Abstract
Vascular-targeting antiangiogenic therapy (VTAT) of cancer can be advantageous over conventional tumor cell targeted cancer therapy if an appropriate target is found. Our hypothesis is that endoglin (ENG; CD105) is an excellent target in VTAT. ENG is selectively expressed on vascular and lymphatic endothelium in tumors. This allows us to target both tumor-associated vasculature and lymphatic vessels to suppress tumor growth and metastasis. ENG is essential for angiogenesis/vascular development and a co-receptor of TGF-β. Our studies of selected anti-ENG monoclonal antibodies (mAbs) in several animal models and in vitro studies support our hypothesis. These mAbs and/or their immunoconjugates (immunotoxins and radioimmunoconjugates) induced regression of preformed tumors as well as inhibited formation of new tumors. In addition, they suppressed metastasis. Several mechanisms were involved in the suppressive activity of the naked (unconjugated) anti-ENG mAbs. These include direct growth suppression of proliferating endothelial cells, induction of apoptosis, ADCC (antibody-dependent cell-mediated cytotoxicity) and induction of T cell immunity. To facilitate clinical application, we generated a human/mouse chimeric anti-ENG mAb termed c-SN6j and performed studies of pharmacokinetics, toxicology and immunogenicity of c-SN6j in nonhuman primates. No significant toxicity was detected by several criteria and minimal immune response to the murine part of c-SN6j was detected after multiple i.v. injections. The results support our hypothesis that c-SN6j can be safely administered in cancer patients. This hypothesis is supported by the ongoing phase 1 clinical trial of c-SN6j (also known as TRC105) in patients with advanced or metastatic solid cancer in collaboration with Tracon Pharma and several oncologists (NCT00582985).
Keywords: Endoglin, CD105, Anti-endoglin antibody, vascular-targeting therapy, antiangiogenic therapy, chimeric antibody
1. Introduction
Vascular-targeting antiangiogenic therapy (VTAT) is highly attractive in treating solid tumors compared with tumor cell-targeted therapy and conventional antiangiogenic therapy [1-6]. For instance, VTAT can potentially minimize the problems of poor delivery, drug resistance and tumor heterogeneity. In addition, a single agent developed for VTAT could be applied to most or all types of solid tumors unlike the case of tumor-cell targeted therapy. VTAT can be more effective for destroying established tumors than conventional antiangiogenic therapy [1]. Nevertheless, a critical issue in VTAT is availability of an appropriate target. We believe that endoglin (ENG; CD105) is a promising target for VTAT.
A homodimeric cell surface glycoprotein, later termed ENG, was initially identified as a human leukemia-associated cell membrane antigen [7-9]. ENG is mainly expressed on immature B-lineage/myeloid leukemia cells and endothelial cells [7]. Although several cell lines and cultured cells were reported to express ENG, it was not detected on these cells in fresh tissues in most cases. This issue is further addressed in the text below. Two isoforms of ENG, L-ENG and S-ENG, differing in the size of their cytoplasmic tails, have been characterized [10]. ENG is a proliferation-associated cell membrane antigen [9, 11-13] and strongly expressed on the tumor-associated vascular and lymphatic endothelium [9, 13-16]. In addition, ENG is essential for angiogenesis/vascular development [17, 18] and a co-receptor of the transforming growth factor (TGF)-β [19]. ENG gene expression was substantially increased in circulating endothelial cells-enriched samples from metastatic carcinoma patients compared with the corresponding samples from healthy donors [20]. Irradiation may upgrade ENG expression [21] and ENG expression was stimulated in the presence of hypoxia and TGF-β [22]. Anti-VEGF therapy may increase ENG expression [23]. Yamashita et al. [24] and others [25] reported that ENG forms a heterodimeric complex with TGF-β receptors I and II. L-ENG and S-ENG may differentially modulate TGF-β signaling [26]. ENG promotes endothelial proliferation and TGF-β/ALK1 signal transduction [27]; ALK1, activin receptor-like kinase 1, is an endothelial specific TGF-β type 1 receptor. Endothelial cells lacking ENG do not grow because TGF-β/ALK1 signaling is reduced and TGF-β/ALK5 signaling is increased [27]; ALK5 is the conventional type 1 TGF-β receptor that is ubiquitously expressed [28]. Conley et al. [29] reported that ENG controls cell migration and composition of focal adhesions. In addition, Lee and Blobe [30] reported that ENG inhibits endothelial cell migration and antagonizes TGF-β-mediated ERK activation by interaction with β-arrestin 2.
Recently, several studies indicated that ENG represents a more specific and sensitive marker for tumor angiogenesis and/or tumor progression than the commonly used pan-endothelial markers such as CD34 and CD31 in various types of human malignancies [31-34].
Previously, we showed that immunoconjugates (immunotoxins and radioimmunoconjugates) and the naked form of selected anti-ENG mAbs were effective for suppression of tumor growth [9, 35-39] and metastasis [40] by targeting angiogenic vasculature in mice. In these studies, we targeted tumors in SCID mice [9, 35, 36], immunocompetent mice [38, 39] and human skin/SCID mouse chimeras in which human tumors were implanted intradermally in human skins grafted into SCID mice [37]. Recently we demonstrated the immune status of the hosts play an important regulatory role in the ENG-targeted vascular targeting therapy [39]. CpG oligodeoxynucleotides synergistically enhanced antitumor efficacy of anti-ENG mAb SN6j, and antitumor efficacy of SN6j in immunocompetent mice was abrogated when CD4+ T cells and/or CD8+ T cells were depleted [39]. More recently we showed that selected anti-ENG mAbs (i.e., SN6j, SN6k and SN6a) were capable of suppressing metastasis in five different metastasis models [40]. These mAbs and SN6f [9] were selected from our 12 anti-ENG mAbs for therapeutic studies in mice partly based on the cross-reactivity with SVEC4-10 murine endothelial cell line [9, 35-37, 39, 40]; the cross-reactivity was measured by flow cytometry [9, 35], a cellular radioimmunoassay [9] and a fluorescence-labeled antibody binding/internalization assay [36, 39]. SVEC4-10 [41] was kindly provided to us by Dr. Kathryn O'Connell of Johns Hopkin's University, and it showed substantial cross-reactivity with the selected anti-human ENG mAbs. However, the properties of SVEC4-10 gradually changed with increased passage number during cell culture as advised by Dr. O'Connell; this type of cell property change with increased cell culture passages is common for endothelial cells. SVEC4-10 from ATCC was not useful and did not show significant cross-reactivity with our selected anti-ENG mAbs. The weak cross-reactivity of the four anti-ENG mAbs with murine endothelial cells was supported by Matrigel plug assay [39, 40] and/or immunohistochemical staining of tissues [9, 35].
To facilitate clinical application, we generated a human/mouse chimeric anti-ENG mAb, termed c-SN6j, and performed studies of pharmacokinetics, toxicology and immunogenicity of c-SN6j in nonhuman primates [42]. No significant toxicity was detected by several criteria and minimal immune response to the murine part of c-SN6j was detected after multiple i.v. injections. The results support our hypothesis that c-SN6j can be safely administered in cancer patients. Indeed, this hypothesis was further supported by the ongoing phase 1 clinical trial of c-SN6j (also known as TRC105) in patients with advanced and/or metastatic solid cancers; this trial is performed in collaboration with Tracon Pharma and several oncologists (see below VIII. Conclusions and Future Directions)
Recently several review articles that address issues relevant to the ENG targeting were published [43-46]. These articles will be useful for understanding ENG further.
