Abstract
Children of depressed parents are significantly more likely to develop depression and other mental health disorders than are children of never-depressed parents. Investigations of the physiological mechanisms underlying this elevated risk have generally focused on basal functioning. It is important to note, however, that physiological reactivity or responses to stress are also critical determinants of mental and physical health. In the current study, we examined whether children of depressed parents exhibit altered physiological responses to stress. In two studies, never-depressed adolescent daughters of either recurrently depressed mothers (RISK) or never-depressed mothers (CTL) underwent social stressors while their physiological responses were measured (cortisol in Study 1, heart rate in Study 2). In both studies, affective responses to the stressors predicted physiological responses in RISK girls, but not in never-depressed girls. For RISK girls, decreased positive affect in response to stress predicted increased cortisol reactivity; in addition, decreased positive affect and increased negative affect were associated with poorer heart rate recovery and habituation, respectively. Future research is needed to examine explicitly whether this coherence between affect and physiology is a mechanism underlying the increased risk for psychopathology in children of depressed parents.
Depressive disorders place a burden of over 40 billion dollars per year on the American economy, accounting for over 20% of costs of all mental illness (Stewart, Ricci, Chee, Hahn, & Morganstein, 2003). It is estimated that 16% of the general population will experience clinically significant depression (Kessler, 2009), and that depression is associated with increases in mortality rates comparable to that of smoking (Mykletun et al., 2009). There is a long history of investigators attempting to identify mechanisms that might underlie risk for this pervasive and life-threatening disorder (e.g., Abramson, Seligman, & Teasdale, 1978; Beck, 1967; Goodman & Gotlib, 1999; Monroe & Simons, 1991). One of the strongest risk factors for becoming depressed is having a parent with major depressive disorder (MDD); children of depressed parents are two to three times more likely to become depressed themselves than are children of never-depressed parents (Hammen, 2009; Joormann, Eugene, & Gotlib, 2008). Certainly, depression in either parent represents a risk factor for the offspring (Lieb, Isensee, Hofler, Pfister, & Wittchen, 2002); most research, however, has focused on the adverse effects of maternal depression (Goodman & Gotlib, 2002; Gotlib & Goodman, 1999). Investigators have estimated that 40% of the offspring of depressed mothers will develop either depression (Hammen, 2009) or other forms of psychopathology (Weissman, Warner, Wickramaratne, Moreau, & Olfson, 1997).
Although it is well established that having a depressed mother is a robust risk factor for developing depression, only recently have researchers begun to elucidate the mechanisms that underlie this elevated risk for depression. Given that both child (Lopez-Duran, Kovacs, & George, 2009) and adult (Burke, Davis, Otte, & Mohr, 2005) depression have been found to be associated with physiological dysregulation, investigators have begun to examine whether this construct might be a risk factor for the development of this disorder, observable before the onset of a first depressive episode. The formulation of physiological dysregulation as a candidate risk mechanism is also consistent with the notion of allostatic load—that the effects on tissue of extended and repeated physiological activation can accrue over time to harm the brain and body (Seeman, McEwen, Rowe, & Singer, 2001). In this paper, we explore two of the physiological systems known to contribute to allostatic load: the hypothalamic–pituitary–adrenal (HPA) axis and the cardiovascular system.
The bulk of the evidence implicating physiological risk mechanisms in children of depressed mothers comes from studies of the HPA axis, particularly disruptions in the diurnal pattern of cortisol secretion, the primary stress hormone of the HPA axis. Children of depressed mothers have been found to exhibit elevated levels of morning cortisol (Halligan, Herbert, Goodyer, & Murray, 2004; Mannie, Harmer, & Cowen, 2007), which in turn predict the onset of subsequent depressive symptoms (Halligan, Herbert, Goodyer, & Murray, 2007).
This evidence suggests that dysregulation of the HPA axis is a risk mechanism underlying the development of depression. It is important to note, however, that the diurnal pattern of cortisol secretion is only one of several indicators of HPA-axis functioning. Another indicator of HPA-axis functioning is cortisol responsivity to acute psychosocial stressors (Kirschbaum, Pirke, & Hellhammer, 1993). According to cognitive theories of risk for depression (e.g., Beck, 1976) and diathesis–stress models of risk for depression (Monroe & Simons, 1991), risk factors for depression emerge in affectively charged stressful situations. Yet despite the evidence that cortisol responses during these types of stressful situations are dysregulated in both adult (Burke et al., 2005; Young, Lopez, Murphy-Weinberg, Watson, & Akil, 2000) and child (Rao, Hammen, Ortiz, Chen, & Poland, 2008) depression, we do not know whether anomalous cortisol responsivity is a risk factor for the development of this disorder. In one of the few studies examining this question, Ashman, Dawson, Panagiotides, Yamada, and Wilkinson (2002) found no difference in cortisol responding between children of depressed mothers (who are themselves at elevated risk for depression) and children of never-depressed mothers. It is important to note, however, that these investigators used a weak stress manipulation that did not produce significant elevations in cortisol from baseline. Given the paucity of research examining whether cortisol responses to stress may be an early biomarker of risk for depression, in the present studies we extend the literature on HPA-axis functioning in children of depressed mothers by examining cortisol responses during a robust stress induction.
Even less is known about the possible role of cardiovascular stress responding as a risk factor in children of depressed mothers. As noted above, like HPA-axis dysfunction, cardiovascular dysregulation is a critical component of allostatic load (Seeman et al., 2001). As is the case with cortisol, cardiovascular responses during stress have also been found to be dysregulated in depressed adults (Salomon, Clift, Karlsdottir, & Rottenberg, 2009). Studies of cardiovascular functioning in children of depressed parents, however, have focused almost exclusively on basal functioning. For example, young children of chronically depressed mothers have been found to be characterized by elevated resting respiratory sinus arrhythmia, a measure of the tonic influence of the parasympathetic system on the heart (Ashman, Dawson, & Panagiotides, 2008). In the present studies, we examine whether children of depressed mothers are also characterized by altered cardiovascular responses to stress.
If altered physiological responding is a risk factor for depression, we would expect to find a difference between children of depressed mothers and children of never-depressed mothers in their physiological responses to stress. Merely being in a stressful situation, however, may not be sufficient to induce these altered physiological responses in children of depressed mothers. Investigators have demonstrated that it is only in the presence of a strong affective experience that some risk mechanisms, such as cognitive biases, are exhibited by children at risk for depression (e.g., Joormann, Talbot, & Gotlib, 2007; Taylor & Ingram, 1999). Thus, as a candidate risk mechanism, altered physiological responding to a stressor may be evident only in those children who exhibit increased negative affect (NA) when experiencing the stressor. In addition to NA, positive affective responses during exposure to a stressful event may also moderate the relation between altered physiological responding and familial risk for depression. Although children of depressed mothers are at increased risk for depression, a significant proportion of these children are resilient and will not develop psychopathology. One influential coping factor that characterizes resilient individuals is their ability to maintain positive affect (PA) during stress (Folkman & Moskowitz, 2000), a characteristic that protects them from experiencing maladaptive physiological stress responses (Tugade & Fredrickson, 2004). Children of depressed mothers who have this ability to maintain relatively high levels of PA during stress may be similarly buffered from altered physiological responses.
In sum, we predict that if altered physiological responses during stressful situations are risk mechanisms for depression, these altered responses should be evident in the children of depressed mothers who exhibit stronger affective responses to stress (i.e., increased NA and/or decreased PA). This formulation does not discount the association between affective and physiological responses to stressors in the children of never-depressed mothers. Many of the above-stated associations between resilience and the use of PA to regulate physiological responses would also hold true for low-risk children. Rather, we propose that, compared with low-risk children, high-risk children’s physiological responses during stressors would be more susceptible to the influence of affective responses during the stressor.
The primary aim of the current studies was to examine whether children at elevated familial risk for depression exhibit altered physiological responses to stressors. In two studies, daughters of mothers with a history of recurrent depression and daughters of never-depressed mothers were exposed to social stressors while their HPA-axis responses (cortisol; Study 1) and cardiovascular responses (heart rate [HR]; Study 2) were recorded. We examined only girls because MDD is twice as prevalent in females than in males (Kessler, 2009), and because daughters of depressed mothers are more likely than are sons to develop depressive symptoms (Davies & Windle, 1997). To ensure that any altered physiological responses would be due to the daughters’ risk for depression and not the symptoms of current disorder or ‘scars’ of past disorder, daughters were screened to ensure that they had no current or lifetime diagnosable psychopathology (Barnett & Gotlib, 1988). We hypothesized that, compared with daughters of never-depressed mothers, daughters of depressed mothers will exhibit altered physiological responding during stress (operationalized in the introduction to each study). Further, we hypothesized that this altered physiological responding will be most pronounced for those daughters of depressed mothers who exhibit strong affective responses to stress (i.e., increased NA and/or decreased PA).
