Abstract
Calcium regulates a wide spectrum of physiological processes such as heartbeat, muscle contraction, neuronal communication, hormone release, cell division, and gene transcription. Major entry-ways for Ca2+ in excitable cells are high-voltage activated (HVA) Ca2+channels. These are plasma membrane proteins composed of several subunits, including α1, α2δ, β, and γ. Although the principal α1 subunit (Cavα1) contains the channel pore, gating machinery and most drug binding sites, the cytosolic auxiliary β subunit (Cavβ) plays an essential role in regulating the surface expression and gating properties of HVA Ca2+ channels. Cavβ is also crucial for the modulation of HVA Ca2+ channels by G proteins, kinases, and the Ras-related RGK GTPases. New proteins have emerged in recent years that modulate HVA Ca2+ channels by binding to Cavβ. There are also indications that Cavβ may carry out Ca2+ channel-independent functions, including directly regulating gene transcription. All four subtypes of Cavβ, encoded by different genes, have a modular organization, consisting of three variable regions, a conserved guanylate kinase (GK) domain, and a conserved Src-homology 3 (SH3) domain, placing them into the membrane-associated guanylate kinase (MAGUK) protein family. Crystal structures of Cavβs reveal how they interact with Cavα1, open new research avenues, and prompt new inquiries. In this article, we review the structure and various biological functions of Cavβ, with both a historical perspective as well as an emphasis on recent advances.
I. INTRODUCTION
Calcium is arguably one of life’s most important elements. Intracellular Ca2+ concentration ([Ca2+]i) is kept at very low levels (~100 nM) under resting conditions, but it rises sharply (to tens or hundreds of µM) upon stimulation. This allows Ca2+ to play a crucial role in numerous biological processes, including neurotransmitter and hormone release, muscle excitation-contraction coupling, cell division, tumorigenesis, differentiation, migration, and cell death. In addition, Ca2+ influx across the plasma membrane causes changes in cellular excitability. Mechanisms that rigorously control intracellular Ca2+ levels are therefore essential for eukaryotic cell function. [Ca2+]i is maintained at low levels by Ca2+-ATPases through active extrusion of cytosolic Ca2+ to the extracellular milieu or into intracellular organelles. On the other hand, Ca2+ entry into cells is mediated primarily by passive flow through voltage-, ligand-, temperature-, and mechanical stretch-gated ion channels.
The principal Ca2+ entryways of nerve, muscle, and some endocrine cells are voltage-gated Ca2+ channels (VGCCs). They were discovered in 1953 with the unexpected observation that crab muscle action potentials (APs) persist in the absence of external Na+, unlike squid nerve APs (145). Muscle APs were then found to increase with increasing extracellular Ca2+ concentration ([Ca2+]o), consistent with a Ca2+ conductance (200). Similar currents were later found in nerve, endocrine, and other tissues in diverse organisms (12, 221,225, 253, 291, 304). Based on the membrane voltage required for activation, VGCCs were subsequently classified into high-voltage activated (HVA) and low-voltage activated (LVA) channels (65, 66,146, 293). Later studies further classified Ca2+ currents into L-, N-, P/Q-, R-, and T-type currents, which exhibit distinct biophysical and pharmacological properties (127, 137,292, 335, 359, 408, 444, 446, 500).
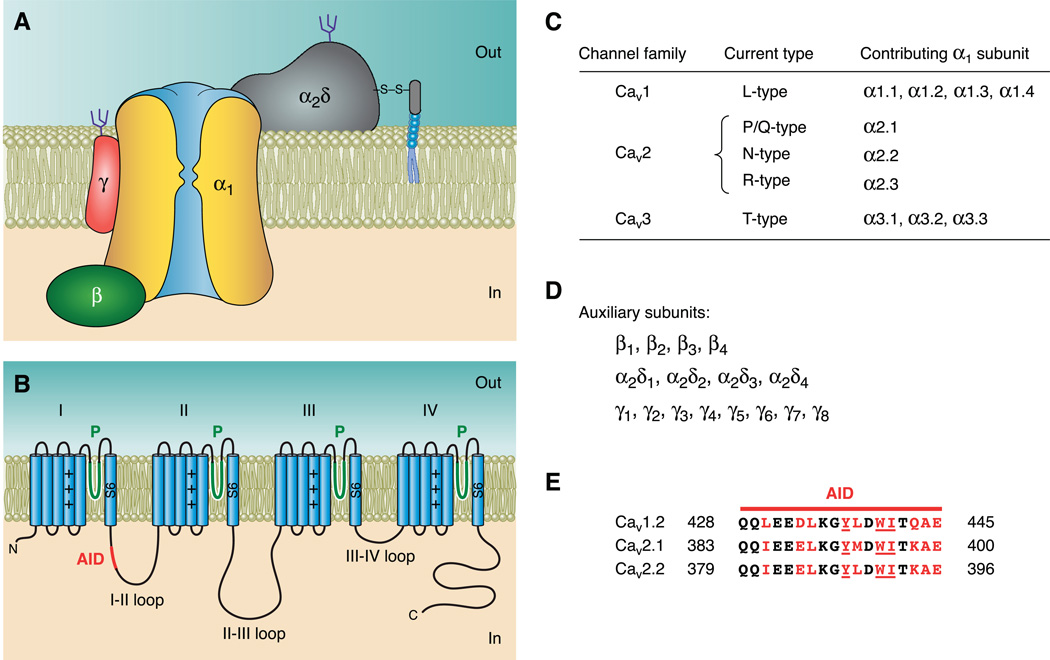
Molecular characterization of VGCCs began with the purification and cloning of the skeletal muscle Ca2+ channel (also called dihydropyridine receptor or DHPR) (107, 430, 434). The purified channel complex is composed of five subunits, termed α1 (175 kDa), α2 (143 kDa), β (54 kDa), δ (24–27 kDa), and γ (30 kDa). α2 and δ are linked posttranslationally by disulfide bonds into a single subunit referred to as α2δ (430). Subsequent research showed that L-, N-, P/Q- and R-type channels are made up of α1, α2δ, β, and, in some tissues, γ subunits (Fig. 1A). T-type channels, on the other hand, appear to require only an α1 subunit (351, 352).
Fig. 1.
Molecular organization of voltage-gated Ca2+ channels. A: subunit composition of high-voltage activated (HVA) Ca2+ channels. B: schematic representation of the predicted transmembrane topology of Cavα1, with the location of the α-interaction domain (AID) marked. C: Ca2+ channel current types and the corresponding α1 subunits of the channels that produce them. D: list of all cloned auxiliary HVA Ca2+ channel subunits. E: amino acid sequence alignment of the AID from the indicated Cavα1. Residues involved in interactions with Cavβ are marked in red, with the most critical residues underlined. Residue numbers are indicated on both sides of the sequence.
The α1 subunit (Cavα1) is the principal component of VGCCs and is responsible for their unique biophysical and pharmacological properties. However, proper trafficking and functioning of L-, N-, P/Q- and R-type channels require the auxiliary subunits. In particular, the β subunit (Cavβ) plays a crucial role in trafficking the channels to the plasma membrane, fine-tuning channel gating, and regulating channel modulation by other proteins and signaling molecules. Crystal structures of the core region of three distinct Cavβs have opened up new avenues for investigating the molecular basis of Cavβ’s actions. There is also emerging evidence that Cavβ may possess functions unrelated to VGCCs. This review focuses on the molecular biology, structure, function, and channelopathy of Cavβ, beginning with a brief overview of all VGCC subunits. Summaries of classical and recent work on VGCC electrophysiology, pharmacology, biochemistry, molecular biology, modulation, cell biology, and pathophysiology can be found in numerous excellent reviews (20, 22,72–74, 108, 126, 138, 189, 216, 220, 234, 237, 246, 318, 351, 389, 423, 440, 445, 495).
A. The α1 Subunit
Cavα1 is the principal subunit of VGCCs. It is a 190- to 250-kDa protein containing four homologous repeats (I–IV) connected through cytoplasmic loops (Fig. 1B). Each repeat has six predicted transmembrane segments (S1–S6) and a reentrant pore-forming loop (P-loop) between S5 and S6. The four P-loops form the ion-selectivity filter, where four highly conserved negatively charged amino acids (glutamate or aspartate), one from each P-loop, form a signature locus that is essential for selecting and conducting Ca2+ (256, 266,389, 482). Similar to K+ channels (128, 243, 290), the S6 segments form the inner pore (505), and the S4 segments’ positively charged amino acids form part of the voltage sensor. The voltage-dependent movement of this sensor results in channel opening and closing. Furthermore, the majority of drug and toxin binding sites are located on Cavα1 (72). Thus Cavα1 possesses all the key features that define a VGCC, including pharmacological and biophysical properties such as gating, ion selectivity, and permeation.
Mammalian Cavα1 are encoded by 10 distinct genes. Based on amino acid sequence similarity, Cavα1 are divided into three subfamilies: Cav1, Cav2, and Cav3 (reviewed in Refs. 10, 72, 141, 486). The Cav1 subfamily includes channels that conduct L-type Ca2+ currents; the Cav2 subfamily includes channels that conduct N-, P/Q-, and R-type Ca2+ currents; and the Cav3 subfamily includes channels that conduct T-type Ca2+ currents (Fig. 1C).
B. The α2δ Subunit
The Cav1 and Cav2 subfamilies contain an auxiliary α2δ subunit (reviewed in Ref. 112). To date, there are four known α2δ subunits, each encoded by a unique gene and all possessing splice variants (Fig. 1D). Each α2δ protein is encoded by a single messenger RNA and is posttranslationally cleaved and then linked by disulfide bonds (259, 367). The δ peptide, originally presumed to be transmembrane but recently shown to be attached to the membrane through a glycosylphosphatidylinositol linker (113), anchors the larger extracellular α2 peptide in place (Fig. 1A). α2δ subunits can modify channel biophysical properties (63, 406, 459), but their main role is to increase Ca2+ channel current (63, 111,174, 259, 260, 322, 406, 459) by promoting trafficking of Cavα1 to the plasma membrane and/or by increasing its retention there (32, 64,194, 385). More recently, it was reported that α2δ functioned as a thrombospondin receptor to regulate excitatory synpatogenesis, independently from its regulation of VGCC activity (140, 267).
In two different mouse strains, naturally occurring mutations that lead to the loss of the full-length α2δ2 protein cause the ducky phenotype. This is characterized by shortened life spans, absence epilepsy, spike wave seizures, cerebellar ataxia, and decreased Purkinje cell dendritic arborization and firing rates (112, 260). α2δ2 knockouts also have abnormalities in the cardiovascular, immune, respiratory, and nervous systems. Irregularities in the cardiovascular system are also found in α2δ1 knockouts (169). α2δ3-null Drosophila are not viable, and the mutants have significantly impaired synaptic transmission (123, 267). Upregulation of α2δ1, on the other hand, is associated with neuropathic pain (283, 284). Importantly, α2δ1 is the main target of the antiepilepsy and antineuropathic pain drugs gabapentin and pregabalin, respectively (150, 169, 254).
C. The γ Subunit
There are eight different γ subunit genes, all yielding proteins with four transmembrane segments and intracellular amino (NH2) and carboxy (COOH) termini. γ1 was the first cloned γ subunit (182, 238, 430) and was copurified with muscle VGCCs, consistent with its primary role as a VGCC subunit. γ1 knockout mice are viable, morphologically indistinguishable from wild-type (WT) mice, but have larger Ca2+ currents with altered inactivation kinetics (168). γ2, γ3, and γ4 also associate with VGCCs (250, 399). γ1–4 subunits have been shown to produce varying effects on VGCC activity, depending on the partnered Cavα1 and Cavβ (134, 168,2017, 250, 258, 277, 379, 406, 467). The most consistent effect is a small reduction of current, caused mainly by a hyperpolarizing shift of the voltage dependence of inactivation and/or a positive shift of the voltage dependence of activation. However, unlike α2δ subunits, whose primary role is regulating VGCCs, γ subunits have more diverse biological functions. Since the discovery that mutations in γ2 underlie the stargazer mouse phenotype (277), which includes absence epilepsy and defects in the cerebellum and inner ear, it has become clear that γ2 and three other closely related γ subunits (γ3, γ4, and γ8) regulate the trafficking, localization, and biophysical properties of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors (41, 82,249, 343, 442). They are therefore referred to as transmembrane AMPA receptor regulatory proteins (TARPs). Indeed, acting as TARPs seems to be the primary role of γ2, γ3, γ4, γ8, and probably γ7 (252). While the function of γ5 remains unknown, γ6 is suggested to inhibit Cav3.1 channels (288), and γ7 is involved in the turnover of the mRNA of Cav2.2 and other proteins (149, 323). For recent reviews on γ subunits, see References 41, 82, 249, 320, 343, 350, 382.
D. The β Subunit
Purified Cav1 and Cav2 channels contain a tightly bound cytosolic Cavβ protein. There are four subfamilies of Cavβs (β1– (β4), each with splice variants, encoded by four distinct genes. All four Cavβs can dramatically enhance Ca2+ channel currents when they are coexpressed in heterologous expression systems along with a Cav1 or Cav2 (α1 subunit (268, 319,322, 361, 405, 450, 467, 470). Cavβs also change the voltage dependence and kinetics of activation and inactivation (247, 268,322, 332, 406, 412, 418, 450, 495); however, they do not affect ion permeation (183, 405,458; but see Ref. 390). Furthermore, Cavβ either regulates or is indispensable for the modulation of Cav1 and Cav2 channels by protein kinases, G proteins, and small RGK (Rem, Rem2, Rad, Gem/Kir) proteins. Not surprisingly, Cavβ knockouts are either nonviable (in the case of (β1 and (β2) or result in a severe pathophysiology (in the case of (β3 and (β4).
The rest of this review is devoted to Cavβ.
II. CLONING OF Cavβ
Molecular studies on Cavβ can be traced back to the first purification and identification of the components of the skeletal muscle DHPR (107). With the use of a combination of chromatography, sucrose gradient sedimentation, and labeling with a high-affinity DHPR-specific ligand, three noncovalently attached subunits were purified: the largest 160-kDa subunit was named (α, a 53-kDa subunit was named (β, and a 32-kDa subunit was named (γ (107). Subsequent purification studies of skeletal and neuronal Ca2+ channel complexes showed the presence of similar protein bands (4, 59,114, 162, 278, 322, 381, 430, 434) and established that the DHPR actually consisted of five subunits, including (α1 (175 kDa), (α2 (143 kDa), (β (54 kDa), (δ (24–27 kDa), and (γ (30 kDa) (430, 434).
Cloning of the first Cavβ was accomplished by Ruth et al. (381) using a classical approach based on peptide sequences derived from a purified skeletal muscle (β subunit. This (β subunit is now referred to as (β1a. This cloning paved the way for the identification of other (β subunits, their genes, and splice variants. Using a labeled skeletal muscle (β1a cDNA, Pragnell et al. (362) screened a rat brain cDNA library and cloned a new (β subunit, which later turned out to be a splice variant of (β1 named (β1b (360) (see sect. IV). Perez-Reyes et al. (353) also screened a rat brain cDNA library with (β1a and, using low-stringency hybridization, uncovered another new (β subunit, which was encoded by a different gene and named (β2 (now named (β2a). Screening a cardiac cDNA library, Hullin et al. (230) found (β2a and two other (β2 splice variants ((β2b and (β2c); in addition, they isolated the cDNA for (β3. Meanwhile, using degenerate primers corresponding to the conserved domains of (β1 and (β2 to perform reverse-transcription PCR, Castellano et al. (67, 68) cloned (β3 and (β4 from a rat brain cDNA library.
The cloning of Cavβs subsequently led to the mapping of the four Cavβ genes (55, 94,143, 347, 438) to chromosomes 17, 10, 12, and 2 for (β1, (β2, (β3, and (β4, respectively, and to the discovery of many other splice variants (see sect. IV).
III. STRUCTURE OF Cavβ
Prior to the determination of the crystal structure of Cavβ, it was already well recognized, based on amino acid sequence alignment, biochemical and functional studies, and molecular modeling, that Cavβ has a modular structure consisting of five distinct regions (40, 93,119, 203, 342, 361). The first, third, and fifth regions are variable in length and amino acid sequence, whereas the second and fourth regions are highly conserved and are homologous to the Src homology 3 (SH3) and guanylate kinase (GK) domains, respectively. The SH3 domain is a common protein interaction module present in diverse groups of proteins (reviewed in Ref. 307). The GK domain, originally found in guanylate kinase from baker’s yeast (416), is also engaged in protein-protein interactions (136, 170, 431). The middle three regions of Cavβ constitute the so-called Cavβ core, which is able to reconstitute many key functions of Cavβ (83, 84,119, 176, 206, 313, 342, 502). In addition, early studies determined that Cavβ binds with high affinity to Cavα1. This high-affinity site is located in the cytoplasmic loop connecting the first two homologous repeats (i.e., the I–II loop) of Cavα1 and was named the (α-interaction domain or AID (121, 361, 472) (Fig. 1E).
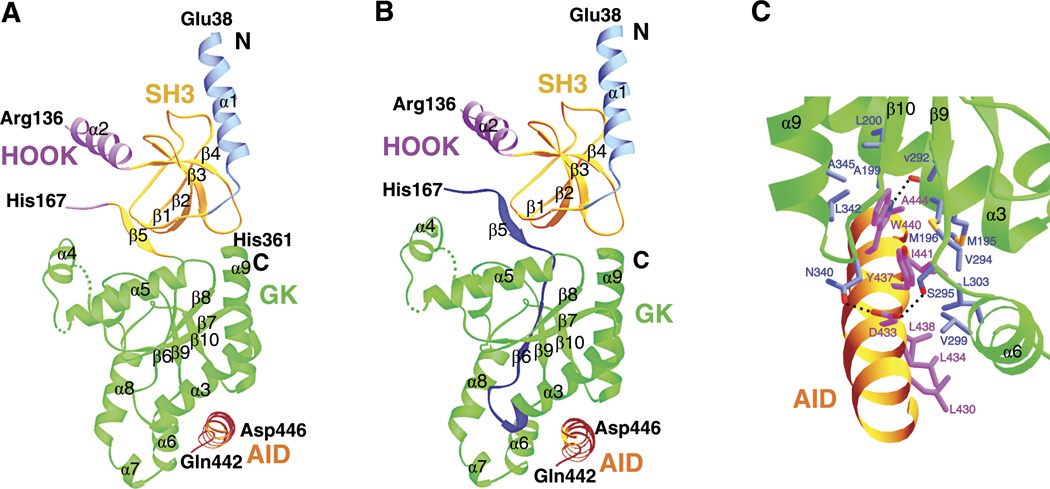
In 2004, three groups simultaneously and independently reported the crystal structure of the core of (β2a, (β3 and (β4, either alone or in complex with the AID (84, 341, 447). The structures show that the Cavβ core indeed contains an SH3 domain and a GK domain, which are connected by a so-called HOOK region (Fig. 2A).
Fig. 2.
Cavβ crystal structure. A: crystal structure of the β3 core in complex with the AID (PDB accession code 1VYT). This structure reveals the following regions: the NH2 terminus (light blue, residues 38–59), an SH3 domain (gold, residues 60–120 and 170–175), a HOOK region (purple, residues 121–169), and a GK domain (green, residues 176–360). Residues 137–166 were disordered and are not included. Residues 226–244 (forming the α4 helix of the GK domain) were disordered in this molecule but were well-resolved in another one in the same asymmetric unit. Residues 422–446 of Cav1.2 containing the entire AID are colored in orange. B: same structure as in A but with the BID (β3 residues K163-T193) highlighted in dark blue. The BID spans parts of the SH3-HOOK-GK motif but is not directly involved in binding the AID. C: close-up of the interface between β3 and AID. Some residues involved in the interactions are shown. [Adapted from Chen et al. (84)].
The existence of an SH3-HOOK-GK module places Cavβ in a family of proteins called the membrane-associated guanylate kinases (MAGUKs). MAGUKs, which include proteins such as PSD95, SAP97, CASK, Shank, and Homer, function as scaffold molecules that play a key role in organizing multiprotein complexes at functionally specialized regions such as synapses and other cellular junctions (136, 170, 431). MAGUKs contain an SH3-HOOK-GK module; in addition, they also contain one or more PDZ domains in the NH2 terminus, which serve protein-protein interaction and oligomerization functions. Cavβ is only partially related to MAGUKs structurally, however, because it does not contain a well-defined PDZ domain. Not surprisingly, the functions of Cavβ are markedly different from those of MAGUKs.
A. The GK Domain
Guanylate kinases are members of the nucleotide monophosphate kinase family that exists in organisms ranging from bacteria to humans. They catalyze the reversible phosphoryl transfer from ATP to GMP to produce ADP and GDP. Crystal structures of yeast guanylate kinases show that these enzymes have a compact structure with well-defined domains and folds and a catalytic site harboring the GMP- and ATP-binding pockets (43, 415, 416). The general structural features of yeast guanylate kinases are preserved in the Cavβ GK domain (Cavβ-GK), but large structural variations exist in the catalytic site, and many key catalytic residues are absent in Cavβ-GK (84, 341, 447). Thus Cavβ-GK is catalytically inactive. Similarly, the GK domain of MAGUKs does not possess catalytic activity, as indicated by the structural changes in the catalytic site and the lack of critical catalytic residues (285, 312, 437). Instead, the GK domains in these proteins have evolved into a protein interaction module. The Cavβ structures show that Cavβ-GK binds tightly to the AID in Cavα1 (84, 341, 447) (Fig. 2A), an interaction that will be further discussed in detail.
B. The SH3 Domain and the HOOK Region
Classical SH3 domains have a well-conserved and compact fold consisting of five sequential β-strands (βstrand 1–5) assembled into two orthogonally packed sheets (271). They mediate specific protein-protein interactions by binding to PxxP-containing motifs in target proteins, through a surface formed by a cluster of highly conserved hydrophobic residues. The Cavβ SH3 domain (Cavβ-SH3) has a similar fold as canonical SH3 domains do, but its last two β sheets are noncontinuous, separated by the HOOK region (84, 341, 447) (Fig. 2A). This split configuration is also shared by the SH3 domain of PSD-95, a MAGUK (312, 437). Cavβ-SH3 contains a well-preserved PxxP motif-binding site and therefore has the potential to bind PxxP motif-containing proteins. However, in the crystal structures, this binding site is partly shielded by the HOOK region and a long loop connecting two of the four continuous β sheets. Thus access to this site requires movement of these two regions. Such conformational changes are conceivable when Cavβ is bound to full-length Cavα1 and/or when it interacts with other partners, but are yet to be demonstrated. In contrast, the PxxP motif-binding site of the SH3 domain of PSD-95 is unobstructed (312, 437), consistent with the observation that the SH3 domain of MAGUKs can associate directly with PxxP motif-containing proteins (177, 306).
The HOOK region is variable in length and amino acid sequence among the Cavβ subfamilies (Fig. 3). In the crystal structures, a large portion of the HOOK is unresolved due to poor electron density, indicating that it has a high degree of flexibility (84, 341, 447). As will be discussed below, the HOOK region plays an important role in regulating channel inactivation.
Fig. 3.
Amino acid sequence alignment of Cavβ subtypes. The four included subtypes are β1b (GenBank accession number, NP-000714), β2a (M80545), β3 (M88751), and β4a (L02315). Light blue indicates the NH2 terminus, gold the SH3 domain, purple the HOOK region, green the GK domain, and gray the COOH terminus. Secondary structure elements are indicated in the top line as arrows for β sheets and solid lines for α helices (based on the crystal structure of β3). Residues involved in interactions with the AID are marked in red.
C. The NH2 Terminus
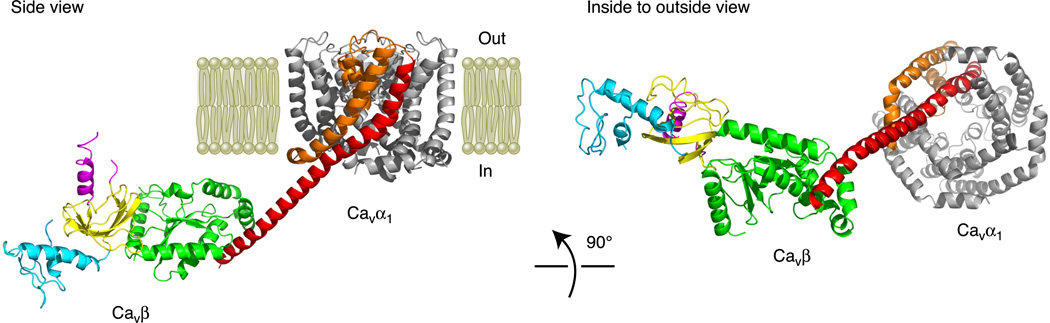
The NH2 and COOH termini of Cavβ (abbreviated as Cavβ-NT and Cavβ-CT) are highly variable in length and amino acid composition (Fig. 3). There is yet no structure available for Cavβ-CT. However, an NMR structure of the NH2 terminus of β4 was solved recently, revealing a fold consisting of two α-helices and two antiparallel β sheets (451). This structure also shows that, unlike previously thought (203), Cavβ-NT does not have a PDZ fold, which consists of five β sheets (380). Incidentally, one of the two α-helices in the NMR structure is equivalent to the very first α-helix in the Cavβ core structures. Superposition of this helix in the two structures reveals that the NH2 terminus is oriented away from the core (Fig. 4).
Fig. 4.
Structural model of a partial Cavα1/Cavβ complex on the plasma membrane. A side view and an inside-to-outside view are presented. The partial structure of Cavα1 includes only the S5, P-loop, and S6 segments and is based on a Cavα1 homology model developed in Stary et al. (411). IS5 is colored orange, and IS6 is red. The IS6-AID linker from Cav1.2 is modeled as an α-helix and is joined with IS6 at its NH2 terminus and the AID at its COOH terminus. The structure of Cavβ is based on the crystal structure of the β4 core region (84) and the NMR structure of the β4 NH2 terminus (451); there is no Cavβ COOH terminus. Since the structure of the β4-AID complex is not available, we docked the AID to β4 based on the crystal structure of the β3 core-AID complex (84). The regions of Cavβ are color coded as in Figures 2 and 3 (NH2 terminus in light blue, SH3 in gold, HOOK in purple, and GK in green).
D. The SH3-GK Intramolecular Interaction
The crystal structures of the Cavβ core show that the SH3 and GK domains interact intramolecularly (84, 341, 447). The affinity of this interaction is unknown, but the interaction is strong enough such that hemi-Cavβ fragments containing the NT-SH3βstrand 1–4-HOOK module and the SH3βstrand 5-GK-CT module can associate biochemically in vitro and reconstitute the functionality of full-length Cavβs when they are coexpressed in cells (298, 313,342, 431, 432, 447). In fact, one of the β2a structures was obtained from cocrystals of two β2a hemifragments truncated at the HOOK region (447).
The last β sheet of Cavβ-SH3 (SH3βstrand 5), which is separated from the rest of the SH3 domain by the HOOK region, is critical for the strong intramolecular SH3-GK interaction (83, 298,313, 342, 431, 432). This β sheet is directly connected to the GK domain, and it interacts extensively with both the GK domain and the rest of the SH3 domain (83). As a result, SH3βstrand5 glues the NT-SH3βstrand1–4-HOOK module and the SH3βstrand5-GK-CT module together and strengthens the otherwise weak interactions at the SH3-GK interface (83).
As in MAGUKs, the SH3-GK intramolecular interaction is important for the function of Cavβ (83, 298,313, 431, 432). Weakening this interaction by mutating the SH3-GK interface or by inserting flexible linkers between the SH3 domain and the GK domain severely compromises the gating effects of Cavβ (83). Thus mutations, modifications, or protein-protein interactions that alter the SH3-GK intramolecular interaction may produce significant functional consequences.
E. The AID-Cavβ Interaction
Which regions anchor Cavβ to Cavα1? By screening an epitope library of 20,000 Cav1.1 fragments, Pragnell et al. (361) identified a region in the I–II loop that binds β1b. This region, known as the AID, is comprised of 18 residues, with a conserved consensus motif (QQxExxLxGYxxWIxxxE) in all Cav1 and Cav2 α1 subunits (Fig. 1E). The AID binds to all four Cavβs (121). The affinity of the AID-Cavβ interaction ranges from 2 to 54 nM, depending on the AID/Cavβ or Cavα1/Cavβ pair and the method of affinity measurement (30, 56,62, 120, 121, 179, 342, 371, 395, 448). Single mutations of several conserved residues in the AID, including Y10, W13, and I14, greatly weaken the AID-Cavβ interaction, as indicated by in vitro binding experiments and by the reduction or abolishment of Cavβ-induced stimulation of Ca2+ channel current in heterologous expression systems (33, 34,56, 120, 181, 185, 206, 218, 276, 361, 448). Thus the role of the AID as the principal interacting domain with Cavβ is firmly established.
Which region(s) of Cavβ interact with the AID? In an influential study, De Waard et al. (119) described a 31-amino acid segment of Cavβ, referred to as the β-interacting domain or BID, as the main binding site for the AID. The BID was able to slightly enhance Ca2+ channel current and modulate gating (119), and several BID point mutations were able to weaken the Cavβ/Cavα1 interaction and reduce BID-stimulated Ca2+ channel currents (119, 120).
For the next decade, it had been generally accepted that Cavβ interacted with Cavα1 primarily through the BID. Surprisingly, however, the crystal structures of two different AID-Cavβ core complexes reveal that the AID does not bind the BID (84, 341, 447). Indeed, the AID and the BID do not come into direct contact (Fig. 2B). Instead, the AID binds to a hydrophobic groove in the GK domain termed the AID-binding pocket (ABP; Fig. 2C) (84, 447, 448). The AID occupies only a tiny fraction of the Cavβ surface area, raising the possibility that other domains of Cavβ are involved in interactions with other regions of Cavα1 or with other proteins. As will be discussed later, both are indeed the case.
The AID-GK domain interactions are extensive and predominantly hydrophobic (Fig. 2C). These interactions account for the 2–54 nM affinity of the AID-Cavβ binding. Functional studies show that mutating two or more key residues in the ABP severely weakens or completely abolishes the AID-Cavβ interaction (206, 502).
The binding of the AID with Cavβ does not significantly change the Cavβ structure, except for some small and localized changes near the ABP. Importantly, however, the AID undergoes a dramatic change in secondary structure when it is engulfed by the ABP. When alone, the AID exists as a random coil in solution, as determined by circular dichroism spectrum measurements (341). When bound to Cavβ, the AID forms a continuous α-helix, as shown in the crystal structures. Together with the observation that the 22-amino acid linker between the AID and the first S6 segment of Cavα1 (i.e., IS6) also forms an α-helix (9), a picture emerges that the entire region encompassing IS6 and the AID adopts a continuous α-helical structure in the presence of Cavβ (Fig. 4). This structural hallmark is crucial for the regulation of Ca2+ channel gating by Cavβ, as will be discussed later.
Since the publication of the Cavβ structures, some investigators have been continuing to perform or interpret experiments based on the notion that the BID interacts with Cavα1 (85, 281,302, 388, 441, 506), so before leaving this section, we briefly revisit the BID. The crystal structures show that the BID spans three different regions of Cavβ (SH3, HOOK and GK) and that most of it is completely buried (Fig. 2B). Thus the BID does not directly interact with Cavα1; rather, it is crucial for maintaining the SH3-GK intramolecular interaction and the structural integrity of Cavβ. Of the four residues in the BID whose mutations weakened the Cavβ/Cavα1 interaction, three were proline and one was tyrosine (119, 120). Mutating these residues most likely alters the folding and/or structure of Cavβ, which explains its inability to bind Cavα1.
But how could the BID enhance Ca2+ channel current (119)? While the mechanism of this action remains unclear, it reminds us of an experiment of our own in which a random 43-amino acid peptide (which has no sequence similarity in GenBank) was coexpressed with Cav2.1 and α2δ in Xenopus oocytes. This random peptide significantly increased Ca2+ channel currents (compared with no Cavβ), to ~50% of β3-induced current. This obvious nonspecific effect, reported in 2004 (84), suggests that the BID-induced current increase may also be a nonspecific effect. Given these structural and functional information, it is prudent to exercise caution when interpreting experimental data concerning the BID.
IV. Cavβ SPLICE VARIANTS AND THEIR TISSUE DISTRIBUTION
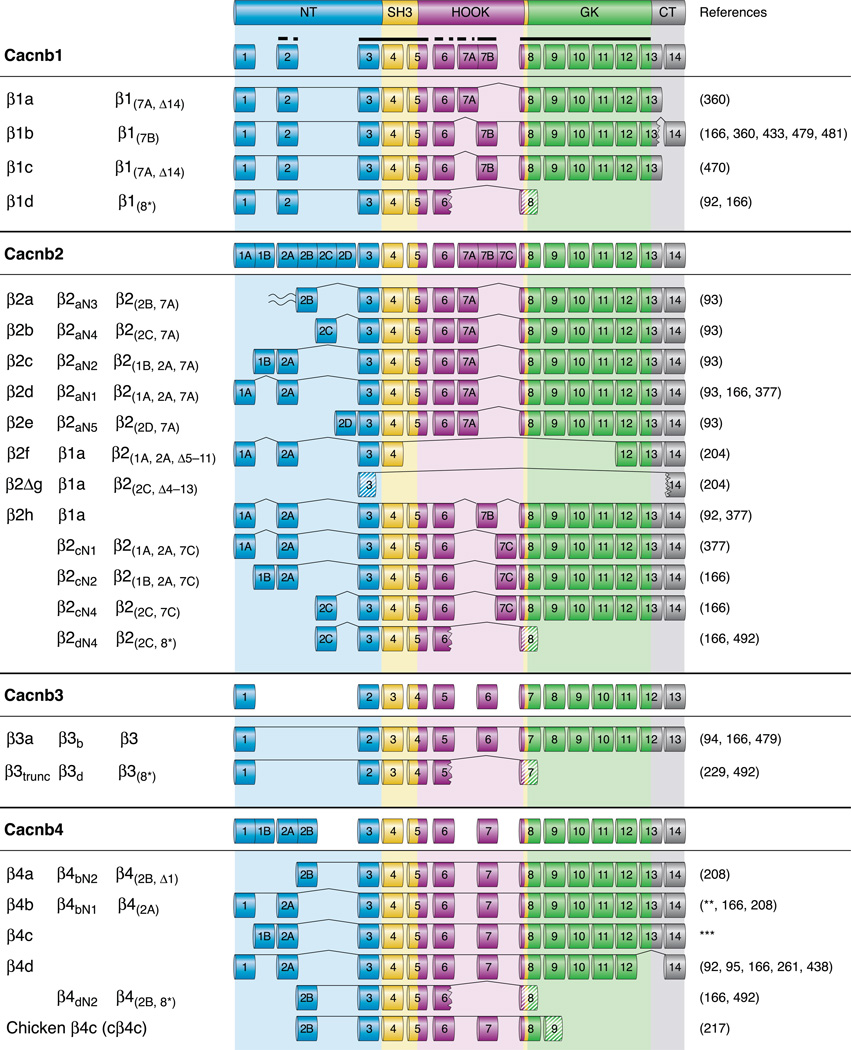
Mammalian Cavβs are encoded by four distinct genes, Cacnb 1–4. They all have 14 exons except Cacnb3, which has 13, and each Cavβ has 2 or more splice variants. Figures 5 and 6 show most of the human Cavβ splice variants found thus far. The five distinct domains and regions of Cavβ are mapped onto their corresponding exons and protein sequences. Alternative splicing occurs in those exons that encode the variable domains or regions, namely, the NH2 and COOH termini and the HOOK region. The four different Cacnb genes utilize different alternative splicing sites. Cacnb1 and Cacnb2, which produce β1 and β2, respectively, exhibit alternative splicing in exon 7, giving rise to divergent HOOK regions. Cacnb1 is also alternatively spliced in the COOH terminus, with exon 14 either included or excluded. On the other hand, Cacnb2 is alternatively spliced extensively in the NH2 terminus, yielding highly diversified NH2 termini. Cacnb4 has no alternative splicing in the HOOK region but has NH2- and possibly COOH-terminal alternative splicing. Cacnb3 has no alternatively spliced exons, but like all other Cacnb genes, produces a truncated isoform.
Fig. 5.