2. Restricted Expression of Eng on Different Cells and Tissues
2.1. Isolated Cells
Our initial test of GP160 [7], that was later proven to be ENG, showed that expression of ENG is highly restricted; the test was performed using a prototype anti-ENG mAb termed SN6. SN6 reacted with immature human B-lineage leukemia cells (mainly acute lymphoblastic leukemia cells) and myelomonocytic leukemia cells, but did not react with other types of leukemia/lymphoma cells [7]. Expression of ENG is highly consistent between fresh leukemia cells from patients and cultured leukemia cell lines of the same phenotypes [7]. Such consistent expression of ENG was not observed for carcinoma cells (see below). SN6 did not show significant reaction with normal peripheral blood cells tested, which included B cells, T cells, granulocytes, monocytes and erythrocytes. However, it reacted with a small population (approximately 1%) of normal bone marrow cells [7]. It is worthy to note that ENG became detectable on the monocyte-derived cells during in vitro culture although ENG was not detectable on freshly isolated monocytes [47]. This finding is consistent with the report of Lastres et al. [48] that ENG is absent from peripheral blood moncytes but it is expressed on in vitro differentiated monocytes.
We did not detect ENG in carcinoma cells in tissues by extensive studies (see below). Nevertheless, we detected ENG on several cultured carcinoma cell lines [49]. We consider therefore that ENG on these cultured cell lines is probably an artifact that was induced by culturing the cells. This type of induced expression of ENG will not be important in therapeutic targeting of ENG in patients. Several cultured cells and cell lines were reported to express ENG [45]. However, it will be important to confirm the ENG expression on fresh cells in tissues before initiation of cell culture.
2.2. Tissues
We tested approximately one-hundred malignant tissues of many different cancers by Immunohistochemical (IHC) staining using anti-ENG mAbs from Seon laboratory (e.g., SN6, SN6h, SN6f, SN6j, SN6k etc.) [47, 49]. An example of such IHC staining is presented in Fig. (1). Two different anti-ENG mAbs SN6 and SN6h that define distinctively different epitopes of ENG [50] reacted strongly with vascular endothelium of tumor tissues. Reactivity of SN6h with blood vessels in tumor tissue was particularly strong (Fig. (1C)) while SN6h showed only marginal reactivity with a few blood vessels in normal breast tissue (Fig. (1D)). SN6h possesses an extremely high binding avidity to ENG on the cell surface (equilibrium association constant K = 1.38 × 1011 liters/mol; [51]. It strongly binds both native and denatured forms of ENG. Therefore, SN6h is suitable for IHC staining of formalin-fixed paraffin-embedded tissues as well as frozen tissues. Our test results of many malignant tissues of a variety of cancers (breast, colorectal, lung, prostate, brain, bladder, ovary, thyroid, lymphoma etc.) revealed that reactivity of our anti-ENG mAbs is restricted to vascular endothelium and these mAbs do not react with tumor cells per se in the tissues [9, 35, 47, 49].
Fig. 1.
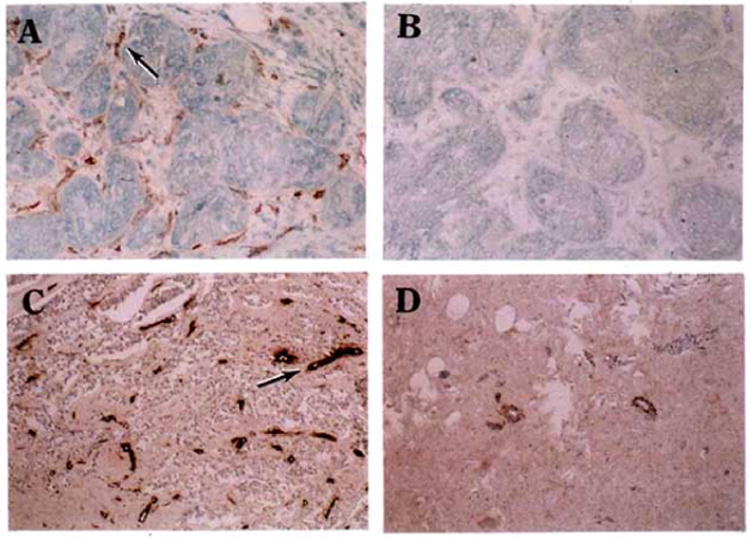
Reactivity of anti-ENG mAbs with malignant and normal breast tissues. Frozen breast carcinoma tissues were allowed to react with anti-ENG mAb SN6 (A) or an isotype-matched control IgG (IgG1-κ) (B) and stained with DAKO staining kits. In an additional test, formalin-fixed paraffin-embedded tissues of breast carcinoma (C) or normal breast (D) were allowed to react with anti-ENG mAb SN6h and stained with DAKO staining kits. An example of the stained blood vessels is indicated by an arrow in panels A and C. SN6 and SN6h show strong staining with multiple blood vessels in malignant breast tissue (A and C). Control IgG did not show any significant staining in each of tested tissues and an example is presented in panel B. SN6h shows marginal staining with a few blood vessels in normal breast tissues (D). It is important to note that reactivity of SN6 and SN6h with malignant breast tissues is restricted to vascular endothelium and no significant reactivity of these mAbs with tumor cells per se is detected.
3. In Vitro Activity of Anti-Eng mAbs
3.1. Antigen-Binding Avidity and Number of ENG Molecules on the Cell
We generated twelve anti-ENG mAbs which are SN6, SN6a, SN6b, SN6c, SN6d, SN6e, SN6f, SN6g, SN6h, SN6i, SN6j and SN6k. Epitopes defined by these mAbs were mapped [50]. Scatchard plot analysis was performed to determine antigen-binding avidity and number of antigen expressed on endothelial/leukemia cells using seven of these mAbs (i.e., SN6a, SN6b, SN6e, SN6f, SN6h, SN6j and SN6k). Antigen-binding avidity varied in the range of 1.2 × 109 – 1.4 × 1011 liters/mol for the equilibrium association constant (K) [37, 51]. There was no significant difference in the antigen-binding avidity of anti-ENG mAbs between ENG on endothelial cells and ENG on leukemia cells [37]. However, endothelial cells (human umbilical vein endothelial cells, HUVECs) express approximately 100-fold as many ENG protein as leukemia cells (KM-3) per cell, i.e., 106 vs 104 ENG/cell [37].
3.2. Suppressive and Cytotoxic Activity
Anti-ENG mAbs SN6, SN6a, SN6h and SN6j showed suppressive activity against proliferation of HUVECs in the absence of any accessory cells [52]. This suppressive activity was synergized with TGF-β. SN6j induced apoptosis of HUVECs in a dose-dependent manner [39]. Test results suggest that SN6j induces T cell immunity in immunocompetent hosts [39]. A human/mouse chimeric antibody c-SN6j (also known as TRC105) showed strong ADCC against HUVECs.
