Abstract
Retinal degenerative diseases, e.g. retinitis pigmentosa, with resulting photoreceptor damage account for the majority of vision loss in the industrial world. Animal models are of pivotal importance to study such diseases. In this regard the photoreceptor-specific toxin N-methyl-N-nitrosourea (MNU) has been widely used in rodents to pharmacologically induce retinal degeneration. Previously, we have established a MNU-induced retinal degeneration model in the zebrafish, another popular model system in visual research.
A fascinating difference to mammals is the persistent neurogenesis in the adult zebrafish retina and its regeneration after damage. To quantify this observation we have employed visual acuity measurements in the adult zebrafish. Thereby, the optokinetic reflex was used to follow functional changes in non-anesthetized fish. This was supplemented with histology as well as immunohistochemical staining for apoptosis (TUNEL) and proliferation (PCNA) to correlate the developing morphological changes.
In summary, apoptosis of photoreceptors occurs three days after MNU treatment, which is followed by a marked reduction of cells in the outer nuclear layer (ONL). Thereafter, proliferation of cells in the inner nuclear layer (INL) and ONL is observed. Herein, we reveal that not only a complete histological but also a functional regeneration occurs over a time course of 30 days. Now we illustrate the methods to quantify and follow up zebrafish retinal de- and regeneration using MNU in a video-format.
Keywords: Cellular Biology, Issue 92, N-methyl-N-nitrosourea (MNU), retina, degeneration, photoreceptors, Müller cells, regeneration, zebrafish, visual function
Introduction
Vision is the most essential sense for the human being and its impairment has a high socio-economic impact. In the developed world, retinal degenerative diseases are the leading cause of vision loss and blindness among older adults 1. The cause of most degenerative retinal diseases is only partly understood and therapeutical solutions to regain lost vision are very limited. Retinitis pigmentosa is a typical example of a retinal degenerative disease with primary photoreceptor loss 2-3. N-methyl-N-nitrosourea (MNU) induces retinal degeneration and is therefore widely used in rodents to model diseases with primary photoreceptor cell death 4. It is an alkylating agent and leads to benign and malignant tumors, which usually appear several months after exposure 5-7. In addition, it causes specific photoreceptor cell death within a short term observation period. Thereby, the loss of the retinal layer structure and significant retinal thinning were observed in a concentration-dependent manner. Retinal glia cells were activated, but no changes in the retinal pigment epithelium (RPE) were found. Endoplasmic reticulum (ER) stress-related apoptosis appears to be the main pathway of MNU action in the retina 8.
We have recently introduced MNU as a chemical model to induce photoreceptor degeneration in zebrafish 9. Amongst other reasons, the zebrafish (Danio rerio) has become important in visual research because of the similarities of its visual system to that of other vertebrates 10. The outer retina contains the photoreceptors, which can be grouped into four different cone types with peak sensitivities in the ultraviolet, short, middle, and long wavelength of the visible spectra and one rod photoreceptor type. In the inner nuclear layer (INL), the cell bodies of bipolar, horizontal, and amacrine interneurons are found, as well as the cell soma of Muller glia cells. In the outer plexiform layer (OPL) the synaptic contacts between photoreceptors and the inner retina are formed, whereas the cell layer closest to the lens is the ganglion cell layer (GC), which components form long axons comprising the optic nerve and the optic tract. Synaptic contacts between ganglion cells and the cells in the inner nuclear layer are formed in the inner plexiform layer (IPL) 11. The RPE lies outside the neurosensory retina and surrounds the photoreceptor outer segments with long apical microvilli 12. Furthermore, the zebrafish is highly regenerative and able to regrow lesioned brain, retina, spinal cord, heart, and other tissues 13. When retinal damage occurs, cells in the INL, which are thought to be Müller cells, are activated and have the potential to differentiate into various retinal cell types. In addition, they also generate rod progenitors, which are located in the ONL. Another source that supplies the retina of adult zebrafish with new cells is the ciliary marginal zone. This source is needed to achieve a constant density of rod photoreceptors in the continuously growing zebrafish eye 14.
