Abstract
The potent tropane analog, WF-23 [2β-propanoyl-3β-(2-naphthyl) tropane], blocks dopamine, serotonin, and norepinephrine transporters with high affinity in vitro and blocks transporters for at least 2 days following a single in vivo administration. Previous studies demonstrated desensitization of monoamine receptor-coupled G-proteins in brain following chronic treatment of rats with WF-23. The current study sought to determine the time course of this desensitization and the behavioral effects of receptor desensitization. Rats were treated with 1 mg/kg WF-23 and injected i.p. every 48 h for 1 to 21 days. Receptor activation of G-proteins was determined by guanosine 5′-O-(3-[35S]thiotriphosphate) ([35S]GTPγS) binding in brain sections for monoamine receptors, as well as μ opioid receptors as a nonmonoamine receptor control. Chronic treatment with WF-23 produced significant reductions in D2, 5-hydroxytryptamine 1A, and α2-adrenergic receptor-stimulated [35S]GTPγS binding; however, the time course of desensitization varied with different receptors. There was no effect of WF-23 treatment on µ opioid-stimulated [35S]GTPγS binding at any time point. Consistent with previous studies, there was no effect of WF-23 treatment on D2 receptor binding, as determined by [3H]spiperone autoradiography. Locomotor activity was significantly increased for up to 48 h following acute administration of WF-23, demonstrated by increased photocell beam interruptions. WF-23-induced increases in locomotor activity occurred following repeated administration, as above, for up to 7 days. Following 7 days of treatment, there was a significant decrease in WF-23-increased locomotor activity. This reduction occurred at the same time point as the decrease in D2 receptor/G-protein coupling, suggesting a role of D2 desensitization in producing tolerance to WF-23-mediated behavior.
Actions of the biogenic amines are terminated via reuptake by specific transporters. Cocaine and many antidepressants exert their actions via blockade of this process (Ritz et al., 1987). Although most studies suggest the reinforcing effects of cocaine are mediated by inhibition of dopamine transporters, cocaine inhibits dopamine, serotonin, and norepinephrine transporters (DAT, SERT, and NET, respectively) with approximately the same affinity (Ritz et al., 1987). Studies involving DAT knockout mice also support the involvement of SERT and NET in the mechanisms of behavioral effects of cocaine (Sora et al., 2001; Mead et al., 2002).
Many laboratories have utilized long-acting biogenic amine transporter blockers (Carroll et al., 1992; Madras et al., 2003; Appell et al., 2004) to investigate the neurobiological actions of cocaine. Davies and colleagues (Davies et al., 1993) have developed a novel series of tropane analogs based on vinylcarbenoid precursors, which exhibit a greater range of structural modification possibilities and increased metabolic stability. The most potent analog to date, WF-23, binds with high affinity at DAT (Ki = 0.12 nM), SERT (Ki = 0.39 nM), or NET (Ki = 2.9 nM) (Bennett et al., 1995). WF-23 has also been shown to produce increases in locomotor activity for up to 24 h following a single injection (1 mg/kg i.p.); the same treatment with WF-23 also blocked DAT binding in rat striatum by at least 48 h after injection (Daunais et al., 1998). The high affinity for biogenic amine transporters and long duration of action of WF-23 in both behavioral and biochemical assays provides a useful pharmacological tool to investigate the effects of long-term blockade of biogenic amine transporters.
Blockade of DAT, SERT, and NET by cocaine or other inhibitors produces increased levels of monoamines in the synaptic cleft and a prolonged exposure of receptors to these neurotransmitters (Hemby et al., 1995). Both receptor desensitization, as defined by prolonged uncoupling of receptors from G-proteins, and down-regulation, defined as a decrease in receptor number, occur in response to chronic agonist exposure (Lefkowitz et al., 1990). One of the advantages of a potent long-acting monoamine transporter inhibitor like WF-23 is the opportunity to examine monoamine receptor desensitization that occurs following chronic increases in extracellular monoamines.
Monoamines exert their actions by binding to specific receptors, most of which belong to the superfamily of seven transmembrane-spanning receptors that couple to G-proteins (Neve, 1997). The receptor G-protein activation cycle has been well characterized to show that binding of agonists to G-protein-coupled receptors dramatically increases the affinity of Gα subunits for GTP (Birnbaumer et al., 1990). This change in Gα can be detected by agonist-stimulated [35S]GTPγS binding, which can be assayed both in brain membranes (Lorenzen et al., 1993; Traynor and Nahorski, 1995) and in brain sections by autoradiography (Sim et al., 1995). Specifically, activation of [35S]GTPγS binding in brain has been specifically measured for monoamine receptors, specifically dopamine D2 (Rinken et al., 1999; O’Connor et al., 2004), α2-adrenergic (Happe et al., 2000), and 5-HT1A receptors (Newman-Tancredi et al., 1996; Sim-Selley et al., 2000b). Agonist-stimulated [35S]GTPγS binding provides an excellent method to determine effects of chronic drug treatment on receptor/G-protein desensitization, i.e., the loss of activation of Gα subunits by receptor agonists (Breivogel et al., 1997a, 1999; Sim-Selley et al., 2000a).
It is clear that chronic treatment with cocaine and other monoamine transporter inhibitors may affect receptor-mediated signal transduction by long-term increases in extrasynaptic monoamines. For example, alterations in several signal transduction mechanisms have been demonstrated following chronic administration of cocaine (Beitner-Johnson et al., 1992; Unterwald et al., 1996; Kushner and Unterwald, 2001). In a previous study (O’Connor et al., 2004), we showed that desensitization of D2 receptors occurred in striatum of rats treated for 15 days with WF-23 (1 mg/kg i.p. every 48 h). The goal of the present study was to characterize the effects of chronic transporter blockade on the rate of desensitization of G-protein-coupled receptors and determine the behavioral consequences of D2 receptor desensitization on locomotor activity. To accomplish this, rats were chronically treated with WF-23 for various time periods to assess the rate of receptor/G-protein desensitization and its effects on spontaneous locomotor activity.
Materials and Methods
Materials
WF-23 was synthesized as previously described (Davies et al., 1994) and was dissolved in phosphate-buffered saline, which served as the vehicle. [35S]GTPγS (1150–1395 Ci/mmol), [125I]RTI-55 (2200 Ci/mmol), and [3H]spiperone (15.7 Ci/mmol), Kodak BioMax MS Films, Kodak BioMax HE TranScreens, and TR tritium sensitive phosphor screens were purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). DAMGO, 8-cyclopentyl-1,3-diproxylxanthine, fluoxetine, GDP, ketanserin, norepinephrine, R(−)-propylnorapomorphine (NPA), spiperone, and 8-OH-DPAT were obtained from Sigma-Aldrich (St. Louis, MO). Xray films were obtained from Phenix Research (Hayward, CA).
