Abstract
When proposing the use of a drug, drug combination, or drug delivery into a novel system, one must assess the pharmacokinetics of the drug in the study model. As the use of mouse models are often a vital step in preclinical drug discovery and drug development1-8, it is necessary to design a system to introduce drugs into mice in a uniform, reproducible manner. Ideally, the system should permit the collection of blood samples at regular intervals over a set time course. The ability to measure drug concentrations by mass-spectrometry, has allowed investigators to follow the changes in plasma drug levels over time in individual mice1, 9, 10. In this study, paclitaxel was introduced into transgenic mice as a continuous arterial infusion over three hours, while blood samples were simultaneously taken by retro-orbital bleeds at set time points. Carotid artery infusions are a potential alternative to jugular vein infusions, when factors such as mammary tumors or other obstructions make jugular infusions impractical. Using this technique, paclitaxel concentrations in plasma and tissue achieved similar levels as compared to jugular infusion. In this tutorial, we will demonstrate how to successfully catheterize the carotid artery by preparing an optimized catheter for the individual mouse model, then show how to insert and secure the catheter into the mouse carotid artery, thread the end of the catheter out through the back of the mouse’s neck, and hook the mouse to a pump to deliver a controlled rate of drug influx. Multiple low volume retro-orbital bleeds allow for analysis of plasma drug concentrations over time.
Keywords: Medicine, Issue 92, pharmacokinetics, paclitaxel, catheter, carotid artery, infusion, tissue distribution
Introduction
Drug infusion through the carotid can be performed reliably and reproducibly by optimizing equipment and technique. The procedure is not intricate, although it does require fine control and attention to detail. Superior care and dexterity are needed to isolate the carotid artery and insert the catheter, which can generally be acquired through practice. Surgery by an experienced technician should not exceed one hour. After successful surgery, the mouse should appear normal and healthy (although the mouse may react to the actual drug infusion), and drug(s) may be administered in a controlled, uniform continuous dosage. Blood samples must be taken from a site other than the carotid artery; retro-orbital bleeds proved easy to collect and satisfactory for analysis of drug concentrations.
Catheters of optimum size and shape are an invaluable asset in performing a successful infusion11. We found the catheters available commercially often to be too large and/or too flexible to allow for convenient access to the mouse carotid artery. It proved preferable to fashion catheters from the polyethylene tubing used to connect the mouse to the infusion syringe. Thus, all the tubing, connectors and needles were of consistent dimensions, which simplified infusion assembly. Using this technique, it is not necessary to push the tip of the catheter into the artery past the point where it is still visible, and blood flow to the carotid artery is not restored until after the catheter is initially secured. This reduces the hazards of puncturing the artery or of having the catheter pushed out by the high pressure of blood flow. The catheter design herein does not incorporate a “bump” to hold it in place, so securing the catheter well with sutures and surgical tape is a priority.
Infusions may be preferable to the common i.v. bolus injections, as a better mimic of clinical administration of drugs such as taxanes3, 12, 13. The technique described here was originally developed to allow infusion into mouse models wherein access to the jugular or femoral vein was precluded by mammary tumor growth and/or excessive vascularization of the insertion area. This method may often be appropriate even in tumor-free mice: although isolating and catheterizing the carotid is slightly more invasive, we found it preferable to the jugular, because the propensity of the jugular wall to rip resulted in more failed insertions and failures to complete the 3 hr time course.
While the results shown here are from C57BL/6J (in-house-bred) mice, we have used this technique to successfully infuse paclitaxel into several strains of mice, including FVB and mixed-strains, to follow the pharmacokinetics in mouse models transgenically manipulated to down-regulate cellular transporter functions. The blood and tissue samples collected showed expected levels of paclitaxel, in the range of the levels seen after jugular infusions1. This technique may be expected to work equally well in other mouse models and with other infusion solutions.
Protocol
This protocol has been approved by the Fox Chase Cancer Center Institutional Animal Care and Use Committee and by the Laboratory Animal Facility, and found to be in accordance with institutional guidelines for humane treatment of animals.
1. Preliminary Preparation
- Preparation of catheter: Prepare a catheter from a short length of polyethylene tubing, modified to form a thinned, blunted end (Figure 3A). Make multiple catheters in advance and save indefinitely.
- Light a Bunsen burner, and adjust to establish a low steady flame. Hold tubing close to flame to soften polyethylene. When tubing begins to melt, slowly pull apart the two ends to create a thinned section of tubing, approximately 0.25 mm OD.
- Cut a beveled end approximately 0.75 cm along thin section. This ensures enough tubing to be secured in artery, without creating an overly long catheter. NOTE: A long thin end on the catheter has a propensity to clog. An overly long end may also hold enough liquid to significantly change the catheter’s liquid volume capacity.
- Blunt the beveled end by passing quickly through the flame — when properly heated, the end becomes slightly rounded and enlarged. The tip hooks back slightly, which helps anchor the tubing while inserting into the artery.
- Cut the tubing 6.0 cm from the point where it begins to thin. This makes a very manageable catheter, that is long enough to thread through to exit from the neck and to hold and work with comfortably, but short enough to prevent the mouse from gnawing on excess tubing or requiring too much extra infusion volume to clear initial saline.