The MNU model can be used as a simple and reproducible degeneration/regeneration approach for retinal tissue. Due to certain similarities of biological processes in zebrafish and in humans this could open the doors to identify involved cell death pathways and to screen potential neuroprotective drugs. Based on a previous study from our group, we now illustrate the methods of this MNU-induced zebrafish model of retinal de- and consequent regeneration including functional changes with according laboratory videos 9.
Protocol
All experiments adhered to the Statement for the Use of Animals in Ophthalmic and Vision Research of the Association for Research in Vision and Ophthalmology (ARVO).
1. Animals
Maintain the wild-type zebrafish (Danio rerio) of the AB (Oregon) strain aged between 6-12 months under standard conditions in water with a temperature of 26.5 °C and a 14/10 hr light/dark cycle 15.
Follow the animal care guidelines of the involved institutions for the animal experiments after approval by the Cantonal Veterinary Office.
2. MNU Treatment
Prepare water containing 150 mg/L dry substance of N-methyl-N-nitrosourea (MNU). CAUTION! MNU is toxic; may cause cancer, heritable genetic damage or harm to the unborn child.
Incubate zebrafish in water containing the MNU for 60 min at room temperature.
Rinse zebrafish quickly with fresh water and put the fish to a new fish tank without MNU. Maintain fish under standard conditions as long as desired for the experiments.
3. Visual Acuity Measurement
Start the optomotor system, choose 'testing' from the menu and set the options to “Simple Staircase”, “Acuity (Freq)” and “Randomized/Separate” 16.
Install an infusion bottle filled with 500 ml water about 1 m above the optomotor system.
Place one zebrafish in the examination chamber and connect it to the infusion bottle. Place the examination chamber in the optomotor system.
Start the measurement and observe the eye movement in real time on the computer screen. Thereby, a positive response (“yes”) is defined as consecutive saccades in the correct direction, whereas a negative response (“no”) represents random eye movements similar to the ones observed with stationary gratings. If the eyes of the zebrafish exhibit three or more subsequent optokinetic responses (OKR), press 'yes'; if not, press 'no'.
Extract the visual acuity, which has been calculated by the software by determining the threshold of the spatial frequency of the optokinetic stimulus, under “Results” in the menu.
4. Histology
Euthanize the zebrafish by an overdose of ethyl 3-aminobenzoate methanesulfonate (200-300 mg/L) and enucleate the eyes.
Fix the whole eyes in 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS) at 4 °C for 12 hr and then dehydrate the samples in a graded alcohol series.
Embed the samples in paraffin, cut 5 µm sections through the optic nerve head (ONH) and mount them on a glass slides.
Stain the deparaffized paraffin sections with hemalum for 4 min and dip the slides two times in distilled water followed by 0.2% hydrochloric acid and 0.8% ammonia. Stain the sections with eosin for 3 min after development of the hemalum staining in tap water for at least 10 min (H&E).
Embed the dehydrated slides in mounting medium and observe the slides under the light microscope.
5. Immunohistochemistry
- Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
- Incubate the deparaffinized sections with 20 µg/ml proteinase K at room temperature for 15 min. Wash the sections three times with Tris buffered saline (TBS) for 5 min each.
- Incubate the sections with 50 µl TUNEL reaction mixture (10% labeling solution and 90% enzyme solution) in a humidified chamber at 37 °C for 60 min. Wash three times with TBS for 5 min each.
- Mount the slides with mounting medium containing DAPI and observe the slides under the fluorescence microscope.
- Proliferating Cell Nuclear Antigen (PCNA) staining
- Boil the deparaffinized sections in antigen retrieval buffer (Tris-EDTA + 0.05% Tween 20, pH 9.0) for 3 min and wash three times with TBS for 5 min each. Block with 100 µl blocking solution (TBS + 10% goat normal serum + 1% bovine serum albumin, pH7.6) at room temperature for 1 hr.
