Abstract
Objective To evaluate the clinical efficacy of an established programme of occupational therapy in maintaining functional activity and reducing further health risks from inactivity in care home residents living with stroke sequelae.
Design Pragmatic, parallel group, cluster randomised controlled trial.
Setting 228 care homes (>10 beds each), both with and without the provision of nursing care, local to 11 trial administrative centres across the United Kingdom.
Participants 1042 care home residents with a history of stroke or transient ischaemic attack, including those with language and cognitive impairments, not receiving end of life care. 114 homes (n=568 residents, 64% from homes providing nursing care) were allocated to the intervention arm and 114 homes (n=474 residents, 65% from homes providing nursing care) to standard care (control arm). Participating care homes were randomised between May 2010 and March 2012.
Intervention Targeted three month programme of occupational therapy, delivered by qualified occupational therapists and assistants, involving patient centred goal setting, education of care home staff, and adaptations to the environment.
Main outcome measures Primary outcome at the participant level: scores on the Barthel index of activities of daily living at three months post-randomisation. Secondary outcome measures at the participant level: Barthel index scores at six and 12 months post-randomisation, and scores on the Rivermead mobility index, geriatric depression scale-15, and EuroQol EQ-5D-3L questionnaire, at all time points.
Results 64% of the participants were women and 93% were white, with a mean age of 82.9 years. Baseline characteristics were similar between groups for all measures, personal characteristics, and diagnostic tests. Overall, 2538 occupational therapy visits were made to 498 participants in the intervention arm (mean 5.1 visits per participant). No adverse events attributable to the intervention were recorded. 162 (11%) died before the primary outcome time point, and 313 (30%) died over the 12 months of the trial. The primary outcome measure did not differ significantly between the treatment arms. The adjusted mean difference in Barthel index score at three months was 0.19 points higher in the intervention arm (95% confidence interval −0.33 to 0.70, P=0.48). Secondary outcome measures also showed no significant differences at all time points.
Conclusions This large phase III study provided no evidence of benefit for the provision of a routine occupational therapy service, including staff training, for care home residents living with stroke related disabilities. The established three month individualised course of occupational therapy targeting stroke related disabilities did not have an impact on measures of functional activity, mobility, mood, or health related quality of life, at all observational time points. Providing and targeting ameliorative care in this clinically complex population requires alternative strategies.
Trial registration Current Controlled Trials ISRCTN00757750.
Introduction
Care homes are residential settings with staff employed to assist with personal care for people who unable to look after themselves. In the United Kingdom, care homes exist with and without the provision of nursing care. For homes providing nursing care, staff must be qualified health professionals. Studying the evidence for efficacious rehabilitation practices in long term institutional care settings is a research priority.1 Recent estimated global figures suggest that despite a decrease in incidence and prevalence of stroke mortality between 1990 and 2010, stroke represents the third most common cause of disability adjusted life years.2 Within the United Kingdom, approximately a quarter of all survivors of stroke are unable to return home and require long term institutional care.3
Care home residents with stroke related disabilities tend to be more physically and cognitively impaired than those living in the community, and consequently have high support needs. A primary objective of this trial was to evaluate the potential benefit of a course of occupational therapy at maintaining or improving functional activity in this population. The focus on functional activity reflects how health and disability are understood in the redrafted international classification of functioning, disability, and health in 2001.4 Disability is discussed in terms of the interaction between an individual’s impairments, limitations of activity, restrictions to participation, and environment.4
After admission to a care home, residents with stroke related disabilities typically follow a downward trajectory in their capacity to engage in functional activity. Observational data suggest that 97% of residents’ days are spent sitting and being inactive.5 Inactivity in this population poses further health risks, including joint contractures, pain, incontinence, pressure ulcers, and low mood.6 Occupational therapy delivered to stroke survivors in their own homes has strong evidence of benefit.7 8 Within its most recent stroke guidelines the UK National Institute for Health and Care Excellence recommends that occupational therapy should be provided for people after stroke who are likely to benefit, to tackle difficulties with personal activities of daily living.9 This form of rehabilitative therapy is rarely available in UK care homes,10 and yet it is arguably more relevant and applicable to a care home setting, where residents’ have higher levels of dependence performing personal activities of daily living than those living in the community. Personal activities of daily living are defined as feeding, bathing, using the toilet, getting dressed, grooming, transfers (for example, from bed to chair and back), and mobility. Owing to the established literature base indicating efficacy of occupational therapy for stroke survivors living within the community,7 8 we deemed it necessary to focus this trial on residents in care homes with stroke sequelae. An objective of the trial was to evaluate whether there is evidence to recommend an improved UK National Health Service provision of this type of therapy for care home residents with stroke related disabilities.
The research team has published a systematic review with the Cochrane Collaboration identifying randomised controlled trials that examined the impact of an occupational therapy intervention, provided by trained therapists, for care home residents with stroke related disabilities, compared with usual care.11 One study was analysed in full.12 This was the phase II cluster randomised controlled trial completed by members of the research team. Overall, 118 participants were recruited from 12 care homes in one region of the United Kingdom. The intervention was similar in style and content to the trial reported here. The primary outcome measure was scores on the Barthel index,13 14 and the secondary outcome measures were scores on the Rivermead mobility index15 and poor global outcome. Poor global outcome was defined as deterioration in Barthel index score or death. Measures were conducted at three and six months after randomisation. Residents receiving the intervention showed a moderate improvement in Barthel index and Rivermead mobility index scores between baseline and the primary endpoint at three months. At the six month follow-up, participants’ level of deterioration was similar between the groups. The proportion of participants with a poor global outcome tended to be higher in the control arm at the three and six month endpoints. The pilot trial showed feasibility and suggested that a course of individualised occupational therapy may provide benefit in maintaining or increasing functional activity for residents in care homes with stroke related disabilities.12 However, owing to the small sample size and high intracluster correlations among the data, no firm conclusions could be drawn.11
We developed pilot findings using a larger sample of care home residents living with stroke related disabilities and to evaluate whether a three month course of occupational therapy would have a significant clinical impact on functional activity compared with usual care. The results aimed to offer a robust assessment of whether occupational therapy should be recommended as part of a routine package to all care home residents living with stroke related disabilities.
Methods
Study design and participants
This study was a phase III pragmatic, parallel group, cluster randomised controlled trial in care homes across the United Kingdom. We chose a cluster design because of the staff education and environmental adaptation components included in the intervention. The trial protocol is summarised here and described in full elsewhere.16 We invited a random selection of care homes with more than 10 beds each to participate in the study, in the geographical vicinity to a trial administrative centre. The trial administrative centres were situated in the south, south west, Midlands, and north west of England, and in Wales. We included all funding models of care home; excluding homes for people with learning disabilities or drug addiction. Care home managers were offered a full explanation of the study. No care homes were actively delivering occupational therapy as a component of standard care.
Once the managers had given informed consent, care home staff searched the residents’ notes to determine confirmed or suspected stroke or transient ischaemic attack. Where a relevant entry was found, the research team sought confirmation from general practice records. Where stroke was suspected, residents were described as having experienced a stroke in care home records; however, these details were not confirmed by a doctor, following multiple attempts. All residents with a history of ischaemic or haemorrhagic stroke or transient ischaemic attack, including those with language and cognitive impairments, were eligible. We excluded residents actively receiving end of life care. Residents with a history of transient ischaemic attack were included owing to the emerging evidence that long term moderate to severe difficulties are experienced in 26% of cases.17 We offered prospective participants (and family members, if appropriate) a full explanation of the study. If prospective participants lacked the capacity to consent, we approached their next of kin to provide consultee agreement on their behalf.18 During a second visit to the care home, trained assessors or care home staff obtained consent from eligible residents.