Study 1
In Study 1, daughters of depressed mothers and of never-disordered mothers participated in a standard social stress task during which they reported their affective responses and provided cortisol samples. We operationalized affective responses as the change in both PA and NA from pretask baseline to immediately after the stressor. Elevated cortisol reactivity is related to social threat and uncertainty, and is one pathway through which psychosocial stressors influence physical and mental health (McEwen, 1998; Sapolsky, Romero, & Munck, 2000). We hypothesized that compared with daughters of never-depressed mothers, daughters of depressed mothers will exhibit greater cortisol reactivity. Further, cortisol reactivity will be greatest for the daughters of depressed mothers who exhibit the strongest affective responses to stress.
Methods
Participants
Participants were daughters who were recruited with their mothers to participate in a larger longitudinal study of emotion dysregulation in girls at risk for depression. Mother/daughter pairs (N = 151) were recruited through advertisements posted in local newspapers, magazines, and local classifieds Websites (e.g., Craigslist). Consent and assent forms were obtained from the mothers and daughters, respectively, and all procedures were in accordance with the institutional review board.
To be eligible, the daughters had to be 9 to 14 years of age with no current or past Axis I disorder. Further, the girls’ biological mothers had to meet one of two diagnostic criteria: recurrent episodes of MDD during the daughters’ lifetime (high risk [RISK]; n = 72) or no current or past Axis I disorder (low risk [CTL]; n = 104). Diagnostic status of the daughters and mothers was assessed using the Schedule for Affective Disorders for School-Age Children—Present and Lifetime version (Kaufman, et al., 1997) and the Structured Clinical Interview for DSM-IV (First, Gibbon, Spitzer, & Williams, 1995), respectively. To assess interrater reliability, an independent rater who was blind to group membership evaluated 30% of our Structured Clinical Interview for DSM-IV and K-SAD-Schedule for Affective Disorders for School-Age Children—Present and Lifetime version interviews by randomly selecting 60 audiotapes of equal numbers of high-risk and control pairs. In all cases these diagnoses matched the original interviewer. Additional details of the larger study including recruitment and assessment of psychopathology as well as demographic characteristics of the mothers and daughters are described elsewhere (e.g., Joormann et al., 2007). In the present study, all participants completed the social stressor described below (cortisol data for a subset of these participants have been presented elsewhere: Gotlib, Joormann, Minor, & Hallmayer, 2008). Because data concerning affective responses to the stressor were collected only for 81 participants (RISK = 30, CTL = 51; mean age = 11.8 years, SD = 1.6), our analyses include only these participants.1
Self-report measures
Body mass index (BMI) and pubertal status
Participants provided their height and weight (from which BMI was calculated) and indicated when they had their first menstrual period.
Depressive symptomatology
Participants completed the 10-item version of the Children’s Depression Inventory (CDI-S; Kovacs, 1985), a self-report measure of depressive symptomatology in children aged 8 to 17. The reliability of this measure was α = 0.63.
Anxiety sensitivity
Participants completed the 16-item Anxiety Sensitivity Index for Children (Laurent, Schmidt, Catanzaro, Joiner, & Kelley, 1998), a measure of sensitivity to anxiety symptoms and physiological arousal. The reliability of this measure was α = 0.78.
PA and NA
Before, during, and after the stressor, participants reported how they felt (from 1 = not at all to 7 = very) using four emotion terms: happy, stressed, excited, and upset. Happy and excited were averaged to create a PA index (rs = .35, .71, .66, for before, during, and after the stressor, respectively; all ps > .002), and stressed and upset were averaged to create a NA index (rs = .39, .42, .50, for before, during, and after the stressor, respectively; all ps > .001).
Procedure
Participants first underwent a pretask baseline period during which they were instructed to relax for 30 min by reading magazines or listening to music. At the end of this 30-min period, saliva samples and baseline emotion ratings were collected. Next, participants underwent a 15-min laboratory stress induction that consisted of two tasks. In the first task, participants completed serial subtraction for three minutes, beginning with the number 400 and counting backward by sevens as quickly and accurately as possible. If they made a mistake, they were told by the experimenter to start over. If participants completed this subtraction task before the 3 min were over, they started over at 4,000 and counted backward by seventeens. Following this task, participants were administered the 12-min Ewart Social Competence Interview (Ewart, Jorgensen, Suchday, Chen, & Matthews, 2002), in which participants discussed details of stressful life situations with the experimenter. This task has been validated as a stressor that induces significant physiological activation (Ewart, Jorgensen, & Kolodner, 1998), and has already been published as a successful stressor in the current sample (Gotlib et al., 2008). Immediately following this stress task, participants provided another saliva sample and completed a second set of emotion ratings. They then watched a neutral video about Denali National Park for 30 min, during which their third (30 min postonset of stressor) and fourth (45 min postonset of stressor) saliva samples were collected. Participants then provided their third and final set of emotion ratings.
Cortisol collection
The four saliva samples (pretask baseline, 15, 30, and 45 min postonset of stressor) were collected using salivettes (Sarstedt, Numbrecht, Germany). A minimum of 0.2 ml of saliva was absorbed into a cotton roll and expressed through a plastic tube into a sterile vial. Cortisol was assayed by luminescence immunoassay reagents using a commercial kit from Immuno-Biological Laboratories (Hamburg, Germany). The sensitivity of the assay was set at 0.015 mg/day; (or 0.414 nmol/l).
Analyses
Outliers for the cortisol samples were winsorized by setting any value over 2 SD above the mean (~7% of responses) to the 2 SD level (Tukey, 1977). All repeated measures analyses of variance (ANOVAs) were Greenhouse–Geisser corrected and these corrected degrees of freedom are reported, as is the test of sphericity (1).
Results
Demographic and clinical variables
There were no significant differences between RISK and CTL girls in age, t (78) = 0.25, p = .81, BMI, t (55) = 0.15, p = .89, CDI-S scores, t (75) = 0.11, p =.11, Anxiety Sensitivity Index for Children scores, t (71) = 0.22, p = .83 or postmenarchal status, χ2 (1, 73) = 2.39, p = .12 (Table 1).2 These data indicate that any differences between the RISK and CTL participants in affective or cortisol responses are not due to differences in demographic variables, pubertal status, severity of depressive symptoms, or anxiety sensitivity.
Table 1.
Study 1 means and standard deviations for clinical and demographic variables
| CTL (n = 51) |
RISK (n = 30) |
|||
|---|---|---|---|---|
| Measure | M | SE | M | SE |
| Age (years) | 11.80 | 0.23 | 11.90 | 0.30 |
| BMIa | 19.03 | 0.40 | 18.92 | 0.75 |
| Postmenarche (%)b | 33 | — | 52 | — |
| Depression (CDI) | 1.23 | 0.21 | 1.61 | 0.52 |
| Anxiety sensitivity (ASIC)b | 0.52 | 0.05 | 0.52 | 0.10 |
| Age (mother)c | 44.36 | 0.73 | 42.67 | 1.32 |
| Marital status (% married)d | 86 | — | 65 | — |
| Household income | ||||
| (% > $100K)e | 75 | — | 46 | — |
Note: BMI, body mass index; CDI, Children’s Depression Inventory; ASIC, anxiety sensitivity in children.
n = 54 (CTL = 39, RISK = 15).
n = 61 (CTL = 46, RISK = 15).
n = 62 (CTL = 44, RISK = 18).
n = 61 (CTL = 44, RISK = 17).
n = 49 (CTL = 36, RISK = 13).
Affective responses
To examine affective responses to the stressor, we conducted separate 2 (Risk Status: RISK, CTL) ×3 (Period: Baseline, Stress, Recovery) mixed analyses of variance (ANOVAs) on PA and NA. For PA, there was only a significant main effect of period, F (2[1.95], 158[153.64]) = 14.87, p > .001, ε = 0.97, which reflected a decrease in PA from baseline (M = 4.76, SE = 0.12, range = 2–7) to stress (M = 4.28, SE = 0.15, range = 1.5–7), t (80) = 5.39, p > .001, d = 0.88, and a subsequent increase in PA from stress to recovery (M = 4.70, SE = 0.15, range = 1.5–7), t (80) = 4.34, p > .001, d = 0.68. There was also a main effect of risk status, F (1, 79) = 10.13, p = .002, d = 0.74. RISK participants reported overall less PA (M = 4.08, SE = 0.18) than CTL participants (M = 4.87, SE = 0.16). The interaction of risk status and period, however, was not significant, F (2[1.95], 158[153.64]) = 0.44, p = .64. For NA, there was also a significant main effect of period, F (2[1.69], 158[133.46]) = 37.12, p > .001, ε = 0.85, which reflected an increase in NA from baseline (M = 1.77, SE = 0.09, range = 1–4) to stress (M = 2.27, SE = 0.13, range = 1–6), t (80) = 4.33, p > .001, d = 0.73, and a subsequent decrease in NA from stress to recovery (M = 1.40, SE = 0.08, range = 1–4), t (80) = 7.61, p > .001, d = 1.32. The main effect of risk status was also significant, F (1, 79) = 4.40, p = .039, d = 0.49: the RISK participants exhibited greater overall NA (M = 2.03, SE = 0.15) than did the CTL participants (M = 1.68, SE = 0.09). The interaction of risk status and period was not significant, F (2[1.61], 158[133.46]) = 1.61, p = .20, ε = 0.85. Thus, although RISK participants exhibited lower overall PA and higher overall NA than did CTL participants, they did not exhibit differential affective responses to the stressor. Taken together, these results support the use of this task as a stressor that induces the predicted changes in affect and show that RISK and CTL girls exhibit similar affective responses to social stressors.