Animals
Male Sprague-Dawley rats (Harlan, Indianapolis, IN), weighing 200 to 300 g at the time of the experiment, were used in all studies. Rats were treated with i.p. injections of WF-23 (1 mg/kg) or saline every 48 h for 3 to 21 days (n = 6 per group). The dose and treatment period were based on previous studies (Daunais et al., 1998) that demonstrated a robust behavioral response and a 50% decrease in [125I]RTI-55 binding 48 h after a single injection of WF-23 (1 mg/kg i.p). Animals were pair-housed in a climate-controlled room with a 12-h light/dark cycle. Food and water were available ad libitum. All animals were adapted to vivarium conditions for 5 days before testing. Injections were given so that all testing occurred during the light phase of the cycle (7:30 AM–4:30 PM). All procedures were carried out in accordance with established practices as described in the National Institutes of Health Guide for Care and Use of Laboratory Animals. In addition, all procedures were reviewed and approved by the Animal Care and Use Committee of Wake Forest University.
[35S]GTPγS Autoradiography
Receptor/G-protein coupling was assayed in rat brain sections using agonist-stimulated [35S]GTPγS autoradiography (Sim et al., 1995; Rinken et al., 1999). Saline- and WF-23-treated animals were euthanized by rapid decapitation 48 h after the last injection on days 3, 7, 15, and 21 (n = 6 per time point). Brains were removed and prepared for sectioning. Rat brain sections were preincubated for 10 min in TME buffer (50 mM Tris-HCl, 3 mM MgCl2, 0.2 mM EGTA, 100 mM NaCl, pH 7.4), then 15 min with 1 to 2 mM GDP and 1 µM 8-cyclopentyl-1,3-diproxylxanthine at 25°C. Sections were incubated for 90 min at 30°C for D2 and 120 min at 25°C for μ, α2, and 5-HT1A. Agonists included: 300 µM norepinephrine (α2), 10 µM NPA (D2), 3 µM DAMGO (μ opioid), and 3 µM 8-OH-DPAT (5-HT1A). The sections were then washed, exposed to X-ray film, and analyzed as described previously (Sim et al., 1995). Agonist-stimulated activity was calculated by subtracting the optical density in basal sections (GDP only) from that of agonist-stimulated sections, and results are expressed as percent stimulation over basal activity. For each agonist, triplicate sections of brain from at least four animals were used.
[3H]Spiperone Autoradiography
D2 receptor binding was assayed in rat brain sections using [3H]spiperone autoradiography (Palacios et al., 1981; Araki et al., 1997). Sections of rat brain at the level of the caudate/putamen from saline- and WF-23-treated animals were prepared as described above. Rat brain sections were preincubated for 10 min in Tris buffer (50 mM Tris-HCl, 1 mM MgCl2, pH 7.6) at 25°C. Sections were incubated in Tris buffer with 0.6 nM [3H]spiperone and 100 nM ketanserin for 60 min at 25°C. Nonspecific binding was assessed in the presence of unlabeled spiperone (0.2 µM). Sections were then washed twice in Tris and once in H2O at 4°C. Sections were then dried and exposed to TR tritium sensitive storage phosphor screens (PerkinElmer Life and Analytical Sciences) for 3 weeks. The Cyclone Storage Phosphor System with OptiQuant image analysis software (version 03.10) was used to scan images from storage phosphor screens. Images were then imported and analyzed in NIH Image J (version 1.30 for MacIntosh). Specific binding was determined by subtracting nonspecific binding from total binding.
[125I]RTI-55 Autoradiography
DAT binding was performed using [125I]RTI-55 autoradiography (Boja et al., 1992; Yoshiyuki and Tsunehiko, 1997) to explore occupancy of DAT by WF-23. Brain sections were incubated in buffer (10 mM sodium phosphate, 0.32 M sucrose, pH 7.4) with 30 nM fluoxetine and 10 pM [125I]RTI-55 (2200 Ci/mmol) at 25°C for 60 min. Nonspecific binding was assessed with 1 µM WF-23. The sections were then washed, exposed to X-ray film (Kodak BioMax MS Film with BioMax HE TranScreen) at −80°C, and analyzed as described previously (Sim et al., 1995). Preincubations of tissue were excluded to minimize washout of bound WF-23.
Behavioral Testing
Locomotor activity was assessed in open-field clear plastic test chambers (42 × 42 × 30 cm). Locomotion was measured by electronic counters that detected interruptions of eight independent photocell beams (Omnitech, Columbus, OH). The following measures were recorded and stored in 10-min intervals: horizontal activity (the total number of horizontal beam interruptions) and forward locomotor or ambulatory activity, vertical activity or rearing and stereotypy (the total number of consecutive breaks of the same beam or two adjacent beams).
Animals were habituated to the chamber for 4 consecutive days before testing, for 60 min each day. On the last 2 days of habituation, animals received saline injections. On the 5th day (test day 1), the animals were placed in the chamber for 30 min where preinjection data were obtained. They were then given a single i.p. injection of WF-23 (1 mg/kg) or saline and returned to the locomotor chamber where their activity was recorded for an additional 2.5 h. This procedure was repeated on days 3, 5, 7, and 15. At each time point, locomotor activity was assessed in treatment groups consisting of WF-23 (n = 8) and saline (n = 8). Animals received injections every 48 h throughout the study. Locomotor activity was assessed on day 21 in the absence of drug for 30 min.
Data Analysis
Unless otherwise indicated, autoradiography data are reported as mean values ± S.E.M. of at least three separate experiments, each of which were performed in triplicate. Net-stimulated [35S]GTPγS binding is defined as stimulated binding minus basal binding. Percent decrease is defined as (100 − [net-stimulated binding in saline-treated animals]/(net-stimulated binding in WF- 23-treated animals) × 100%). Statistical significance of all data was determined by one-way analysis of variance with repeated measures, followed by Tukey-Kramer t test for multiple comparisons using JMP (SAS Institute, Cary, NC). Locomotor activity data are reported as mean values ± S.E.M. of eight animals at each time point (either as the total photocell beam interruptions over a session or per 10-min blocks within a session across the duration of treatment). Data were analyzed with one-way analysis of variance followed by Tukey- Kramer t test for multiple comparisons to compare WF-23 to vehicle-treated animals at each time point and to compare WF-23-treated animals across treatment duration.
Results
Effects of Chronic WF-23 Treatment on Receptor-Activated G-Proteins
The dose of WF-23 and treatment period were based on previous studies (Daunais et al., 1998) that evaluated the effects of a single administration of WF-23 on horizontal locomotor activity and [125I]RTI-55 binding in caudate/putamen. These studies had demonstrated a 50% decrease in [125I]RTI-55 binding 48 h after a single injection of WF-23 (1 mg/kg i.p.). More recent results showed that chronic treatment with WF-23 (injected every 48 h for 15 days) produced significant desensitization of monoamine receptor- activated G-proteins (O’Connor et al., 2004). Therefore, in our study, injections were given every 48 h and brains removed 48 h following the last injection after various durations of treatment with WF-23.
The time course effects of chronic WF-23 treatment on receptor-activated G-proteins were examined for three monoamine receptors, D2, α2-adrenergic, and 5-HT1A, as well as μ opioid receptors as a nonmonoamine control. Figure 1 compares typical autoradiograms from saline-treated rats and from rats treated with WF-23 for 3 and 21 days with D2- and μ opioid-stimulated [35S]GTPγS binding in caudate/putamen, α2-adrenergic activity in amygdala, and 5-HT1A activity in hippocampus. In caudate, a robust decrease in D2-stimulated binding was observed after 21 days of chronic WF-23 treatment with no effect observed after only 3 days of treatment. In contrast, there was no effect on µ opioid-stimulated [35S]GTPγS binding in caudate after either 3 or 21 days of WF-23 treatment. In amygdala, chronic WF-23 administration produced some reduction in α2-adrenergic-stimulated [35S]GTPγS binding after 3 days of treatment, with even further reduction evident after 21 days. Similarly, in hippocampus, chronic WF-23 administration also produced reduction in 5-HT1A receptor-stimulated [35S]GTPγS binding after 3 days of treatment, with even further reductions after 21 days (Fig. 1).