Randomisation and masking
To reduce bias, independent assessors administered baseline assessments before randomisation.19 No additional participants joined the study after randomisation. If a care home had at least one consenting resident, it was eligible for randomisation. We stratified care homes by type of care provided (nursing or residential) and trial administrative centre (11 centres), and then randomised them 1:1 to either the intervention arm or the control arm. An independent statistician generated an allocation sequence in nQuery Advisor, version 7.0 (Statistical Solutions, Cork, Republic of Ireland) using randomised blocks (size=2) within strata. To reduce predictability we randomised homes in batches across the strata. The sequence was concealed from the research team and held in a secure database. Homes were randomised once the study coordinator logged the details about the stratification factors and received notification that all consenting participants in a care home had completed baseline measures. Care home allocation was revealed to the study coordinator, who then informed the care home manager and corresponding site therapist. The study coordinator was not involved in data collection or data analysis. Independent assessors were masked to the treatment allocation of care homes.
Intervention and control
Residents in the control arm received usual care. This did not involve an occupational therapy component. The occupational therapy intervention at the level of the care home resident followed a client centred approach, involving task specific training delivered by qualified occupational therapists (box).20
Summary of occupational therapy intervention
Therapy followed a patient centred goal setting approach, aiming to improve or maintain functional capacity in personal activities of daily living
-
Therapy was administered according to categories:
Assessment and goal setting—involving the assessment of a resident’s current level of functional activity in personal activities of daily living and mutually identifying functional goals of therapy
Personal activities of daily living training—involving techniques to assist with feeding, bathing, using the toilet, getting dressed, and grooming
Transfers and mobility—involving walking, standing, moving around in bed, and transfers to and from a chair
Communication—involving the provision of information and guidance (to staff, residents, or relatives), referrals to other agencies, ordering equipment, and listening to residents’ concerns about personal activities of daily living
Environment (including adaptive equipment and seating posture)—involving provision of items such as adaptive cutlery, palm protectors, wheelchair cushions, walking aids, chair raisers, grab rails, raised toilet seats, and bed levers
Other—involving treating impairments directly, such as joint contracture
Frequency and duration of visits depended on agreed goals between therapist and resident
Workshops for care home staff focused on facilitating residents’ functional activity, mobility, and use of adaptive equipment
The intervention package was developed using evidence and expert consensus opinion from occupational therapists, trialled previously in a stroke population,12 and described in detail in previous publications.21 22 The intervention was customised to each resident and aimed to augment or maintain functional capacity in personal activities of daily living, such as dressing, grooming, bathing, using the toilet, feeding, and mobility. Therapists made appropriate environmental adaptations where necessary, to promote safe and effective practice of personal activities of daily living (for example, the installation of bed levers, grab rails, raised toilet seats). Environmental adaptations were made according to each therapist’s professional opinion.
Occupational therapists assessed residents in the intervention group to establish baseline functional ability and identify areas of activity limitation that could be dealt with during treatment. Task performance goals for the intervention were mutually agreed between the therapist and resident. For residents with communication or cognitive difficulties the therapist included family members or care home staff to agree the shared goals of therapy. The frequency and duration of therapy sessions depended on the resident’s wishes and the agreed goals of therapy.
We provided specific training workshops as part of the intervention package to staff in homes randomised to the intervention group.21 The workshops aimed to increase awareness of stroke related disabilities and provide advice on their management in relation to long term care. Risks associated with inactivity were highlighted, as well as the carer’s role in supporting mobility (for example, safe and effective methods of transfer), preventing accumulative problems from poor positioning (for example, unsuitable seating), and facilitating resident participation in self care activities.5 For staff in care homes randomised to the control arm, we offered a training workshop after the 12 month follow-up. The training offered to care home staff received endorsement from the UK Stroke Forum Education and Training (www.stroke-education.org.uk/).
Outcome measures
The primary outcome measure was the Barthel index score at the participant level, three months post-randomisation.13 14 We conducted secondary follow-up assessments of the Barthel index score at six and 12 months post-randomisation. The Barthel index consists of a scale between 0 and 20, 20 signifying maximum ability.13 22 It assesses levels of dependency in 10 categories of self care (for example, dressing, feeding). An increase of 2 points is accepted as being clinically significant.23 A 2 point change equates to a perceptible step change in function. For example, in the self care dimensions an increase of 2 points may indicate a change from being unable to dress and feed oneself to managing with some form of help. Care home staff assisted residents who were unable to complete the Barthel index. At baseline the Sheffield screening test for acquired language disorders was administered along with the mini-mental state examination.24 25 The tests provided an indication of the participant’s capacity to understand instructions and directly engage in therapy, and informed the research team whether they required consultee assistance during recruitment.
Secondary outcome measures included the Rivermead mobility index,15 geriatric depression scale-15,26 and EuroQol EQ-5D-3L questionnaire.27 All secondary outcome measures were assessed at all follow-up time points. Independent assessors, blinded to treatment allocation, were trained in conducting all outcome measures and completed all assessments in their allocated homes. Recorded personal data included age, sex, ethnicity, comorbidities, and history of falls before the onset of the trial. Adverse events were defined as a fall that led to a consultation with a general practitioner or visit to an emergency department as a result of participating in the study, including cases of adaptive equipment failure (for example, breakage of a walking aid).
Statistical analyses
To observe a clinically significant 2 point increase in mean Barthel index score at three months,23 we estimated that we would require a sample size of 330 residents in each treatment arm. This estimate was based on a standard deviation of 3.7, with 90% power at the 5% significance level, and an intracluster correlation coefficient of 0.37.28 The sample size calculation was estimated from data related to several pilot studies.12 28 29 We used the larger estimate to calculate sample size in the interest of adequate power. Assuming an attrition rate of 26%12 and a total of 10 residents per home, we estimated that 45 care homes would be required in each treatment arm (n=900 residents).
Analyses were performed in Stata (version 12.1) and SAS (version 9.2) software using mixed and glimmix procedures. All analyses were performed using an intention to treat approach, whereby participants were analysed by the arm to which their initial home of residence was randomised. The primary linear mixed model analysis compared Barthel index scores between groups at three months. We adjusted the analysis by care home (as a random factor), baseline Barthel index score, and stratification factors: trial administrative centre (11 centres) and type of care home (residential or nursing). Participants who died before a follow-up assessment were assigned a Barthel index score of zero for all subsequent follow-up assessments. In the primary analysis we excluded participants with missing or incomplete data for Barthel index scores.
In addition, participants were categorised into three outcome groups based on their change in Barthel index score at three months from baseline (<0 or death=poor, 0-1=moderate, ≥2=good). We used a non-linear mixed effects model to compare this ordinal outcome between the groups. Sensitivity analyses excluded clusters with fewer than three participants and examined the effects of missing data using best case (last value carried forward), worst case (zero), and multiple imputation methods. Participants who died were imputed with a zero score for all sensitivity analyses. We performed further secondary analyses on the Barthel index data to assess the effects of the three month intervention over time. A repeated measures mixed model analysis of Barthel index scores was performed across all endpoints, adjusted using an identical method to the primary analysis. We also performed secondary between group analyses on the Rivermead mobility index, geriatric depression scale-15, and EQ-5D-3L data at all endpoints. In addition to the evaluation of clinical efficacy we also performed an economic analysis that assessed the cost of the intervention per quality adjusted life year.