- Prepare a syringe of approximately 0.2 ml heparin solution, topped with a blunted needle. Insert needle into the wide end of the catheter (Figure 3B). Fill catheter with heparin, being careful to ensure that there are no bubbles in the tubing. Set the heparin syringe and catheter aside on a sterile area. Sterilize catheters by gamma irradiation, by placing catheter(s) in a petri dish, and exposing to 20 Gy of gamma irradiation. If you do not have access to an gamma radiation source, check with your animal facility to investigate other means of sterilization, such as gas- or chemical-sterilization. Do not autoclave, as polyethylene cannot be heat sterilized.
- Creation of a saline lead (Figure 3B).
- Prepare a second syringe of approximately 0.5 ml sterile saline, being careful to ensure a bubble-free line.
- Cut a second piece of tubing, approximately 15 cm, and slip onto the blunted syringe needle. Attach a connector port to the free end of the tubing.
- Test that flow of saline is unobstructed by smoothly advancing a small volume of saline through the lead. Use this saline lead after catheter insertion, to check the flow through the catheter, and to flush the line of blood. Set the saline syringe aside on a sterile area.
Prior to surgery, sterilize equipment by autoclaving, or alternatively by gas sterilization or glass bead sterilization.
- Prepare sterile surgical area.
- Wipe bench and microscope surfaces with disinfectant such as 70% ethanol or chlorine dioxide. Cover bench and microscope base with a clean disposable, absorbent pad.
- Prepare a surgical board by covering with two layers of clean, absorbent paper, securely attached with adhesive tape.
- Lay out all surgical supplies (as cataloged in Materials List) so that they are readily accessible.
- Cut three lengths of sterile suture, 8 cm each, and set aside with other supplies. Place a port where it will be easily available (Figure 3B).
2. Surgery
- Preparation of the animal
- Anesthetize mouse by exposure to 2-3% isoflurane in anesthesia chamber connected to precision vaporizer. Withdraw mouse from the chamber, and shave hair from the mouse’s neck / upper torso, and below the right ear (site of catheter exit). Administer veterinary petrolatum ophthalmic ointment to eyes to prevent dryness while under anesthesia. Ensure the animal does not awaken during surgical preparations by allowing mouse sufficient time in inhalation chamber before prep (at least two min), or by administering isoflurane to mouse by means of a nose cone during prep. Return mouse to anesthesia chamber.
- When mouse is sufficiently inert, move to sterile surgical area, place anesthesia nose cone over the mouse’s nose and mouth, and divert the flow of isoflurane to nose cone. Confirm proper anesthetization by pinching paw with forceps; when the mouse shows no reaction, proceed to next step.
- Position the mouse on its back, with the head facing toward the investigator. Secure the ears, fore-paws and hind-paws to surgical board with adhesive tape or other restraining device to keep the mouse steady. Clean the incision area with povidone-iodine and 70% ethanol.
- Isolation of carotid artery
- Make a 1 cm longitudinal cut slightly to the right of the midline of the neck. Use forceps to separate fat and muscle to expose the trachea (Figure 4A). Locate the carotid artery running parallel to the trachea (Figure 4B).
- Carefully use forceps to separate fascia overlying the artery (Figure 4C). Lightly pull the vagus nerve aside from the carotid, and insert forceps into the space between. Gently open forceps to create a gap in the fascia, and carefully pull away the nerve from the artery, from the fork in the artery near the larynx (anterior end), up (posterior) as far as possible (at least 3 mm) (Figure 4D).
- Clear away any remaining fascia until the artery is well isolated (Figure 4E). Add a drop of saline to the surgery area occasionally to keep tissue moist, and thus less brittle and less prone to tearing randomly.
- Suture placement and artery preparation for catheter insertion (Figures 4, 5).
- Use forceps to draw a silk suture thread under the artery. Tie a secure knot to close off the artery as far toward the anterior as possible (Figure 4F).
- Draw a second thread under the artery. Tie a retractable knot to temporarily close off the artery as far toward the posterior as possible (Figure 4F).
- Draw a third thread under the artery. Tie a very loose knot between the first two sutures, to be used to quickly secure the catheter after placement (Figure 4G).
- Hold ends of all sutures out of the way by wetting them with a bit of 70% ethanol.
- Catheter insertion
- With the suture, grab the lower knot to pull the artery slightly taut. Nick the artery above, but very close to, the anterior suture (Figure 4H). Be careful not to cut too deeply, but check the slit to make sure the opening is unobstructed.
- Remove heparin-filled catheter from syringe needle, trying to avoid creating large pockets of air at either end. Manipulate the catheter to position bevel at a comfortable angle, generally downward, and slightly to the right (for right-handers).
- While holding on to the suture to keep the artery remaining slightly taut, gently insert catheter into slit (Figure 4I). Use the forceps to hold the anterior suture and pull the artery down over catheter (pushing up excessively with the catheter may cause the beveled end to puncture the artery). Carefully release the catheter and anterior suture.
- Securing of catheter and initiation of blood flow (Figures 4J, 5B).
