Abstract
Background
Human hip morphology is variable, and some variations (or hip morphotypes) such as coxa profunda and coxa recta (cam-type hip) are associated with femoroacetabular impingement and the development of osteoarthrosis. Currently, however, this variability is unexplained. A broader perspective with background information on the morphology of the proximal femur of nonhuman apes is lacking. Specifically, no studies exist of nonhuman ape femora that quantify concavity and its variability.
Questions/purposes
We hypothesized that, when compared with modern humans, the nonhuman apes would show (1) greater proximal femoral concavity; (2) less variability in concavity; and (3) less sexual dimorphism in proximal femoral morphology.
Methods
Using identical methods, we compared 10 morphological parameters in 375 human femora that are part of the Hamann-Todd collection at the Cleveland Museum of Natural History with 210 nonhuman ape femora that are part of the collection of the Royal Museum for Central Africa, Tervuren, Belgium, and the Muséum National d’Histoire Naturelle, Paris, France.
Results
The nonhuman apes have larger proximal femoral concavity than modern humans. This morphology is almost uniform without large variability or large differences neither between species nor between sexes.
Conclusions
Variability is seen in human but not in nonhuman ape proximal femoral morphology. An evolutionary explanation can be that proximal femoral concavity is more important for the nonhuman apes, for example for climbing, than for modern humans, where a lack of concavity may be related to high loading of the hip, for example in running.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-014-3754-z) contains supplementary material, which is available to authorized users.
Introduction
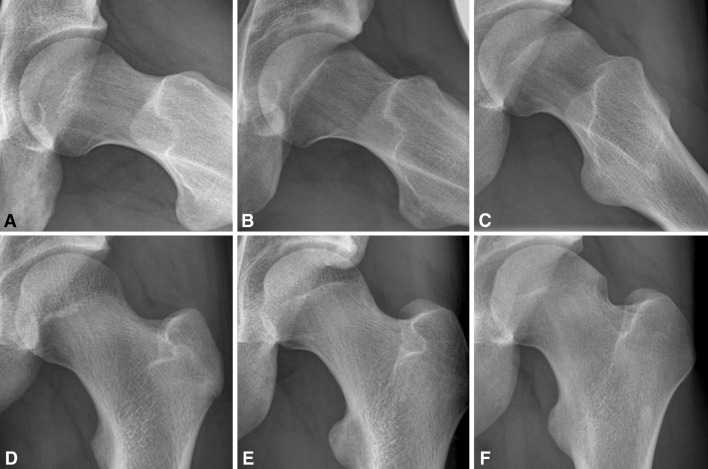
Human hip morphology is variable (Fig. 1), and some variations (or hip morphotypes) such as coxa profunda and coxa recta (cam type hip) are associated with femoroacetabular impingement and the development of osteoarthrosis [6, 7]. Currently, however, this variability is unexplained.
Fig. 1A–F.
Frog leg lateral (A–C) and AP radiographs of asymptomatic adolescent soccer players illustrate coxa rotunda (A, D) and coxa recta (B–C, E–F). Figures provided with courtesy of R. Agricola and used with the author’s permission.
One explanation may be that the existence of hip morphotypes is an expression of normal genic variability, and hence a normal feature of all hominids (modern humans and their ancestors, but also chimpanzees, bonobo’s, gorilla, and orangutan; we refer to the nonhuman hominids as “nonhuman apes”). Another explanation may be that proximal femoral morphology is, at least partly, determined by the mechanical loading history of the hip during growth and development. Although the number of studies suggesting a relation between hip loading and morphology is increasing [1, 12, 14, 15], the presence or absence of variability in the nonhuman apes is unknown. The nonhuman apes are climbers, quadrupedal walkers/runners, and facultative bipedal walkers [5, 8, 13], creating a very different loading history during hip ontogenesis.
Perhaps surprisingly, a comprehensive quantitative description of human proximal femoral morphology and its variability was published only in 2009 [17]. Of particular interest for the definition of morphotypes is the shape of the proximal femur, which can be defined by the concavity of the head and neck junction. This attribute is determined not only by femoral head sphericity, but also by the dimension and position of the femoral head relative to the neck. In a sample of 375 human femora, Toogood et al. [17] found variability in concavity at the superior and anterior head-neck junction.
For the nonhuman apes, given the importance of hip ROM for climbing, specifically rotations and abduction, the presence or absence of large concavity likely has a real effect on evolutionary fitness. Given these considerations, we hypothesized that, when compared with modern humans, the nonhuman apes would show (1) greater proximal femoral concavity; (2) less variability in concavity; and (3) less sexual dimorphism in proximal femoral concavity.