Results
Participants
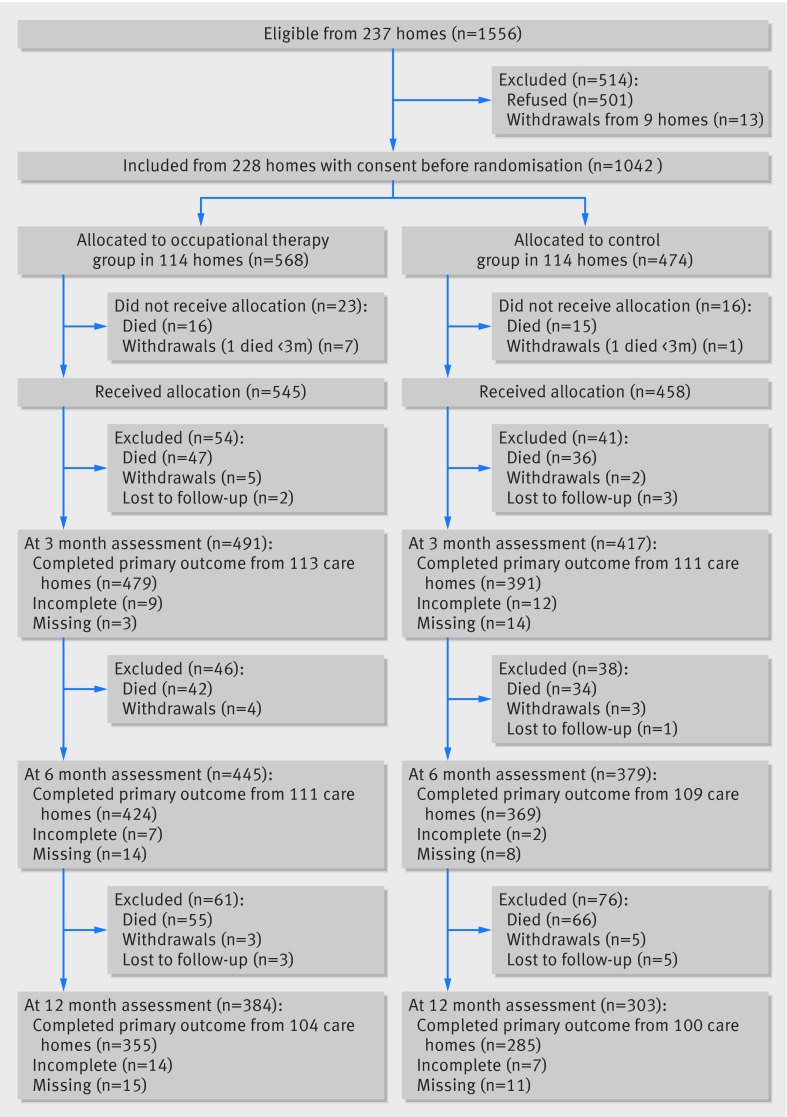
Figure 1 shows the flow of participants through the study. A total of 237 care homes offered consent at a managerial level across the 12 administrative centres. Within the consenting care homes 1556 out of 9840 (16%) residents were eligible for the study; 1055 of the 1556 (68%) provided consent to participate. One trial administrative centre, involving four care homes and 11 consenting residents withdrew before the randomisation stage owing to problems during the start-up phase. Eleven trial administrative centres were involved in the remainder of trial. Four care homes with consent at a managerial level across two administrative centres did not recruit any consenting participants and were withdrawn. Two consenting participants in a single care home were withdrawn by the care home manager before randomisation to receive end of life care. As a result the care home was withdrawn. A total of nine care homes, involving 13 consenting participants, did not proceed to the randomisation stage (fig 1).
Fig 1 Flow of participants through study
The trial was planned for four years but the anticipated end date was brought forward from December 2014 to February 2013 owing to high recruitment levels. Randomisation of participating care homes occurred between May 2010 and March 2012. Recruitment exceeded the target, with 1042 participants from 228 care homes (114 homes in each arm) local to 11 trial administrative centres across England and Wales. We recruited more care homes because the average cluster size was lower than predicted but comparable between the two arms (table 1). The median size of clusters was 4 (interquartile range 2-6). Of the care homes recruited, 121 (53%) provided nursing care. Most participants resided in homes with nursing care (64%). More eligible residents resided in clusters randomised to the intervention arm (n=568) than the control arm (n=474). This was a chance occurrence as consent was obtained before randomisation. Overall, 64% of the participants were female, and the mean age was 82.9 (SD 9.2) years.
Table 1.
Details of clusters and personal and baseline assessment information for participants. Values are numbers (percentages) unless stated otherwise
| Characteristics | Intervention group | Control group |
|---|---|---|
| Care home type | ||
| Residential care | 53/114 (46) | 54/114 (47) |
| Nursing care | 61/114 (54) | 60/114 (53) |
| Mean (SD) cluster size | 5 (3.7) | 4.2 (3.0) |
| Personal details | ||
| Mean (SD) age (years) | 83.1 (9.9) | 83.6 (9.5) |
| Men | 203/568 (36) | 174/474 (37) |
| White | 517/568 (91) | 445/474 (94) |
| Comorbidities | ||
| Cardiovascular disease | 342/530 (65) | 278/446 (62) |
| Respiratory disease | 90/484 (19) | 76/415 (18) |
| Hepatic disease | 6/471 (1) | 8/406 (2) |
| Gastrointestinal disease | 96/485 (20) | 78/421 (19) |
| Renal disease | 38/461 (8) | 51/410 (12) |
| Urological disease | 92/475 (19) | 80/411 (19) |
| Neurological disease | 371/505 (73) | 296/424 (70) |
| Musculoskeletal disease | 214/474 (45) | 199/425 (47) |
| Dermatological problems | 86/459 (19) | 71/403 (18) |
| Fall history | 203/495 (41) | 200/427 (47) |
| Stroke data (confirmed by general practice) | ||
| Confirmed stroke | 329/568 (58) | 317/474 (67) |
| Confirmed transient ischaemic attack | 47/568 (8) | 28/474 (6) |
| Suspected stroke | 73/568 (13) | 66/474 (14) |
| Missing confirmation | 119/568 (21) | 63/474 (13) |
| Left sided stroke | 161/318 (51) | 154/283 (54) |
| Right sided stroke | 148/318 (46) | 108/283 (38) |
| Bilateral stroke | 9/318 (3) | 21/283 (7) |
| Assessment data | ||
| Mean (SD) Sheffield screening test* (0-20) | 10.9 (7.1) | 11.0 (6.9) |
| Language impairment (<15) | 245/424 (58) | 213/374 (57) |
| Barthel index (0-20): | ||
| Mean (SD) score | 6.5 (5.8) | 6.3 (5.7) |
| Very severe (0-4) | 268/562 (48) | 234/467 (50) |
| Severe (5-9) | 129/562 (23) | 104/467 (22) |
| Moderate (10-14) | 91/562 (16) | 76/467 (16) |
| Mild (15-19) | 64/562 (11) | 46/467 (10) |
| Independent (20) | 10/562 (2) | 7/467 (1) |
| Mini-mental state examination (0-30) | 13.6 (9.5) | 13.2 (9.0) |
| Cognitive impairment (0-20) | 279/398 (70) | 263/362 (73) |
| Borderline (21-23) | 40/398 (10) | 42/362 (12) |
| Mean (SD) Rivermead mobility index (0-15) | 3.1 (3.8) | 2.8 (3.7) |
| Mean (SD) geriatric depression scale-15 (0-15) | 6.8 (3.9) | 6.4 (3.5) |
| Mild (0-4) | 157/498 (32) | 131/415 (32) |
| Moderate (5-9) | 205/498 (41) | 200/425 (48) |
| Severe (10-15) | 136/498 (27) | 84/415 (20) |
| Mean (SD) EQ-5D-3L† | 0.20 (0.4) | 0.24 (0.4) |
*Sheffield screening test for acquired language disorders.
†EuroQol group 5-dimension self report questionnaire (three levels).
