Abstract
Intestinal resections are frequently required for treatment of diseases involving the gastrointestinal tract, with Crohn’s disease and colon cancer being two common examples. Despite the frequency of these procedures, a significant knowledge gap remains in describing the inherent effects of intestinal resection on host physiology and disease pathophysiology. This article provides detailed instructions for an ileocolic resection with primary end-to-end anastomosis in mice, as well as essential aspects of peri-operative care to maximize post-operative success. When followed closely, this procedure yields a 95% long-term survival rate, no failure to thrive, and minimizes post-operative complications of bowel obstruction and anastomotic leak. The technical challenges of performing the procedure in mice are a barrier to its wide spread use in research. The skills described in this article can be acquired without previous surgical experience. Once mastered, the murine ileocolic resection procedure will provide a reproducible tool for studying the effects of intestinal resection in models of human disease.
Keywords: Medicine, Issue 92, Ileocolic resection, anastomosis, Crohn's disease, mouse models, intestinal adaptation, short bowel syndrome
Introduction
Ileocolic resection (ICR) is a common procedure performed in both emergent and elective situations for a variety of illnesses. Crohn’s disease and colon cancers are the two most common indications for ICR. In both illnesses, recurrence in the bowel at the site of surgery represents a major clinical problem. Local recurrence rates for colon cancer remain an issue even with the most aggressive resections1. Following ICR in Crohn’s disease, the disease most frequently (in up to 80%) recurs in the neo-terminal ileum at 1 year after surgery2. Given the impact of these two illnesses and their recurrence after surgery, it is important to understand local intestinal factors after ICR, which may have intrinsic influences on the natural history of these diseases. Further, it is also important to consider anastomotic healing after ICR. In the early post-operative period, anastomotic leaks can have devastating consequences for patients resulting in repeat surgeries, stoma creations, significant morbidity, and even mortality3. Despite the importance of this topic, our current understanding of anastomotic healing remains in its infancy as a subject of research. Animal models of ICR, in particular mice, are an excellent platform for studying the intestinal and anastomotic healing following surgery.
A mouse model of ICR was initially developed by Helmrath et al. to be used as a model of short gut syndrome4. The authors experimented with various diet regiments and suture sizes to optimize animal survival following ICR. They concluded that feeding with liquid diet in the perioperative period and using 9-0 monofilament sutures resulted in an optimal post-operative survival of 88%. Since this initial publication, ICR in mice removing 50% of the small bowel has been used in several studies to explore the dynamics of massive small bowel resection and the adaptive growth response in attempt to develop new therapies for short gut syndrome5,6.
The first application of the ICR mouse model to Crohn’s disease used the IL-10-/- mouse model, which spontaneously develops colitis7. The authors found that after ICR these animals developed inflammation in the neo-terminal ileum similar to that seen in post-operative Crohn’s disease patients, and that this inflammation was dependent on the presence of bacteria7. More recently, this model was used to explore bacterial changes induced by ICR. In Crohn’s disease there is an associated dysbiosis with relative decreases in bacteria known to have anti-inflammatory properties and increases in invasive species of bacteria8,9. The association holds true in cases of post-operative recurrence10. Two studies sought to identify microbial changes resulting from ICR. The first used IL-10 null mice, and performed denaturing gel electrophoresis to compare bacterial similarity between the small bowel and colon after ICR11. This study demonstrated that bacterial populations became similar in the small intestine and colon following ICR. A subsequent study used wild type mice and 16s pyrosequencing for phylogenetic classification of bacterial species post-operatively. This study demonstrated a marked shift in bacterial species resulting from surgery alone with Clostridium species becoming dominant as well as an increase in ϒ-proteobacteria. The results also confirmed the findings of the previous study with similar populations found in the small bowel and colon after ICR12.