Fig. 1.
Effects of chronic WF-23 treatment on receptor-stimulated [35S]GTPγS binding. Shown are representative autoradiograms of sections of rat brain following 21 days of saline (top), 3 days (1 injection) of WF-23 (middle), and 21 days (10 injections) of WF-23 treatment (bottom). Coronal sections were assayed for agonist-stimulated [35S]GTPγS binding as follows (left to right): D2 using 10 µM NPA (caudate/putamen), μ opioid using 3 µM DAMGO (caudate/putamen), α2-adrenergic using 300 µM norepinephrine (amygdala), and 5-HT1A using 3 µM 8-OH-DPAT (hippocampus).
Densitometric analysis of these results confirmed these time-dependent reductions in agonist-stimulated [35S]GTPγS binding for all three monoamine receptor systems (Table 1). Quantification of autoradiograms at the level of the caudate/ putamen showed that D2-stimulated [35S]GTPγS binding was not affected after 3 days of chronic WF-23 treatment, but was significantly reduced (by 23 ± 4% and 34 ± 7%) following 7 and 15 days of treatment, respectively, and further reduced (by 72 ± 10%) following 21 days of treatment. α2-Adrenergic-stimulated [35S]GTPγS binding in amygdala was significantly reduced following 7 days of treatment (by 21 ± 7%), with further reductions occurring with longer treatment (46 ± 9% after 15 days; 61 ± 4% after 21 days). Reductions in α2-adrenergic-stimulated activity in amygdala were observed in some animals after 3 days of WF-23 treatment, but were not significant in the whole treated group. Densitometric analysis of 5-HT1A receptor-stimulated [35S]GTPγS binding in hippocampus revealed significant reduction following a single injection of WF-23 (reduced by 33 ± 6%), with further reductions following longer treatment with WF-23 (reduced by 59 ± 6% following 21 days). Although 5-HT1A-stimulated [35S]GTPγS binding was also observed in septum and dorsal raphe, no significant effect of chronic WF-23 was observed in either region (data not shown). In contrast, there was no effect of chronic WF-23 treatment on µ opioid-stimulated [35S]GTPγS binding in caudate/putamen at any time point.
TABLE 1. Effect of chronic WF-23 treatment on receptor-stimulated [35S]GTPγS binding.
Brain sections from saline- and WF-23-treated rats were assayed for agonist-stimulated [35S]GTPγS binding as described under Materials and Methods. Data represent nanocurie per gram of [35S] from densitometric analysis of autoradiograms and are mean values ± S.E.M. of triplicate sections of brains from six treated and six control animals.
| Region | Agonist | Net-Stimulated [35S]GTPγS Binding (nCi/g, % Saline) |
||||
|---|---|---|---|---|---|---|
| Saline | Day 3 | Day 7 | Day 15 | Day 21 | ||
| CPu | NPA | 252 ± 18 | 227 ± 18 | 186 ± 10* | 166 ± 17* | 70 ± 24* |
| (100 ± 7%) | (90 ± 7%) | (77 ± 4%) | (66 ± 7%) | (28 ± 10%) | ||
| CPu | DAMGO | 225 ± 18 | 218 ± 27 | 207 ± 22 | 205 ± 35 | 200 ± 14 |
| (100 ± 8%) | (100 ± 12%) | (92 ± 10%) | (106 ± 15%) | (93 ± 6%) | ||
| Amyg | NE | 271 ± 14 | 219 ± 36* | 180 ± 18* | 161 ± 25* | 78 ± 11* |
| (100 ± 5%) | (89 ± 13%) | (79 ± 7%) | (54 ± 9%) | (39 ± 4%) | ||
| Hippo | 8-OH-DPAT | 342 ± 33 | 189 ± 20* | 181 ± 19* | 173 ± 11* | 163 ± 21* |
| (100 ± 10%) | (67 ± 6%) | (52 ± 6%) | (42 ± 3%) | (41 ± 6%) | ||
CPu, caudate/putamen; Amyg, amygdala; Hippo, hippocampus.
p ≤ 0.05, significantly different from control by Tukey-Kramer t test for multiple comaparisons.
Effects of Chronic WF-23 Treatment on D2 Receptor and DAT Binding in Caudate/Putamen
These experiments were designed to determine whether chronic WF-23 treatment affected D2 receptor binding and to use DAT binding in brains from WF-23-treated animals as a measure of occupancy of DAT by WF-23 after chronic treatment. The observed decrease in receptor-stimulated [35S]GTPγS binding after chronic WF-23 treatment may be attributable to a functional uncoupling of the receptor/G-protein unit, a reduction in D2 receptor number, or a combination of both mechanisms. We have previously demonstrated that chronic WF-23 treatment produced desensitization of D2 receptor-activated G-proteins in the absence of down-regulation of D2 receptors following 15 days of WF-23 treatment (O’Connor et al., 2004). However, it is possible that the longer treatment with WF-23 in the current study may result in alterations in receptor number due to the prolonged period of transporter blockade.