Baseline characteristics
Baseline characteristics for all personal characteristics and diagnostic tests were similar between the treatment groups (table 1). Data on length of stay in the care home were available for 562/568 (99%) participants in the intervention arm and 467/474 (99%) participants in the control arm. The median length of stay between care home admission and trial randomisation was 2.35 (interquartile range 0.96-4.49) years for the intervention group and 2.16 (1.04-4.12) years for the control group. We attempted to retrieve the exact dates of participants’ stroke from medical records, but responses from general practices were limited to approximately 46% of all participants. Date of stroke was confirmed for 225 participants in the intervention arm and 250 participants in the control arm. The median duration between residents’ stroke and trial randomisation was 3.17 (1.30-7.12) years in the intervention arm and 2.82 (1.18-5.83) years in the control arm.
Most participants (542/760, 71%) who completed the mini-mental state examination scored between 0 and 20 out of 30, indicating significant cognitive impairment.24 30 For the Sheffield screening test, 458/798 (57%) scored below 15, indicating impairment of communication.25 In addition, 404/913 (44%) participants scored in the range signifying moderate depression on the geriatric depression scale-15 at baseline, and 220/913 (24%) scored in the range indicative of severe depression (table 1).26
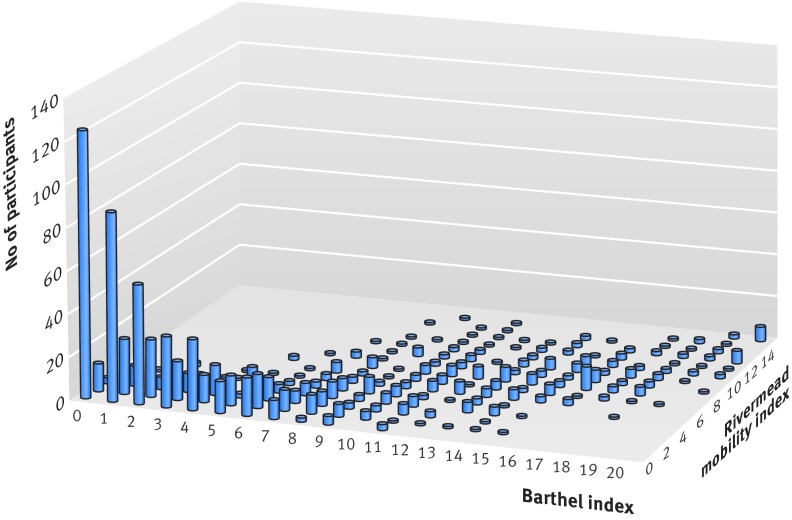
Baseline data on the Barthel index for the primary analysis were recorded for 562/568 (99%) residents in the intervention group and 467/474 (99%) in the control group (table 1). Over 70% of all participants were categorised as severe or very severe using the Barthel index. For the secondary analyses, baseline assessments were administered with a high completion rate for the Rivermead mobility index (97%), geriatric depression scale-15 (88%), and EQ-5D-3L (89%) and were comparable between groups (table 1). Figure 2 displays a plot of the relation between baseline Barthel index scores and baseline Rivermead mobility index scores for 1012 participants across both treatment arms. A total of 493/1012 (49%) participants scored less than 4 on both the Rivermead mobility index and Barthel index scales, suggesting that approximately half of the sampled population had both severe limitations on functional activity when engaging in personal activities of daily living, and severe limitations with mobility.
Fig 2 Relation between baseline Barthel index scores and baseline Rivermead mobility index scores across both treatment arms (n=1012). Barthel index scores 0-20, 20 signifying maximum ability; Rivermead mobility index scores 0-15, 15 signifying maximum ability
Intervention
Overall, 2538 visits were made to 498 residents in the intervention arm: mean 5.1 (SD 3.0) visits. Total therapy time was 1724 hours. The median session duration was 30 (interquartile range 15-60) minutes. Therapy was administered according to categories: 23% of therapy time was spent on individual assessment, 49% on communication, 7% on activities of daily living training, 8% on mobility training, 7% on the provision of adaptive equipment, and 6% on treating specific impairments. Time spent on communication involved the provision of information and guidance for staff, residents, or relatives; referrals to other agencies; and ordering relevant equipment. Table 2 shows examples of treatment plans for personal activities of daily living and mobility training or the use of adaptive equipment in three hypothetical residents.
Table 2.
Three examples of treatment plans, with recommendations left for care home staff
| Resident and problem identified | Goals | Actions | Outcome (including recommendations for staff) |
|---|---|---|---|
| Resident A: | |||
| Dressing | To dress top half of body independently | Assessment of perception and motor skills | Able to dress and undress top half safely and with minimal assistance but requires time and prompting, although he tires quickly. Encourage resident A to participate in dressing whenever possible |
| Feeding | To feed independently | Issued right angled light weight spoon | Managed independently with the spoon but tired quickly and had difficulty finding food on the plate. Encourage independence with feeding within resident A’s stamina levels. Resident A still requires supervision and assistance. Position in wheelchair to facilitate independent function when eating at the table. Placement of feet on the floor may assist with sitting balance |
| Transfer from chair | Standing from a chair | Supply chair raisers to facilitate standing from a chair | Ensure height of chair is correct. Resident A requires constant prompting and may require assistance to position feet before standing. Use hoist if unable to weight bear or to follow instructions to stand |
| Resident B: | |||
| Mobility/transfers | Standing from a chair | Practice transferring from wheelchair to chair | Resident B was able to transfer between two chairs safely and independently. Ensure height of chair is correct. May require prompting to push up from the chair and may require assistance to position feet before standing |
| Walking with three wheeled walker | Walking practice, replace ferrules on walking aid | Resident B leans heavily on walking frame when mobilising, but mobilises safely and independently with walking frame. However, some supervision and prompting required because of difficulty anticipating manoeuvres required to sit in a chair safely. Continue to use walking frame | |
| Resident C: | |||
| Dressing | To participate in dressing | Dressing assessment | Encourage resident C to continue dressing independently |
| Mobility | To maintain mobility | Assessment of walking aid | Replace ferrules. Check ferrules regularly for wear |
| Transfers | To maintain safe and independent transfers | Advise on use of bed lever | Encourage correct transfer technique. Prompt resident C to come to the front of the chair and to push up to stand from the bed and chair; encourage use of the bed lever when sitting up in bed and pushing up to stand |
Attrition and cases of unblinding
Retention of care home participation was good throughout the study (fig 1). Completion rates for all primary and secondary measures were balanced between the two groups at each of the follow-up assessments. Primary outcome data were recorded in 224/228 (98%) care homes at the three month follow-up. Outcome measures were completed in 220/228 (96%) care homes at six months and 204/228 (89%) care homes at 12 months. The reason for withdrawal for 15/24 (63%) care homes was because all participating residents had died during follow-up. This occurred in 7/10 homes in the intervention arm and 8/14 homes in the control arm over the 12 month duration of the trial. Of the remaining withdrawals, the care home manager withdrew consent, with the exception of one care home in the intervention arm that was withdrawn before the primary endpoint owing to the treatment allocation being revealed to the assessor. In addition to this case of unblinding, five further cases occurred; one after the final 12 month assessment and the remainder after the primary outcome time point. All cases of unblinding occurred in homes allocated to the intervention.
Before the primary outcome endpoint at three months 64/568 (11%) participants died in the intervention arm and 52/474 (11%) in the control arm (116 participants in total). Between the three and six month follow-up 42/504 (8%) participants died in the intervention arm and 34/422 (8%) in the control arm (76 participants in total). Between the six and 12 month follow-up 55/462 (12%) participants died in the intervention arm and 66/388 (17%) in the control arm (121 participants in total). A total of 313/1042 (30%) participants died during the trial.
