Abstract
Macrophages are key phagocytic innate immune cells. When macrophages encounter a pathogen, they produce antimicrobial proteins and compounds to kill the pathogen, produce various cytokines and chemokines to recruit and stimulate other immune cells, and present antigens to stimulate the adaptive immune response. Thus, being able to efficiently manipulate macrophages with techniques such as RNA-interference (RNAi) is critical to our ability to investigate this important innate immune cell. However, macrophages can be technically challenging to transfect and can exhibit inefficient RNAi-induced gene knockdown. In this protocol, we describe methods to efficiently transfect two mouse macrophage cell lines (RAW264.7 and J774A.1) with siRNA using the Amaxa Nucleofector 96-well Shuttle System and describe procedures to maximize the effect of siRNA on gene knockdown. Moreover, the described methods are adapted to work in 96-well format, allowing for medium and high-throughput studies. To demonstrate the utility of this approach, we describe experiments that utilize RNAi to inhibit genes that regulate lipopolysaccharide (LPS)-induced cytokine production.
Keywords: Immunology, Issue 93, macrophage, RAW264.7, J774A.1, lipopolysaccharide, LPS, innate immunity, RNAi, siRNA, cytokines
Introduction
In this protocol, we describe efficient methods to inhibit genes in mouse macrophage cell lines using siRNAs and monitor the effects of these treatments on the innate immune response. These procedures are performed in 96-well format, allowing for RNAi screens in medium- or high-throughput fashion.
In response to infection, humans mount an immediate innate immune response and a slower but more specific adaptive immune response. This rapid innate immune response involves the recruitment and activation of phagocytic innate immune cells including macrophages1. Classically activated macrophages are involved in acute inflammatory responses and produce antimicrobial proteins and compounds, cytokines and chemokines, and present antigens2,3. Alternatively activated macrophages play a role in regulating immunity, maintaining tolerance, tissue repair, and wound healing4-8. Because of their wide array of functions, macrophages can play a role in numerous diseases including atherosclerosis, arthritis, and cancer9. Thus, the study of macrophages has been a key area of research for some time in a wide variety of disease fields.
Despite their importance in the innate immune response, macrophages can be challenging cells to work with. In particular, it is difficult to obtain efficient transfection using lipid reagents in macrophages without associated toxicity10,11. Moreover, even when siRNA is efficiently delivered to macrophages, the robustness of RNAi-induced gene knockdown often can be fairly moderate and can vary from gene to gene.
To overcome these technical challenges, we have optimized transfection and knockdown techniques12-16 in two mouse macrophage cell lines, RAW264.717 and J774A.118. This approach uses the Amaxa Nucleofector 96-well Shuttle System for transfection; this system uses a combination of specialized reagents and electroporation to transfect cells in 96-well format19. Following transfection, we describe methods to maximize cell recovery and viability and to maximize subsequent siRNA-induced gene knockdown. To illustrate the utility of this approach, we describe a protocol for siRNA delivery to these macrophage cell lines, stimulate these cells with lipopolysaccharide (LPS), and monitor the innate immune response at the level of production of several pro-inflammatory cytokines. We provide sample data in which we target the Toll-like receptor (TLR) family, whose members sense LPS and other pathogen associated molecular patterns (PAMPs), to regulate innate immunity.
Protocol
1. Maintenance of Macrophage Cell Lines
Grow RAW264.7 and J774A.1 cell lines in DMEM supplemented with 10% fetal bovine serum (FBS) at 37 ºC in the presence of 5% CO2. To help maintain sterility, add 1% penicillin/streptomycin (pen/strep) to the media (although this is not strictly necessary).
Critical step: Ensure that the macrophages are growing in a healthy state for efficient transfection and siRNA-induced gene knockdown. Use cells between passages 3 and 10 after fresh thawing, as these macrophage lines exhibit the best transfection efficiency and maintain robust post-transfection survival at this stage; do not use a higher passage number (>20), as transfection efficiency and gene knockdown both diminish greatly.
For optimized siRNA transfection, plate cells at 20% confluency and grow one or two days until the cells reach no more than 80% confluence. Overgrown cells will not transfect efficiently.
