, and date and time of administration. To quantify initial fluid resuscitation during the first 24 hours from admission, the subject's daily flow sheet was reviewed and all intravenous fluid boluses were recorded.
For antimicrobial therapy, data recorded included the name of antimicrobial agent ordered, date/time of physician order, date/time of pharmacist verification, and date/time of administration to the subject. Time to first antimicrobial administration from sepsis onset was calculated as the time from the first fluid bolus order to the time that the first antimicrobial was administered. Time to appropriate antimicrobial administration was calculated as the time from first fluid bolus order to the time that the first appropriate antimicrobial, as defined below, was administered. Primary site of infection was determined by manual review of the subject's medical record and progress notes. Microbiologic data included the date/time of initial cultures, date/time of any repeat cultures within the first 48 hours from hospital admission, and the identification and susceptibility information for all positive cultures. The 2012 Surviving Sepsis Campaign Guidelines recommend initial empiric anti-infective therapy of 1 or more drugs that have activity against all likely pathogens and that penetrate in adequate concentrations into tissues presumed to be the source of infection.2 For the purposes of this investigation, appropriate empiric antimicrobial treatment was defined as the microbiological documentation of an infection (positive culture result) that was being effectively treated based on in vitro susceptibility results at the time of its identification. For patients without microbiological documentation of an infection (no positive culture result) appropriateness of empiric antimicrobial regimen was determined by review of the subject's medical record for pertinent risk factors such as age, presence of immunosuppression, history of colonization with an organism, history of previous infection with a multidrug resistant organism, and likely source of infection. Length of stay in hospital and PICU were calculated for each subject and survival time to hospital discharge was calculated as well.
All data were analyzed using descriptive statistics, which included means and standard deviations for parametric continuous data and medians and interquartile ranges (IQRs) for non-parametric continuous data. Student's t-tests were used to compare means, and Mann-Whitney U tests were used to compare medians. Chi-square and Fisher's exact test were used to compare demographic data. Statistical analyses were performed using Microsoft Excel 2011 (Microsoft Corporation, Redmond, WA).
RESULTS
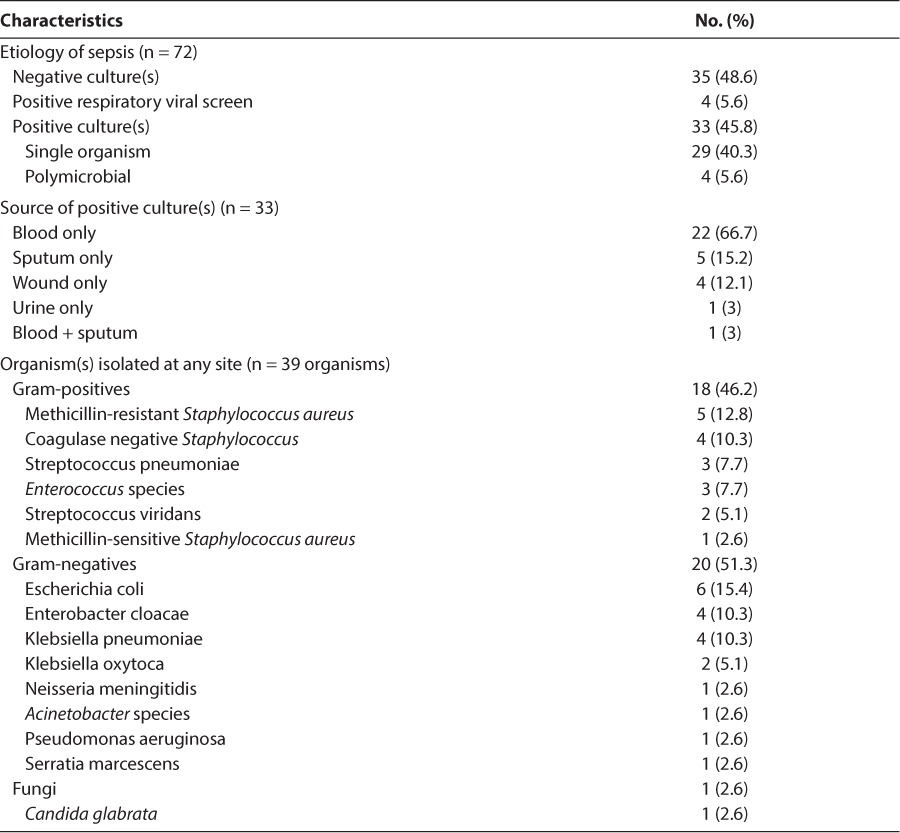
A total of 72 subjects met the inclusion criteria during the study period and were analyzed. Subjects had a median (IQR) age of 5 years (0.9–16.2). Median (IQR) height was 97.5 cm (65.5–138.8), and median (IQR) weight was 15.6 kg (7.1–34.7). Forty-seven (65.3%) of subjects were male. The primary site of infection for each subject is summarized in Table 1. The majority of subjects (70.8%) had at least 1 prior medical condition diagnosed before their admission for sepsis. Microbiological data for the study population are summarized in Table 2. Of the 4 subjects who had multiple organisms isolated, 2 subjects had multiple gram-negative organisms and 2 subjects had both gram-positive and gram-negative organisms. Four subjects (5.6%) died during the study period. Two (2.8%) subjects did not have an organism identified, and 2 (2.8%) subjects had 1 or more organisms identified. Of the 2 subjects who died with 1 or more organisms identified, 1 subject had a positive blood culture for Pseudomonas aeruginosa and Serratia marcescens and the other subject had a positive blood culture for Neisseria meningitides.