Human Cavβ splice variants. Fourteen Cavβ exons (13 for β3) are color-coded based on the regions they give rise to: the NH2 terminus (light blue), the SH3 domain (gold), the HOOK (purple), the GK domain (green), and the COOH terminus (gray). Exons are numbered, and some exons have additional letters to indicate alternatively spliced variants. The thick full and dashed lines at the very top indicate highly or somewhat conserved exons, respectively. Of the weakly conserved regions, similar exons are placed in the same column (e.g., β1 exon 2 is homologous to β2 exon 2A). Exons 13 and 14 of β1 were originally designated as 13a and 13b, respectively (222). The names of splice variants are, from left to right columns, those used in this article, those proposed by Foell et al. (166), and those proposed by Yang and Berggren (486). β2a is the only splice variant that can be palmitoylated (wave). The jagged edge (e.g., exon 6 of β1d) indicates missing amino acids resulting from exon skipping and/or frame-shifts. Striped exons (e.g., exon 8 of β1d) are translated with a frame shift; hence, their amino acid sequence is unrelated to the “conventional” sequence produced by that exon. **Direct submission by M. E. Williams, 1997. ***AK316045; direct submission by T. Isogai and J. Yamamoto, 2008.
Fig. 6.
Amino acid sequence alignment of Cavβ splice variants. The 5 Cavβ regions, their corresponding exons, and the exon boundaries are marked. Color coding follows the same scheme as in previous figures, with the NH2 terminus in light blue, the SH3 domain in gold, the HOOK in purple, the GK domain in green, and the COOH terminus in gray. Exon numbers are indicated in the color bar, and some exons have additional letters to indicate alternatively spliced variants. Arrows and bold amino acids mark exon boundaries. A single bold residue indicates that exon splicing occurs within its codon, whereas two bold residues indicate that splicing occurs between their codons. Shaded in black are missense sequences resulting from a frame-shift. # Indicates a premature stop codon. The GenBank accession number of each sequence is indicated at the end of the sequence, except for two sequences where the original reference is given. All sequences are from human except Cβ4c, which is a chicken isoform. In regions where alternative splicing occurs (e.g., the NH2 terminus of β2), the amino acid sequence is aligned with its parent exon; thus the alignment in these regions does not necessarily indicate amino acid sequence similarity.
Table 1 shows the tissue distribution of some Cavβ splice variants. As expected, Cavβs are abundantly expressed in excitable tissues such as the brain, heart, and muscles. While some splice variants (e.g., β1b and β2b) are widely expressed, others (e.g., β1a, β2d, and β2e) have a more restricted expression. The expression of some splice variants is developmentally regulated. For example, β1b and β4 expression increases with development, whereas β2c, β2d, and β2e expression decreases with development. It is important to note that, in most cases, protein but not mRNA expression was listed. Immunolocalization experiments should be interpreted cautiously since an antibody may recognize several splice variants, for example, an antibody against the β2 COOH terminus will recognize all β2 splice variants.
TABLE 1.
Tissue distribution of CaVβ
| CaVβ | Tissue Distribution | Reference Nos. |
|---|---|---|
| β1 | Expressed in brain (cerebral cortex, habenula, hippocampus, and olfactory bulb), heart, skeletal muscle, spleen, and T cells, but not in kidney, liver, or stomach. | 360, 419, 435 |
| β1a | Expressed only in skeletal muscle (but see Ref. 89). Exclusive partner of CaV1.1 and irreplaceable for excitation-contraction coupling. | 360, 381, 395, 472 |
| β1b | Expressed in brain (cerebellum and cerebral cortex), nerve endings at the NMJ, and pancreas. Expression is detected at P0 in rat brains and increases from P7 to adulthood by ~3-fold. | 311, 344, 353, 355, 360, 395, 456, 472 |
| β1c | Expressed in brain and spleen, but not in kidney, liver, muscle, or stomach. | 360 |
| β1d | Expressed in heart. | 92 |
| β2 | Expressed in brain (hippocampus–becoming the most abundant isoform there, cerebellum, pontine nucleus, susbtantia nigra, central grey, habenula, pineal gland, thalamic nuclei, cerebrum), heart, lung, nerve endings at the NMJ, T cells, and osteoblasts, but not in kidney, liver, pancreas, or spleen. Brain expression is constant during development, but see hippocampus data (391). | 121, 294, 311, 344, 353, 355, 391, 398, 419, 435, 456 |
| β2a | Expressed in brain, heart, and aorta; its heart and brain levels seem lower than other β subunits and isoforms. | 215, 229, 230 |
| β2b | Expressed in brain, heart, and aorta. It is the most abundant CaVβ in human heart. | 89, 215, 230 |
| β2c | Expressed in brain and heart, where it is the second most abundant CaVβ. Its expression declines in adults. | 89, 215, 230 |
| β2d,e | Expressed in heart. β2e expression is robust only in young animals. | 89, 215 |
| β3 | Expressed mostly in brain (cerebellum, cerebral cortex, habenula, hippocampus, olfactory bulb, and striatum), but also in heart, aorta, kidney, lung, skeletal muscle, smooth muscle, spleen, thalamus, T cells, and trachea, but not in liver, pancreas, or testis. Expression remains constant in the brain and heart during development. It is the most predominant partner of CaV2.2 (N-type) channels in the brain, and it pairs with ~40% of brain L-type channels. | 58, 68, 89, 230, 294, 310, 329, 344, 355, 395, 419, 435, 449, 456, 472, 473 |
| β3trunc | Expressed in brain, heart, and aorta. | 230 |
| β4 | Expressed in brain (cerebellum–the most abundant Cavβ there, brain stem, cerebral cortex, dentate gyrus, habenula, hippocampus, olfactory bulb, striatum, thalamus, and hypothalamus), kidney, nerve endings at the NMJ, ovary, skeletal muscle, spinal cord, T cells, and testis, but not detected in heart (except in young animals, Ref. 89), liver, lung, spleen, or thymus. The expression increases in rat brain by 10-fold from P0 to adult. It is the most prevalent partner of CaV2.1 (P/Q-type) channels in brain, and, like β3, it pairs with ~40% of brain L-type channels. | 55, 67, 294, 311, 355, 391, 395, 419, 435, 449, 456, 472 |
| β4a | Expressed in spinal cord and cerebellum. | 208, 209, 452 |
| β4b | Expressed in spinal cord and forebrain. | 209 |
NMJ, neuromuscular junction.
Since the association between Cavβ and Cavα1 is promiscuous (i.e., any full-length Cavβ can associate with any Cav1 or Cav2 α1 subunit), alternative splicing greatly increases the molecular diversity and functionality of HVA Ca2+ channels. Furthermore, some splice variants may take on functions other than regulating HVA Ca2+ channels (see sect. XII). Thus a major future challenge (and a fruitful area of research) is to determine how alternative splicing is regulated in various tissues and at different developmental stages.
V. Cavβ REGULATES THE SURFACE EXPRESSION OF HIGH-VOLTAGE ACTIVATED Ca2+ CHANNELS
The α1 subunit of Cav1 and Cav2 channels cannot reach the membrane by itself; it shows no surface expression and produces very small or no currents when expressed without auxiliary subunits. Coexpression of Cavβ with Cavα1 increases currents by orders of magnitude, depending on factors such as the expression system, DNA or RNA concentration, VGCC turnover rate, inhibitory factors present, the α1/β combination, etc. (reviewed in Refs. 10, 40, 125, 237, 495). The current increase reflects enhanced channel expression on the plasma membrane and also an increase in channel open probability. In this section we discuss the evidence and mechanisms of increased channel surface expression.
A. Cavβ Is Required for Normal Channel Expression
It has been well established that Cavβ can function as a chaperone to dramatically increase the surface expression of Cav1 and Cav2 channels. This is observed in various heterologous expression systems with all four subfamilies of Cavβ and all Cav1 and Cav2 subunits (14, 50,88, 93, 191, 245, 247, 248, 276, 322, 353, 458, 481, 495). The increased surface expression can be detected by Cavα1 epitope tag staining, surface biotinylation, gating charge measurements, or increased Ca2+ channel current. An important point to mention is that Xenopus oocytes, a widely used expression system for studies of VGCCs, have two endogenous β subunits that share 98% homology with β3 (436). These endogenous subunits are expressed at sufficient levels to transport a small number of exogenously expressed Cavα1 to the plasma membrane and hence lead to small Ca2+ channel currents in the absence of an exogenous Cavβ. Antisense oligonucleotides against endogenous β3 are able to suppress these currents (62, 436). Little or no endogenous Cavβ was detected in widely used mammalian cell lines such as HEK 293 cells, COS cells, and CHO cells (276, 315). Nevertheless, expression of Cavα1 alone in these cells can produce measureable, albeit miniscule, Ca2+ channel currents (245, 247,248, 303, 407, 418, 436), suggesting that either a very small fraction of Cavα1 can be trafficked to the plasma membrane in the absence of Cavβ or these cells contain low levels of endogenous Cavβs.
Cavβ also enhances Ca2+ channel surface expression in vivo. For example, β1 and β2 knockout mice have severely reduced Ca2+ currents in muscle and heart (see sect. XIII). Knockdown of Cavβ also decreases endogenous Ca2+ currents in neuronal cells (35, 279). Conversely, overexpression of Cavβ using adenoviruses increases Ca2+ channel current density in native cardiac cells, suggesting that Ca2+ channel surface expression may be limited by the availability of Cavβ (336, 465).
Binding of Cavβ to the AID of Cav1 and Cav2 is essential for its chaperone effect. Point mutations in the AID that weaken or abolish the AID-Cavβ interaction severely reduce or abolish Cavβ-stimulated Ca2+ channel current (33, 34,56, 120, 181, 185, 206, 218, 276, 336, 361, 448). Deleting the AID altogether, not surprisingly, abolishes Cavβ-induced current enhancement (185, 298). Likewise, mutations in the ABP that weaken or abolish the AID-Cavβ interaction also reduce or abolish Cavβ-stimulated Ca2+ channel expression and current (206, 502). Recent studies show that the GK domain itself can largely recapitulate the chaperone function of full-length Cavβs, greatly increasing Ca2+ channel surface expression and current in Xenopus oocytes and mammalian cells (129, 206).
How does Cavβ enhance Ca2+ channel surface expression? One hypothesis is that Cavβ shields or disrupts one or more ER retention signals on the I–II loop of Cavα1 (39), and several lines of evidence support this hypothesis. The I–II loop of Cav1.2 and Cav2 can trap α1 subunits in the ER (except Cav1.1), but the I–II loop of Cav3.1 (a T channel) fails to do so. Also, tagging a Shaker K+ channel with the I–II loop of Cav1.2 or Cav2.1 decreases its expression by approximately sevenfold, while coexpression of Cavβ prevents this downregulation (39). Moreover, deleting the I–II loop from Cav1.2 (Δ389–423) increases its surface expression in the absence of Cavβ (39).
However, some results are inconsistent with this hypothesis. 1) The I–II loop of Cav1.1 does not cause ER retention of a CD8 peptide (99). 2) CD4 fusion constructs of the I–II loop of Cav1.2 and Cav2.2 are trafficked efficiently to the plasma membrane, rather than being retained in the ER (5). 3) Transplanting the I–II loop of Cav2.2 into Cav3.1 causes Cavβ-independent current upregulation instead of downregulation (9).
An alternative possibility is that additional trafficking signals exist in the NH2 and COOH termini of Cavα1 (99, 163,175, 262, 466). However, the NH2 and COOH termini of Cav1 and Cav2 are not conserved, and yet, the chaperone function of Cavβ is universal, suggesting that any ER retention signals in the NH2 and COOH termini may only be modulatory.
Recently, a new study suggested that Cavβ increases Cavα1 expression on the plasma membrane by preventing its ubiquitination and proteasomal degradation (5). Thus Cavβ may simply be required to help Cavα1 escape the degradation pathway.
B. Membrane Association and Subcellular Targeting of Cavβ
Cavβs are expected to have a cytosolic localization based on analyses of their amino acid sequence (353, 381). This is true for the majority of Cavβ splice variants when they are expressed alone, without a Cavα1 (with a few exceptions discussed below; Refs. 176, 181). However, some Cavβs, most notably β2a, can be localized to the plasma membrane on their own. β2a is linked to the plasma membrane through palmitoyl groups that are covalently attached to two cysteines (Cys 3, 4) in the NH2 terminus (86, 87). When palmitoylation is abolished, in a double Cys→Ser mutant, membrane localization disappears (87). Importantly, β2a palmitoylation can be dynamically regulated in vivo, adding a layer of physiological control (232, 464). However, palmitoylation alone may not be sufficient for membrane localization because implanting the β2a NH2 terminus into other Cavβs does not yield membrane localization (87, but see Ref. 369). Thus β2a probably possesses additional determinants that help target it to the plasma membrane. Another β2 subunit, β2e, is not palmitoylated but is found at the plasma membrane (433). The underlying mechanism is yet unknown. Finally, β1b is localized to the plasma membrane in COS-7 cells (44, 50), but this is not observed in tsA201 cells (87) or primary cardiomyocytes (93). The reason for the discrepancy is unclear, but in COS-7 cells, the membrane localization is attributed to a COOH-terminal acidic motif (WEEEEDYEEE) whose deletion diminishes membrane localization. When this motif is fused to β3, which is normally cytosolic, it migrates to the plasma membrane (44, 50). As will be discussed in section VI, membrane localization of Cavβ coincides with many functional effects, especially slowed inactivation.
In the presence of Cavα1, all Cavβs localize to the plasma membrane through their association with Cavα1; however, they may be targeted to different subcellular locations depending on which Cavα1 they associate with. For example, β3 and β4, which predominantly associate with presynaptic Cav2 channels, can be found in axons, whereas β1 is scarce in this compartment (336); instead, β1 is found in postsynaptic compartments (soma and dendrites). In skeletal muscle, β1a is targeted to the triads through its association with Cav1.1 (333). When exogenously expressed in epithelial cells, β1b is localized on the apical membrane with Cav2.1 but on the basolateral membrane with Cav1.2 (44). Conversely, Cavβ may affect the subcellular localization of Cavα1. For example, β1a helps arrange L-type Ca2+ channels as tetrads in the t tubules of skeletal muscles (see sect. XIA; Refs. 164, 191, 394, 507), and β4 is implicated in the synaptic localization of P/Q-type channels in cultured hippocampal neurons (474). Furthermore, through interactions with different proteins, Cavβ helps attach Ca2+ channels to synaptic vesicles (257), the cytoskeleton (223), or the surface of sarcoplasmic reticulum (333). These examples illustrate the role of Cavβ as a scaffold protein.
VI. Cavβ REGULATION OF Ca2+ CHANNEL GATING
Once the Ca2+ channel complex reaches the plasma membrane, Cavβ powerfully modulates its gating. The main features of gating modulation are the enhancement of voltage-dependent activation (VDA) and voltage-dependent inactivation (VDI). β2a is unique in that it inhibits VDI. This section describes these Cavβ effects and their mechanisms.
A. Cavβ Enhances Voltage-Dependent Activation
All Cavβs shift the voltage dependence of activation to more hyperpolarized voltages (by ~10–15 mV, Table 2 and Fig. 7). This was shown for both Cav1 and Cav2 channels in various expression systems (61, 116,229, 245, 268, 322, 406, 412, 414, 443, 495). The shift can also be observed in vivo in some knockout mice (191, 325, 468), while in some other cases, it is probably obscured by the compensatory effects of other Cavβ genes (31, 330, 331). In addition, the speed of activation is increased in general (268, 412), but it could appear slower depending on the stimulus voltage (353) and the particular α1/β pair (184, 245,443, 459).
TABLE 2.
Effect of CaVβ on HVA Ca2+ channel gating properties
| CaVβ | Kinetics of Activation | Voltage Dependence of Activation |
Kinetics of Inactivation | Voltage Dependence of Inactivation |
|---|---|---|---|---|
| β1 | Accelerates activation by 2- to 100-fold; acceleration is weaker for CaV2 compared with Cav1 channels (40, 119, 206, 245, 268, 406, 412, 443, 450, 467). | Hyperpolarizing shift of −10 to −15 mV (40, 44, 92, 206, 245, 417, 450, 467). | Accelerates inactivation by 2- to 10-fold (40, 206, 218, 276, 340, 406, 417, 443, 450). α2δ may be required in some instances. | Hyperpolarizing shift of −5 to −30 mV (40, 44, 119, 206, 218, 245, 276, 314, 340, 406, 443). α2δ or γ may promote larger shifts. |
| β2a | Accelerates activation by 2- to 4-fold; acceleration is less evident with CaV2 channels (40, 119, 206, 230, 245, 353). | Hyperpolarizing shift of −5 to −20 mV (40, 206, 230, 245, 276, 340, 353, 417). | Slows inactivation by ~10-fold (except for the unaffected CaV1.4 channels) (40, 58, 206, 218, 265, 276, 340, 369). | Depolarizing shift of 10 to 40 mV with CaV2 channels; much less or no effect on L-type channels (40, 119, 132, 206, 218, 245, 276, 314, 340, 369). |
| Other β2 | Accelerate activation kinetics ~2- to 4-fold (110, 215, 230, 433) | Hyperpolarizing shift of −7 to −20 mV (40, 110, 215, 230, 340, 433). | Inactivation is accelerated except with β2e (58, 215, 340). | Hyperpolarizing shift of approximately −10 mV except β2e (215, 340, 433). |
| β3 | Accelerates activation by 0 to 3.5-fold; acceleration is less evident with CaV2 channels (68, 119, 206, 230, 245). | Hyperpolarizing shift of −6 to −15 mV (44, 68, 206, 245, 276, 386, 417, 477). | Accelerates inactivation by 2- to 7-fold (68, 206, 265, 329). | Hyperpolarizing shift of −5 to −30 mV; α2δ may lessen the shift (44, 68, 119, 206, 245, 276, 386). |
| β4 | Accelerates activation by ~2- to 3-fold; acceleration is less evident with CaV2 channels (67, 119, 206, 208, 209, 245). | Hyperpolarizing shift of −5 to −25 mV (67, 159, 179, 206, 208, 245, 386, 452). For differences between splice variants, see Refs. 208, 209. | Accelerates inactivation by 2- to 4-fold (67, 206, 208), but see (417). | Hyperpolarizing shift of −5 to −30 mV; less evident for L-type channels (67, 119, 179, 206, 245, 386). For some differences between splice variants, see Refs. 208, 209. |
Reference numbers are given in parentheses.
Fig. 7.
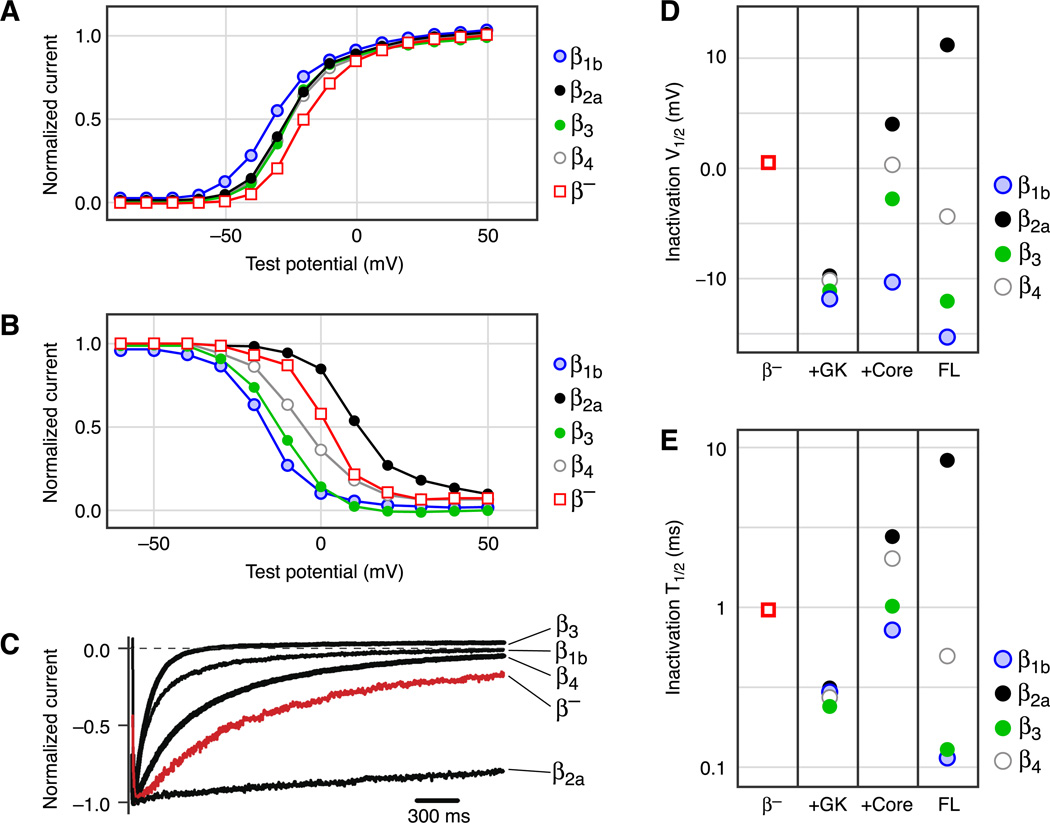
Modulation of Ca2+ channel gating by Cavβ. A: voltage dependence of activation of P/Q-type Ca2+ channels containing β1b, β2a, β3, or β4 or no β (β−). In this and all other panels, currents were recorded in cell-attached macropatches from oocytes expressing Cav2.1 and α2δ, without or with the indicated β subunit. B: voltage dependence of inactivation. C: representative current traces evoked by a depolarization to ~30 mV, showing the kinetics of voltage-dependent inactivation. Currents are shown only from the first 2.5 s of a 25-s pulse. D and E: comparison of V1/2 and t1/2 of voltage-dependent inactivation of P/Q-type Ca2+ channels containing no β (β−) or the indicated β module: the GK domain, β core (SH3-HOOK-GK), or full-length (FL) β. V1/2 is the membrane voltage at the midpoint of voltage-dependent inactivation, and t1/2 is the time for the current to inactivate to 50% of the peak value in C. Note the logarithmic scale of the y-axis in E. [All data from He et al. (206).]
These effects are also visible at the single-channel level. Thus channels without a Cavβ tend to open less frequently, open for a shorter duration, and require more positive activation voltages. Cavβ coexpression greatly increases channel open probability (Po) and shortens the latency to first opening (93, 125,215, 229, 295, 457). Notably, β2a produces the most dramatic increase in Po (76, 93, 132).
Normal VDA is largely reconstituted by the core region of Cavβ (206). Deleting the entire Cavβ COOH terminus has no effect on VDA, at least for Cav2.1 channels expressed in Xenopus oocytes (206). The NH2 terminus, however, appears to have a small role in modulating VDA. For example, β4b, which has a longer NH2 terminus compared with β4a, induces a larger hyperpolarizing shift in the activation of some Cavα1 (208).
B. Cavβ Promotes Voltage-Dependent Inactivation, Except β2a
VDI reduces the amount of Ca2+ entering the cell following depolarization and decreases the number of channels responsive to subsequent depolarizations. Cavβ is a key modulator of VDI, as first demonstrated in 1991 (268, 406, 450) and subsequently confirmed for various α1/β combinations in different expression systems (116, 137,245, 340, 348, 414, 417, 458). Several aspects of VDI are affected by Cavβ. 1) β1, most β2 splice variants, β3, and β4 shift the voltage dependence of inactivation to more hyperpolarized voltages (by ~10–20 mV; Table 2 and Fig. 7), whereby weaker depolarizations are able to inactivate the channels. β2a, however, causes a shift to more depolarized voltages (by ~10 mV) (40, 93,119, 132, 206, 218, 245, 276, 314, 340, 369). 2) Cavβs (except β2a) promote the process of “closed state” inactivation exhibited by Cav2 channels when they rapidly transition between closed and open states, such as during a train of action potentials (β3 > β1b = β4 >> β2a; Refs. 348, 491). Similarly, a large hyperpolarization of steady-state inactivation (approximately ~40 mV) is observed when β3 is overexpressed with N- and R-type channels, dramatically increasing the population of inactivated channels at resting conditions (491). 3) β1, most β2 splice variants, β3, and β4 speed up the inactivation kinetics, whereas β2a and β2e slow down inactivation (Table 2 and Fig. 7).
The unique effects of β2a on VDI are largely abolished when palmitoylation of β2a is disrupted by mutating its two NH2-terminal cysteine residues to serine (β2a C3,4S) (365, 369). WT β2a-like properties can be restored when a transmembrane segment of an unrelated membrane protein is fused to this mutant, suggesting that membrane anchorage rather than palmitoylation per se is critical for β2a’s unique functions (369). Supporting this idea, the nonpalmitoylated but membrane-attached β2e has properties similar to β2a (433).
Multiple domains and regions of Cavβ are involved in the regulation of VDI. The GK domain alone, when expressed together with Cav2.1 and α2δ in Xenopus oocytes, has been shown to speed up VDI and hyperpolarize the voltage dependence of VDI (206). The GK domain of all four subfamilies of Cavβ produces the same effects (Fig. 7, D and E; Ref. 206), as expected from its high degree of amino acid conservation. Similarly, the GK domain of β2a greatly accelerates VDI and hyperpolarizes the voltage dependence of VDI of Cav2.2 channels expressed in oocytes and tsA-201 cells (129, 372). On the other hand, it has been reported that refolded and purified proteins of β2a and β1b GK domains slow down VDI and depolarize the voltage dependence of Cav2.3 channels expressed in oocytes (187). The discrepancy between these studies may result from the use of different Cavα1 or from RNA versus protein injection, but it should be noted that the refolded and purified GK domains appear to be dimerized proteins (187), and it is unknown whether and how dimerization changes the function of the GK domain.
The HOOK plays an important role in regulating VDI, as first suggested by chimeric studies between different Cavβs (364, 420). Two recent studies based on structurally defined Cavβ domains provide more definitive evidence. 1) Swapping the HOOK between the core regions (SH3-HOOK-GK) of β1b and β2a, which have opposite effects on VDI, also swaps their effects on VDI (206). 2) Deleting the HOOK in either β2a core or full-length β2a results in increased VDI (372). These studies, in conjunction with those discussed earlier, indicate that both membrane attachment through palmitoylation and a long HOOK region contribute to the unique effects of β2a on VDI.
The role of the NH2 terminus of Cavβ in regulating VDI has long been established. Deleting or shortening the NH2 terminus, or swapping the NH2 terminus of different Cavβs markedly alters VDI (236, 340,364, 420). β2 or β4 splice variants differing in the NH2 terminus exhibit markedly different VDI (208, 209,215, 433). As discussed above, the palmitoylation site of β2a is in the NH2 terminus.
Surprisingly, the COOH terminus of Cavβ seems to play a very limited or no role in regulating VDI, even though it is highly variable among the four Cavβ subfamilies. Thus, although a very small change in the inactivation kinetics of Cav2.1 channels is observed when the COOH terminus of β4 is deleted (460), exchanging the COOH terminus between β3 and β4 or deleting the entire COOH terminus of any of the four Cavβs has little effect on VDI of Cav2.2 channels or Cav2.1 channels (206, 420). It remains to be determined whether the COOH terminus exerts a more prominent effect on VDI under other conditions and for certain combinations of Cavα1 and Cavβ. Intriguingly, a β4 COOH-terminal truncation mutant missing the last 38 amino acids, which causes slightly faster inactivation of Cav2.1 channels at moderate depolarizations, was identified in a juvenile myoclonic epilepsy patient (142). Whether the very subtle change in Ca2+ channel inactivation underlies the disease is unclear.
C. A Unified Model for Cavβ Regulation of Ca2+ Channel Gating
How does Cavβ regulate VDA and VDI of Cav1 and Cav2 channels? Before addressing this question, we first briefly discuss the pore structure, the location of the activation gate, and the mechanism of VDI of VGCCs.
The external pore, including the ion selectivity filter, of VGCCs is formed by the pore loop between the S5 and S6 transmembrane segments of each of the four homologous repeats of Cavα1; point mutations in this region, especially of the four conserved glutamate or aspartate residues, drastically alter ion selectivity, permeation, and pore blockage (256, 266,389, 482). The inner pore is formed by all four S6 segments of Cavα1, as demonstrated by the substituted cystine accessibility method (505). Cystine accessibility studies also indicate that the activation gate is located at the cytoplasmic end of the S6 segments (476). The S6 segments, together with the I–II loop and the NH2 and COOH termini of Cavα1, are involved in controlling or regulating VDI (for review, see Refs. 212, 422). Although the precise molecular mechanism of VDI is unknown, a prevalent model is that the I–II loop of Cavα1 functions as a “hinged lid” to physically occlude the pore by binding to the cytoplasmic ends of the S6 segments (421, 422), reminiscent of VDI of voltage-gated Na+ channels (71). Which amino acids form the inactivation gate and its receptor site remain unknown. An alternative model is that VDI is produced by a constriction of the pore (151). Either way, the S6 segments constitute a converging point through which both VDA and VDI are controlled and regulated.
The biochemical, functional, and structural studies presented above support a unified model for Cavβ regulation of VDA and VDI of VGCCs (9, 125,151, 206, 298, 341, 448, 455, 461). This model has two central components.
First, the high-affinity AID-GK domain interaction and a rigid IS6-AID linker are essential for Cavβ regulation of VGCC gating. As mentioned in section IIIE, in the presence of Cavβ, through the AID-GK domain interaction, the entire region encompassing the IS6 segment and the end of the AID becomes a continuous α-helix (9, 151, 341). Via this rigid structure, Cavβ gains a lever with which to regulate both activation and inactivation (Fig. 4). Thus Cavβ binding adds mass and tension to IS6 and the I–II loop, which most likely affects the energetics of voltage-dependent movement of both IS6 and the inactivation gate, thereby directly changing the voltage dependence and kinetics of activation and inactivation. This explains why the GK domain alone is capable of affecting both activation and inactivation (129, 206, 372). Equally important, the AID-GK domain interaction anchors Cavβ to Cavα1, thereby enabling interactions between Cavβ and other parts of Cavα1 that are of intrinsic low affinity but are important for Cavβ’s gating effects (see below). Supporting an essential role of the AID-GK domain interaction, many studies show that Cavβ regulation of gating is abolished by mutations in the AID (33, 34,56, 120, 181, 185, 206, 218, 276, 361, 448) or in the ABP (206, 502). However, one difficulty in interpreting these and similar experiments is that those mutations dramatically reduce or abolish Cavβ-stimulated Ca2+ channel surface expression, leaving minuscule currents to be scrutinized. This problem is circumvented in several recent studies where the rigid α-helical structure of the IS6-AID linker was disrupted by substituting linker residues with glycines, or inserting multiple glycines in the linker, while leaving the AID-GK domain interaction intact. These substitutions or insertions do not affect Cavβ-enhanced Ca2+ channel surface expression, but they severely compromise or eliminate the ability of Cavβ to regulate Cav1 and Cav2 channel activation and inactivation (151, 455, 502). These results underscore the essential role of a rigid IS6-AID linker in Cavβ regulation of VGCC gating.
An additional factor that is important for Cavβ regulation of gating is the orientation of Cavβ relative to Cavα1 (455, 502). Inserting five alanine residues in the IS6-AID linker, which is expected to maintain the α-helical structure of the linker but induce a 180° rotation of Cavβ with respect to Cavα1, markedly diminishes Cavβ regulation of activation, while insertion of seven alanines, which produces two full turns, has no significant detrimental effect (502). Similarly, deleting one or three residues in the IS6-AID linker totally abolishes Cavβ regulation of both activation and inactivation (455). These studies are consistent with the notion that additional contacts between Cavβ and Cavα1 besides the AID-GK domain interaction are critical for Cavβ regulation of VGCC gating.
Second, intrinsically low-affinity interactions between Cavβ and Cavα1 are crucial for Cavβ regulation of VGCC gating (especially VDI), and these interactions confer each Cavβ its distinct modulatory effect and α1/β pair-specific gating properties. Besides the AID-GK domain interaction, other direct contacts between Cavβ and Cavα1 have been observed in vitro. For example, the Cavβ SH3 domain interacts with the I–II loop, but at a region different from the AID (298), and a COOH-terminal region conserved only in β2 binds to a COOH-terminal region of Cav1.2 where calmodulin (CaM) also binds (270). The same Cav1.2 COOH-terminal region also binds to a β2a construct containing the N-SH3βstrand1–4-HOOK module (501). Other regions of Cavα1, including the NH2 and COOH termini and the III–IV loop, have also been shown to interact directly with Cavβ (366, 436,460, 461). It remains to be determined which regions of Cavβ they associate with, but the Cavβ NH2 terminus and HOOK are prime candidates since they are critically involved in regulating VDI. These additional α1/β interactions have intrinsic low affinity, and on their own, do not produce significant gating effects. However, the strength of these interactions increases dramatically when Cavβ is anchored to Cavα1 by the AID-GK domain interaction. These notions are supported by the aforementioned mutagenesis/insertion studies in the AID, the ABP, and the IS6-AID linker. Further supporting these ideas, Chen et al. (83) reported that, without changing the AID-GK domain interaction, splitting β2a into two connected modules (N-SH3-HOOK and GK-C) through the insertion of increasingly longer flexible linkers between the SH3 and GK domains leads to a gradual diminishment of the effect of the N-SH3-HOOK module on VDI (83). This result indicates that keeping the N-SH3-HOOK module near Cavα1 is essential for its modulatory effect. A future challenge is to develop ways to precisely map the interface of intrinsically low-affinity Cavα1/Cavβ interactions, which might be too weak to be identified biochemically and might require more than one Cavα1 region.
How low-affinity α1/β interactions regulate gating is unclear. These interactions could pull on Cavβ and thereby modulate the movement of IS6 and the presumed inactivation gate in the I–II loop. They may also interfere with intramolecular interactions between the I–II loop and other parts of Cavα1, such as the NH2 and COOH termini and the III–IV loop, where point mutations and deletions cause marked changes of VDI (for review, see Refs. 212, 422). These intramolecular interactions, as well as the low-affinity α1/β interactions, are α1 or α1/β pair specific (2, 99,178, 262, 369, 386, 404, 436, 460, 501). Thus, to fully appreciate the physiological importance of Cavβ regulation of VGCC gating, it is crucial to examine the pairing of Cavα1 and Cavβ in different tissues and cell types, in different subcellular locations, and at different developmental stages.
A final point that should be mentioned here is that many proteins that interact directly with Cavβ have been shown to regulate VGCC gating, such as RGK proteins (see sect. IX), Best1 (493), and RIM1 (257) (see sect. XI).
D. Can Cavβ Produce AID-Independent Gating Effects?
Several reports, which at first seemed to contradict the model presented above, are in fact in accord with the model upon closer examination. It has been shown that β2a is able to modulate VDA and VDI of Ca2+ channels formed by a mutant Cav2.1 subunit (Cav2.1_ΔAID) whose AID is deleted (298). This result led the authors to conclude that essential Cavβ modulatory properties are AID independent. This result, however, has an alternative explanation: β2a can be anchored to the plasma membrane through palmitoylation, and this membrane tethering might mimic, at least partially, the anchoring role of the AID-GK domain interaction, bringing β2a near Cav2.1_ΔAID subunits and promoting the functionally important low-affinity α1/β interactions alluded to above. Indeed, this result lends strong support to the second part of the model discussed above, i.e., there are low-affinity interactions between Cavβ and Cavα1 that are crucial for Cavβ regulation of VGCC gating.
Several studies reported that a 41-amino acid β2 COOH-terminal fragment and some Cavβ splice variants, including β2f, β2Δg, β1d, and chicken β4c, all of which lack most or the entire GK domain (and hence cannot bind the AID), are all able to enhance Ca2+ channel currents and/or regulate their gating (92, 204, 270). However, these effects are much weaker than those produced by full-length Cavβs. Moreover, the specificity of these effects is called into question by the clear nonspecific effects of two short peptides that do not exist in nature: a 35-amino acid peptide containing the BID and a 43-amino acid peptide with a random sequence, both of which are able to stimulate Ca2+ channel expression and weakly modulate gating (84, 119, 120). Nevertheless, given that β2f and β2Δg are found in native cells (204, 270), they could affect Ca2+ channel gating through low-affinity α1/β interactions if they are expressed at very high levels. At present, the physiological role of β2f and β2Δg remains unknown.
E. Cavβ Regulation of Ca2+-Dependent Inactivation and Facilitation
HVA Ca2+ channels are strongly regulated by another type of inactivation that depends on Ca2+ influx, namely, Ca2+-dependent inactivation (CDI) (for reviews, see Refs. 53, 78, 201, 358), which serves as a negative-feedback mechanism. CDI is mediated by the ubiquitous Ca2+-sensing protein CaM, which is constitutively bound to the Cavα1 COOH terminus (494, 509). The exact molecular mechanism of CDI is unclear, as is the relationship between CDI and VDI, but a recent study shows that two of the elements critical for VDI, Cavβ and a rigid IS6-AID linker, are also essential for CDI (151). Glycine (but not alanine) substitutions that disrupt the α-helix of the IS6-AID linker dramatically slow CDI. The absence of Cavβ binding to Cavα1, ensured by mutating the AID, produces similar results (151). Thus CDI and VDI appear to share a common mechanism by which conformational changes caused by CaM-Cavα1 interactions or Cavβ-Cavα1 interactions are transmitted to the pore through the rigid IS6-AID linker.