Burrows et al. [12] reported that an anti-ENG immunotoxin TEC-11-dgRA showed selective toxicity against proliferating endothelial cells in vitro. Volkel et al. [53] generated immunoliposomes (ILA5) targeting proliferating endothelial cells by chemically coupling a single-chain Fv fragment directed against human endoglin to the liposomal surface. In vitro, doxorubicin-loaded ILA5 showed an increased cytotoxicity towards endothelial cells compared to untargeted liposomes and free doxorubicin. Munoz et al. [54] generated an immunotoxin by conjugating nigrin b to an anti-ENG mAb which killed specifically ENG-expressing target cells in vitro.
4. Correlation between Endoglin-Stained Intratumoral Microvessel Density and Prognosis
Several groups including us reported that high microvessel density in tumor tissues determined using an anti-ENG mAb correlated with poor prognosis in patients with various solid cancers; these cancers included breast cancer [31, 34], lung cancer [32], colorectal cancer [33, 55], prostate cancer [56] gastric cancer [57], endometrial cancer [58], hepatocellular carcinoma [59], ovarian cancer [60], cervical cancer [61], head and neck cancer [62, 63] and glioblastoma [64]. In these tests, SN6h has been most commonly used because it effectively immunostains formalin-fixed paraffin-embedded tissues as well as frozen tissues.
5. Endoglin as a Target for Tumor Imaging
Fonsatti et al. [65] reported that ENG was effectively targeted for radioimaging in a canine mammary carcinoma model. Others also targeted ENG for rarioimaging in mice bearing B16 melanoma xenografts [66], in excised kidneys from renal carcinoma patients [67], and in mice bearing Calu6 lung cancer xenografts [68]. Korpanty et al. [69] targeted ENG for imaging with microbubbles conjugated to mAbs in a mouse model of pancreatic carcinoma.
6. Therapeutic Targeting of Endoglin in Animal Models
6.1. SCID Mice Bearing Human Tumors
In our study, MCF-7 human breast cancer cell line was used to generate xenograft tumors in SCID mice [9, 35-38]. Many human breast cancer cell lines (e.g., MDA-MB-231 and MCF-7 AR) express varying degrees of ENG on the cell surface. However, ENG was not detected on wild type MCF-7 cells. Systemic (i.v.) administration of deglycosylated ricin A-chain (dgRA) conjugates of SN6f (SN6f-dgRA) inhibited the growth of tumors from s.c. inoculated MCF-7 [9]. Systemic administration of SN6j-dgRA or SN6k-dgRA induced regression of established MCF-7 tumors [35]. In addition, 125I-labeled SN6f and SN6j inhibited growth of MCF-7 tumors [36].
6.2. Human Skin/SCID Mouse Chimera
To facilitate antitumor therapy with naked anti-ENG mAbs in animal models, we used human skin/SCID mouse chimeras bearing MCF-7 tumors [37]. Blood vessels in the chimeras were analyzed by immunostaining with species (human or mouse)-specific anti-CD31 and anti-ENG mAbs including an anti-human ENG mAb SN6h. Blood vessels in the completely healed grafted human skins consisted of a mixture of human (43.5%) and murine (56.5%) vessels while only murine vessels were detected in the adjacent murine skins and subcutaneous tissues. Therefore, murine vessels infiltrate into the human skin grafts from the adjacent murine tissues whereas the growth of human vessels is limited within the boundary of human skins. Growth of human MCF-7 tumors in the human skin grafts increased the ratio of human vessels to murine vessels. Analyses of the grafted skins before and after tumor transplantation showed that SN6h reacted with tumor-induced angiogenic blood vessels but not with non-angiogenic vessels, whereas anti-human CD31 mAb reacted with both angiogenic and non-angiogenic vessels. The results show that SN6h is capable of distinguishing the tumor-induced angiogenic vasculature from the non-angiogenic vasculature in the present model. Vascular-targeting antiangiogenic therapy of the chimeras bearing established MCF-7 tumors was carried out by i.v. administration of a mAb via the tail vein of mice. SN6j and SN6k were effective for suppressing the established tumors while tumor suppression was weaker with SN6f. The results indicate the absence of a direct correlation between antigen-binding avidity and in vivo antitumor efficacy of anti-ENG mAbs, and suggest importance of other factors (e.g., epitopes) in antitumor efficacy. No significant toxicity of the mAbs was detected. Combination of SN6j and cyclophosphamide using an antiangiogenic schedule (continuous low-dose; [70, 71] of drug dosing showed synergistic antitumor efficacy. The combination therapy induced lasting complete regression of the established tumors in two of the eight treated chimeras. The results show that systemic administration of naked anti-human ENG mAbs can suppress established tumors and the efficacy was markedly enhanced by combining a chemotherapeutic drug using an antiangiogenic schedule of drug dosing. Multiple mechanisms may contribute to the enhanced antitumor efficacy by combining an anti-ENG mAb with cyclophosphamide or other chemotherapeutic drugs. One possible mechanism is that anti-ENG mAb normalizes tumor vasculature in vivo which will lead to enhancement of the antitumor efficacy of cyclophosphamide and other drugs [72, 73].
We examined human and murine blood vessels in large human tumors from the chimeras at the end of the therapeutic experiment. The test showed that SN6j therapy resulted in complete or nearly complete suppression of human vessels in the tumors but resulted in only weak suppression of murine vessels. The results indicate that these mAbs should show stronger antitumor efficacy in patients whose tumors depend entirely on human blood vessels.
6.3. Immunocompetent Mice Bearing Murine Tumors
6.3.1. Mechanisms by Which Naked Anti-ENG mAbs Suppress Tumor Growth
In this study, we investigated the mechanisms by which anti-ENG mAbs suppress angiogenesis and tumor growth. Anti-human ENG mAbs effectively suppressed angiogenesis in mice in the Matrigel plug assay [39, 40]. The test results of SN6j are illustrated in Fig. (2). Microvessel density of Matrigel plugs of the SN6j-treated mice was significantly lower than that of the isotype-matched control IgG-treated mice (p<0.05; Fig. 2A and 2B). Moreover, vessels in the plugs of the SN6j-treated mice showed shrinkage and attenuation compared with those of the control IgG-treated mice.
Fig. 2.
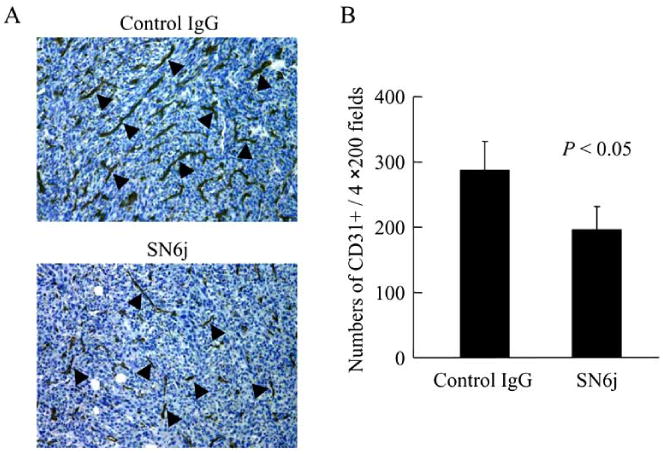
Effect of SN6j on the Matrigel plug angiogenesis in mice. Matrigel Matrix mixed with 1.25×105 colon 26 cells was injected s.c. in the left frank of BALB/c mice on day 0. The mice were treated with 1.8 μg/g body weight of SN6j or isotype-matched control IgG via tail vein on day 1, 4 and 7. The Matrigel plugs were fixed on day 10 for immunohistochemical staining. A, examples of rat anti-mouse CD31 (PECAM-1) mAb staining of Matrigel plug sections from SN6j-treated and control IgG-treated mice. The sections were counterstained with hematoxylin. Arrowheads in the figure indicate representative CD31 positive vessels. B, the CD31 positive vessels in hotspots were counted in four separate ×200 fields from 3 samples per group.