Table 1.
Demographics of Study Population (n = 72)
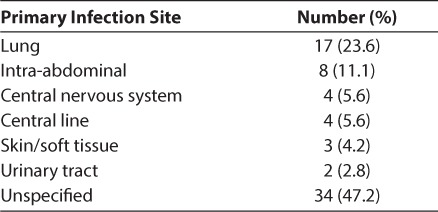
Table 2.
Microbiologic Data of Study Population
Outcome data are summarized in Table 3. For the primary outcome, median time from first fluid bolus order to first antimicrobial administration was 2.7 hours (0.5–5.1). Median time from triage to first antimicrobial administration was 5.3 hours (1.3–23.8). Twenty-four subjects (33.3%) received their first dose of antimicrobials within 1 hour of sepsis onset. Time to first antimicrobial administration was analyzed based on the time of day that the subject was admitted to the hospital. Subjects were divided into 2 groups, those admitted during the daytime (between 7 am and 6:59 pm) and those admitted during the night (between 7 pm and 6:59 am). Median time to first antimicrobial administration during the daytime was 2.9 hours (0.3–5.9) and during the night was 2.7 hours (1.4–4.7) (p = 0.87).
Table 3.
Management of Sepsis in Study Population (n = 72)
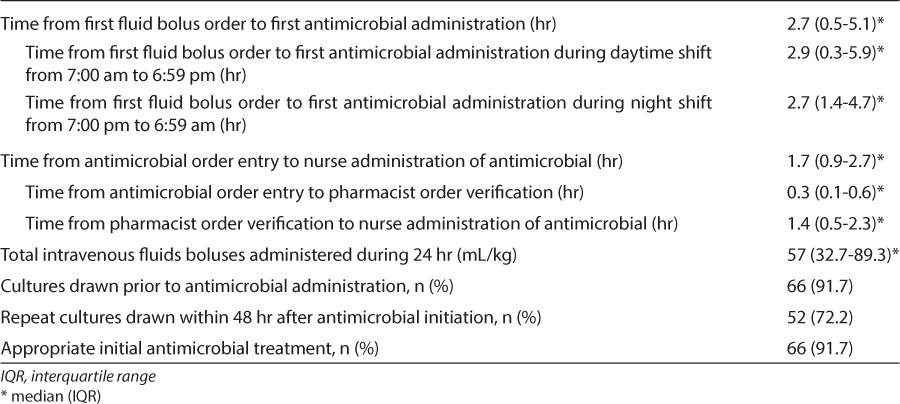
Sixty-six subjects (91.7%) had a culture drawn from any site prior to the initiation of antimicrobials. Of the 72 empiric antimicrobial regimens analyzed, 66 (91.7%) were deemed appropriate and 6 (8.3%) were not. Four of these regimens were deemed inappropriate based on isolation of an organism that was not covered by the empiric antimicrobial regimen. The 2 remaining regimens were deemed inappropriate based on the review of the subjects' past medical history and identification of specific risk factors that made the empiric antimicrobial regimen inadequate. Median time from triage to appropriate antibiotics was 5.6 hours (1.6–30.4) and time from first fluid bolus to appropriate antibiotics was 3 hours (0.6–6.6). The antimicrobial agents included as part of initial sepsis regimens are summarized in Table 4. Empiric antimicrobial therapy for 16 subjects consisted of monotherapy and for 56 subjects consisted of combination therapy with 2 or more antimicrobials. The most common antimicrobial combinations included piperacillin/tazobactam plus vancomycin in 19 subjects (26.4%) and ceftriaxone plus vancomycin in 15 subjects (20.8%).