HVA Ca2+ channels also undergo Ca2+-dependent facilitation (CDF), which occurs during repetitive channel activation, such as during a train of action potentials (for reviews, see Refs. 53, 201, 358). This process, which is dependent on CaM binding to the Cavα1 COOH terminus, also requires Cavβ binding to the AID and an intact IS6-AID α-helix (151). Interestingly, CDF is readily observed with β2a but not with β1b or β4 (80, 272). The main reason for this difference is probably that channels with β2a inactivate much slower; slow inactivation not only allows the unmasking of CDF but also further stimulates CDF by permitting a larger Ca2+ influx.
F. Cavβ Regulation of Voltage-Dependent Facilitation
L-type Ca2+ channels exhibit voltage-dependent facilitation (VDF) (47). VDF is manifested as a gradual increase in L-type current during high-frequency action potentials, and it partly explains activity-dependent enhancement of L-type currents in skeletal muscle, brain, and heart. VDF can be differentiated from CDF by using Ba2+ as the charge carrier; it is accompanied by an increase in high Po gating (357) and may be dependent on phosphorylation (273). Like CDF, VDF depends on the presence of Cavβ (47, 75; but see Ref. 274); it is supported by β1 and β3 but not β2a (47, 75,365; but see Ref. 109). Some of the discrepancies in the literature may result from the following reasons. 1) Differences in L-type channel splice variants and the α2δ subunits used affect the results. For example, α2δ1 and α2δ3 seem to mask VDF by increasing inactivation (109). 2) The β2a-containing channels already have a high Po, so VDF is harder to observe in these channels. 3) Nonpalmitoylated β2a mutants can restore VDF (365), suggesting that different levels of palmitoylation may contribute some variations in the results.
The GK domain alone appears to be necessary and sufficient to confer VDF; deleting other domains, including the SH3 domain, separately or in combination, spares VDF (77). Hence, it is possible that VDF, just like CDF, CDI, VDI, and VDA, relies on the rigid IS6-AID linker and Cavβ to affect gating. It would be of interest to investigate whether glycine substitution or insertion in the IS6-AID linker also affects VDF.
VII. STOICHIOMETRY AND REVERSIBILITY OF THE Cavα1-Cavβ INTERACTION
How many β subunits need to bind to each Cavα1 to bring about the aforementioned trafficking and gating effects? Is the Cavα1-Cavβ interaction reversible? This section discusses these two important issues.
A. Cavα1 and Cavβ Are Paired With a 1:1 Stoichiometry
Early biochemical studies suggest that skeletal and neuronal VGCCs contain a single Cavα1 and a single Cavβ (430, 473). This remains the prevalent view today, but it comes after a brief competition with the idea of a 1 Cavα1:2 or more Cavβ stoichiometry (62, 436).
As mentioned in section VA, Xenopus oocytes express two endogenous β3-like subunits, called β3xo (436). When Cavα1 cRNA is injected into Xenopus oocytes alone, a small fraction of the Cavα1 is transported to the plasma membrane by β3xo (436). Coinjection of a mammalian Cavβ or either of the two Xenopus β subunits greatly increases Ca2+ channel current and changes its gating properties. These results led to the proposal that the “Cavα1-alone” channels in fact contained a β3xo and that one or more exogenous Cavβ bind the Cavα1/β3xo complex to form a higher order complex with modulated gating (436). Subsequently, by varying the concentration of coexpressed β3, it was found that β3 produced the trafficking effect with a sevenfold higher apparent affinity than it did gating modulation (17 vs. 120 nM) (62). This result was initially explained by one of two hypotheses: either two Cavβs bind a single Cavα1 or the mature Cavα1 on the plasma membrane has a lower affinity for Cavβ than the nascent Cavα1 does (62).
Subsequent extensive studies indicate that Cavβ associates with Cavα1 in a 1:1 stoichiometry and that this stoichiometry is determined by the AID-GK domain inter- action. 1) Channels coexpressed with a mixture of β2a and β3 form two biophysically distinct channel populations, rather than a single population of “mixed”-channel type (245). 2) Colecraft and colleagues (110) covalently linked a single β2b to the COOH terminus of Cav1.2 (creating Cav1.2-β2b) and found that the channels formed by Cav1.2-β2b exhibited the same gating properties as channels formed by the coexpression of Cav1.2 and β2b did. Moreover, coexpression of β2a and Cav1.2-β2b did not further change channel gating. 3) The crystal structures of the AID-Cavβ core complexes clearly show that each Cavβ binds a single AID (84, 341, 447). 4) Mutations of key residues in the AID or the ABP abolish both Cavβ-mediated Ca2+ channel surface expression and gating modulation (33, 34,56, 120, 181, 185, 206, 218, 276, 361, 448, 502).
B. The Cavα1-Cavβ Interaction Is Reversible
The affinity of the AID-Cavβ interaction measured in vitro is very high, with a Kd ranging from 2 to 54 nM (30, 56,62, 120, 121, 179, 342, 371, 395, 448). The affinity of Cavα1-Cavβ interactions in cells is less certain but seems to be lower (218), probably partly due to competition for Cavα1 and Cavβ binding by other proteins. The lower affinity likely permits a more dynamic Cavα1-Cavβ interaction. Indeed, several studies support the notion that the Cavα1-Cavβ interaction is reversible in intact cells. 1) Injection of β3 protein into oocytes expressing L-type Cavα1 alone quickly alters Ca2+ channel gating properties, suggesting that some channels on the plasma membrane are devoid of Cavβ (480). 2) A synthetic AID peptide can significantly reduce the Po of channels formed by L-type Cavα1 and β2a in HEK 293 cells when it is applied to the cytoplasmic side of inside-out membrane patches, but it has no effect on channels containing no β2a, suggesting that the AID peptide can compete off bound β2a (224). 3) Injection of β2a protein into oocytes expressing Cav2.3 and β1b results in a dramatic inhibition and slowing down of inactivation, consistent with β2a replacing previously bound β1b and overtaking the channel (218).
That Xenopus oocytes have endogenous Cavβs and that the Cavα1-Cavβ interaction has a 1:1 stoichiometry and is reversible provide a straightforward explanation for why Ca2+ currents can be recorded in oocytes expressing Cavα1 alone, and why the gating properties of these currents can be modulated by exogenous Cavβ: the endogenous β3XO subunits are expressed at high enough levels to interact with a small fraction of the nascent Cavα1 in the ER and transport them to the plasma membrane; there, β3XO eventually dissociates from Cavα1, leaving most of the channels devoid of a β subunit because the cytoplasmic concentration of β3XO is too low to rebind these β-less channels. The β-less channels, however, can associate with exogenously overexpressed Cavβ to form a stable Cavα1/Cavβ complex, as long as the cytoplasmic concentration of Cavβ is a few fold higher than the Kd of the Cavα1-Cavβ interaction. It is likely that this is also the scenario in mammalian expression systems.
A dynamic and reversible Cavα1-Cavβ association might play an important role in regulating Ca2+ channel activity, especially during development when changes in the expression level of different Cavβ isoforms occur (311, 435, 449). It has been shown that the Cavβ component of N-type Ca2+ channels changes during postnatal development, from β1b > β3 >> β2 at P2 to β3 > β1b = β4 at P14 and adult age (449). This study further shows that although no N-type channels associate with β4 at P2, 14 and 25% of N-type channels contain β4 at P14 and adult age, respectively.
VIII. ROLE OF Cavβ IN G PROTEIN INHIBITION OF CaV2 CHANNELS
VGCCs are susceptible to negative-feedback inhibition by hormones and neurotransmitters through the activation of G protein-coupled receptors (GPCRs). An extensively studied form of inhibition is the G protein-mediated, membrane-delimited, and voltage-dependent inhibition of members of the Cav2 channel family (i.e., N-, PQ-, and R-type channels). It is believed that this inhibition contributes to presynaptic inhibition and short-term synaptic plasticity (36, 52, 126, 219, 440, 471). This inhibition is mediated by the direct binding of G protein Gβγ subunits to the channel (213, 233), and it demonstrates three hallmarks: 1) it shifts channel activation to more depolarized potentials (23); 2) it is accompanied by a slowing of channel activation (23), resulting from latent Gβγ unbinding from the channel (139, 244, 349); and 3) it can be reversed by a strong conditioning depolarizing prepulse, which accelerates Gβγ dissociation from the channel in a phenomenon known as prepulse facilitation, or PPF (23, 139, 251). Below we discuss the role of Cavβ in the Gβγ-mediated, voltage-dependent inhibition. For in-depth reviews on other aspects of this inhibition, see References 117, 126, 138, 424, 440, and 497.
A. Cavβ Is Required for Voltage-Dependent Gβγ Inhibition
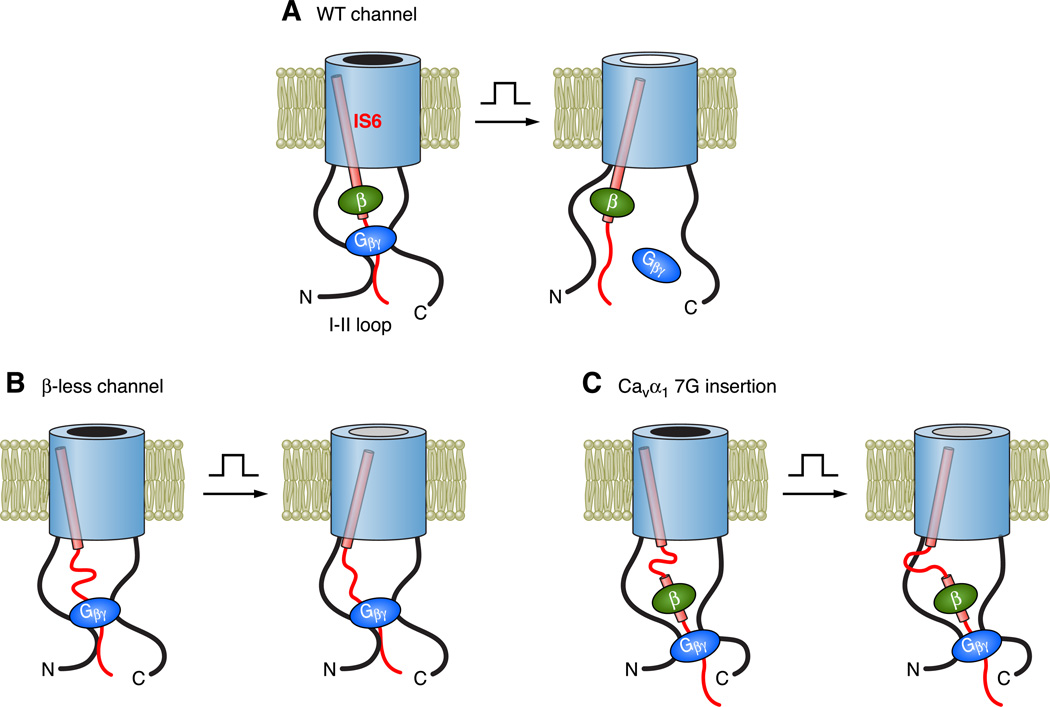
It has long been observed that some effects of Gβγ on VGCCs, such as the slowing of activation and the depolarizing shift of the voltage dependence of activation, are opposite to those of Cavβ, raising the possibility that Gβγ and Cavβ compete with each other (48, 60). Supporting this idea, early studies found that knockdown of endogenous Cavβ in neurons increased GPCR-induced inhibition of Ca2+ currents (60), and coexpression of Cavβ with Cavα1 in oocytes decreased G protein-mediated inhibition (48, 366). However, later studies showed that in COS-7 cells G protein inhibition of N-type Ca2+ channels was markedly enhanced by coexpressed Cavβs (315), and that in tsA-201 cells, a mutant Cav2.2 that contained a point mutation in the AID (W391A) and was unable to associate with Cavβ could no longer display voltage-dependent G protein inhibition (276). The latter studies indicate that Cavβ is essential for voltage-dependent G protein inhibition of N-type Ca2+ channels.
The discrepancy among these studies could arise from many factors. In particular, in the early studies (48, 60, 366), G protein inhibition was examined at a single voltage, which could complicate the interpretation because Gβγ and Cavβ both shift the voltage dependence of channel activation, but in opposite directions. Another factor could be the difficulty of 1) characterizing inhibition of tiny Ca2+ channel currents typically recorded in the absence of coexpressed Cavβ, and 2) excluding the contribution of endogenous Cavβs. To overcome these difficulties, a mutant β2a subunit (named β2a_Mut2) was created by mutating two key AID-binding residues (M245 and L249) to alanine (502). When coexpressed with Cav2.1 in Xenopus oocytes, β2a_Mut2 is still capable of promoting channel trafficking, but owing to its reduced affinity for the AID, it can be washed off from the surface of Ca2+ channels in excised membrane patches (502). With the use of this approach, large populations of Ca2+ channels devoid of Cavβ can be generated on the plasma membrane. Such β-less channels are still inhibited by purified Gβγ protein applied to the cytoplasmic side of the channels; however, all the hallmarks of voltage-dependent inhibition are absent (502). This finding strongly supports the notion that Cavβ is indispensible for voltage-dependent Gβγ inhibition.
Although β-less channels do not display voltage-dependent G protein inhibition, they can still be inhibited by G proteins in a voltage-independent manner. For example, the mutant Cav2.2 harboring the W391A mutation is still susceptible to voltage-independent G protein inhibition (276). Likewise, the β-less Cav2.1 channels (produced by washing off the bound β2a_Mut2 in inside-out membrane patches) are also still inhibited by Gβγ, but without any voltage-dependent features (502). These findings indicate that Gβγ can bind Cavα1 in the absence of Cavβ. Thus the essential role of Cavβ is to enable voltage-dependent dissociation of Gβγ from the inhibited channels, a process that gives rise to the voltage dependence of Gβγ inhibition (45).
B. Gβγ Does Not Displace Cavβ
Another important question is whether Cavβ and Gβγ coexist on Cavα1 during voltage-dependent inhibition. The apparent opposing actions of Gβγ and Cavβ prompted the hypothesis that Gβγ displaces Cavβ from Cavα1 (48, 60, 366, 375). This conclusion was also reached in a study showing that Förster resonance energy transfer (FRET) signals between Cavβ and Cavα1 change during Gβγ inhibition (387). However, functional antagonism does not necessarily indicate direct competition, and, while FRET signal changes are indicative of protein conformational changes, they are inadequate in demonstrating protein dissociation (3, 231, 490).
On the contrary, several lines of evidence indicate that Cavβ remains associated with Cavα1 during Gβγ modulation. 1) Different subfamilies of Cavβ have different effects on the magnitude and properties of voltage-dependent Gβγ inhibition, with β2a being the least effective in promoting this inhibition (61, 129, 147, 316, 376). 2) Cavβ increases the rate of Gβγ dissociation (as determined by the time constant of PPF) from the inhibited channels, but the efficacy of the four Cavβs is different (with a rank order of β3 > β4 > β1b > β2a; Refs. 61, 147). These observations (1 and 2) are most easily explained if Cavα1, Cavβ, and Gβγ form a tripartite complex during Gβγ modulation. 3) Since Cavβ critically affects VDA and VDI, these properties are expected to change if Cavβ were dislodged from the channel. However, the voltage dependence and kinetics of VDI remain unchanged before, during, and after Gβγ modulation (23, 316, 502). Similarly, the voltage dependence of activation is unchanged before and after Gβγ modulation (502). These results support the notion that Cavβ is not dislodged by Gβγ from the inhibited channels.
C. The Rigid IS6-AID α-Helix Is Necessary for Voltage-Dependent Gβγ Inhibition
The antagonistic effects of Cavβ and Gβγ on channel activation suggest that their actions are related structurally and mechanistically. As mentioned in section VIC, disruption of the α-helical structure of the IS6-AID linker (by inserting 3–7 glycine residues in the linker or by substituting 3 linker residues with glycine) abolishes Cavβ modulation of VGCC gating (151, 455, 502). The same maneuver also completely eliminates voltage-dependent Gβγ inhibition but spares voltage-independent Gβγ inhibition (502), indicating that a rigid IS6-AID helix is not necessary for Gβγ binding to Cavα1 but is essential for voltage-dependent dissociation of Gβγ. Strikingly, both voltage-dependent and -independent Gβγ inhibition are abolished when five alanine residues are inserted into the IS6-AID linker of Cav2.1, which is likely to maintain the β-helical structure of the linker but produce a ~180° rotation of Cavβ with respect to Cavα1 (502). It is possible that Gβγ can no longer bind to this mutant Cav2.1, but further investigation is necessary to confirm this speculation.
Consistent with the requirement of a rigid IS6-AID linker, two recent studies show that the GK domain alone is sufficient to support voltage-dependent G protein inhibition in both N- and P/Q-type channels (129, 502). On the other hand, the observation that different isoforms of Cavβ differentially modulate voltage-dependent G protein inhibition indicates that other Cavβ domains and regions can fine-tune this process. Indeed, the HOOK region has been suggested to play a role in enhancing the voltage-dependent dissociation of Gβγ (129).
On another note, the differential effect of different Cavβs on Gβγ-mediated inhibition can be further exposed by the expression of other proteins. For example, RGS2, which is a member of the regulators of G protein signaling that catalyze GTP hydrolysis and terminate G protein signaling (498), can unmask differences in G-protein modulation of P/Q-type channels containing different types of Cavβ (300).
D. Model for the Voltage Dependence of Gβγ Inhibition
Before presenting a model for voltage-dependent Gβγ inhibition, we first consider the molecular components involved in this process. 1) Several distinct regions in Cavα1, all of which bind Gβγ in vitro, play a role in voltage-dependent Gβγ inhibition, including the NH2 terminus (3, 345), the I–II loop (118, 346, 439, 496), and to a lesser extent, the COOH terminus (3, 189, 231, 282, 366, 499). The NH2 terminus of Cavα1 binds directly to the I–II loop, and together they form a Gβγ-gated inhibitory module (3). 2) The I–II loop has two Gβγ binding sites: one extends from the COOH-terminal end of IS6 to the NH2-terminal end of the AID and contains a signature Gβγ-interacting QxxER motif (QQIER in Cav2.1), and the other is located further downstream of the AID (118, 439, 496). The downstream site, termed the G protein interaction domain or GID, is likely to be an anchoring site for Gβγ. It has a ~20 nM affinity for Gβγ (118, 496), and when applied as a 21-amino acid peptide, it can prevent PPF. The upstream site containing the QxxER motif has a ~60 nM affinity for Gβγ, and its mutations attenuate Gβγ modulation (214). However, this site may serve only as a secondary Gβγ-binding site in the holo-channel for three reasons. 1) It is partially buried by Cavβ, as shown by the AID-Cavβ core crystal structures (84, 341, 447). Hence, Gβγ binding to the upstream site is significantly weaker in the presence of Cavβ than in the absence of Cavβ (502). 2) The QxxER motif is unlikely to become completely available, since Cavβ does not vacate from the Gβγ-bound channels (61, 231, 315, 502), as discussed above. When seven alanine residues are inserted in the upstream site, which is expected to prevent Gβγ binding to this site, voltage-dependent Gβγ inhibition remains intact (502). 3) As discussed in section IIIE, Cavβ binding to the AID results in the formation of an α-helix extending continuously from IS6 to the AID. The integrity of this α-helix is critical for voltage-dependent Gβγ inhibition, as mentioned above.
Figure 8 depicts an allosteric model proposed recently for the origin of the voltage dependence of Gβγ inhibition of Cav2 channels (502). This model links voltage-dependent dissociation of Gβγ to the voltage-dependent movement of IS6 and to the obligatory role of Cavβ. The pocket where Gβγ binds in the holo-channel to produce the voltage-dependent inhibition is still unknown, but it is postulated to be downstream of the COOH-terminal end of the AID and is formed collectively by portions in the NH2 terminus, the I–II loop, and the COOH terminus (and possibly yet unknown regions). Under the resting condition and in the presence of Gβγ, the channel is inhibited (Fig. 8A, left). Upon depolarization, the S6 segments of Cavα1 move, and owing to the continuous rigid α-helical structure of the IS6-AID linker, this movement is transmitted to and beyond the AID, resulting in a movement of the distal I–II loop and, consequently, a conformational change of the Gβγ-binding pocket. Such a chain of events ultimately leads to the disassembly of the NH2 terminus-I–II loop inhibitory module and the dissociation of Gβγ from the channel (Fig. 8A, right), which account for the slowing of the activation kinetics and prepulse facilitation. In the absence of Cavβ, Gβγ can still bind to the holo-channel but cannot be discharged by the depolarizing potential, because the Gβγ-binding pocket is uncoupled from IS6 as a result of the unwinding of the AID into a random coil (Fig. 8B). Such uncoupling can also be produced by glycine insertions in the IS6-AID linker (Fig. 8C). In summary, this model postulates that the voltage dependence of Gβγ inhibition of Cav2 channels arises from the voltage-dependent movement of IS6 and that Cavβ and a rigid IS6-AID linker play a pivotal role in translating this movement to Gβγ dissociation.
Fig. 8.
Model for the voltage dependence of Gβγ inhibition. The Gβγ-binding pocket in the holo-channel is postulated to be formed by a region of the I–II loop distal to the AID, the NH2 terminus, and the COOH terminus of Cavα1. A: WT channel: depolarization moves IS6; this movement is propagated through the rigid IS6-AID α-helix, consequently altering the conformation of the Gβγ-binding pocket and resulting in Gβγ dissociation. B: β-less channel: the AID relaxes into a random coil in the absence of Cavβ, uncoupling IS6 from the Gβγ-binding pocket. Gβγ can bind and inhibit the channel but does not dissociate in a voltage-dependent way. C: channel containing Cavβ but with a flexible IS6-AID linker: insertion of 3–7 glycine residues in the IS6-AID linker disrupts the α-helix, uncoupling IS6 from the Gβγ-binding pocket and abolishing voltage-dependent dissociation of Gβγ. [Adapted from Zhang et al. (502)].
IX. ROLE OF Cavβ IN RGK INHIBITION OF HIGH-VOLTAGE ACTIVATED Ca2+ CHANNELS
The RGK (Rad, Rem, Rem2, Gem/Kir) family of Ras-related monomeric small GTP-binding proteins has emerged as potent inhibitors of HVA Ca2+ channels (27, 155). There are four members in this family: Rad (Ras associated with diabetes; Ref. 370), Rem (or Ges = human ortholog; Ref. 153), Rem2 (157), and Gem/Kir (91, 296). They share a conserved Ras-like core but differ from other Ras members in that their GTP/GDP-binding domains have nonconserved mutations that alter or abolish the GTP/GDP cycle (410, 489). They also contain extended NH2 and COOH termini. The COOH terminus has a motif that can anchor them to the membrane (reviewed in Refs. 81, 104, 211, 296) and is critical for their function (81, 104, 105, 488). RGK proteins have two known functions: shaping cytoskeletal dynamics and inhibiting HVA Ca2+ channels (24, 27, 104, 255, 324). These two functions can be differentially regulated; for example, RGK modification of cytoskeletal reorganization, but not inhibition of HVA Ca2+ channels, is attenuated by dephosphorylation of certain RGK residues (152, 463). The physiological importance of RGK inhibition of HVA Ca2+ channels is illustrated by recent in vivo studies that manipulate endogenous Rad levels with consequences for the heart (79, 462, 478). For example, dominant negative suppression of endogenous Rad in the heart increases L-type Ca2+ channel currents and action potential duration in cardiac cells and causes longer QT intervals and arrhythmias (478). Here, we only discuss RGK inhibition of HVA Ca2+ channels and the role of Cavβ in this process. For more comprehensive reviews of RGK proteins and their functions, see References 104 and 255.
A. Cavβ Is Essential for RGK Inhibition of HVA Ca2+ Channels
All members of the RGK family are able to inhibit, in a voltage-independent manner, HVA Ca2+ channels when expressed in various heterologous expression systems (15, 24–27, 81, 101, 102, 154–156, 165, 281, 397, 478, 487, 488). This inhibition depends on Cavβ, as RGK proteins do not affect Ca2+ channel currents recorded in cells expressing only Cavα1 (27, 155, 397 but see Ref. 106). Consistent with this notion, RGK proteins do not affect the activity of T-type Ca2+ channels, which do not associate with Cavβ nor require Cavβ for their activity (81, 155). Furthermore, RGK proteins interact directly with Cavβ, both in vitro and in cells (24–28, 101, 102, 154–156, 165, 281, 487), and this interaction seems to be promiscuous whereby any RGK protein can interact with any full-length Cavβ. A structural model of Gem-β3 interaction has been recently developed (28) based on systematic mutagenesis analysis and homology modeling based on the crystal structure of the AID-β3 core complex (84) and a crystal structure of GDP-bound Gem (PDB 2G3Y). This model shows that Gem binds to the β3 GK domain at a site distinct from the AID-binding pocket and that residues D194, D270, and D272 in β3 and R196, V223, and H225 in Gem are critical for this interaction. Supporting this model, mutating these residues individually or in combination severely weakens or abolishes in vitro binding of Gem and β3 (28, 144).
Three mechanisms of RGK inhibition have been reported. The first mechanism, reported in the first study on RGK regulation of HVA Ca2+ channels (27) and advanced in subsequent studies mostly from the same groups (24–26, 28, 388, 478), is that all RGK proteins disrupt the trafficking of HVA Ca2+ channels to the plasma membrane and hence reduce the number of surface Ca2+ channels, as determined primarily by imaging the subcellular localization of epitope-tagged Cavα1. It was hypothesized that RGK proteins compete with Cavα1 by sequestering Cavβ in the cytoplasm and/or the nucleus, thus leaving Cavα1 trapped in the ER. Several observations are consistent with this hypothesis. 1) Nuclear targeting of Rem and Rad causes nuclear sequestration of Cavβ (24). 2) In addition to Cavβ, all RGK proteins also interact with CaM and 14 –3-3 (24 –26, 158, 297). Abolishing CaM and 14 –3-3 binding or CaM binding alone results in nuclear accumulation of RGK proteins (24 –26), suggesting that these interactions regulate the subcellular localization of RGK proteins. Moreover, Rem2 and Gem (but not Rad and Rem) mutants deficient in CaM binding are unable to inhibit HVA Ca2+ channel currents (24 –27, 463).
On the other hand, other findings are inconsistent with the aforementioned mechanism (i.e., sequestration of Cavβ and disruption of channel surface expression). 1) Cavβ-Cavα1 binding through the AID-GK domain interaction is much stronger than RGK-Cavβ binding (154), making it unlikely for RGK proteins to compete off Cavβ from Cavα1. 2) Several studies show that Cavα1, Cavβ, and RGK proteins form a trimeric complex in vitro and in cells (28, 101, 144, 154, 487). 3) Work from several groups show that RGK proteins can inhibit HVA Ca2+ channels without affecting their surface expression, with the latter determined by surface binding of a radioactive toxin (81), surface biotinylation (144, 154, 156), or gating charge measurement (487). These observations suggest that cytoplasmic and/or nuclear sequestration of Cavβ by RGK proteins occurs via a mechanism other than competition with the AID and may, if at all, only partially account for the observed RGK inhibition of HVA Ca2+ channels.
The second mechanism, closely related to the first one, is that RGK proteins decrease the number of surface Ca2+ channels by increasing their internalization. A recent study shows that Rem enhances dynamin-mediated endocytosis of L-type channels expressed in HEK 293 cells (488).
The third mechanism of RKG inhibition is the suppression of the activity of channels already on the plasma membrane. Such direct inhibition has been observed for different RGK proteins and different HVA Ca2+ channels in a variety of expression systems. For example, Rem2 inhibits endogenous surface N-type channels in native neurons (81), Rem inhibits surface L-type channels expressed in pancreatic β-cells (154, 156) and HEK 293 cells (488), and rapid translocation of a recombinant Rem derivative acutely inhibits L- and N-type channels expressed in tsA201 cells (487). Recently, Gem inhibition of P/Q-type channels was reconstituted in inside-out membrane patches by direct application of a purified Gem protein domain (144). Furthermore, it was found that this acute inhibition was completely abolished when Cavβ was washed away from the patch (using the strategy mentioned in sect. VIIIA), leaving P/Q channels β-less, but it was fully restored after application of a purified Cavβ protein, demonstrating that Cavβ is indispensable for Gem inhibition of surface P/Q channels. Finally, in agreement with a recent study (281), it was found that the Cavβ GK domain alone was sufficient to support Gem inhibition of P/Q channels expressed in Xenopus oocytes (144).
The absolute requirement of Cavβ for RGK-mediated inhibition of HVA Ca2+ channels is reminiscent of such a requirement for voltage-dependent Gβγ inhibition of N- and P/Q-type channels. The latter process further requires a rigid α-helical structure of the IS6-AID linker. It was shown, however, that disrupting the IS6-AID α-helix by glycine insertions did not affect Gem inhibition of P/Q channels (144). This result suggests that Gem inhibition uses a fundamentally different mechanism from voltage-dependent Gβγ inhibition and may not involve IS6-transmitted conformational changes in the pore.
The three mechanisms of RGK inhibition discussed above operate on different time scales and are not mutually exclusive. For example, in the case of Rem inhibition of L-type channels expressed in HEK 293 cells, both reduction of surface channel density and direct inhibition of surface channels occur (488). Which mechanism dominates or is utilized in native cells remains to be determined, but it likely depends on the type and expression level of RGK proteins as well as Ca2+ channel subunits.
B. A New Paradigm for RGK Inhibition of HVA Ca2+ Channels
As mentioned above, all members of the RGK family can interact with all four subfamilies of Cavβ. The RGK-Cavβ interaction has been widely presumed to be essential for RGK inhibition of HVA Ca2+ channels (15, 24–27, 81, 101, 102, 104, 154–156, 165, 206, 281, 397, 478). However, it was recently reported that while Cavβ is required for Gem inhibition of surface P/Q-type channels, the interaction between Cavβ and Gem is not (144). In this study, residues D194, D270, and D272 in β3 and R196, V223, and H225 in Gem were simultaneously mutated to alanine; these residues are predicted and shown to be critical for the Gem-β3 interaction (28, 144). This combination of mutations completely abolished binding of Gem and β3, and yet, the mutant Gem (named Gem_Mut3) was still fully capable of inhibiting P/Q channels containing either the mutant or WT β3 (144). Mean-while, it was found that Cav2.1 could be coimmunoprecipitated by WT Gem or Gem_Mut3, either in the presence or absence of β3, suggesting that Gem directly interacts with Cav2.1. Chimeric studies with PQ- and the RGK-insensitive T-type channels indicate that the IIS1–IIS3 region of Cavα1 is essential for gem inhibition (144).
Based on these results and those discussed above, we propose a “Cavβ-priming” model for Gem inhibition of P/Q-type Ca2+ channels on the plasma membrane (Fig. 9; Ref 144). (This model may be applicable to Rem and Rem2 since they also inhibit surface Ca2+ channels.) A distinct feature of this model is that the interaction between Gem and Cavβ is not necessary for Gem’s inhibitory effect, but a direct association between Gem and Cav2.1 is essential. In this model, Gem interacts directly with Cav2.1 through an anchoring site, with or without Cavβ being present. In the presence of Cavβ and Gem, Cav2.1 forms a multimeric complex with both proteins on the plasma membrane (Fig. 9A). Binding of Cavβ to Cav2.1 produces a conformational change, resulting in the formation of an inhibitory site in Cav2.1 where Gem binds to produce inhibition (Fig. 9A). When Cavβ dissociates or is washed off from surface Cav2.1, the inhibitory site disappears, rendering Gem unable to inhibit Cav2.1, even though it can remain attached to Cav2.1 via the anchoring site (Fig. 9B). When Cavβ and Gem mutants that cannot bind to each other are used (Fig. 9C), inhibition can nevertheless proceed since the ability of Cavβ and Gem to bind Cav2.1 is not compromised. Thus the essential role of Cavβ is to convert Cav2.1 into a state permissive for Gem inhibition.
Fig. 9.
The “Cavβ-priming” model of Gem inhibition of surface HVA Ca2+ channels. Gem associates directly with Cavα1 via an anchoring site in Cavα1 (indicated by the purple patch). A: WT channel: binding of Cavβ to Cavα1 induces an inhibitory site in Cavα1 (indicated by the red patch), where Gem binds to induce inhibition. B: β-less channel: Gem can still associate with Cavα1 via the anchoring site, but it does not inhibit the channel because Cavα1 lacks the Cavβ-induced inhibitory site. C: WT channel with mutually noninteracting Cavβ and Gem: disrupting the interaction between Cavβ/Gem with mutations in the Cavβ/Gem interface does not affect Gem inhibition, since the interactions between Cavβ and Cavα1 and between Gem and Cavα1 remain intact. [Modified from Fan et al. (144).]
At present, this model remains speculative, and many questions remain unanswered. For example, where is the anchoring site for Gem on Cav2.1? Where is the inhibitory site? How does Cavβ binding to Cav2.1 create the inhibitory site? How does Gem binding to the inhibitory site lead to channel inhibition? Does the Gem-Cavβ interaction play any role at all? With regard to the last question, we speculate that in native cells, with physiological levels of Gem protein, the Gem-Cavβ interaction may increase the effective concentration of Gem near surface Ca2+ channels and thereby facilitate Gem inhibition.
X. ROLE OF Cavβ IN PHOSPHO- AND LIPID REGULATION OF HIGH-VOLTAGE ACTIVATED Ca2+ CHANNELS
Phosphorylation allows dynamic regulation of protein functions, including those of HVA Ca2+ channels. During the “fight-or-flight” response, for example, β-adrenergic stimulation leads to PKA-dependent upregulation of L-type Ca2+ channel currents, which results in a faster and stronger heartbeat. The activity of HVA Ca2+ channels is regulated by a variety of protein kinases and phosphatases. While Cavα1 is often the target of phosphorylation, Cavβ can nevertheless modulate the effect of such phosphorylation, and in some cases, Cavβ itself is the target. HVA Ca2+ channels can also be regulated by membrane lipids and their metabolic products. This section discusses the role of Cavβ in phospho- and lipid regulation of HVA Ca2+ channels.
A. Ca2+/Calmodulin-Dependent Kinase II
Ca2+/calmodulin-dependent kinase II (CaMKII) is among the most abundant enzymes in many cell types. Recent studies show that CaMKII can interact directly with Cavα1 of HVA Ca2+ channels and regulate their activities (242, 273, 358). In cardiac and smooth muscle cells, CaMKII plays a role in the facilitation of L-type Ca2+ channels (133, 192, 308). In cardiomyocytes, the molecular mechanism partly involves CaMKII-mediated phosphorylation of the β2a subunit (192). CaMKII binds to the COOH terminus of β2a and phosphorylates it at T498, which leads to an upregulation of L-type currents (192). This upregulation is not observed in the absence of β2a or when a nonphosphorylatable β2a mutant, β2a (T498A), is coexpressed in tsA201 cells. This mutant can also act as a dominant negative, preventing CaMKII-mediated facilitation of endogenous Ca2+ currents. Moreover, T498 phosphorylation promotes the dissociation of CaMKII from β2a, which may serve as a negative-feedback mechanism (193). Since most β2 splice variants have a common COOH terminus identical to that of β2a (Fig. 6), it would be interesting to examine whether CaMKII also interacts with and phosphorylates these β2 variants.
CaMKII has also been shown to associate with β1b in vitro, but not with β3 and β4 (193). A recent study further shows that CaMKII coimmunoprecipitates with forebrain L-type Ca2+ channel complexes containing β1 or β2 but not β4 (1). β1b, β3, and β4 have also been shown to be phosphorylated by CaMKII (193), but the physiological consequences remain to be determined.
B. Mitogen-Activated Protein Kinase
Mitogen-activated protein kinase (MAPK) is a member of a signaling network that responds to extracellular stimuli and induces diverse physiological and pathological processes. The small monomeric G protein, Ras, can upregulate Ca2+ currents in dorsal root ganglion neurons through the activation of the MAPK signaling pathway (160). In COS-7 cells, this upregulation is shown to require Cavβ, because in the absence of Cavβ, MAPK-dependent upregulation is abolished (159). Furthermore, different Cavβs support different degrees of upregulation. For example, in the presence of β2a, but not other Cavβs, Ca2+ channels are partially resistant to inhibition by an antagonist of MAPKK, the exclusive activator of MAPK (159). It is speculated that MAPK directly phosphorylates the channel complex (159). While both Cavα1 and Cavβ have consensus MAPK phosphorylation sites, it is unclear which, if any, are phosphorylated in cells.