We found that SN6j is more effective for tumor suppression in immunocompetent mice than in SCID mice. We hypothesized that T cell immunity is important for effective antitumor efficacy of SN6j in vivo. To test this hypothesis, we investigated effects of depletion of CD4+ T cells and/or CD8+ T cells on antitumor efficacy of SN6j in mice [39]. In addition, we investigated effects of CpG oligodeoxynucleotides (ODN) on the antitumor efficacy of SN6j [39]. Systemic (i.v.) administration of a relatively small dose (0.6 μg/g body weight/dose) of SN6j suppressed the growth of established s.c. tumors of colon-26 in BALB/c mice and improved survival of the tumor-bearing mice. Addition of CpG ODN to SN6j synergistically enhanced antitumor efficacy of SN6j. In contrast, such enhancing effects of CpG ODN were not detected in SCID mice. Antitumor efficacy of SN6j in BALB/c mice was abrogated when CD4+ T cells and/or CD8+ T cells were depleted; effect of CD8+ T cell depletion was stronger. Interestingly, CD4-depletion decreased tumor growth while CD8-depletion enhanced tumor growth in the absence of SN6j. The results suggest that SN6j induces T cell immunity probably by antigen cross-presentation [39]. SN6j induced apoptosis in HUCECs in a dose-dependent manner which indicates an additional mechanism of antiangiogenesis by SN6j [39].
6.3.2. Suppression of Metastasis
Anti-metastatic activity of an antitumor agent is exceedingly important because metastasis is the primary cause of death for most solid cancer patients. We found that three anti-ENG mAbs SN6a, SN6j and SN6k which define individually distinct epitopes of ENG are capable of suppressing tumor metastases in the multiple metastasis models [40]. The metastasis models were generated by i.v., s.c. (into the flank) or mammary gland fat pad injection of 4T1 murine mammary carcinoma cells and splenic injections of two types of colon 26 murine colorectal carcinoma cells. Individual mAbs were injected i.v. via the tail vein of mice. SN6a and SN6j effectively suppressed formation of metastatic colonies of 4T1 in the lung in all of the three 4T1 metastatic models. In addition, these mAbs were effective for suppressing the primary tumors of 4T1 in the skin and mammary fat pad. These mAbs effectively suppressed microvessel density and angiogenesis in tumors as measured by the Matrigel plug assay in mice. No significant side effects of the administered mAbs were detected. Furthermore, SN6a and SN6j extended survival of the tumor-bearing mice. SN6j, SN6k and their immunoconjugates with deglycosylated ricin A-chain were all effective for suppressing hepatic metastasis of colon 26. These findings in the present study are clinically relevant in view of the ongoing clinical trial of a chimerized form of SN6j in patients with metastatic and/or advanced solid caners.
7. Generation of a Human/Mouse Chimeric mAb c-SN6j and Test of c-SN6j in Nonhuman Primates
7.1. Generation and Characterization of c-SN6j
To facilitate clinical application of anti-ENG mAbs, we generated a human/mouse chimeric antibody c-SN6j from the parental murine anti-ENG mAb SN6j by replacing the constant regions of the light chain and the heavy chain (i.e., CL and CH) of mouse IgG with CL and CH of human IgG. We used human Cκ as CL and human Cγ1 or Cγ3 as CH. In generation of c-SN6j, we used the cassette expression vectors that were reported by Norderhaug et al. [74]. We generated both IgG1 and IgG3 chimeric mAbs to obtain γ1-c-SN6j and γ3-c-SN6j. The latter showed a slightly higher antigen-binding avidity than the former, but it showed substantially weaker ADCC than the former. In addition, human IgG1 is known to have a longer plasma half life in vivo than human IgG3 [75, 76]. In view of these facts, we selected γ1-c-SN6j (simply termed as c-SN6j) for the clinical application. The primary sequences of the variable regions of the light chain (VL) and the heavy chain (VH) of c-SN6j (also of SN6j) are shown in Figs. (3) and (4) respectively. The sequence analyses show that SN6j-VL belongs to Vκ subgroup VI while SN6j-VH belongs to VH subgroup IIIC.
Fig. 3.
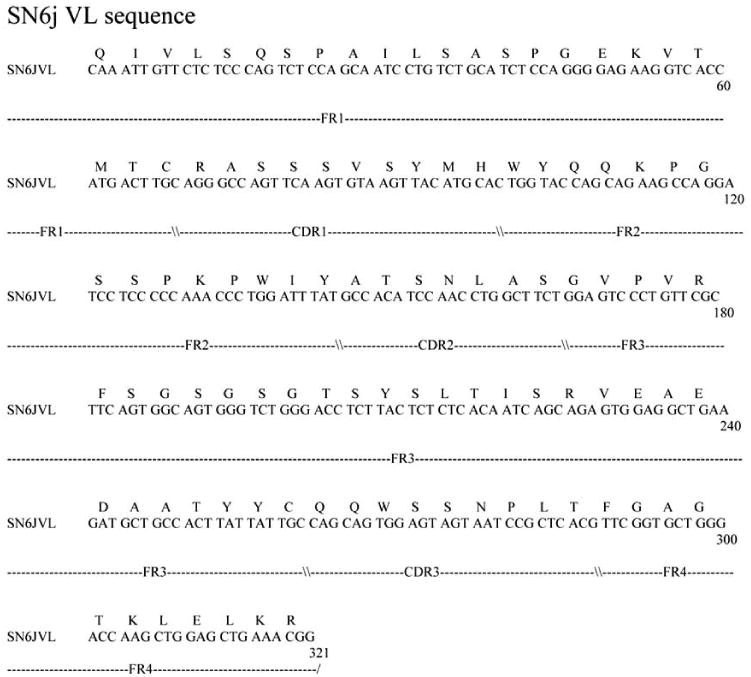
Nucleotide sequence of the gene encoding VL of c-SN6j (also of SN6j) and the deduced amino acid sequence of VL of c-SN6j. FR and CDR denote the framework region and the complementarity-determining region of antibody, respectively.
Fig. 4.