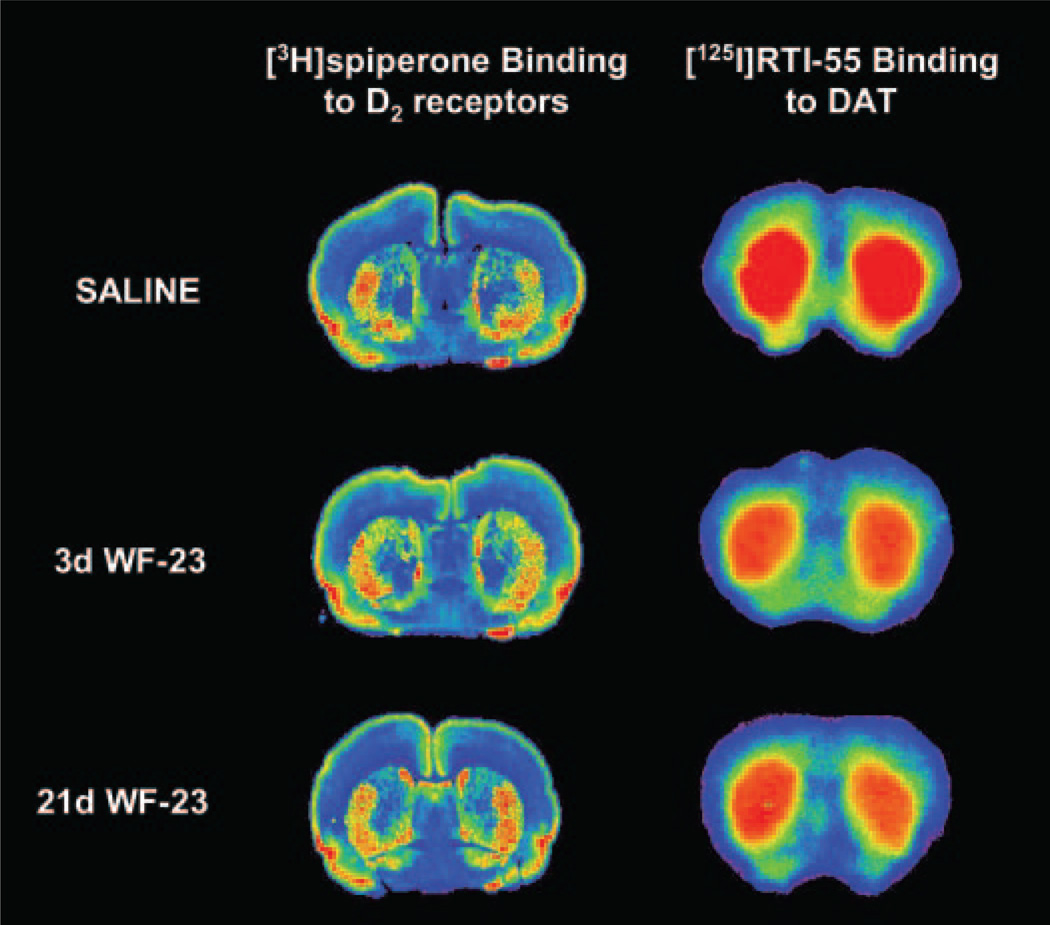
Therefore, [3H]spiperone binding was performed following 21 days of WF-23 treatment to assess D2 receptor density. Representative autoradiograms of saline and WF-23-treated animals (Fig. 2, left) show significant binding of [3H]spiperone in caudate/putamen, with no discernible effect of chronic WF-23 treatment. Densitometric analysis of saline- and WF-23-treated sections (n = 6 per group) revealed no significant difference between groups (WF-23 treated animals = 100 ± 3% of saline values). Therefore the decrease in D2-activated G-proteins occurred in the absence of down-regulation of D2 receptors in caudate/putamen following 15 to 21 days of WF-23 treatment.
Fig. 2.
Effects of chronic WF-23 treatment on D2 receptor and DAT binding. Shown are representative autoradiograms of coronal sections of rat brain at the level of the striatum following 21 days of saline (top), 3 days of WF-23 (middle), and 21 days of WF-23 treatment (bottom). D2 receptor binding was assessed using [3H]spiperone (left), and occupancy of dopamine transporters by WF-23 was measured using [125I]RTI-55 binding (right).
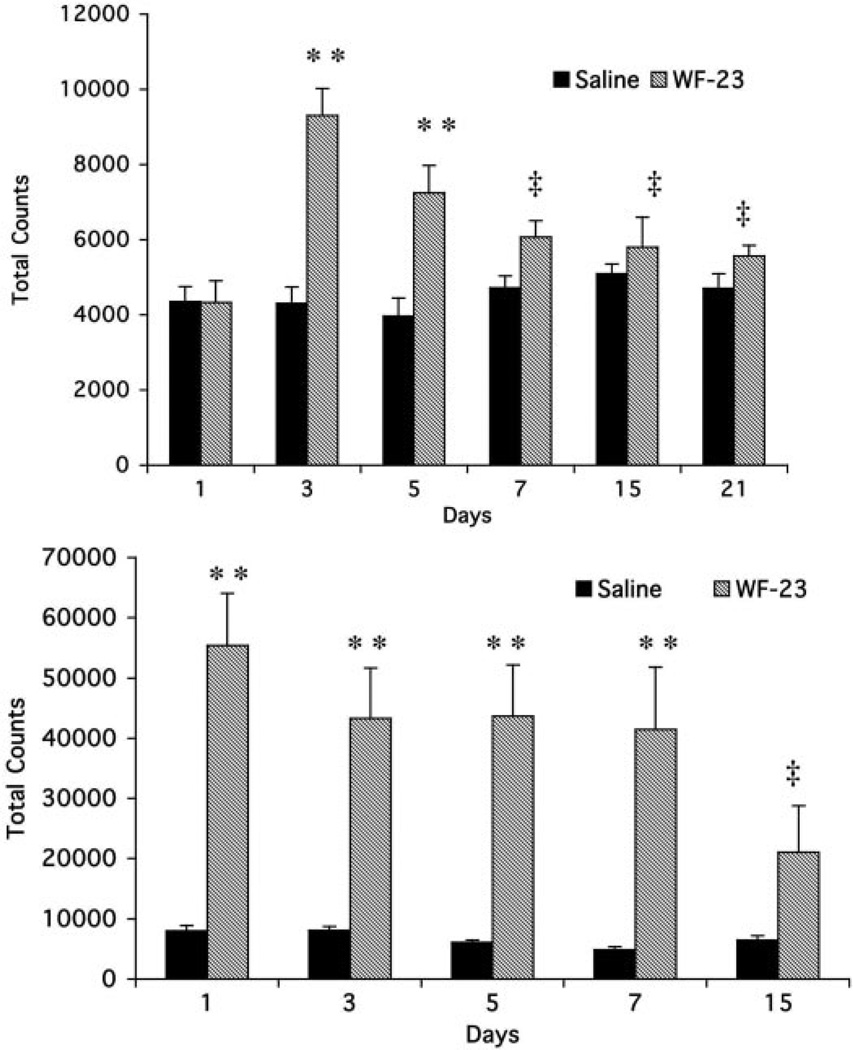
In addition, occupancy of DAT by WF-23 during the chronic treatment paradigm was assessed in striatal sections from rats treated chronically with saline or WF-23 using [125I]RTI-55 autoradiography. Preliminary experiments using naive animals demonstrated that addition of 30 nM fluoxetine was sufficient to block binding of [125I]RTI-55 to SERT (data not shown). Reductions in [125I]RTI-55 binding were observed following a single injection of WF-23 as assessed on day 3, and this reduction appeared to be relatively constant regardless of the duration of treatment (Fig. 2, right). Densitometric analysis showed that [125I]RTI-55 binding to DAT was decreased on average by 32 ± 5% in caudate/putamen of rats chronically treated with WF-23 up to 48 h after each administration when compared with controls (Table 2).
TABLE 2. Effects of chronic WF-23 treatment on [125I]RTI-55 binding.
Brain sections from control and WF-23-treated rats were assayed for [125I]RTI-55 binding as described under Materials and Methods. Data represent ROD of [125I]RTI-55-specific binding from densitometric analysis of autoradiograms and are mean values ± S.E.M. of triplicate sections of brains from six treated and six control animals.
| Specific Binding of [125I]RTI-55 (ROD, % Saline) | ||||
|---|---|---|---|---|
| Saline | Day 3 | Day 7 | Day 15 | Day 21 |
| 64 ± 5 | 53 ± 4* | 42 ± 2* | 31 ± 1* | 50 ± 3* |
| (100 ± 8%) | (72 ± 6%) | (63 ± 3%) | (62 ± 2%) | (72 ± 5%) |
ROD, relative optical densities.
p ≤ 0.001, significantly different from control by Tukey-Kramer t test for multiple comparisons.