- Secure the catheter by tightening the knot of the middle suture, close to the entrance of the catheter into the artery. Make a tight triple knot, but be sure not to pull so tight as to obstruct flow through the catheter. Further secure the catheter by tying it down with the anterior suture, below the entrance to the artery.
- Attach the saline lead to the catheter by means of the connector plug, again trying to avoid introducing air bubbles into the line.
- Grasp the ends of the posterior suture and gently pull to release the knot. Maneuver the suture down the artery, over the end of the catheter (do not remove the thread). Blood should flow into the catheter; if there is no blood flow, gently wiggle catheter to attempt to remove the constriction.
- When flow appears unobstructed, use the last thread (from the posterior suture) to tie an additional knot, slightly above the middle suture.
Temporary sealing of catheter. Flush the catheter of blood, then use a hemostat to clamp the end of the catheter close to the connector plug. Remove the connector and replace it with the port plug to seal off the end of the catheter, and remove the hemostat.
- Repositioning catheter to exit from back of neck.
- With forceps in each hand, use one pair of forceps to hold on to the catheter just below the anterior suture, and with the other, press a kink into the catheter so it will easily bend to the side. Repeat to create a second kink. This allows the free end of the catheter to be pulled toward the back of the mouse’s head, without forcing the tip of the catheter to turn laterally against the wall of the artery.
- Turn the mouse onto its (left) side, keeping the nose cone positioned over the mouth and nose, and clean the incision area with 70% ethanol and povidone-iodine. Make a small incision (approximately 4 mm) below and behind the right ear.
- Use forceps to hold the open flap of skin, while working the blunt hollow probe under skin to create a channel through the cheek, to the cavity at the neck. It is advisable to bring the probe around the salivary gland, instead of trying to go between the gland and the skin. Use forceps to carefully free a space for the probe to exit.
- Thread port plug/catheter through probe to exit in neck. Do not pull too hard; be sure the catheter is not crushing or constricting blood vessels or organs.
- Closure and recovery. Administer topical analgesic (e.g. bupivacaine) to the shoulder incision, and cover the wound with water-proof, surgical adhesive tape. Apply a second piece of adhesive tape to further secure the catheter.
- Administer topical analgesic to chest incision, and close wound with silk or staples.
- Remove mouse from anesthesia, and allow animal to recover in a clean, warmed space (place cage on top of a heating pad or under a heat lamp), for at least 30 min.
3. Infusion
- Prepare aliquots of 5 mg/ml solution of paclitaxel / methanol.
- Measure 50 mg of paclitaxel into a 15 ml centrifuge tube. Add 10 ml of sterile methanol. Cap tube. Rotate by hand or on a rotating shaker at room temperature until powder is dissolved.
- Aliquot 500 µl of solution into 20 small, freezer safe tubes, and store at -20 °C.
- Thaw individual aliquot at room temperature or in 37 °C water bath, immediately before infusion.
- Prepare the infusion pump (Figure 6).
- Cut a long length of polyethylene tubing of approximately 40 cm. Attach a blunted needle to one end, and a connector port to the other.
- Draw up drug into a syringe with a known inner-diameter (most programmable pumps will require the diameter of the syringe barrel, in order to calculate the speed of the pump arm). Attach the needle to the syringe and load the drug through the needle and tubing.
- Situate the syringe into the pump according to the manufacturer’s directions. Prime the pump so that the drug is flowing smoothly out the connector plug, and it is ready for infusion.
- Attach mouse to pump.
- Hold the mouse steady and use the hemostat to clamp catheter, close to the port plug. Remove plug and replace with the connector attached to the syringe and tubing. NOTE: Blood may start to flow back through tubing.
- Quickly administer a fast pump to clear the volume of the catheter (calculated empirically through observing the volume of 6 cm of tubing), then immediately switch to the desired infusion rate.
- Continue Paclitaxel infusion for three hour time course.
- Monitor tubing occasionally to check for leaks at junctions as this is often a sign of a blockage in the flow to the mouse. Watch the mouse for expected or unexpected reactions to infusion (lethargy or hyperactivity, signs of discomfort).
- Depending on the length and nature of the infusion, the mouse might not eat or drink, but be sure to provide access to food and water according to the institution’s established policy. Be aware of the potential for dehydrating the mouse through collection of large quantities of blood.
- Continue to keep the cage warm with a heating pad or lamp, unless the mouse appears to desire to stay away from the heat. If animal is not euthanized within several hr after surgery, implement plan for post-surgical treatment of animal, including sterile housing conditions and treatment for post-surgical pain.
- Keep a close watch on the mouse, especially in the first few min, to ensure it does not pull out the tubing through hyperactivity, or irritation from tubing (which may be a sign of a poor insertion). If the mouse is not over-active, constant monitoring may not be necessary, but check the mouse routinely to be sure the animal does not get tangled in the tubing. Harness and tether system are available commercially, but their use is beyond the scope of this protocol.
- Collection of samples.
- Collect blood samples at regular intervals by submandibular or retro-orbital bleeds (If your protocol does not make use of mammary tumor models, consider collection of blood via a jugular catheter inserted at the same time as the carotid catheter). Be careful not to pull on the infusion line. If collecting by retro-orbital bleeds, lightly anesthetize the mouse with an inhalational anesthetic (e.g. methoxyflurane) so one need not grab by the scruff to secure the mouse.