2. Programming the 96-well Shuttle System for Transfection
Start the 96-well shuttle software on the computer connected to the shuttle. Select new parameter file from the top left file menu.
Highlight the wells that will be transfected on the displayed 96-well plate schematic. A green box will indicate which wells are selected.
Select the program that corresponds to the macrophage cell line being used (DS-136 for RAW264.7 or DS-130 for J774A.1) and select apply, located below the diagramed 96-well plate. Note that after selecting a program, the wells on the schematic plate will be colored dark blue. Empty circles indicate wells that have not been assigned a program and will not be shocked.
3. Preparing the Reagents for Transfection
Prepare the working Nucleofector™ solution by mixing SF cell line 96-well Nucleofector™ solution with the entire contents of the enclosed supplement tube. Allow the mixed reagents to equilibrate at room temperature prior to use. Store excess complete Nucleofector™ solution at 4 ºC for up to 3 months for future use.
Pre-warm the DMEM, RPMI-1640, 0.25% trypsin/EDTA, and phosphate-buffered saline (PBS) at 37 ºC prior to preparing the macrophages for transfection.
Prepare the siRNA for transfection by thawing on ice. In order to minimize the number of freeze thaws of the siRNA, freeze new siRNAs in aliquots the first time they are used.
In initial experiments, use siRNA at a relatively high dose of 2 µM (diluted 1:10 from 20 µM stocks). Subsequently titrate siRNAs that induce robust gene knockdown down to lower doses, although typically higher doses are needed for efficient gene knockdown in macrophages compared to other cell types. NOTE: siRNAs are available from many sources; fairly robust gene knockdown can be obtained using siGenome SMARTPOOLs, which are pools of four siRNAs targeting each gene. As a negative control, use any of various non-targeting siRNA pools or siRNAs.
To monitor transfection efficiency, transfect either the pmaxGFP control plasmid (provided in the Amaxa kits) or fluorescently-labeled siRNAs in parallel to the experimental siRNAs. Expect 30-50% transfection of the GFP plasmid (assayed by microscopy or flow cytometry the day after transfection) and >99% transfection of fluorescently-labeled siRNA (assayed by flow cytometry immediately after transfection and recovery).
4. Preparing the Macrophages for Transfection
Detach adherent macrophages from the cell culture dishes by trypsinization. Aspirate the media and wash the cells twice with 10 ml PBS. After removing the PBS, incubate the cells with pre-warmed 0.25% trypsin/EDTA (1 ml/10 cm plate) for 1-2 min until cells begin to detach. Gently tap the plate and move the solution during the incubation to maximize trypsinization.
After the incubation, add 9 ml DMEM + FBS media, mix gently up and down, and collect the cells by pipetting into a sterile tube.
Count the number of isolated macrophages using a hemocytometer. Expect roughly 10 million macrophages per 10 cm plate. Calculate the total number of macrophages needed; each well on the 96-well shuttle requires 200,000 cells. Remember to add a few extra wells' worth of cells to allow for pipetting losses.
Pipette the calculated number of cells into a centrifuge tube. Centrifuge the cells for 10 min at 150 x g at room temperature. Do not centrifuge too quickly as this will diminish macrophage health and transfection efficiency.
5. Nucleofection of Macrophages with siRNA
Using a sterile 96-well round bottom plate (which facilitates mixing while minimizing reagent loss), add 2 µl of each siRNA to each well to be transfected. Note: Depending on the number of siRNAs, this can be accomplished during the macrophage centrifugation step.
Aspirate the media from the centrifuged cells. Gently resuspend the cells in Nucleofector™ solution SF (containing the supplement solution), using 20 µl per 200,000 cells pelleted. Once resuspended, use the cells immediately for optimal transfection.
Transfer resuspended cells into a sterile trough; using a multichannel pipette, transfer 20 µl of the resuspended cell mixture into each well of the 96-well plate containing the siRNAs. Gently mix by pipetting up and down.
Transfer 20 µl of the cell/siRNA mixture into the 96-well Nucleocuvette™ plate. Critical step: avoid generating bubbles during the transfer step as this can induce errors during Nucleofection.