Effects of WF-23 on Locomotor Activity
Daunais et al. (1998) showed that a single dose (1 mg/kg) of WF-23 produced robust increases in locomotor activity, beginning as early as 60 min after injection of WF-23 and lasting 24 h. In the current study, locomotor activity was measured to determine changes occurring after chronic treatment with WF-23. Data were collected in both pre- and postinjection sessions to assess the locomotor activity 48 h after each injection of drug (Fig. 3). In the preinjection phase, where animals were tested for 30 min before injection of drug, data were used to assess the locomotor activity 48 h after each injection, as well as to establish a baseline on each injection day. After injection of drug, postinjection data were collected for 150 min. These data reflect both the direct behavioral response to WF-23, as well as the cumulative effects of WF-23 following chronic treatment for up to 21 days. Preinjection testing at day 21 occurred 48 h after the final WF-23 injection so that this group matched the treatment paradigm of animals for biochemical testing; this accounts for the fact that preinjection data were analyzed for 21 days, whereas postinjection data were analyzed for only 15 days of WF-23 treatment.
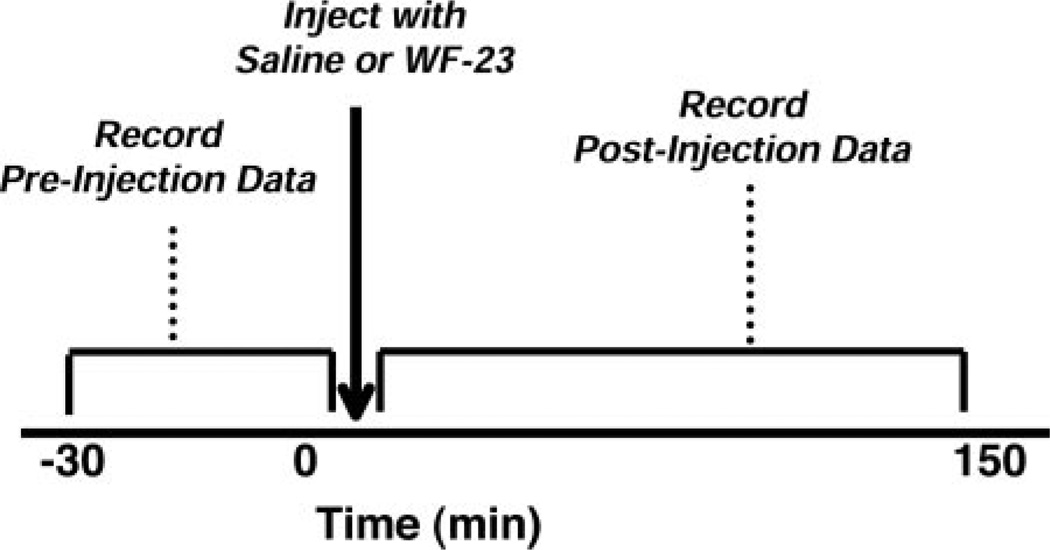
Fig. 3.
Schematic illustrating the procedure for collecting locomotor activity data in chronic WF-23-treated rats during pre- and postinjection sessions. Preinjection data were collected for 30 min immediately prior to each drug injection on days 1, 3, 5, 7, 15, and 21. Postinjection data were obtained immediately following injection on days 1, 3, 5, 7, and 15.
Preinjection data (Fig. 4, top) showed that WF-23 produced significant elevations (215 ± 16%) in horizontal activity for up to 48 h after a single injection when compared with saline-treated rats. However, 48 h after the second injection of WF-23 (day 5), preinjection values were reduced (180 ± 18%). Following 7 days of treatment, these preinjection increases were no longer significant (119 ± 9%).
Fig. 4.
Effects of WF-23 on spontaneous locomotor activity. Preinjection (top) and postinjection (bottom) data are expressed as total photocell beam interruptions over a 30-min (preinjection) and 150-min (postinjection) session, respectively, across the duration of treatment. Data are mean ± S.E.M. analyzed with one-way analysis of variance followed by Tukey-Kramer t test for multiple comparisons comparing the effects of WF-23 treatment time point with vehicle at each time point. **, p < 0.01 different from saline; ‡, p < 0.05 different from WF-23 on day 3 for preinjection and WF-23 on day 1 for postinjection.
Postinjection data (Fig. 4, bottom) showed that the first injection of WF-23 produced significant peak elevations in locomotor activity (690 ± 10%). Subsequent injections on day 3, 5, and 7 continued to produce increases in locomotor activity (531 ± 10%, 584 ± 35%, and 518 ± 50%). In contrast, WF-23-produced increases in locomotor activity were severely attenuated after 15 days (327 ± 13%), with levels significantly lower (40% of day 1 levels) than levels observed on day 1.
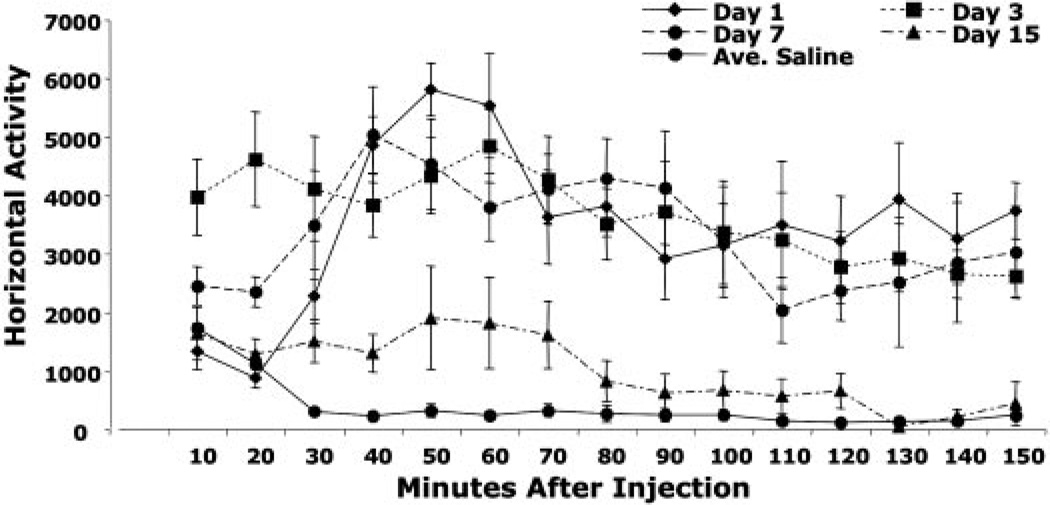
Figure 5 shows the time course of locomotor activity within each session after 1, 5, 7, and 15 days of treatment. Peak activity levels occurred 60 min after the first injection, consistent with the findings ofDaunais et al. (1998). These levels decreased slightly after 60 min, but remained significantly elevated for the duration of the session. Following subsequent injections on days 3 and 7, the peak increase in activity occurred more rapidly (usually within 20 min) and also remained elevated for the duration of the session. In contrast, on day 15, locomotor activity was elevated for only 70 min, followed by a reduction in activity to levels not significantly different from saline after 80 min which remained low for the duration of the session. Moreover, the peak of activity (which occurred at 50–60 min) was significantly lower after 15 days of treatment compared with 1, 3, and 7 days.
Fig. 5.
