Abstract
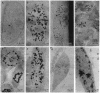
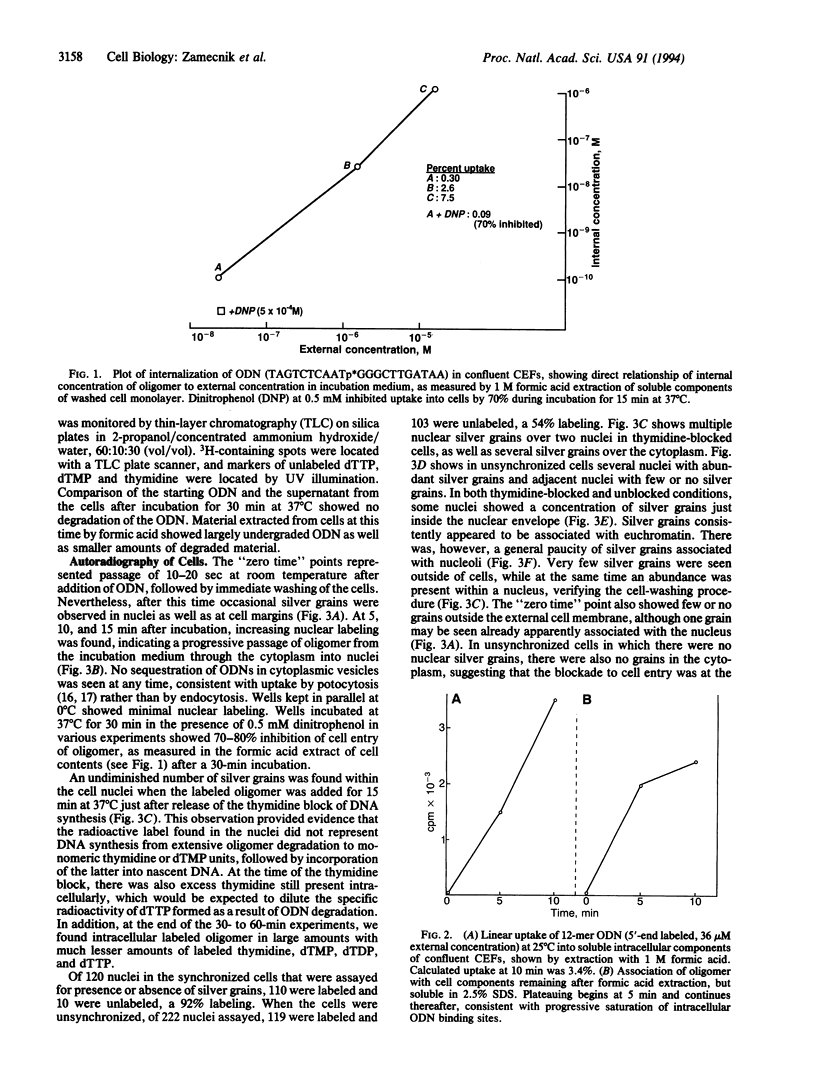
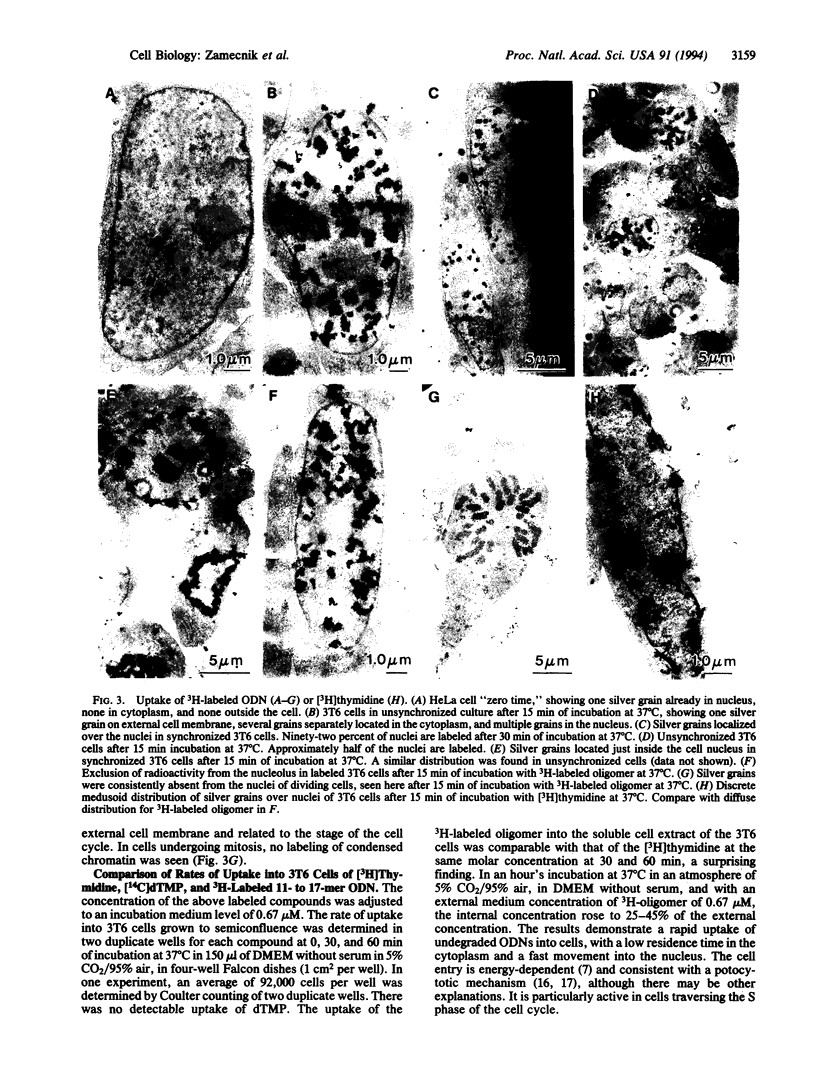
Unmodified oligodeoxynucleotides (ODNs) were synthesized and tested for their ability to cross external eukaryotic cell membranes and to enter the cytosol and nucleus in tissue cultures. The ODNs were labeled with high-specific-activity [3H]thymidine (> or = 100 Ci/mmol), or [ alpha-32P]ATP or [ gamma-32P]ATP (300-1000 Ci/mmol; 1 Ci = 37 GBq), and the label was either in the central portion of the molecule or at the 3' or 5' end. The cells employed were for the most part 3T6 murine fibroblasts, grown in monolayers, either semiconfluent or confluent, but some experiments were carried out with chicken embryo fibroblasts or human HeLa cells. Parallel wells in the same experiment were prepared for electron microscopy or for cell fractionation and radioactivity assays. Electron microscopic autoradiography indicated that ODNs cross the external cell membrane, traverse the cytosol, and begin to enter the cell nucleus within a few seconds to 5 min at 37 degrees C in Dulbecco's medium without added serum. After 30-60 min of incubation with ODNs, abundant silver grains were observed at or just inside the nuclear membrane or well distributed across the nucleus, particularly in association with euchromatin. There was a paucity of silver grains associated with nucleoli. Cell entry of oligomer was related to cell cycling events and was energy dependent. Degradation of oligomer to monomers, with reincorporation into DNA, does not appear to explain these results. No sequestration of labeled oligomer in cytoplasmic vesicles en route from the exterior of the cell to the nucleus was observed. The observations are more suggestive of internalization of oligonucleotide by a mechanism as yet unclear or, alternatively, by a caveolar, potocytotic mechanism rather than by endocytosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar S., Juliano R. L. Cellular uptake and intracellular fate of antisense oligonucleotides. Trends Cell Biol. 1992 May;2(5):139–144. doi: 10.1016/0962-8924(92)90100-2. [DOI] [PubMed] [Google Scholar]
- Anderson R. G. Caveolae: where incoming and outgoing messengers meet. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10909–10913. doi: 10.1073/pnas.90.23.10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu R. W., Baril E. F. Nuclear DNA polymerases and the HeLa cell cycle. J Biol Chem. 1975 Oct 10;250(19):7951–7957. [PubMed] [Google Scholar]