We used a standardized measurement technique [17] to compare 10 parameters of proximal femoral morphology between modern humans and the nonhuman apes.
Materials and Methods
Specimens
All specimens studied (Table 1) are part of the collection of the Royal Museum for Central Africa, Tervuren, Belgium, and the Muséum National d’Histoire Naturelle, Paris, France. We examined 210 femora of three ape species: chimpanzee (Pan troglodytes, n = 80), bonobo (Pan paniscus, n = 38), and gorilla (Gorilla gorilla, n = 92). Femora with the femoral neck physis not fully closed, osteoarthritic changes, fractures, or other anatomical abnormalities on visual inspection were excluded.
Table 1.
Overview of specimens studied
| Specimen type | Gorilla | Pan | Human |
|---|---|---|---|
| Male | 46 | 54 | 188 |
| Female | 28 | 51 | 187 |
| Unknown sex | 18 | 13 | 0 |
| Left | 47 | 57 | 182 |
| Right | 45 | 61 | 193 |
| Total | 92 | 118 | 375 |
No differences were found between bonobo and chimpanzee in any of the parameters studied (data not shown); therefore, the two species were analyzed as one group, Pan.
In 96 cases, left and right femurs of one individual were available for analysis. In 18 cases, only one femur was. Comparison of means was used to assess if left and right could be treated as individual specimens. No difference was found between left and right femora in any of the parameters studied. Therefore, left and right femora were analyzed as individual specimens.
Human Specimens
Measurements of the ape femora were compared with measurements of 375 human femora, as published earlier [17]. These femora were randomly selected from the 3000 skeletons of the Hamann-Todd collection at the Cleveland Museum of Natural History and represent 375 femora from the normal human population. The sample was diversified in terms of race, age, and sex by dividing equally among sexes and available races (45 white males, 46 white females, 49 black males, 48 black females). Femora not fully mature at the time of death and femora from individuals younger than 18 years were excluded. Also, femora with anatomic abnormalities on visual inspection were excluded as well as femora affected by disease such as osteoarthrosis, osteonecrosis, or other deformity.
Photography
We used the technique and terminology as described by Toogood et al. [17]. Briefly, photographs were taken in two standardized positions, termed AP and lateral. For the AP photographs, we placed each femur in a supine position on a flat laboratory bench with anterior surfaces directed toward the ceiling. In this position, the specimen rested distally on the medial and lateral condyles and proximally on the greater trochanter. The femoral neck then was made parallel to the superior surface of the laboratory bench by either rotating the femoral shaft internally and supporting the lateral condyle if the neck axis was anteverted or rotating the femoral shaft externally and supporting the medial condyle if the neck axis was retroverted. Parallelism between the femoral neck and laboratory bench was determined through visual inspection. The investigator taking the photographs used square cards, approximately 1 mm in thickness, to increasingly support the medial or lateral condyle until the axis of the neck appeared parallel to the laboratory bench surface. By taking a photograph from directly overhead (camera lens parallel to the laboratory bench and femoral neck axis as confirmed by a level), we obtained accurate AP pictures; any potential distortion resulting from neck version was eliminated by making all components of the setup parallel.
For the lateral photographs, we again placed each femur on the flat laboratory bench surface with anterior surfaces facing up. The femur then was abducted until the femoral neck was parallel with the plane produced by the edge of the laboratory bench. Parallelism again was determined through visual inspection. The investigator taking the photographs increasingly abducted the femoral shaft until the axis of the neck appeared parallel to the laboratory bench edge from overhead. Additionally, each femur was checked to ensure the medial and lateral condyles rested on the surface of the laboratory bench distally, allowing the table surface to represent the transcondylar axis. By taking pictures with the lens of the camera parallel to the edge of the laboratory bench (as confirmed using a T-square ruler) and even with its surface (as confirmed through the camera’s view finder), we obtained accurate lateral images. Any distortion produced by the angle of inclination was eliminated by making the neck axis and camera parallel.