Time course of effects of chronic WF-23 on spontaneous locomotor activity. The effects of chronic i.p. administration of WF-23 (1 mg/kg) or saline on horizontal activity within sessions on treatment days 1, 3, 7, and 15 are expressed as total photocell beam interruptions per 10-min bin. Data are mean ± S.E.M. analyzed with one-way analysis of variance followed by Tukey-Kramer t test for multiple comparisons comparing the effects of WF-23 treatment time point with vehicle at each time point. Postinjection, WF-23-treated animals were significantly different from saline on day 1 from 30 to 150 min (p < 0.01), on day 3 from 10 to 150 min (p < 0.01), and on day 15 between 30 and 70 min (p < 0.01). Additionally, WF-23-treated animals were significantly different postinjection on day 15 compared with day 1 and day 3 throughout the duration of the session (10–150 min, p < 0.05).
Discussion
Previous results showing that a single administration of WF-23 produced long-term blockade of both DAT and SERT binding (Daunais et al., 1998) suggested that this tropane analog would be useful in producing long-term increases in biogenic amines after chronic administration. Furthermore, our previous studies (O’Connor et al., 2004) showed that chronic WF-23 treatment reduced monoamine receptor/G-protein coupling in specific areas of the brain. Although desensitization of G-protein-coupled receptors in response to chronic agonist exposure has been extensively documented, these previous studies (O’Connor et al., 2004) were the first to demonstrate similar effects in response to chronic transporter blockade. The current study shows that transporter blockade-mediated desensitization of dopamine receptors occurs with specific regional and temporal patterns that closely parallel the time course of changes in locomotor activity.
These effects on receptor/G-protein coupling by chronic WF-23 treatment appeared to be specific reductions to monoamine receptor-stimulated [35S]GTPγS binding. Chronic treatment with WF-23 did not alter µ opioid-stimulated [35S]GTPγS binding in the caudate/putamen at any time point examined in the same animals where significant decreases were observed in D2 receptor-stimulated [35S]GTPγS binding in the same region. These results are similar to homologous desensitization observed after chronic opioid and cannabinoid treatment (Sim et al., 1996; Breivogel et al., 1999) but in contrast to reports of chronic cocaine treatment, where increases have been shown in both monoamine and nonmonoamine receptor signal transduction (Unterwald et al., 1996; Kushner and Unterwald, 2001; Schroeder et al., 2003). It is unlikely that these differences are due to pharmacological differences in the specificity of cocaine and WF-23, since both drugs block all three monoamine transporters, but may involve pharmacokinetic differences between the two transport inhibitors. The cocaine binge treatment used (Unterwald et al., 1996; Kushner and Unterwald, 2001; Schroeder et al., 2003) by many investigators produces temporary high increases in monoamine levels, whereas the long-acting WF-23 tropane analog may produce sustained increases in DA, 5-HT, and NE. Sustained elevations of these monoamines, in contrast to periodic high concentrations, may result in different responses by the receptors.
Desensitization in the absence of down-regulation of dopamine D2 receptors was previously demonstrated following 15 days of WF-23 treatment (O’Connor et al., 2004). In the present study, no decreases in D2 receptor binding, assessed by [3H]spiperone binding in caudate/putamen, were observed following 21 days of treatment. These data demonstrate that uncoupling of the receptor/G-protein complex, or desensitization, occur in the absence of receptor down-regulation following long-term transporter blockade by WF-23. This same result was observed after chronic heroin treatment, where µ opioid-stimulated [35S]GTPγS binding was reduced with no change in µ receptor binding (Sim-Selley et al., 2000a) and in contrast to chronic Δ9-tetrahydrocannabinol treatment, which reduced CB1 receptor number as well as CB1-activated G-proteins (Breivogel et al., 1999).
Chronic administration of WF-23 produced specific time-dependent effects which varied across the three monoamine receptors. For example, although significant reductions in D2 receptor-stimulated [35S]GTPγS binding in striatum were not observed until 15 days of treatment, significant reductions in 5-HT1A activity (in hippocampus) and α2-adrenergic activity (in amygdala) were observed following a single injection of WF-23. Previous studies have demonstrated similar regional differences in time-dependent desensitization of cannabinoid CB1-stimulated [35S]GTPγS binding following chronic treatment with Δ9-tetrahydrocannabinol. It has been suggested that regional differences in intracellular regulatory mechanisms of signal transduction may contribute to these effects (Breivogel et al., 1997b).
However, in addition to these potential receptor G-protein regulatory mechanisms, there is an extrasynaptic component mediating desensitization of monoamine receptors following chronic WF-23 treatment. Synaptic levels of monoamines are dependent upon both release and uptake processes. Thus, effects of WF-23 on extrasynaptic levels of monoamines will depend on the level of presynaptic activity and may therefore produce larger reductions in receptor-stimulated [35S]GTPγS binding in areas of higher monoamine release. Furthermore, monoamine receptors, specifically D2 and 5-HT1A, function both post- and presynaptically. For example, D2 autoreceptors may be present in a higher proportion in the caudate/ putamen than D2 postsynaptic receptors (Neve, 1997). Although NPA-stimulated [35S]GTPγS binding cannot differentiate D2 autoreceptors from postsynaptic receptors, it is possible that the relative contribution of autoreceptors to postsynaptic receptors may contribute to the time-dependent differences observed in D2 desensitization compared with 5-HT1A and α2-adrenergic receptors.
One of the goals of the present study was to examine the effects of chronic WF-23 treatment on locomotor activity. The cocaine-like behavioral effects of WF-23 have been shown in previous studies, which demonstrated that WF-23 substituted for cocaine in self-administration paradigms (Lile et al., 2003; Roberts et al., 2003) and increased locomotor activity for up to 24 h following a single i.p. injection (Daunais et al., 1998). This long duration of action was in contrast to cocaine, whose effects on locomotor activity lasted only 1 h after a single injection (Daunais et al., 1998). Qualitatively, the stereotypic behaviors elicited by WF-23 were indistinguishable from those elicited by cocaine and included rearing, head-bobbing, increased sniffing, and gnawing.
The locomotor assays in the present study revealed an overall loss of WF-23 effects after chronic administration of this drug compared with its acute effects. These data demonstrate that chronic treatment with long-acting psychostimulants produces significant behavioral tolerance, at least in terms of locomotor activity. For example, the preinjection data revealed elevations in horizontal activity 48 h following the previous injection of WF-23 for the first 7 days of treatment, consistent with prolonged blockade of DAT for at least 48 h. However, the preinjection increases in horizontal activity diminished during chronic WF-23 treatment, with increases of 215 ± 16% (versus controls) on day 3, 182 ± 18% on day 5, and 119 ± 9% on day 7 (the last value significantly lower than the increase on day 3, p ≤ 0.05). Postinjection locomotor data revealed a similar tolerance after chronic WF-23 treatment; horizontal activity measured during the 150-min session after WF-23 injection revealed elevations after each injection until day 15 where the WF-23-induced elevations in activity were severely reduced. These effects were not simply limited to horizontal activity, but were also observed when stereotypic activity was evaluated (not shown).