After placing all of the experimental samples in the transfection plate, cover the 96-well plate with the provided lid and very gently tap on a hard surface to remove any bubbles from the samples.
Place 96-well plate with lid into the 96-well Nucleofector shuttle and select 'upload and start' on the bottom right of the program screen. Prior to Nucleofection, follow the program prompt to save the results. Note: During the Nucleofection process, wells successfully tranfected will be depicted on the 96-well schematic on the screen by a green circle with a plus sign. A red circle with minus signs indicates an error, most likely due to bubbles or mis-pipetting the correct volume of reagents.
6. Recovery and Plating of Macrophages
Immediately after Nucleofection, using a multichannel pipette, add 80 µl of pre-warmed (37 ºC) RMP1-1640 media to each well. Add the media to the side of each well, allowing the media to slowly mix with the transfected cells, which are very delicate at this stage. Do not add the media too fast, as this will greatly decrease viability.
Place the 96-well plates with transfected samples and RPMI-1640 into a 37 ºC incubator with 5% CO2 for 2 min; this is critical to allow the cells to recover prior to further manipulation.
Remove the 96-well plate from the incubator and carefully transfer the mixture from each well into a 96-well tissue culture plate using a multichannel pipette.
Using a multichannel pipette, add 100 µl of pre-warmed (37 ºC) DMEM+FBS medium to each well.
Incubate the 96-well tissue culture plate with samples in a 37 ºC incubator with 5% CO2 for 24-36 hr (optimize the exact timing for individual siRNAs, although this time range generally works well).
7. Stimulation of Macrophages with LPS
Following the 24-36 hr post-Nucleofection incubation, prepare LPS (or other PAMPs) in fresh media. Prepare enough fresh medium for all the wells (200 µl media/well of the 96-well plate plus a little extra for pipetting). Dilute LPS to a final concentration of 20 ng/ml in DMEM+FBS.
Carefully aspirate media from the 96-well plate and add the fresh media containing the LPS to the cells.
Monitor the response to LPS or other PAMPs at various times after stimulation. 6 hr is sufficient to generate a robust inflammatory cytokine response for cytokines such as IL-6 or TNFα.
8. Monitoring the Induced Innate Immune Response, Gene Knockdown, and Viability in Macrophages
To monitor LPS-induced cytokine production, pipette the supernatant from the cells into a 96-well storage plate. Cytokine production can then be measured by ELISA.
- Once the supernatant is removed, monitor cell viability using fluorescein diacetate (when cleaved by cellular esterases in live cells, this compound becomes fluorescent). Identify siRNAs that target genes that control cell viability using this approach. Note: the transfection process itself should have little effect on viability if performed properly.
- Add fluorescein diacetate (5 mg/ml stock in acetone) to each well (1:100 final dilution in PBS).
- Incubate at room temperature for one to five min and then monitor fluorescence in the cells using a plate reader (460 nm excitation, 515 nm emission).
- Optional: To ensure that the fluorescence in the wells is uniform, lyse the cells using buffers such as RIPA; this provides a uniform fluorescent signal in the well, as some plate readers are configured to monitor only a small portion of the well; if the cells are not plated uniformly, the fluorescein diacetate could give an inaccurate reading.
- As an alternative to monitoring cell viability, monitor siRNA-induced gene knockdown by qPCR using RNA derived from the adherent cells.
- After the supernatant is removed from the cells (step 8.1 above), add RLT buffer (which is used for lysis and preservation of RNA) directly to the adherent cells to lyse them.
- Isolate RNA using a RNA preparation kit such as the RNeasy mini-kit, and use qPCR with gene-specific primers to monitor gene knockdown relative to control siRNA treatment. Use primers to Βactin or GAPDH for normalization.
As another alternative to monitor other aspects of the innate immune response, monitor the phagocytic ability of the macrophages by adding FITC-labeled E. coli particles directly onto the adherent cells using a kit. Following the manufacturer's protocol, which takes only a few hours, monitor phagocytic uptake using a plate reader or microscopy.
Representative Results
To demonstrate the efficiency of transfection using this approach, we monitored uptake of FITC-labeled siRNA using a flow cytometer (Figure 1).