C. Phosphoinositide 3-Kinase and Protein Kinase B (or Akt)
Some external stimuli (e.g., insulin-like growth factor) activate receptors that are associated with tyrosine kinases and upregulate L- and N-type Ca2+ channel currents (42). The mechanism likely involves phosphoinositide 3-kinase (PI3K) activation and subsequent production of phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphatidylinositol 3,4,5-trisphosphate (PIP3), known regulators of HVA Ca2+ channels (for reviews, see Refs. 122, 318). Increased PIP3 levels recruit protein kinase B (PKB) to the membrane, which can phosphorylate β2a at S574 (454). This results in an upregulation of currents conducted by channels containing β2a and Cav1.2 or Cav2.2, mainly by increasing surface expression (454). Ca2+ channels containing an unphosphorylatable β2a (bearing the S574A mutation) are resistant to upregulation by PI3K/PKB, whereas those containing a phosphorylation-mimic β2a (bearing the S574E mutation) exhibit tonically increased current and are insensitive to upregulation by PI3K. The permissive role of β2a is not shared by other Cavβs and does not depend on palmitoylation (454).
A similar PI3K/PKB signaling pathway may play a role in maintaining normal cardiac function. Thus, in the absence of active PKB (created in a cardiac-specific conditional knockout), L-type Ca2+ channel surface expression is greatly reduced, leading to severe cardiomyopathy (70). This deficit results from lysosomal degradation of L-type channels, initiated by conserved PEST sequences (signals for rapid protein degradation) in Cav1.2. When PKB is active, it binds and phosphorylates β2, which in turn masks the degradation signals and leads to an increased channel surface expression (70).
D. cAMP-Dependent Protein Kinase
cAMP-dependent protein kinase (PKA)-mediated upregulation of cardiac L-type Ca2+ channel currents was one of the first examples of ion channel modulation. During the “fight-or-flight” response, β-adrenergic stimulation, through G proteins and adenylate cyclase, results in increased levels of cAMP and subsequent activation of PKA, eventually leading to dramatic increases of cardiac L-type Ca2+ currents and the consequent faster and stronger heartbeat (for reviews, see Refs. 72, 309). In spite of intense research, the target of PKA phosphorylation that underlies L-type current upregulation still remains obscure, partly owing to the difficulty of reconstituting this regulation in heterologous expression systems. In vivo, this upregulation is accompanied by increased phosphorylation of both Cav1.2 and β2 (54, 72, 114, 115, 180, 197). A PKA phosphorylation site on Cav1.2 (S1928) has been proposed to mediate the β-adrenergic response (115). However, a recent study using the Cav1.2 (S1928A) knock-in mice shows that basal L-type currents and the upregulation of L-type currents by PKA and β-adrenergic receptor stimulation are unchanged (275), indicating that PKA phosphorylation of S1928 is not the underlying cause for L-type current upregulation.
On the other hand, it has been shown that, in vitro, PKA phosphorylates three sites on β2a: S459, S478, and S479 (180). It was initially proposed that β2a phosphorylation at S478 and S479 is critical for L-type channel upregulation (54, 173). This was based on the lack of or reduced upregulation of currents conducted by L-type channels containing β2a (S478A/S479A) (54, 173). A caveat is that these studies were done either with a truncated Cav1.2 (54) or without comparative experiments with WT β2a (173). The role of β2a phosphorylation in L-type current upregulation has been strongly challenged by a recent study, which shows that in cardiac muscle cells L-type channels containing β2a(S459A/S478A/S479A) exhibit the same degree of PKA-mediated upregulation as channels containing WT β2a (321). This study further demonstrates that the extent of PKA modulation is influenced by the associated Cavβ. Thus channels containing β1b show the strongest upregulation, followed by those containing β3 and β4, whereas channels containing β2a show the least modulation (probably because β2a dramatically increases channel Po in the first place, as mentioned in sect. VIA). Since β1b, β3, and β4 do not share the aforementioned PKA phosphorylation sites in β2a, the latter observation further strengthens the notion that PKA phosphorylation of β2a does not play an essential role in the upregulation of cardiac L-type currents. Thus identifying the site(s) of PKA phosphorylation that give rise to this event remains a stubborn challenge.
Lastly, Cav1.3 channels can also be potentiated by PKA phosphorylation (287). The extent and duration of this potentiation also depend on the identity of the associated Cavβ (287).
E. Protein Kinase C
Protein kinase C (PKC) can enhance some neuronal L- and N-type Ca2+ channel currents (483). In Cav2 channels, the enhancement partly results from the disruption of Gβγ inhibition (19, 21, 96, 202, 426, 496). This is achieved by phosphorylating the I–II loop of Cavα1, which may block Gβγ binding, considering that the I–II loop contributes to form the Gβγ-binding pocket (see sect. VIIID). Since Cavβ also binds to the I–II loop, it is conceivable that its binding is sensitive to PKC phosphorylation, or vice versa. In fact, pharmacological activation of PKC can increase Cav2.2 and Cav2.3 channel currents, but only in the presence of Cavβ, and transferring the I–II loop from these channels to the unresponsive Cav2.1 and Cav1.2 channels can transfer PKC sensitivity (49, 413). Thus it seems that Cavβ is permissive for PKC modulation of certain Cavα1. However, the effects of PKC phosphorylation of Cavβ itself (e.g., β2a; Ref. 180) are unknown.
F. cGMP-Dependent Protein Kinase
cGMP-dependent protein kinase (PKG) is activated by cGMP and can phosphorylate both Cav1.2 and β2a-Cav1.2 at S1928 (the same residue that can also be phsophorylated by PKA) and β2a at S496 (484, 485). Activation of PKG results in inhibition of channels containing Cav1.2 and β2a in HEK cells (484). This inhibition is abolished by the β2a S496A mutation, suggesting that PKG phosphorylation of β2a is critical for this process. The physiological consequences of this inhibition remain to be determined.
G. Arachidonic Acid
Like many other types of ion channels, the activity of HVA Ca2+ channels can be regulated by membrane lipids and their metabolic products (for reviews, see Refs. 46, 122, 172, 318, 373). One example is the voltage-independent inhibition of HVA Ca2+ channels upon PIP2 depletion from the plasma membrane (171, 475). Another example is channel inhibition by arachidonic acid (AA), an unsaturated fatty acid released from phospholipids (including PIP2) by the action of some phospholipases (18, 289, 374, 403). The magnitude of AA inhibition depends on the partnered Cavβ in the channel (374). In particular, β2a seems to dampen AA inhibition of Cav1.3 channels. This unique effect of β2a is abolished when palmitoylation is eliminated in a mutant β2a (β2a C3,4S). Attaching a transmembrane segment of an unrelated membrane protein to the NH2 terminus of this mutant β2a does not restore the dampening effect, suggesting that palmitoylation rather than membrane anchorage per se is responsible for the antagonizing effect of β2a on AA-mediated inhibition. It is proposed that the palmitoyl groups of β2a compete with AA for a common binding site on Cav1.3 (374). This unique ability of β2a has also been proposed to underlie the enhancement of Cav2.2 channels containing β2a by the stimulation of Gq-coupled receptors, which, in contrast, causes inhibition of channels containing β1b, β3, or β4 (210).
XI. INTERACTION OF Cavβ WITH OTHER PROTEINS
For many years, Cavα1 was the only known interacting partner for Cavβ. In recent years, however, a growing number of proteins have been found to interact with Cavβ, in some cases with significant functional impact. This section reviews some of these interactions and their functional consequences. Two more examples are discussed in section XII, in the context of a potential role of Cavβ in transcriptional regulation.
A. Ryanodine Receptors
In skeletal muscle, Ca2+ channel complexes (DHPRs), which are made up of Cav1.1, β1a, α2δ1, and γ1 subunits, are arranged on the plasma membrane of t tubules in tetrads, which are in contact with ordered RyRs in the membrane of adjoining sarcoplasmic reticulum (SR) (for reviews, see Refs. 167, 363). This physical arrangement is required for efficient excitation-contraction (EC) coupling. It has been shown that β1a, which is expressed exclusively in skeletal muscle, is indispensible for EC coupling (for reviews, see Refs. 100, 164). This is because β1a is essential not only for the surface expression of DHPRs (37, 38, 191, 393, 394, 402) but also for the tetrad formation (393, 394). It seems that β1a allosterically primes Cav1.1 to properly interact with RyRs to form the tetrads (393). Expression of exogenous Cavβ in skeletal muscle cells isolated from β1a-null mice or zebrafish can fully rescue L-type Ca2+ channel current, but only β1a (and β1c) can normalize EC coupling (38, 393, 402). This unique ability is due to the presence of a heptad repeat (L478-V485-L492) in the distal COOH terminus of β1a (393, 401, 402). When this region is deleted from β1a, EC coupling is lost, and when it is transferred to β2a, EC coupling is observed. It is unclear, however, where the heptad repeat binds (402). What is certain is that β1a can bind directly to the RyR (85, 164). On the RyR, β1a binds to a highly charged region (KKKRRxxR), whose mutation attenuates EC coupling (85). Interestingly, two pathogenic mutations, R3348H and P3527S, occur in this region of the RyR and cause malignant hyperthermia susceptibility (384) and multi-minicore congenital myopathy (148), respectively. It remains to be determined whether the loss of interaction with β1a is the underlying cause.
B. Ahnak
Ahnak, a ubiquitous large (700 kDa) signaling and scaffolding protein involved in diverse aspects of cell physiology and pathophysiology (reviewed in Refs. 7, 195), has been shown to interact with Cavβ in several distinct cell types, including cardiac cells, osteoblasts, and T lymphocytes (6, 199, 223, 305, 398). This interaction provides a potential link between Ca2+ channels, the cytoskeleton, and cellular organelles. Multiple regions in the COOH terminus of Ahnak can bind β2a in vitro, with apparent affinities ranging from 50 to ~300 nM (196, 199, 223). The Ahnak-interacting region on β2a is unknown, but since Ahnak coimmunoprecipitates with β1b, β3, and β2a (6, 199, 398), it is likely to be in the conserved GK or SH3 domains.
It has been proposed that the Ahnak-β2a interaction plays a role in PKA-mediated upregulation of cardiac L-type currents (195, 196). Both β2a and Ahnak can be phosphorylated by PKA, and this phosphorylation weakens the binding between the two proteins by ~50% (196). This effect is accompanied by L-type current upregulation, a hyperpolarizing shift in the voltage dependence of activation, and an occlusion of subsequent PKA effects (196, 199). Thus it was suggested that under basal conditions, L-type channel activity is suppressed by the Ahnak-β2a interaction and that PKA phosphorylation of both proteins disengages β2a from Ahnak and hence relieves this tonic inhibition (195). However, a challenge to this proposal is that PKA phosphorylation of β2a does not appear to play a role in the β-adrenergic receptor stimulation-induced upregulation of cardiac L-type currents, as discussed in section XD.
In the immune system, T cells are central in cell-mediated immunity. T cells are nonexcitable cells, yet they express all four Cav1 channels and all four Cavβs (241, 419). Activation of T-cell antigen receptors (TCR) causes Ca2+ influx, which is key for T-cell activation. This Ca2+ influx is thought to be mediated in part by Cav1 channels, although the mechanism of channel activation remains unclear (11, 241,305, 419). CD4+ T lymphocytes isolated from β3- and β4-null mice and CD8+ T cells isolated from β3-null mice display impaired TCR-triggered Ca2+ response (11, 241), presumably because of deficient surface expression of Cav1 channels. T cells from Ahnak1 knockout mice also respond poorly to TRC stimulation and have impaired Ca2+ influx (305). It is proposed that this deficit results from decreased plasma membrane expression of Cav1 channels, owing to the lack of the Ahnak1-Cavβ interaction (305); however, this hypothesis needs further testing.
C. BKCa Potassium Channels
Large-conductance Ca2+-activated K+ channels (BKCa) are synergistically activated by membrane depolarization and intracellular Ca2+ and play important roles in various physiological processes (383). Even though BKCa channels have their own auxiliary subunits, it has been shown recently that BKCa channels bind directly to the Ca2+ channel β1 subunit (508). In HEK 293T cells, this interaction dampens the Ca2+ sensitivity of BKCa channels and slows their activation and deactivation. The GK domain of β1 is necessary and sufficient for these effects, suggesting that other Cavβs may share this β1 function, but this remains to be determined. It is also unknown whether the Cavβ-BKCa channel interaction occurs in native cells, and whether and how this interaction affects HVA Ca2+ channels.
D. Bestrophin
Bestrophin (Best1) is a 585– 604 amino acid chloride channel expressed in the retinal pigment epithelium (RPE) whose mutations cause Best’s and other retinopathies (see review in Ref. 205). It modulates L-type Ca2+ channel gating and blocks L-type channel-mediated rises in [Ca2+]i in RPE (301, 378). Recently, it was shown that Best1 binds the Ca2+ channel β4 subunit and that its effects disappear in the absence of β4, suggesting that Best1 may be acting through β4 on Cav1.3 channels (493). Befittingly, β4 knockout mice also have rethinopathies (301). The COOH terminus of Best1, which on its own does not generate Cl− currents, can also inhibit L-type channels. It contains a predicted proline-rich domain (PRD) whose mutation abolishes the effects of Best1. It is proposed that this PRD binds to the SH3 domain of β4, disrupts the GK-SH3 interaction, and causes L-type channel inhibition (493). However, direct evidence for such a mechanism is still lacking. It would be interesting to examine whether the PxxP-binding region of β4 binds Best1. As discussed in section IIIB, although this region is occluded in the Cavβ crystal structures (84, 341, 447), it could conceivably become accessible when Cavβ is bound to Cavα1 and other proteins.
E. Dynamin
A recent study reported that full-length β2a interacts in vitro with dynamin, a multi-partner GTPase involved in endocytosis (186). This interaction was presumed to involve a PRD of dynamin and the SH3 domain of β2a, since a purified β2a fragment (amino acids 24–136) containing the four contiguous β sheets of the SH3 domain was found to interact with dynamin and this interaction was partially blocked by the dynamin PRD. This β2a fragment was able to markedly suppress the surface expression of Cav1.2 channels, and this suppression depended on dynamin. It was proposed that the dynamin-SH3 domain interaction links HVA Ca2+ channels to the endocytotic machinery (186). It should be noted, however, that the β2a fragment used in this study lacks the fifth (i.e., the last) β sheet of the SH3 domain and the HOOK region, which hinders access to the PxxP-binding region of Cavβ (84, 341, 447). To determine whether the PxxP-binding region of β2a is involved in the interaction between dynamin and full-length β2a, it would be useful to examine whether this interaction is abolished by selective mutations of β2a residues that are presumably directly involved in binding PRDs.
F. Synaptic Proteins: Synaptotagmin I and RIM1
The α1 subunit of presynaptic HVA Ca2+ channels physically interacts with presynaptic proteins, including syntaxin, SNAP-25, and synaptotagmin I; these interactions are important for synaptic vesicle docking and fusion (reviewed in Ref. 400). Recent studies show that Cavβ can also interact with synaptic proteins (257, 452). For example, the NH2 terminus of β3 and β4a (but not β4b) binds to synaptotagmin I, and this interaction is abolished by a high concentration (10 mM) of Ca2+ (452). However, the physiological importance of this interaction is yet unknown. Another study shows that RIM1, a presynaptic protein critical for synaptic transmission and plasticity (69, 392), binds directly and with a high affinity (35 nM) to β4b and β2a. This interaction is mediated by the COOH terminus of RIM1, and the SH3-HOOK-GK module of Cavβ is sufficient for binding to occur. The most prominent effect of the RIM1-Cavβ interaction on HVA Ca2+ channels is the slowing of VDI and a hyperpolarizing shift of the voltage dependence of inactivation. This effect is observed on recombinant L-, N-, P/Q-, and R-type channels containing β4b and on recombinant P/Q-type channels containing β1a, β2a, β3, or β4b. RIM1 may also play a role in anchoring synaptic vesicles to presynaptic VGCCs through binding to the synaptic vesicle protein Rab3. Consequently, overexpression of a mutant Cavβ that is unable to bind Cavα1 can attenuate vesicle docking at the presynaptic membrane in PC12 cells, presumably by competing with the WT Cavα1/β complex for RIM1 binding. Furthermore, overexpression of RIM1 in PC12 cells and cultured cerebellar neurons enhances neurotransmitter release. This study establishes a direct role of Cavβ in the physical organization of the synaptic vesicle release machinery (257).
G. Zinc Transporter 1
Zinc transporter 1 (ZnT-1), a ubiquitous transmembrane protein involved in zinc transport and metabolism, binds directly to β2a (280) and has been shown to inhibit L-type Ca2+ channels in heterologous expression systems and native cells (29, 280,337, 396). When coexpressed with Cav1.2 and β2a in Xenopus oocytes, ZnT-1 reduced Cav1.2 channel currents (280). This inhibitory effect disappeared in the absence of β2a or in the presence of an excess amount of β2a. ZnT-1 reduced the surface expression of Cav1.2 without changing its total expression level when they were coexpressed in HEK 293T cells (280). The authors proposed that ZnT-1, through direct binding to Cavβ, inhibits L-type channels by reducing their trafficking to the plasma membrane (280). It remains to be examined whether ZnT-1 physically interacts with other types of Cavβs and whether it inhibits Cav2 channels.
XII. Ca2+ CHANNEL-INDEPENDENT FUNCTIONS OF Cavβ
Until recently, the functions of Cavβ have been exclusively linked to VGCCs. However, a stream of recent studies suggests that Cavβ may possess functions independent of their association with VGCCs. This line of inquiry began with the cloning of various short isoforms of Cavβ, some of which lacked the GK domain. This inability to engage in the high-affinity AID-GK domain interaction with Cavα1 raised questions about their functions (92, 166,204, 217, 229). The first study examining possible alternative functions of truncated splice variants of Cavβ centered on a β4 splice variant expressed in chicken cochlea and brain, termed β4c (217); to avoid confusion, we refer to it as cβ4c. cβ4c is truncated after exon 8 and thus lacks 90% of the GK domain and the entire COOH terminus (Fig. 6). As expected, cβ4c barely affects Cav2.1 channels coexpressed with α2δ in Xenopus oocytes. However, cβ4c interacts directly with the scaffolding domain of heterochromatin protein 1 (HP1), a nuclear protein involved in gene silencing and transcriptional regulation. Both proteins are colocalized in the nuclei of cochlear hair cells, and their coexpression in tsA201 cells causes translocation of cβ4c from the cytoplasm to the nucleus. Moreover, cβ4c attenuates the repressor function of HP1 in a dose-response manner. The effects on HP1 are specific since a longer isoform, β4a, has no effect. These findings suggest that cβ4c may function as a transcription regulator.
In a recent study, Zhang et al. (504) reported that full-length β3 could directly interact with a new splicing isoform of Pax6, a transcription factor critical for the development of the eye and nervous system. The new isoform, named Pax6(S), has a truncated COOH terminus with a unique serine-rich tail. The interaction between Pax6(S) and β3 is conferred mainly by the S tail and the SH3-HOOK-GK module of β3. Since the other three subtypes of Cavβ can also interact with Pax6(S), the binding site for the S tail likely resides in the conserved SH3 or GK domain. Coexpression of Pax6(S) with Cav2.1 channels containing β3 in Xenopus oocytes does not alter channel properties; however, the in vitro transcriptional activity of Pax6(S) is markedly suppressed by β3. Furthermore, co-expression of β3 and Pax6(S) in HEK 293T cells results in the translocation of β3 from the cytoplasm to the nucleus. These results suggest that full-length Cavβs may function as transcription regulators (504).
This notion is further supported by other recent studies (16, 427), which show that, upon neuronal differentiation, full-length β4a physically interacts with B56δ, a nuclear regulatory subunit of phosphatase 2A (PP2A). The β4a/B56δ complex relocates to the nucleus, where it associates with nucleosomes and regulates the dephosphorylation of histones, a key mechanism in transcriptional regulation. A mutant β4 lacking the last 38 COOH-terminal residues and associated with a case of juvenile myoclonic epilepsy (142), can neither associate with B56δ nor translocate to the nucleus, and its in vitro transcriptional regulation activity is different from that of WT β4 (16, 427). Formation of the β4a/B56δ complex requires an intact intramolecular SH3-GK interaction.
Consistent with the notion that full-length Cavβs may function as transcription regulators, it has been shown that full-length Cavβs can be targeted to the nucleus in native cells. For example, β4, and to a lesser extent, β1b and β3, are translocated into the nucleus when they are exogenously expressed in cardiac cells (93). A recent study reports that endogenous β4 is present in the nuclei of cerebellar granule cells and Purkinje cells (425). When heterologously expressed in skeletal myotubes or cultured hippocampal neurons, β4b is robustly targeted to the nucleus, whereas other Cavβs are not. Nuclear localization of β4b is dependent on an Arg-Arg-Ser motif in the NH2 terminus, which is necessary since deleting this motif decreases nuclear targeting of β4b. This motif is also sufficient since fusing it to β4a increases nuclear targeting of the resulting chimera. Importantly, nuclear targeting of β4b is diminished upon increased electrical activity and Ca2+ influx through L-type Ca2+ channels, suggesting a potential physiological function (425).
Another possible VGCC-independent function for β4 is demonstrated by a recent study in zebrafish (135), which express all four subtypes of Cavβs (506). Morpholino knockdown of zebrafish β4 abolishes or retards epiboly, an early development process, due to disturbances in mitotic and postmitotic cytoskeletal rearrangements. Epiboly can be rescued by coinjecting full-length human β4a or β4b cRNA. Interestingly, epiboly can also be rescued upon coinjection of a mutant β4a, which contains a triple mutation (M204A/L208A/L350A) in its AID-binding pocket and cannot bind Cavα1 or enhance Ca2+ channel currents in Xenopus oocytes. These results suggest an involvement of β4 in zebrafish early development, probably through VGCC-independent actions. It remains to be determined how β4 is involved and whether this function is shared by other Cavβs.
In a study using β3 knockout mice, high glucose conditions caused pancreatic β cells to produce twofold more insulin than their WT counterparts (31). No change in VGCC currents was detected, but the β3-deficient cells exhibited a higher frequency of glucose-induced intracellular [Ca2+] oscillations, accompanied by increased IP3 production and increased Ca2+ release from intracellular stores. On the basis of the colocalization of these proteins in pancreatic β cells, it was hypothesized that β3 may directly interact with IP3 receptors to cause some of these effects (31).
In snails (Lymnaea), the expression of the sole Cavβ (LCavβ) is temporally and spatially uncoupled from the expression of LCav2, a Lymnaea homolog of the mammalian Cav2 family of VGCCs (409). Functionally, LCavβ does not modulate the surface expression or gating of LCav2 when they are coexpressed in tsA201 cells, even though they show current upregulation and gating modulation when they are partnered with rat Cavα1 and Cavβ, respectively (409). Furthermore, knockdown of LCav2, but not LCavβ, alters neurite morphology. These results suggest that LCavβ may have VGCC-independent functions, which remain to be elucidated. This work illustrates that studies in simple model organisms might be beneficial to our understanding of the full spectrum of Cavβ functions.
XIII. Cavβ KNOCKOUTS AND PATHOPHYSIOLOGY
As expected, because of the essential role of Cavβ in the surface expression and functional modulation of HVA Ca2+ channels, Cavβ knockouts or mutations can produce severe functional deficits and, in some cases, are lethal. The phenotype of Cavβ knockout mice depends on the ability of the remaining three Cavβ genes to compensate. This section discusses the phenotypes and pathophysiology of Cavβ knockouts and mutations.
A. Gene Knockouts and Mutants
1. β1
As mentioned in section XIA, β1a is irreplaceable in partnering with Cav1.1 channels to enable skeletal muscle EC coupling. Thus β1 knockout mice, similar to Cav1.1 knockouts, are born motionless and die immediately from asphyxiation (191). Skeletal muscles isolated from β1-null mice are twitchless upon electrical stimulation, and action potentials do not elicit Ca2+ transients. L-type Ca2+ channel currents and the surface expression of Cav1.1 subunits are much reduced in these muscles, but caffeine can still cause contractions, indicating that internal Ca2+ stores are intact (191). Transgenic expression of β1a exclusively in the skeletal muscle of β1-null mice rescues the mice, which exhibit no obvious phenotype, suggesting that the remaining Cavβ genes can compensate for the functions of β1 in other tissues (14).
Zebrafish β1 knockouts also exist (393, 394). They have the Relaxed phenotype and die paralyzed days after hatching, with completely deficient EC coupling. Skeletal muscles from β1-null zebrafish have no tetrads and show reduced depolarization-induced Ca2+ transients, but exhibit normal caffeine-induced Ca2+ transients (394). Unlike in β1-null mice, targeting of Cav1.1 subunits to t tubules and the formation of triads are preserved (394), suggesting a nonessential role of β1 in these processes in zebrafish. It remains to be determined whether other Cavβs are expressed in β1-null zebrafish skeletal muscles or whether zebrafish Cav1.1 is able to traffic to the plasma membrane on its own.
2. β2
Several β2 splice variants are the predominant Cavβs expressed in the heart (Table 1). Thus it is no surprise that β2 knockouts have no cardiac contractions and are nonviable beyond embryonic day 10.5 (14, 468). This is due to diminished L-type Ca2+ channel currents in cardiomyocytes and cardiac failure-associated defective remodeling of blood vessels. The β2-null phenotype can be rescued by the expression of β2 under a cardiac muscle-specific promoter (14). These partial knockouts revealed an essential role of β2 in tissues besides the heart: such mice (lacking β2 in all but cardiac tissues) are deaf due to a dramatic reduction in the membrane expression of Cav1.3 channels in inner hair cells, coupled with decreased exocytosis, improper hair cell development, and defective cochlear amplification (331). These “rescued” mice also have defects in vision with a phenotype similar to human patients with congenital stationary night blindness (13).
Given the knockout results, genetic mutations in β1 and β2 are expected to affect mainly skeletal and cardiac muscles, respectively. While no β1 mutations have been associated with genetic diseases thus far, β2 mutations have. Thus a mutation in the COOH terminus of β2b (CACNB2b), S481L, contributes to a type of sudden death syndrome characterized by a short QT interval and an elevated ST segment (8), which are categorized into a group of genetic heart diseases called the Brugada syndrome. This mutation decreases Cav1.2 currents by ~75% in an expression system (CHO-K1 cells). Another mutation, in the β2b NH2 terminus (T11I), causes accelerated inactivation of cardiac L-type channels and is also linked to the Brugada syndrome (97). This mutation occurs in exon 2C of the CACNB2 gene and only affects β2b, the most abundant Cavβ isoform in the heart (93). A recent study suggests that variations in CACNB2 may be also associated with a heightened risk for Alzheimer’s disease (286).
3. β3
β3 Knockouts are viable and were initially found to be normal (328, 330). Later studies, however, uncovered a wide spectrum of abnormalities, especially under stress conditions. For example, at high glucose concentrations, the frequency of [Ca2+]i oscillations and the resulting insulin secretion from pancreatic β cells are potentiated (31). This is likely due to the attenuation of β3-mediated inhibition of IP3 production (31, 339). Also, a high-salt diet causes elevation of blood pressure, reduction in plasma catecholamine levels, and a hypertrophy of heart and aortic smooth muscle (328, ). The latter effects, as well as observations from mice overexpressing β3 (327), are consistent with a function of β3 in sympathetic control. In this regard, β3 knockouts resemble N-type channel (Cav2.2) knockouts (428, ), which is not surprising since N-type channels preferably partner with β3 (294, 325–327, 395). Indeed, sympathetic neurons from β3-null mice have reduced N- and L-type channels activity (330). N-type current is also decreased in dorsal root neurons, which dampens inflammatory pain, but not mechanical or thermal pain (325). In the brain, N-type channel expression is reduced by ~40% (326); in the hippocampus, expression of NR2B (an NMDA receptor subunit), NMDA receptor currents, and long-term potentiation are all increased (239). Some forms of hippocampus-dependent learning and memory appear to be enhanced, but working memory is impaired (239, ). Furthermore, pruning of visual retinocollicular pathways is developmentally reduced and delayed (98). Behaviorally, β3-null mice have lower anxiety, increased aggression, and increased night-time activity (326). Finally, β3-null mice show abnormal signaling in CD4 T-cells, where receptor-mediated Ca2+ responses, nuclear translocation of NFAT, and cytokine production are all attenuated (11).
4. β4
Mutated β4 was first reported in lethargic mice (55, 130, 131). The naturally occurring null mutation is a four nucleotide insertion in Cacnb4, causing a translational frame shift and a premature stop codon. Lethargic mice have ataxia, seizures, absence epilepsy, and paroxysmal dyskinesia (17, 55, 227). The abnormal phenotype appears after postnatal day 15, a time when WT animals have an increase in β4 expression in the brain (311). The upregulation of β4 in WT mice is particularly robust in cerebellar granule and Purkinje neurons, which likely explains the ataxia in null mice (55). T-type Ca2+ channel upregulation (by ~50%) in thalamic neurons of lethargic mice likely contributes to the seizures (503). It is not clear why the remaining Cavβs fail to compensate for the lack of β4, but this could be partly because of the unique interactions between the NH2 and COOH termini of β4 with other proteins (for examples, see Refs. 51, 121, 420, 460, 461, 474). Nevertheless, in lethargic mice, there is increased pairing of Cav2.2 and Cav2.1 with other Cavβs; in particular, both β1b and Cav2.2/β1b complexes are upregulated, similar to what is found in the developing brain (310, 311). Some other characteristics of lethargic mice include lower N-type channel expression in the forebrain and cerebellum (310), reduced excitatory neurotransmission in the thalamus (57), a modified electro-oculogram (301), splenic and thymic involution (130, 131), and renal cysts (130). Similar to β3 knockouts, CD4+ T-cells have attenuated receptor-mediated Ca2+ responses, nuclear translocation of NFAT, and cytokine production (11).
Since β4 is the predominant partner for P/Q-type (Cav2.1) channels in brain (311), it is no surprise that tottering mice (161), with mutations in Cav2.1, have a phenotype very similar to lethargic mice (356). Both tottering and lethargic mice are also models for epilepsy (17, 226). Indeed, there are examples where mutations in β4 precipitate epilepsy and ataxia in humans. In one case, an R468Q mutation in CACNB4, which enhances Cav2.1 current, was associated with a history of febrile seizures (338). In another, truncated β4 (R482x) that only has a very minor effect on HVA Ca2+ channel properties was found in a juvenile myoclonic epilepsy patient (142). In yet another case, a mutation in the SH3 domain of β4 (C104F) causes different symptoms in two different families: episodic ataxia in one and generalized epilepsy and praxis-induced seizures in the other, presumably as a result of different genetic backgrounds (142).
B. Cavβ in Pathophysiology
Changes in the expression level of various Cavβs have been reported in certain pathological conditions. For example, in hypertrophic obstructive cardiomyopathy, β2 is upregulated, which likely drives the observed increase in Ca2+ channels (198, 465). Downregulation of Cavβ is observed in allografts from diastolically failing hearts (228), in pancreatic islets from type 2 diabetic rats (235), and during atrial fibrillation (188). However, these observations are only correlative, and it remains unclear whether these changes are causative or incidental to the disease.
In the Lambert-Eaton myasthenic syndrome (LEMS), an autoimmune disease, autoantibodies against the extracellular loops of presynaptic Ca2+ channels disrupt channel arrays at the neuromuscular junction and impair synaptic transmission (269, 299). However, antibodies against β3 and β4 are also common in sera from LEMS patients (55% of the time), including in five of five LEMS patients who also had small-cell lung carcinoma (368). In some instances, the Cavβ autoantibodies can prevent Cavα1-Cavβ binding (368, 377). It is unclear, however, how Cavβ autoantibodies contribute to the disease, since Cavβ is an intracellular protein and is unlikely to be a target of the Cavβ autoantibodies in the intact muscle. Indeed, immunization of rats with a purified Cavβ protein causes no neuropathy in spite of the induction of high antibody titers (453). These observations and considerations suggest that Cavβ autoantibodies do not directly contribute to the pathology of the disease, but their presence can serve as an additional diagnostic tool (368).
Finally, schistosomiasis, or bilharzia, is a parasitic disease caused by Schistosoma flatworms, which infect ~200 million people in the developing world, damaging the nervous system and internal organs. It is relatively successfully cured with Praziquantel (PZQ). The exact mechanism of action is still unclear, but PZQ seems to target a variant of Schistostoma Cavβ (reviewed in Refs. 124, 190, 240). Schistosomas have one “conventional” Cavβ and one, named βvar, with a long COOH terminus and nonconserved changes in both the SH3 and GK domains (240, 263,264, 334). When expressed with a mammalian Cavα1, βvar modulates gating as expected for a Cavβ, but it causes a decrease in current amplitude (263). PZQ recovers current amplitude (263), consistent with results showing that PZQ causes a Ca2+ influx into worms, followed by a sustained muscular contraction and paralysis (240). It is not clear, however, whether βvar associates with Schistosoma Cavα1 since expressing them has been difficult (190). A new study shows that Cavβ knockdown in Schistosomas, using siRNA, confers resistance to PZQ, further implicating Cavβ (334). Thus PZQ likely targets Schistosoma Cavβ, but the downstream events remain to be elucidated (124, 190). They may involve an increase in Ca2+ channel currents but may also include other pathways. Recently, it was suggested that Ca2+ influx on its own is not sufficient to kill Schistosomas, because cytochalasin D, an inhibitor of actin polymerization, can rescue Schistosomas from PZQ, in spite of cellular Ca2+ overload (354).
XIV. PERSPECTIVES
Great strides have been made in the last two decades in our understanding of the molecular biology, structure, function, and regulation of Cavβ. An emerging theme is that Cavβ is a multifunction protein, acting primarily as a Ca2+ channel regulatory subunit but also performing Ca2+ channel-independent functions. Although much is known, many important questions and issues remain to be elucidated, some of which we highlight here.
Although it is well established that Cavβ is essential for the surface expression of HVA Ca2+ channels, it is yet unclear why Cavβ is required. The traditional view is that Cavβ facilitates the export of Cavα1 from the ER. However, no definitive ER retention signals have been found on Cavα1 that are blocked by the binding of Cavβ. An alternative possibility is that Cavα1 can traffic to the plasma membrane on its own, but its continued presence there requires Cavβ. Further studies on the role of Cavβ in Cavα1 internalization, ubiquitination, and proteasomal degradation may shed light on this issue.
Given that many isoforms of Cavβ exist, that the association between Cavβ and Cavα1 is promiscuous, and that Cavβ regulates channel gating in a Cavα1-Cavβ pair-specific manner, there is enormous combinatorial complexity. Furthermore, the reversible nature of Cavα1-Cavβ association provides a means for dynamic regulation of HVA Ca2+ channel activity. Thus, to better understand the function and regulation of HVA Ca2+ channels in native cells, it is necessary to examine the spatial and temporal expression of different Cavβ isoforms, not only at cellular levels but also at subcellular levels, as exemplified by the work in neurons (317, 336). Currently, we know very little about the molecular mechanisms governing the splicing and expression of Cavβ in different tissues, cell types, and subcellular locations (e.g., soma vs. dendrites vs. axon terminals).
Although the high-affinity interaction between the Cavβ GK domain and the AID is essential for Cavβ regulation of HVA Ca2+ channel gating, it is the interactions among other regions of Cavβ and Cavα1 that confer distinct Cavα1-Cavβ pair-specific characteristics to Cavβ regulation. We do not yet have a full grasp of the molecular determinants involved in these interactions, owing to their intrinsically low affinity. Characterizing these low-affinity interactions will remain a difficult challenge, as conventional biochemical approaches may not be sufficient to uncover the underlying molecular components and mechanisms.
Useful knowledge has been gained from Cavβ knockouts; however, lethal phenotypes and/or compensation by other Cavβs have limited the amount of information gleaned from systemic knockouts. It would be desirable to achieve inducible and tissue-specific knockout, knockdown, or overexpression of a particular Cavβ. A recent study (90) suggests that such approaches may even become useful venues for gene therapy of certain forms of cardiovascular or neurological disorders.
The list of Cavβ-interacting proteins continues to grow, but in most cases, the physiological importance of their interactions with Cavβ in native cells remains unclear. Some VGCC-independent functions of Cavβ are presented in this review, but almost certainly more are to be discovered. In this regard, it would be particularly interesting to investigate whether, and under what conditions, full-length Cavβs participate in regulating gene expression in native cells.
To better understand the function of Cavβ, it would be valuable to obtain high-resolution structures of full-length Cavβs, by themselves and in complex with their various interacting partners. These are clearly long-term goals, and there will undoubtedly be technical challenges in such endeavors, but the successful determination of the crystal structure of three different Cavβ cores’ and two different AID-Cavβ core complexes warrants optimism.