Cortisol responses
Next, we examined cortisol responses. To calculate affective responses to the stressor, we regressed stress PA and NA on baseline PA and NA, respectively, and used the residuals as indices of change in affect. PA and NA change were negatively correlated with each other (r = −.44, p > .001). Next, we calculated the area under the curve interval (AUCi; Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003), an index of cortisol change from baseline to the other three cortisol measurement periods (15, 30, and 45 min postonset of stresser). An ANOVA with AUCi as the dependent variable and risk status as the categorical factor and PA and NA change as the continuous factors allowed to interact with risk status yielded the predicted interaction of risk status and PA change, F (1, 75) = 5.88, p = .018.3 Confirming our hypothesis, this interaction was due to a strong inverse correlation between PA change and cortisol responses to the stressor for the RISK group (r = −.43, p = .018), but not for the CTL group (r = .11, p = .43). There were no main effects of risk status, PA, NA, or interactions between risk status and NA on cortisol responses to the stressor.
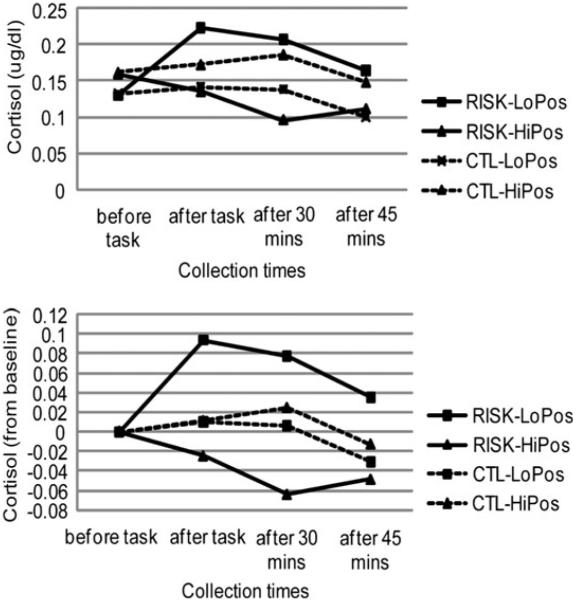
To examine which cortisol collection periods produced this interaction between risk status and change in PA, we calculated change scores from pretask baseline for the other three cortisol periods (15, 30, and 45 min postonset of stressor). There were strong inverse correlations between change in PA and changes in cortisol for the RISK group at 15 min (r = −.39, p = .035), 30 min (r = −.46, p = .011), and a trend at 45 min (r = −.34, p = .068) poststressor onset (Figure 1). There were no significant correlations between changes in PA and changes in cortisol for the CTL group (rs < .12, ps > .05). This pattern of results indicates that decreases in PA are correlated with both heightened and sustained cortisol reactivity in daughters of depressed mothers, but not in daughters of never-depressed mothers.
Figure 1.
Study 1. Cortisol reactivity in girls at high (RISK) and low (CTL) risk for depression by virtue of the presence or absence of maternal depression, respectively. The line graphs show estimates of (a) raw cortisol levels and (b) cortisol increases from baseline estimated at high (+1 SD) and low (−1 SD) positive affect reactivity for each of the groups. PA, positive affect; AUCi, area under the curve interval (i.e., from baseline).
We further characterized the positive affective responses of the daughters of depressed mothers who exhibited elevated cortisol reactivity and those who did not. We conducted simple slopes analyses on the high-risk daughters’ cortisol AUCi, and found that at high levels of cortisol AUCi (+1 SD above mean), RISK participants reported a significant decrease in PA (β = −1.74, p = .001), whereas at low levels of AUCi (−1 SD below mean), there was no significant change in PA (β = 0.68, p = .20). This pattern suggests that the daughters of depressed mothers who were able to maintain PA throughout the stressor exhibited lower cortisol reactivity. We also examined whether this lower cortisol reactivity was significantly different from that observed in CTL participants, as the pattern of results in Figure 1b seems to suggest. This was not the case: simple slopes analyses on the daughters’ cortisol AUCi revealed that only at low levels of PA (−1 SD below mean) did RISK participants exhibit significantly different cortisol responses than did CTL participants (β = 0.175, p = .024). At high levels of PA (+1 SD above mean), there was no significant difference between RISK and CTL participants in their cortisol responses (β = −0.129, p = .124).
Discussion
The data from Study 1 extend the literature examining diurnal levels of cortisol by demonstrating that cortisol reactivity is another index of HPA axis functioning that is anomalous in children at elevated familial risk for depression. Unlike dysregulation of basal cortisol levels, which has been found to characterize familial risk for depression (Ashman et al., 2002), child depression (Lopez-Duran et al., 2009), and adult depression (Parker, Schatzberg, & Lyons, 2003), elevated cortisol reactivity relatively early after the onset of a stressor seems to characterize only familial risk for depression in children (in conjunction with lowered PA; present study) and, to a lesser degree, child depression (Rao et al., 2008). This evidence raises the possibility that elevated cortisol reactivity is a cumulative risk mechanism for depression. In daughters of depressed mothers, it is possible that increased cortisol reactivity in stressful situations that elicit increases in NA, coupled with increased frequency of these stressful situations (Goodman & Gotlib, 1999), will alter basal HPA axis functioning over time, leading to hypercortisolemia and, perhaps, depression. This formulation is consistent with the notion of allostatic load, in which maladaptive physiological processes accumulate to produce deleterious effects on physical and mental health (Seeman et al., 2001). Future investigations examining changes in HPA-axis functioning and onset of depression in children of depressed mothers are needed to test this formulation more explicitly.
Elevated cortisol reactivity only characterized those girls at elevated familial risk for depression who exhibited the greatest changes in stress-induced affect. As hypothesized from previous work on resilience (Tugade & Fredrickson, 2004), greater decreases in PA during the stressor corresponded to greater increases in cortisol reactivity for the daughters of depressed mothers. Those daughters of depressed mothers who exhibited lower cortisol reactivity were characterized by the ability to maintain their PA during the stressor, a finding also consistent with research on resilience (e.g., Folkman & Moskowitz, 2000; Tugade & Fredrickson, 2004). Notably, and contrary to our predictions stemming from research on negative cognitive biases, there was no relation between NA responses to the stressor and cortisol reactivity. A simple explanation for this finding may be that the majority of cognitive vulnerability studies either do not measure decreases in PA or combine low PA and high NA into a single measure of “mood” (e.g., Miranda, Persons, & Byers, 1990). An alternative explanation, however, may be that cognitive biases and cortisol reactivity are risk mechanisms that are activated by different emotional responses to stressful situations. For example, one study that did separate low PA and high NA found that the cognitive biases in remitted depressives were apparent only in the presence of high NA and not low PA (Roberts & Kassel, 1996). On the other hand, meta-analyses have shown that cortisol reactivity is more associated with decreases in PA than increases in NA (Chida & Hamer, 2008). Future investigations that examine both cognitive biases and cortisol reactivity will be integral to exploring this formulation.
Study 2
The aims of Study 2 were to replicate and extend the results of Study 1 by assessing altered cardiovascular responses (HR) to stress in daughters of depressed mothers. We focused on cardiovascular recovery as our primary variable of interest. Cardiovascular recovery is a critical determinant of both mental (Salomon et al., 2009; Tugade & Fredrickson, 2004) and physical (Lauer & Froelicher, 2002) health. Particularly relevant to the current study, depressed adults show slower cardiovascular recovery in stressful situations (Salomon et al., 2009), highlighting poor cardiovascular recovery as a potential risk mechanism for depression present in daughters of depressed mothers.
In addition to recovery, we also assessed habituation to repeated stress by having participants undergo three successive mental arithmetic sessions. Habituation is a process by which responses to repeated stimuli decrease with each subsequent presentation of the stimulus (Thompson & Spencer, 1966). Successful habituation to stressful situations is a marker of positive mental health (Dienstbier, 1989), and is a critical process in improving mental health (Foa & Kozak, 1986). Relevant to the current study, previous work has shown that offspring of anxious parents are less likely to habituate to repeated fear-relevant stimuli than are offspring of nonanxious parents (Turner, Beidel, & Roberson-Nay, 2005). The current study examines whether daughters of depressed mothers also exhibit altered cardiovascular habituation to repeated stress.