Measurements
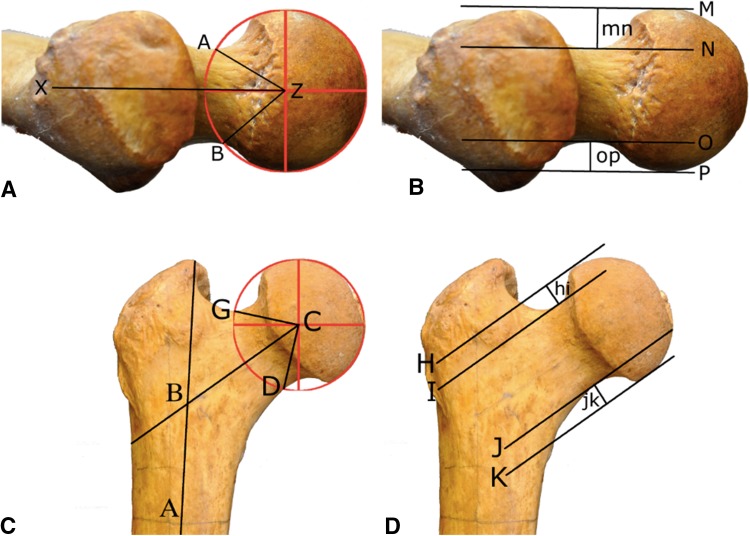
Ten measurements were used to describe proximal femoral morphology in the nonhuman apes (Fig. 2). Proximal femoral concavity was quantified with four angles: alpha, beta, gamma, and delta, describing concavity at the anterior, posterior, superior, and inferior aspects of the head-neck junction, respectively. The position of the femoral head relative to the neck was quantified with four translational measurements: anterior, posterior, superior, and inferior offset. To overcome size differences between species, offset of the femoral head was expressed as a ratio, ie, anterior offset/posterior offset and superior offset/inferior offset. In addition, the relation of the femoral neck to the femoral shaft was quantified with neck version (anteversion or retroversion) and neck-shaft angle.
Fig. 2A–D.
Lateral view (A–B) (perpendicular to the cranial marginal) showing the femoral neck in a chimpanzee with the posterior surface of the femoral condyles parallel to the horizontal plane/examination table. (A) Center of femoral head (Z); femoral neck axis (line XZ). Femoral neck version is the angle between XZ and the horizontal plane with anteversion represented as a positive value and retroversion as a negative value. Alpha angle is the angle between AZ and XZ; beta angle is the angle between BZ and XZ. (B) Anterior offset (mn) is the perpendicular distance between lines M and N; posterior offset (op) is the perpendicular distance between lines O and P. Lines M, N, O, and P are all parallel to the femoral neck axis (XZ). Lines M and P are tangential to the femoral head. Lines N and O are tangential to the femoral neck. Anterior view (C–D) of the proximal femur in a chimpanzee shows the femoral neck axis parallel to the horizontal plane/examination table. (C) Center of femoral head (C); femoral shaft axis (line AB); femoral neck axis (line BC); neck-shaft angle is the angle between AB and BC; gamma angle is the angle between BC and CG; delta angle is the angle between BC and CD. (D) Superior offset (hi) is the perpendicular distance between lines H and I; inferior offset (jk) is the perpendicular distance between line J and K. Lines H, I, J, and K are all parallel to the femoral neck axis (BC). Lines H and K are tangential to the femoral head. Lines I and J are tangential to the femoral neck.
Gimp 2.6 (http://www.gimp.org/) was used to perform all measurements.
Interobserver analysis showed moderate/good agreement (intraclass correlation coefficient [ICC], alpha 0.68; beta 0.75; delta 0.75; posterior offset 0.79; inferior offset 0.78) and strong agreement (ICC, gamma 0.81; neck-version 0.94; neck-shaft angle 0.94; anterior offset 0.93; superior offset 0.84) in the parameters used.
Statistical Analysis
For all statistics, we used IBM SPSS statistics 20.0 (IBM, Armonk, NY, USA). Range, mean, and SD were determined for each of the parameters measured, for all specimens as one group, and divided into subgroups by species (Gorilla, Pan, and Human).
We compared the three subgroups with a one-way analysis of variance (ANOVA). T-tests were not sufficient because multiple tests on the same data increased the chance of falsely rejecting the null hypothesis. A one-way ANOVA corrects for this Type I error.
To explore variables between subgroups, Hochberg’s GT2 post hoc tests were used as a result of differences in sample size. Variables included were alpha, beta, gamma, and delta angles, anterior offset/posterior offset, superior offset/inferior offset, femoral neck version, and neck-shaft angle.
Levene’s tests for homogeneity of variance were performed to examine variability in alpha, beta, gamma, and delta angles in Gorilla, Pan, and Human.
Independent t-tests were used to determine sex differences for the parameters measured. For this analysis, 18 Gorilla and 13 Pan specimens were excluded because of unknown sex.