- Stain with anti-PCNA primary antibodies in a 1:200 dilution in a humidified chamber at 4 °C overnight. Wash three times with TBS + Tween 20 for 5 min each.
- Finish the detection with the appropriate secondary antibodies in a 1:500 dilution at room temperature for 1 hr.
- Mount the slides with mounting medium containing DAPI and observe the slides under the fluorescence microscope.
Representative Results
Visual acuity:
The experimental set-up [spatial frequency: 0.042 circles/degree (c/d); contrast: 100%; drift speed: 20 degrees/second (d/src); back light luminance: 152 cd/m2] of this study enabled OKR assessment of adult zebrafish. The mean duration of VA measurement was about 5 – 10 minutes for each zebrafish, which tolerated the procedure well. Visual acuity before MNU exposure was 0.577 ± 0.014 cycles/degree (c/d). Figure 1 shows the visual acuity course after the application of 150 mg/L MNU. Starting from day 1, the measurements revealed a marked decrease in visual acuity, reaching minimum values at day 3. Beginning from day 8, an increase of visual acuity occurs, showing a full recovery of visual acuity 30 days after MNU exposure. For statistical analysis a one-way ANOVA followed by Bonferroni‘s multiple comparison test has been applied. Thereby, the differences in visual acuity between baseline and measurements after treatment was significant for days 1, 3, 5, and 8 (p <0.001, each), but not for days 15 and 30.
Histological assessment of the retinal degeneration:
H&E staining was used to quantify morphological changes in one eye of the zebrafish per time point (n = 3) at baseline as well as 3, 8, 15, and 30 days after treatment (Figure 2). Histology was performed as described by Tappeiner et al.9. Thereby, retinal degeneration including disruption of the INL and cavity formation of the ONL was observed starting at day 3. The number of cells in the ganglion cell layer (GC), the inner nuclear layer (INL) and the number of rod (RN) and cone (CN) photoreceptors were determined manually 250 µm from the center of the ONH on both sides of the eye section (size of the counted area refers to a retinal section of 100 µm length). The degeneration was followed for 30 days after treatment with the maximum of rod ONL loss found on day 8. Thereby, the number of rod photoreceptors (RN) decreased to 82% on day 3, 71% on day 8 and 77% on days 15 and 30 of the original number. Furthermore, accumulation of cell clusters was found, mainly in the INL.
Quantification of apoptosis:
TUNEL staining was performed according to the manufacturer (In situ Cell Death Detection Kit; Roche Applied Sciences, Rotkreuz, Switzerland). The observed retinal degeneration after MNU treatment was caused by apoptosis. This was revealed by positive TUNEL staining in different cell layers in the sensory retina. For quantification, the number of TUNEL-positive cells was determined manually at two retinal areas of 450 μm length each, starting at the periphery of the retina. Thereby, the majority of TUNEL-positive cells was found in the ONL at day 3. However, dying cells were also seen in the INL at that time point (Figure 3). No relevant TUNEL-positive cells were detectable before treatment.
Quantification of cell proliferation:
To assess proliferation after cell death, retinas were stained for proliferating cell nuclear antigen (PCNA) as described by Tappeiner et al. 9. For quantification, the number of PCNA-positive cells was determined manually as described above. Cell death was followed by induced proliferation seen as PCNA-positive cells (Figure 4). Thereby, the maximum of proliferating cells was measured in the INL on day 5. Additionally, cells in the ONL (mainly rods) showed positive staining for PCNA until the end of the study (day 30). Few PCNA-positive cells in the ciliary marginal zone were detectable before and at all time-points after treatment.