Affective responses were operationalized as in Study 1, except for two changes. First, to mirror our interest in cardiovascular recovery, we assessed affect during the recovery period. Measuring affect during the recovery period and not immediately after the stressor also allowed us to avoid potentially intrusive effects of rating one’s emotion on physiology (Lieberman et al., 2007; Taylor, Phan, Decker, & Liberzon, 2003). This is especially important given that cardiovascular recovery from stress tends to be fast—on the order of minutes (e.g., Fredrickson, Mancuso, Branigan, & Tugade, 2000). Second, to mirror our interest in cardiovascular habituation, we measured this affective recovery after each of the three stress tasks. Our affective responses of primary interest, therefore, are the relative decreases in PA and increases in NA during recovery periods across repeated stressors. We hypothesized that compared with daughters of never-depressed mothers, daughters of depressed mothers will exhibit poorer (slower or less complete) cardiovascular recovery and/or habituation. Further, poor cardiovascular recovery and/or habituation will be most apparent for the daughters of depressed mothers who exhibit the strongest affective responses to stress.
Methods
Participants
Mother–daughter dyads from the larger study were recontacted and invited to participate in the present psychophysiology study, resulting in 53 participants (18 RISK, 35 CTL; M age = 13.67 years, SD = 1.96 years).4 The length of time since initial assessment ranged from 2 weeks to 29 months. Daughters for whom more than 1 month had passed since their last assessment, and their mothers, were reassessed with the Affective Disorders for School-Age Children—Present and Lifetime version with reference to the daughter at the end of the psychophysiology session to ensure that none of the daughters in this sample met criteria for any current or past Axis I disorder.
Self-report measures
Depressive symptomatology
As in Study 1, participants completed the CDI-S (Kovacs, 1985). Reliability of this measure was α = 0.63.
Anxiety symptomatology
Participants completed the 41-item version of the Screen for Child Anxiety Related Emotional Disorders (Birmaher et al., 1997), a self-report measure of anxiety symptoms for children and adolescents ages 8 and older. Reliability of this measure was α = 0.90.
PA and NA
At various times in the procedure, participants were asked to rate how they felt “right now” on a scale from 1 (not at all) to 7 (very) for each of four different emotion words comprising a PA subscale (happy, excited; rs = .65, .59, .72, .72, for pretask and each measurement posttask, respectively; all ps > .001) and a NA subscale (stressed, upset; rs = .52, .38, .42, .61, for pretask and each measurement posttask, respectively; all ps > .006).
Physiological measures
Acquisition
Physiological activity was recorded at a sampling rate of 1 kHz with an integrated system and software package (Biopac MP150, AcqKnowledge; Biopac Systems, Goleta, CA). Cardiovascular activity was recorded with the electrocardiogram (ECG) amplifier module and disposable snap ECG electrodes using a modified lead II configuration.
Signal processing
Physiological data were scored in 60-s intervals using the Mindware software package (HRV 2.51; Mindware, Westerville, OH). We inspected the cardiovascular data for artifacts and missing R-peaks (based on improbable interbeat intervals). For each minute, if one R-peak was missing, an R-peak was inserted at a time point halfway in between the two neighboring R-peaks. If more than one R-peak was missing, that minute was not scored (Berntson et al., 1997). After correcting for artifacts and missing R-peaks, we calculated HR in beats per minute (bpm).
Procedure
Pretask
Upon arrival, participants sat in a comfortable armchair and the experimenter described the task to them in general terms to minimize possible anticipatory effects during the pretask baseline physiology recording. The experimenter then attached the ECG sensors as well as skin conductance sensors and a respiration band (data not presented here). After a 10-min acclimation period during which participants completed the self-report measures including baseline PA/NA (first assessment), we recorded baseline ECG for 5 min.
Mental arithmetic stressors
Following pretask baseline, participants completed three versions of the serial subtraction task presented in Study 1 using different starting numbers and subtraction values (4,672 by 17s, 3,412 by 13s, 5,870 by 19s; counterbalanced). After each 3-minute subtraction task period, the experimenter left the room, and participants sat quietly for a 5-min recovery period. After each recovery period, participants reported their current PA and NA (second through fourth assessments). These self-reports were completed after recovery instead of immediately after the stress tasks so that our measure of affective recovery mirrored our interest in cardiovascular recovery. In addition, having participants report their affect at the end of each recovery period avoided confounding the HR data with physical and mental processes associated with reporting on one’s emotions (Lieberman et al., 2007; Taylor et al., 2003).5
Postsession
All sensors were removed and the participants were debriefed.
Statistical analysis strategy
Following previous research (Kristjansson, Kircher, & Webb, 2007; Waugh, Panage, Mendes, & Gotlib, 2010), we used hierarchical linear modeling (HLM6; Raudenbush, Bryk, & Congdon, 2008) to analyze our HR data. We specified a two-level HLM model. Level 1 of the model consisted of data points for each of the 29 min within the experimental session. Level 2 of the model consisted of participant-level predictors of Level 1 slopes and intercepts.
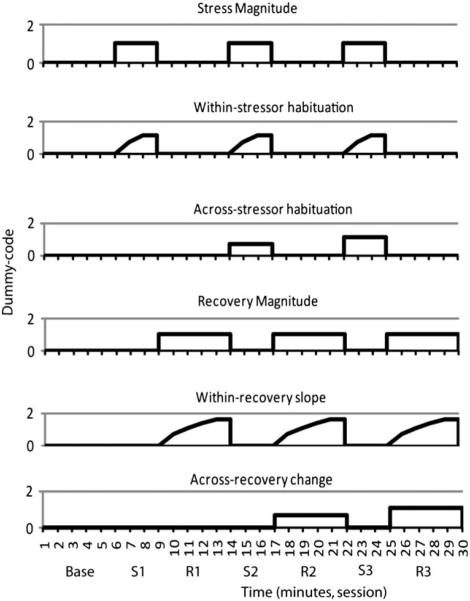
We took the following steps to build each of the HLM models. First, at Level 1 we fit a series of dummy-coded variables that corresponded to six possible patterns in the data (Figure 2). Stress and recovery magnitude change assessed overall changes in HR during the first minutes of the stress period and of the recovery period, respectively. Within-stressor habituation and within-recovery slope assessed changes in HR within the stress and recovery periods, respectively. Finally, across-stressor habituation and recovery magnitude change assessed changes in HR across the three stress or recovery periods, respectively. To do this, we used a piecewise regression approach (Llabre, Spitzer, Saab, & Schneiderman, 2001), in which we simultaneously fit these dummy-coded variables to the data as multiple regression lines within one continuous time series. The stress and recovery magnitude change predictors were dummy coded as 1s during the respective task period and 0s everywhere else. These predictors represent change in physiology during the first minute of the task periods relative to baseline. Habituation is theorized to follow a negative exponential curve (Thompson & Spencer, 1966); consequently, the within- and across-stressor habituation predictors were dummy coded as the natural log of each of the 3 min within each stress task or the three task periods, respectively. Within-recovery slope and across-recovery change were similarly dummy coded with exponential predictors for the recovery periods. Each of the predictors significantly reduced overall deviance in the model, thus justifying our inclusion of all of the Level 1 predictors in the final model (Table 2).
Figure 2.
Study 2. Pictoral representation of the dummy codes used in the hierarchical linear model to estimate possible patterns in the heart rate (HR) response to repeated stressors. S1, S2, S3, stress periods 1, 2, and 3, respectively; R1, R2, R3, recovery periods 1, 2, and 3, respectively.
Table 2.
Model fit and remaining variance for Level 1 hierarchical linear modeling model
| Model Fit |
Remaining Variance (Final Level 1 Model) |
||||
|---|---|---|---|---|---|
| Level 1 Parameters | Deviance | Parameters | χ2 (df) | Variance | χ2 (49) |
| Baselinea | 9457.15 | 2 | — | 124.38 | 4158.00** |
| Stress magnitudea | 8314.06 | 4 | 1143.1 (2)** | 74.80 | 758.23** |
| Recovery magnitudeb | 8232.10 | 7 | 82.00 (3)** | 19.00 | 269.36** |
| Between-stressors habituationb | 8081.08 | 11 | 151.01 (4)** | 12.89 | 206.61** |
| Within-recovery slopeb | 8043.15 | 16 | 37.93 (5)** | 4.23 | 183.87** |
| Within-stressors habituation | 7834.60 | 22 | 208.55 (6)** | 19.05 | 281.94** |
| Across-recovery change | 7779.53 | 29 | 55.06 (7)** | 4.91 | 148.59** |
Note: Level 1 parameters were entered sequentially to test the additional fit provided to the model. The remaining variance was calculated after all Level 1 parameters were entered.
p < .01.