Results
Comparing between populations of the nonhuman apes and modern humans, the latter showed larger alpha (mean 45.6, SD 10.5 versus mean 27.4, SD 3.4 [Gorilla subgroup] and mean 29.4, SD 4.7 [Pan subgroup]), equal beta (mean 41.9, SD 6.9 versus mean 41.0, SD 4.2 [Gorilla subgroup] and mean 41.7, SD 4.9 [Pan subgroup]), larger gamma (mean 53.5, SD 12.7 versus 43.7, SD 3.9 [Gorilla subgroup] and mean 36.5, SD 4.2 [Pan subgroup]), and smaller delta angles (mean 43.0, SD 4.9 versus mean 48.7, SD 5.6 [Gorilla subgroup] and mean 47.0, SD 4.1 [Pan subgroup]). Smaller anterior offset/posterior offset- (mean 1.1, SD 0.4 versus mean 1.5, SD 0.3 [Gorilla subgroup] and mean 1.4, SD 0.3 [Pan subgroup]) and superior offset/inferior offset ratios (mean 0.9, SD 0.4 versus mean 1.3, SD 0.3 [Gorilla subgroup] and mean 1.5, SD 0.4 [Pan subgroup]) were found as well as a higher neck version (mean 9.7, SD 9.3 versus mean 4.6, SD 7.0 [Gorilla subgroup] and mean 5.3, SD 6.9 [Pan subgroup]) and neck-shaft angle (mean 129.2, SD 6.3 versus mean 123.0, SD 5.7 [Gorilla subgroup] and mean 125.6, SD 5.2 [Pan subgroup]) (Table 2).
Table 2.
The mean and SD of the different species in all measurements*
| Parameter measured | Species | One-way ANOVA difference | |||
|---|---|---|---|---|---|
| Gorilla Mean (SD) |
Pan Mean (SD) |
Human Mean (SD) |
F | p value | |
| Alpha | 27.4 (3.4) | 29.4 (4.7) | 45.6 (10.5) | 259.5 | < 0.001 |
| Beta | 41.0 (4.2) | 41.7 (4.9) | 41.9 (6.9) | 0.7 | > 0.05 |
| Gamma | 43.7 (3.9) | 36.5 (4.2) | 53.5 (12.7) | 129.8 | < 0.001 |
| Delta | 48.7 (5.6) | 47.0 (4.1) | 43.0 (4.9) | 68.5 | < 0.001 |
| Anterior offset/posterior offset | 1.5 (0.3) | 1.4 (0.3) | 1.1 (0.4) | 54.0 | < 0.001 |
| Superior offset/inferior offset | 1.3 (0.3) | 1.5 (0.4) | 0.9 (0.4) | 140.4 | < 0.001 |
| Neck version | 4.6 (7.0) | 5.3 (6.9) | 9.7 (9.3) | 21.3 | < 0.001 |
| Neck-shaft angle | 123.0 (5.7) | 125.6 (5.2) | 129.2 (6.3) | 47.5 | < 0.001 |
* The one-way analysis of variance (ANOVA) shows whether significant differences exist between the species.