ICR is a common procedure for patients with colon cancer involving the cecum and ascending colon, and it is becoming increasingly recognized that the host-response to surgery likely contributes to both local and distant tumor recurrence13. Despite this observation, models of ICR have not been utilized for the study of colorectal cancer and post-operative recurrence. Understanding both the systemic and local immunologic changes resulting from ICR will be important in investigating future therapies. Potential pathways involved in cancer recurrence post ICR include up regulation of growth factors, which may rescue cells from apoptosis and stimulate proliferation, mechanical tumor disruption with cell shedding, and loss of immune surveillance through post-operative immunosuppression13,14.
Mouse models of ICR have the potential to be a powerful tool for the investigation of short bowel syndrome, Crohn’s disease, and colon cancer. They may also provide lessons on how to prevent early post-operative anastomotic complications by further defining the cellular and biochemical pathways involved in healing the newly constructed anastomosis. A major barrier in utilizing the murine ICR model is the technical difficulty. The intestinal anastomosis requires the use of 8-0 or 9-0 suture, an operating microscope, and training in microsurgical techniques. The goal of this article is to provide clear instructions on how to perform ICR in mice with the goal of utilizing this procedure in models of disease.
Protocol
Animal use protocols were approved by the Health Science Animal Care and Use committee at the University of Alberta.
1. Preparation of Instruments, Animals and Operative Setup
Transfer animals to a new, clean cage absent of all solid food 24 hr prior to the procedure. They may have free access to water, and liquid diet ad lib until the time of the procedure.
Autoclave all instruments required for the procedure. Clean operating surface and anesthetic nose cone with 70% ethanol.
Set up the operating surface with operating microscope, anesthetic machine and supplies in a manner which is comfortable for the operating surgeon. The elbows of the surgeon should be allowed to rest comfortably on the operating table, with hands and arms unobstructed by equipment. Instruments, sutures, cotton swabs, and a 10 ml syringe should be placed in a location that permits easy access during the procedure.
Set up overhead heat lamps to provide both warmth for the animal during the procedure and light for the operating surface.
Fill a 50 ml conical tube with 0.9% saline, and a 1.5 ml tube with petroleum jelly and place near the operating surface. NOTE: Sterilize all instruments and surgical supplies. Because the intestine is transected, the procedure itself is not sterile. It is considered clean-contaminated. Take measures to avoid the introduction of exogenous sources of infection.
2. Ileocolic Resection with Anastomosis
Induce anesthesia by administering 4% isoflurane with an oxygen flow rate of 2 L/min via nose cone from the isoflurane vaporizer until the animals respiratory rate slows to approximately 30-40 breaths/min. Apply moderate pressure to the hind foot of the mouse to ensure there is no pain response prior to initiating the procedure. At this point, turn down isoflurane to 2% and oxygen flow to 0.5 L/min. Intermittently check pain response during the procedure and adjust isoflurane flow rate accordingly.
Apply petroleum jelly to the eyes to prevent drying during surgery, and immobilize the mouse in the supine position with limbs secured using transparent tape.
Clean the abdomen with povodine/iodine solution, and change into new sterile gloves.
Make a 1.5 cm skin incision in the upper midline of the abdomen using sharp point dissecting scissors to expose the fascia and peritoneum. Open the fascial/peritoneal layer in a similar fashion through the linea alba to expose the peritoneal contents.
In contrast to humans, the mouse cecum is typically found in the left upper quadrant of the abdomen. Once identified, gently grasp the cecum with forceps and deliver it through the incision. Use moistened cotton swabs to fan out approximately 3 cm of terminal ileum extending from the cecum over a sterile gauze draped on the abdominal surface (Figure 1A). Ensure the exposed bowel is kept moist with 0.9% saline during the entirety of the procedure.
Identify the ileocecal artery branching off the superior mesenteric artery along the colon (Figure 1A) using the operating microscope. Dissect out the avascular tissues adjacent to the ileocecal artery, encircle and ligate the artery with a 5-0 silk tie. Next, locate the regional blood supply to the terminal ileum and choose a transection point 1.5-2 cm proximal to the ileocecal junction. Ligate the branches to this section of ileum as above. Divide the arteries with micro dissecting scissors.