Primary outcome
The estimated sample size listed in the protocol was 900 participants at the primary endpoint. The trial over-recruited to allow for an increased number of small clusters (total recruited n=1042). Of the participants alive at three months, the Barthel index was completed by 479/504 (95%) from 113 care homes in the intervention arm, and 391/422 (93%) from 111 care homes in the control arm (870 residents in total). The adjusted mean difference in Barthel index score between groups at three months was 0.19 points higher in the intervention arm (95% confidence interval −0.33 to 0.70, P=0.48). This difference did not reach statistical significance at the 0.05 level nor did it represent a significant clinical impact (table 3).23
Table 3.
Comparison of primary and secondary outcome measures at three month follow-up assessment
| Outcome | Intervention | Control | Baseline ICC (95% CI) | Adjusted ICC† (95% CI) | Difference in adjusted means (95% CI) | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| Adjusted mean* (SE) | No | Adjusted mean* (SE) | No | ||||||
| Primary: | |||||||||
| Barthel index‡ | 5.47 (0.20) | 540 | 5.29 (0.21) | 436 | 0.36 (0.29 to 0.43) | 0.09 (0.05 to 0.17) | 0.19 (−0.33 to 0.70) | 0.48 | |
| Secondary: | |||||||||
| Rivermead mobility index | 2.74 (0.11) | 465 | 2.73 (0.12) | 382 | 0.28 (0.21 to 0.36) | 0.04 (0.01 to 0.15) | 0.02 (−0.28 to 0.31) | 0.90 | |
| Geriatric depression scale-15 | 6.09 (0.21) | 383 | 6.30 (0.22) | 324 | 0.11 (0.06 to 0.18) | 0.07 (0.03 to 0.17) | −0.21 (−0.76 to 0.33) | 0.44 | |
| EQ-5D-3L§ | 0.24 (0.02) | 409 | 0.23 (0.02) | 338 | 0.25 (0.18 to 0.33) | 0.06 (0.02 to 0.17) | 0.01 (−0.04 to 0.06) | 0.65 | |
ICC=model based intracluster correlation coefficient.
*Adjusted for care home as random effect, and baseline score, type of care home, and administrative centre as fixed effects.
†Adjusted for baseline score, treatment arm, type of care home, and administrative centre.
‡Participants who died before follow-up are given a Barthel score of zero.
§EuroQol group 5-dimension self report questionnaire (three levels).
Secondary outcomes
At the two subsequent follow-up endpoints, the Barthel index data showed no significant differences between groups (table 4). Of the participants alive at six months, the Barthel index was completed by 424/462 (92%) from 111 care homes in the intervention arm and 369/388 (95%) from 109 care homes in the control arm (793 residents in total). At six months the adjusted mean difference in Barthel index score was 0.004 points (95% confidence interval −0.52 to 0.53, P=0.99). Of the participants alive at 12 months, the Barthel index was completed by 355/407 (87%) from 104 care homes in the intervention arm and 285/322 (89%) from 100 care homes in the control arm (640 residents in total). At 12 months the adjusted mean difference in Barthel index score was 0.16 (−0.40 to 0.72, P=0.58). The results from the secondary analyses assessing mobility, mood, and health related quality of life also showed no statistically significant or clinically important differences between groups, at each follow-up time point (tables 3 and 4).
Table 4.
Comparison of primary and secondary outcomes at six and 12 month follow-up assessments
| Outcome by follow-up | Intervention | Control | Difference in adjusted means (95% CI)† | P value† | Group×time interaction | |||
|---|---|---|---|---|---|---|---|---|
| Adjusted mean* (SE) | No | Adjusted mean* (SE) | No | |||||
| Primary | ||||||||
| Barthel index‡: | ||||||||
| 6 months | 4.78 (0.20) | 525 | 4.78 (0.22) | 448 | 0.004 (−0.52 to 0.53) | 0.99 | 0.35 | |
| 12 months | 3.93 (0.21) | 512 | 3.77 (0.22) | 430 | 0.16 (−0.40 to 0.72) | 0.58 | ||
| Secondary | ||||||||
| Rivermead mobility index: | ||||||||
| 6 months | 2.64 (0.11) | 421 | 2.67 (0.12) | 346 | −0.03 (−0.33 to 0.27) | 0.84 | 0.23 | |
| 12 months | 2.19 (0.13) | 354 | 2.46 (0.14) | 271 | −0.26 (−0.62 to 0.09) | 0.15 | ||
| Geriatric depression scale-15: | ||||||||
| 6 months | 6.20 (0.21) | 338 | 6.68 (0.22) | 284 | −0.48 (−1.04 to 0.09) | 0.10 | 0.57 | |
| 12 months | 6.22 (0.22) | 297 | 6.40 (0.25) | 219 | −0.18 (−0.80 to 0.43) | 0.56 | ||
| EQ-5D-3L§: | ||||||||
| 6 months | 0.22 (0.02) | 363 | 0.23 (0.02) | 315 | −0.01 (−0.05 to 0.04) | 0.72 | 0.56 | |
| 12 months | 0.20 (0.02) | 316 | 0.18 (0.02) | 244 | 0.02 (−0.03 to 0.07) | 0.48 | ||
*Adjusted by care home as a random effect, and baseline score, type of care home and centre as fixed effects.
†Tukey-Kramer adjusted confidence intervals and P values.
‡Participants who died before follow-up are given a Barthel score of zero.
§EuroQol group 5-dimension self report questionnaire (three levels).
Adverse events
No adverse events attributable to the intervention were recorded. A significantly higher fall rate per resident was reported in the intervention arm at three months (rate ratio 1.74, 95% confidence interval 1.09 to 2.77, P=0.02). The mean number of falls per resident during the first three months in the intervention arm was 0.18 (SD 0.52) compared with 0.11 (SD 0.40) in the control arm. The number of residents who had a fall resulting in injury or medical attention during the first three months was 63/482 (13%) in the intervention arm and 35/408 (9%) in the control arm. When adjustment was made for care home, trial administrative centre, and type of care home there was a suggestion of increased odds of experiencing at least one fall in the intervention arm (odds ratio 1.55, 95% confidence interval 0.96 to 2.53, P=0.07).
Subgroup analyses
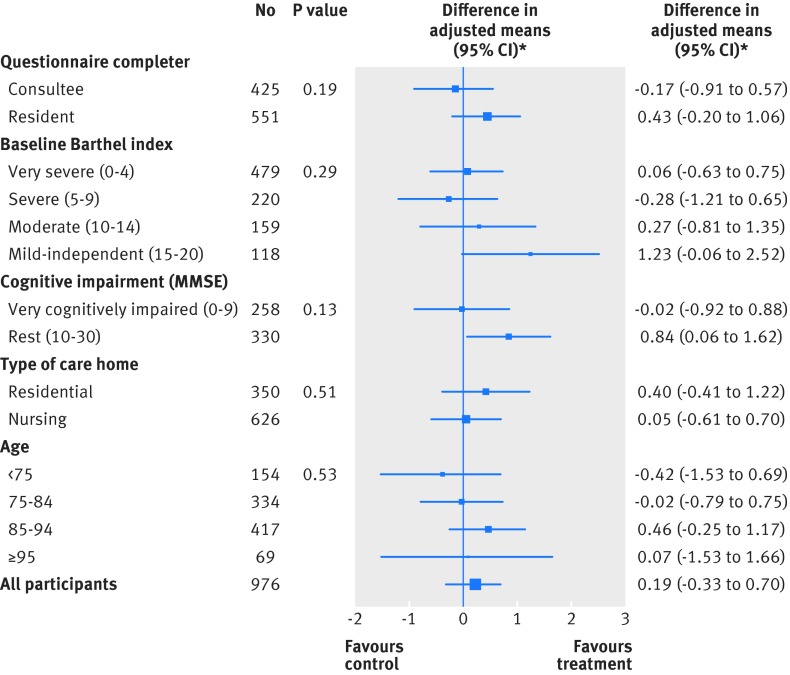
Exploratory subgroup analyses were performed to assess whether response to the intervention at the primary endpoint differed according to a predefined list of variables, compared with a balanced control population. Analyses considered participants’ age, type of care home, Barthel index severity rating, level of cognitive impairment, and whether the measures were completed by the participant or a consultee. None of the exploratory subgroup analyses provided evidence of a significant difference between groups (fig 3). The effect sizes, based on change in Barthel index score between baseline and three months, were also similar between groups (table 5).