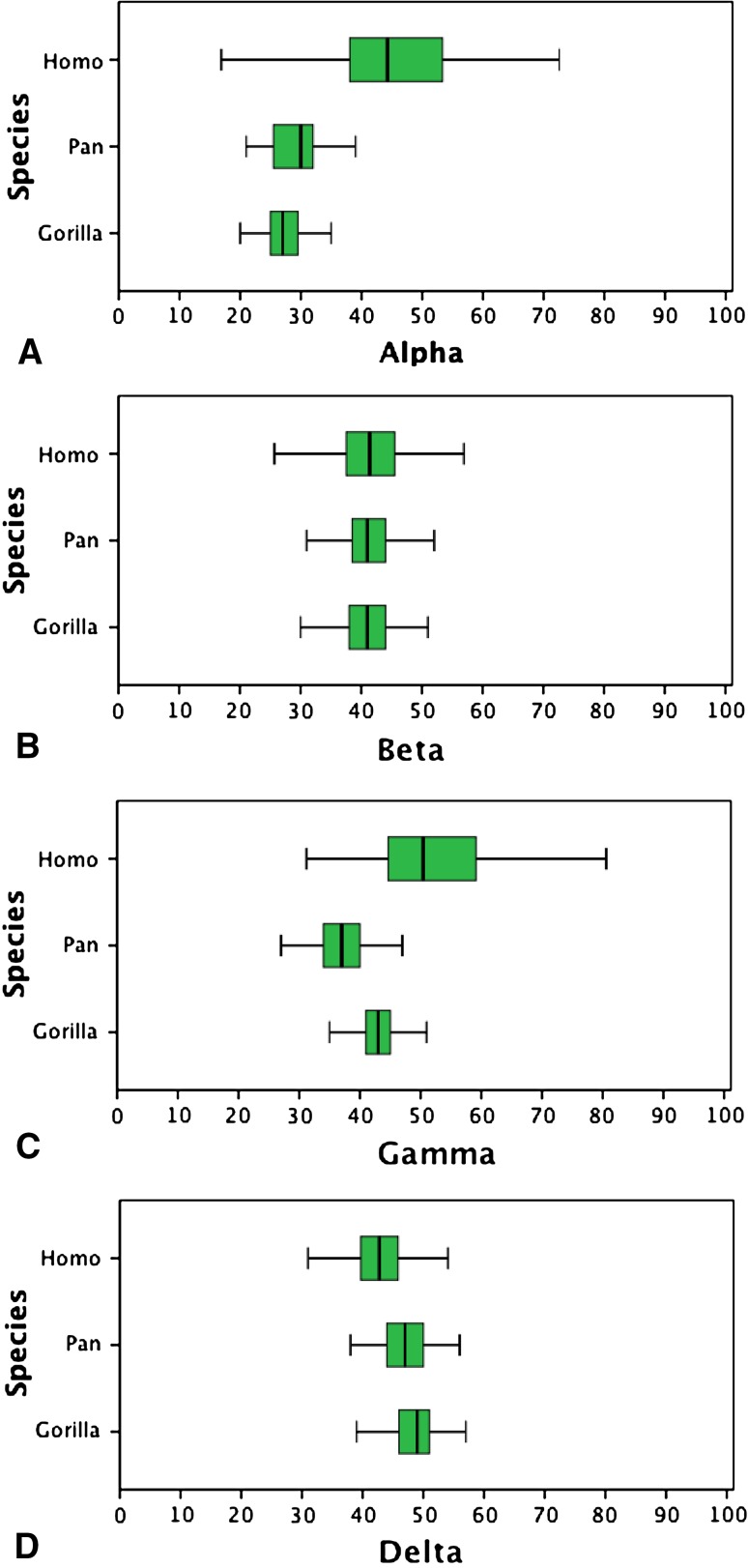
The most striking difference between the human and nonhuman ape proximal femora was lower concavity in the anterosuperior quadrant of the head-neck junction as evidenced by higher alpha (mean 45.6, SD 10.5) and gamma angles (mean 53.5, SD 12.7) in modern humans compared with the Pan subgroup (alpha mean 29.4, SD 4.7; gamma mean 36.5, SD 4.2) and the Gorilla subgroup (alpha mean 27.4, SD 3.4; gamma mean 43.7, SD 3.9) (Fig. 3). Compared with the nonhuman apes, this lower concavity in modern humans is the result of a combination of a wider femoral neck, a more posteroinferior position of the femoral head on the neck, and reduced femoral head sphericity, as quantified by the superior offset/inferior offset and anterior offset/posterior offset, alpha and gamma angles, respectively (Table 2; Fig. 3).
Fig. 3A–D.
Large variability in human but not Pan and Gorilla for the alpha angle (A) and gamma (C) angle. By contrast, beta angle (B) and delta angle (D) display negligible variability. Median, interquartile range (green box), and minimum/maximum excluding outliers (bars) are shown.
Furthermore, in modern humans, variability in alpha and gamma angles was much larger than in the nonhuman apes, as quantified by Levene statistics of Gorilla versus Pan: 12.076, p = 0.001 (alpha angle), 1.235, p > 0.05 (gamma angle) compared with Gorilla versus human: 82.644, p < 0.001 (alpha angle), 58.057, p < 0.001 (gamma angle), and Pan versus human: 66.001, p < 0.001 (alpha angle), 65.797, p < 0.001 (gamma angle). Variability for all other parameters was more or less similar (Table 3).
Table 3.