Furthermore, the time course of behavioral tolerance produced by chronic treatment with WF-23 paralleled the observed desensitization of D2 receptor-coupled G-proteins, suggesting that this tolerance may be mediated, in part, by desensitization of monoamine receptor-coupled G-proteins. As shown in Table 1, D2-stimulated [35S]GTPγS binding in caudate/putamen remained relatively constant throughout the chronic WF-23 treatment until day 7, where D2 activity was reduced to 77% (versus control) and further decreased on day 15 and day 21 to 66 and 28%, respectively. As discussed above, significant loss in preinjection effects of WF-23 was also observed on day 7, whereas loss in postinjection effects was observed on day 15. The difference in these two time courses may simply reflect the large difference in WF-23 present during the preinjection and postinjection phases, i.e., loss in postinjection effects of WF-23 may be occurring at day 7, but masked by the large effects of the drug itself on locomotor activity.
Therefore, these data demonstrate that chronic treatment with a long-acting psychostimulant produces significant behavioral tolerance and suggest that this tolerance may be mediated, in part, by desensitization of monoamine receptor-coupled G-proteins. This is in contrast to the effects of cocaine, a short-acting psychostimulant, which has been shown to produce sensitization, rather than tolerance, following chronic treatment (Post et al., 1979). Locomotor sensitization has been shown to be produced following specific treatment paradigms (Post et al., 1992). In general, intermittent, rather than continuous, exposure of animals to cocaine has been shown to produce behavioral sensitization (Reith et al., 1987). Therefore, it is not surprising that WF-23, which produces a sustained blockade of DAT (Fig. 2), results in tolerance and not sensitization to the locomotor effects of this psychostimulant in contrast to cocaine.
Acknowledgments
This work was supported in part by DA-06634 (S.R.C., H.M.L.D.), DA-07246 (K.A.O.), and DA-16840 (K.A.O.) from the National Institute on Drug Abuse.
ABBREVIATIONS
- DAT
dopamine transporter
- SERT
serotonin transporter
- NET
norepinephrine transporter
- WF-23
2β-propanoyl-3β-(2-naphthyl) tropane
- [35S]GTPγS
guanosine 5′-O-(3-[35S]thiotriphosphate)
- 5-HT1A
5-hydroxytryptamine 1A
- DAMGO
[d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin
- NPA
R(−)-propylnorapomorphine
- 8-OH-DPAT
8-hydroxy-2-dipropylaminotetralin
- [125I]RTI-55
3β-(4-[125I]iodophenyl)-tropane-2-carboxylic acid methyl ester.
Contributor Information
Kerry A. O’Connor, Department of Physiology/Pharmacology, Center for the Neurobiological Investigation of Drug Abuse, Wake Forest University Health Sciences, Winston-Salem, North Carolina
Linda J. Porrino, Department of Physiology/Pharmacology, Center for the Neurobiological Investigation of Drug Abuse, Wake Forest University Health Sciences, Winston-Salem, North Carolina
Huw M. L. Davies, Department of Chemistry, State University of New York at Buffalo, Buffalo, New York
Steven R. Childers, Department of Physiology/Pharmacology, Center for the Neurobiological Investigation of Drug Abuse, Wake Forest University Health Sciences, Winston-Salem, North Carolina
References
- Appell M, Berfield JL, Wang LC, Dunn WJ, Chen N, Reith ME. Structure-activity relationships for substrate recognition by the human dopamine transporter. Biochem Pharmacol. 2004;67:293–302. doi: 10.1016/j.bcp.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Araki T, Kato H, Shuto K, Fujimwara T, Itoyama Y. Effect of aging on dopaminergic receptors and uptake sites in the rat brain studied by receptor autoradiography. J Neurol Sci. 1997;148:131–137. doi: 10.1016/s0022-510x(96)05343-9. [DOI] [PubMed] [Google Scholar]
- Beitner-Johnson D, Guitart X, Nestler EJ. Common intracellular actions of chronic morphine and cocaine in dopaminergic brain reward regions. In: Kalivas PW, Samson HH, editors. The Neurobiology of Drug and Alcohol Addiction. New York, NY: New York Academy of Sciences; 1992. pp. 70–87. [DOI] [PubMed] [Google Scholar]
- Bennett BA, Wichems CH, Hollingsworth CK, Davies HML, Thornley C, Sexton T, Childers SR. Novel 2-substituted cocaine analogs: uptake and ligand binding studies at dopamine, serotonin and norepinephrine transport sites in the rat brain. J Pharmacol Exp Ther. 1995;272:1176–1186. [PubMed] [Google Scholar]
- Birnbaumer L, Abramowitz J, Brown AM. Receptor-effector coupling by G proteins. Biochim Biophys Acta. 1990;1031:163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Boja JW, Mitchell WM, Patel A, Kopajtic TA, Carroll FI, Lewin AH, Abraham P, Kuhar MJ. High-affinity binding of [125I]RTI-55 to dopamine and serotonin transporters in rat brain. Synapse. 1992;12:27–36. doi: 10.1002/syn.890120104. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ. Chronic Δ9-tetrahydrocannabinol produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G-proteins in rat brain. J Neurochem. 1999;73:2447–2459. doi: 10.1046/j.1471-4159.1999.0732447.x. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Selley DE, Childers SR. Acute and chronic effects of opioids on delta and mu receptor activation of G-proteins in NG108-15 and SK-N-SH cell membranes. J Neurochem. 1997a;68:1462–1472. doi: 10.1046/j.1471-4159.1997.68041462.x. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Sim LJ, Childers SR. Regional differences in cannabinoid receptor/G-protein coupling in rat brain. J Pharmacol Exp Ther. 1997b;282:1632–1642. [PubMed] [Google Scholar]
- Carroll FI, Abraham P, Lewin AH, Parham K, Boja JW, Kuhar MJ. Isopropyl and phenyl esters of 3β-(p-substituted phenyl)-tropane-2 β-carboxylic acids. Potent and selective compounds for the dopamine transporter. J Med Chem. 1992;35:2497–2500. doi: 10.1021/jm00091a019. [DOI] [PubMed] [Google Scholar]
- Daunais JB, Hart SL, Smith HR, Letchworth SR, Davies HML, Sexton T, Bennett B, Childers SR, Porrino LJ. Long-acting blockade of biogenic amine transporters in rat brain by administration of the potent novel tropane 2β-propranoyl-3β-(2-naphthyl)-tropane. J Pharmacol Exp Ther. 1998;285:1246–1254. [PubMed] [Google Scholar]
- Davies HML, Saikali E, Huby NSJ, Gilliat VJ, Matasi J, Sexton T, Childers SR. Synthesis of 2-beta-acyl-aryl-8-azabicyclo[3.2.1]octanes and their binding affinities at dopamine and serotonin transport sites in rat striatum and frontal cortex. J Med Chem. 1994;37:1262–1268. doi: 10.1021/jm00035a005. [DOI] [PubMed] [Google Scholar]
- Davies HML, Saikali E, Sexton T, Childers SR. Novel 2-substituted cocaine analogs: binding properties at dopamine transport sites in rat striatum. Eur J Pharmacol. 1993;244:93–97. doi: 10.1016/0922-4106(93)90063-f. [DOI] [PubMed] [Google Scholar]