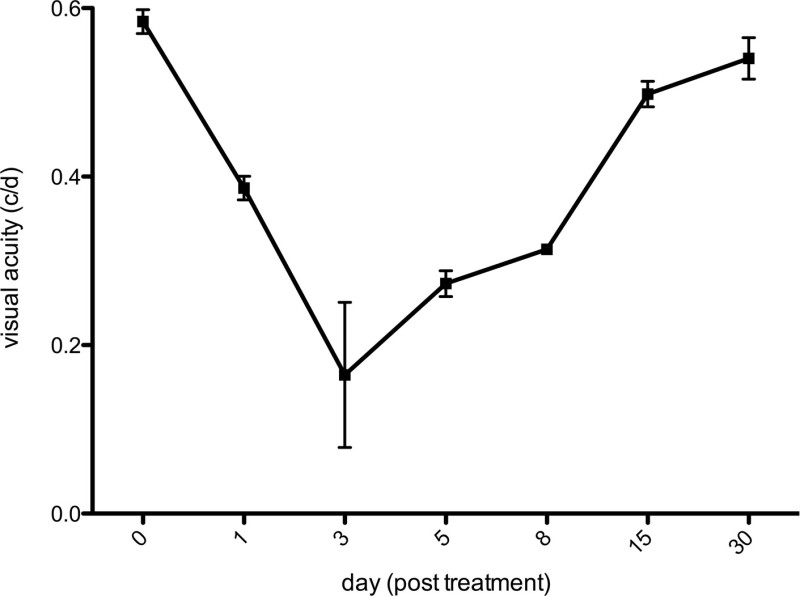 Figure 1: Visual acuity. Measurement of the visual acuity in adult zebrafish after the application of 150 mg/L MNU. Significant decrease of visual acuity with a minimum at day 3 post treatment was followed by full recovery, beginning on day 5, until day 30 (mean ± SD; n = 3).
Figure 1: Visual acuity. Measurement of the visual acuity in adult zebrafish after the application of 150 mg/L MNU. Significant decrease of visual acuity with a minimum at day 3 post treatment was followed by full recovery, beginning on day 5, until day 30 (mean ± SD; n = 3).
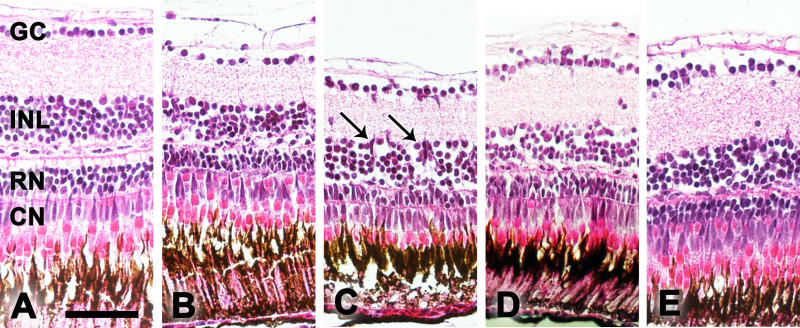 Figure 2: Histology. Examples of H&E stained histological sections of the zebrafish retina at baseline (A) and 3 (B), 8 (C), 15 (D), and 30 (E) days after MNU treatment. CN, cone nuclei; RN, rod nuclei; INL, inner nuclear layer; GC, ganglion cell layer. The arrows mark cell clusters in the INL. Scale bar equals 50 µm.
Figure 2: Histology. Examples of H&E stained histological sections of the zebrafish retina at baseline (A) and 3 (B), 8 (C), 15 (D), and 30 (E) days after MNU treatment. CN, cone nuclei; RN, rod nuclei; INL, inner nuclear layer; GC, ganglion cell layer. The arrows mark cell clusters in the INL. Scale bar equals 50 µm.