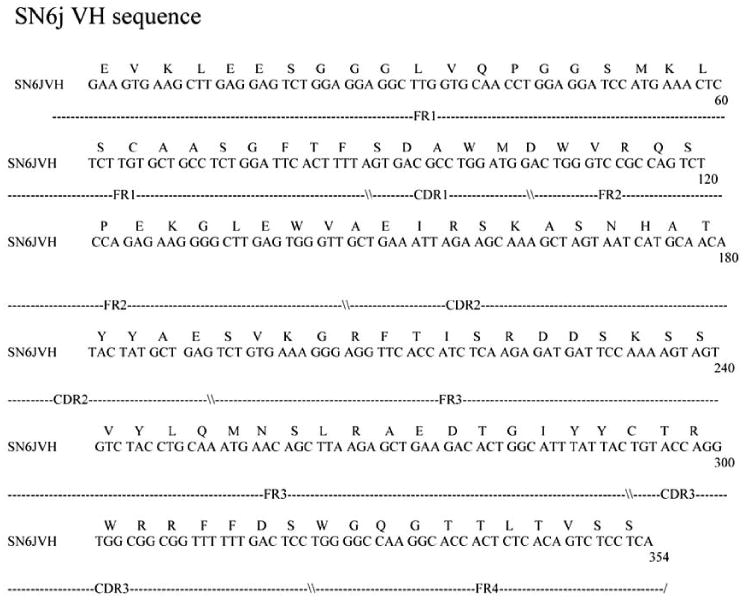
Nucleotide sequence of the gene encoding VH of c-SN6j (also of SN6j) and the deduced amino acid sequence of VH of c-SN6j. FR and CDR denote as described in the legend to Fig. 3.
7.2. Production of GLP (Good Laboratory Practice)-Compliant c-SN6j and Test of the GLP c-SN6j in Non-human Primates
The genes for c-SN6j were transfected into NS0 mouse myeloma cells, and stable and high antibody-producing clones were selected. Gram quantities of GLP c-SN6j were produced using a selected clone at a commercial facility (Unisyn Technologies, Hopkinton, MA). We performed studies of pharmacokinetics (PKs), immunogenicity and toxicology of c-SN6j in monkeys after multiple i.v. injections [42]. A dose-escalation study was performed by administration of c-SN6j into six monkeys at the dose of 1 mg, 3 mg and 10 mg per kg body weight. In addition, both c-SN6j (3 mg/kg) and doxorubicin (0.275 mg/kg) were injected into two monkeys. c-SN6j and doxorubicin were injected twice a week for three weeks. We developed a unique and sensitive ELISA by sequentially targeting the common and idiotypic epitopes of c-SN6j-Fv to quantify plasma c-SN6j. Application of this ELISA showed that an increase in the c-SN6j dose resulted in a proportional increase in the circulating c-SN6j after the first injection. In addition, the estimated AUC (area under the curve) for the first injection of c-SN6j is proportional to the dose. We carried out detailed analyses of PKs of c-SN6j during and after the repeated injections. Our model of PKs fitted the empirical data well. Addition of doxorubicin modulated the PK parameters. We developed two new ELISAs to separately determine the immune responses to the murine part and the human part of c-SN6j in monkeys. Interestingly, the murine part induced a weaker immune response than the human part. Increases in the c-SN6j dose increased plasma levels of c-SN6j but did not increase the immune responses to c-SN6j. No significant toxicity of c-SN6j was detected by several criteria that included blood chemistry, observation of monkeys during and after the c-SN6j injections, and tissue histology after the SN6j-injected monkeys were sacrificed. An additional large scale dose-escalation study of c-SN6j was performed in monkeys in collaboration with Tracon Pharma. Results of this study were consistent with those of the earlier study [42] that are described above.
8. Conclusions and Future Directions
Tissue distribution pattern, molecular nature (e.g., a transmembrane glycoprotein), functional properties suggested us that endoglin (ENG) might be a promising target in vascular-targeting antiangiogenesis therapy (VTAT) of solid tumors and other angiogenesis-associated diseases such as hemangiomas, psoriasis and age-related macular degeneration [3, 77]. This hypothesis was supported by our studies of selected anti-ENG mAbs (SN6j, SN6a, SN6f and SN6k) and their immunoconjugates in tumor-bearing mice. To facilitate clinical application, we generated a human/mouse chimeric mAb termed c-SN6j and performed studies of pharmacokinetics (PKs), toxicology and immune response in nonhuman primates. No significant toxicity was detected by several criteria after repeated i.v. injections of c-SN6j and we detected minimal immune response to the murine part of c-SN6j. PK analysis indicated that the administered c-SN6j circulates in monkeys in a similar manner to other reported chimeric and humanized mAbs of IgG1 isotype in humans. The results suggest that c-SN6j can be safely administered in patients. This hypothesis is supported by an ongoing multicenter phase 1 clinical trial of GMP (Good Manufacturer Practice)-compliant c-SN6j (also known as TRC105; NCT00582985). This trial is conducted in collaboration with Tracon Pharma and oncologists at Premiere Oncology (Santa Monica, CA and Scottsdale, AZ), Roswell Park Cancer Institute and Duke University Medical Center [78]. Expansion of this clinical trial is under way.
It has been a long time since we first identified ENG as a tumor-associated homodimeric cell membrane glycoprotein in 1986 [7, 9]. Our long journey still continues and we hope that our efforts will lead to reductions of suffering of many cancer patients and their families, and will ultimately lead to saving of the lives of many cancer patients.
Acknowledgments
We wish to thank Dr. Kathryn O'Connell and Dr. Lars Norderhaug for providing us with SVEC4-10 and cassette expression vectors, respectively. We thank Dr. Maurice Barcos, Dr. Masahiro Tabata, Dr. Ken Shiozaki, Ms. Betsy Spaulding, Ms. Jill Duzen and Ms. Kanako Fujita for their help in the presented works. We wish to acknowledge the major contribution of Dr. Charles Theuer, Dr. Bryan Leigh, Dr. Sharon Real, and Ms. Bonne Adams of Tracon Pharma in application of TRC105 to clinical trials which will be the major focus of our future effort.
The presented works were supported by grants R01 CA19304, R01 CA37131, P01 CA42683 and CA016156 from NCI, grants CH-514, IM741A and IM741B from ACS, a New York State CABET grant, and Translational Research grant BC960497 and Clinical Translational Research grant BC020043 from the Department of Defense Breast Cancer Research Program.