Levene’s test for homogeneity of variances*
| Parameter measured | Gorilla versus Pan | Gorilla versus human | Pan versus human | |||
|---|---|---|---|---|---|---|
| Levene statistic | p value | Levene statistic | p value | Levene statistic | p value | |
| Alpha | 12.076 | 0.001 | 82.644 | < 0.001 | 66.006 | < 0.001 |
| Beta | 1.027 | > 0.05 | 13.182 | < 0.001 | 9.486 | < 0.001 |
| Gamma | 1.235 | > 0.05 | 58.057 | < 0.001 | 65.797 | < 0.001 |
| Delta | 0.658 | > 0.05 | 0.028 | > 0.05 | 1.991 | > 0.05 |
* Probability values < 0.05 correspond to a significant difference in variance between species. Higher Levene statistics indicate a stronger difference in variance.
Sex differences were found in all parameters for the group human, except for beta and gamma angle (Table 4). In Pan, none of the parameters showed a sex difference, whereas in Gorilla, only alpha angle and neck-shaft angle showed a sex difference.
Table 4.
Sex differences within species
| Parameter measured | Sex | Gorilla | Pan | Human | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Difference within sex p value |
Mean (SD) | Difference within sex p value |
Mean (SD) | Difference within sex p value |
||
| Alpha | M | 27.9 (3.6) | < 0.05 | 29.4 (3.8) | > 0.05 | 47.5 (10.7) | < 0.001 |
| F | 26.2 (2.2) | 29.8 (5.1) | 43.7 (9.9) | ||||
| Beta | M | 40.7 (4.5) | > 0.05 | 41.1 (5.3) | > 0.05 | 41.5 (7.6) | > 0.05 |
| F | 41.5 (3.7) | 42.7 (4.9) | 42.2 (6.1) | ||||
| Gamma | M | 43.7 (3.8) | > 0.05 | 36.0 (4.2) | > 0.05 | 54.3 (12.4) | > 0.05 |
| F | 43.9 (3.4) | 36.9 (4.2) | 52.7 (12.9) | ||||
| Delta | M | 48.3 (3.9) | > 0.05 | 46.9 (3.8) | > 0.05 | 42.4 (5.1) | < 0.05 |
| F | 50.1 (7.9) | 47.4 (4.2) | 43.5 (4.5) | ||||
| Anterior offset/posterior offset | M | 1.5 (0.3) | > 0.05 | 1.4 (0.3) | > 0.05 | 1.1 (0.4) | < 0.05 |
| F | 1.6 (0.2) | 1.5 (0.4) | 1.2 (0.4) | ||||
| Superior offset/inferior offset | M | 1.3 (0.3) | > 0.05 | 1.5 (0.3) | > 0.05 | 0.8 (0.4) | < 0.01 |
| F | 1.2 (0.3) | 1.6 (0.5) | 1.0 (0.4) | ||||
| Neck version | M | 4.2 (6.9) | > 0.05 | 6.0 (8.3) | > 0.05 | 9.6 (9.8) | > 0.05 |
| F | 5.8 (4.8) | 4.5 (5.5) | 9.8 (8.7) | ||||
| Neck-shaft angle | M | 120.7 (4.7) | < 0.001 | 125.2 (5.2) | > 0.05 | 129.7 (6.6) | > 0.05 |
| F | 127.8 (4.0) | 125.9 (5.4) | 128.8 (6.0) | ||||
Discussion
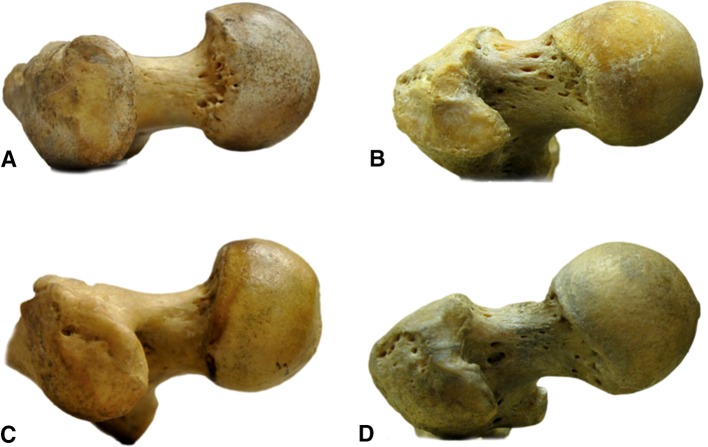
There is substantial variability in the shape of the human proximal femur with certain variations (or morphotypes) predisposing to long-term clinical sequelae. For example, both coxa profunda and coxa recta are morphotypes associated with femoroacetabular impingement [6, 7]. However, such variability in the shape of the human proximal femur does not appear to be present in nonhuman apes (Figs. 3, 4). The presence of such variability in nonhuman apes would contradict explanations for hip morphotypes that focus on the hip’s loading history. The absence of this variability, however, does not invalidate a genetic component in the explanation of how these morphotypes come about. One reason for this can be that, unlike anthropocentrism may lead us to believe, a “perfectly round” femoral head with large concavity may actually be more important in evolutionary terms for the nonhuman apes than for modern humans [11].
