DNA damage signaling is a critical component of the regulatory network that protects cells from genome instability and cell death. In an elegant study published in Cell Cycle,1 Hanzlikova and colleagues have investigated the role of several critical protein components of this signaling network. In particular, this new work resolves the controversy concerning the impact of the protein DAXX (Death-Associated Protein 6) on p53 transcriptional activity and identifies a novel mechanistic facet of DAXX regulation. Numerous biological roles have been assigned to DAXX, which possesses histone chaperone activity and is a transcriptional regulator. However, the role of DAXX as a modulator of p53-mediated transcriptional activity and apoptosis has become the most controversial one, with a variety of published reports presenting conflicting evidence.2 Here, in a technically elegant and comprehensive series of experiments, Brazina et al.1 resolve this controversy and demonstrate unequivocally in their experimental model that DAXX has little if any role in p53-mediated transcriptional activation.
While the new work refutes a role for DAXX in regulating p53, it supports a role for DAXX in signaling networks regulated by p53. For example, Brazina et al.1 neatly demonstrate that DAXX is a target of the same regulatory feedback loop that activates p53 after DNA damage, which is driven by the opposing “Yin and Yang” activities of the protein kinase ATM and the protein phosphatase Wip1 (PPM1D) (Fig. 1). Brazina et al.1 confirm that DAXX is rapidly phosphorylated at Ser564 in an ATM-dependent manner following DNA damage. Moreover, they demonstrate that this phosphorylation event is subsequently reversed by Wip1 phosphatase, thereby restoring DAXX phosphorylation status at Ser564 to a pre-DNA damage ‘ground state’. The importance of ATM and Wip1 to genome stability and cancer is well established, with the former functioning as a classical tumor suppressor and the latter as a classical oncoprotein.3 For example, while ATM is mutated in multiple cancers, Wip1 is required for cellular transformation and is amplified in breast cancer. The regulatory roles played by the ATM/Wip1 axis in controlling protein phosphorylation are thus critical determinants of genome stability in response to endogenous and exogenous genotoxic stress, and the current work identifies DAXX as a novel protein target of this axis.
Figure 1.
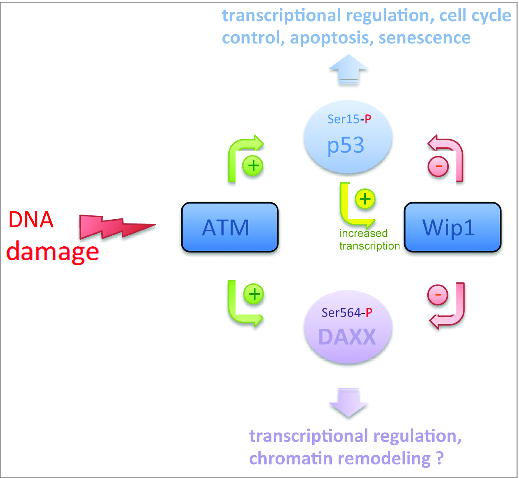
Regulation of DAXX phosphorylation by the ATM/Wip1 Axis. ATM protein kinase and Wip1 protein phosphatase promote (green arrows) or reduce (red arrows) phosphorylation of p53 and DAXX, respectively. Notice the negative feedback loop linking p53 to Wip1 (yellow arrow) and by which p53 is autoregulated.
Like most interesting studies, the work by Hanzlikova and colleagues raises a number of important additional and unanswered questions, not least of which is what is the molecular role of DAXX phosphorylation? The authors speculate that the phosphorylation status of Ser564 may modulate interaction with one or more protein partners of DAXX, perhaps in combination with one or more other phosphorylated residues or protein modifications. This is a plausible scenario and is consistent with other phosphorylation events that are controlled by the ATM/Wip1 axis. For example, the phosphorylation status of p53 at Ser15, which is similarly regulated by ATM/Wip1, influences binding by the E3 ubiquitin ligase Mdm2 and thereby modulates the stability and transcriptional activity of p53.4 The identities of the DAXX protein partners that might be affected by phosphorylation of Ser564 are unclear, but their identification will likely yield important insights into the critical role(s) of DAXX during the cellular response to DNA damage. Given the importance of chromatin remodeling in the DNA damage response, and the activity of DAXX as a histone chaperone, protein partners that are regulators of chromatin structure are plausible candidates. The observation that DAXX interacts with several effectors of chromatin remodeling, including ATRX and both histone acetyltransferase and deacetylase activities2 is consistent with this idea, and should be a fruitful avenue for future investigation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Brazina J, et al. DNA damage-induced regulatory interplay between DAXX, p53, ATM kinase and Wip1 phosphatase. Cell Cycle. 2014; 14(3):397-91; http://dx.doi.org/ 10.4161/15384101.2014.988019 [DOI] [Google Scholar]
- 2. Salomoni P, Khelifi AF. Trends Cell Biol 2006; 16:97-104; PMID:16406523; http://dx.doi.org/ 10.1016/j.tcb.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 3. Zhu Y-H, Bulavin DV. Prog Mol Biol Transl Sci 2012; 106:307-25; PMID:22340722; http://dx.doi.org/ 10.1016/B978-0-12-396456-4.00001-8 [DOI] [PubMed] [Google Scholar]
- 4. Eliaš J, et al. Biochim Biophys Acta 2014; 1844:232-47; PMID:24113167; http://dx.doi.org/ 10.1016/j.bbapap.2013.09.019 [DOI] [PubMed] [Google Scholar]