To illustrate the utility of our approach for monitoring the innate immune response, we transfected siRNAs targeting known innate immune regulatory genes into the RAW264.7 macrophage cell line, stimulated the cells with LPS, and then monitored production of the pro-inflammatory cytokines IL-6 and TNFα. Different TLRs recognize different PAMPs, with LPS from Gram negative bacteria recognized by TLR420, lipopeptides such as PAM3CSK4 recognized by TLR221, and dsRNA from viruses such as the mimic poly(I:C) recognized by TLR322,23. As shown in Figure 2, inhibition of TLR4 (but not other TLRs using siRNA) strongly diminishes production of LPS-induced IL-6 and TNFα. Moreover, inhibition of IL-6 with siRNA also diminishes IL-6 production while not affecting TNFα production (compare panel A to B in Figure 2).
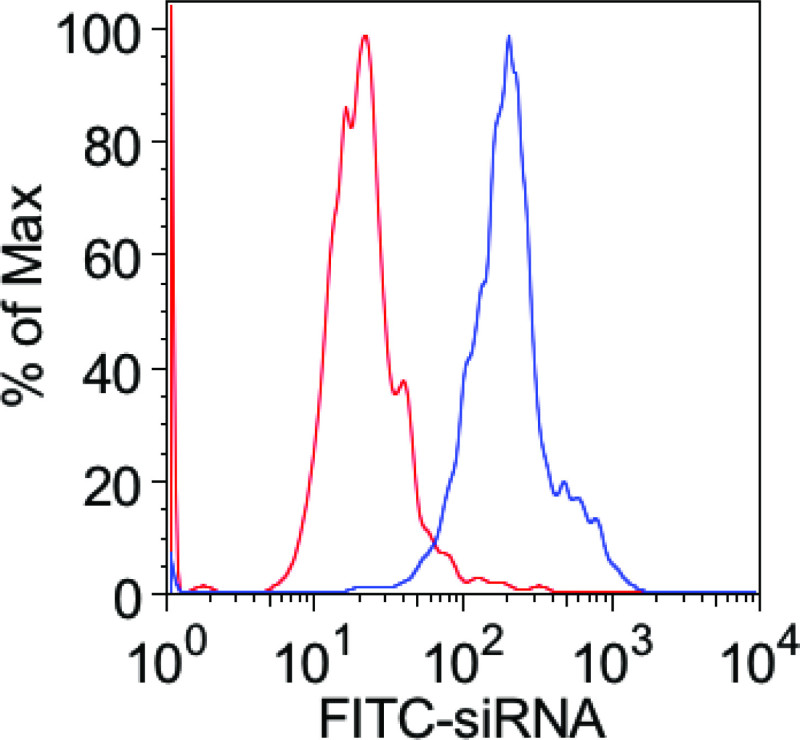 Figure 1: FITC-labeled siRNA is efficiently transfected into the mouse macrophage cell line J774A.1 using the described methods. Immediately after transfection, the cells were washed several times, fixed in paraformaldehyde, and fluorescence was monitored by flow cytometry. The figure compares cells incubated in the presence of FITC-siRNA that were either transfected (blue curve) or not (red curve).
Figure 1: FITC-labeled siRNA is efficiently transfected into the mouse macrophage cell line J774A.1 using the described methods. Immediately after transfection, the cells were washed several times, fixed in paraformaldehyde, and fluorescence was monitored by flow cytometry. The figure compares cells incubated in the presence of FITC-siRNA that were either transfected (blue curve) or not (red curve).