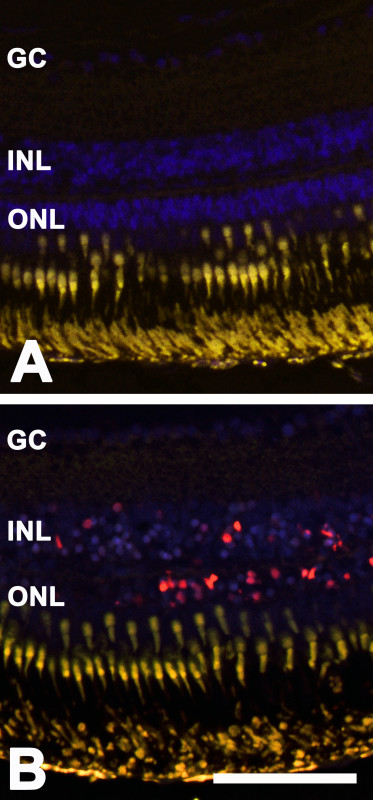 Figure 3: TUNEL-positive cells. TUNEL-positive cells (red) in the zebrafish retina at day 3 after MNU treatment. The cells were mainly located in the ONL but were also found in the INL. Panel A depicts the control, whereas panel B shows the images after MNU treatment. Scale bar indicates 50 µm.
Figure 3: TUNEL-positive cells. TUNEL-positive cells (red) in the zebrafish retina at day 3 after MNU treatment. The cells were mainly located in the ONL but were also found in the INL. Panel A depicts the control, whereas panel B shows the images after MNU treatment. Scale bar indicates 50 µm.
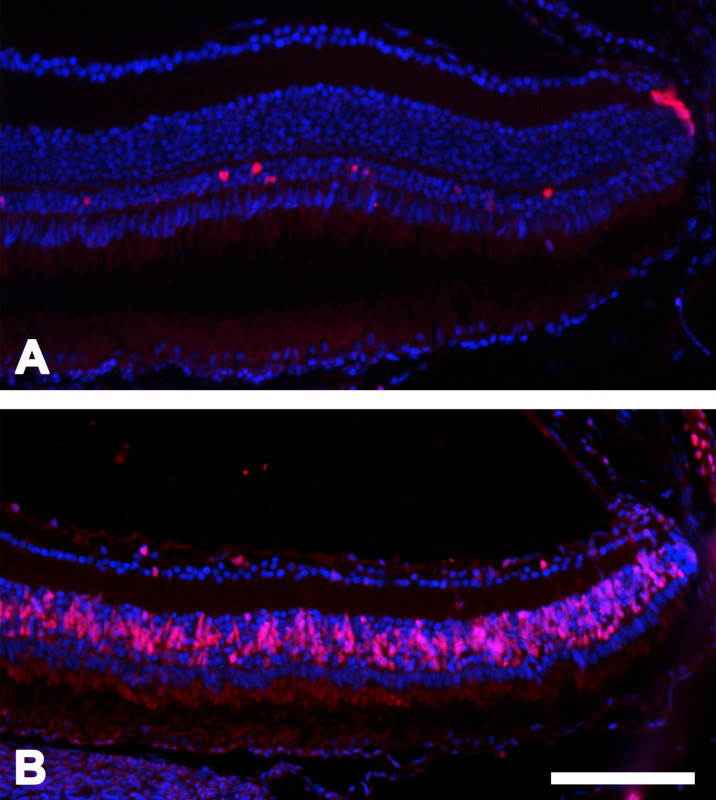 Figure 4: PCNA-positive cells. PCNA-positive cells (red) in the zebrafish retina at day 5 after MNU treatment compared to the control sample. Panel A depicts the control, whereas panel B shows the images after MNU treatment. Scale bar indicates 100 µm.
Figure 4: PCNA-positive cells. PCNA-positive cells (red) in the zebrafish retina at day 5 after MNU treatment compared to the control sample. Panel A depicts the control, whereas panel B shows the images after MNU treatment. Scale bar indicates 100 µm.