- Spin blood in hemocrit centrifuge to separate blood cells from plasma. Use a file or diamond-tip pen to score tube at face of phase separation. Break tube and collect plasma only, into small, freezer-safe tube. Store at -80 °C until analysis. NOTE: If a hemocrit centrifuge is not available, transfer blood sample into micro-centrifuge tube, and spin in a micro-centrifuge at high speed to separate blood cells from plasma. Collect plasma into a second tube, and store at -80 °C.
- Euthanize mouse by CO2 asphyxiation. Collect tissue (about 20 – 50 mg) from organs of interest and flash freeze in liquid nitrogen. Store at -80 °C until analysis.
4. Sample Analysis
NOTE: All samples for this protocol were analyzed through an outside laboratory by liquid chromatography-tandem mass spectrometry (LC-MS/MS), who calculated the paclitaxel concentrations as follows:
Extract paclitaxel from samples. Homogenize tissue sample in 0.1% acetic acid, 50% methanol prior to extraction. Extract paclitaxel by liquid/liquid extraction, using methyl tert-butyl ether (MTBE) fortified with an internal analogue (docetaxel) standard. Remove MTBE and dry samples. Resuspend in 50% acetonitrile, 0.1% acetic acid solution.
Prepare calibration standards. Add a known concentration of paclitaxel to appropriate C57BL/6 matrix to obtain a final range of standards (from 1 to 20,000 ng/ml for plasma samples, from 0.1 to 5,000 ng/ml for tissue samples). Extract standards in duplicate, using the same method as for the study samples above. Measure paclitaxel peak in samples by HPLC/MS/MS utilizing electrospray ionization.
Calculate concentration using the area ratio of paclitaxel to internal standard. Use calibration standards to create a standard curve, and interpolated study samples by fit on the curve. Normalize concentration of study samples by the starting weight of sample before homogenization.
Representative Results
Paclitaxel distribution follows predictable patterns during a 3 hr dosing regimen of a 15 min high-speed infusion, followed by a 165 min low-speed infusion.
Figure 1 shows a comparison of jugular vein-infusion plasma paclitaxel concentrations and carotid artery-infusions. The paclitaxel concentrations drop quickly in the first 15 min following an initial high volume infusion, and then level off over the next 150 min. By comparison, paclitaxel levels in a poor infusion start off relatively low, and hover up and down throughout the assay. This was most likely caused by a blockage in the line early in the infusion. Records of the assay show the mouse had little to no external reaction to the infusion, corroborating the idea of an inferior administration of drug. Figure 2 shows relative levels of paclitaxel in liver and brain tissue, as well as blood plasma, at the end of the 3 hr infusion.
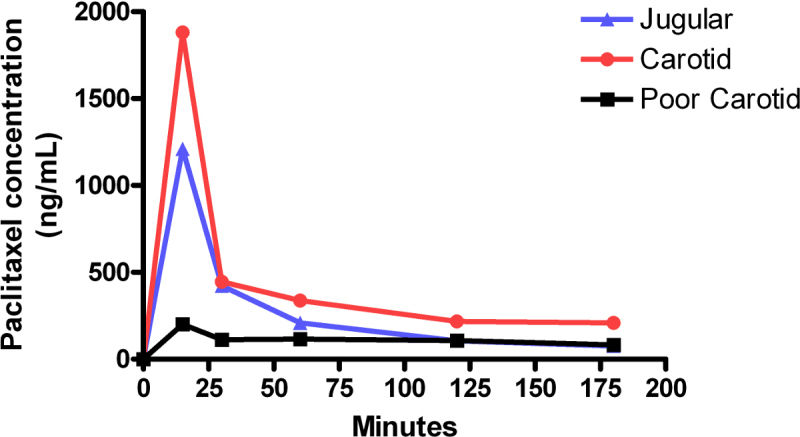 Figure 1: Plasma paclitaxel levels during carotid and jugular infusions. Curves represent plasma paclitaxel concentrations in individual mice. Each mouse received a biphasic infusion, consisting of an initial high-speed, 15 min infusion of 0.42 mg/kg/min, immediately followed by a low-speed, 165 min infusion of 0.021mg/kg/min. The area under the curve (AUC) for carotid infusion was approximately 59 μg/ml∙min versus an AUC for jugular infusion of approximately 37 μg/ml∙min. The half-life of paclitaxel calculated from the curves generated for carotid infusion was 10 min and for jugular infusion was 11 min. Carotid infusion shows roughly equivalent levels of drug concentration compared to jugular infusion. Continuous low concentrations, or concentrations that cycle up and down, often represent a poor infusion.
Figure 1: Plasma paclitaxel levels during carotid and jugular infusions. Curves represent plasma paclitaxel concentrations in individual mice. Each mouse received a biphasic infusion, consisting of an initial high-speed, 15 min infusion of 0.42 mg/kg/min, immediately followed by a low-speed, 165 min infusion of 0.021mg/kg/min. The area under the curve (AUC) for carotid infusion was approximately 59 μg/ml∙min versus an AUC for jugular infusion of approximately 37 μg/ml∙min. The half-life of paclitaxel calculated from the curves generated for carotid infusion was 10 min and for jugular infusion was 11 min. Carotid infusion shows roughly equivalent levels of drug concentration compared to jugular infusion. Continuous low concentrations, or concentrations that cycle up and down, often represent a poor infusion.