There was significant remaining variance for each of the Level 1 predictors (Table 2), so we next added predictors at Level 2 of the model. First, we added risk status at Level 2 of the model predicting each of the Level 1 intercepts and slopes. We dummy coded risk status as 1 (CTL) and 2 (RISK) and then standardized this variable so that the intercepts represented the weighted mean of all participants. To assess the relation between affective and cardiovascular habituation, at Level 2 we added PA and NA change variables. Affective change was calculated as the fitted exponential curve across each participant’s affective reports (pretask baseline affect and the three poststressor affective responses). To control for overall magnitude of affect in the HLM model, we regressed out the intercept of the exponential curves and used the residuals as the affective change scores. The positive and negative change scores were not correlated (r = .08, p = .59). To examine whether the relation between affective change and cardiovascular habituation was moderated by risk status, we multiplied the standardized risk status variable with each affective change variable and added these interaction terms to Level 2. Finally, we included age (also standardized) as a covariate of noninterest, but to conserve degrees of freedom, age was excluded when it did not significantly predict the Level 1 variable.
Level 2 predictors were treated as random effects; that is, error terms were estimated at each Level 2 equation to allow for randomly varying slopes (Bryk & Raudenbush, 1992). We used restricted maximum likelihood to estimate the coefficients. Because of equipment malfunctioning, 3 participants (all CTL) were excluded from data analysis, leaving 18 RISK and 32 CTL participants. This is the resulting model:
Level 1:
Level 2:
The subscript i corresponds to each parameter at Level 1; Rec refers to the recovery periods; ΔPA and ΔNA refer to changes across stressors in PA and NA, respectively; Mag, Hab, and Slp refer to magnitude change, habituation, and slope, respectively; and Age is in parentheses because it is only included as a covariate for Level 1 variables for which it is a significant predictor.
Results
Anxiety and depression
There were no significant differences between RISK and CTL daughters in age or in self-reported anxiety or depressive symptoms, ts (48) = 1.05, 0.17, 1.1, respectively, all ps > .05 (Table 3). As in Study 1, these data indicate that any differences between these groups in affective change or cardiovascular habituation were not due to age or to levels of anxiety or depression.6
Table 3.
Study 2 means and standard errors for age, clinical variables, and self-reported affect
| CTL (n = 32) |
RISK (n = 18) |
|||
|---|---|---|---|---|
| Measure | M | SE | M | SE |
| Age (years) | 13.59 | 0.34 | 14.06 | 0.43 |
| Depression (CDI) | 1.13 | 0.31 | 1.67 | 0.38 |
| Anxiety (SCARED) | 16.16 | 1.94 | 15.67 | 1.91 |
| NA change | −0.24 | 0.13 | −0.20 | 0.12 |
| PA change | −0.29 | 0.10 | −0.31 | 0.14 |
| NA magnitude | 2.22 | 0.23 | 2.28 | 0.24 |
| PA magnitude | 4.36 | 0.22 | 4.24 | 0.32 |
| Age (mother) | 44.62 | 0.68 | 42.44 | 1.18 |
| Marital status (% married)a | 85 | — | 59 | — |
| Household income | ||||
| (% > $100K)b | 69 | — | 47 | — |
Note: CDI, Children’s Depression Inventory; SCARED, Screen for Child Anxiety Related Emotional Disorders; NA, negative affect; PA, positive affect. There were no significant differences on any of these measures between girls at low risk (CTL) and high risk (RISK) for depression.
n = 51 (CTL = 34, RISK = 17).
n = 41 (CTL = 26, RISK = 15).
Affective responses.7
Affective change
We examined whether the CTL and RISK daughters differed in positive or negative affective change. Overall, there was significant change in both PA (M = −0.30, SE = 0.08), t (49) = 3.65, p = .001, d = 0.52, and NA (M = 20.22, SE = 0.09), t (49) = 2.43, p = .019, d = 0.34. There were no significant differences between the CTL and RISK daughters, however, in either positive or negative affective change, ts (48) = 0.09, −0.21, ps > .05, respectively (Table 3).
Affective magnitude
Similar to affective change, there were no significant differences between CTL and RISK daughters in the magnitude of their PA and NA across the session, ts (48) = 0.33, 20.17, ps > .05, respectively (Table 3).
Cardiovascular responses
Stress reactivity
On average, participants’ HR increased by 11.3 bpm during the first minute of the first mental arithmetic stressor (Table 4). There was a significant within-stressor habituation of 3.32 bpm per log(min) and between-stressor habituation of 3.07 bpm per log(stressor period). There were main effects of NA change on initial stress magnitude and within-stressor habituation such that NA change was positively associated with a larger HR increase to the initial stressor (γ13 = 3.29) coupled with a steeper within-stressor habituation slope (γ23 = −1.13). There were no main effects of risk status. There was, however, an interaction of risk status and NA change in predicting between-stressor HR habituation. Simple-slopes analyses reveal that this interaction was due to a significant correlation between NA change and between-stressor HR habituation in the RISK group, B = 3.28, t (44) = 2.50, p = .016, but not in the CTL group, B = −0.72, t (44) = −1.01, p = .318.
Table 4.
Study 2 hierarchical linear modeling of heart rate in repeated mental arithmetic task
| Coefficient | SE | t | p | |
|---|---|---|---|---|
| Baselinea | ||||
| Intercept, γ00 | 76.93 | 1.36 | 56.58 | <.001 |
| Risk status, γ01 | −0.87 | 1.39 | −0.63 | .533 |
| PA change, γ01 | 1.62 | 1.41 | 1.15 | .256 |
| NA change, γ03 | −1.57 | 1.46 | −1.08 | .288 |
| PA Change × Risk Status, γ04 | −0.41 | 1.41 | −0.29 | .771 |
| NA Change × Risk Status, γ05 | −2.14 | 1.63 | −1.31 | .197 |
| Age, γ06 | −2.45 | 0.74 | −3.31 | .002 |
| Stress magnitudea | ||||
| Intercept, γ10 | 11.31 | 1.04 | 10.86 | <.001 |
| Risk status, γ11 | −1.02 | 1.06 | −0.96 | .343 |
| PA change, γ12 | −0.42 | 1.08 | −0.39 | .700 |
| NA change, γ13 | 3.29 | 1.12 | 2.95 | .006 |
| PA Change × Risk Status, γ14 | −1.43 | 1.07 | −1.33 | .191 |
| NA Change × Risk Status, γ15 | 0.98 | 1.25 | 0.79 | .436 |
| Age, γ16 | 1.98 | 0.53 | 3.72 | .001 |
| Within-stressor habituationb | ||||
| Intercept, γ20 | −3.32 | 0.52 | −6.44 | <.001 |
| Risk status, γ21 | 0.48 | 0.53 | 0.90 | .371 |
| PA change, γ22 | −0.16 | 0.53 | −0.30 | .768 |
| NA change, γ23 | −1.13 | 0.55 | −2.03 | .048 |
| PA Change × Risk Status, γ24 | 0.20 | 0.53 | 0.38 | .706 |
| NA Change × Risk Status, γ25 | 0.29 | 0.62 | 0.47 | .642 |
| Age, γ26 | −0.74 | 0.28 | −2.69 | .011 |
| Between-stressors habituationb | ||||
| Intercept, γ30 | −3.07 | 0.61 | −5.03 | <.001 |
| Risk status, γ31 | 0.73 | 0.62 | 1.18 | .244 |
| PA change, γ32 | 0.27 | 0.62 | 0.43 | .669 |
| NA change, γ33 | 0.72 | 0.65 | 1.11 | .276 |
| PA Change × Risk Status, γ34 | −0.78 | 0.62 | −1.24 | .220 |
| NA Change × Risk Status, γ35 | 1.94 | 0.72 | 2.68 | .011 |
| Recovery magnitudeb | ||||
| Intercept, γ40 | 0.12 | 0.62 | 0.20 | .846 |
| Risk status, γ41 | 0.34 | 0.63 | 0.55 | .586 |
| PA change, γ42 | −0.60 | 0.63 | −0.94 | .351 |
| NA change, γ43 | 0.97 | 0.66 | 1.46 | .151 |
| PA Change × Risk Status, γ44 | −1.26 | 0.63 | −1.99 | .053 |
| NA Change × Risk Status, γ45 | 1.21 | 0.73 | 1.64 | .107 |
| Within-recovery slopeb | ||||
| Intercept, γ50 | 0.11 | 0.31 | 0.36 | .717 |
| Risk status, γ51 | −0.27 | 0.31 | −0.87 | .390 |
| PA change, γ52 | 0.11 | 0.31 | 0.34 | .738 |
| NA change, γ53 | −0.43 | 0.33 | −1.31 | .196 |
| PA Change × Risk Status, γ54 | 0.73 | 0.31 | 2.34 | .024 |
| NA Change × Risk Status, γ55 | −0.55 | 0.36 | −1.50 | .140 |
| Between-recoveries changeb | ||||
| Intercept, γ60 | −0.17 | 0.32 | −0.53 | .597 |
| Risk status, γ61 | 0.18 | 0.32 | 0.58 | .567 |
| PA change, γ62 | 1.03 | 0.32 | 3.21 | .003 |
| NA change, γ63 | 1.13 | 0.34 | 3.34 | .002 |
| PA Change × Risk Status, γ64 | 0.13 | 0.32 | 0.40 | .693 |
| NA Change × Risk Status, γ65 | 0.10 | 0.37 | 0.28 | .780 |
Note: PA, positive affect; NA, negative affect.
df=43.
df=44.