Table 4.
Antimicrobials Included As Part of Initial Sepsis Regimen for Study Population

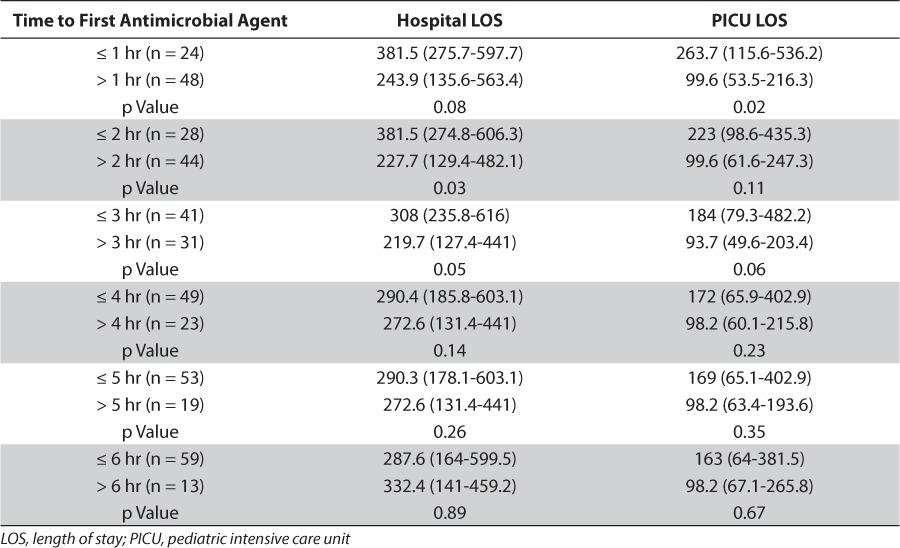
Median (IQR) PICU length of stay was 129 hours (64.6–370.9), approximately 5 days, and median (IQR) hospital length of stay was 289 hours (162.5–597.1), approximately 12 days. Length of stay in both the PICU and hospital were analyzed based on when subjects received their first antimicrobial agent. These data are summarized in Table 5.
Table 5.
Impact of Time to First Antimicrobial Administration on Length of Stay
DISCUSSION
Prompt recognition and treatment of sepsis is imperative to optimizing patient outcomes and survival. Our study found a median of 2.7 hours elapsed between the time the septic subject received their first fluid bolus order to the time they received their first antimicrobial. International guidelines for the management of sepsis, both in pediatric and adult subjects, advocate for administration of effective antimicrobial agents within 1 hour of sepsis presentation.2,3 In our study population, 24 subjects (33.3%) received antimicrobial agents within 1 hour of their first fluid bolus. The time of admission, either during the day or at night, did not impact the time to first antibiotic administration. Recently published data suggest that compliance with sepsis guidelines may be higher at night, but our study did not find a difference.9 In adult subjects, each hour delay in administration of effective antimicrobial agents is associated with an approximate 7.6% increased risk of mortality.7 Our study included subjects from sepsis onset, which we defined as the time at which subjects received their first fluid bolus order. Although it is difficult to estimate the time of actual sepsis onset, as fluid boluses are generally a first-line intervention for managing sepsis, the approximate time at which subjects received their first bolus order should be an indicator that the provider was preparing to treat sepsis. Since antimicrobial therapy should also be a part of empiric sepsis management, they should be implemented shortly after the diagnosis of sepsis is considered in an effort to reduce progression to severe sepsis and septic shock.
Empiric antimicrobial therapy was deemed appropriate in 66 (91.7%) subjects and inappropriate in 6 (8.3%). Four of the empiric antimicrobial regimens were deemed inappropriate based on the isolation of an organism that was not covered by the empiric regimen. Two subjects grew strains of Enterococcus species for which they were not being covered for, 1 subject had a strain of multidrug resistant Escherichia coli, and the fourth subject had grown Candida glabrata and was not receiving antifungal therapy empirically. Of the remaining 2 subjects with inappropriate empiric antimicrobial regimens, 1 subject had a history of colonization with multidrug resistant organisms for which they were not currently being covered and the second subject was immunosuppressed and did not have adequate antipseudomonal coverage. Almost all (91.7%) of the subjects had blood cultures drawn prior to antimicrobial administration. Although the goal for all subjects would be to obtain necessary cultures prior to initiation of antimicrobial therapy, if there is difficulty obtaining cultures, then antimicrobial therapy should not be delayed for this reason alone. Repeat cultures were only done in 72.2% of subjects, which could be due to rapid recovery or that sepsis was ruled out and no further workup was warranted.