Divide the ischemic portions of ileum and colon ensuring there is adequate blood supply to the transected ends (Figure 1B). It is often helpful to spatulate the ileum by dividing it at a 30 degree angle to increase the diameter of the lumen so it more closely matches the colon. Once the ileocecal portion of bowel has been removed, align the transected ends of ileum and colon on the gauze, ensuring the mesenteric borders of each are aligned.
Construct the anastomosis by approximating the transected end of ileum to the transected end of colon using interrupted 8-0 polypropylene sutures (Figure 1C). The first stitch is placed at the mesenteric border, with subsequent sutures placed every 0.5 mm until the Ileocolic anastomosis is watertight. When passing the suture needle through the ileum and colon, ensure that the cut edge is not rolled, and needle bites are 0.5 mm from the cut edges of the bowel. A typical anastomosis will require 14 to 16 interrupted sutures. Test the integrity and patency of the anastomosis upon completion by rolling a cotton swab proximal to distal over the ileum to force contents through the anastomosis. Small bowel contents should freely pass into the colon without anastomotic leakage.
Rinse the exposed bowel with 3-4 ml of 0.9% saline from the 10 ml syringe to wash away stool from the surface of the bowel, and deliver bowel back into the peritoneal cavity. Using 2 ml of 0.9% saline flush the peritoneal cavity, and then drain this fluid by applying gentle pressure to the abdominal wall laterally.
Close the incision with a 3-0 silk running suture, and discontinue the flow of isoflurane. Administer 0.1 mg/kg of the long acting opiate –buprenorphine- subcutaneously for post-operative pain control.
Observe the animals under the heat lamp until they are mobile then transfer them to a continually warmed cage.
3. Post-operative Care and Monitoring
Monitor animals in a continually warmed cage for signs of distress for the remainder of the day. Transfer animals back to the animal care facility in a new sterile cage with access to liquid diet and water ad lib. Animals may be housed in groups of 3-4.
Perform a check on the post-operative animals the following morning, ensure the animals do not appear in distress. Feed only liquid diet. If they appear uncomfortable (i.e., hunched posture or minimal activity) administer an additional dose of subcutaneous buprenorphine. Check on the animals once more in the afternoon on post-operative day 1.
On the morning of post-operative day 2 the animals should appear fully recovered. Evidence of food consumption and stooling are positive signs of recovery. Now, resume a solid chow diet for the animals. NOTE: Signs of distress include hunched posture, poor grooming, and minimal activity. If signs of distress are prominent the animals should be euthanized.
Euthanize the animals by inducing deep anesthesia with 4% isoflurane at an O2 flow rate of 2 L/min until animals are unresponsive to foot pressure. Perform cervical dislocation, and observe for signs of proper euthanization.
Guidelines vary, so refer to the institutions recommendations regarding indications for and appropriate methods of euthanization in mice.
Representative Results
Mortality rates and weight change post-op. Mortality rates following ICR in 129S1 wild type mice are generally ~ 5%. The most common cause of morality is bowel obstruction at the anastomosis. Other causes of mortality include anastomotic leak and internal hernia leading to bowel obstruction.
Weight loss can be seen up to 14 days post-operatively, but is generally non-significant. Mice tend to completely regain pre-operative weight by post-operative day 28 (Figure 2).
Transferability of technique A new graduate student (BM) was taught to perform the ICR procedure to determine if an operator with no previous surgical training could learn the technique. Training began with learning how to perform the single interrupted sutures analogous to those used in the anastomosis using 8-0 nylon suture. Sutures were performed to close an incision made across a latex glove. After BM was able to efficiently place sutures through the glove without tearing, they observed three complete procedures prior to attempting one. After the first procedure was completed, they then observed another procedure and attempted two more. Of the three animals completed, the last two went on to survive and thrive. The first animal was euthanized due to complications at the anastomosis.