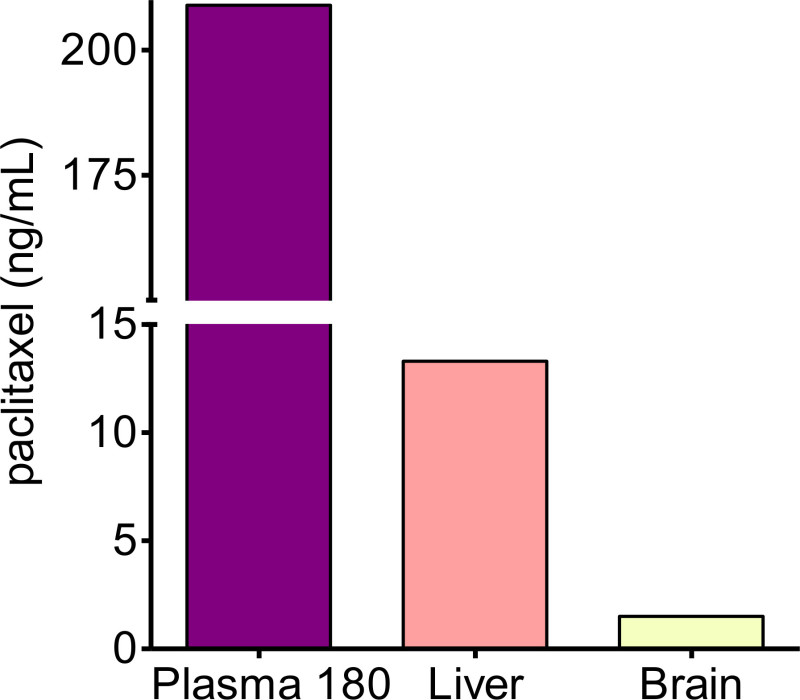 Figure 2: Paclitaxel concentration by tissue. Immediately following the 3 hr paclitaxel infusion and collection of the last blood sample, the mouse was euthanized, and liver and brain tissue samples were collected. Paclitaxel concentration levels in plasma and tissues were acquired by mass-spec analysis. This data represents samples collected from the Carotid Infusion-Mouse in Figure 1.
Figure 2: Paclitaxel concentration by tissue. Immediately following the 3 hr paclitaxel infusion and collection of the last blood sample, the mouse was euthanized, and liver and brain tissue samples were collected. Paclitaxel concentration levels in plasma and tissues were acquired by mass-spec analysis. This data represents samples collected from the Carotid Infusion-Mouse in Figure 1.
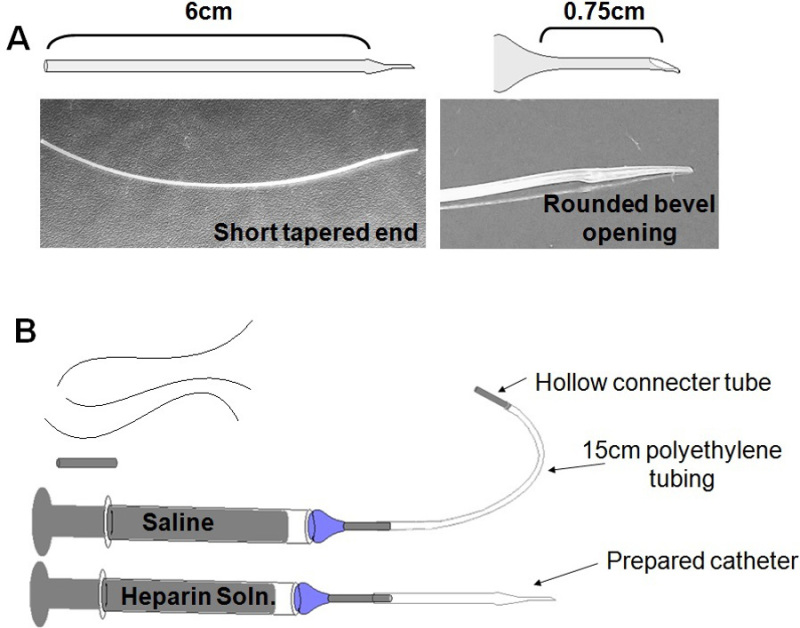 Figure 3: Surgical paraphernalia. (A)Catheter Production: Pulling ones own catheters keeps down material expenses, while allowing the researcher to tailor size and shape of catheter to mouse age and size. (B) Prepare before Surgery: Three (3) silk sutures, approximately 8 cm each; Sterile port plug; Saline syringe and lead; Catheter, attached to heparin syringe.
Figure 3: Surgical paraphernalia. (A)Catheter Production: Pulling ones own catheters keeps down material expenses, while allowing the researcher to tailor size and shape of catheter to mouse age and size. (B) Prepare before Surgery: Three (3) silk sutures, approximately 8 cm each; Sterile port plug; Saline syringe and lead; Catheter, attached to heparin syringe.