ACKNOWLEDGMENTS
We thank Kathryn Abele for reading and commenting on a draft of this article, Minghui Li and Yong Yu for helping with figures, and Linling He, Yun Zhang, and Mingming Fan for discussion.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-53494 and NS-45383 (to J. Yang).
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Abiria SA, Colbran RJ. CaMKII associates with Cav1.2 L-type calcium channels via selected β subunits to enhance regulatory phosphorylation. J Neurochem. 2010;112:150–161. doi: 10.1111/j.1471-4159.2009.06436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams B, Tanabe T. Structural regions of the cardiac Ca channel α subunit involved in Ca-dependent inactivation. J Gen Physiol. 1997;110:379–389. doi: 10.1085/jgp.110.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agler HL, Evans J, Tay LH, Anderson MJ, Colecraft HM, Yue DT. G protein-gated inhibitory module of N-type Cav2.2 Ca2+ channels. Neuron. 2005;46:891–904. doi: 10.1016/j.neuron.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Ahlijanian MK, Westenbroek RE, Catterall WA. Subunit structure and localization of dihydropyridine-sensitive calcium channels in mammalian brain, spinal cord, and retina. Neuron. 1990;4:819–832. doi: 10.1016/0896-6273(90)90135-3. [DOI] [PubMed] [Google Scholar]
- 5.Altier C, Garcia-Caballero A, Simms B, Walcher J, Tedford HW, Hermosilla G, Zamponi GW. Program No. 519.13.2009 Neuroscience meeting planner. Chicago, IL: Society for Neuroscience; 2009. The Cav β subunit prevents Nedd4 mediated ubiquitination and proteasomal degradation of L-type calcium channels via the Derlin-1/p97 ERAD protein complex. Online. [Google Scholar]
- 6.Alvarez J, Hamplova J, Hohaus A, Morano I, Haase H, Vassort G. Calcium current in rat cardiomyocytes is modulated by the carboxyl-terminal ahnak domain. J Biol Chem. 2004;279:12456–12461. doi: 10.1074/jbc.M312177200. [DOI] [PubMed] [Google Scholar]
- 7.Amagai M. A mystery of AHNAK/desmoyokin still goes on. J Invest Dermatol. 2004;123:xiv–xv. doi: 10.1111/j.0022-202X.2004.23432.x. [DOI] [PubMed] [Google Scholar]
- 8.Antzelevitch C, Pollevick GD, Cordeiro JM, Casis O, Sanguinetti MC, Aizawa Y, Guerchicoff A, Pfeiffer R, Oliva A, Wollnik B, Gelber P, Bonaros EP, Jr, Burashnikov E, Wu Y, Sargent JD, Schickel S, Oberheiden R, Bhatia A, Hsu LF, Haissaguerre M, Schimpf R, Borggrefe M, Wolpert C. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation. 2007;115:442–449. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arias JM, Murbartian J, Vitko I, Lee JH, Perez-Reyes E. Transfer of β subunit regulation from high to low voltage-gated Ca2+ channels. FEBS Lett. 2005;579:3907–3912. doi: 10.1016/j.febslet.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol. 2003;13:298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 11.Badou A, Jha MK, Matza D, Mehal WZ, Freichel M, Flockerzi V, Flavell RA. Critical role for the β regulatory subunits of Cav channels in T lymphocyte function. Proc Natl Acad Sci USA. 2006;103:15529–15534. doi: 10.1073/pnas.0607262103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker PF, Meves H, Ridgway EB. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973;231:511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ball SL, Gregg RG. Using mutant mice to study the role of voltage-gated calcium channels in the retina. Adv Exp Med Biol. 2002;514:439–450. doi: 10.1007/978-1-4615-0121-3_26. [DOI] [PubMed] [Google Scholar]
- 14.Ball SL, Powers PA, Shin HS, Morgans CW, Peachey NS, Gregg RG. Role of the β2 subunit of voltage-dependent calcium channels in the retinal outer plexiform layer. Invest Ophthalmol Visual Sci. 2002;43:1595–1603. [PubMed] [Google Scholar]
- 15.Bannister RA, Colecraft HM, Beam KG. Rem inhibits skeletal muscle EC coupling by reducing the number of functional L-type Ca2+ channels. Biophys J. 2008;94:2631–2638. doi: 10.1529/biophysj.107.116467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbado M, Fablet K, Ronjat M, De Waard M. Gene regulation by voltage-dependent calcium channels. Biochim Biophys Acta. 2009;1793:1096–1104. doi: 10.1016/j.bbamcr.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Barclay J, Rees M. Mouse models of spike-wave epilepsy. Epilepsia. 1999;40(Suppl 3):17–22. doi: 10.1111/j.1528-1157.1999.tb00894.x. [DOI] [PubMed] [Google Scholar]
- 18.Barrett CF, Liu L, Rittenhouse AR. Arachidonic acid reversibly enhances N-type calcium current at an extracellular site. Am J Physiol Cell Physiol. 2001;280:C1306–C1318. doi: 10.1152/ajpcell.2001.280.5.C1306. [DOI] [PubMed] [Google Scholar]
- 19.Barrett CF, Rittenhouse AR. Modulation of N-type calcium channel activity by G-proteins and protein kinase C. J Gen Physiol. 2000;115:277–286. doi: 10.1085/jgp.115.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bean BP. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- 21.Bean BP. Modulating modulation. J Gen Physiol. 2000;115:273–275. doi: 10.1085/jgp.115.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bean BP. Multiple types of calcium channels in heart muscle and neurons. Modulation by drugs and neurotransmitters. Ann NY Acad Sci. 1989;560:334–345. doi: 10.1111/j.1749-6632.1989.tb24113.x. [DOI] [PubMed] [Google Scholar]
- 23.Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- 24.Beguin P, Mahalakshmi RN, Nagashima K, Cher DH, Ikeda H, Yamada Y, Seino Y, Hunziker W. Nuclear sequestration of β-subunits by Rad and Rem is controlled by 14–3-3 and calmodulin and reveals a novel mechanism for Ca2+ channel regulation. J Mol Biol. 2006;355:34–46. doi: 10.1016/j.jmb.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Beguin P, Mahalakshmi RN, Nagashima K, Cher DH, Kuwamura N, Yamada Y, Seino Y, Hunziker W. Roles of 14–3-3 and calmodulin binding in subcellular localization and function of the small G-protein Rem2. Biochem J. 2005;390:67–75. doi: 10.1042/BJ20050414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beguin P, Mahalakshmi RN, Nagashima K, Cher DH, Takahashi A, Yamada Y, Seino Y, Hunziker W. 14–3-3 and calmodulin control subcellular distribution of Kir/Gem and its regulation of cell shape and calcium channel activity. J Cell Sci. 2005;118:1923–1934. doi: 10.1242/jcs.02321. [DOI] [PubMed] [Google Scholar]
- 27.Beguin P, Nagashima K, Gonoi T, Shibasaki T, Takahashi K, Kashima Y, Ozaki N, Geering K, Iwanaga T, Seino S. Regulation of Ca2+ channel expression at the cell surface by the small G-protein kir/Gem. Nature. 2001;411:701–706. doi: 10.1038/35079621. [DOI] [PubMed] [Google Scholar]
- 28.Beguin P, Ng YJ, Krause C, Mahalakshmi RN, Ng MY, Hunziker W. RGK small GTP-binding proteins interact with the nucleotide kinase domain of Ca2+-channel β-subunits via an uncommon effector binding domain. J Biol Chem. 2007;282:11509–11520. doi: 10.1074/jbc.M606423200. [DOI] [PubMed] [Google Scholar]
- 29.Beharier O, Etzion Y, Katz A, Friedman H, Tenbosh N, Zacharish S, Bereza S, Goshen U, Moran A. Crosstalk between L-type calcium channels and ZnT-1, a new player in rate-dependent cardiac electrical remodeling. Cell Calcium. 2007;42:71–82. doi: 10.1016/j.ceca.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Bell DC, Butcher AJ, Berrow NS, Page KM, Brust PF, Nesterova A, Stauderman KA, Seabrook GR, Nurnberg B, Dolphin AC. Biophysical properties, pharmacology, modulation of human, neuronal L-type α1D, and Cav1.3 voltage-dependent calcium currents. J Neurophysiol. 2001;85:816–827. doi: 10.1152/jn.2001.85.2.816. [DOI] [PubMed] [Google Scholar]
- 31.Berggren PO, Yang SN, Murakami M, Efanov AM, Uhles S, Kohler M, Moede T, Fernstrom A, Appelskog IB, Aspinwall CA, Zaitsev SV, Larsson O, de Vargas LM, Fecher-Trost C, Weissgerber P, Ludwig A, Leibiger B, Juntti-Berggren L, Barker CJ, Gromada J, Freichel M, Leibiger IB, Flockerzi V. Removal of Ca2+ channel β3 subunit enhances Ca2+ oscillation frequency and insulin exocytosis. Cell. 2004;119:273–284. doi: 10.1016/j.cell.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 32.Bernstein GM, Jones OT. Kinetics of internalization and degradation of N-type voltage-gated calcium channels: role of the α2/δ subunit. Cell Calcium. 2007;41:27–40. doi: 10.1016/j.ceca.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Berrou L, Dodier Y, Raybaud A, Tousignant A, Dafi O, Pelletier JN, Parent L. The C-terminal residues in the α-interacting domain (AID) helix anchor Cavβ subunit interaction and modulation of Cav2.3 channels. J Biol Chem. 2005;280:494–505. doi: 10.1074/jbc.M410859200. [DOI] [PubMed] [Google Scholar]
- 34.Berrou L, Klein H, Bernatchez G, Parent L. A specific tryptophan in the I–II linker is a key determinant of β-subunit binding and modulation in Cav2.3 calcium channels. Biophys J. 2002;83:1429–1442. doi: 10.1016/S0006-3495(02)73914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berrow NS, Campbell V, Fitzgerald EM, Brickley K, Dolphin AC. Antisense depletion of β-subunits modulates the biophysical and pharmacological properties of neuronal calcium channels. J Physiol. 1995;482:481–491. doi: 10.1113/jphysiol.1995.sp020534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertram R, Swanson J, Yousef M, Feng ZP, Zamponi GW. A minimal model for G protein-mediated synaptic facilitation and depression. J Neurophysiol. 2003;90:1643–1653. doi: 10.1152/jn.00190.2003. [DOI] [PubMed] [Google Scholar]
- 37.Beurg M, Ahern CA, Vallejo P, Conklin MW, Powers PA, Gregg RG, Coronado R. Involvement of the carboxy-terminus region of the dihydropyridine receptor β1a subunit in excitation-contraction coupling of skeletal muscle. Biophys J. 1999;77:2953–2967. doi: 10.1016/S0006-3495(99)77128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beurg M, Sukhareva M, Ahern CA, Conklin MW, Perez-Reyes E, Powers PA, Gregg RG, Coronado R. Differential regulation of skeletal muscle L-type Ca2+ current and excitation-contraction coupling by the dihydropyridine receptor β subunit. Biophys J. 1999;76:1744–1756. doi: 10.1016/S0006-3495(99)77336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bichet D, Cornet V, Geib S, Carlier E, Volsen S, Hoshi T, Mori Y, De Waard M. The I–II loop of the Ca2+ channel α1 subunit contains an endoplasmic reticulum retention signal antagonized by the β subunit. Neuron. 2000;25:177–190. doi: 10.1016/s0896-6273(00)80881-8. [DOI] [PubMed] [Google Scholar]
- 40.Birnbaumer L, Qin N, Olcese R, Tareilus E, Platano D, Costantin J, Stefani E. Structures and functions of calcium channel β subunits. J Bioenerg Biomembr. 1998;30:357–375. doi: 10.1023/a:1021989622656. [DOI] [PubMed] [Google Scholar]
- 41.Black JL., 3rd The voltage-gated calcium channel γ subunits: a review of the literature. J Bioenerg Biomembr. 2003;35:649–660. doi: 10.1023/b:jobb.0000008029.22650.c5. [DOI] [PubMed] [Google Scholar]
- 42.Blair LA, Marshall J. IGF-1 modulates N and L calcium channels in a PI 3-kinase-dependent manner. Neuron. 1997;19:421–429. doi: 10.1016/s0896-6273(00)80950-2. [DOI] [PubMed] [Google Scholar]
- 43.Blaszczyk J, Li Y, Yan H, Ji X. Crystal structure of unligated guanylate kinase from yeast reveals GMP-induced conformational changes. J Mol Biol. 2001;307:247–257. doi: 10.1006/jmbi.2000.4427. [DOI] [PubMed] [Google Scholar]
- 44.Bogdanov Y, Brice NL, Canti C, Page KM, Li M, Volsen SG, Dolphin AC. Acidic motif responsible for plasma membrane association of the voltage-dependent calcium channel β1b subunit. Eur J Neurosci. 2000;12:894–902. doi: 10.1046/j.1460-9568.2000.00981.x. [DOI] [PubMed] [Google Scholar]
- 45.Boland LM, Bean BP. Modulation of N-type calcium channels in bullfrog sympathetic neurons by luteinizing hormone-releasing hormone: kinetics and voltage dependence. J Neurosci. 1993;13:516–533. doi: 10.1523/JNEUROSCI.13-02-00516.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boland LM, Drzewiecki MM. Polyunsaturated fatty acid modulation of voltage-gated ion channels. Cell Biochem Biophys. 2008;52:59–84. doi: 10.1007/s12013-008-9027-2. [DOI] [PubMed] [Google Scholar]
- 47.Bourinet E, Charnet P, Tomlinson WJ, Stea A, Snutch TP, Nargeot J. Voltage-dependent facilitation of a neuronal α 1C L-type calcium channel. EMBO J. 1994;13:5032–5039. doi: 10.1002/j.1460-2075.1994.tb06832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bourinet E, Soong TW, Stea A, Snutch TP. Determinants of the G protein-dependent opioid modulation of neuronal calcium channels. Proc Natl Acad Sci USA. 1996;93:1486–1491. doi: 10.1073/pnas.93.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bouron A, Soldatov NM, Reuter H. The β1-subunit is essential for modulation by protein kinase C of an human and a non-human L-type Ca2+ channel. FEBS Lett. 1995;377:159–162. doi: 10.1016/0014-5793(95)01327-x. [DOI] [PubMed] [Google Scholar]
- 50.Brice NL, Berrow NS, Campbell V, Page KM, Brickley K, Tedder I, Dolphin AC. Importance of the different β subunits in the membrane expression of the α1A and α2 calcium channel subunits: studies using a depolarization-sensitive α1A antibody. Eur J Neurosci. 1997;9:749–759. doi: 10.1111/j.1460-9568.1997.tb01423.x. [DOI] [PubMed] [Google Scholar]
- 51.Brice NL, Dolphin AC. Differential plasma membrane targeting of voltage-dependent calcium channel subunits expressed in a polarized epithelial cell line. J Physiol. 1999;515:685–694. doi: 10.1111/j.1469-7793.1999.685ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brody DL, Patil PG, Mulle JG, Snutch TP, Yue DT. Bursts of action potential waveforms relieve G-protein inhibition of recombinant P/Q-type Ca2+ channels in HEK 293 cells. J Physiol. 1997;499:637–644. doi: 10.1113/jphysiol.1997.sp021956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Budde T, Meuth S, Pape HC. Calcium-dependent inactivation of neuronal calcium channels. Nat Rev Neurosci. 2002;3:873–883. doi: 10.1038/nrn959. [DOI] [PubMed] [Google Scholar]
- 54.Bunemann M, Gerhardstein BL, Gao T, Hosey MM. Functional regulation of L-type calcium channels via protein kinase A-mediated phosphorylation of the β2 subunit. J Biol Chem. 1999;274:33851–33854. doi: 10.1074/jbc.274.48.33851. [DOI] [PubMed] [Google Scholar]
- 55.Burgess DL, Jones JM, Meisler MH, Noebels JL. Mutation of the Ca2+ channel β subunit gene Cchb4 is associated with ataxia and seizures in the lethargic (lh) mouse. Cell. 1997;88:385–392. doi: 10.1016/s0092-8674(00)81877-2. [DOI] [PubMed] [Google Scholar]
- 56.Butcher AJ, Leroy J, Richards MW, Pratt WS, Dolphin AC. The importance of occupancy rather than affinity of Cavβ subunits for the calcium channel I–II linker in relation to calcium channel function. J Physiol. 2006;574:387–398. doi: 10.1113/jphysiol.2006.109744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caddick SJ, Wang C, Fletcher CF, Jenkins NA, Copeland NG, Hosford DA. Excitatory but not inhibitory synaptic transmission is reduced in lethargic [Cacnb4(lh)] and tottering (Cacna1atg) mouse thalami. J Neurophysiol. 1999;81:2066–2074. doi: 10.1152/jn.1999.81.5.2066. [DOI] [PubMed] [Google Scholar]
- 58.Cahill AL, Hurley JH, Fox AP. Coexpression of cloned α1B, β2a, α2/δ subunits produces non-inactivating calcium currents similar to those found in bovine chromaffin cells. J Neurosci. 2000;20:1685–1693. doi: 10.1523/JNEUROSCI.20-05-01685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campbell KP, Leung AT, Imagawa T. Structural characterization of the nitrendipine receptor of the voltage-dependent Ca2+ channel: evidence for a 52,000 dalton subunit. J Cardiovasc Pharmacol. 1988;12(Suppl 4):S86–S90. doi: 10.1097/00005344-198806124-00017. [DOI] [PubMed] [Google Scholar]
- 60.Campbell V, Berrow NS, Fitzgerald EM, Brickley K, Dolphin AC. Inhibition of the interaction of G protein Go with calcium channels by the calcium channel β-subunit in rat neurones. J Physiol. 1995;485:365–372. doi: 10.1113/jphysiol.1995.sp020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Canti C, Bogdanov Y, Dolphin AC. Interaction between G proteins and accessory subunits in the regulation of 1B calcium channels in Xenopus oocytes. J Physiol. 2000;527:419–432. doi: 10.1111/j.1469-7793.2000.t01-1-00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Canti C, Davies A, Berrow NS, Butcher AJ, Page KM, Dolphin AC. Evidence for two concentration-dependent processes for β-subunit effects on α1B calcium channels. Biophys J. 2001;81:1439–1451. doi: 10.1016/S0006-3495(01)75799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Canti C, Davies A, Dolphin AC. Calcium channel α2δ subunits: structure, functions and target site for drugs. Curr Neuropharmacol. 2003;1:209–217. [Google Scholar]
- 64.Canti C, Nieto-Rostro M, Foucault I, Heblich F, Wratten J, Richards MW, Hendrich J, Douglas L, Page KM, Davies A, Dolphin AC. The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of α2δ subunits is key to trafficking voltage-gated Ca2+ channels. Proc Natl Acad Sci USA. 2005;102:11230–11235. doi: 10.1073/pnas.0504183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carbone E, Lux HD. A low voltage-activated calcium conductance in embryonic chick sensory neurons. Biophys J. 1984;46:413–418. doi: 10.1016/S0006-3495(84)84037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carbone E, Lux HD. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984;310:501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- 67.Castellano A, Wei X, Birnbaumer L, Perez-Reyes E. Cloning and expression of a neuronal calcium channel β subunit. J Biol Chem. 1993;268:12359–12366. [PubMed] [Google Scholar]
- 68.Castellano A, Wei X, Birnbaumer L, Perez-Reyes E. Cloning and expression of a third calcium channel β subunit. J Biol Chem. 1993;268:3450–3455. [PubMed] [Google Scholar]
- 69.Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. RIM1α is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- 70.Catalucci D, Zhang DH, DeSantiago J, Aimond F, Barbara G, Chemin J, Bonci D, Picht E, Rusconi F, Dalton ND, Peterson KL, Richard S, Bers DM, Brown JH, Condorelli G. Akt regulates L-type Ca2+ channel activity by modulating Cavα1 protein stability. J Cell Biol. 2009;184:923–933. doi: 10.1083/jcb.200805063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Catterall WA. Molecular mechanisms of gating and drug block of sodium channels. Novartis Found Symp. 2002;241:206–232. [PubMed] [Google Scholar]
- 72.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 73.Catterall WA, Dib-Hajj S, Meisler MH, Pietrobon D. Inherited neuronal ion channelopathies: new windows on complex neurological diseases. J Neurosci. 2008;28:11768–11777. doi: 10.1523/JNEUROSCI.3901-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59:882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 75.Cens T, Mangoni ME, Richard S, Nargeot J, Charnet P. Coexpression of the β2 subunit does not induce voltage-dependent facilitation of the class C L-type Ca channel. Pflügers Arch. 1996;431:771–774. [PubMed] [Google Scholar]
- 76.Cens T, Restituito S, Galas S, Charnet P. Voltage and calcium use the same molecular determinants to inactivate calcium channels. J Biol Chem. 1999;274:5483–5490. doi: 10.1074/jbc.274.9.5483. [DOI] [PubMed] [Google Scholar]
- 77.Cens T, Restituito S, Vallentin A, Charnet P. Promotion and inhibition of L-type Ca2+ channel facilitation by distinct domains of the subunit. J Biol Chem. 1998;273:18308–18315. doi: 10.1074/jbc.273.29.18308. [DOI] [PubMed] [Google Scholar]
- 78.Cens T, Rousset M, Leyris JP, Fesquet P, Charnet P. Voltage-and calcium-dependent inactivation in high voltage-gated Ca2+ channels. Prog Biophys Mol Biol. 2006;90:104–117. doi: 10.1016/j.pbiomolbio.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 79.Chang L, Zhang J, Tseng YH, Xie CQ, Ilany J, Bruning JC, Sun Z, Zhu X, Cui T, Youker KA, Yang Q, Day SM, Kahn CR, Chen YE. Rad GTPase deficiency leads to cardiac hypertrophy. Circulation. 2007;116:2976–2983. doi: 10.1161/CIRCULATIONAHA.107.707257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chaudhuri D, Alseikhan BA, Chang SY, Soong TW, Yue DT. Developmental activation of calmodulin-dependent facilitation of cerebellar P-type Ca2+ current. J Neurosci. 2005;25:8282–8294. doi: 10.1523/JNEUROSCI.2253-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen H, Puhl HL, 3rd, Niu SL, Mitchell DC, Ikeda SR. Expression of Rem2, an RGK family small GTPase, reduces N-type calcium current without affecting channel surface density. J Neurosci. 2005;25:9762–9772. doi: 10.1523/JNEUROSCI.3111-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen RS, Deng TC, Garcia T, Sellers ZM, Best PM. Calcium channel γ subunits: a functionally diverse protein family. Cell Biochem Biophys. 2007;47:178–186. doi: 10.1007/s12013-007-0002-0. [DOI] [PubMed] [Google Scholar]
- 83.Chen YH, He LL, Buchanan DR, Zhang Y, Fitzmaurice A, Yang J. Functional dissection of the intramolecular Src homology 3-guanylate kinase domain coupling in voltage-gated Ca2+ channel β-subunits. FEBS Lett. 2009;583:1969–1975. doi: 10.1016/j.febslet.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen YH, Li MH, Zhang Y, He LL, Yamada Y, Fitzmaurice A, Shen Y, Zhang H, Tong L, Yang J. Structural basis of the α1-β subunit interaction of voltage-gated Ca2+ channels. Nature. 2004;429:675–680. doi: 10.1038/nature02641. [DOI] [PubMed] [Google Scholar]
- 85.Cheng W, Altafaj X, Ronjat M, Coronado R. Interaction between the dihydropyridine receptor Ca2+ channel β-subunit and ryanodine receptor type 1 strengthens excitation-contraction coupling. Proc Natl Acad Sci USA. 2005;102:19225–19230. doi: 10.1073/pnas.0504334102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chien AJ, Carr KM, Shirokov RE, Rios E, Hosey MM. Identification of palmitoylation sites within the L-type calcium channel β2a subunit and effects on channel function. J Biol Chem. 1996;271:26465–26468. doi: 10.1074/jbc.271.43.26465. [DOI] [PubMed] [Google Scholar]
- 87.Chien AJ, Gao T, Perez-Reyes E, Hosey MM. Membrane targeting of L-type calcium channels. Role of palmitoylation in the sub-cellular localization of the β2a subunit. J Biol Chem. 1998;273:23590–23597. doi: 10.1074/jbc.273.36.23590. [DOI] [PubMed] [Google Scholar]
- 88.Chien AJ, Zhao X, Shirokov RE, Puri TS, Chang CF, Sun D, Rios E, Hosey MM. Roles of a membrane-localized β subunit in the formation and targeting of functional L-type Ca2+ channels. J Biol Chem. 1995;270:30036–30044. doi: 10.1074/jbc.270.50.30036. [DOI] [PubMed] [Google Scholar]
- 89.Chu PJ, Larsen JK, Chen CC, Best PM. Distribution and relative expression levels of calcium channel β subunits within the chambers of the rat heart. J Mol Cell Cardiol. 2004;36:423–434. doi: 10.1016/j.yjmcc.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 90.Cingolani E, Ramirez Correa GA, Kizana E, Murata M, Cho HC, Marban E. Gene therapy to inhibit the calcium channel β subunit: physiological consequences and pathophysiological effects in models of cardiac hypertrophy. Circ Res. 2007;101:166–175. doi: 10.1161/CIRCRESAHA.107.155721. [DOI] [PubMed] [Google Scholar]
- 91.Cohen L, Mohr R, Chen YY, Huang M, Kato R, Dorin D, Tamanoi F, Goga A, Afar D, Rosenberg N. Transcriptional activation of a ras-like gene (kir) by oncogenic tyrosine kinases. Proc Natl Acad Sci USA. 1994;91:12448–12452. doi: 10.1073/pnas.91.26.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cohen RM, Foell JD, Balijepalli RC, Shah V, Hell JW, Kamp TJ. Unique modulation of L-type Ca2+ channels by short auxiliary β1d subunit present in cardiac muscle. Am J Physiol Heart Circ Physiol. 2005;288:H2363–H2374. doi: 10.1152/ajpheart.00348.2004. [DOI] [PubMed] [Google Scholar]
- 93.Colecraft HM, Alseikhan B, Takahashi SX, Chaudhuri D, Mittman S, Yegnasubramanian V, Alvania RS, Johns DC, Marban E, Yue DT. Novel functional properties of Ca2+ channel β subunits revealed by their expression in adult rat heart cells. J Physiol. 2002;541:435–452. doi: 10.1113/jphysiol.2002.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Collin T, Lory P, Taviaux S, Courtieu C, Guilbault P, Berta P, Nargeot J. Cloning, chromosomal location and functional expression of the human voltage-dependent calcium-channel β3 subunit. Eur J Biochem. 1994;220:257–262. doi: 10.1111/j.1432-1033.1994.tb18621.x. [DOI] [PubMed] [Google Scholar]
- 95.Collin T, Wang JJ, Nargeot J, Schwartz A. Molecular cloning of three isoforms of the L-type voltage-dependent calcium channel β subunit from normal human heart. Circ Res. 1993;72:1337–1344. doi: 10.1161/01.res.72.6.1337. [DOI] [PubMed] [Google Scholar]
- 96.Cooper CB, Arnot MI, Feng ZP, Jarvis SE, Hamid J, Zamponi GW. Cross-talk between G-protein and protein kinase C modulation of N-type calcium channels is dependent on the G-protein β subunit isoform. J Biol Chem. 2000;275:40777–40781. doi: 10.1074/jbc.C000673200. [DOI] [PubMed] [Google Scholar]
- 97.Cordeiro JM, Marieb M, Pfeiffer R, Calloe K, Burashnikov E, Antzelevitch C. Accelerated inactivation of the L-type calcium current due to a mutation in CACNB2b underlies Brugada syndrome. J Mol Cell Cardiol. 2009;46:695–703. doi: 10.1016/j.yjmcc.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cork RJ, Namkung Y, Shin HS, Mize RR. Development of the visual pathway is disrupted in mice with a targeted disruption of the calcium channel β3-subunit gene. J Comp Neurol. 2001;440:177–191. doi: 10.1002/cne.1378. [DOI] [PubMed] [Google Scholar]
- 99.Cornet V, Bichet D, Sandoz G, Marty I, Brocard J, Bourinet E, Mori Y, Villaz M, De Waard M. Multiple determinants in voltage-dependent P/Q calcium channels control their retention in the endoplasmic reticulum. Eur J Neurosci. 2002;16:883–895. doi: 10.1046/j.1460-9568.2002.02168.x. [DOI] [PubMed] [Google Scholar]
- 100.Coronado R, Ahern CA, Sheridan DC, Cheng W, Carbonneau L, Bhattacharya D. Functional equivalence of dihydropyridine receptor α1S and β1a subunits in triggering excitation-contraction coupling in skeletal muscle. Biol Res. 2004;37:565–575. doi: 10.4067/s0716-97602004000400010. [DOI] [PubMed] [Google Scholar]
- 101.Correll RN, Botzet GJ, Satin J, Andres DA, Finlin BS. Analysis of the Rem2-voltage dependant calcium channel β subunit interaction and Rem2 interaction with phosphorylated phosphatidylinositide lipids. Cell Signal. 2008;20:400–408. doi: 10.1016/j.cellsig.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Correll RN, Pang C, Finlin BS, Dailey AM, Satin J, Andres DA. Plasma membrane targeting is essential for Rem-mediated Ca2+ channel inhibition. J Biol Chem. 2007;282:28431–28440. doi: 10.1074/jbc.M706176200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Correll RN, Pang C, Niedowicz DM, Finlin BS, Andres DA. The RGK family of GTP-binding proteins: regulators of voltage-dependent calcium channels and cytoskeleton remodeling. Cell Signal. 2008;20:292–300. doi: 10.1016/j.cellsig.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Correll RN, Pang C, Niedowicz DM, Satin J, Andres DA. Calmodulin binding is dispensable for Rem-mediated Ca2+ channel inhibition. Mol Cell Biochem. 2008;310:103–110. doi: 10.1007/s11010-007-9670-8. [DOI] [PubMed] [Google Scholar]
- 106.Crump SM, Correll RN, Schroder EA, Lester WC, Finlin BS, Andres DA, Satin J. L-type calcium channel α-subunit and protein kinase inhibitors modulate Rem-mediated regulation of current. Am J Physiol Heart Circ Physiol. 2006;291:H1959–H1971. doi: 10.1152/ajpheart.00956.2005. [DOI] [PubMed] [Google Scholar]
- 107.Curtis BM, Catterall WA. Purification of the calcium antagonist receptor of the voltage-sensitive calcium channel from skeletal muscle transverse tubules. Biochemistry. 1984;23:2113–2118. doi: 10.1021/bi00305a001. [DOI] [PubMed] [Google Scholar]
- 108.Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dai S, Klugbauer N, Zong X, Seisenberger C, Hofmann F. The role of subunit composition on prepulse facilitation of the cardiac L-type calcium channel. FEBS Lett. 1999;442:70–74. doi: 10.1016/s0014-5793(98)01632-9. [DOI] [PubMed] [Google Scholar]
- 110.Dalton S, Takahashi SX, Miriyala J, Colecraft HM. A single Cavβ can reconstitute both trafficking and macroscopic conductance of voltage-dependent calcium channels. J Physiol. 2005;567:757–769. doi: 10.1113/jphysiol.2005.093195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Davies A, Douglas L, Hendrich J, Wratten J, Tran Van Minh A, Foucault I, Koch D, Pratt WS, Saibil HR, Dolphin AC. The Calcium Channel α2δ–2 Subunit Partitions with Cav2.1 into Lipid Rafts in Cerebellum: Implications for Localization and Function. J Neurosci. 2006;26:8748–8757. doi: 10.1523/JNEUROSCI.2764-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC. Functional biology of the α2δ subunits of voltage-gated calcium channels. Trends Pharmacol Sci. 2007;28:220–228. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 113.Davies A, Kadurin I, Alvarez-Laviada A, Douglas L, Nieto-Rostro M, Bauer CS, Pratt WS, Dolphin AC. The α2δ subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function. Proc Natl Acad Sci USA. 2010;107:1654–1659. doi: 10.1073/pnas.0908735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.De Jongh KS, Merrick DK, Catterall WA. Subunits of purified calcium channels: a 212-kDa form of α1 and partial amino acid sequence of a phosphorylation site of an independent β subunit. Proc Natl Acad Sci USA. 1989;86:8585–8589. doi: 10.1073/pnas.86.21.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.De Jongh KS, Murphy BJ, Colvin AA, Hell JW, Takahashi M, Catterall WA. Specific phosphorylation of a site in the full-length form of the α1 subunit of the cardiac L-type calcium channel by adenosine 3’,5’-cyclic monophosphate-dependent protein kinase. Biochemistry. 1996;35:10392–10402. doi: 10.1021/bi953023c. [DOI] [PubMed] [Google Scholar]
- 116.De Waard M, Campbell KP. Subunit regulation of the neuronal α1A Ca2+ channel expressed in Xenopus oocytes. J Physiol. 1995;485:619–634. doi: 10.1113/jphysiol.1995.sp020757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.De Waard M, Hering J, Weiss N, Feltz A. How do G proteins directly control neuronal Ca2+ channel function? Trends Pharmacol Sci. 2005;26:427–436. doi: 10.1016/j.tips.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 118.De Waard M, Liu H, Walker D, Scott VE, Gurnett CA, Campbell KP. Direct binding of G-protein βγ complex to voltage-dependent calcium channels. Nature. 1997;385:446–450. doi: 10.1038/385446a0. [DOI] [PubMed] [Google Scholar]
- 119.De Waard M, Pragnell M, Campbell KP. Ca2+ channel regulation by a conserved β subunit domain. Neuron. 1994;13:495–503. doi: 10.1016/0896-6273(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 120.De Waard M, Scott VE, Pragnell M, Campbell KP. Identification of critical amino acids involved in α1-β interaction in voltage-dependent Ca2+ channels. FEBS Lett. 1996;380:272–276. doi: 10.1016/0014-5793(96)00007-5. [DOI] [PubMed] [Google Scholar]
- 121.De Waard M, Witcher DR, Pragnell M, Liu H, Campbell KP. Properties of the α1-β anchoring site in voltage-dependent Ca2+ channels. J Biol Chem. 1995;270:12056–12064. doi: 10.1074/jbc.270.20.12056. [DOI] [PubMed] [Google Scholar]
- 122.Delmas P, Coste B, Gamper N, Shapiro MS. Phosphoinositide lipid second messengers: new paradigms for calcium channel modulation. Neuron. 2005;47:179–182. doi: 10.1016/j.neuron.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 123.Dickman DK, Kurshan PT, Schwarz TL. Mutations in a Drosophila α2δ voltage-gated calcium channel subunit reveal a crucial synaptic function. J Neurosci. 2008;28:31–38. doi: 10.1523/JNEUROSCI.4498-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Doenhoff MJ, Cioli D, Utzinger J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr Opin Infect Dis. 2008;21:659–667. doi: 10.1097/QCO.0b013e328318978f. [DOI] [PubMed] [Google Scholar]
- 125.Dolphin AC. β Subunits of voltage-gated calcium channels. J Bioenerg Biomembr. 2003;35:599–620. doi: 10.1023/b:jobb.0000008026.37790.5a. [DOI] [PubMed] [Google Scholar]
- 126.Dolphin AC. G protein modulation of voltage-gated calcium channels. Pharmacol Rev. 2003;55:607–627. doi: 10.1124/pr.55.4.3. [DOI] [PubMed] [Google Scholar]
- 127.Dolphin AC. A short history of voltage-gated calcium channels. Br J Pharmacol. 2006;147(Suppl 1):S56–S62. doi: 10.1038/sj.bjp.0706442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 129.Dresviannikov AV, Page KM, Leroy J, Pratt WS, Dolphin AC. Determinants of the voltage dependence of G protein modulation within calcium channel β subunits. Pflügers Arch. 2009;457:743–756. doi: 10.1007/s00424-008-0549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dung HC, Swigart RH. Experimental studies of “lethargic” mutant mice. Texas Rep Biol Med. 1971;29:273–288. [PubMed] [Google Scholar]