References
- 1.Denekamp J. Review Article: Angiogenesis, neovascular proliferation and vascular pathophysiology as targets for cancer therapy. Br J Radiol. 1993;66:181–196. doi: 10.1259/0007-1285-66-783-181. [DOI] [PubMed] [Google Scholar]
- 2.Bicknell R, Harris AL. Anticancer strategies involving the vasculature: vascular targeting and the inhibition of angiogenesis. Semin Cancer Biol. 1992;3:399–407. [PubMed] [Google Scholar]
- 3.Seon BK, Kumar S. CD105 antibody for targeting of tumor vascular endothelial cells. In: Fan TP, Kohn E, editors. New Angiotherapy. Humana Press; Totowa, New Jersey: 2001. pp. 499–515. [Google Scholar]
- 4.Brekken RA, Li C, Kumar S. Strategies for Vascular Targeting In Tumors. Int J Cancer. 2002;100:123–130. doi: 10.1002/ijc.10462. [DOI] [PubMed] [Google Scholar]
- 5.Thorpe PE. Vascular targeting agents as cancer therapeutics. Clin Cancer Res. 2004;10:415–427. doi: 10.1158/1078-0432.ccr-0642-03. [DOI] [PubMed] [Google Scholar]
- 6.Horsman MR, Siemann DW. Pathophysiologic effects of vascular-targeting agents and the implications for combination with conventional therapies. Cancer Res. 2006;66:11520–11539. doi: 10.1158/0008-5472.CAN-06-2848. [DOI] [PubMed] [Google Scholar]
- 7.Haruta Y, Seon BK. Distinct human leukemia-associated cell surface glycoprotein GP160 defined by monoclonal antibody SN6. Proc Natl Acad Sci USA. 1986;83:7898–7902. doi: 10.1073/pnas.83.20.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gougos A, Letarte M. Identification of a human endothelial cell antigen with monoclonal antibody 44G4 produced against a pre-B leukemic cell line. J Immunol. 1988;141:1925–1933. [PubMed] [Google Scholar]
- 9.Seon BK, Matsuno F, Haruta Y, Kondo M, Barcos M. Long-lasting complete inhibition of human solid tumors in SCID mice by targeting endothelial cells of tumor vasculature with anti-human endoglin immunotoxin. Clin Cancer Res. 1997;3:1031–1044. [PubMed] [Google Scholar]
- 10.Bellon T, Corbi A, Lastres P, Cales C, Cebrian M, Vera S, Cheifetz S, Massague J, Letarte M, Bernabeu C. Identification and expression of two forms of the human transforming growth factor-beta-binding protein endoglin with distinct cytoplasmic regions. Eur J Immunol. 1993;23:2340–2345. doi: 10.1002/eji.1830230943. [DOI] [PubMed] [Google Scholar]
- 11.Westphal JR, Willems HW, Schalkwijk CJ, Ruiter DJ, de Waal RM. A new 180-kDa dermal endothelial cell activation antigen: in vitro and in situ characteristics. J Invest Dermatol. 1993;100:27–34. doi: 10.1111/1523-1747.ep12349946. [DOI] [PubMed] [Google Scholar]
- 12.Burrows FJ, Derbyshire EJ, Tazzari PL, Amlot P, Gazdar AF, King SW, Letarte M, Vitetta ES, Thorpe PE. Up-regulation of endoglin on vascular endothelial cells in human solid tumors: implications for diagnosis and therapy. Clin Cancer Res. 1995;1:1623–1634. [PubMed] [Google Scholar]
- 13.Miller DW, Graulich W, Karges B, Stahl S, Ernst M, Ramaswamy A, Sedlacek HH, Muller R, Adamkiewicz J. Elevated expression of endoglin, a component of the TGF-beta-receptor complex, correlates with proliferation of tumor endothelial cells. Int J Cancer. 1999;81:568–572. doi: 10.1002/(sici)1097-0215(19990517)81:4<568::aid-ijc11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 14.Wang JM, Kumar S, Pye D, van Agthoven AJ, Krupinski J, Hunter RD. A monoclonal antibody detects heterogeneity in vascular endothelium of tumours and normal tissues. Int J Cancer. 1993;54:363–370. doi: 10.1002/ijc.2910540303. [DOI] [PubMed] [Google Scholar]
- 15.Clasper S, Royston D, Baban D, Cao Y, Ewers S, Butz S, Vestweber D, Jackson DG. A novel gene expression profile in lymphatics associated with tumor growth and nodal metastasis. Cancer Res. 2008;68:7293–7303. doi: 10.1158/0008-5472.CAN-07-6506. [DOI] [PubMed] [Google Scholar]
- 16.Yoshitomi H, Kobayashi S, Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Miyazaki M. Specific expression of endoglin (CD105) in endothelial cells of intratumoral blood and lymphatic vessels in pancreatic cancer. Pancreas. 2008;37:275–281. doi: 10.1097/mpa.0b013e3181690b97. [DOI] [PubMed] [Google Scholar]
- 17.Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 18.Arthur HM, Ure J, Smith AJ, Renforth G, Wilson DI, Torsney E, Charlton R, Parums DV, Jowett T, Marchuk DA, Burn J, Diamond AG. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol. 2000;217:42–53. doi: 10.1006/dbio.1999.9534. [DOI] [PubMed] [Google Scholar]
- 19.Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J, Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992;267:19027–19030. [PubMed] [Google Scholar]
- 20.Smirnov DA, Foulk BW, Doyle GV, Connelly MC, Terstappen LWMM, O'Hara SM. Global gene expression profiling of circulating endothelial cells in patients with metastatic carcinomas. Cancer Res. 2006;66:2918–2922. doi: 10.1158/0008-5472.CAN-05-4003. [DOI] [PubMed] [Google Scholar]
- 21.Wang JM, Kumar S, Agthoven AV, Kumar P, Pye D, Hunter RD. Irradiation induces up-regualtion of E9 protein (CD105) in human vascular endothelial cells. Int J Cancer. 1995;62:791–796. doi: 10.1002/ijc.2910620624. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Elsner T, Botella LM, Velaco B, Langa C, Bernabeu C. Endoglin expression is regulated by transcriptonal cooperation between the hypoxia and transforming growth factor-β pathways. J Biol Chem. 2002;277:43799–43808. doi: 10.1074/jbc.M207160200. [DOI] [PubMed] [Google Scholar]
- 23.Bockhorn M, Tsuzuki Y, Xu L, Frilling A, Broelsch CE, Fukumura D. Differential vascualr and transcritional rsponses to anti-vascular endothelial growth factor antibody in orthotopic human pancreatic cancer xenografts. Clin Cancer Res. 2003;9:4221–4226. [PubMed] [Google Scholar]
- 24.Yamashita H, Ichijo H, Grimsby S, Moren A, ten Dijke P, Miyazono K. Endoglin forms a heteromeric complex with the signaling receptors for transforming growth factor-beta. J Biol Chem. 1994;269:1995–2001. [PubMed] [Google Scholar]
- 25.Guerrero-Esteo M, Sanchez-Elsner T, Letamendia A, Bernabeu C. Extracellular and cytoplasmic domains of endoglin interact with the transforming growth factor- beta receptors I and II. J Biol Chem. 2002;277:29197–29209. doi: 10.1074/jbc.M111991200. [DOI] [PubMed] [Google Scholar]
- 26.Velasco S, Alvarez-Munoz P, Pericacho M, ten Dijke P, Bernabeu C, Lopez-Novoa JM. Rodriguez-Barbero, AL- and S-endoglin differentially modulate TGFbeta1 signaling mediated by ALK1 and ALK5 in L6E9 myoblasts. J Cell Sci. 2008;121:913–919. doi: 10.1242/jcs.023283. [DOI] [PubMed] [Google Scholar]