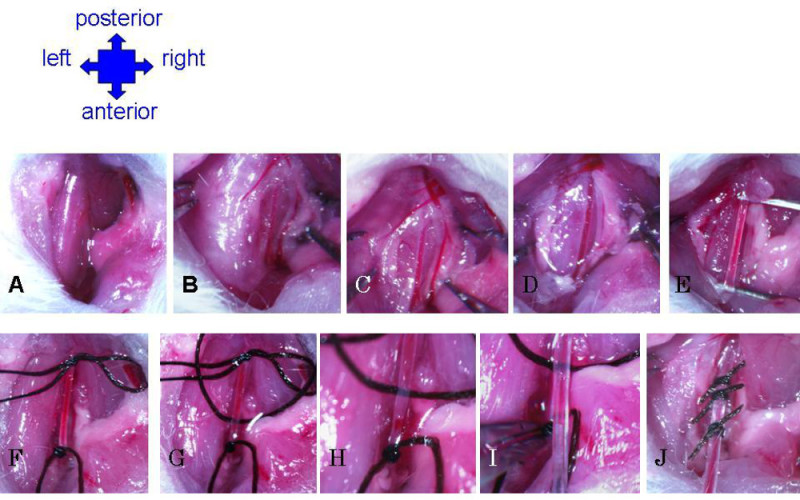 Figure 4: Preparation of carotid artery and catheter insertion. (A) Cut through skin, move aside glands and use forceps to grossly separate fat to expose muscle. (B) Use forceps to gently separate muscle to expose right side of trachea. Carotid artery will become visible as largest, thick-walled vessels, running parallel to trachea. (C) Break fascia around artery. (D) Separate vagus nerve from carotid artery. (E) Continue removing fascia until carotid is completely isolated along cavity. (F) Suture permanent knot at anterior extremity, and slip knot at posterior extremity. (G) Third suture is threaded under carotid and very loosely knotted. (H) Artery is nicked just above anterior suture. (I) Insert catheter into nick in artery. Grab anterior suture with forceps to pull artery down over catheter. (J) Secure catheter in carotid artery with all three sutures.
Figure 4: Preparation of carotid artery and catheter insertion. (A) Cut through skin, move aside glands and use forceps to grossly separate fat to expose muscle. (B) Use forceps to gently separate muscle to expose right side of trachea. Carotid artery will become visible as largest, thick-walled vessels, running parallel to trachea. (C) Break fascia around artery. (D) Separate vagus nerve from carotid artery. (E) Continue removing fascia until carotid is completely isolated along cavity. (F) Suture permanent knot at anterior extremity, and slip knot at posterior extremity. (G) Third suture is threaded under carotid and very loosely knotted. (H) Artery is nicked just above anterior suture. (I) Insert catheter into nick in artery. Grab anterior suture with forceps to pull artery down over catheter. (J) Secure catheter in carotid artery with all three sutures.
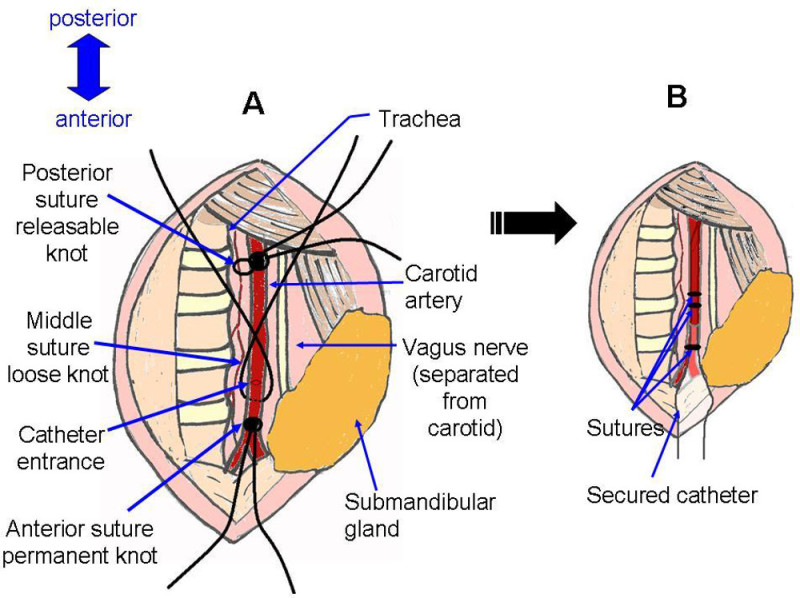 Figure 5: Suture placement. Schematic representation of surgical site before and after catheter installation. A corresponds with photograph Figure 4G, with the addition of a nick in the artery, as in Figure 4H. B corresponds with photograph Figure 4J.
Figure 5: Suture placement. Schematic representation of surgical site before and after catheter installation. A corresponds with photograph Figure 4G, with the addition of a nick in the artery, as in Figure 4H. B corresponds with photograph Figure 4J.