- 131.Dung HC, Swigart RH. Histo-pathologic observations of the nervous and lymphoid tissues of “lethargic” mutant mice. Texas Rep Biol Med. 1972;30:23–39. [PubMed] [Google Scholar]
- 132.Dzhura I, Neely A. Differential modulation of cardiac Ca2+ channel gating by β-subunits. Biophys J. 2003;85:274–289. doi: 10.1016/S0006-3495(03)74473-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nature Cell Biol. 2000;2:173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- 134.Eberst R, Dai S, Klugbauer N, Hofmann F. Identification and functional characterization of a calcium channel γ subunit. Pflügers Arch. 1997;433:633–637. doi: 10.1007/s004240050324. [DOI] [PubMed] [Google Scholar]
- 135.Ebert AM, McAnelly CA, Srinivasan A, Linker JL, Horne WA, Garrity DM. Ca2+ channel-independent requirement for MAGUK family CACNB4 genes in initiation of zebrafish epiboly. Proc Natl Acad Sci USA. 2008;105:198–203. doi: 10.1073/pnas.0707948105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Elias GM, Nicoll RA. Synaptic trafficking of glutamate receptors by MAGUK scaffolding proteins. Trends Cell Biol. 2007;17:343–352. doi: 10.1016/j.tcb.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 137.Ellinor PT, Zhang JF, Randall AD, Zhou M, Schwarz TL, Tsien RW, Horne WA. Functional expression of a rapidly inactivating neuronal calcium channel. Nature. 1993;363:455–458. doi: 10.1038/363455a0. [DOI] [PubMed] [Google Scholar]
- 138.Elmslie KS. Neurotransmitter modulation of neuronal calcium channels. J Bioenerg Biomembr. 2003;35:477–489. doi: 10.1023/b:jobb.0000008021.55853.18. [DOI] [PubMed] [Google Scholar]
- 139.Elmslie KS, Zhou W, Jones SW. LHRH and GTP-γ-S modify calcium current activation in bullfrog sympathetic neurons. Neuron. 1990;5:75–80. doi: 10.1016/0896-6273(90)90035-e. [DOI] [PubMed] [Google Scholar]
- 140.Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, Green EM, Lawler J, Dolmetsch R, Garcia KC, Smith SJ, Luo ZD, Rosenthal A, Mosher DF, Barres BA. Gabapentin receptor α2δ-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, Catterall WA. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- 142.Escayg A, De Waard M, Lee DD, Bichet D, Wolf P, Mayer T, Johnston J, Baloh R, Sander T, Meisler MH. Coding and non-coding variation of the human calcium-channel β4-subunit gene CACNB4 in patients with idiopathic generalized epilepsy and episodic ataxia. Am J Hum Genet. 2000;66:1531–1539. doi: 10.1086/302909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Escayg A, Jones JM, Kearney JA, Hitchcock PF, Meisler MH. Calcium channel β4 (CACNB4): human ortholog of the mouse epilepsy gene lethargic. Genomics. 1998;50:14–22. doi: 10.1006/geno.1998.5311. [DOI] [PubMed] [Google Scholar]
- 144.Fan M, Buraei Z, Luo HR, Levenson-Palmer R, Yang J. Direct inhibition of P/Q-type voltage-gated Ca2+ channels by Gem does not require a direct Gem/Cavβ interaction. Proc Natl Acad Sci USA. 2010;107:14887–14892. doi: 10.1073/pnas.1007543107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fatt P, Katz B. The electrical properties of crustacean muscle fibres. J Physiol. 1953;120:171–204. doi: 10.1113/jphysiol.1953.sp004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fedulova SA, Kostyuk PG, Veselovsky NS. Two types of calcium channels in the somatic membrane of new-born rat dorsal root ganglion neurones. J Physiol. 1985;359:431–446. doi: 10.1113/jphysiol.1985.sp015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Feng ZP, Arnot MI, Doering CJ, Zamponi GW. Calcium channel β subunits differentially regulate the inhibition of N-type channels by individual Gβ isoforms. J Biol Chem. 2001;276:45051–45058. doi: 10.1074/jbc.M107784200. [DOI] [PubMed] [Google Scholar]
- 148.Ferreiro A, Monnier N, Romero NB, Leroy JP, Bonnemann C, Haenggeli CA, Straub V, Voss WD, Nivoche Y, Jungbluth H, Lemainque A, Voit T, Lunardi J, Fardeau M, Guicheney P. A recessive form of central core disease, transiently presenting as multi-minicore disease, is associated with a homozygous mutation in the ryanodine receptor type 1 gene. Ann Neurol. 2002;51:750–759. doi: 10.1002/ana.10231. [DOI] [PubMed] [Google Scholar]
- 149.Ferron L, Davies A, Page KM, Cox DJ, Leroy J, Waithe D, Butcher AJ, Sellaturay P, Bolsover S, Pratt WS, Moss FJ, Dolphin AC. The stargazin-related protein γ7 interacts with the mRNA-binding protein heterogeneous nuclear ribonucleoprotein A2 and regulates the stability of specific mRNAs, including Cav2.2. J Neurosci. 2008;28:10604–10617. doi: 10.1523/JNEUROSCI.2709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Field MJ, Cox PJ, Stott E, Melrose H, Offord J, Su TZ, Bramwell S, Corradini L, England S, Winks J, Kinloch RA, Hendrich J, Dolphin AC, Webb T, Williams D. Identification of the α2-δ-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci USA. 2006;103:17537–17542. doi: 10.1073/pnas.0409066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Findeisen F, Minor DL., Jr Disruption of the IS6-AID linker affects voltage-gated calcium channel inactivation and facilitation. J Gen Physiol. 2009;133:327–343. doi: 10.1085/jgp.200810143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Finlin BS, Andres DA. Phosphorylation-dependent association of the Ras-related GTP-binding protein Rem with 14–3-3 proteins. Arch Biochem Biophys. 1999;368:401–412. doi: 10.1006/abbi.1999.1316. [DOI] [PubMed] [Google Scholar]
- 153.Finlin BS, Andres DA. Rem is a new member of the Rad- and Gem/Kir Ras-related GTP-binding protein family repressed by lipopolysaccharide stimulation. J Biol Chem. 1997;272:21982–21988. doi: 10.1074/jbc.272.35.21982. [DOI] [PubMed] [Google Scholar]
- 154.Finlin BS, Correll RN, Pang C, Crump SM, Satin J, Andres DA. Analysis of the complex between Ca2+ channel β-subunit and the Rem GTPase. J Biol Chem. 2006;281:23557–23566. doi: 10.1074/jbc.M604867200. [DOI] [PubMed] [Google Scholar]
- 155.Finlin BS, Crump SM, Satin J, Andres DA. Regulation of voltage-gated calcium channel activity by the Rem and Rad GTPases. Proc Natl Acad Sci USA. 2003;100:14469–14474. doi: 10.1073/pnas.2437756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Finlin BS, Mosley AL, Crump SM, Correll RN, Ozcan S, Satin J, Andres DA. Regulation of L-type Ca2+ channel activity and insulin secretion by the Rem2 GTPase. J Biol Chem. 2005;280:41864–41871. doi: 10.1074/jbc.M414261200. [DOI] [PubMed] [Google Scholar]
- 157.Finlin BS, Shao H, Kadono-Okuda K, Guo N, Andres DA. Rem2, a new member of the Rem/Rad/Gem/Kir family of Ras-related GTPases. Biochem J. 2000;347:223–231. [PMC free article] [PubMed] [Google Scholar]
- 158.Fischer R, Wei Y, Anagli J, Berchtold MW. Calmodulin binds to and inhibits GTP binding of the ras-like GTPase Kir/Gem. J Biol Chem. 1996;271:25067–25070. doi: 10.1074/jbc.271.41.25067. [DOI] [PubMed] [Google Scholar]
- 159.Fitzgerald EM. The presence of Ca2+ channel β subunit is required for mitogen-activated protein kinase (MAPK)-dependent modulation of α1B Ca2+ channels in COS-7 cells. J Physiol. 2002;543:425–437. doi: 10.1113/jphysiol.2002.022822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fitzgerald EM. Regulation of voltage-dependent calcium channels in rat sensory neurones involves a Ras-mitogen-activated protein kinase pathway. J Physiol. 2000;527:433–444. doi: 10.1111/j.1469-7793.2000.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fletcher CF, Lutz CM, O’Sullivan TN, Shaughnessy JD, Jr, Hawkes R, Frankel WN, Copeland NG, Jenkins NA. Absence epilepsy in tottering mutant mice is associated with calcium channel defects. Cell. 1996;87:607–617. doi: 10.1016/s0092-8674(00)81381-1. [DOI] [PubMed] [Google Scholar]
- 162.Flockerzi V, Oeken HJ, Hofmann F, Pelzer D, Cavalie A, Trautwein W. Purified dihydropyridine-binding site from skeletal muscle t-tubules is a functional calcium channel. Nature. 1986;323:66–68. doi: 10.1038/323066a0. [DOI] [PubMed] [Google Scholar]
- 163.Flucher BE, Kasielke N, Grabner M. The triad targeting signal of the skeletal muscle calcium channel is localized in the COOH terminus of the α1S subunit. J Cell Biol. 2000;151:467–478. doi: 10.1083/jcb.151.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Flucher BE, Obermair GJ, Tuluc P, Schredelseker J, Kern G, Grabner M. The role of auxiliary dihydropyridine receptor subunits in muscle. J Muscle Res Cell Motil. 2005;26:1–6. doi: 10.1007/s10974-005-9000-2. [DOI] [PubMed] [Google Scholar]
- 165.Flynn R, Chen L, Hameed S, Spafford JD, Zamponi GW. Molecular determinants of Rem2 regulation of N-type calcium channels. Biochem Biophys Res Commun. 2008;368:827–831. doi: 10.1016/j.bbrc.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 166.Foell JD, Balijepalli RC, Delisle BP, Yunker AM, Robia SL, Walker JW, McEnery MW, January CT, Kamp TJ. Molecular heterogeneity of calcium channel β-subunits in canine and human heart: evidence for differential subcellular localization. Physiol Genomics. 2004;17:183–200. doi: 10.1152/physiolgenomics.00207.2003. [DOI] [PubMed] [Google Scholar]
- 167.Franzini-Armstrong C, Protasi F, Ramesh V. Comparative ultrastructure of Ca2+ release units in skeletal and cardiac muscle. Ann NY Acad Sci. 1998;853:20–30. doi: 10.1111/j.1749-6632.1998.tb08253.x. [DOI] [PubMed] [Google Scholar]
- 168.Freise D, Held B, Wissenbach U, Pfeifer A, Trost C, Himmerkus N, Schweig U, Freichel M, Biel M, Hofmann F, Hoth M, Flockerzi V. Absence of the γ subunit of the skeletal muscle dihydropyridine receptor increases L-type Ca2+ currents and alters channel inactivation properties. J Biol Chem. 2000;275:14476–14481. doi: 10.1074/jbc.275.19.14476. [DOI] [PubMed] [Google Scholar]
- 169.Fuller-Bicer GA, Varadi G, Koch SE, Ishii M, Bodi I, Kadeer N, Muth JN, Mikala G, Petrashevskaya NN, Jordan MA, Zhang SP, Qin N, Flores CM, Isaacsohn I, Varadi M, Mori Y, Jones WK, Schwartz A. Targeted disruption of the voltage-dependent calcium channel α2/δ-1-subunit. Am J Physiol Heart Circ Physiol. 2009;297:H117–H124. doi: 10.1152/ajpheart.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Funke L, Dakoji S, Bredt DS. Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu Rev Biochem. 2005;74:219–245. doi: 10.1146/annurev.biochem.74.082803.133339. [DOI] [PubMed] [Google Scholar]
- 171.Gamper N, Reznikov V, Yamada Y, Yang J, Shapiro MS. Phosphatidylinositol 4,5-bisphosphate signals underlie receptor-specific Gq/11-mediated modulation of N-type Ca2+ channels. J Neurosci. 2004;24:10980–10992. doi: 10.1523/JNEUROSCI.3869-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gamper N, Shapiro MS. Regulation of ion transport proteins by membrane phosphoinositides. Nat Rev Neurosci. 2007;8:921–934. doi: 10.1038/nrn2257. [DOI] [PubMed] [Google Scholar]
- 173.Ganesan AN, Maack C, Johns DC, Sidor A, O’Rourke B. B-adrenergic stimulation of L-type Ca2+ channels in cardiac myocytes requires the distal carboxyl terminus of α1C but not serine 1928. Circ Res. 2006;98:e11–e18. doi: 10.1161/01.RES.0000202692.23001.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gao B, Sekido Y, Maximov A, Saad M, Forgacs E, Latif F, Wei MH, Lerman M, Lee JH, Perez-Reyes E, Bezprozvanny I, Minna JD. Functional properties of a new voltage-dependent calcium channel α2δ auxiliary subunit gene (CACNA2D2) J Biol Chem. 2000;275:12237–12242. doi: 10.1074/jbc.275.16.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gao T, Bunemann M, Gerhardstein BL, Ma H, Hosey MM. Role of the C terminus of the α1C (Cav1.2) subunit in membrane targeting of cardiac L-type calcium channels. J Biol Chem. 2000;275:25436–25444. doi: 10.1074/jbc.M003465200. [DOI] [PubMed] [Google Scholar]
- 176.Gao T, Chien AJ, Hosey MM. Complexes of the α1C and β subunits generate the necessary signal for membrane targeting of class C L-type calcium channels. J Biol Chem. 1999;274:2137–2144. doi: 10.1074/jbc.274.4.2137. [DOI] [PubMed] [Google Scholar]
- 177.Garcia EP, Mehta S, Blair LA, Wells DG, Shang J, Fukushima T, Fallon JR, Garner CC, Marshall J. SAP90 binds and clusters kainate receptors causing incomplete desensitization. Neuron. 1998;21:727–739. doi: 10.1016/s0896-6273(00)80590-5. [DOI] [PubMed] [Google Scholar]
- 178.Geib S, Sandoz G, Cornet V, Mabrouk K, Fund-Saunier O, Bichet D, Villaz M, Hoshi T, Sabatier JM, De Waard M. The interaction between the I–II loop and the III–IV loop of Cav2.1 contributes to voltage-dependent inactivation in a β-dependent manner. J Biol Chem. 2002;277:10003–10013. doi: 10.1074/jbc.M106231200. [DOI] [PubMed] [Google Scholar]
- 179.Geib S, Sandoz G, Mabrouk K, Matavel A, Marchot P, Hoshi T, Villaz M, Ronjat M, Miquelis R, Leveque C, de Waard M. Use of a purified and functional recombinant calcium-channel β4 subunit in surface-plasmon resonance studies. Biochem J. 2002;364:285–292. doi: 10.1042/bj3640285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gerhardstein BL, Puri TS, Chien AJ, Hosey MM. Identification of the sites phosphorylated by cyclic AMP-dependent protein kinase on the β2 subunit of L-type voltage-dependent calcium channels. Biochemistry. 1999;38:10361–10370. doi: 10.1021/bi990896o. [DOI] [PubMed] [Google Scholar]
- 181.Gerster U, Neuhuber B, Groschner K, Striessnig J, Flucher BE. Current modulation and membrane targeting of the calcium channel α1C subunit are independent functions of the β subunit. J Physiol. 1999;517:353–368. doi: 10.1111/j.1469-7793.1999.0353t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Glossmann H, Striessnig J, Hymel L, Schindler H. Purified L-type calcium channels: only one single polypeptide (α1-subunit) carries the drug receptor domains and is regulated by protein kinases. Biomed Biochim Acta. 1987;46:S351–S356. [PubMed] [Google Scholar]
- 183.Gollasch M, Ried C, Liebold M, Haller H, Hofmann F, Luft FC. High permeation of L-type Ca2+ channels at physiological [Ca2+]: homogeneity and dependence on the α1-subunit. Am J Physiol Cell Physiol. 1996;271:C842–C850. doi: 10.1152/ajpcell.1996.271.3.C842. [DOI] [PubMed] [Google Scholar]
- 184.Gonzalez-Gutierrez G, Miranda-Laferte E, Contreras G, Neely A, Hidalgo P. Swapping the I–II intracellular linker between L-type Cav1.2 and R-type Cav2.3 high-voltage gated calcium channels exchanges activation attributes. Channels. 2010;4:42–50. doi: 10.4161/chan.4.1.10562. [DOI] [PubMed] [Google Scholar]
- 185.Gonzalez-Gutierrez G, Miranda-Laferte E, Naranjo D, Hidalgo P, Neely A. Mutations of nonconserved residues within the calcium channel α1-interaction domain inhibit β-subunit potentiation. J Gen Physiol. 2008;132:383–395. doi: 10.1085/jgp.200709901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gonzalez-Gutierrez G, Miranda-Laferte E, Neely A, Hidalgo P. The Src homology 3 domain of the β-subunit of voltage-gated calcium channels promotes endocytosis via dynamin interaction. J Biol Chem. 2007;282:2156–2162. doi: 10.1074/jbc.M609071200. [DOI] [PubMed] [Google Scholar]
- 187.Gonzalez-Gutierrez G, Miranda-Laferte E, Nothmann D, Schmidt S, Neely A, Hidalgo P. The guanylate kinase domain of the β-subunit of voltage-gated calcium channels suffices to modulate gating. Proc Natl Acad Sci USA. 2008;105:14198–14203. doi: 10.1073/pnas.0806558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Grammer JB, Zeng X, Bosch RF, Kuhlkamp V. Atrial L-type Ca2+-channel, β-adrenorecptor, 5-hydroxytryptamine type 4 receptor mRNAs in human atrial fibrillation. Basic Res Cardiol. 2001;96:82–90. doi: 10.1007/s003950170081. [DOI] [PubMed] [Google Scholar]
- 189.Gray AC, Raingo J, Lipscombe D. Neuronal calcium channels: splicing for optimal performance. Cell Calcium. 2007;42:409–417. doi: 10.1016/j.ceca.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Greenberg RM. Are Ca2+ channels targets of praziquantel action? Int J Parasitol. 2005;35:1–9. doi: 10.1016/j.ijpara.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 191.Gregg RG, Messing A, Strube C, Beurg M, Moss R, Behan M, Sukhareva M, Haynes S, Powell JA, Coronado R, Powers PA. Absence of the β subunit (cchb1) of the skeletal muscle dihydropyridine receptor alters expression of the α1 subunit and eliminates excitation-contraction coupling. Proc Natl Acad Sci USA. 1996;93:13961–13966. doi: 10.1073/pnas.93.24.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Grueter CE, Abiria SA, Dzhura I, Wu Y, Ham AJ, Mohler PJ, Anderson ME, Colbran RJ. L-type Ca2+ channel facilitation mediated by phosphorylation of the β subunit by CaMKII. Mol Cell. 2006;23:641–650. doi: 10.1016/j.molcel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 193.Grueter CE, Abiria SA, Wu Y, Anderson ME, Colbran RJ. Differential regulated interactions of calcium/calmodulin-dependent protein kinase II with isoforms of voltage-gated calcium channel β subunits. Biochemistry. 2008;47:1760–1767. doi: 10.1021/bi701755q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gurnett CA, Felix R, Campbell KP. Extracellular interaction of the voltage-dependent Ca2+ channel α2δ and α1 subunits. J Biol Chem. 1997;272:18508–18512. doi: 10.1074/jbc.272.29.18508. [DOI] [PubMed] [Google Scholar]
- 195.Haase H. Ahnak, a new player in β-adrenergic regulation of the cardiac L-type Ca2+ channel. Cardiovasc Res. 2007;73:19–25. doi: 10.1016/j.cardiores.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 196.Haase H, Alvarez J, Petzhold D, Doller A, Behlke J, Erdmann J, Hetzer R, Regitz-Zagrosek V, Vassort G, Morano I. Ahnak is critical for cardiac Cav1.2 calcium channel function and its β-adrenergic regulation. FASEB J. 2005;19:1969–1977. doi: 10.1096/fj.05-3997com. [DOI] [PubMed] [Google Scholar]
- 197.Haase H, Bartel S, Karczewski P, Morano I, Krause EG. In-vivo phosphorylation of the cardiac L-type calcium channel β-subunit in response to catecholamines. Mol Cell Biochem. 1996:163–164. 99, 106. doi: 10.1007/BF00408645. [DOI] [PubMed] [Google Scholar]
- 198.Haase H, Kresse A, Hohaus A, Schulte HD, Maier M, Osterziel KJ, Lange PE, Morano I. Expression of calcium channel subunits in the normal and diseased human myocardium. J Mol Med. 1996;74:99–104. doi: 10.1007/BF00196785. [DOI] [PubMed] [Google Scholar]
- 199.Haase H, Podzuweit T, Lutsch G, Hohaus A, Kostka S, Lindschau C, Kott M, Kraft R, Morano I. Signaling from β-adrenoceptor to L-type calcium channel: identification of a novel cardiac protein kinase A target possessing similarities to AHNAK. FASEB J. 1999;13:2161–2172. doi: 10.1096/fasebj.13.15.2161. [DOI] [PubMed] [Google Scholar]
- 200.Hagiwara S, Naka KI. The initiation of spike potential in barnacle muscle fibers under low intracellular Ca2+ J Gen Physiol. 1964;48:141–162. doi: 10.1085/jgp.48.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Halling DB, Aracena-Parks P, Hamilton SL. Regulation of voltage-gated Ca2+ channels by calmodulin. Sci STKE. 2006;2006:er1. doi: 10.1126/stke.3182006er1. [DOI] [PubMed] [Google Scholar]
- 202.Hamid J, Nelson D, Spaetgens R, Dubel SJ, Snutch TP, Zamponi GW. Identification of an integration center for cross-talk between protein kinase C and G protein modulation of N-type calcium channels. J Biol Chem. 1999;274:6195–6202. doi: 10.1074/jbc.274.10.6195. [DOI] [PubMed] [Google Scholar]
- 203.Hanlon MR, Berrow NS, Dolphin AC, Wallace BA. Modelling of a voltage-dependent Ca2+ channel β subunit as a basis for understanding its functional properties. FEBS Lett. 1999;445:366–370. doi: 10.1016/s0014-5793(99)00156-8. [DOI] [PubMed] [Google Scholar]
- 204.Harry JB, Kobrinsky E, Abernethy DR, Soldatov NM. New short splice variants of the human cardiac Cavβ2 subunit: redefining the major functional motifs implemented in modulation of the Cav1.2 channel. J Biol Chem. 2004;279:46367–46372. doi: 10.1074/jbc.M409523200. [DOI] [PubMed] [Google Scholar]
- 205.Hartzell HC, Qu Z, Yu K, Xiao Q, Chien LT. Molecular physiology of bestrophins: multifunctional membrane proteins linked to best disease and other retinopathies. Physiol Rev. 2008;88:639–672. doi: 10.1152/physrev.00022.2007. [DOI] [PubMed] [Google Scholar]
- 206.He LL, Zhang Y, Chen YH, Yamada Y, Yang J. Functional modularity of the β-subunit of voltage-gated Ca2+ channels. Biophys J. 2007;93:834–845. doi: 10.1529/biophysj.106.101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Held B, Freise D, Freichel M, Hoth M, Flockerzi V. Skeletal muscle L-type Ca2+ current modulation in γ1-deficient and wild-type murine myotubes by the γ1 subunit and cAMP. J Physiol. 2002;539:459–468. doi: 10.1113/jphysiol.2001.012745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Helton TD, Horne WA. Alternative splicing of the β4 subunit has α1 subunit subtype-specific effects on Ca2+ channel gating. J Neurosci. 2002;22:1573–1582. doi: 10.1523/JNEUROSCI.22-05-01573.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Helton TD, Kojetin DJ, Cavanagh J, Horne WA. Alternative splicing of a β4 subunit proline-rich motif regulates voltage-dependent gating and toxin block of Cav2.1 Ca2+ channels. J Neurosci. 2002;22:9331–9339. doi: 10.1523/JNEUROSCI.22-21-09331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Heneghan JF, Mitra-Ganguli T, Stanish LF, Liu L, Zhao R, Rittenhouse AR. The Ca2+ channel β subunit determines whether stimulation of Gq-coupled receptors enhances or inhibits N current. J Gen Physiol. 2009;134:369–384. doi: 10.1085/jgp.200910203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hering S, Berjukow S, Sokolov S, Marksteiner R, Weiss RG, Kraus R, Timin EN. Molecular determinants of inactivation in voltage-gated Ca2+ channels. J Physiol. 2000;528:237–249. doi: 10.1111/j.1469-7793.2000.t01-1-00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein β γ subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 214.Herlitze S, Hockerman GH, Scheuer T, Catterall WA. Molecular determinants of inactivation and G protein modulation in the intracellular loop connecting domains I and II of the calcium channel α1A subunit. Proc Natl Acad Sci USA. 1997;94:1512–1516. doi: 10.1073/pnas.94.4.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Herzig S, Khan IF, Grundemann D, Matthes J, Ludwig A, Michels G, Hoppe UC, Chaudhuri D, Schwartz A, Yue DT, Hullin R. Mechanism of Cav1.2 channel modulation by the amino terminus of cardiac β2-subunits. FASEB J. 2007;21:1527–1538. doi: 10.1096/fj.06-7377com. [DOI] [PubMed] [Google Scholar]
- 216.Hess P. Calcium channels in vertebrate cells. Annu Rev Neurosci. 1990;13:337–356. doi: 10.1146/annurev.ne.13.030190.002005. [DOI] [PubMed] [Google Scholar]
- 217.Hibino H, Pironkova R, Onwumere O, Rousset M, Charnet P, Hudspeth AJ, Lesage F. Direct interaction with a nuclear protein and regulation of gene silencing by a variant of the Ca2+-channel β4 subunit. Proc Natl Acad Sci USA. 2003;100:307–312. doi: 10.1073/pnas.0136791100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hidalgo P, Gonzalez-Gutierrez G, Garcia-Olivares J, Neely A. The α1-β-subunit interaction that modulates calcium channel activity is reversible and requires a competent α-interaction domain. J Biol Chem. 2006;281:24104–24110. doi: 10.1074/jbc.M605930200. [DOI] [PubMed] [Google Scholar]
- 219.Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 220.Hille B, Beech DJ, Bernheim L, Mathie A, Shapiro MS, Wollmuth LP. Multiple G-protein-coupled pathways inhibit N-type Ca channels of neurons. Life Sci. 1995;56:989–992. doi: 10.1016/0024-3205(95)00038-8. [DOI] [PubMed] [Google Scholar]
- 221.Hodgkin AL, Keynes RD. Movements of labelled calcium in squid giant axons. J Physiol. 1957;138:253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hogan K, Greg RG, Powers PA. Structure and alternative splicing of the gene encoding the human β1 subunit of voltage dependent calcium channels. Neurosci Lett. 1999;277:111–114. doi: 10.1016/s0304-3940(99)00851-4. [DOI] [PubMed] [Google Scholar]
- 223.Hohaus A, Person V, Behlke J, Schaper J, Morano I, Haase H. The carboxyl-terminal region of ahnak provides a link between cardiac L-type Ca2+ channels and the actin-based cytoskeleton. FASEB J. 2002;16:1205–1216. doi: 10.1096/fj.01-0855com. [DOI] [PubMed] [Google Scholar]
- 224.Hohaus A, Poteser M, Romanin C, Klugbauer N, Hofmann F, Morano I, Haase H, Groschner K. Modulation of the smooth-muscle L-type Ca2+ channel α1 subunit (α1C-b) by the β2a subunit: a peptide which inhibits binding of β to the I–II linker of α1 induces functional uncoupling. Biochem J. 2000;348:657–665. [PMC free article] [PubMed] [Google Scholar]
- 225.Horn R. Propagating calcium spikes in an axon of Aplysia. J Physiol. 1978;281:513–534. doi: 10.1113/jphysiol.1978.sp012437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hosford DA, Clark S, Cao Z, Wilson WA, Jr, Lin FH, Morrisett RA, Huin A. The role of GABAB receptor activation in absence seizures of lethargic (lh/lh) mice. Science. 1992;257:398–401. doi: 10.1126/science.1321503. [DOI] [PubMed] [Google Scholar]
- 227.Hosford DA, Lin FH, Wang Y, Caddick SJ, Rees M, Parkinson NJ, Barclay J, Cox RD, Gardiner RM, Hosford DA, Denton P, Wang Y, Seldin MF, Chen B. Studies of the lethargic (lh/lh) mouse model of absence seizures: regulatory mechanisms and identification of the lh gene. Adv Neurol. 1999;79:239–252. [PubMed] [Google Scholar]
- 228.Hullin R, Asmus F, Ludwig A, Hersel J, Boekstegers P. Subunit expression of the cardiac L-type calcium channel is differentially regulated in diastolic heart failure of the cardiac allograft. Circulation. 1999;100:155–163. doi: 10.1161/01.cir.100.2.155. [DOI] [PubMed] [Google Scholar]
- 229.Hullin R, Khan IF, Wirtz S, Mohacsi P, Varadi G, Schwartz A, Herzig S. Cardiac L-type calcium channel β-subunits expressed in human heart have differential effects on single channel characteristics. J Biol Chem. 2003;278:21623–21630. doi: 10.1074/jbc.M211164200. [DOI] [PubMed] [Google Scholar]
- 230.Hullin R, Singer-Lahat D, Freichel M, Biel M, Dascal N, Hofmann F, Flockerzi V. Calcium channel β subunit heterogeneity: functional expression of cloned cDNA from heart, aorta and brain. EMBO J. 1992;11:885–890. doi: 10.1002/j.1460-2075.1992.tb05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hummer A, Delzeith O, Gomez SR, Moreno RL, Mark MD, Herlitze S. Competitive and synergistic interactions of G protein β2 and Ca2+ channel β1b subunits with Cav2.1 channels, revealed by mammalian two-hybrid and fluorescence resonance energy transfer measurements. J Biol Chem. 2003;278:49386–49400. doi: 10.1074/jbc.M306645200. [DOI] [PubMed] [Google Scholar]
- 232.Hurley JH, Cahill AL, Currie KP, Fox AP. The role of dynamic palmitoylation in Ca2+ channel inactivation. Proc Natl Acad Sci USA. 2000;97:9293–9298. doi: 10.1073/pnas.160589697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein β γ subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 234.Ikeda SR, Dunlap K. Voltage-dependent modulation of N-type calcium channels: role of G protein subunits. Adv Second Messenger Phosphoprotein Res. 1999;33:131–151. doi: 10.1016/s1040-7952(99)80008-1. [DOI] [PubMed] [Google Scholar]
- 235.Iwashima Y, Abiko A, Ushikubi F, Hata A, Kaku K, Sano H, Eto M. Downregulation of the voltage-dependent calcium channel (VDCC) β-subunit mRNAs in pancreatic islets of type 2 diabetic rats. Biochem Biophys Res Commun. 2001;280:923–932. doi: 10.1006/bbrc.2000.4122. [DOI] [PubMed] [Google Scholar]
- 236.Jangsangthong W, Kuzmenkina E, Khan IF, Matthes J, Hullin R, Herzig S. Inactivation of L-type calcium channels is determined by the length of the N terminus of mutant β1 subunits. Pflügers Arch. 2010;459:399–411. doi: 10.1007/s00424-009-0738-z. [DOI] [PubMed] [Google Scholar]
- 237.Jarvis SE, Zamponi GW. Trafficking and regulation of neuronal voltage-gated calcium channels. Curr Opin Cell Biol. 2007;19:474–482. doi: 10.1016/j.ceb.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 238.Jay SD, Ellis SB, McCue AF, Williams ME, Vedvick TS, Harpold MM, Campbell KP. Primary structure of the γ subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1990;248:490–492. doi: 10.1126/science.2158672. [DOI] [PubMed] [Google Scholar]
- 239.Jeon D, Song I, Guido W, Kim K, Kim E, Oh U, Shin HS. Ablation of Ca2+ channel β3 subunit leads to enhanced N-methyl-d-aspartate receptor-dependent long term potentiation and improved long term memory. J Biol Chem. 2008;283:12093–12101. doi: 10.1074/jbc.M800816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Jeziorski MC, Greenberg RM. Voltage-gated calcium channel subunits from platyhelminths: potential role in praziquantel action. Int J Parasitol. 2006;36:625–632. doi: 10.1016/j.ijpara.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Jha MK, Badou A, Meissner M, McRory JE, Freichel M, Flockerzi V, Flavell RA. Defective survival of naive CD8+ T lymphocytes in the absence of the β3 regulatory subunit of voltage-gated calcium channels. Nat Immunol. 2009;10:1275–1282. doi: 10.1038/ni.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Jiang X, Lautermilch NJ, Watari H, Westenbroek RE, Scheuer T, Catterall WA. Modulation of Cav2.1 channels by Ca2+/calmodulin-dependent protein kinase II bound to the C-terminal domain. Proc Natl Acad Sci USA. 2008;105:341–346. doi: 10.1073/pnas.0710213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 2002;417:515–522. doi: 10.1038/417515a. [DOI] [PubMed] [Google Scholar]
- 244.Jones LP, Patil PG, Snutch TP, Yue DT. G-protein modulation of N-type calcium channel gating current in human embryonic kidney cells (HEK 293) J Physiol. 1997;498:601–610. doi: 10.1113/jphysiol.1997.sp021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Jones LP, Wei SK, Yue DT. Mechanism of auxiliary subunit modulation of neuronal α1E calcium channels. J Gen Physiol. 1998;112:125–143. doi: 10.1085/jgp.112.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jones SW. Calcium channels: unanswered questions. J Bioenerg Biomembr. 2003;35:461–475. doi: 10.1023/b:jobb.0000008020.86004.28. [DOI] [PubMed] [Google Scholar]
- 247.Josephson IR, Varadi G. The β subunit increases Ca2+ currents and gating charge movements of human cardiac L-type Ca2+ channels. Biophys J. 1996;70:1285–1293. doi: 10.1016/S0006-3495(96)79685-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kamp TJ, Perez-Garcia MT, Marban E. Enhancement of ionic current and charge movement by coexpression of calcium channel β1A subunit with α1C subunit in a human embryonic kidney cell line. J Physiol. 1996;492:89–96. doi: 10.1113/jphysiol.1996.sp021291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kang MG, Campbell KP. Γ subunit of voltage-activated calcium channels. J Biol Chem. 2003;278:21315–21318. doi: 10.1074/jbc.R300004200. [DOI] [PubMed] [Google Scholar]
- 250.Kang MG, Chen CC, Felix R, Letts VA, Frankel WN, Mori Y, Campbell KP. Biochemical and biophysical evidence for γ2 subunit association with neuronal voltage-activated Ca2+ channels. J Biol Chem. 2001;276:32917–32924. doi: 10.1074/jbc.M100787200. [DOI] [PubMed] [Google Scholar]
- 251.Kasai H, Aosaki T. Modulation of Ca-channel current by an adenosine analog mediated by a GTP-binding protein in chick sensory neurons. Pflügers Arch. 1989;414:145–149. doi: 10.1007/BF00580956. [DOI] [PubMed] [Google Scholar]
- 252.Kato AS, Zhou W, Milstein AD, Knierman MD, Siuda ER, Dotzlaf JE, Yu H, Hale JE, Nisenbaum ES, Nicoll RA, Bredt DS. New transmembrane AMPA receptor regulatory protein isoform, γ-7, differentially regulates AMPA receptors. J Neurosci. 2007;27:4969–4977. doi: 10.1523/JNEUROSCI.5561-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Katz B, Miledi R. Tetrodotoxin-resistant electric activity in pre-synaptic terminals. J Physiol. 1969;203:459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kavoussi R. Pregabalin: from molecule to medicine. Eur Neuropsychopharmacol. 2006;16(Suppl 2):S128–S133. doi: 10.1016/j.euroneuro.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 255.Kelly K. The RGK family: a regulatory tail of small GTP-binding proteins. Trends Cell Biol. 2005;15:640–643. doi: 10.1016/j.tcb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 256.Kim MS, Morii T, Sun LX, Imoto K, Mori Y. Structural determinants of ion selectivity in brain calcium channel. FEBS Lett. 1993;318:145–148. doi: 10.1016/0014-5793(93)80009-j. [DOI] [PubMed] [Google Scholar]
- 257.Kiyonaka S, Wakamori M, Miki T, Uriu Y, Nonaka M, Bito H, Beedle AM, Mori E, Hara Y, De Waard M, Kanagawa M, Itakura M, Takahashi M, Campbell KP, Mori Y. RIM1 confers sustained activity and neurotransmitter vesicle anchoring to pre-synaptic Ca2+ channels. Nature Neurosci. 2007;10:691–701. doi: 10.1038/nn1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Klugbauer N, Dai S, Specht V, Lacinova L, Marais E, Bohn G, Hofmann F. A family of γ-like calcium channel subunits. FEBS Lett. 2000;470:189–197. doi: 10.1016/s0014-5793(00)01306-5. [DOI] [PubMed] [Google Scholar]