Four subjects died during our study period. Overall, severe sepsis mortality in this study (5.6%) was lower than that reported (10.3%) in epidemiologic data of pediatric severe sepsis.1 Two subjects did not have an organism; however, their empiric antimicrobial regimen was deemed to be appropriate. The remaining 2 subjects did have organisms identified, which included Pseudomonas aeruginosa and Serratia marcescens in 1 subject and Neisseria meningitidis in the other, and were considered to be on appropriate empiric antimicrobial therapy. Three of the 4 subjects, however, did not receive their initial antimicrobial therapy until approximately 2.5 hours after the onset of sepsis. Although this study did not address this association, as discussed above, delays in effective antimicrobial therapy can impact the risk of mortality. The remaining subject did receive their initial antimicrobial therapy within 1 hour of sepsis onset.
Median PICU length of stay was significantly longer in patients who received their first antibiotic within 1 hour of sepsis onset compared to subjects who received their first antibiotic greater than 1 hour of sepsis onset. Similarly, hospital length of stay was significantly longer in patients who received their first antibiotic ≤ 2 hours and ≤ 3 hours from sepsis onset compared to > 2 hours and > 3 hours, respectively. The findings of our study seem counter to expectations and it was unclear why this occurred. A possible explanation for this could be that the caring provider could have deemed subjects who received antimicrobials within 1 hour as sicker and therefore their care was expedited. We did not assess disease severity, so we could not test this hypothesis. Gaieski et al5 also suggested that in their study they could not rule out the possibility that sicker patients received antibiotics sooner. Paul et al6 reported that 88 (70%) of subjects studied received an antibiotic within 60 minutes, and when combined with compliance with other sepsis bundle end points, a 57% reduction in hospital length of stay was reported. With the relatively small sample size we had and low number of subjects (n = 24, 33.3%) receiving antibiotics within 1 hour, we may not have been able to demonstrate the impact that time to antibiotic administration could have on length of stay in a larger sample. In addition to disease severity, we also did not assess eligibility for goal-directed therapy as has been done previously.5 It is possible that not all subjects were candidates for early goal-directed therapy; however, they were included in our analysis based on our inclusion criteria of ICD-9 codes reflective of sepsis.
Our study did have limitations. As a retrospective analysis, the exact time at which the onset of sepsis occurred, or when the provider was considering sepsis as a diagnosis, had to be estimated. This could affect how time to first antimicrobial administration influences PICU and hospital length of stay. Median time from triage to first antimicrobial was 5.3 hours (1.3–23.8) where median time from first fluid bolus to first antimicrobial was 2.7 (0.5–5.1), indicating that there was a delay between triage and first fluid bolus. This delay could be multifactorial, including delayed intravenous access or a delay in the senior provider (i.e. attending) assessment of the patient. One of the challenges noted with pediatric sepsis management is the actual recognition of sepsis.10,11 Signs and symptoms of pediatric sepsis vary from that of adult subjects, particularly with regard to pediatric subjects' ability to maintain their blood pressures until very late stages of sepsis. Provider inexperience may cause additional delays in the implementation of time-sensitive therapies and could impact outcomes including length of stay and mortality. At our institution, there is no protocol for triaging a septic patient, which other studies have reported to be beneficial in implementing early goal-directed therapy.10,11 Secondly, we relied on ICD-9 codes to identify patients with sepsis. It is possible that not all subjects included in our data analysis were candidates for early goal-directed therapy and therefore their treatments may not be consistent with guidelines we used to assess our end points. Lastly, the provision of care for a septic subject involves multiple steps and health-care team members. Our study took a more global view of the time to initiation of antimicrobial therapy. There are many steps that occur from drug order entry to drug administration. Once an order is entered, it needs to be verified by a pharmacist, prepared by a pharmacy technician or pharmacist, delivered to the patient's unit, and then administered by a nurse or other qualified healthcare team member. Our data found the longest delay in this process occurred from time of antimicrobial order verification by a pharmacist to antimicrobial administration by a nurse. Further investigations should be directed toward analyzing the various steps of the process in an effort to identify and resolve barriers to implementing time-sensitive interventions.