- Happe HK, Bylund DB, Murrin LC. Alpha(2)-adrenoceptor-stimulated GTPγS binding in rat brain: an autoradiographic study. Eur J Pharmacol. 2000;399:17–27. doi: 10.1016/s0014-2999(00)00380-0. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Co C, Reboussin D, Davies HM, Dworkin SI, Smith JE. Comparison of a novel tropane analog of cocaine: 2β-propanoyl-3β-(4-tolyl)tropane (PTT) with cocaine HCl in rats: nucleus accumbens extracellular dopamine concentration and motor activity. J Pharmacol Exp Ther. 1995;272:656–666. [PubMed] [Google Scholar]
- Kushner SA, Unterwald EM. Chronic cocaine administration decreases the functional coupling of GABABreceptors in the rat ventral tegmental area as measured by baclofen-stimulated35S-GTPγS binding. Life Sci. 2001;69:1093–1102. doi: 10.1016/s0024-3205(01)01203-6. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Hausdorff WP, Caron MG. Role of phosphorylation in desensitization of the β-adrenoceptor. Trends Pharmacol. 1990;11:190–194. doi: 10.1016/0165-6147(90)90113-m. [DOI] [PubMed] [Google Scholar]
- Lile JA, Wang Z, Woolverton WL, France JE, Gregg TC, Davies HML, Nader MA. The reinforcing efficacy of psychostimulants in rhesus monkeys: the role of pharmacokinetics and pharmacodynamics. J Pharmacol Exp Ther. 2003;307:356–366. doi: 10.1124/jpet.103.049825. [DOI] [PubMed] [Google Scholar]
- Lorenzen A, Fuss M, Vogt H, Schwabe U. Measurement of guanine nucleotide-binding protein activation by A1adenosine receptor agonists in bovine brain membranes: stimulation of guanosine-5′-O-(3-[35S]thio)triphosphate binding. Mol Pharmacol. 1993;44:115–123. [PubMed] [Google Scholar]
- Madras BK, Fahey MA, Miller GM, De La Garza R, Goulet M, Spealman RD, Meltzer PC, George SR, O’Dowd BF, Bonab AA, et al. Non-amine-based dopamine transporter (reuptake) inhibitors retain properties of amine-based progenitors. Eur J Pharmacol. 2003;479:41–51. doi: 10.1016/j.ejphar.2003.08.055. [DOI] [PubMed] [Google Scholar]
- Mead AN, Rocha BA, Donovan DM, Katz JL. Intravenous cocaine inducedactivity and behavioural sensitization in norepinephrine-, but not dopamine-transporter knockout mice. Eur J Neurosci. 2002;16:514–520. doi: 10.1046/j.1460-9568.2002.02104.x. [DOI] [PubMed] [Google Scholar]
- Neve KA, Neve RL. The Dopamine Receptors. Totowa, NJ: Humana Press, Inc; 1997. [Google Scholar]
- Newman-Tancredi A, Chaput C, Verriele L, Millan MJ. S 15535 and WAY 100,635 antagonise 5-HT-stimulated [35S]GTPγS binding at cloned human 5-HT1Areceptors. Eur J Pharmacol. 1996;307:107–111. doi: 10.1016/0014-2999(96)00303-2. [DOI] [PubMed] [Google Scholar]
- O’Connor KA, Gregg TC, Davies HML, Childers SR. Effects of long-term biogenic amine transporter blockade on receptor/G-protein coupling in rat brain. Neuropharmacology. 2004;48:62–71. doi: 10.1016/j.neuropharm.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Palacios JM, Niehoff DL, Kuhar MJ. [3H]Spiperone binding sites in brain: autoradiographic localization of multiple receptors. Brain Res. 1981;213:277–289. doi: 10.1016/0006-8993(81)90234-1. [DOI] [PubMed] [Google Scholar]
- Post RM, Smith CC, Squillace KM, Tallman JF. Effect of chronic cocaine on behavior and cyclic AMP in cerebrospinal fluid of rhesus monkeys. Commun Psychopharmacol. 1979;3:143–152. [PubMed] [Google Scholar]
- Post RM, Weiss SRB, Pert A. Sensitization and kindling effects of chronic cocaine administration. In: Lakoski JM, Galloway MP, White FJ, editors. Cocaine: Pharmacology, Physiology and Clinical Strategies. Boca Raton, FL: CRC Press, LLC; 1992. pp. 115–162. [Google Scholar]
- Reith ME, Benuck M, Lajtha A. Cocaine disposition in the brain after continuous or intermittent treatment and locomotor stimulation in mice. J Pharmacol Exp Ther. 1987;243:281–287. [PubMed] [Google Scholar]
- Rinken A, Finnman U, Fuxe K. Pharmacological characterization of dopamine-stimulated [35S]GTPγS binding in rat striatal membranes. Biochem Pharmacol. 1999;57:155. doi: 10.1016/s0006-2952(98)00287-1. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science (Wash DC) 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Jungersmith KR, Phelan R, Gregg TM, Davies HML. Effect of HD-23, a potent long acting cocaine-analog, on cocaine self-administration in rats. Psychopharmacology. 2003;167:386–392. doi: 10.1007/s00213-003-1424-z. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Niculescu M, Unterwald EM. Cocaine alters mu but not delta or kappa opioid receptor-stimulate in situ [35S]GTPγS binding in rat brain. Synapse. 2003;47:26–32. doi: 10.1002/syn.10148. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Childers SR. In vitro autoradiography of receptor-activated G-proteins in rat brain by agonist-stimulated guanylyl 5′-[γ-[35S]thio]-triphosphate binding. Proc Natl Acad Sci USA. 1995;92:7242–7246. doi: 10.1073/pnas.92.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Dworkin SI, Childers SR. Effects of chronic morphine administration on mu opioid receptor-stimulated [35S]GTPγS autoradiography in rat brain. J Neurosci. 1996;16:2684–2692. doi: 10.1523/JNEUROSCI.16-08-02684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Selley DE, Childers SR, Martin TJ. Chronic heroin self-administration desensitizes mu opioid receptor-activated G-proteins in specific regions of rat brain. J Neurosci. 2000a;20:4555–4562. doi: 10.1523/JNEUROSCI.20-12-04555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Vogt LJ, Xiao R, Childers SR, Selley DE. Region-specific changes in 5-HT1Areceptor-activated G-proteins in rat brain following chronic buspirone. Eur J Pharmacol. 2000b;389:147–153. doi: 10.1016/s0014-2999(99)00875-4. [DOI] [PubMed] [Google Scholar]
- Sora I, Wichems C, Takahashi H, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR. Cocaine reward models: conditioned place preference can be established in dopamine-and in serotonin-transporter knockout mice. Proc Natl Acad Sci USA. 2001;95:7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor JR, Nahorski SR. Modulation by mu-opioid agonists of guanosine-5′-O-(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol Pharmacol. 1995;47:848–854. doi: 10.1016/S0026-895X(25)08634-1. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Fillmore J, Kreek MJ. Chronic repeated cocaine administration increases dopamine D1receptor-mediated signal transduction. Eur J Pharmacol. 1996;318:31–35. doi: 10.1016/s0014-2999(96)00841-2. [DOI] [PubMed] [Google Scholar]
- Yoshiyuki W, Tsunehiko N. Brain dopamine transporter in spontaneously hypertensive rats. J Nucl Med. 1997;38:470–474. [PubMed] [Google Scholar]