Discussion
Previously, our group has transferred the MNU model of photoreceptor degeneration from rodents into the zebrafish system 9. The ensuing events were described and followed for up to 30 days. In this time period complete retinal morphological degeneration and regeneration occurred after the initial treatment. First, histology reveals a reduced rod cell count from day 3 on with a minimum at day 8. Correspondingly, TUNEL staining identifies apoptosis of rod photoreceptors 3 days after MNU treatment. Regeneration starts in the INL at day 3, with a maximum at day 5. In the ONL, proliferating cells can be observed until the end of the observation period with a maximum at day 5. This is in contrast to the INL where no increased proliferation was observed after day 15. The ciliary marginal zone is the only location of the retina where some PCNA positive cells were found in the untreated retina. This indicates an area with constantly retinal neurogenesis throughout lifetime 14. The identification of cell types involved in the regeneration of pharmacologically damaged photoreceptors in zebrafish was not in the focus of this manuscript. However, in previous publications the process has been traced to regenerating cells in the INL that have been suggested to be Müller cells 9,17,18.
In the present study, we have also evaluated functional changes in the MNU-induced zebrafish model. To quantify visual function in adult fish, assessment of the OKR has been employed, one of several techniques to reliably measure the visual acuity (VA) of small animals 19. Thereby, the OKR, based on an innate behavior, does not need prior time-consuming training of the animals 20. Furthermore, as the OKR can be repeatedly elicited independently of the animals’ cooperation, and the large eyes can easily be observed, assessment of the OKR is an ideal method to follow visual acuity during degeneration and regeneration in zebrafish. OKR measurements in the present study revealed a decreasing visual acuity until day 3, followed by a complete restoration of the visual function on day 30. This is in accordance with the histological de- and regenerative changes after MNU application that shows a maximum of apoptosis at day 3.
A benefit of the model is its simplicity as MNU is fast to prepare and can be directly dissolved in the water tank of the zebrafish. Treatment of zebrafish with MNU is performed in one hour. The number of fish that can be treated en masse is only limited by the size of the fish tank. Overall, this allows simple induction of retina degeneration in a great number of zebrafish without the need of manipulation of individual fish. Therefore, this model is less invasive than other models of retina degeneration that use intravitreal or surgical induced techniques 21-23 and it may thereby exhibit a better reproducibility.
In general, this zebrafish MNU model is a versatile tool to induce photoreceptor-specific degeneration and to visualize the following regeneration not only morphologically but also functionally. Therefore, we are confident that the model helps to better understand the degeneration and regeneration process in the sensory retina in general and opens the possibility to compare its outcome with the mammalian system.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We thank Monika Kilchenmann, Federica Bisignani and Agathe Duda for their excellent technical assistance.
References
- Haddad S, Chen CA, Santangelo SL, Seddon JM. The genetics of age-related macular degeneration: a review of progress to date. Surv. Ophthalmol. 2006;51(4):316–363. doi: 10.1016/j.survophthal.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Bhatti MT. Retinitis pigmentosa, pigmentary retinopathies, and neurologic diseases. Curr. Neurol. Neurosci. Rep. 2006;6(5):403–413. doi: 10.1007/s11910-996-0021-z. [DOI] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- Tsubura A, Yoshizawa K, Kuwata M, Uehara N. Animal models for retinitis pigmentosa induced by MNU; disease progression, mechanisms and therapeutic trials. Histol. Histopathol. 2010;25:233–248. doi: 10.14670/HH-25.933. [DOI] [PubMed] [Google Scholar]