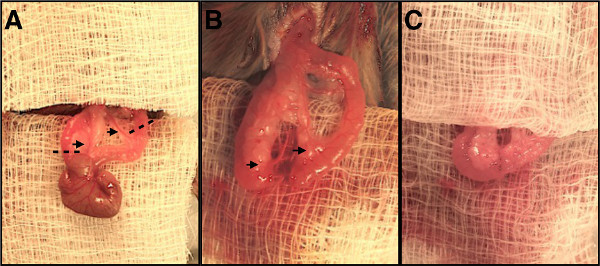 Figure 1. Stages of ileocolic resection and anastomosis. (A) Ileocecal region of bowel delivered out of abdomen. Black arrows indicate vessels to be ligated, dashed lines indicate transection points for colon and terminal ileum. (B) Ileocecal region removed, cut ends of bowel are aligned, remaining blood supply is demonstrated by black arrows. (C) Completed ileocolic anastomosis. Please click here to view a larger version of this figure.
Figure 1. Stages of ileocolic resection and anastomosis. (A) Ileocecal region of bowel delivered out of abdomen. Black arrows indicate vessels to be ligated, dashed lines indicate transection points for colon and terminal ileum. (B) Ileocecal region removed, cut ends of bowel are aligned, remaining blood supply is demonstrated by black arrows. (C) Completed ileocolic anastomosis. Please click here to view a larger version of this figure.
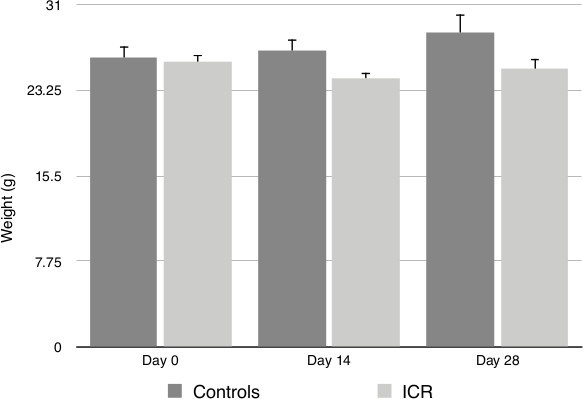 Figure 2. Animal weights at baseline, post-operative day 14 and post-operative day 28. No significant changes in weights were observed post-procedure as determined by the Mann-Whitney U statistical test. ICR (Ileocolic resection group), Control (Non-operative controls).
Figure 2. Animal weights at baseline, post-operative day 14 and post-operative day 28. No significant changes in weights were observed post-procedure as determined by the Mann-Whitney U statistical test. ICR (Ileocolic resection group), Control (Non-operative controls).
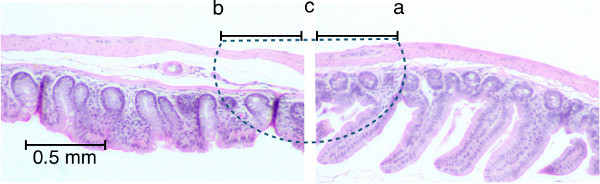 Figure 3. Representative path of sutures through intestinal tissues. H&E stained histologic sections of ileum (right) and colon (left) are lined up to demonstrate the trajectory of the suture needle through the tissues (dashed line) starting at point (a) through to point (b). The knot should be tied at point (c). Note that less mucosa is included in the anastomosis than other tissues. Please click here to view a larger version of this figure.
Figure 3. Representative path of sutures through intestinal tissues. H&E stained histologic sections of ileum (right) and colon (left) are lined up to demonstrate the trajectory of the suture needle through the tissues (dashed line) starting at point (a) through to point (b). The knot should be tied at point (c). Note that less mucosa is included in the anastomosis than other tissues. Please click here to view a larger version of this figure.
Discussion
The murine ICR is a powerful model that can be used to study the effects of surgery in bowel diseases. This article describes a method of performing ICR in mice with a success rate of 95% and no issues with failure to thrive as reflected by stable weights up to 28 days post-procedure. The most significant challenges to successful ICR include avoiding bowel obstructions at the anastomosis and anastomotic leaks.