- 259.Klugbauer N, Lacinova L, Marais E, Hobom M, Hofmann F. Molecular diversity of the calcium channel α2δ subunit. J Neurosci. 1999;19:684–691. doi: 10.1523/JNEUROSCI.19-02-00684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Klugbauer N, Marais E, Hofmann F. Calcium channel α2δ subunits: differential expression, function, and drug binding. J Bioenerg Biomembr. 2003;35:639–647. doi: 10.1023/b:jobb.0000008028.41056.58. [DOI] [PubMed] [Google Scholar]
- 261.Kobayashi T, Yamada Y, Fukao M, Shiratori K, Tsutsuura M, Tanimoto K, Tohse N. The GK domain of the voltage-dependent calcium channel β subunit is essential for binding to the α subunit. Biochem Biophys Res Commun. 2007;360:679–683. doi: 10.1016/j.bbrc.2007.06.112. [DOI] [PubMed] [Google Scholar]
- 262.Kobrinsky E, Tiwari S, Maltsev VA, Harry JB, Lakatta E, Abernethy DR, Soldatov NM. Differential role of the α1C subunit tails in regulation of the Cav1.2 channel by membrane potential, β subunits, and Ca2+ ions. J Biol Chem. 2005;280:12474–12485. doi: 10.1074/jbc.M412140200. [DOI] [PubMed] [Google Scholar]
- 263.Kohn AB, Anderson PA, Roberts-Misterly JM, Greenberg RM. Schistosome calcium channel β subunits. Unusual modulatory effects and potential role in the action of the antischistosomal drug praziquantel. J Biol Chem. 2001;276:36873–36876. doi: 10.1074/jbc.C100273200. [DOI] [PubMed] [Google Scholar]
- 264.Kohn AB, Roberts-Misterly JM, Anderson PA, Greenberg RM. Creation by mutagenesis of a mammalian Ca2+ channel β subunit that confers praziquantel sensitivity to a mammalian Ca2+ channel. Int J Parasitol. 2003;33:1303–1308. doi: 10.1016/s0020-7519(03)00209-1. [DOI] [PubMed] [Google Scholar]
- 265.Koschak A, Reimer D, Walter D, Hoda JC, Heinzle T, Grabner M, Striessnig J. Cav1.4 α1 subunits can form slowly inactivating dihydropyridine-sensitive L-type Ca2+ channels lacking Ca2+-dependent inactivation. J Neurosci. 2003;23:6041–6049. doi: 10.1523/JNEUROSCI.23-14-06041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Kuo CC, Hess P. Ion permeation through the L-type Ca2+ channel in rat pheochromocytoma cells: two sets of ion binding sites in the pore. J Physiol. 1993;466:629–655. [PMC free article] [PubMed] [Google Scholar]
- 267.Kurshan PT, Oztan A, Schwarz TL. Presynaptic α2δ-3 is required for synaptic morphogenesis independent of its Ca2+-channel functions. Nature Neurosci. 2009;12:1415–1423. doi: 10.1038/nn.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Lacerda AE, Kim HS, Ruth P, Perez-Reyes E, Flockerzi V, Hofmann F, Birnbaumer L, Brown AM. Normalization of current kinetics by interaction between the α1 and β subunits of the skeletal muscle dihydropyridine-sensitive Ca2+ channel. Nature. 1991;352:527–530. doi: 10.1038/352527a0. [DOI] [PubMed] [Google Scholar]
- 269.Lang B, Newsom-Davis J, Wray D, Vincent A, Murray N. Autoimmune aetiology for myasthenic (Eaton-Lambert) syndrome. Lancet. 1981;2:224–226. doi: 10.1016/s0140-6736(81)90474-8. [DOI] [PubMed] [Google Scholar]
- 270.Lao QZ, Kobrinsky E, Harry JB, Ravindran A, Soldatov NM. New Determinant for the Cavβ2 subunit modulation of the Cav1.2 calcium channel. J Biol Chem. 2008;283:15577–15588. doi: 10.1074/jbc.M802035200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Larson SM, Davidson AR. The identification of conserved interactions within the SH3 domain by alignment of sequences and structures. Protein Sci. 2000;9:2170–2180. doi: 10.1110/ps.9.11.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lee A, Scheuer T, Catterall WA. Ca2+/calmodulin-dependent facilitation and inactivation of P/Q-type Ca2+ channels. J Neurosci. 2000;20:6830–6838. doi: 10.1523/JNEUROSCI.20-18-06830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lee TS, Karl R, Moosmang S, Lenhardt P, Klugbauer N, Hofmann F, Kleppisch T, Welling A. Calmodulin kinase II is involved in voltage-dependent facilitation of the L-type Cav1.2 calcium channel: identification of the phosphorylation. J Biol Chem. 2006;281:25560–25567. doi: 10.1074/jbc.M508661200. [DOI] [PubMed] [Google Scholar]
- 274.Lee TS, Ono K, Hadama T, Uchida Y, Arita M. Roles of α1 and α1/β subunits derived from cardiac L-type Ca2+ channels on voltage-dependent facilitation mechanisms. Jpn J Physiol. 2001;51:337–344. doi: 10.2170/jjphysiol.51.337. [DOI] [PubMed] [Google Scholar]
- 275.Lemke T, Welling A, Christel CJ, Blaich A, Bernhard D, Lenhardt P, Hofmann F, Moosmang S. Unchanged β-adrenergic stimulation of cardiac L-type calcium channels in Ca v 1.2 phosphorylation site S1928A mutant mice. J Biol Chem. 2008;283:34738–34744. doi: 10.1074/jbc.M804981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Leroy J, Richards MW, Butcher AJ, Nieto-Rostro M, Pratt WS, Davies A, Dolphin AC. Interaction via a key tryptophan in the I–II linker of N-type calcium channels is required for β1 but not for palmitoylated β2, implicating an additional binding site in the regulation of channel voltage-dependent properties. J Neurosci. 2005;25:6984–6996. doi: 10.1523/JNEUROSCI.1137-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Letts VA, Felix R, Biddlecome GH, Arikkath J, Mahaffey CL, Valenzuela A, Bartlett FS, 2nd, Mori Y, Campbell KP, Frankel WN. The mouse stargazer gene encodes a neuronal Ca2+-channel γ subunit. Nature Genet. 1998;19:340–347. doi: 10.1038/1228. [DOI] [PubMed] [Google Scholar]
- 278.Leung AT, Imagawa T, Block B, Franzini-Armstrong C, Campbell KP. Biochemical and ultrastructural characterization of the 1,4-dihydropyridine receptor from rabbit skeletal muscle. Evidence for a 52,000 Da subunit. J Biol Chem. 1988;263:994–1001. [PubMed] [Google Scholar]
- 279.Leuranguer V, Bourinet E, Lory P, Nargeot J. Antisense depletion of β-subunits fails to affect T-type calcium channels properties in a neuroblastoma cell line. Neuropharmacology. 1998;37:701–708. doi: 10.1016/s0028-3908(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 280.Levy S, Beharier O, Etzion Y, Mor M, Buzaglo L, Shaltiel L, Gheber LA, Kahn J, Muslin AJ, Katz A, Gitler D, Moran A. The molecular basis for ZnT-1 action as an endogenous inhibitor of L-type calcium channels. J Biol Chem. 2009;284:32434–32443. doi: 10.1074/jbc.M109.058842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Leyris JP, Gondeau C, Charnet A, Delattre C, Rousset M, Cens T, Charnet P. RGK GTPase-dependent Cav2.1 Ca2+ channel inhibition is independent of Cavβ-subunit-induced current potentiation. FASEB J. 2009;23:2627–2638. doi: 10.1096/fj.08-122135. [DOI] [PubMed] [Google Scholar]
- 282.Li B, Zhong H, Scheuer T, Catterall WA. Functional role of a C-terminal Gβγ-binding domain of Cav2.2 channels. Mol Pharmacol. 2004;66:761–769. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 283.Li CY, Song YH, Higuera ES, Luo ZD. Spinal dorsal horn calcium channel α2δ-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia. J Neurosci. 2004;24:8494–8499. doi: 10.1523/JNEUROSCI.2982-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Li CY, Zhang XL, Matthews EA, Li KW, Kurwa A, Boroujerdi A, Gross J, Gold MS, Dickenson AH, Feng G, Luo ZD. Calcium channel α2δ1 subunit mediates spinal hyperexcitability in pain modulation. Pain. 2006;125:20–34. doi: 10.1016/j.pain.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Li Y, Spangenberg O, Paarmann I, Konrad M, Lavie A. Structural basis for nucleotide-dependent regulation of membrane-associated guanylate kinase-like domains. J Biol Chem. 2002;277:4159–4165. doi: 10.1074/jbc.M110792200. [DOI] [PubMed] [Google Scholar]
- 286.Liang X, Slifer M, Martin ER, Schnetz-Boutaud N, Bartlett J, Anderson B, Zuchner S, Gwirtsman H, Gilbert JR, Pericak-Vance MA, Haines JL. Genomic convergence to identify candidate genes for Alzheimer disease on chromosome 10. Hum Mutat. 2009;30:463–471. doi: 10.1002/humu.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Liang Y, Tavalin SJ. Auxiliary β subunits differentially determine pka utilization of distinct regulatory sites on Cav1.3 L type Ca2+ channels. Channels. 2007;1:102–112. doi: 10.4161/chan.4284. [DOI] [PubMed] [Google Scholar]
- 288.Lin Z, Witschas K, Garcia T, Chen RS, Hansen JP, Sellers ZM, Kuzmenkina E, Herzig S, Best PM. A critical GxxxA motif in the γ6 calcium channel subunit mediates its inhibitory effect on Cav3.1 calcium current. J Physiol. 2008;586:5349–5366. doi: 10.1113/jphysiol.2008.159111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Liu L, Barrett CF, Rittenhouse AR. Arachidonic acid both inhibits and enhances whole cell calcium currents in rat sympathetic neurons. Am J Physiol Cell Physiol. 2001;280:C1293–C1305. doi: 10.1152/ajpcell.2001.280.5.C1293. [DOI] [PubMed] [Google Scholar]
- 290.Liu Y, Holmgren M, Jurman ME, Yellen G. Gated access to the pore of a voltage-dependent K+ channel. Neuron. 1997;19:175–184. doi: 10.1016/s0896-6273(00)80357-8. [DOI] [PubMed] [Google Scholar]
- 291.Llinas R, Hess R. Tetrodotoxin-resistant dendritic spikes in avian Purkinje cells. Proc Natl Acad Sci USA. 1976;73:2520–2523. doi: 10.1073/pnas.73.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Llinas R, Sugimori M, Lin JW, Cherksey B. Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc Natl Acad Sci USA. 1989;86:1689–1693. doi: 10.1073/pnas.86.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Llinas R, Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol. 1981;315:569–584. doi: 10.1113/jphysiol.1981.sp013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ludwig A, Flockerzi V, Hofmann F. Regional expression and cellular localization of the α1 and β subunit of high voltage-activated calcium channels in rat brain. J Neurosci. 1997;17:1339–1349. doi: 10.1523/JNEUROSCI.17-04-01339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Luvisetto S, Fellin T, Spagnolo M, Hivert B, Brust PF, Harpold MM, Stauderman KA, Williams ME, Pietrobon D. Modal gating of human Cav2.1 (P/Q-type) calcium channels: I The slow and the fast gating modes and their modulation by β subunits. J Gen Physiol. 2004;124:445–461. doi: 10.1085/jgp.200409034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Maguire J, Santoro T, Jensen P, Siebenlist U, Yewdell J, Kelly K. Gem: an induced, immediate early protein belonging to the Ras family. Science. 1994;265:241–244. doi: 10.1126/science.7912851. [DOI] [PubMed] [Google Scholar]
- 297.Mahalakshmi RN, Nagashima K, Ng MY, Inagaki N, Hunziker W, Beguin P. Nuclear transport of Kir/Gem requires specific signals and importin α5 and is regulated by calmodulin and predicted serine phosphorylations. Traffic. 2007;8:1150–1163. doi: 10.1111/j.1600-0854.2007.00598.x. [DOI] [PubMed] [Google Scholar]
- 298.Maltez JM, Nunziato DA, Kim J, Pitt GS. Essential Cavβ modulatory properties are AID-independent. Nat Struct Mol Biol. 2005;12:372–377. doi: 10.1038/nsmb909. [DOI] [PubMed] [Google Scholar]
- 299.Mareska M, Gutmann L. Lambert-Eaton myasthenic syndrome. Semin Neurol. 2004;24:149–153. doi: 10.1055/s-2004-830900. [DOI] [PubMed] [Google Scholar]
- 300.Mark MD, Wittemann S, Herlitze S. G protein modulation of recombinant P/Q-type calcium channels by regulators of G protein signalling proteins. J Physiol. 2000;528:65–77. doi: 10.1111/j.1469-7793.2000.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Marmorstein LY, Wu J, McLaughlin P, Yocom J, Karl MO, Neussert R, Wimmers S, Stanton JB, Gregg RG, Strauss O, Peachey NS, Marmorstein AD. The light peak of the electroretinogram is dependent on voltage-gated calcium channels and antagonized by bestrophin (best-1) J Gen Physiol. 2006;127:577–589. doi: 10.1085/jgp.200509473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Marsh JD, Telemaque S, Rhee SW, Stimers JR, Rusch NJ. Delivery of ion channel genes to treat cardiovascular diseases. Trans Am Clin Climatol Assoc. 2008;119:171–182. [PMC free article] [PubMed] [Google Scholar]
- 303.Massa E, Kelly KM, Yule DI, MacDonald RL, Uhler MD. Comparison of fura-2 imaging and electrophysiological analysis of murine calcium channel α1 subunits coexpressed with novel β2 subunit isoforms. Mol Pharmacol. 1995;47:707–716. [PubMed] [Google Scholar]
- 304.Matthews EK, Sakamoto Y. Electrical characteristics of pancreatic islet cells. J Physiol. 1975;246:421–437. doi: 10.1113/jphysiol.1975.sp010897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Matza D, Badou A, Kobayashi KS, Goldsmith-Pestana K, Masuda Y, Komuro A, McMahon-Pratt D, Marchesi VT, Flavell RA. A scaffold protein, AHNAK1, is required for calcium signaling during T cell activation. Immunity. 2008;28:64–74. doi: 10.1016/j.immuni.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Maximov A, Sudhof TC, Bezprozvanny I. Association of neuronal calcium channels with modular adaptor proteins. J Biol Chem. 1999;274:24453–24456. doi: 10.1074/jbc.274.35.24453. [DOI] [PubMed] [Google Scholar]
- 307.Mayer BJ. SH3 domains: complexity in moderation. J Cell Sci. 2001;114:1253–1263. doi: 10.1242/jcs.114.7.1253. [DOI] [PubMed] [Google Scholar]
- 308.McCarron JG, McGeown JG, Reardon S, Ikebe M, Fay FS, Walsh JV., Jr Calcium-dependent enhancement of calcium current in smooth muscle by calmodulin-dependent protein kinase II. Nature. 1992;357:74–77. doi: 10.1038/357074a0. [DOI] [PubMed] [Google Scholar]
- 309.McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- 310.McEnery MW, Copeland TD, Vance CL. Altered expression and assembly of N-type calcium channel α1B and β subunits in epileptic lethargic (lh/lh) mouse. J Biol Chem. 1998;273:21435–21438. doi: 10.1074/jbc.273.34.21435. [DOI] [PubMed] [Google Scholar]
- 311.McEnery MW, Vance CL, Begg CM, Lee WL, Choi Y, Dubel SJ. Differential expression and association of calcium channel subunits in development and disease. J Bioenerg Biomembr. 1998;30:409–418. doi: 10.1023/a:1021997924473. [DOI] [PubMed] [Google Scholar]
- 312.McGee AW, Dakoji SR, Olsen O, Bredt DS, Lim WA, Prehoda KE. Structure of the SH3-guanylate kinase module from PSD-95 suggests a mechanism for regulated assembly of MAGUK scaffolding proteins. Mol Cell. 2001;8:1291–1301. doi: 10.1016/s1097-2765(01)00411-7. [DOI] [PubMed] [Google Scholar]
- 313.McGee AW, Nunziato DA, Maltez JM, Prehoda KE, Pitt GS, Bredt DS. Calcium channel function regulated by the SH3-GK module in β subunits. Neuron. 2004;42:89–99. doi: 10.1016/s0896-6273(04)00149-7. [DOI] [PubMed] [Google Scholar]
- 314.McRory JE, Hamid J, Doering CJ, Garcia E, Parker R, Hamming K, Chen L, Hildebrand M, Beedle AM, Feldcamp L, Zamponi GW, Snutch TP. The CACNA1F gene encodes an L-type calcium channel with unique biophysical properties and tissue distribution. J Neurosci. 2004;24:1707–1718. doi: 10.1523/JNEUROSCI.4846-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Meir A, Bell DC, Stephens GJ, Page KM, Dolphin AC. Calcium channel β subunit promotes voltage-dependent modulation of α1 B by G β γ. Biophys J. 2000;79:731–746. doi: 10.1016/S0006-3495(00)76331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Meir A, Dolphin AC. Kinetics and Gβγ modulation of Cav2.2 channels with different auxiliary β subunits. Pflügers Arch. 2002;444:263–275. doi: 10.1007/s00424-002-0803-3. [DOI] [PubMed] [Google Scholar]
- 317.Mermelstein PG, Foehring RC, Tkatch T, Song WJ, Baranauskas G, Surmeier DJ. Properties of Q-type calcium channels in neostriatal and cortical neurons are correlated with β subunit expression. J Neurosci. 1999;19:7268–7277. doi: 10.1523/JNEUROSCI.19-17-07268.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Michailidis IE, Zhang Y, Yang J. The lipid connection-regulation of voltage-gated Ca2+ channels by phosphoinositides. Pflügers Arch. 2007;455:147–155. doi: 10.1007/s00424-007-0272-9. [DOI] [PubMed] [Google Scholar]
- 319.Mikami A, Imoto K, Tanabe T, Niidome T, Mori Y, Takeshima H, Narumiya S, Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989;340:230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- 320.Milstein AD, Nicoll RA. Regulation of AMPA receptor gating and pharmacology by TARP auxiliary subunits. Trends Pharmacol Sci. 2008;29:333–339. doi: 10.1016/j.tips.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Miriyala J, Nguyen T, Yue DT, Colecraft HM. Role of Cavβ subunits, lack of functional reserve, in protein kinase A modulation of cardiac Cav1.2 channels. Circ Res. 2008;102:e54–e64. doi: 10.1161/CIRCRESAHA.108.171736. [DOI] [PubMed] [Google Scholar]
- 322.Mori Y, Friedrich T, Kim MS, Mikami A, Nakai J, Ruth P, Bosse E, Hofmann F, Flockerzi V, Furuichi T. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991;350:398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- 323.Moss FJ, Viard P, Davies A, Bertaso F, Page KM, Graham A, Canti C, Plumpton M, Plumpton C, Clare JJ, Dolphin AC. The novel product of a five-exon stargazin-related gene abolishes Cav2.2 calcium channel expression. EMBO J. 2002;21:1514–1523. doi: 10.1093/emboj/21.7.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Moyers JS, Bilan PJ, Zhu J, Kahn CR. Rad and Rad-related GTPases interact with calmodulin and calmodulin-dependent protein kinase II. J Biol Chem. 1997;272:11832–11839. doi: 10.1074/jbc.272.18.11832. [DOI] [PubMed] [Google Scholar]
- 325.Murakami M, Fleischmann B, De Felipe C, Freichel M, Trost C, Ludwig A, Wissenbach U, Schwegler H, Hofmann F, Hescheler J, Flockerzi V, Cavalie A. Pain perception in mice lacking the β3 subunit of voltage-activated calcium channels. J Biol Chem. 2002;277:40342–40351. doi: 10.1074/jbc.M203425200. [DOI] [PubMed] [Google Scholar]
- 326.Murakami M, Nakagawasai O, Yanai K, Nunoki K, Tan-No K, Tadano T, Iijima T. Modified behavioral characteristics following ablation of the voltage-dependent calcium channel β3 subunit. Brain Res. 2007;1160:102–112. doi: 10.1016/j.brainres.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 327.Murakami M, Ohba T, Xu F, Satoh E, Miyoshi I, Suzuki T, Takahashi Y, Takahashi E, Watanabe H, Ono K, Sasano H, Kasai N, Ito H, Iijima T. Modified sympathetic nerve system activity with overexpression of the voltage-dependent calcium channel β3 subunit. J Biol Chem. 2008;283:24554–24560. doi: 10.1074/jbc.M802319200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Murakami M, Yamamura H, Murakami A, Okamura T, Nunoki K, Mitui-Saito M, Muraki K, Hano T, Imaizumi Y, Flockerzi T, Yanagisawa T. Conserved smooth muscle contractility and blood pressure increase in response to high-salt diet in mice lacking the β3 subunit of the voltage-dependent calcium channel. J Cardiovasc Pharmacol. 2000;36(Suppl 2):S69–S73. doi: 10.1097/00005344-200000006-00015. [DOI] [PubMed] [Google Scholar]
- 329.Murakami M, Yamamura H, Suzuki T, Kang MG, Ohya S, Murakami A, Miyoshi I, Sasano H, Muraki K, Hano T, Kasai N, Nakayama S, Campbell KP, Flockerzi V, Imaizumi Y, Yanagisawa T, Iijima T. Modified cardiovascular L-type channels in mice lacking the voltage-dependent Ca2+ channel β3 subunit. J Biol Chem. 2003;278:43261–43267. doi: 10.1074/jbc.M211380200. [DOI] [PubMed] [Google Scholar]
- 330.Namkung Y, Smith SM, Lee SB, Skrypnyk NV, Kim HL, Chin H, Scheller RH, Tsien RW, Shin HS. Targeted disruption of the Ca2+ channel β3 subunit reduces N- and L-type Ca2+ channel activity and alters the voltage-dependent activation of P/Q-type Ca2+ channels in neurons. Proc Natl Acad Sci USA. 1998;95:12010–12015. doi: 10.1073/pnas.95.20.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Neef J, Gehrt A, Bulankina AV, Meyer AC, Riedel D, Gregg RG, Strenzke N, Moser T. The Ca2+ channel subunit β2 regulates Ca2+ channel abundance and function in inner hair cells and is required for hearing. J Neurosci. 2009;29:10730–10740. doi: 10.1523/JNEUROSCI.1577-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Neely A, Wei X, Olcese R, Birnbaumer L, Stefani E. Potentiation by the β subunit of the ratio of the ionic current to the charge movement in the cardiac calcium channel. Science. 1993;262:575–578. doi: 10.1126/science.8211185. [DOI] [PubMed] [Google Scholar]
- 333.Neuhuber B, Gerster U, Doring F, Glossmann H, Tanabe T, Flucher BE. Association of calcium channel α1S and β1a subunits is required for the targeting of β1a but not of α1S into skeletal muscle triads. Proc Natl Acad Sci USA. 1998;95:5015–5020. doi: 10.1073/pnas.95.9.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Nogi T, Zhang D, Chan JD, Marchant JS. A novel biological activity of praziquantel requiring voltage-operated Ca channel Β subunits: subversion of flatworm regenerative polarity. PLoS Negl Trop Dis. 2009;3:e464. doi: 10.1371/journal.pntd.0000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985;316:440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- 336.Obermair GJ, Schlick B, Di Biase V, Subramanyam P, Gebhart M, Baumgartner S, Flucher BE. Reciprocal interactions regulate targeting of calcium channel β subunits and membrane expression of α1 subunits in cultured hippocampal neurons. J Biol Chem. 2010;285:5776–5791. doi: 10.1074/jbc.M109.044271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ohana E, Sekler I, Kaisman T, Kahn N, Cove J, Silverman WF, Amsterdam A, Hershfinkel M. Silencing of ZnT-1 expression enhances heavy metal influx and toxicity. J Mol Med. 2006;84:753–763. doi: 10.1007/s00109-006-0062-4. [DOI] [PubMed] [Google Scholar]
- 338.Ohmori I, Ouchida M, Miki T, Mimaki N, Kiyonaka S, Nishiki T, Tomizawa K, Mori Y, Matsui H. A CACNB4 mutation shows that altered Cav2.1 function may be a genetic modifier of severe myoclonic epilepsy in infancy. Neurobiol Dis. 2008;32:349–354. doi: 10.1016/j.nbd.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 339.Ohta T, Ohba T, Suzuki T, Watanabe H, Sasano H, Murakami M. Decreased calcium channel currents and facilitated epinephrine release in the Ca2+ channel β3 subunit-null mice. Biochem Biophys Res Commun. 2010;394:464–469. doi: 10.1016/j.bbrc.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 340.Olcese R, Qin N, Schneider T, Neely A, Wei X, Stefani E, Birnbaumer L. The amino terminus of a calcium channel β subunit sets rates of channel inactivation independently of the subunit’s effect on activation. Neuron. 1994;13:1433–1438. doi: 10.1016/0896-6273(94)90428-6. [DOI] [PubMed] [Google Scholar]
- 341.Opatowsky Y, Chen CC, Campbell KP, Hirsch JA. Structural analysis of the voltage-dependent calcium channel β subunit functional core and its complex with the α1 interaction domain. Neuron. 2004;42:387–399. doi: 10.1016/s0896-6273(04)00250-8. [DOI] [PubMed] [Google Scholar]
- 342.Opatowsky Y, Chomsky-Hecht O, Kang MG, Campbell KP, Hirsch JA. The voltage-dependent calcium channel β subunit contains two stable interacting domains. J Biol Chem. 2003;278:52323–52332. doi: 10.1074/jbc.M303564200. [DOI] [PubMed] [Google Scholar]
- 343.Osten P, Stern-Bach Y. Learning from stargazin: the mouse, the phenotype and the unexpected. Curr Opin Neurobiol. 2006;16:275–280. doi: 10.1016/j.conb.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 344.Pagani R, Song M, McEnery M, Qin N, Tsien RW, Toro L, Stefani E, Uchitel OD. Differential expression of α1 and β subunits of voltage dependent Ca2+ channel at the neuromuscular junction of normal and P/Q Ca2+ channel knockout mouse. Neuroscience. 2004;123:75–85. doi: 10.1016/j.neuroscience.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 345.Page KM, Canti C, Stephens GJ, Berrow NS, Dolphin AC. Identification of the amino terminus of neuronal Ca2+ channel α1 subunits α1B and α1E as an essential determinant of G-protein modulation. J Neurosci. 1998;18:4815–4824. doi: 10.1523/JNEUROSCI.18-13-04815.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Page KM, Stephens GJ, Berrow NS, Dolphin AC. The intracellular loop between domains I and II of the B-type calcium channel confers aspects of G-protein sensitivity to the E-type calcium channel. J Neurosci. 1997;17:1330–1338. doi: 10.1523/JNEUROSCI.17-04-01330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Park SH, Suh YS, Kim H, Rhyu IJ, Kim HL. Chromosomal localization and neural distribution of voltage dependent calcium channel β3 subunit gene. Mol Cell. 1997;7:200–203. [PubMed] [Google Scholar]
- 348.Patil PG, Brody DL, Yue DT. Preferential closed-state inactivation of neuronal calcium channels. Neuron. 1998;20:1027–1038. doi: 10.1016/s0896-6273(00)80483-3. [DOI] [PubMed] [Google Scholar]
- 349.Patil PG, de Leon M, Reed RR, Dubel S, Snutch TP, Yue DT. Elementary events underlying voltage-dependent G-protein inhibition of N-type calcium channels. Biophys J. 1996;71:2509–2521. doi: 10.1016/S0006-3495(96)79444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Payne HL. The role of transmembrane AMPA receptor regulatory proteins (TARPs) in neurotransmission and receptor trafficking. Mol Membr Biol. 2008;25:353–362. doi: 10.1080/09687680801986480. [DOI] [PubMed] [Google Scholar]
- 351.Perez-Reyes E. Molecular characterization of T-type calcium channels. Cell Calcium. 2006;40:89–96. doi: 10.1016/j.ceca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 352.Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 353.Perez-Reyes E, Castellano A, Kim HS, Bertrand P, Baggstrom E, Lacerda AE, Wei XY, Birnbaumer L. Cloning and expression of a cardiac/brain β subunit of the L-type calcium channel. J Biol Chem. 1992;267:1792–1797. [PubMed] [Google Scholar]
- 354.Pica-Mattoccia L, Orsini T, Basso A, Festucci A, Liberti P, Guidi A, Marcatto-Maggi AL, Nobre-Santana S, Troiani AR, Cioli D, Valle C. Schistosoma mansoni: lack of correlation between praziquantel-induced intra-worm calcium influx and parasite death. Exp Parasitol. 2008;119:332–335. doi: 10.1016/j.exppara.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 355.Pichler M, Cassidy TN, Reimer D, Haase H, Kraus R, Ostler D, Striessnig J. Β subunit heterogeneity in neuronal L-type Ca2+ channels. J Biol Chem. 1997;272:13877–13882. doi: 10.1074/jbc.272.21.13877. [DOI] [PubMed] [Google Scholar]
- 356.Pietrobon D. Calcium channels and channelopathies of the central nervous system. Mol Neurobiol. 2002;25:31–50. doi: 10.1385/MN:25:1:031. [DOI] [PubMed] [Google Scholar]
- 357.Pietrobon D, Hess P. Novel mechanism of voltage-dependent gating in L-type calcium channels. Nature. 1990;346:651–655. doi: 10.1038/346651a0. [DOI] [PubMed] [Google Scholar]
- 358.Pitt GS. Calmodulin and CaMKII as molecular switches for cardiac ion channels. Cardiovasc Res. 2007;73:641–647. doi: 10.1016/j.cardiores.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 359.Plummer MR, Logothetis DE, Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989;2:1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- 360.Powers PA, Liu S, Hogan K, Gregg RG. Skeletal muscle and brain isoforms of a β-subunit of human voltage-dependent calcium channels are encoded by a single gene. J Biol Chem. 1992;267:22967–22972. [PubMed] [Google Scholar]
- 361.Pragnell M, De Waard M, Mori Y, Tanabe T, Snutch TP, Campbell KP. Calcium channel β-subunit binds to a conserved motif in the I–II cytoplasmic linker of the α1-subunit. Nature. 1994;368:67–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- 362.Pragnell M, Sakamoto J, Jay SD, Campbell KP. Cloning and tissue-specific expression of the brain calcium channel β-subunit. FEBS Lett. 1991;291:253–258. doi: 10.1016/0014-5793(91)81296-k. [DOI] [PubMed] [Google Scholar]
- 363.Protasi F. Structural interaction between RYRs and DHPRs in calcium release units of cardiac and skeletal muscle cells. Front Biosci. 2002;7:d650–d658. doi: 10.2741/A801. [DOI] [PubMed] [Google Scholar]
- 364.Qin N, Olcese R, Zhou J, Cabello OA, Birnbaumer L, Stefani E. Identification of a second region of the β-subunit involved in regulation of calcium channel inactivation. Am J Physiol Cell Physiol. 1996;271:C1539–C1545. doi: 10.1152/ajpcell.1996.271.5.C1539. [DOI] [PubMed] [Google Scholar]
- 365.Qin N, Platano D, Olcese R, Costantin JL, Stefani E, Birnbaumer L. Unique regulatory properties of the type 2a Ca2+ channel β subunit caused by palmitoylation. Proc Natl Acad Sci USA. 1998;95:4690–4695. doi: 10.1073/pnas.95.8.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Qin N, Platano D, Olcese R, Stefani E, Birnbaumer L. Direct interaction of gβγ with a C-terminal gβγ-binding domain of the Ca2+ channel α1 subunit is responsible for channel inhibition by G protein-coupled receptors. Proc Natl Acad Sci USA. 1997;94:8866–8871. doi: 10.1073/pnas.94.16.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Qin N, Yagel S, Momplaisir ML, Codd EE, D’Andrea MR. Molecular cloning and characterization of the human voltage-gated calcium channel α2δ-4 subunit. Mol Pharmacol. 2002;62:485–496. doi: 10.1124/mol.62.3.485. [DOI] [PubMed] [Google Scholar]
- 368.Raymond C, Walker D, Bichet D, Iborra C, Martin-Moutot N, Seagar M, De Waard M. Antibodies against the β subunit of voltage-dependent calcium channels in Lambert-Eaton myasthenic syndrome. Neuroscience. 1999;90:269–277. doi: 10.1016/s0306-4522(98)00378-9. [DOI] [PubMed] [Google Scholar]
- 369.Restituito S, Cens T, Barrere C, Geib S, Galas S, De Waard M, Charnet P. The [β]2a subunit is a molecular groom for the Ca2+ channel inactivation gate. J Neurosci. 2000;20:9046–9052. doi: 10.1523/JNEUROSCI.20-24-09046.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Reynet C, Kahn CR. Rad: a member of the Ras family overexpressed in muscle of type II diabetic humans. Science. 1993;262:1441–1444. doi: 10.1126/science.8248782. [DOI] [PubMed] [Google Scholar]
- 371.Richards MW, Butcher AJ, Dolphin AC. Ca2+ channel β-subunits: structural insights AID our understanding. Trends Pharmacol Sci. 2004;25:626–632. doi: 10.1016/j.tips.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 372.Richards MW, Leroy J, Pratt WS, Dolphin AC. The HOOK-domain between the SH3 and the GK domains of Cavβ subunits contains key determinants controlling calcium channel inactivation. Channels. 2007;1:92–101. doi: 10.4161/chan.4145. [DOI] [PubMed] [Google Scholar]
- 373.Roberts-Crowley ML, Mitra-Ganguli T, Liu L, Rittenhouse AR. Regulation of voltage-gated Ca2+ channels by lipids. Cell Calcium. 2009;45:589–601. doi: 10.1016/j.ceca.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Roberts-Crowley ML, Rittenhouse AR. Arachidonic acid inhibition of L-type calcium (Cav1.3b) channels varies with accessory Cavβ subunits. J Gen Physiol. 2009;133:387–403. doi: 10.1085/jgp.200810047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Roche JP, Anantharam V, Treistman SN. Abolition of G protein inhibition of α1A and α1B calcium channels by co-expression of the β3 subunit. FEBS Lett. 1995;371:43–46. doi: 10.1016/0014-5793(95)00860-c. [DOI] [PubMed] [Google Scholar]
- 376.Roche JP, Treistman SN. The Ca2+ channel β3 subunit differentially modulates G-protein sensitivity of α1A and α1B Ca2+ channels. J Neurosci. 1998;18:878–886. doi: 10.1523/JNEUROSCI.18-03-00878.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Rosenfeld MR, Wong E, Dalmau J, Manley G, Posner JB, Sher E, Furneaux HM. Cloning and characterization of a Lambert-Eaton myasthenic syndrome antigen. Ann Neurol. 1993;33:113–120. doi: 10.1002/ana.410330126. [DOI] [PubMed] [Google Scholar]
- 378.Rosenthal R, Bakall B, Kinnick T, Peachey N, Wimmers S, Wadelius C, Marmorstein A, Strauss O. Expression of bestrophin-1, the product of the VMD2 gene, modulates voltage-dependent Ca2+ channels in retinal pigment epithelial cells. FASEB J. 2006;20:178–180. doi: 10.1096/fj.05-4495fje. [DOI] [PubMed] [Google Scholar]
- 379.Rousset M, Cens T, Restituito S, Barrere C, Black JL, 3rd, McEnery MW, Charnet P. Functional roles of γ2, γ3 and γ4, three new Ca2+ channel subunits, in P/Q-type Ca2+ channel expressed in Xenopus oocytes. J Physiol. 2001;532:583–593. doi: 10.1111/j.1469-7793.2001.0583e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Rousset M, Charnet P, Cens T. Structure of the calcium channel β subunit: the place of the β-interaction domain. Med Sci. 2005;21:279–283. doi: 10.1051/medsci/2005213279. [DOI] [PubMed] [Google Scholar]
- 381.Ruth P, Rohrkasten A, Biel M, Bosse E, Regulla S, Meyer HE, Flockerzi V, Hofmann F. Primary structure of the β subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1989;245:1115–1118. doi: 10.1126/science.2549640. [DOI] [PubMed] [Google Scholar]
- 382.Sager C, Tapken D, Kott S, Hollmann M. Functional modulation of AMPA receptors by transmembrane AMPA receptor regulatory proteins. Neuroscience. 2009;158:45–54. doi: 10.1016/j.neuroscience.2007.12.046. [DOI] [PubMed] [Google Scholar]