- 27.Lebrin F, Goumans MJ, Jonker L, Carvalho RLC, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM, ten Dijke P. Endoglin promotes endothelial cell proliferation and TGF-β/ALK1 signal transduction. EMBO J. 2004;23:4018–4028. doi: 10.1038/sj.emboj.7600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massague J. TGF-β Signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 29.Conley BA, Koleva R, Smith JD, Kacer D, Zhang D, Bernabeu C, Vary CPH. Endoglin controls cell migration and composition of focal adhesions. J Biol Chem. 2004;279:27440–27449. doi: 10.1074/jbc.M312561200. [DOI] [PubMed] [Google Scholar]
- 30.Lee NY, Blobe GC. The interaction of endoglin with β-arrestin2 regulates transforming growth factor-β-mediated ERK activation and migration in endothelial cells. J Biol Chem. 2007;282:21507–21517. doi: 10.1074/jbc.M700176200. [DOI] [PubMed] [Google Scholar]
- 31.Kumar S, Ghellal A, Li C, Byrne G, Haboubi N, Wang JM, Bundred N. Breast carcinoma: vascular density determined using CD105 antibody correlates with tumor prognosis. Cancer Res. 1999;59:856–861. [PubMed] [Google Scholar]
- 32.Tanaka F, Otake Y, Yanagihara K, Kawano Y, Miyahara R, Li M, Yamada T, Hanaoka N, Inui K, Wada H. Evaluation of angiogenesis in non-small cell lung cancer: comparison between anti-CD34 antibody and anti-CD105 antibody. Clin Cancer Res. 2001;7:3410–3415. [PubMed] [Google Scholar]
- 33.Li C, Gardy R, Seon BK, Duff SE, Abdalla S, Renehan A, O'Dwyer ST, Haboubi N, Kumar S. Both high intratumoral microvessel density determined using CD105 antibody and elevated plasma levels of CD105 in colorectal cancer patients correlate with poor prognosis. Br J Cancer. 2003;88:1424–1431. doi: 10.1038/sj.bjc.6600874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charpin C, Dales JP, Garcia S, Carpentier S, Djemli A, Andrac L, Lavaut MN, Allasia C, Bonnier P. Tumor neoangiogenesis by CD31 and CD105 expression evaluation in breast carcinoma tissue microarrays. Clin Cancer Res. 2004;10:5815–5819. doi: 10.1158/1078-0432.CCR-04-0021. [DOI] [PubMed] [Google Scholar]
- 35.Matsuno F, Haruta Y, Kondo M, Tsai H, Barcos M, Seon BK. Induction of lasting complete regression of preformed distinct solid tumors by targeting the tumor vasculature using two new antiendoglin monoclonal antibodies. Clin Cancer Res. 1999;5:371–382. [PubMed] [Google Scholar]
- 36.Tabata M, Kondo M, Haruta Y, Seon BK. Antiangiogenic radioimmunotherapy of human solid tumors in SCID mice using 125I-labeled anti-endoglin monoclonal antibodies. Int J Cancer. 1999;82:737–742. doi: 10.1002/(sici)1097-0215(19990827)82:5<737::aid-ijc18>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi N, Haba A, Matsuno F, Seon BK. Antiangiogenic therapy of established tumors in human skin/severe combined immunodeficiency mouse chimeras by anti-endoglin (CD105) monoclonal antibodies, and synergy between anti-endoglin antibody and cyclophosphamide. Cancer Res. 2001;61:846–854. [PubMed] [Google Scholar]
- 38.Tsujie M, Uneda S, Tsai H, Seon BK. Effective antiangiogenic therapy of established tumors in mice by naked anti-human endoglin (CD105) antibody: differences in growth rate and therapeutic response between tumors growing at different sites. Int J Oncol. 2006;29:1087–1094. [PubMed] [Google Scholar]
- 39.Tsujie M, Tsujie T, Toi H, Uneda S, Shiozaki K, Tsai H, Seon BK. Anti-tumor activity of an anti-endoglin monoclonal antibody is enhanced in immunocompetent mice. Int J Cancer. 2008;122:2266–2273. doi: 10.1002/ijc.23314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uneda S, Toi H, Tsujie T, Tsujie M, Harada N, Tsai H, Seon BK. Anti-endoglin monoclonal antibodies are effective for suppressing metastasis and the primary tumors by targeting tumor vasculature. Int J Cancer. 2009;125:1446–1453. doi: 10.1002/ijc.24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Connell KA, Edidin M. A mouse lymphoid endothelial cell line immortalized by simian Virus 40 binds lymphocytes and retains functional characteristics of normal endothelial cells. J Immunol. 1990;144:521–525. [PubMed] [Google Scholar]
- 42.Shiozaki K, Harada N, Greco WR, Haba A, Uneda S, Tsai H, Seon BK. Antiangiogenic chimeric anti-endoglin (CD105) antibody: pharmacokinetics and immunogenicity in nonhuman primates and effects of doxorubicin. Cancer Immunol Immunother. 2006;55:140–150. doi: 10.1007/s00262-005-0691-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dallas NA, Samuel S, Xia L, Fan F, Gray MJ, Lim SJ, Ellis LM. Endoglin (CD105): A marker of tumor vasculature and potential target for therapy. Clin CancerRes. 2008;14:1931–1937. doi: 10.1158/1078-0432.CCR-07-4478. [DOI] [PubMed] [Google Scholar]
- 44.ten Dijke P, Goumans MJ, Pardali E. Endoglin in angiogenesis and vascular diseases. Angiogenesis. 2008;11:79–89. doi: 10.1007/s10456-008-9101-9. [DOI] [PubMed] [Google Scholar]
- 45.Bernabeu C, Lopez-Novoa JM, Quintanilla M. The emerging role of TGF-β superfamily coreceptors in cancer. Biochim Biophys Acta. 2009;1792:954–973. doi: 10.1016/j.bbadis.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Fonsatti E, Nicolay HJ, Altomonte M, Covre A, Maio M. Targeting cancer vasculature via endoglin/CD105: a novel antibody-based diagnostic and therapeutic strategy in solid tumours. Cardiovasc Res. 2010;86:12–19. doi: 10.1093/cvr/cvp332. [DOI] [PubMed] [Google Scholar]
- 47.Seon BK, Haruta Y, Chervinsky D, Barcos M, Spaulding B. Restricted expression of endoglin (CD105) in tumor tissues and minimal effects of anti-endoglin immunotoxins on normal hematopoietic progenitors. Proc Am Assoc Cancer Res. 2004;45:948. [Google Scholar]
- 48.Lastres P, Bellon T, Cabanas C, Sanchez-Madrid F, Acevedo A, Gougos A, Letarte M, Bernabeu C. Regulated expression on human macrophages of endoglin, an Arg-Gly-Asp-containing surface antigen. Eur J Immunol. 1992;22:393–397. doi: 10.1002/eji.1830220216. [DOI] [PubMed] [Google Scholar]
- 49.Tsujie T, Tsujie M, Haruta Y, Barcos M, Spaulding B, Tsai H, Seon BK. Differential expression of endoglin (CD105) between human malignant epithelial tissues and cultured epithelial cell lines. Proc Am Assoc Cancer Res. 2007;48:907. [Google Scholar]
- 50.She XW, Seon BK. Epitope mapping of endoglin, a TGF-β receptor, using recombinant fragments and twelve monoclonal antibodies. Proc Am Assoc Cancer Res. 2001;42:825. [Google Scholar]
- 51.Takahashi N, Kawanishi-Tabata R, Haba A, Tabata M, Haruta Y, Tsai H, Seon BK. Association of serum endoglin with metastasis in patients with colorectal, breast, and other solid tumors, and suppressive effect of chemotherapy on the serum endoglin. Clin Cancer Res. 2001;7:524–532. [PubMed] [Google Scholar]