Fig 3 Exploratory subgroup analysis: comparison of Barthel index at three months. *Type of care home means were adjusted for care home as a random effect and baseline Barthel index score and trial administrative centre as fixed effects. All other subgroup means were adjusted for care home as a random effect and baseline Barthel index score, trial administrative centre, and type of care home as fixed effects. MMSE=mini-mental state examination
Table 5.
Comparison of Barthel index grouped outcome at all follow-up time points. Values are numbers (percentages) unless stated otherwise
| Barthel index grouped outcome* by follow-up | Intervention group | Control group | Odds ratio† (95% CI) | P value |
|---|---|---|---|---|
| 3 months: | ||||
| Poor | 293/540 (54) | 227/436 (52) | 0.96 (0.70 to 1.33) | 0.81 |
| Moderate | 164/540 (30) | 150/436 (34) | ||
| Good | 83/540 (15) | 59/436 (14) | ||
| 6 months: | ||||
| Poor | 306/526 (58) | 269/449 (60) | 0.95 (0.71 to 1.27) | 0.74 |
| Moderate | 161/526 (31) | 122/449 (27) | ||
| Good | 59/526 (11) | 58/449 (13) | ||
| 12 months: | ||||
| Poor | 350/513 (68) | 314/432 (73) | 0.84 (0.61 to 1.15) | 0.27 |
| Moderate | 121/513 (24) | 77/432 (18) | ||
| Good | 42/513 (8) | 41/432 (9) |
*Based on change in Barthel index score from baseline (<0 or death=poor, 0-1=moderate, ≥2=good).
†Proportional odds of improvement in outcome after intervention compared with control; adjusted by care home as a random effect and type of care home and centre as fixed effects.
Sensitivity analyses
A sensitivity analysis excluding small clusters with fewer than three residents did not alter the results. Similarly, imputation of missing Barthel index scores using three methods (best case, worst care, and multiple imputation) did not change the conclusions. A further complete case analysis tested the robustness of the reported Barthel index analysis and gave similar results. The complete case analysis did not involve imputing zero for those with missing data due to death. The difference in adjusted mean Barthel index score for the complete case analysis between the groups at three months was 0.15 higher in the intervention arm (95% confidence interval −0.33 to 0.64, P=0.53). To examine the influence of a potential ceiling effect, we excluded the 52/1042 (5%) participants who had a baseline Barthel index score of 18 or more. The result was unchanged (difference in adjusted means between the two groups was 0.12, −0.38 to 0.61, P=0.64).
Economic analysis
Based on cost per quality adjusted life year, we found it unlikely that the trialled intervention was more cost effective than usual treatment. We did not find a reduction in health resource use that could have outweighed the cost of the intervention. Therefore, although outcomes were virtually equivalent in both arms, costs were higher in the intervention arm.
Discussion
In this phase III cluster randomised controlled trial we found no evidence of benefit of a three month course of individualised occupational therapy, involving patient centred goal setting, education of staff, and appropriate adaptation of the environment, for care homes residents with stroke related disabilities. The intervention did not have an impact on participants’ level of functional activity, as measured by the Barthel index at each endpoint. Furthermore, subgroup analyses found no evidence of a difference in Barthel index scores in any subgroup (fig 3). Removal of clusters with fewer than three residents, or imputation of missing data, did not change this result. We also found no evidence of any influence of the intervention on the secondary measures (mood, mobility, and health related quality of life). A process evaluation examining the fidelity of the occupational therapy intervention for residents with stroke related disabilities living in UK care homes is presented elsewhere.31 The indications of promise observed during the pilot phase were not substantiated.12
A fundamental difference between this trial and the pilot trial was the mean Barthel index score at baseline. In the pilot trial, the mean baseline Barthel score in the intervention arm was in the moderate range, whereas the mean baseline score in the intervention arm of the larger trial was in the severe range, with 268 out of 562 (47%) participants graded as very severe (table 1). The observed prevalence of severe limitations on functional activity among UK care home residents with stroke related disabilities is one of the results that deserves attention from this trial.
No adverse events attributable to the intervention were reported; however, a significantly higher rate of falls per resident was reported in the intervention arm than in the control arm (482 v 408) during the first three months. The mean number of falls per resident during the first three months in the intervention arm was 0.18 (SD 0.52). According to recently published figures,32 33 34 the average fall risk for older adults living in long term care institutions varies from 1.49-2.5 per annum. This suggests that the quarterly fall rate of 0.18 observed in the intervention arm was within the normal range.
Strengths and weaknesses of this study
This is the largest cluster randomised controlled trial of occupational therapy conducted in care homes. Recruitment levels were high and a large number of care homes indicated interest. We found that many care home managers were receptive to research activity seeking to benefit residents. The large geographical distribution of different types of care home, combined with the involvement of a high number of qualified therapists and a protocol that did not exclude resident survivors of stroke with cognitive and communication impairments, increase the potential for generalisability of the observed results to all care homes within the United Kingdom. Tolerance of the intervention was good, resulting in no adverse events and high completion rates for all assessments at each endpoint. Participant baseline characteristics were representative of the UK care home population for age, sex balance, and levels of support needed.35 The occupational therapy offered to participants was similar to that shown to benefit survivors of stroke living in their own homes, and similar to a standard NHS intervention.7 The sample size calculation was based on an intracluster correlation coefficient of 0.37 from a previous pilot study.28 The unadjusted intracluster correlation coefficient for Barthel index scores in this trial was 0.36 at baseline; however, for the change in scores from baseline to three months, allowing for the effect of treatment, trial administrative centre, and type of care home, it decreased to 0.09 (table 3).
Several potential limitations are acknowledged. Firstly, the percentage of care home residents affected by stroke was less than expected, which resulted in a larger number of small clusters than was originally anticipated. It has been noted elsewhere that the incidence of stroke in UK care homes decreased between 2009 and 2012, from 20% to 16%.36 The 16% incidence of stroke in the current study concurred with this finding. Despite multiple attempts at contacting general practices, confirmation of participants’ stroke was missing in 17% of cases across both treatment arms, indicating a lack of integration between care homes and local health services.37 The mean age of participants (82.9 years) was also lower than expected. There were six reported cases of treatment allocation being revealed to the assessors by residents or staff. The potential influence of these cases of unblinding on the overall result is regarded to be minimal.
The high proportion of participants with cognitive impairment and depression scores indicative of moderate to severe depression may have potentially limited engagement in therapy. Similarly, most participants (>70%) were graded as severe or very severe on the Barthel index at baseline, which may also have limited the participants’ capacity to engage in therapy (table 1). It is possible the occupational therapy intervention was more suited to participants graded as less severe on the Barthel index scale at baseline (fig 3). However, the current data did not support this assertion. The severity rating of Barthel index score at baseline had no significant mediating influence on participants’ response to the intervention at three months according to the exploratory subgroup analysis. Despite these potential limitations, the estimates of the potential effect of the intervention are regarded as precise. The evidence presented here does not support the use of a routine occupational therapy intervention to maintain levels of functional activity for older care home residents with stroke related disabilities. However, it may be the case that individual referrals within a care home setting may be of benefit to residents with lower levels of impairment.