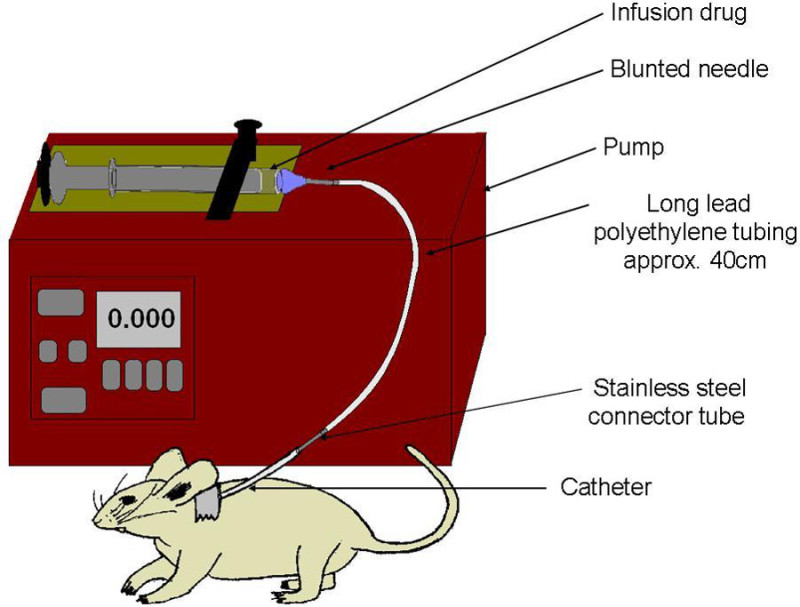 Figure 6: Schematic of infusion set-up. Syringe is filled with drug, and capped with a blunt needle. Polyethylene line attaches syringe to carotid catheter. Pump slowly compresses syringe, to deliver uniform dosage directly into the bloodstream.
Figure 6: Schematic of infusion set-up. Syringe is filled with drug, and capped with a blunt needle. Polyethylene line attaches syringe to carotid catheter. Pump slowly compresses syringe, to deliver uniform dosage directly into the bloodstream.
Discussion
Carotid artery infusion is a significant technique in this study of paclitaxel pharmacokinetics. Carotid artery infusion is a method to quickly distribute drug throughout the circulatory system14. The 3 hr infusion is a closer mimic of clinical administration of drugs such as taxanes than bolus injections. The surgery can be reliably performed by a single individual, surgery time is relatively short, and success rates are >75%. After samples are collected, they must be analyzed by the appropriate methods. We used mass spectrometry to determine the paclitaxel concentration in plasma and tissue samples. To further validate this technique, we sent blood and tissue samples to an independent lab for analysis. This data was plotted as individual plasma-paclitaxel concentration curves for each animal tested (Figure 1), and the distribution of paclitaxel was compared in different tissues (Figure 2). In each case, it is important to consider the best method to analyze drug distribution and/or metabolism, depending on the drug and system of interest. Other options for measurement of different drugs may include HPLC-UV or immunoassays 2.
Two primary factors essential for successful carotid catheterization are well fashioned catheters and superior artery isolation. Fashioning catheters according to the size of the mouse model is paramount. If the catheter diameter is too thick, insertion into the artery will be overly difficult, while a too thin catheter will be harder to secure and likely to clog before or during infusion. The angle and sharpness of the catheter tip must also be in a moderate range; a tip that is too sharp may puncture the artery wall, while a tip that is too dull will be difficult to insert into the artery. The measurements given here were derived using ten-week-old C57BL/6J mice, approximately 20 g, as a model template. Measurements must be scaled up or down empirically to fit individual models.
Isolation of the carotid artery must be a delicate, deliberate process to avoid needless damage to tissue and to prevent large-scale bleeding. Subcutaneous-fat can generally be easily separated with low to medium sharp forceps. Muscle tissue over the carotid should be separated with medium to fine tipped forceps along the bias of the muscle fibers. If a more extensive gap is necessary, the technician must be extremely careful to avoid rupturing small blood vessels. Once the carotid is visible, there will still be a fair amount of fascia that needs to be tweezed away from the artery with fine tipped forceps. Finally, the vagus nerve must be separated from the carotid artery without damage to either. When the carotid is properly isolated, it should be possible to insert the forceps underneath, with an empty space on either side of the artery (see Figure 4E).
When trouble shooting poor infusions, start by reviewing the instructions on the pump to be sure that the investigator has properly programmed the pump to deliver the expected dosage. Then, carefully consider changing the volume that will be introducing into the test animal. The dilution of drug must be calculated so that the dosage volume is appropriate: the volume must not be too large for the animal to tolerate, and ideally will not significantly affect blood pressure; yet the volume must be large enough for the pump to deliver reliably, and will create a steady flow to avoid clogs at junctions. If clogs become a regular occurrence, consider switching to a smaller gauge (larger diameter) needle and tubing. Further, if plasma drug content does not reach expected levels, the researcher should check the mice post mortem to determine whether the catheter remains well placed in the artery and free-flowing, and modify the shape/size of catheter as necessary.
The usefulness of this method may be limited by such factors as the size and general health of the subject, and the intended length of time of the infusion. The surgery and infusion can overtax an already distressed subject. Even in a healthy animal, carotid artery catheter is only appropriate for short term infusions, generally several hours to several days. Consider what methods of pain-relief will be used if mice show signs of discomfort in response to the drug infusion, such as repeated applications of topical anesthesia to wound sites, or pre-emptive systemic analgesics. It will be necessary to have all animal work approved by the local animal regulatory organization or IACUC, to obtain the appropriate permissions to perform this procedure. If it is necessary to have a longer infusion, or to have the mouse survive the infusion for an extended period of time, alternative infusion methods must be explored.