- Iwanaga T., Ferriola P. C. Cellular uptake of phosphorothioate oligodeoxynucleotides is negatively affected by cell density in a transformed rat tracheal epithelial cell line: implication for antisense approaches. Biochem Biophys Res Commun. 1993 Mar 31;191(3):1152–1157. doi: 10.1006/bbrc.1993.1337. [DOI] [PubMed] [Google Scholar]
- Plesner P., Goodchild J., Kalckar H. M., Zamecnik P. C. Oligonucleotides with rapid turnover of the phosphate groups occur endogenously in eukaryotic cells. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1936–1939. doi: 10.1073/pnas.84.7.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu M., Simionescu N., Palade G. E. Differentiated microdomains on the luminal surface of capillary endothelium: distribution of lectin receptors. J Cell Biol. 1982 Aug;94(2):406–413. doi: 10.1083/jcb.94.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. A., Tonkinson J. L., Zhang L. M., Yakubov L., Gervasoni J., Taub R., Rotenberg S. A. Dynamics of the internalization of phosphodiester oligodeoxynucleotides in HL60 cells. Biochemistry. 1993 May 11;32(18):4855–4861. doi: 10.1021/bi00069a022. [DOI] [PubMed] [Google Scholar]
- Stephenson M. L., Zamecnik P. C. Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc Natl Acad Sci U S A. 1978 Jan;75(1):285–288. doi: 10.1073/pnas.75.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M., Takayama K. RNA. Deviants--or emissaries. Nature. 1994 Jan 6;367(6458):17–18. doi: 10.1038/367017a0. [DOI] [PubMed] [Google Scholar]
- Wu-Pong S., Weiss T. L., Hunt C. A. Antisense c-myc oligodeoxyribonucleotide cellular uptake. Pharm Res. 1992 Aug;9(8):1010–1017. doi: 10.1023/a:1015846209681. [DOI] [PubMed] [Google Scholar]
- Zamecnik P. C., Goodchild J., Taguchi Y., Sarin P. S. Inhibition of replication and expression of human T-cell lymphotropic virus type III in cultured cells by exogenous synthetic oligonucleotides complementary to viral RNA. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4143–4146. doi: 10.1073/pnas.83.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci U S A. 1978 Jan;75(1):280–284. doi: 10.1073/pnas.75.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]