Fig. 4A–D.
Lateral view shows a gorilla femur (A), chimpanzee femur (C), modern human femur with coxa recta morphotype (B), and modern human femur with coxa rotunda morphotype (D).
We therefore explored the level of variability in femoral concavity in modern humans and in the nonhuman apes, using bony specimens, with a view toward establishing a broader perspective on modern human morphology.
Our study is limited by the relative paucity of ape specimens (n = 210) in contrast to a robust collection of 375 human specimens. Although we obtained access to six fossil specimens of early homo species, we could not draw any conclusions from the data as a result of the limited number of specimens. For the same reason, we have not included our measurements of 11 orangutan (Pongo pygmaeus) specimens described elsewhere [4]. We also have no data on acetabular and pelvic anatomy; thus, we cannot conclude that variability may be substantially greater in sites other than the proximal femur. In addition, we cannot answer whether morphologic differences are the result of evolutionary and developmental processes or purely from the hip activity and subsequent loading of an individual specimen. Alternatively, it may reflect differences in nonbony anatomy such as differences in labral structure and cartilage thickness, which would not be apparent in a study focusing purely on osteology.
Our first hypothesis, that nonhuman apes would display greater concavity of the femoral neck and head than modern humans was confirmed. Clearly, the wider femoral neck of modern humans reduces head/neck ratio and concavity compared with the nonhuman apes. However, our measurements show it is not neck width alone that explains the reduced concavity in modern humans. Reduced anterosuperior femoral head sphericity (Fig. 1) and a femoral head offset posteroinferior relative to the femoral neck can distinguish the modern human femur from the nonhuman apes (Table 4). We speculate that the reduced concavity in the anterosuperior head-neck region in modern humans is an adaptation to better resist the higher loads of obligate bipedal upright gait and running [5]. Decreasing concavity may increase tensile strength in the anterosuperior femoral head-neck junction [2], ie, the region where the femoral neck fracture line starts [2, 9].
We also confirmed our second hypothesis of decreased variability in proximal femoral concavity in nonhuman apes when compared with humans. Comparing our findings with other studies, considerable variability in human proximal femoral morphology was also documented, albeit qualitatively, in a sample of 532 human femora [3]. We are not aware of reports that documented morphological variability of the proximal femur in other mammals than modern humans. To explain the uniformity of the nonhuman ape proximal femoral morphology, and specifically the lack of variability in concavity, we suggest this reflects the importance of this trait for these climbing apes. From an evolutionary perspective, a large proximal femoral concavity and lack of variability may provide nonhuman apes with an evolutionary advantage in terms of survival, and thus represents a Type I trait in the classification by Lovejoy et al. [11] (Appendix 1 [Supplemental materials are available with the online version of CORR®.]). In contrast, the lower concavity in humans, and the variability seen, may reflect the relative lack of an evolutionary advantage for this particular anatomic trait in humans.
We found no evidence of sexual dimorphism in the proximal femora of nonhuman apes, in contrast to our findings in modern humans. Sexual dimorphism is well documented for the pelvis in both humans and the nonhuman apes with females having larger relative and/or absolute pelvic dimensions related to overall body size [10, 16]. The absence of sex differences in proximal femoral morphology in the nonhuman apes suggests sex hormones do not play an important role in their hip ontogeny, whereas they likely do in pelvic ontogeny [16]. Of interest, higher alpha and gamma angles and lower anterior offset/posterior offset and superior offset/inferior offset in human males (Table 4) all correspond to coxa recta (cam-type hip) associated with femoroacetabular impingement and the development of osteoarthrosis.
In conclusion, there are clear differences in the anatomy of the proximal femur between the modern human and our most closely related evolutionary cousins, the nonhuman apes. The degree of variability within humans is substantially higher as well. Whether this reflects a true evolutionary difference or an adaptation to walking on two legs remains to be seen. A broader perspective of proximal femoral morphology, obtained from nonhuman apes, may help to formulate explanations for human hip morphotypes.