- 52.She X, Matsuno F, Harada N, Tsai H, Seon BK. Synergy between anti-endoglin (CD105) monoclonal antibodies and TGF-beta in suppression of growth of human endothelial cells. Int J Cancer. 2004;108:251–257. doi: 10.1002/ijc.11551. [DOI] [PubMed] [Google Scholar]
- 53.Volkel T, Holig P, Merdan T, Muller R, Kontermann RE. Targeting of immunoliposomes to endothelial cells using a single-chain Fv fragment directed against human endoglin (CD105) Biochim Biophys Acta. 2004;1663:158–166. doi: 10.1016/j.bbamem.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 54.Munoz R, Arias Y, Ferreras JM, Rojo MA, Gayoso MJ, Nocito M, Benitez J, Jimenez P, Bernabeu C, Girbes T. Targeting a marker of the tumour neovasculature using a novel anti human CD105-immunotoxin containing the non-toxic type 2 ribosome-inactivating protein nigrin b. Cancer Lett. 2007;256:73–80. doi: 10.1016/j.canlet.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 55.Saad RS, Liu YL, Nathan G, Celebrezze J, Medich D, Silverman JF. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in colorectal cancer. Mod Pathol. 2004;17:197–203. doi: 10.1038/modpathol.3800034. [DOI] [PubMed] [Google Scholar]
- 56.El-Gohary YM, Silverman JF, Olson PR, Liu YL, Cohen JK, Miller R, Saad RS. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in prostatic adenocarcinoma. Am J Clin Pathol. 2007;127(4):572–579. doi: 10.1309/X6NXYE57DLUE2NQ8. [DOI] [PubMed] [Google Scholar]
- 57.Ding S, Li C, Lin S, Yang Y, Liu D, Han Y, Zhang Y, Li L, Zhou L, Kumar S. Comparative evaluation of microvessel density determined by CD34 or CD105 in benign and malignant gastric lesions. Hum Pathol. 2006;37(7):861–866. doi: 10.1016/j.humpath.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 58.Erdem O, Taskiran C, Onan MA, Erdem M, Guner H, Ataoglu O. CD105 expression is an independent predictor of survival in patients with endometrial cancer. Gynecol Oncol. 2006;103(3):1007–1011. doi: 10.1016/j.ygyno.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 59.Yang LY, Lu WQ, Huang GW, Wang W. Correlation between CD105 expression and postoperative recurrence and metastasis of hepatocellular carcinoma. BMC Cancer. 2006;6:110–119. doi: 10.1186/1471-2407-6-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taskiran C, Erdem O, Onan A, Arisoy O, Acar A, Vural C, Erdem M, Ataoglu O, Guner H. The prognostic value of endoglin (CD105) expression in ovarian carcinoma. Int J Gynecol Cancer. 2006;16:1789–1793. doi: 10.1111/j.1525-1438.2006.00658.x. [DOI] [PubMed] [Google Scholar]
- 61.Zijlmans HJ, Fleuren GJ, Hazelbag S, Sier CF, Dreef EJ, Kenter GG, Gorter A. Expression of endoglin (CD105) in cervical cancer. Br J Cancer. 2009;100:1617–1626. doi: 10.1038/sj.bjc.6605009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martone T, Rosso P, Albera R, Migliaretti G, Faire F, Pignataro L, Pruneri G, Bellone G, Cortesina G. Prognostic relevance of CD105+ microvessel density in HNSCC patient outcome. Oral Oncol. 2005;41:147–155. doi: 10.1016/j.oraloncology.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 63.Kyzas PA, Agnantis NJ, Stefanou D. Endoglin (CD105) as prognostic factor in head and neck squamous cell carcinoma. Virchows Arch. 2006;448:768–775. doi: 10.1007/s00428-006-0195-4. [DOI] [PubMed] [Google Scholar]
- 64.Behrem S, Zarkovic K, Eskinha N, Jonjic N. Endoglin is a better marker than CD31 in evaluation of angiogenesis in glioblastoma. Croat Med J. 2005;46:417–422. [PubMed] [Google Scholar]
- 65.Fonsatti E, Jekunen AP, Kairemo KJ, Coral S, Snellman M, Nicotra MR, Natali PG, Altomonte M, Maio M. Endoglin is a suitable target for efficient imaging of solid tumors: in vivo evidence in a canine mammary carcinoma model. Clin Cancer Res. 2000;6:2037–2043. [PubMed] [Google Scholar]
- 66.Bredow S, Lewin M, Hofmann B, Marecos E, Weissleder R. Imaging of tumour neovasculature by targeting the TGF-beta binding receptor endoglin. Eur J Cancer. 2000;36:675–681. doi: 10.1016/s0959-8049(99)00335-4. [DOI] [PubMed] [Google Scholar]
- 67.Costello B, Li C, Duff S, Butterworth D, Khan A, Perkins M, Owens S, Al- Mowallad AF, O'Dwyer S, Kumar S. Perfusion of 99Tcm-labeled CD105 Mab into kidneys from patients with renal carcinoma suggests that CD105 is a promising vascular target. Int J Cancer. 2004;109:436–441. doi: 10.1002/ijc.11699. [DOI] [PubMed] [Google Scholar]
- 68.Lee SY, Hong YD, Felipe PM, Pyun MS, Choi SJ. Radio-labeling of monoclonal anti-CD105 with (177)Lu for potential use in radioimmunotherapy. Appl Radiat Isot. 2009 doi: 10.1016/j.apradiso.2009.02.071. Epub. [DOI] [PubMed] [Google Scholar]
- 69.Korpanty G, Carbone JG, Grayburn PS, Fleming JB, Brekken RA. Monitoring response to anticancer therapy by targeting microbubbles to tumor vasculature. Clin Cancer Res. 2007;13:323–330. doi: 10.1158/1078-0432.CCR-06-1313. [DOI] [PubMed] [Google Scholar]
- 70.Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O'Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 71.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kerbel RS. Antiangiogenic therapy: A universal chemosensitization strategy for cancer? Science. 2006;312:1171–1175. doi: 10.1126/science.1125950. [DOI] [PubMed] [Google Scholar]
- 73.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 74.Norderhaug L, Olafsen T, Michaelsen TE, Sandlie I. Versatile vectors for transient and stable expression of recombinant antibody molecules in mammalian cells. J Immunol Methods. 1997;204:77–87. doi: 10.1016/s0022-1759(97)00034-3. [DOI] [PubMed] [Google Scholar]
- 75.Spiegelberg HL, Fishkin BG, Grey HM. Catabolism of human γG-immunoglobulins of different heavy chain subclasses I. Catabolism of γG-myeloma proteins in man. J Clin Invest. 1968;47:2323–2330. doi: 10.1172/JCI105917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morell A, Terry WD, Waldmann TA. Metabolic properties of IgG sublasses in man. J Clin Invest. 1970;49:673–680. doi: 10.1172/JCI106279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seon BK. Expression of endoglin (CD105) in tumor blood vessels. Int J Cancer. 2002;99:310–311. doi: 10.1002/ijc.10378. [DOI] [PubMed] [Google Scholar]
- 78.Rosen L, Gordon MS, Hurwitz HI, Wong MK, Adams BJ, Alvarez D, Seon BK, Leigh BR, Theuer CP. Early evidence of tolerability and clinical activity from phase I study of TRC105 (anti-CD105 antibody) in patients with advanced refractory cancer. J Clin Oncol. 2009;27 No 15s, 150s. [Google Scholar]