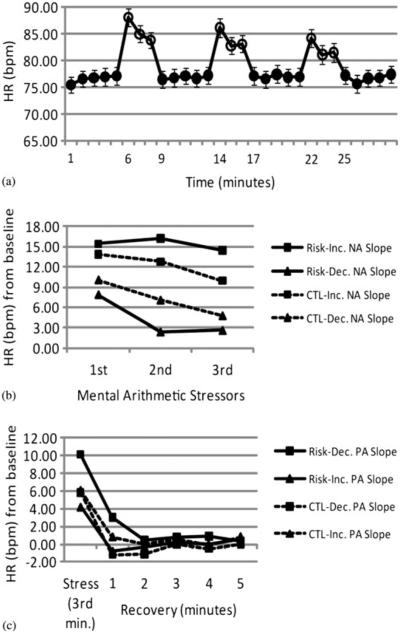
We explored this interaction further by using simple slope analyses in which we evaluated between-stressor HR habituation at the upper (+1 SD) and lower (−1 SD) levels of NA change, which correspond to positive NA slopes (increases in NA; ω = 0.43), t (49) = 4.64, p > .001, and negative NA slopes (decreases in NA, ω = −0.87), t (49) = −9.05, p > .001, over the course of the experiment, respectively. Only when evaluated at upper levels of NA change (increases in NA) did the RISK group fail to show significant HR habituation between the stressors, B = 1.18, t (44) = 0.74, p = .463 (Figure 3). At lower levels of NA change (decreases in NA) the RISK group exhibited significant HR habituation between stressors, B = −5.38, t (44) = −3.12, p = .004, as did the CTL group evaluated at both the upper and lower levels of NA change, Bs = −4.32, −2.89, ts (44) = −4.08, −2.83, and ps = .001, .007, respectively. Taken together, these results support the hypothesis that daughters of depressed mothers will exhibit poor cardiovascular habituation coupled with affective responses to the stressor—in this case, maintaining NA.
Figure 3.
Study 2. (a) Heart rate (HR) responses to the repeated stressor task. (b, c) HR habituation across (b) repeated stressors and (c) HR recovery averaged for all the stressors in girls at high (RISK) and low (CTL) risk for depression by virtue of the presence or absence of maternal depression, respectively. The line graphs show estimates of HR estimated at decreasing (dec; +1 SD) and increasing (inc; −1 SD) negative affect (NA) and positive affect (PA) change slopes across repeated stressors for each of the groups.
Stress recovery
On average, participants’ HR recovered to pretask baseline levels during the first minute after each stressor (Table 4). There were main effects of PA and NA change on between-recovery change such that decreases in both PA and NA were associated with decreases in initial HR during recovery after successive stressors. There was no main effect of risk status; there was, however, an interaction of risk status and PA change on within-recovery slope. Simple-slopes analyses reveal that this interaction was due to a significant correlation between PA change and within-recovery HR slope for the RISK group, B = 1.07, t (44) = 2.10, p = .042, but not for the CTL group, B = −0.44, t (44) = −1.11, p = .273. For the RISK group, larger decreases in PA over the course of the experiment were associated with a steeper within-recovery slope, which in turn seemed to be at least partially due to higher HR during the first minute of recovery, B = −2.26, t (44) = −2.18, p = .034 (Figure 3). We explored this interaction further by using simple slope analyses in which we evaluated HR recovery at the upper (+1 SD) and lower (−1 SD) levels of PA change, which correspond to positive PA slopes (increases in PA; ω = 0.28), t (49) = 3.42, p = .001, and negative PA slopes (decreases in PA, ω = −0.88), t (49) = −10.72, p > .001, over the course of the experiment, respectively. Only the RISK group at the lower levels of PA change (decreases in PA) showed a trend toward incomplete HR recovery during the first minute of recovery, B = 2.83, t (44) = 1.96, p = .056. The RISK group at the upper levels of PA change (increases in PA) and CTL participants at both the upper and lower levels of PA change exhibited complete HR recovery during the first minute of recovery (ts < −1.14, ps > .10). Taken together, these findings support the hypothesis that daughters of depressed mothers will exhibit poor cardiovascular recovery coupled with affective responses to the stressor, in this case, lowered PA.
Mental arithmetic performance
Finally, we examined whether the interactions between risk status and affective change on HR could be due to differential engagement with and/or performance on the mental arithmetic task. Engagement was operationalized as the number of arithmetic opera tions attempted within each 3-min task period and performance was operationalized as the percentage of errors per arithmetic operation attempted. Engagement and performance were each submitted to a 3 (Task Period: First, Second, Third) × 2 (Risk Status: CTL, RISK) mixed analysis of covariance with PA and NA change as continuous factors (allowed to interact with risk status) and age as a covariate of noninterest. For neither variable were there main effects of risk status or interactions between risk status and affective change, suggesting that the previously obtained risk status effects on HR habituation and recovery were not due to differential engagement with or performance on the mental arithmetic task.
Discussion
The results of Study 2 provide evidence that, in addition to altered HPA-axis functioning, daughters of depressed mothers also exhibit an increased coupling between their affective and cardiovascular responses to stress. Decreasing PA was correlated with poorer HR recovery in daughters of depressed mothers but not in daughters of never-depressed mothers. Impaired cardiovascular recovery is a robust predictor of cardiovascular disease and all-cause mortality (Lauer & Froelicher, 2002). Moreover, impaired cardiovascular recovery is also associated with adult depression (Salomon et al., 2009). The present data indicate that impaired cardiovascular recovery is not only a symptom of depression, but it may also be present in those at elevated risk for depression when they experience decreases in PA.
We obtained a significant correlation between maintaining NA and poor HR habituation in daughters of depressed mothers but not in daughters of never-depressed mothers. These results are consistent with reviews showing that heart rate and NA exhibit similar patterns of habituation in repeated stress situations (Foa & Kozak, 1986). Compared to cardiovascular recovery, less is known about whether poor cardiovascular habituation co-occurs with depression. Thus, the pathway through which poor cardiovascular habituation may be a risk mechanism that predicts the onset of depression is also unclear. Therefore, it will be important in future research to elucidate whether and how poor cardiovascular habituation predicts the onset of depression.
General Discussion
The data from these studies provide evidence that a subset of children at elevated familial risk for depression exhibit not only altered basal physiological functioning, but also altered physiological responding to stress. In two studies, never-depressed daughters of depressed mothers who experienced decreased PA in response to stressors exhibited heightened and sustained cortisol reactivity (Study 1) and sustained cardiovascular responses (Study 2). Moreover, daughters of depressed mothers who did not show decreases in NA across repeated stressors exhibited poor cardiovascular habituation (Study 2). These data suggest that, as with negative cognitive biases (Dearing & Gotlib, 2009; Joormann et al., 2007; Taylor & Ingram, 1999), altered physiological responses are coactivated with affective responses to stress in those at elevated risk for depression. Still unknown, however, is whether these physiological responses to stress underlie the elevated risk for depression in children of depressed mothers. It will be important for future studies to extend these findings by using longitudinal designs to assess the onset of depressive disorder as an outcome measure.
The daughters with elevated familial risk who were able to maintain PA during stress did not exhibit altered physiological responses. Notably, had we not taken into account self-reported affective responses, we would not have observed any group differences between daughters of depressed mothers and daughters of never-depressed mothers in their physiological responses to the stressors. Although it is possible that we would have found group differences if we had had a larger sample, or if we had permitted the inclusion of girls with past or current depression, this pattern of correlations suggests that these physiological stress responses are not present in all individuals at elevated risk for depression. Instead, daughters of depressed mothers likely represent a heterogeneous population. At the highest estimates, 40% of these daughters will develop some mental health disorder (Hammen, 2009). A majority of these daughters, therefore, will not succumb to psychopathology despite being raised in a stressful environment (Goodman & Gotlib, 1999). Daughters of depressed mothers who were better able to maintain high PA and low NA in the face of stressors may be more likely to belong to this resilient group (Folkman & Moskowitz, 2000; Fredrickson, Tugade, Waugh, & Larkin, 2003). Only by following these daughters over time will we be able to test this formulation.