Findings in context
These findings concur with the results of other recently published large randomised control trials conducted in care homes.38 39 Furthermore, a recent meta-analysis that focused on the influence of physical rehabilitation on the performance of personal activities of daily living for residents in long term institutional care reported the potential for relatively small effect sizes overall.40 In light of these neutral results, it prompts the question “Do we expect too much from this predominantly frail population, with a high incidence of cognitive impairment, depression, and dementia, to respond to individual activity based interactive interventions (fig 2)?” A reasonable conclusion from this trial is that most participants were incapable of engaging in therapy. A recent review by the Care Quality Commission highlighted the need for residents in care homes to exert choice and control over their healthcare, whenever possible, and to promote care activities that are, most importantly, safe and that respect residents’ dignity.41 These are values that the intervention was attempting to promote, but the findings suggest that this inactive population, with low autonomy, may need alternative approaches. Furthermore, the concept and application of patient centred goal setting may require further scrutiny in the context of this clinically complex population.
A changing role of care homes is acknowledged in recent reports from the Centre for Policy and Aging.35 36 The emphasis now is more on providing care for residents with high support needs for a short period towards the end of life. Attention must be given to how the care home environment can be suitably modified to tackle these needs. Observations from therapists administering the intervention were that the level of adaptive equipment in use in the participating care homes was relatively low and highly variable. As a result of the number of patients after stroke transferring directly from hospital to a care home environment,3 as opposed to returning home, it is necessary for rehabilitation and social care services to achieve equivalent standards, especially for those patients living with high support needs.9 42 It is predicted that by 2031, 22% of the population will be more than 65 years old, and the over 85s age bracket is the fastest growing sector.43 44 The prevalence of stroke and dementia in this population suggests a huge demand for long term care facilities both now and in the future.45 Future research needs to identify applicable criteria to promote an enabling environment within care homes.
What is already known on this topic
Survivors of stroke residing in care homes tend to have increased cognitive and physical impairments than those living in the community
Occupational therapy provided to survivors of stroke living at home has good evidence of benefit
Evidence in the literature on the efficacy of occupational therapy for older residents of care homes is conflicting, but until now the randomised controlled trials have been small and underpowered
What this study adds
The results of this large phase III cluster randomised controlled trial found no evidence of benefit of a three month course of individualised occupational therapy, involving patient centred goal setting, education of staff, and adaptations to the environment for care home residents with stroke related disabilities
Observed limitations on functional activity in this population were more severe than anticipated
Providing and targeting ameliorative care in this clinically complex population requires alternative strategies
We thank the participants, care home staff, care home managers, owners, and providers involved in the trial; and the occupational therapists who delivered the intervention: A Lake, S Kilmister, V Blakemore, R Merrick, B Jenkins, H Nicholls, L Whitfield, J Mackenzie, K Wood, B Lang, S Baker, S Rundle, A McMichael, S Evans, J Shirley, and M March.
Participating centres and collaborative group members: S Bevan, M Feltham, G Yao, S Herron-Marx, N Russell, L M Harris, and P Bradburn (Birmingham); E Roberts, H Owen, and J Shirley (Bangor); K Saunders, B Longland, A Orpen, J Bell, and C Ovington (Bournemouth); H Wright and C Randall (Coventry); M Auton, D Fawshaw, and K Patel (Lancashire); G Sands, K Mortimer, G Barton, E Costello, D Kelly, and P Sharp (Norwich); A Moody, C Coole, and C Edwards (Nottingham); L Househam, R Truscott, and C Brown (Plymouth); J Williams, L Burton, and C Colwell (Portsmouth); H Mackey, K Finney, K Townshend, and S Lyjko (Staffordshire); S Glanfield, L Caudwell, J Homan, and S Edwards (Taunton); and J Bisiker and K Preece (Wolverhampton).
OTCH trial investigators: C M Sackley, M F Walker, A K Roalfe, C R Burton, J Mant, C L Watkins, B Sheehan, K Wheatley, J Fletcher-Smith, L Sharp, K E Stant, K Wilde, K Steele, L Irvine, G Peryer, along with K Lett (Primary Care Clinical Sciences, School of Health and Population Sciences, University of Birmingham); Jane Williams (Portsmouth Hospitals NHS Trust, Queen Alexandra Hospital, Portsmouth); Farzana Rashid (Primary Care Clinical Sciences, University of Birmingham); Garry Barton (Faculty of Medical and Health Sciences, University of East Anglia, Norwich); and Patricia Masterson-Algar (School of Healthcare Sciences, Bangor University).
Trial steering committee (independent members): Helen Dawes (Oxford Brookes University, Oxford); Rowan Harwood (Nottingham University Hospitals NHS Trust); Pip Logan (Queen’s Medical Centre, Nottingham); Norman Phillips (patient representative); and Martin Underwood (Clinical Trials Unit, University of Warwick).
Data monitoring committee: David Barer (Newcastle Biomedicine, Medical School, Newcastle University); Gail Mountain (ScHARR, University of Sheffield); and John Norrie (Health Services Research Unit, University of Aberdeen).
Contributors: CMS was chief investigator. CMS, MFW, CRB, CLW, JM, KWh, BS, KL, JW, and LS were coapplicants for funding. CMS, MFW, JF-S, and KES developed the intervention. KWi, KES, FR, and JF-S were involved in recruitment. AR was the trial statistician. LI was the health economist. AR and GP analysed data and created the figures. GP and CMS drafted the paper. All authors contributed to developing, and approved, the submitted article. CMS is the guarantor.
Funding: The OTCH study was funded by the UK National Institute for Health Research Health Technology Assessment programme (08/14/30). The results will be published in full in Health Technology Assessment. All researchers acted independently to the funding body. The funding body had no role in designing the study, data collection, analysing and interpreting the data, or writing the report. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the funding body, the NHS, or the Department of Health. The research team acknowledge the support of the NIHR, through the Comprehensive Clinical Research Network, in particular, Jacqueline Briggs and Rhian Hughes. The corresponding author (GP) confirms full access to all the data in the study and had final responsibility for the decision to submit for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: CMS, LS, CRB, JF-S, CLW, KWh, LI, and GP had financial support from an NIHR HTA research grant for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: This study was approved by Coventry research ethics committee (09/H1210/88).
Data sharing: Dataset available from the trial statistician (a.k.roalfe@bham.ac.uk). Data sharing consent was not obtained. All data are anonymised with a low risk of identification.
Transparency: The lead author (CMS) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Cite this as: BMJ 2015;350:h468
References
- 1.Harwood RH. Do we still need care homes? Age Ageing 2004;33:529-30. [DOI] [PubMed] [Google Scholar]
- 2.Hankey GJ. The global and regional burden of stroke. Lancet Glob Health 2013;1:e239-e40. [DOI] [PubMed] [Google Scholar]
- 3.Stroke Association. Don’t forget about care home residents. 2014. www.stroke.org.uk/research/achievements/carehomes.
- 4.World Health Organization. International classification of functioning, disability and health (ICF). 2001. www.who.int/classifications/icf/icf_more/en/.