A previous study at our institution specifically involving subjects with septic shock requiring vasopressors identified more than a 7-hour delay in the administration of effective antimicrobial agents from the time of vasopressor initiation.8 The present study included all patients with sepsis, not just those requiring vasopressors, and used an earlier definition of time of sepsis onset. Although our study reported an improved median time to first antimicrobial agent (2.7 hours), only 33.3% of subjects received an antimicrobial agent within 1 hour of sepsis onset. At the time of the previous study, CPOE was being implemented and unfamiliarty with the system could have led to delays in treatment. Since the previous study, more providers have become comfortable with the CPOE system, standardized order sentences for treatments (fluids, antibiotics, vasopressors) have been created, and functions such as “STAT” orders have been linked to time-sensitive interventions. One area that we have not addressed is improving the recognition of sepsis and triaging septic patients. Studies from pediatric EDs that have implemented protocols for triaging and treating septic patients have reported a reduction in time to administration of drug therapy, decreased lengths of stay, and decreased ED and hospital costs.10,11 Our study did identify delays in triage to first antimicrobial administration, and a more critical evaluation of the triage process should be performed.
The results of our study suggest that time to first antimicrobial administration after onset of sepsis is not optimal at our institution and exceeds the recommendation set forth in international guidelines. Despite this, time to first antimicrobial administration did not have an impact on outcomes such as mortality or length of stay in a manner consistent with previous studies.5,6,9 The delay in implementation of antimicrobial therapy may be the result of many factors including triage and the process from antimicrobial order entry to antimicrobial administration. Institutions caring for pediatric patients should review their processes for managing sepsis, and future investigations should be directed toward identifying and correcting barriers to rapid implementation of life-saving interventions, particularly effective antimicrobial therapy, in pediatric patients suffering from sepsis.
ACKNOWLEDGMENT
Results of this study were presented at the 21st Pediatric Pharmacy Advocacy Group (PPAG) Annual Meeting in Houston, Texas, on April 19, 2012.
ABBREVIATIONS
- CPOE
computerized physician order entry
- ED
emergency department
- ICD-9
International Classification of Diseases Ninth Revision
- IQR
interquartile range
- NICU
neonatal intensive care unit
- PICU
pediatric intensive care unit
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Watson RS, Carcillo JA, Linde-Zwirble WT et al. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167(5):695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 2.Dellinger RP, Levy MM, Rhodes A et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 3.Brierley J, Carcillo JA, Choong K et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37(2):666–688. doi: 10.1097/CCM.0b013e31819323c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivers E, Nguyen B, Havstad S et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 5.Gaieski DF, Mikkelsen ME, Band RA et al. Impact of time to antibiotics on survival in patients with severe sepsis or spetic shock in whom early goal-directed therapy was initiated in the emegency department. Crit Care Med. 2010;38(4):1045–1053. doi: 10.1097/CCM.0b013e3181cc4824. [DOI] [PubMed] [Google Scholar]
- 6.Paul R, Neuman MI, Monuteaux MC et al. Adherence to PALS sepsis guidelines and hospital length of stay. Pediatrics. 2012;130(2):e273–e280. doi: 10.1542/peds.2012-0094. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Roberts D, Wood KE et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 8.LaRochelle J, Morgan JA, Parbuoni KA. Retrospective analysis of the initiation of antimicrobial therapy in severe sepsis in pediatric patients. J Pediatr Pharmacol Ther. 2009;14(4):221–225. doi: 10.5863/1551-6776-14.4.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almeida M, Ribeiro O, Aragão I et al. Differences in compliance with Surviving Sepsis Campaign recommendations according to hospital entrance time: day versus night. Crit Care. 2013;17(2):R79. doi: 10.1186/cc12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruz AT, Perry AM, Williams EA et al. Implementation of goal-directed therapy for children with suspected sepsis in the emergency department. Pediatrics. 2011;127(3):e758–e766. doi: 10.1542/peds.2010-2895. [DOI] [PubMed] [Google Scholar]
- 11.Larsen GY, Mecham N, Greenberg R. An emergency department septic shock protocol and care guideline for children initiated at triage. Pediatrics. 2011;127(6):e1585–e1592. doi: 10.1542/peds.2010-3513. [DOI] [PubMed] [Google Scholar]