One caveat to this interpretation of our results is that the significant correlations between affective and physiological responses were between subjects and not within subjects. Therefore, it is not clear whether the propensity to maintain high PA and/or low NA during stress is a reliable resilience-related trait that would be consistent across different types of stressors, as suggested above, or whether it is a specific response to the stressors used in this study. This latter interpretation would posit that these physiological responses are present in most, if not all, individuals at elevated risk for depression, but that the stressors used in the current study failed to induce robust affective responses and associated physiological responses in a uniform manner. The stressors used in this study elicited relatively mild affective and physiological responses in a majority of the girls. To test this formulation, future investigations should use more intense stressors and assess whether the strength of within-person covariation of affect and physiological responding (Mauss, Levenson, McCarter, Wilhelm, & Gross, 2005) varies as a function of familial risk for depression. Alternatively, future investigations could also test this formulation with an experimental design that compared an affectively charged physiological stressor with a nonaffectively charged physiological stressor. Such an experimental design could examine the direction of causality between affective and physiological responding, and how this causal relation might differ as a function of familial risk for depression.
We had hypothesized that the association between affect and physiology would be greater for daughters of depressed mothers than for daughters of never-disordered mothers. One intriguing finding of the present study, however, was that these responses were uncorrrelated in the daughters of never-disordered mothers. One possible explanation for this finding involves an assumption that all of the low-risk daughters are resilient. If this were the case, all of the low-risk girls might use positive emotions to regulate physiological responses to stress, but there may not have been enough within-group variation in their coupling of positive affective and physiological responses to be detected by between-subjects analyses. This possibility is supported in part by the finding in the second study that the low-risk group as a whole exhibited similar cardiovascular recovery to the daughters of depressed mothers who reported high PA. A study examining the coupling of these responses over time within subjects could address this possibility. An alternative possibility is that this coupling between PA and physiology is not present in the low-risk girls because it is a mechanism for coping with stressors that is forged through stress inoculation (Parker, Buckmaster, Schatzberg, & Lyons, 2004) and physiological toughening (Dienstbier, 1989). In this case, it may be that because daughters of never-disordered mothers typically experience lower levels of life stress than do daughters of depressed mothers (Goodman & Gotlib, 1999), they have not needed to develop such a coping mechanism. This stress inoculation formulation is partially supported by the pattern of findings from the first study in which the high-risk girls with high PA seem to exhibit relatively decreased cortisol responses during the stressor (although this effect was not statistically significant in the current study).
These results showed for the first time that maintaining NA across repeated stressors is associated with poor cardiovascular habituation in daughters of depressed mothers. These data are particularly important for two reasons. First, little is known about the psychosocial causes and consequences of poor cardiovascular habituation. The present findings illustrate that, in addition to cortisol reactivity and poor cardiovascular recovery, poor cardiovascular habituation is also associated with having a depressed mother. Second, there is not a reliable association between state NA and cardiovascular responses to stressors (Waugh et al., 2010), perhaps because studies in this area have typically measured affective and cardiovascular responses only to single stressors. Our findings indicate that examining changes in NA across repeated stressors reveal otherwise undiscovered associations with physiological responses to those stressors.
An important feature of our participant recruitment was that none of the girls in our study met diagnostic criteria for any current or past Axis I disorder. It is important that this absence of disorder in the girls allows us to postulate that the coupling of affect and physiology in the risk girls may be a risk mechanism for depression a consequence of current or past depression. It is possible, however, that using these exclusion criteria may have inadvertently yielded a sample that is more resilient than the sample population. It might prove interesting, then, for future studies to compare the affective and physiological responses in these high-risk daughters with girls who have already experienced an episode of major depression.
In the current studies, we replicated the relation between elevated risk for depression and affect across two relatively independent physiological systems. We should point out, however, that both studies drew on the same pool of participants; these results should be replicated in independent samples. We excluded girls who had experienced a current or past Axis I disorder; we did not, however, include or exclude girls from participation in this study on the basis of their pubertal status. Although we did not find that pubertal status influenced our cortisol findings, it is important that future studies examining the relation between stress physiology and affect in adolescence consider temporal and contextual aspects of puberty that may be related to the onset of depression (Conley & Rudolph, 2009). In the same vein, it is also important to include sons of depressed parents and to examine other populations at elevated risk for depression, such as abused children (Brown, Cohen, Johnson, & Smailes, 1999). Future studies should use samples larger than that assessed in the current study to examine more reliably both within- and between-subject correlations between affect and physiology. It is also important for future studies to examine these effects in real-life stressors and to unpack the mechanisms by which these physiological risk factors are transmitted from parents to their offspring. Despite these limitations, the current studies provide evidence for the coupling of affect and physiological responding in children of depressed mothers and highlight altered physiological responding as a possible mechanism by which risk for depression may be conferred by parents to their offspring.
Acknowledgments
This research was supported by a Young Investigator Award from the National Alliance for Research on Schizophrenia and Affective Disorders (NARSAD; to J.J.) and by a Distinguished Scientist Award from NARSAD and Grant MH74849 from the National Institute of Mental Health (to I.H.G.). The authors thank Kirsten Gilbert, Yamanda Wright, and Hannah Burley for their help in recruiting and running the participants in this study. The authors also thank Wendy Berry Mendes for her comments on earlier versions of this manuscript.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Although we collected cortisol from all of the participants in our larger sample, we only began to collect affective responses to the stressor partway through our recruitment. It is important, though, that individuals who participated in the present study did not differ from individuals from the larger sample who did not participate in this study with respect to the proportion of participants who were CTL versus RISK (χ2 = 0.93, p = .335) or cortisol responses to the stressor, t (152) = 1.17, p = .243. Nevertheless, it is possible that there are other unmeasured systematic biases due to this temporal difference in the samples that may limit the generalizability of these findings.
Although there were no significant demographic differences between the RISK and CTL daughters, there were trends in expected directions (i.e., RISK > CTL in CDI and rates of maternal divorce) that might have been reliable given a larger sample size. It is important, however, that including these variables as covariates in our analyses did not appreciably change any of the results.
There were no significant differences between RISK and CTL daughters in the time of day at which their cortisol was collected (M ~ 12:30 pm, both ts < 0.43, ps > .05), and adding this as a covariate did not alter the results.
All of these daughters participated in the cortisol stress session described in Study 1; only 32 of the daughters in Study 2, however (10 RISK, 22 CTL) were included in Study 1 because they had also provided affective responses to the stressor. Thus, of the 176 girls who participated in the parent project, 32 girls participated in both Study 1 and Study 2, 49 girls participated only in Study 1, 21 girls participated only in Study 2, and 74 girls did not participate in either Study 1 or Study 2.
Because recovery affect was assessed immediately prior to the start of the next stressor, it is possible that this recovery affect is confounded with anticipatory affect. Although we cannot disentangle this in our study, both of these types of affective responses may contribute to our conceptualization of these participants as either “maintaining” or not maintaining their affective responses over time.
Unlike in Study 1, we did not reassess menarche status, and so cannot determine whether including postmenarchal status in the analyses would change these results.
Although affective magnitude was regressed out of affective change variables in the HLM model, we present group statistics here for each of these variables separately.
References
- Abramson LY, Seligman MEP, Teasdale JD. Learned helpless in humans: Critique and reformulation. Journal of Abnormal Psychology. 1978;87:49–74. [PubMed] [Google Scholar]
- Ashman SB, Dawson G, Panagiotides H. Trajectories of maternal depression over 7 years: Relations with child psychophysiology and behavior and role of contextual risks. Development and Psychopathology. 2008;20:55–77. doi: 10.1017/S0954579408000035. [DOI] [PubMed] [Google Scholar]
- Ashman SB, Dawson G, Panagiotides H, Yamada E, Wilkinson CW. Stress hormone levels of children of depressed mothers. Development and Psychopathology. 2002;14:333–349. doi: 10.1017/s0954579402002080. [DOI] [PubMed] [Google Scholar]
- Barnett PA, Gotlib IH. Psychosocial functioning and depression: Distinguishing among antecedents, concomitants, and consequences. Psychological Bulletin. 1988;104:97–126. doi: 10.1037/0033-2909.104.1.97. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression: Causes and treatment. University of Pennsylvania Press; Philadelphia, PA: 1967. [Google Scholar]
- Beck AT. Cognitive therapy and the emotional disorders. New American Library; New York: 1976. [Google Scholar]
- Berntson GG, Bigger JT, Jr., Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, et al. The screen for child anxiety related emotional disorders (SCARED): Scale construction and psychometric characteristics. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Brown J, Cohen P, Johnson JG, Smailes EM. Childhood abuse and neglect: Specificity of effect on adolescent and young depression and suicidality. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38:1490–1496. doi: 10.1097/00004583-199912000-00009. [DOI] [PubMed] [Google Scholar]
- Bryk A, Raudenbush S. Hierarchical linear models: Applications and data analysis methods. Sage; Newbury Park, NJ: 1992. [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Chida Y, Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: A quantitative review of 30 years of investigations. Psychological Bulletin. 2008;134:829–885. doi: 10.1037/a0013342. [DOI] [PubMed] [Google Scholar]
- Conley CS, Rudolph KD. The emerging sex difference in adolescent depression: Interacting contributions of puberty and peer stress. Development and Psychopathology. 2009;21:593–620. doi: 10.1017/S0954579409000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PT, Windle M. Gender-specific pathways between maternal depressive symptoms, family discord, and adolescent adjustment. Developmental Psychology. 1997;33:657–668. doi: 10.1037//0012-1649.33.4.657. [DOI] [PubMed] [Google Scholar]
- Dearing KF, Gotlib IH. Interpretation of ambiguous information in girls at risk for depression. Journal of Abnormal Child Psychology. 2009;37:79–91. doi: 10.1007/s10802-008-9259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienstbier RA. Arousal and physiological toughness: Implications for mental and physical health. Psychological Review. 1989;96:84–100. doi: 10.1037/0033-295x.96.1.84. [DOI] [PubMed] [Google Scholar]
- Ewart CK, Jorgensen RS, Kolodner KB. Sociotropic cognition moderates blood pressure response to interpersonal stress in high-risk adolescent girls. International Journal of Psychophysiology. 1998;28:131–142. doi: 10.1016/s0167-8760(97)00091-3. [DOI] [PubMed] [Google Scholar]
- Ewart CK, Jorgensen RS, Suchday S, Chen E, Matthews KA. Measuring stress resilience and coping in vulnerable youth: The social competence interview. Psychological Assessment. 2002;14:339–352. doi: 10.1037//1040-3590.14.3.339. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. User’s guide for the Structured Clinical Interview for DSM-IV Axis I disorders. 1995. (version 2.0). Unpublished manuscript.