Technical elements to the surgery for preventing obstruction are aimed at maximizing lumen diameter at the anastomosis. Spatulation of the ileum at the transection point increases the lumen diameter by dividing the bowel at a 30 degree angle as depicted in Figure 1A. When taking bites of intestinal tissue with the suture needle, it is essential to unroll the edges of the bowel and pass the needle approximately 0.5 mm from the cut edge. Excessively large bites of tissue will narrow the lumen. An option to avoid luminal narrowing when constructing the anastomosis is to employ the use of a digestible stent as described by Kiernan et al.15. Non-technical aspects to avoid obstruction include selecting a sufficiently small suture4 and avoiding bulky luminal contents by feeding animals a liquid diet one day prior to surgery and 2 days following surgery.
The second most common cause of post-operative mortality is anastomotic leak. Avoiding this complication requires strict adherence to the principles of intestinal anastomosis. The first being adequate blood supply. After the vessels supplying blood to the region of bowel being resected have been ligated, the bowel should be visually inspected prior to transection. The ischemic segment of bowel will appear dusky; this should be completely removed leaving healthy edges. It is important to visualize a small amount of bleeding from the cut edges of the remaining intestine. A water tight anastomosis is also essential to avoid spillage of luminal contents leading to sepsis. This is achieved by avoiding large gaps between sutures, and ensuring that all layers of the intestine are included in the stitch (Figure 3). Wound strength is greatly diminished in the first two post-operative days, thus properly placed sutures are essential to provide strength16,17. A potential pitfall is inadvertently only including the mucosa as the mucosa can pucker out of the lumen of the transected colon. This can be avoided by visualizing the cut edge of serosa and gently reducing prolapsed mucosa back into the lumen with forceps.
Sham surgical controls should be considered in the experimental design of studies using the ICR model to control for the potential confounding effects of surgical stress, intestinal manipulation, and/or loss of the ileocecal valve. The choice of sham procedure will depend on the primary question being asked in the study. For example, if the research objective is to evaluate the host response after losing the ileocecal valve, then it would be prudent to include a sham surgery group in which an intestinal anastomosis is performed while leaving the ileocecal valve in place. This is typically done by transecting the small intestine without resection then performing a small bowel anastomosis in the same fashion as the ileocolic anastomosis7,11. This would allow investigators to comment specifically on the effects of ileocecal valve loss by controlling for the effects of intestinal anastomosis. Alternatively, if the goal is only to study the effects of intestinal resection and anastomosis without commenting specifically on loss of the ileocecal valve, then a sham surgery should include a laparotomy, and the bowel should be treated as it would for ICR, but without transection and anastomosis. This would control for surgical stress and intestinal manipulation. The techniques described in the protocol above can be easily adapted for performing sham procedures.
This protocol describes an ileocolic resection, which removes 1.5-2 cm of terminal ileum and the entirety of the cecum, and closely mimics an operation frequently performed in patients with ileocolic Crohn’s disease. Similar procedures have been used in the IL-10-/- colitis model after which mice developed spontaneous inflammation and fibrosis in the small bowel analogous to Crohn’s disease recurrence7,11. The principles of this procedure may also apply to other models of disease. By removing 50% of the small bowel with ileocolic anastomosis, mouse models have been used to investigate the adaptive intestinal growth response in short gut syndrome4-6. In the future this may also become a useful model for studying the systemic and local effects of surgery in colorectal cancer.
Disclosures
The authors have nothing to disclose
Acknowledgments
We would like to acknowledge the funding contributions of the Canadian Surgical Research Fund, the Edmonton Civic Employees Research Assistance Fund, and the Alberta IBD Consortium through a grant from Alberta Innovates.