- Machida K, Urano K, Yoshimura M, Tsutsumi H, Nomura , Usui T. Carcinogenic comparative study on rasH2 mice produced by two breeding facilities. J. Toxicol. Sci. 2008;33:493–501. doi: 10.2131/jts.33.493. [DOI] [PubMed] [Google Scholar]
- Morton D, et al. N-Methyl-N-Nitrosourea (MNU): A positive control chemical for p53+/- mouse carcinogenicity studies. Toxicol. Pathol. 2008;36:926–931. doi: 10.1177/0192623308324959. [DOI] [PubMed] [Google Scholar]
- Terracini B, Testa MC. Carcinogenicity of a single administration of N-nitrosomethylurea: a comparison between newborn and 5-week-old mice and rats. 1970;24:588–598. doi: 10.1038/bjc.1970.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulliger R, Lecaudé S, Eigeldinger-Berthou S, Wolf-Schnurrbusch UEK, Enzmann V. Caspase-3-independent photoreceptor degeneration by N-methyl-N-nitrosourea (MNU) induces morphological and functional changes in the mouse retina. Graefes Arch. Clin. Exp. Ophthalmol. 2011;249:859–869. doi: 10.1007/s00417-010-1584-6. [DOI] [PubMed] [Google Scholar]
- Tappeiner C, et al. Characteristics of rod regeneration in a novel zebrafish retinal degeneration model using N-methyl-N-nitrosourea (MNU) PLOS One. 2013;12 doi: 10.1371/journal.pone.0071064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilotta J, Saszik S. The zebrafish as a model visual system. Int. J. Dev. Neurosci. 2001;19:621–629. doi: 10.1016/s0736-5748(01)00050-8. [DOI] [PubMed] [Google Scholar]
- Fleisch C, Neuhauss S. Visual Behavior in Zebrafish. Zebrafish. 2006;3:191–201. doi: 10.1089/zeb.2006.3.191. [DOI] [PubMed] [Google Scholar]
- Hodel C, Neuhauss SCF, Biehmaier O. Time course and development of light adaptation processes in the outer zebrafish retina. InterScience. 2006;288:653–662. doi: 10.1002/ar.a.20329. [DOI] [PubMed] [Google Scholar]
- Gemberling M, Bailey TJ, Hyde DR, Poss KD. The zebrafish as a model for complex tissue regeneration. Trends Genet. 2013;29(11):611–620. doi: 10.1016/j.tig.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockerhoff SE, Fadool JM. Genetics of photoreceptor degeneration and regeneration in zebrafish. Cell Mol. Life Sci. 2011;68:651–659. doi: 10.1007/s00018-010-0563-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Granato M, Nüsslein-Volhard C. Keeping and raising zebrafish. In: Nüsslein-Volhard C, Dahm R, editors. Zebrafish: A Practical Approach. IRL Press; 2002. pp. 7–38. [Google Scholar]
- Tappeiner C, Gerber S, Enzmann V, Balmer J, Jazwinska A, Tschopp M. Visual acuity and contrast sensitivity of adult zebrafish. Front. Zool. 2012;9(1):10. doi: 10.1186/1742-9994-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TJ, Fossum SL, Fimbel SM, Montgomery JE, Hyde DR. The inhibitor of phagocytosis, O-phospho-L-serine, suppresses Müller glia proliferation and cone cell regeneration in the light-damaged zebrafish retina. Exp. Eye Res. 2010;91:601–612. doi: 10.1016/j.exer.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Hyde DR. Müller glia as a source of neuronal progenitor cells to regenerate the damaged zebrafish retina. Adv. Exp. Med. Biol. 2012;723:425–430. doi: 10.1007/978-1-4614-0631-0_54. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest. Ophthalmol Vis. Sci. 2004;45(12):4611–4616. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- Beck JC, Gilland E, Tank DW, Baker R. Quantifying the ontogeny of optokinetic and vestibulo-ocular behaviors in zebrafish, medaka, and goldfish. J. Neurophysiol. 2004;92(6):3546–3561. doi: 10.1152/jn.00311.2004. [DOI] [PubMed] [Google Scholar]
- Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of Inner Retinal Neurons after Intravitreal Injection of Ouabain in. Zebrafish. J. Neurosci. 2007;27:1712–1724. doi: 10.1523/JNEUROSCI.5317-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherpa T, et al. Ganglion cell regeneration following whole-retina destruction in zebrafish. Dev. Neurobiol. 2008;68:166–181. doi: 10.1002/dneu.20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurco P, Cameron DA. Responses of Müller glia to retinal injury in adult zebrafish. Vision Res. 2005;45:991–1002. doi: 10.1016/j.visres.2004.10.022. [DOI] [PubMed] [Google Scholar]