- 5.Sackley C, Levin S, Cardoso K, Hoppitt T. Observations of activity levels and social interaction in a residential care setting. Int J Ther Rehabil 2006;13:370-3. [Google Scholar]
- 6.Sackley C, Brittle N, Patel S, Ellins J, Scott M, Wright C, et al. The prevalence of joint contractures, pressure sores, painful shoulder, other pain, falls, and depression in the year after a severely disabling stroke. Stroke 2008;39:3329-34. [DOI] [PubMed] [Google Scholar]
- 7.Legg L, Drummond A, Leonardi-Bee J, Gladman JRF, Corr S, Donkervoort M, et al. Occupational therapy for patients with problems in personal activities of daily living after stroke: systematic review of randomised trials. BMJ 2007;335:922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker MF, Leonardi-Bee J, Bath P, Langhorne P, Dewey M, Corr S, et al. Individual patient data meta-analysis of randomized controlled trials of community occupational therapy for stroke patients. Stroke 2004;35:2226-32. [DOI] [PubMed] [Google Scholar]
- 9.National Institute for Health and Care Excellence. Stroke rehabilitation: long term rehabilitation after stroke. (Clinical guideline 162.) 2013. http://guidance.nice.org.uk/CG162.
- 10.Barodawala S, Kesavan S, Young J. A survey of physiotherapy and occupational therapy provision in UK nursing homes. Clin Rehabil 2001;15:607-10. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher-Smith JC, Walker MF, Cobley CS, Steultjens EM, Sackley CM. Occupational therapy for care home residents with stroke. Cochrane Database Syst Rev 2013;6:CD010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sackley C, Wade D, Mant D, Atkinson J, Yudkin P, Cardoso K, et al. Cluster randomized pilot controlled trial of an occupational therapy intervention for residents with stroke in UK care homes. Stroke 2006;37:2336-41. [DOI] [PubMed] [Google Scholar]
- 13.Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud 1988;10:61-3. [DOI] [PubMed] [Google Scholar]
- 14.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965;14:61-5. [PubMed] [Google Scholar]
- 15.Collen FM, Wade DT, Robb GF, Bradshaw CM. The Rivermead Mobility Index: a further development of the Rivermead Motor Assessment. Int Disabil Stud 1991;13:50-4. [DOI] [PubMed] [Google Scholar]
- 16.Sackley C, Burton C, Herron-Marx S, Lett K, Mant J, Roalfe A, et al. A cluster randomised controlled trial of an occupational therapy intervention for residents with stroke living in UK care homes (OTCH): study protocol. BMC Neurol 2012;12. [DOI] [PMC free article] [PubMed]
- 17.Van Wijk I, Lindeman E, Kappelle LJ, van Gijn J, Koudstaal PJ, Gorter JW, et al. Functional status and use of healthcare facilities in long-term survivors of transient ischaemic attack or minor ischaemic stroke. JNNP 2006;77:1238-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ministry of Justice. Mental Capacity Act. Stationery Office, 2005.
- 19.Puffer S, Torgerson DJ, Watson J. Evidence for risk of bias in cluster randomised trials: review of recent trials published in three general medical journals. BMJ 2003;327:785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paterson M, Higgs J, Wilcox S. Developing expertise in judgement artistry in occupational therapy practice. Br J Occup Ther 2006;69:115-23. [Google Scholar]
- 21.Van den Berg M, Lett K, Sackley C. A workshop to maintain residents’ mobility and activity. Nurs Times 2006;102:32-4. [PubMed] [Google Scholar]
- 22.Gompertz P, Pound P, Ebrahim S. A postal version of the Barthel Index. Clin Rehabil 1994;8:233-9. [Google Scholar]
- 23.Hsieh YW, Wang CH, Wu SC, Chen PC, Sheu CF, Hsieh CL. Establishing the minimal clinically important difference of the Barthel Index in stroke patients. Neurorehabil Neural Repair 2007;21:233-8. [DOI] [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. [DOI] [PubMed] [Google Scholar]
- 25.Syder D Body R, Parker M, Boddy M. Sheffield screening test for acquired language disorders. NFER Nelson, 1993.
- 26.Sheikh J, Yesavage J. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol 1986;5:165-73. [Google Scholar]
- 27.EuroQol-a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy 1990;16(3):199-208. [DOI] [PubMed] [Google Scholar]
- 28.Sackley C, Rodriguez N, van den Berg M, Badger F, Wright C, Besemer J, et al. A phase II exploratory cluster randomized controlled trial of a group mobility training and staff education intervention to promote urinary continence in UK care homes. Clin Rehabil 2008;22:714-21. [DOI] [PubMed] [Google Scholar]
- 29.Sackley C, van den Berg M, Lett K, Patel S, Hollands K, Wright C, et al. Effects of a physiotherapy and occupational therapy intervention on mobility and activity in care home residents: a cluster randomised controlled trial. BMJ 2009;339:670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR, Fanjiang G. Mini-Mental State Examination: user’s guide. Psychological Assessment Resources, 2001.
- 31.Masterson-Algar P, Burton CR, Rycroft-Malone J, Sackley CM, Walker MF. Towards a programme theory for fidelity in the evaluation of complex interventions. J Eval Clin Pract 2014;20:445-52. [DOI] [PubMed] [Google Scholar]
- 32.Damian J, Pastor-Barriuso R, Valderrama-Gama E, de Pedro-Cuesta J. Factors associated with falls among older adults living in institutions. BMC Geriatr 2013;13:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rapp K, Becker C, Cameron ID, König H-H, Büchele G. Epidemiology of falls in residential aged care: analysis of more than 70,000 falls from residents of Bavarian nursing homes. J Am Med Dir Assoc 2012;13:187.e1-.e6. [DOI] [PubMed] [Google Scholar]
- 34.Whitney J, Close JCT, Jackson SHD, Lord SR. Understanding risk of falls in people with cognitive impairment living in residential care. J Am Med Dir Assoc 2012;13:535-40. [DOI] [PubMed] [Google Scholar]
- 35.Centre for Policy and Aging. A profile of residents in Bupa care homes: results from the 2012 Bupa census. 2012. www.cpa.org.uk/information/reviews/Bupa-Census-2012.pdf.
- 36.Lievesley N, Bowman C, Crosby G. The changing role of care homes. 2011. www.cpa.org.uk/information/reviews/changingroleofcarehomes.pdf.
- 37.Gage H, Dickinson A, Victor C, Williams P, Cheynel J, Davies S, et al. Integrated working between residential care homes and primary care: a survey of care homes in England. BMC Geriatr 2012;12:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Underwood M, Lamb SE, Eldridge S, Sheehan B, Slowther AM, Spencer A, et al. Exercise for depression in elderly residents of care homes: a cluster-randomised controlled trial. Lancet 2013;382:41-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kerse N, Peri K, Robinson E, Wilkinson T, Von Randow M, Kiata L, et al. Does a functional activity programme improve function, quality of life, and falls for residents in long term care? Cluster randomised controlled trial. BMJ 2008;337:912-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crocker T, Young J, Forster A, Brown L, Ozer S, Greenwood DC. The effect of physical rehabilitation on activities of daily living in older residents of long-term care facilities: systematic review with meta-analysis. Age Ageing 2013;42:682-8. [DOI] [PubMed] [Google Scholar]
- 41.Care Quality Commission. Health care in care homes: a special review of the provision of health care to those in care homes 2012. www.cqc.org.uk/sites/default/files/media/documents/health_care_in_care_homes_cqc_march_2012.pdf.
- 42.Dworzynski K, Ritchie G, Fenu E, MacDermott K, Playford ED. Rehabilitation after stroke: summary of NICE guidance. BMJ 2013;346:f3615. [DOI] [PubMed] [Google Scholar]
- 43.Bray H. Office of National Statistics. 2006-based national population projections for the UK and constituent countries. Population Trends 2008;131:8-18. [PubMed] [Google Scholar]
- 44.Department of Health, OPD: a new ambition for old age—next steps in implementing the National Service Framework for Older People. 2006. www.psige.org/public/files/A%20New%20Ambition%20for%20Old%20Age.pdf.
- 45.Macdonald A, Cooper B. Long-term care and dementia services: an impending crisis. Age Ageing 2007;36:16-22. [DOI] [PubMed] [Google Scholar]