References
- Hallet J, Zih FS, Lemke M, Milot L, Smith AJ, Wong CS. Neo-adjuvant chemoradiotherapy and multivisceral resection to optimize R0 resection of locally recurrent adherent colon cancer. European Journal of Surgical Oncology. 2014;40(6) doi: 10.1016/j.ejso.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P, Geboes K, Vantrappen G. Natural history of recurrent Crohn's disease at the ileocolonic anastomosis after curative surgery. Gut. 1984;25(6):665–672. doi: 10.1136/gut.25.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D, Rivadeneira D. Complications of colorectal anastomoses: leaks, strictures, and bleeding. The Surgical clinics of North America. 2013;93(1):61–87. doi: 10.1016/j.suc.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Helmrath MA, VanderKolk WE, Can G, Erwin CR, Warner BW. Intestinal adaptation following massive small bowel resection in the mouse. Journal of the American College of Surgeons. 1996;183(5):441–449. [PubMed] [Google Scholar]
- Dekaney CM, Fong JJ, Rigby RJ, Lund PK, Henning SJ, Helmrath MA. Expansion of intestinal stem cells associated with long-term adaptation following ileocecal resection in mice. American journal of physiology. Gastrointestinal and liver physiology. 2007;293(5) doi: 10.1152/ajpgi.00218.2007. [DOI] [PubMed] [Google Scholar]
- Speck KE, De Cruz P, et al. Inflammation enhances resection-induced intestinal adaptive growth in IL-10 null mice. The Journal of surgical research. 2011;168(1):62–69. doi: 10.1016/j.jss.2009.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby RJ, Hunt MR, et al. A new animal model of postsurgical bowel inflammation and fibrosis: the effect of commensal microflora. Gut. 2009;58(8):1104–1112. doi: 10.1136/gut.2008.157636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic AD, Xavier RJ, Gevers D. The Microbiome in Inflammatory Bowel Disease: Current Status and the Future Ahead. Gastroenterology. 2014. pp. 1–11. [DOI] [PMC free article] [PubMed]
- Gevers D, Kugathasan S, et al. The Treatment-Naive Microbiome in New-Onset Crohn's Disease. Cell host & microbe. 2014;15(3):382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed T, Rieder F, Fiocchi C, Achkar JP. Pathogenesis of postoperative recurrence in Crohn's disease. Gut. 2011;60(4):553–562. doi: 10.1136/gut.2010.221705. [DOI] [PubMed] [Google Scholar]
- Borowiec A, Sydora B, et al. Small bowel fibrosis and systemic inflammatory response after ileocolonic anastomosis in IL-10 null mice. Journal of Surgical Research. 2012;178(1):147–154. doi: 10.1016/j.jss.2012.01.048. [DOI] [PubMed] [Google Scholar]
- Devine AA, Gonzalez A, et al. Impact of Ileocecal Resection and Concomitant Antibiotics on the Microbiome of the Murine Jejunum and Colon. 2013;8(8) doi: 10.1371/journal.pone.0073140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bij GJ, Oosterling SJ, Beelen RHJ, Meijer S, Coffey JC, van Egmond M. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Annals of surgery. 2009;249(5) doi: 10.1097/SLA.0b013e3181a3ddbd. [DOI] [PubMed] [Google Scholar]
- Scott AD, Uff C, Phillips RK. Suppression of macrophage function by suture materials and anastomotic recurrence of Crohn's disease. The British journal of surgery. 1993;80(3) doi: 10.1002/bjs.1800800342. [DOI] [PubMed] [Google Scholar]
- Kiernan JA. Intestinal anastomosis in the rat facilitated by a rapidly digested internal splint and indigestible but absorbable sutures. Journal of Surgical Research. 1988;45:427–431. doi: 10.1016/0022-4804(88)90192-8. [DOI] [PubMed] [Google Scholar]
- Andersen TL, et al. Action of matrix metalloproteinases at restricted sites in colon anastomosis repair: an immunohistochemical and biochemical study. Surgery. 2006;140(1):72–82. doi: 10.1016/j.surg.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Thompson SK, Chang EY, Jobe BA. Clinical review: Healing in gastrointestinal anastomoses, Part I. Microsurgery. 2006;26(3):131–136. doi: 10.1002/micr.20197. [DOI] [PubMed] [Google Scholar]