- Foa EB, Kozak MJ. Emotional processing of fear: Exposure to corrective information. Psychological Bulletin. 1986;99:20–35. [PubMed] [Google Scholar]
- Folkman S, Moskowitz JT. Positive affect and the other side of coping. American Psychologist. 2000;55:647–654. doi: 10.1037//0003-066x.55.6.647. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Mancuso RA, Branigan C, Tugade MM. The undoing effect of positive emotions. Motivation and Emotion. 2000;24:237–258. doi: 10.1023/a:1010796329158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Tugade MM, Waugh CE, Larkin GR. What good are positive emotions in crisis? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001. Journal of Personality and Social Psychology. 2003;84:365–376. doi: 10.1037//0022-3514.84.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: A developmental model for understanding mechanisms of transmission. Psychological Review. 1999;106:458–490. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Gotlib IH. Transmission of risk to children of depressed parents: Integration and conclusions. In: Goodman SH, Gotlib IH, editors. Children of depressed parents: Mechanisms of risk and implications for treatment. American Psychological Association; Washington, DC: 2002. pp. 307–326. [Google Scholar]
- Gotlib IH, Goodman SH. Children of parents with depression. In: Silverman WK, Ollendick TH, editors. Developmental issues in the clinical treatment of children. Allyn & Bacon; Needham Heights, MA: 1999. pp. 415–432. [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer I, Murray L. Disturbances in morning cortisol secretion in association with maternal postnatal depression predict subsequent depressive symptomatology in adolescents. Biological Psychiatry. 2007;62:40–46. doi: 10.1016/j.biopsych.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer IM, Murray L. Exposure to postnatal depression predicts elevated cortisol in adolescent offspring. Biological Psychiatry. 2004;55:376–381. doi: 10.1016/j.biopsych.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Hammen CL. Children of depressed parents. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2nd Guilford Press; New York: 2009. pp. 275–297. [Google Scholar]
- Joormann J, Eugene F, Gotlib IH. Parental depression: Impact on offspring and mechanisms underlying transmission of risk. In: Nolen-Hoeksema S, editor. Handbook of adolescent depression. Guilford Press; New York: 2008. [Google Scholar]
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. Journal of Abnormal Psychology. 2007;116:135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The epidemiology of depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2nd Guilford Press; New York: 2009. pp. 5–22. [Google Scholar]
- Kirschbaum C, Pirke K-M, Hellhammer DH. The “Trier Social Stress Test”: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The children’s depression inventory. Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Kristjansson SD, Kircher JC, Webb AK. Multilevel models for repeated measures research designs in psychophysiology: An introduction to growth curve modeling. Psychophysiology. 2007;44:728–736. doi: 10.1111/j.1469-8986.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- Lauer MS, Froelicher V. Abnormal heart-rate recovery after exercise. Lancet. 2002;360:1176–1177. doi: 10.1016/S0140-6736(02)11224-4. [DOI] [PubMed] [Google Scholar]
- Laurent J, Schmidt NB, Catanzaro SJ, Joiner TE, Kelley AM. Factor structure of a measure of anxiety sensitivity in children. Journal of Anxiety Disorders. 1998;12:307–331. doi: 10.1016/s0887-6185(98)00017-6. [DOI] [PubMed] [Google Scholar]
- Lieb R, Isensee B, Hofler M, Pfister H, Wittchen H-U. Parental major depression and the risk of depression and other mental disorders in offspring. Archives of General Psychiatry. 2002;59:365–374. doi: 10.1001/archpsyc.59.4.365. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberg NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: Affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Llabre MM, Spitzer SB, Saab PG, Schneiderman N. Piecewise latent growth curve modeling of systolic blood pressure reactivity and recovery from the cold pressor test. Psychophysiology. 2001;38:951–960. doi: 10.1111/1469-8986.3860951. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic–pituitary–adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology. 2009;34:1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannie ZN, Harmer CJ, Cowen PJ. Increased waking salivary cortisol levels in young people at familial risk of depression. American Journal of Psychiatry. 2007;164:617–621. doi: 10.1176/ajp.2007.164.4.617. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5:175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Miranda J, Persons JB, Byers CN. Endorsement of dysfunctional beliefs depend on current mood state. Journal of Abnormal Psychology. 1990;99:237–241. doi: 10.1037//0021-843x.99.3.237. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Simons AD. Diathesis–stress theories in the context of life stress research: Implications for the depressive disorders. Psychological Bulletin. 1991;110:406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Mykletun A, Bjerkeset O, Overland S, Prince M, Dewey M, Stewart R. Levels of anxiety and depression as predictors of mortality: The HUNT study. British Journal of Psychiatry. 2009;195:118–125. doi: 10.1192/bjp.bp.108.054866. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Prospective investigation of stress inoculation in young monkeys. Archives of General Psychiatry. 2004;61:933–941. doi: 10.1001/archpsyc.61.9.933. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Hormones & Behavior. 2003;43:60–66. doi: 10.1016/s0018-506x(02)00016-8. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Rao U, Hammen CL, Ortiz LR, Chen L, Poland RE. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biological Psychiatry. 2008;64:521–526. doi: 10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush S, Bryk A, Congdon R. HLM6 [Computer software] Scientific Software International; Chicago: 2008. [Google Scholar]
- Roberts JE, Kassel JD. Mood-state dependence in cognitive vulnerability to depression: The roles of positive and negative affect. Cognitive Therapy and Research. 1996;20:1–12. [Google Scholar]
- Salomon K, Clift A, Karlsdottir M, Rottenberg J. Major depressive disorder is associated with attenuated cardiovascular reactivity and impaired recovery among those free of cardiovascular disease. Health Psychology. 2009;28:157–165. doi: 10.1037/a0013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Review. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W, Ricci J, Chee E, Hahn S, Morganstein D. Cost of lost productive work time among US workers with depression. Journal of American Medical Association. 2003;289:3135–3144. doi: 10.1001/jama.289.23.3135. [DOI] [PubMed] [Google Scholar]
- Taylor L, Ingram RE. Cognitive reactivity and depressotypic information processing in children of depressed mothers. Journal of Abnormal Psychology. 1999;108:202–210. doi: 10.1037//0021-843x.108.2.202. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. NeuroImage. 2003;18:650–659. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychological Review. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Tugade MM, Fredrickson BL. Resilient individuals use positive emotions to bounce back from negative emotional experiences. Journal of Personality and Social Psychology. 2004;86:320–333. doi: 10.1037/0022-3514.86.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey J. Exploratory data analysis. Addison–Wesley; Reading, MA: 1977. [Google Scholar]
- Turner SM, Beidel DC, Roberson-Nay R. Offspring of anxious parents: Reactivity, habituation, and anxiety-proneness. Behaviour Research & Therapy. 2005;43:1263–1279. doi: 10.1016/j.brat.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Waugh CE, Panage S, Mendes WB, Gotlib IH. Cardiovascular and affective recovery from anticipatory threat. Biological Psychology. 2010;84:169–175. doi: 10.1016/j.biopsycho.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Warner V, Wickramaratne P, Moreau D, Olfson M. Offspring of depressed parents: 10 years later. Archives of General Psychiatry. 1997;54:932–940. doi: 10.1001/archpsyc.1997.01830220054009. [DOI] [PubMed] [Google Scholar]
- Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H. Hormonal evidence for altered responsiveness to social stress in major depression. Neuropsychopharmacology. 2000;23:411–418. doi: 10.1016/S0893-133X(00)00129-9. [DOI] [PubMed] [Google Scholar]