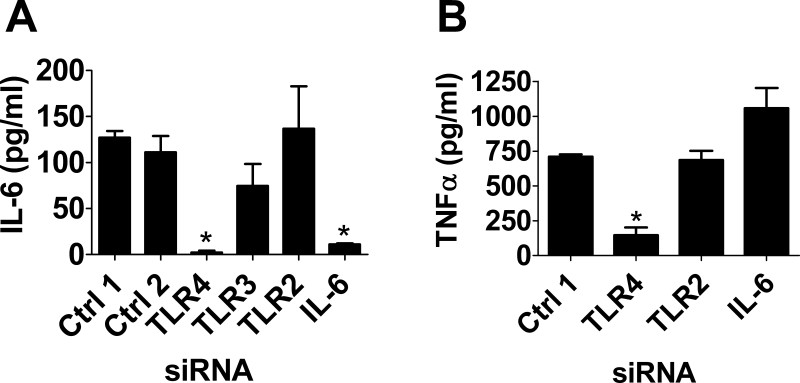 Figure 2: Efficacy and specificity of siRNA in the mouse macrophage cell line RAW264.7. RAW264.7 cells were transfected with the indicated siRNAs (either control non-targetting siRNA pools #1 or 2 or siRNAs that target TLR4, TLR3, TLR2, or IL-6 as indicated). 24 hr later, cells were stimulated with 20 ng/ml LPS for 6 hr and cytokine production (either IL-6 in panel A or TNFα in panel B was subsequently monitored by ELISA. Asterisks indicate value significantly different from control treatment (p <0.05, t-test).
Figure 2: Efficacy and specificity of siRNA in the mouse macrophage cell line RAW264.7. RAW264.7 cells were transfected with the indicated siRNAs (either control non-targetting siRNA pools #1 or 2 or siRNAs that target TLR4, TLR3, TLR2, or IL-6 as indicated). 24 hr later, cells were stimulated with 20 ng/ml LPS for 6 hr and cytokine production (either IL-6 in panel A or TNFα in panel B was subsequently monitored by ELISA. Asterisks indicate value significantly different from control treatment (p <0.05, t-test).
Discussion
Numerous studies have been published in which individual genes have been targeted by siRNA in murine macrophages. While lipid-mediated transfection has been used to deliver siRNA to macrophage cell lines on an individual basis, these methods suffer from potential effects on viability, limited gene knockdown, and variability from gene to gene. To develop more robust assays that could be used to target genes in medium- or high-throughput fashion, we optimized techniques using the Amaxa nucleofector 96-well shuttle system, which exhibits more consistency in gene knockdown and limited effects on viability (this more robust system is more expensive than lipid-mediated approaches). Many variables were tested in order to optimize these procedures. We were able to use this system to efficiently deliver siRNA to many different primary macrophages and immortalized macrophage cell lines (in some cases this necessitated using the Amaxa 96-well cell line optimization kit). In preliminary tests with these different mouse macrophage cell lines (RAW264.7, J774A.1, and MH-S) and primary cells (thioglycollate-elicited and bone marrow-derived macrophages), we found that RAW264.7 and J774A.1 cells exhibited the most robust siRNA-induced gene knockdown (followed by MH-S and primary cells, respectively, data not shown). As these two immortalized macrophage cell lines are commonly used for study, we optimized our RNAi procedures further using these lines.
To monitor transfection efficiency, we either use the control GFP plasmid in the nucleofector kits or FITC-labeled siRNA. The GFP plasmid exhibits strong fluorescence the day after transfection, with transfection efficiency of 30-50% as assayed microscopically or by flow cytometry (data not shown). With FITC-labeled siRNA, we observe >99% transfection efficiency as assayed by flow cytometry (dsRNA transfects better than plasmid DNA). While it is important to wait for fluorescence to develop when using the GFP plasmid, the fluorescent siRNA should be monitored relatively soon after transfection because the fluorescent signal weakens with time.
Transfection under the described conditions does not have a significant impact on cell viability. We typically monitor cell viability following our siRNA treatments using fluorescein diacetate. This compound is not fluorescent; however, when cleaved by cellular esterases in live cells, it becomes fluorescent. Moreover, the fluorescent form is then retained in cells with an intact plasma membrane24. This fluorescence-based assay is a relatively straightforward, rapid, and cheap assay to monitor cell viability, although there are other more sophisticated assays that for example monitor cellular ATP levels.