Electronic supplementary material
Acknowledgments
We thank Dr Christine Lefèvre (MNHN, Paris, France) and Wim Wendelen (Royal Museum for Central Africa, Tervuren, Belgium) for their indispensable and very kind cooperation in examining the ape collections. We thank Dr Evie Vereecke, Department of Development and Regeneration, University of Leuven, Leuven, Belgium, for her valuable suggestions on the manuscript.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Agricola R, Bessems JH, Ginai AZ, Heijboer MP, van der Heijden RA, Verhaar JA, Weinans H, Waarsing JH. The development of cam-type deformity in adolescent and young male soccer players. Am J Sports Med. 2012;40:1099–1106. doi: 10.1177/0363546512438381. [DOI] [PubMed] [Google Scholar]
- 2.Alonso-Rasgado T, Jimenez-Cruz D, Bailey CG, Mandal P, Board T. Changes in the stress in the femoral head neck junction after osteochondroplasty for hip impingement: a finite element study. J Orthop Res. 2012;30:1999–2006. doi: 10.1002/jor.22164. [DOI] [PubMed] [Google Scholar]
- 3.Asfaw B. Proximal femur articulation in Pliocene hominids. Am J Phys Anthropol. 1985;68:535–538. doi: 10.1002/ajpa.1330680409. [DOI] [PubMed] [Google Scholar]
- 4.Bouma HW, De Boer SF, De Vos J, Van Kampen PM, Hogervorst T. Mammal hip morphology and function: coxa recta and coxa rotunda. Anat Rec (Hoboken). 2013;296:250–256. doi: 10.1002/ar.22634. [DOI] [PubMed] [Google Scholar]
- 5.Doran DM. Ontogeny of locomotion in mountain gorillas and chimpanzees. J Hum Evol. 1997;32:323–344. doi: 10.1006/jhev.1996.0095. [DOI] [PubMed] [Google Scholar]
- 6.Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of osteoarthritis of the hip: an integrated mechanical concept. Clin Orthop Relat Res. 2008;466:264–272. doi: 10.1007/s11999-007-0060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogervorst T, Bouma H, de Boer SF, de Vos J. Human hip impingement morphology: an evolutionary explanation. J Bone Joint Surg Br. 2011;93:769–776. doi: 10.1302/0301-620X.93B6.25149. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins FA., Jr Chimpanzee bipedalism: cineradiographic analysis and implications for the evolution of gait. Science. 1972;178:877–879. doi: 10.1126/science.178.4063.877. [DOI] [PubMed] [Google Scholar]
- 9.Juszczyk MM, Cristofolini L, Viceconti M. The human proximal femur behaves linearly elastic up to failure under physiological loading conditions. J Biomech. 2011;44:2259–2266. doi: 10.1016/j.jbiomech.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 10.Kurki HK. Pelvic dimorphism in relation to body size and body size dimorphism in humans. J Hum Evol. 2011;61:631–643. doi: 10.1016/j.jhevol.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Lovejoy CO, Meindl RS, Ohman JC, Heiple KG, White TD. The Maka femur and its bearing on the antiquity of human walking: applying contemporary concepts of morphogenesis to the human fossil record. Am J Phys Anthropol. 2002;119:97–133. doi: 10.1002/ajpa.10111. [DOI] [PubMed] [Google Scholar]
- 12.Murray RO, Duncan C. Athletic activity in adolescence as an etiological factor in degenerative hip disease. J Bone Joint Surg Br. 1971;53:406–419. [PubMed] [Google Scholar]
- 13.Schultz AH. The Life of Primates. New York, NY, USA: Universe Books; 1969. [Google Scholar]
- 14.Siebenrock KA, Ferner F, Noble PC, Santore RF, Werlen S, Mamisch TC. The cam-type deformity of the proximal femur arises in childhood in response to vigorous sporting activity. Clin Orthop Relat Res. 2011;469:3229–3240. doi: 10.1007/s11999-011-1945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siebenrock KA, Kaschka I, Frauchiger L, Werlen S, Schwab JM. Prevalence of cam-type deformity and hip pain in elite ice hockey players before and after the end of growth. Am J Sports Med. 2013;41:2308–2313. doi: 10.1177/0363546513497564. [DOI] [PubMed] [Google Scholar]
- 16.Tague RG. Big-bodied males help us recognize that females have big pelves. Am J Phys Anthropol. 2005;127:392–405. doi: 10.1002/ajpa.20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toogood PA, Skalak A, Cooperman DR. Proximal femoral anatomy in the normal human population. Clin Orthop Relat Res. 2009;467:876–885. doi: 10.1007/s11999-008-0473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.