After having mastered carotid artery perfusions in the study of the pharmacokinetics of paclitaxel, we plan to use this technique in the future to investigate the effects of other drugs, and Abcc10 modulators in the C57BL/6J and FVB mice, and other mouse models.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We would like to acknowledge the FCCC Laboratory Animal Facility for their support in this project. We thank Wolfe Laboratories, Inc. for their assistance in analyzing paclitaxel levels in plasma and tissue. This work was supported by National Institutes of Health grants K01CA120091 to E.H.B., and CA06927 to Fox Chase Cancer Center.
References
- Gallo JM, Li S, Guo P, Ma Reed K, J The Effect of P-Glycoprotein on Paclitaxel Brain and Brain Tumor Distribution in Mice. Cancer Research. 2003;63(16):5114–5117. [PubMed] [Google Scholar]
- Sonnichen DS, Relling MV. Clinical Pharmacokinetics of Paclitaxel. Clinical Pharmacokinetics. 1994;27(4):256–269. doi: 10.2165/00003088-199427040-00002. [DOI] [PubMed] [Google Scholar]
- Gianni L, et al. Nonlinear Pharmacokinetics and Metabolism of Paclitaxel and Its Pharmacokinetic / Pharmacodynamic Relationship in Humans. Journal Clinical Oncology. 1995;13(1):180–190. doi: 10.1200/JCO.1995.13.1.180. [DOI] [PubMed] [Google Scholar]
- Sparreboom A, Van Tellingen O, Nooijen WJ, Beijnen JH. Nonlinear Pharmacokinetics of Paclitaxel in Mice Results from the Pharmaceutical Vehicle Cremophor EL. Cancer Research. 1996;56(9):2112–2115. [PubMed] [Google Scholar]
- Sparreboom A, Van Tellingen O, Nooijen WJ, Beijnen JH. Determination of paclitaxel and metabolites in mouse plasma, tissues, urine and faeces by semi-automated reversed-phase high-performance liquid chromatography. Journal of Chromatography B: Biomedical Sciences and Applications. 1995;664:383–391. doi: 10.1016/0378-4347(94)00495-q. [DOI] [PubMed] [Google Scholar]
- Bradshaw-Peirce EL, Eckhardt SG, Gustafson DL. A Physiologically Based Pharmacokinetic Model of Docetaxel Disposition: from Mouse to Man. Clinical Cancer Research. 2007;13:2768–2778. doi: 10.1158/1078-0432.CCR-06-2362. [DOI] [PubMed] [Google Scholar]
- Eiseman JL, et al. Plasma Pharmacokinetics and Tissue Distribution of Paclitaxel in CD2F1 Mice. Cancer Chemotherapy and Pharmacology. 1994;34(6):465–471. doi: 10.1007/BF00685656. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, et al. Normal Viability and Altered Pharmacokinetics in Mice Lacking mdr1-type (drug-transporting) P-glycoproteins. Proc. Nat. Acad. Sci. USA. 1997;94:4028–4033. doi: 10.1073/pnas.94.8.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser IJ, Dear GJ, Plumb R, L’Affineur M, Fraser D, Skippen AJ. The Use of Capillary High Performance Liquid Chromatography with Electrospray Mass Spectrometry for the Analysis of Small Volume Blood Samples from Serially Bled Mice to Determine the Pharmacokinetics of Early Discovery Compounds. Rapid Communications in Mass Spectrometry. 1999;13(23):2366–2375. doi: 10.1002/(SICI)1097-0231(19991215)13:23<2366::AID-RCM800>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Bateman KP, et al. Reduction of Animal Usage by Serial Bleeding of Mice for Pharmacokinetic Studies: Application of Robotic Sample Preparation and Fast Liquid Chromatography – Mass Spectrometry. Journal of Chromatography B: Biomedical Sciences and Applications. 2001;754(1):245–251. doi: 10.1016/s0378-4347(00)00612-5. [DOI] [PubMed] [Google Scholar]
- Berndt K, Vogel J, Buehler C, Vogt P, Born W, Fuchs B. A new method for repeated drug infusion into the femoral artery of mice. J. Am. Assoc. Lab Animal Sci. 2012;51(6):825–831. [PMC free article] [PubMed] [Google Scholar]
- Drug Information for TAXOL (Paclitaxel) Injection. Bristol-Myers Squibb Company; 2011. Squibb & Sons, L.L.C. [Google Scholar]
- Full Prescribing Information ABRAXANE for Injectable suspension (paclitaxel protein-bound particles for injectable suspension) (albumin-bound) Celgene Corporation; 2013. Abraxis BioScience L.L.C. [Google Scholar]
- Fergusson G, Ethier M, Zarrouki B, Fontés G, Poitout V. A Model of Chronic Nutrient Infusion in the Rat. J. Vis. Exp. 2013. [DOI] [PMC free article] [PubMed]