We typically assay our cells for innate immune function 24-36 hr following transfection, which is on the early side for RNAi compared to other cell lines. While one has to wait for endogenous proteins to turn over to facilitate gene knockdown, one must balance this with loss of siRNA potency, and we find more robust knockdown in these macrophage lines at slightly early times than the typical 48-72 hr used in other cell types. While it is preferable to monitor protein levels by western blot, we typically monitor mRNA levels by qPCR, with the goal of studying multiple genes at once, for which multiple antibodies and western blots may not be practical. We use LPS at the minimal dose that maximizes inflammatory cytokine production.
When targeting new genes with siRNA, we start with the relatively high siRNA dose described in the Protocol and then titrate down siRNAs that induce a phenotype. Subsequent controls designed to avoid off-target effects include the use of multiple siRNA duplexes to determine if more than one siRNA can induce the same phenotype, verifying gene knockdown by qPCR or western blot, and using gene overexpression studies to complement the RNAi approach. These approaches should allow for rapid identification of genes that regulate innate immunity in mouse macrophages.
Disclosures
The authors declare that they have no competing financial interests. This article was sponsored by Lonza, Inc.
Acknowledgments
Thanks to Brad Lackford for assistance optimizing some of the techniques described in this manuscript.
References
- Kaufmann SHE, Medzhitov R, Gordon S. The innate immune response to infection. ASM Press; 2004. [Google Scholar]
- Adams DO, Hamilton TA. The cell biology of macrophage activation. Annual review of immunology. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Fujiwara N, Kobayashi K. Macrophages in inflammation. Current drug targets. Inflammation and allergy. 2005;4:281–286. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annual review of immunology. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Cihakova D. Alternatively activated macrophages in infection and autoimmunity. Journal of autoimmunity. 2009;33:222–230. doi: 10.1016/j.jaut.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. Journal of immunology. 2008;181:3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Burke B, Lewis CE. The macrophage. 2nd edn. Oxford University Press; 2002. [Google Scholar]
- Lee G, Santat LA, Chang MS, Choi S. RNAi methodologies for the functional study of signaling molecules. PloS one. 2009;4 doi: 10.1371/journal.pone.0004559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carralot JP, et al. Automated high-throughput siRNA transfection in raw 264.7 macrophages: a case study for optimization procedure. Journal of biomolecular screening. 2009;14:151–160. doi: 10.1177/1087057108328762. [DOI] [PubMed] [Google Scholar]
- Alper S, et al. Identification of innate immunity genes and pathways using a comparative genomics approach. ProcNatl Acad Sci USA. 2008;105:7016–7021. doi: 10.1073/pnas.0802405105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Arras L, et al. An evolutionarily conserved innate immunity protein interaction network. J. Bio. Chem. 2013:1967–1978. doi: 10.1074/jbc.M112.407205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang IV, et al. Novel regulators of the systemic response to lipopolysaccharide. Am J Respir Cell Mol Biol. 2011;45:393–402. doi: 10.1165/rcmb.2010-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang IV, et al. Identification of novel innate immune genes by transcriptional profiling of macrophages stimulated with TLR ligands. Molecular immunology. 2011;48:1886–1895. doi: 10.1016/j.molimm.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang IV, et al. Identification of novel genes that mediate innate immunity using inbred mice. Genetics. 2009;183:1535–1544. doi: 10.1534/genetics.109.107540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschke WC, Baird S, Ralph P, Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978;15:261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- Ralph P, Moore MA, Nilsson K. Lysozyme synthesis by established human and murine histiocytic lymphoma cell lines. The Journal of experimental medicine. 1976:1528–1533. doi: 10.1084/jem.143.6.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumbansen M, et al. First siRNA library screening in hard-to-transfect HUVEC cells. Journal of RNAi and gene silencing. 2010;6:354–360. [PMC free article] [PubMed] [Google Scholar]
- Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Aliprantis AO, et al. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Conforti R, et al. Opposing effects of toll-like receptor (TLR3) signaling in tumors can be therapeutically uncoupled to optimize the anticancer efficacy of TLR3 ligands. Cancer Res. 2010;70:490–500. doi: 10.1158/0008-5472.CAN-09-1890. [DOI] [PubMed] [Google Scholar]
- Fernandez-Botran R, Větvička V. Methods in Cellular Immunology. Vol. 8. CRC Press; 2001. p. 8. [Google Scholar]