- 383.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 384.Sambuughin N, Holley H, Muldoon S, Brandom BW, de Bantel AM, Tobin JR, Nelson TE, Goldfarb LG. Screening of the entire ryanodine receptor type 1 coding region for sequence variants associated with malignant hyperthermia susceptibility in the North American population. Anesthesiology. 2005;102:515–521. doi: 10.1097/00000542-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 385.Sandoval A, Oviedo N, Andrade A, Felix R. Glycosylation of asparagines 136 and 184 is necessary for the α2δ subunit-mediated regulation of voltage-gated Ca2+ channels. FEBS Lett. 2004;576:21–26. doi: 10.1016/j.febslet.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 386.Sandoz G, Bichet D, Cornet V, Mori Y, Felix R, De Waard M. Distinct properties and differential β subunit regulation of two C-terminal isoforms of the P/Q-type Ca2+-channel α1A subunit. Eur J Neurosci. 2001;14:987–997. doi: 10.1046/j.0953-816x.2001.01728.x. [DOI] [PubMed] [Google Scholar]
- 387.Sandoz G, Lopez-Gonzalez I, Grunwald D, Bichet D, Altafaj X, Weiss N, Ronjat M, Dupuis A, De Waard M. Cavβ-subunit displacement is a key step to induce the reluctant state of P/Q calcium channels by direct G protein regulation. Proc Natl Acad Sci USA. 2004;101:6267–6272. doi: 10.1073/pnas.0306804101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Sasaki T, Shibasaki T, Beguin P, Nagashima K, Miyazaki M, Seino S. Direct inhibition of the interaction between α-interaction domain and α-interaction domain of voltage-dependent Ca2+ channels by Gem. J Biol Chem. 2005;280:9308–9312. doi: 10.1074/jbc.M413773200. [DOI] [PubMed] [Google Scholar]
- 389.Sather WA, McCleskey EW. Permeation and selectivity in calcium channels. Annu Rev Physiol. 2003;65:133–159. doi: 10.1146/annurev.physiol.65.092101.142345. [DOI] [PubMed] [Google Scholar]
- 390.Schjott JM, Hsu SC, Plummer MR. The neuronal β4 subunit increases the unitary conductance of L-type voltage-gated calcium channels in PC12 cells. J Biol Chem. 2003;278:33936–33942. doi: 10.1074/jbc.M302059200. [DOI] [PubMed] [Google Scholar]
- 391.Schlick B, Flucher BE, Obermair GJ. Voltage-activated calcium channel expression profiles in mouse brain and cultured hippocampal neurons. Neuroscience. 2010;167:786–798. doi: 10.1016/j.neuroscience.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, Schmitz F, Malenka RC, Sudhof TC. RIM1α forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002;415:321–326. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- 393.Schredelseker J, Dayal A, Schwerte T, Franzini-Armstrong C, Grabner M. Proper restoration of excitation-contraction coupling in the dihydropyridine receptor β1-null zebrafish relaxed is an exclusive function of the β1a subunit. J Biol Chem. 2009;284:1242–1251. doi: 10.1074/jbc.M807767200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Schredelseker J, Di Biase V, Obermair GJ, Felder ET, Flucher BE, Franzini-Armstrong C, Grabner M. The β1a subunit is essential for the assembly of dihydropyridine-receptor arrays in skeletal muscle. Proc Natl Acad Sci USA. 2005;102:17219–17224. doi: 10.1073/pnas.0508710102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Scott VE, De Waard M, Liu H, Gurnett CA, Venzke DP, Lennon VA, Campbell KP. Β subunit heterogeneity in N-type Ca2+ channels. J Biol Chem. 1996;271:3207–3212. doi: 10.1074/jbc.271.6.3207. [DOI] [PubMed] [Google Scholar]
- 396.Segal D, Ohana E, Besser L, Hershfinkel M, Moran A, Sekler I. A role for ZnT-1 in regulating cellular cation influx. Biochem Biophys Res Commun. 2004;323:1145–1150. doi: 10.1016/j.bbrc.2004.08.211. [DOI] [PubMed] [Google Scholar]
- 397.Seu L, Pitt GS. Dose-dependent and isoform-specific modulation of Ca2+ channels by RGK GTPases. J Gen Physiol. 2006;128:605–613. doi: 10.1085/jgp.200609631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Shao Y, Czymmek KJ, Jones PA, Fomin VP, Akanbi K, Duncan RL, Farach-Carson MC. Dynamic interactions between L-type voltage-sensitive calcium channel Cav1.2 subunits and ahnak in osteoblastic cells. Am J Physiol Cell Physiol. 2009;296:C1067–C1078. doi: 10.1152/ajpcell.00427.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Sharp AH, Black IJL, Dubel SJ, Sundarraj S, Shen JP, Yunker AMR, Copeland TD, McEnery MW. Biochemical and anatomical evidence for specialized voltage-dependent calcium channel [γ] isoform expression in the epileptic and ataxic mouse, stargazer. Neuroscience. 2001;105:599–617. doi: 10.1016/s0306-4522(01)00220-2. [DOI] [PubMed] [Google Scholar]
- 400.Sheng ZH, Westenbroek RE, Catterall WA. Physical link and functional coupling of presynaptic calcium channels and the synaptic vesicle docking/fusion machinery. J Bioenerg Biomembr. 1998;30:335–345. doi: 10.1023/a:1021985521748. [DOI] [PubMed] [Google Scholar]
- 401.Sheridan DC, Cheng W, Ahern CA, Mortenson L, Alsammarae D, Vallejo P, Coronado R. Truncation of the carboxyl terminus of the dihydropyridine receptor β1a subunit promotes Ca2+ dependent excitation-contraction coupling in skeletal myotubes. Biophys J. 2003;84:220–237. doi: 10.1016/S0006-3495(03)74844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Sheridan DC, Cheng W, Carbonneau L, Ahern CA, Coronado R. Involvement of a heptad repeat in the carboxyl terminus of the dihydropyridine receptor β1a subunit in the mechanism of excitation-contraction coupling in skeletal muscle. Biophys J. 2004;87:929–942. doi: 10.1529/biophysj.104.043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Shimada T, Somlyo AP. Modulation of voltage-dependent Ca channel current by arachidonic acid and other long-chain fatty acids in rabbit intestinal smooth muscle. J Gen Physiol. 1992;100:27–44. doi: 10.1085/jgp.100.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Shistik E, Ivanina T, Blumenstein Y, Dascal N. Crucial role of N terminus in function of cardiac L-type Ca2+ channel and its modulation by protein kinase C. J Biol Chem. 1998;273:17901–17909. doi: 10.1074/jbc.273.28.17901. [DOI] [PubMed] [Google Scholar]
- 405.Shistik E, Ivanina T, Puri T, Hosey M, Dascal N. Ca2+ current enhancement by α2/δ and β subunits in Xenopus oocytes: contribution of changes in channel gating and α1 protein level. J Physiol. 1995;489:55–62. doi: 10.1113/jphysiol.1995.sp021029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, Dascal N. The roles of the subunits in the function of the calcium channel. Science. 1991;253:1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- 407.Skryma R, Prevarskaya N, Vacher P, Dufy B. Voltage-dependent Ca2+ channels in Chinese hamster ovary (CHO) cells. FEBS Lett. 1994;349:289–294. doi: 10.1016/0014-5793(94)00690-3. [DOI] [PubMed] [Google Scholar]
- 408.Soong TW, Stea A, Hodson CD, Dubel SJ, Vincent SR, Snutch TP. Structure and functional expression of a member of the low voltage-activated calcium channel family. Science. 1993;260:1133–1136. doi: 10.1126/science.8388125. [DOI] [PubMed] [Google Scholar]
- 409.Spafford JD, Van Minnen J, Larsen P, Smit AB, Syed NI, Zamponi GW. Uncoupling of calcium channel α1 and β subunits in developing neurons. J Biol Chem. 2004;279:41157–41167. doi: 10.1074/jbc.M403781200. [DOI] [PubMed] [Google Scholar]
- 410.Splingard A, Menetrey J, Perderiset M, Cicolari J, Regazzoni K, Hamoudi F, Cabanie L, El Marjou A, Wells A, Houdusse A, de Gunzburg J. Biochemical and structural characterization of the gem GTPase. J Biol Chem. 2007;282:1905–1915. doi: 10.1074/jbc.M604363200. [DOI] [PubMed] [Google Scholar]
- 411.Stary A, Shafrir Y, Hering S, Wolschann P, Guy HR. Structural model of the Cav1.2 pore. Channels. 2008;2:210–215. doi: 10.4161/chan.2.3.6158. [DOI] [PubMed] [Google Scholar]
- 412.Stea A, Dubel SJ, Pragnell M, Leonard JP, Campbell KP, Snutch TP. A β-subunit normalizes the electrophysiological properties of a cloned N-type Ca2+ channel α1-subunit. Neuropharmacology. 1993;32:1103–1116. doi: 10.1016/0028-3908(93)90005-n. [DOI] [PubMed] [Google Scholar]
- 413.Stea A, Soong TW, Snutch TP. Determinants of PKC-dependent modulation of a family of neuronal calcium channels. Neuron. 1995;15:929–940. doi: 10.1016/0896-6273(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 414.Stea A, Tomlinson WJ, Soong TW, Bourinet E, Dubel SJ, Vincent SR, Snutch TP. Localization and functional properties of a rat brain α1A calcium channel reflect similarities to neuronal Q- and P-type channels. Proc Natl Acad Sci USA. 1994;91:10576–10580. doi: 10.1073/pnas.91.22.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Stehle T, Schulz GE. Refined structure of the complex between guanylate kinase and its substrate GMP at 2.0 A resolution. J Mol Biol. 1992;224:1127–1141. doi: 10.1016/0022-2836(92)90474-x. [DOI] [PubMed] [Google Scholar]
- 416.Stehle T, Schulz GE. Three-dimensional structure of the complex of guanylate kinase from yeast with its substrate GMP. J Mol Biol. 1990;211:249–254. doi: 10.1016/0022-2836(90)90024-G. [DOI] [PubMed] [Google Scholar]
- 417.Stephens GJ, Page KM, Bogdanov Y, Dolphin AC. The α1B Ca2+ channel amino terminus contributes determinants for β subunit-mediated voltage-dependent inactivation properties. J Physiol. 2000;525:377–390. doi: 10.1111/j.1469-7793.2000.t01-1-00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Stephens GJ, Page KM, Burley JR, Berrow NS, Dolphin AC. Functional expression of rat brain cloned α1E calcium channels in COS-7 cells. Pflügers Arch. 1997;433:523–532. doi: 10.1007/s004240050308. [DOI] [PubMed] [Google Scholar]
- 419.Stokes L, Gordon J, Grafton G. Non-voltage-gated L-type Ca2+ channels in human T cells: pharmacology and molecular characterization of the major α pore-forming and auxiliary β-subunits. J Biol Chem. 2004;279:19566–19573. doi: 10.1074/jbc.M401481200. [DOI] [PubMed] [Google Scholar]
- 420.Stotz SC, Barr W, McRory JE, Chen L, Jarvis SE, Zamponi GW. Several structural domains contribute to the regulation of N-type calcium channel inactivation by the β3 subunit. J Biol Chem. 2004;279:3793–3800. doi: 10.1074/jbc.M308991200. [DOI] [PubMed] [Google Scholar]
- 421.Stotz SC, Hamid J, Spaetgens RL, Jarvis SE, Zamponi GW. Fast inactivation of voltage-dependent calcium channels. A hinged-lid mechanism? J Biol Chem. 2000;275:24575–24582. doi: 10.1074/jbc.M000399200. [DOI] [PubMed] [Google Scholar]
- 422.Stotz SC, Jarvis SE, Zamponi GW. Functional roles of cytoplasmic loops and pore lining transmembrane helices in the voltage-dependent inactivation of HVA calcium channels. J Physiol. 2004;554:263–273. doi: 10.1113/jphysiol.2003.047068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Striessnig J, Koschak A. Exploring the function and pharmacotherapeutic potential of voltage-gated Ca2+ channels with gene knockout models. Channels. 2008;2:233–251. doi: 10.4161/chan.2.4.5847. [DOI] [PubMed] [Google Scholar]
- 424.Strock J, Diverse-Pierluissi MA. Ca2+ channels as integrators of G protein-mediated signaling in neurons. Mol Pharmacol. 2004;66:1071–1076. doi: 10.1124/mol.104.002261. [DOI] [PubMed] [Google Scholar]
- 425.Subramanyam P, Obermair GJ, Baumgartner S, Gebhart M, Striessnig J, Kaufmann WA, Geley S, Flucher BE. Activity and calcium regulate nuclear targeting of the calcium channel β4b subunit in nerve and muscle cells. Channels. 2009;3 doi: 10.4161/chan.3.5.9696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Swartz KJ, Merritt A, Bean BP, Lovinger DM. Protein kinase C modulates glutamate receptor inhibition of Ca2+ channels and synaptic transmission. Nature. 1993;361:165–168. doi: 10.1038/361165a0. [DOI] [PubMed] [Google Scholar]
- 427.Tadmouri A, Kiyonaka S, Barbado M, Rousset M, Arnoult C, Dolmetsch RE, Ronjat M, Mori Y, de Waard M. 10th ECS Meeting. Belgium: Leuven; 2008. The calcium channel Cavβ4 subunit acts as an independent transcription factor. poster A-7. [Google Scholar]
- 428.Takahashi E, Ino M, Miyamoto N, Nagasu T. Increased expression of P/Q-type Ca2+ channel α1A subunit mRNA in cerebellum of N-type Ca2+ channel α1B subunit gene-deficient mice. Brain Res. 2004;124:79–87. doi: 10.1016/j.molbrainres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 429.Takahashi E, Nagasu T. Enhanced expression of Ca2+ channel α1A and β4 subunits and phosphorylated tyrosine hydroxylase in the adrenal gland of N-type Ca2+ channel α1B subunit-deficient mice with a CBA/JN genetic background. Comp Med. 2006;56:168–175. [PubMed] [Google Scholar]
- 430.Takahashi M, Seagar MJ, Jones JF, Reber BF, Catterall WA. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc Natl Acad Sci USA. 1987;84:5478–5482. doi: 10.1073/pnas.84.15.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Takahashi SX, Miriyala J, Colecraft HM. Membrane-associated guanylate kinase-like properties of β-subunits required for modulation of voltage-dependent Ca2+ channels. Proc Natl Acad Sci USA. 2004;101:7193–7198. doi: 10.1073/pnas.0306665101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Takahashi SX, Miriyala J, Tay LH, Yue DT, Colecraft HM. A Cavβ SH3/guanylate kinase domain interaction regulates multiple properties of voltage-gated Ca2+ channels. J Gen Physiol. 2005;126:365–377. doi: 10.1085/jgp.200509354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Takahashi SX, Mittman S, Colecraft HM. Distinctive modulatory effects of five human auxiliary β2 subunit splice variants on L-type calcium channel gating. Biophys J. 2003;84:3007–3021. doi: 10.1016/S0006-3495(03)70027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Tanabe T, Takeshima H, Mikami A, Flockerzi V, Takahashi H, Kangawa K, Kojima M, Matsuo H, Hirose T, Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987;328:313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- 435.Tanaka O, Sakagami H, Kondo H. Localization of mRNAs of voltage-dependent Ca2+-channels: four subtypes of α1- and β-subunits in developing and mature rat brain. Brain Res. 1995;30:1–16. doi: 10.1016/0169-328x(94)00265-g. [DOI] [PubMed] [Google Scholar]
- 436.Tareilus E, Roux M, Qin N, Olcese R, Zhou J, Stefani E, Birnbaumer L. A Xenopus oocyte β subunit: evidence for a role in the assembly/expression of voltage-gated calcium channels that is separate from its role as a regulatory subunit. Proc Natl Acad Sci USA. 1997;94:1703–1708. doi: 10.1073/pnas.94.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Tavares GA, Panepucci EH, Brunger AT. Structural characterization of the intramolecular interaction between the SH3 and guanylate kinase domains of PSD-95. Mol Cell. 2001;8:1313–1325. doi: 10.1016/s1097-2765(01)00416-6. [DOI] [PubMed] [Google Scholar]
- 438.Taviaux S, Williams ME, Harpold MM, Nargeot J, Lory P. Assignment of human genes for β2 and β4 subunits of voltage-dependent Ca2+ channels to chromosomes 10p12 and 2q22–q23. Hum Genet. 1997;100:151–154. doi: 10.1007/pl00008704. [DOI] [PubMed] [Google Scholar]
- 439.Tedford HW, Kisilevsky AE, Vieira LB, Varela D, Chen L, Zamponi GW. Scanning mutagenesis of the I–II loop of the Cav2.2 calcium channel identifies residues Arginine 376 and Valine 416 as molecular determinants of voltage dependent G protein inhibition. Mol Brain. 2010;3:6. doi: 10.1186/1756-6606-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Tedford HW, Zamponi GW. Direct G protein modulation of Cav2 calcium channels. Pharmacol Rev. 2006;58:837–862. doi: 10.1124/pr.58.4.11. [DOI] [PubMed] [Google Scholar]
- 441.Telemaque S, Sonkusare S, Grain T, Rhee SW, Stimers JR, Rusch NJ, Marsh JD. Design of mutant β2 subunits as decoy molecules to reduce the expression of functional Ca2+ channels in cardiac cells. J Pharmacol Exp Ther. 2008;325:37–46. doi: 10.1124/jpet.107.128215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Tomita S, Fukata M, Nicoll RA, Bredt DS. Dynamic interaction of stargazin-like TARPs with cycling AMPA receptors at synapses. Science. 2004;303:1508–1511. doi: 10.1126/science.1090262. [DOI] [PubMed] [Google Scholar]
- 443.Tomlinson WJ, Stea A, Bourinet E, Charnet P, Nargeot J, Snutch TP. Functional properties of a neuronal class C L-type calcium channel. Neuropharmacology. 1993;32:1117–1126. doi: 10.1016/0028-3908(93)90006-o. [DOI] [PubMed] [Google Scholar]
- 444.Tsien RW, Ellinor PT, Horne WA. Molecular diversity of voltage-dependent Ca2+ channels. Trends Pharmacol Sci. 1991;12:349–354. doi: 10.1016/0165-6147(91)90595-j. [DOI] [PubMed] [Google Scholar]
- 445.Tsien RW, Hess P, McCleskey EW, Rosenberg RL. Calcium channels: mechanisms of selectivity, permeation, and block. Annu Rev Biophys Biophys Chem. 1987;16:265–290. doi: 10.1146/annurev.bb.16.060187.001405. [DOI] [PubMed] [Google Scholar]
- 446.Tsien RW, Lipscombe D, Madison DV, Bley KR, Fox AP. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988;11:431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- 447.Van Petegem F, Clark KA, Chatelain FC, Minor DL., Jr Structure of a complex between a voltage-gated calcium channel β-subunit and an α-subunit domain. Nature. 2004;429:671–675. doi: 10.1038/nature02588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Van Petegem F, Duderstadt KE, Clark KA, Wang M, Minor DL., Jr Alanine-scanning mutagenesis defines a conserved energetic hotspot in the Cavα1 AID-Cavβ interaction site that is critical for channel modulation. Structure. 2008;16:280–294. doi: 10.1016/j.str.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Vance CL, Begg CM, Lee WL, Haase H, Copeland TD, McEnery MW. Differential expression and association of calcium channel α1B and β subunits during rat brain ontogeny. J Biol Chem. 1998;273:14495–14502. doi: 10.1074/jbc.273.23.14495. [DOI] [PubMed] [Google Scholar]
- 450.Varadi G, Lory P, Schultz D, Varadi M, Schwartz A. Acceleration of activation and inactivation by the β subunit of the skeletal muscle calcium channel. Nature. 1991;352:159–162. doi: 10.1038/352159a0. [DOI] [PubMed] [Google Scholar]
- 451.Vendel AC, Rithner CD, Lyons BA, Horne WA. Solution structure of the N-terminal A domain of the human voltage-gated Ca2+channel β4a subunit. Protein Sci. 2006;15:378–383. doi: 10.1110/ps.051894506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Vendel AC, Terry MD, Striegel AR, Iverson NM, Leuranguer V, Rithner CD, Lyons BA, Pickard GE, Tobet SA, Horne WA. Alternative splicing of the voltage-gated Ca2+ channel β4 subunit creates a uniquely folded N-terminal protein binding domain with cell-specific expression in the cerebellar cortex. J Neurosci. 2006;26:2635–2644. doi: 10.1523/JNEUROSCI.0067-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Verschuuren JJ, Dalmau J, Tunkel R, Lang B, Graus F, Schramm L, Posner JB, Newsom-Davis J, Rosenfeld MR. Antibodies against the calcium channel β-subunit in Lambert-Eaton myasthenic syndrome. Neurology. 1998;50:475–479. doi: 10.1212/wnl.50.2.475. [DOI] [PubMed] [Google Scholar]
- 454.Viard P, Butcher AJ, Halet G, Davies A, Nurnberg B, Heblich F, Dolphin AC. PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. Nature Neurosci. 2004;7:939–946. doi: 10.1038/nn1300. [DOI] [PubMed] [Google Scholar]
- 455.Vitko I, Shcheglovitov A, Baumgart JP, Arias O, II, Murbartian J, Arias JM, Perez-Reyes E. Orientation of the calcium channel β relative to the α122 subunit is critical for its regulation of channel activity. PLoS One. 2008;3:e3560. doi: 10.1371/journal.pone.0003560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Volsen SG, Day NC, McCormack AL, Smith W, Craig PJ, Beattie RE, Smith D, Ince PG, Shaw PJ, Ellis SB, Mayne N, Burnett JP, Gillespie A, Harpold MM. The expression of voltage-dependent calcium channel β subunits in human cerebellum. Neuroscience. 1997;80:161–174. doi: 10.1016/s0306-4522(97)00115-2. [DOI] [PubMed] [Google Scholar]
- 457.Wakamori M, Mikala G, Mori Y. Auxiliary subunits operate as a molecular switch in determining gating behaviour of the unitary N-type Ca2+ channel current in Xenopus oocytes. J Physiol. 1999;517:659–672. doi: 10.1111/j.1469-7793.1999.0659s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Wakamori M, Mikala G, Schwartz A, Yatani A. Single-channel analysis of a cloned human heart L-type Ca2+ channel α1 subunit and the effects of a cardiac β subunit. Biochem Biophys Res Commun. 1993;196:1170–1176. doi: 10.1006/bbrc.1993.2374. [DOI] [PubMed] [Google Scholar]
- 459.Wakamori M, Niidome T, Furutama D, Furuichi T, Mikoshiba K, Fujita Y, Tanaka I, Katayama K, Yatani A, Schwartz A. Distinctive functional properties of the neuronal BII (class E) calcium channel. Receptors Channels. 1994;2:303–314. [PubMed] [Google Scholar]
- 460.Walker D, Bichet D, Campbell KP, De Waard M. A β4 isoform-specific interaction site in the carboxyl-terminal region of the voltage-dependent Ca2+ channel α1A subunit. J Biol Chem. 1998;273:2361–2367. doi: 10.1074/jbc.273.4.2361. [DOI] [PubMed] [Google Scholar]
- 461.Walker D, Bichet D, Geib S, Mori E, Cornet V, Snutch TP, Mori Y, De Waard M. A new β subtype-specific interaction in α1A subunit controls P/Q-type Ca2+ channel activation. J Biol Chem. 1999;274:12383–12390. doi: 10.1074/jbc.274.18.12383. [DOI] [PubMed] [Google Scholar]
- 462.Wang G, Zhu X, Xie W, Han P, Li K, Sun Z, Wang Y, Chen C, Song R, Cao C, Zhang J, Wu C, Liu J, Cheng H. Rad as a novel regulator of excitation-contraction coupling and β-adrenergic signaling in heart. Circ Res. 2010;106:317–327. doi: 10.1161/CIRCRESAHA.109.208272. [DOI] [PubMed] [Google Scholar]
- 463.Ward Y, Spinelli B, Quon MJ, Chen H, Ikeda SR, Kelly K. Phosphorylation of critical serine residues in Gem separates cytoskeletal reorganization from down-regulation of calcium channel activity. Mol Cell Biol. 2004;24:651–661. doi: 10.1128/MCB.24.2.651-661.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Washbourne P. Greasing transmission: palmitoylation at the synapse. Neuron. 2004;44:901–902. doi: 10.1016/j.neuron.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 465.Wei SK, Colecraft HM, DeMaria CD, Peterson BZ, Zhang R, Kohout TA, Rogers TB, Yue DT. Ca2+ channel modulation by recombinant auxiliary β subunits expressed in young adult heart cells. Circ Res. 2000;86:175–184. doi: 10.1161/01.res.86.2.175. [DOI] [PubMed] [Google Scholar]
- 466.Wei X, Neely A, Olcese R, Lang W, Stefani E, Birnbaumer L. Increase in Ca2+ channel expression by deletions at the amino terminus of the cardiac α1C subunit. Receptors Channels. 1996;4:205–215. [PubMed] [Google Scholar]
- 467.Wei XY, Perez-Reyes E, Lacerda AE, Schuster G, Brown AM, Birnbaumer L. Heterologous regulation of the cardiac Ca2+ channel α1 subunit by skeletal muscle β and γ subunits. Implications for the structure of cardiac L-type Ca2+ channels. J Biol Chem. 1991;266:21943–21947. [PubMed] [Google Scholar]
- 468.Weissgerber P, Held B, Bloch W, Kaestner L, Chien KR, Fleischmann BK, Lipp P, Flockerzi V, Freichel M. Reduced cardiac L-type Ca2+ current in Cavβ2−/− embryos impairs cardiac development and contraction with secondary defects in vascular maturation. Circ Res. 2006;99:749–757. doi: 10.1161/01.RES.0000243978.15182.c1. [DOI] [PubMed] [Google Scholar]
- 470.Williams ME, Feldman DH, McCue AF, Brenner R, Velicelebi G, Ellis SB, Harpold MM. Structure and functional expression of α1, α2, and β subunits of a novel human neuronal calcium channel subtype. Neuron. 1992;8:71–84. doi: 10.1016/0896-6273(92)90109-q. [DOI] [PubMed] [Google Scholar]
- 471.Williams S, Serafin M, Muhlethaler M, Bernheim L. Facilitation of N-type calcium current is dependent on the frequency of action potential-like depolarizations in dissociated cholinergic basal forebrain neurons of the guinea pig. J Neurosci. 1997;17:1625–1632. doi: 10.1523/JNEUROSCI.17-05-01625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Witcher DR, De Waard M, Liu H, Pragnell M, Campbell KP. Association of native Ca2+ channel β subunits with the α1 subunit interaction domain. J Biol Chem. 1995;270:18088–18093. doi: 10.1074/jbc.270.30.18088. [DOI] [PubMed] [Google Scholar]
- 473.Witcher DR, De Waard M, Sakamoto J, Franzini-Armstrong C, Pragnell M, Kahl SD, Campbell KP. Subunit identification and reconstitution of the N-type Ca2+ channel complex purified from brain. Science. 1993;261:486–489. doi: 10.1126/science.8392754. [DOI] [PubMed] [Google Scholar]
- 474.Wittemann S, Mark MD, Rettig J, Herlitze S. Synaptic localization and presynaptic function of calcium channel β4-subunits in cultured hippocampal neurons. J Biol Chem. 2000;275:37807–37814. doi: 10.1074/jbc.M004653200. [DOI] [PubMed] [Google Scholar]
- 475.Wu L, Bauer CS, Zhen XG, Xie C, Yang J. Dual regulation of voltage-gated calcium channels by PtdIns(4,5)P2. Nature. 2002;419:947–952. doi: 10.1038/nature01118. [DOI] [PubMed] [Google Scholar]
- 476.Xie C, Zhen XG, Yang J. Localization of the activation gate of a voltage-gated Ca2+ channel. J Gen Physiol. 2005;126:205–212. doi: 10.1085/jgp.200509293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Xu W, Lipscombe D. Neuronal Cav1.3α1 L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Yada H, Murata M, Shimoda K, Yuasa S, Kawaguchi H, Ieda M, Adachi T, Murata M, Ogawa S, Fukuda K. Dominant negative suppression of Rad leads to QT prolongation and causes ventricular arrhythmias via modulation of L-type Ca2+ channels in the heart. Circ Res. 2007;101:69–77. doi: 10.1161/CIRCRESAHA.106.146399. [DOI] [PubMed] [Google Scholar]
- 479.Yamada Y, Masuda K, Li Q, Ihara Y, Kubota A, Miura T, Nakamura K, Fujii Y, Seino S, Seino Y. The structures of the human calcium channel α1 subunit (CACNL1A2) and β subunit (CACNLB3) genes. Genomics. 1995;27:312–319. doi: 10.1006/geno.1995.1048. [DOI] [PubMed] [Google Scholar]
- 480.Yamaguchi H, Hara M, Strobeck M, Fukasawa K, Schwartz A, Varadi G. Multiple modulation pathways of calcium channel activity by a β subunit. Direct evidence of β subunit participation in membrane trafficking of the α1C subunit. J Biol Chem. 1998;273:19348–19356. doi: 10.1074/jbc.273.30.19348. [DOI] [PubMed] [Google Scholar]
- 481.Yamaguchi H, Okuda M, Mikala G, Fukasawa K, Varadi G. Cloning of the β2a subunit of the voltage-dependent calcium channel from human heart: cooperative effect of α2/δ and β2a on the membrane expression of the α1C subunit. Biochem Biophys Res Commun. 2000;267:156–163. doi: 10.1006/bbrc.1999.1926. [DOI] [PubMed] [Google Scholar]
- 482.Yang J, Ellinor PT, Sather WA, Zhang JF, Tsien RW. Molecular determinants of Ca2+ selectivity and ion permeation in L-type Ca2+ channels. Nature. 1993;366:158–161. doi: 10.1038/366158a0. [DOI] [PubMed] [Google Scholar]
- 483.Yang J, Tsien RW. Enhancement of N- and L-type calcium channel currents by protein kinase C in frog sympathetic neurons. Neuron. 1993;10:127–136. doi: 10.1016/0896-6273(93)90305-b. [DOI] [PubMed] [Google Scholar]
- 484.Yang L, Liu G, Zakharov SI, Bellinger AM, Mongillo M, Marx SO. Protein kinase G phosphorylates Cav1.2 α1c and β2 subunits. Circ Res. 2007;101:465–474. doi: 10.1161/CIRCRESAHA.107.156976. [DOI] [PubMed] [Google Scholar]
- 485.Yang L, Liu G, Zakharov SI, Morrow JP, Rybin VO, Steinberg SF, Marx SO. Ser1928 is a common site for Cav1.2 phosphorylation by protein kinase C isoforms. J Biol Chem. 2005;280:207–214. doi: 10.1074/jbc.M410509200. [DOI] [PubMed] [Google Scholar]
- 486.Yang SN, Berggren PO. The role of voltage-gated calcium channels in pancreatic β-cell physiology and pathophysiology. Endocr Rev. 2006;27:621–676. doi: 10.1210/er.2005-0888. [DOI] [PubMed] [Google Scholar]
- 487.Yang T, Suhail Y, Dalton S, Kernan T, Colecraft HM. Genetically encoded molecules for inducibly inactivating Cav channels. Nat Chem Biol. 2007;3:795–804. doi: 10.1038/nchembio.2007.42. [DOI] [PubMed] [Google Scholar]
- 488.Yang T, Xu X, Kernan T, Wu V, Colecraft H. Rem inhibits recombinant Cav1.2 channels using multiple mechanisms that require distinct configurations of the GTPase. J Physiol. 2010;588:1665–1681. doi: 10.1113/jphysiol.2010.187203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Yanuar A, Sakurai S, Kitano K, Hakoshima T. Crystal structure of human Rad GTPase of the RGK-family. Genes Cells. 2006;11:961–968. doi: 10.1111/j.1365-2443.2006.00994.x. [DOI] [PubMed] [Google Scholar]
- 490.Yasuda R. Imaging spatiotemporal dynamics of neuronal signaling using fluorescence resonance energy transfer and fluorescence lifetime imaging microscopy. Curr Opin Neurobiol. 2006;16:551–561. doi: 10.1016/j.conb.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 491.Yasuda T, Lewis RJ, Adams DJ. Overexpressed Cavβ3 inhibits N-type (Cav2.2) calcium channel currents through a hyperpolarizing shift of ultra-slow and closed-state inactivation. J Gen Physiol. 2004;123:401–416. doi: 10.1085/jgp.200308967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Yu AS, Boim M, Hebert SC, Castellano A, Perez-Reyes E, Lytton J. Molecular characterization of renal calcium channel β-subunit transcripts. Am J Physiol Renal Fluid Electrolyte Physiol. 1995;268:F525–F531. doi: 10.1152/ajprenal.1995.268.3.F525. [DOI] [PubMed] [Google Scholar]
- 493.Yu K, Xiao Q, Cui G, Lee A, Hartzell HC. The best disease-linked Cl− channel hBest1 regulates Ca V 1 (L-type) Ca2+ channels via src-homology-binding domains. J Neurosci. 2008;28:5660–5670. doi: 10.1523/JNEUROSCI.0065-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Zamponi GW. Calmodulin lobotomized: novel insights into calcium regulation of voltage-gated calcium channels. Neuron. 2003;39:879–881. doi: 10.1016/s0896-6273(03)00564-6. [DOI] [PubMed] [Google Scholar]
- 495.Zamponi GW. Voltage-Gated Calcium Channels. New York: Landes Bioscience/Eurekah; 2005. p. 377. [Google Scholar]
- 496.Zamponi GW, Bourinet E, Nelson D, Nargeot J, Snutch TP. Crosstalk between G proteins and protein kinase C mediated by the calcium channel α1 subunit. Nature. 1997;385:442–446. doi: 10.1038/385442a0. [DOI] [PubMed] [Google Scholar]
- 497.Zamponi GW, Snutch TP. Decay of prepulse facilitation of N type calcium channels during G protein inhibition is consistent with binding of a single Gβ subunit. Proc Natl Acad Sci USA. 1998;95:4035–4039. doi: 10.1073/pnas.95.7.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Zerangue N, Jan LY. G-protein signaling: fine-tuning signaling kinetics. Curr Biol. 1998;8:R313–R316. doi: 10.1016/s0960-9822(98)70196-4. [DOI] [PubMed] [Google Scholar]
- 499.Zhang JF, Ellinor PT, Aldrich RW, Tsien RW. Multiple structural elements in voltage-dependent Ca2+ channels support their inhibition by G proteins. Neuron. 1996;17:991–1003. doi: 10.1016/s0896-6273(00)80229-9. [DOI] [PubMed] [Google Scholar]
- 500.Zhang JF, Randall AD, Ellinor PT, Horne WA, Sather WA, Tanabe T, Schwarz TL, Tsien RW. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993;32:1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]
- 501.Zhang R, Dzhura I, Grueter CE, Thiel W, Colbran RJ, Anderson ME. A dynamic α-β inter-subunit agonist signaling complex is a novel feedback mechanism for regulating L-type Ca2+ channel opening. FASEB J. 2005;19:1573–1575. doi: 10.1096/fj.04-3283fje. [DOI] [PubMed] [Google Scholar]
- 502.Zhang Y, Chen YH, Bangaru SD, He L, Abele K, Tanabe S, Kozasa T, Yang J. Origin of the voltage dependence of G-protein regulation of P/Q-type Ca2+ channels. J Neurosci. 2008;28:14176–14188. doi: 10.1523/JNEUROSCI.1350-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Zhang Y, Mori M, Burgess DL, Noebels JL. Mutations in high-voltage-activated calcium channel genes stimulate low-voltage-activated currents in mouse thalamic relay neurons. J Neurosci. 2002;22:6362–6371. doi: 10.1523/JNEUROSCI.22-15-06362.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Zhang Y, Yamada Y, Fan M, Bangaru SD, Lin B, Yang J. The β subunit of voltage-gated Ca2+ channels interacts with and regulates the activity of a novel isoform of Pax6. J Biol Chem. 2010;285:2527–2536. doi: 10.1074/jbc.M109.022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Zhen XG, Xie C, Fitzmaurice A, Schoonover CE, Orenstein ET, Yang J. Functional architecture of the inner pore of a voltage-gated Ca2+ channel. J Gen Physiol. 2005;126:193–204. doi: 10.1085/jgp.200509292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Zhou W, Horstick EJ, Hirata H, Kuwada JY. Identification and expression of voltage-gated calcium channel β subunits in zebrafish. Dev Dyn. 2008;237:3842–3852. doi: 10.1002/dvdy.21776. [DOI] [PubMed] [Google Scholar]
- 507.Zhou W, Saint-Amant L, Hirata H, Cui WW, Sprague SM, Kuwada JY. Non-sense mutations in the dihydropyridine receptor β1 gene, CACNB1, paralyze zebrafish relaxed mutants. Cell Calcium. 2006;39:227–236. doi: 10.1016/j.ceca.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 508.Zou S, Jha S, Kim EY, Dryer SE. The β1 subunit of L-type voltage-gated Ca2+ channels independently binds to and inhibits the gating of large-conductance Ca2+-activated K+ channels. Mol Pharmacol. 2008;73:369–378. doi: 10.1124/mol.107.040733. [DOI] [PubMed] [Google Scholar]
- 509.Zuhlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 1999;399:159–162. doi: 10.1038/20200. [DOI] [PubMed] [Google Scholar]