Abstract
Muscle microvascular surface area determines substrate and hormonal exchanges between plasma and muscle interstitium. GLP-1 (glucagon-like peptide-1) regulates glucose-dependent insulin secretion and has numerous extrapancreatic effects, including a salutary vascular action. To examine whether GLP-1 recruits skeletal and cardiac muscle microvasculature in healthy humans, 26 overnight-fasted healthy adults received a systemic infusion of GLP-1 (1.2 pmol/kg of body mass per min) for 150 min. Skeletal and cardiac muscle MBV (microvascular blood volume), MFV (microvascular flow velocity) and MBF (microvascular blood flow) were determined at baseline and after 30 and 150 min. Brachial artery diameter and mean flow velocity were measured and total blood flow was calculated before and at the end of the GLP-1 infusion. GLP-1 infusion raised plasma GLP-1 concentrations to the postprandial levels and suppressed plasma glucagon concentrations with a transient increase in plasma insulin concentrations. Skeletal and cardiac muscle MBV and MBF increased significantly at both 30 and 150 min (P < 0.05). MFV did not change in skeletal muscle, but decreased slightly in cardiac muscle. GLP-1 infusion significantly increased brachial artery diameter (P < 0.005) and flow velocity (P = 0.05) at 150 min, resulting in a significant increase in total brachial artery blood flow (P < 0.005). We conclude that acute GLP-1 infusion significantly recruits skeletal and cardiac muscle microvasculature in addition to relaxing the conduit artery in healthy humans. This could contribute to increased tissue oxygen, nutrient and insulin delivery and exchange and therefore better prandial glycaemic control and tissue function in humans.
Keywords: conduit artery, contrast ultrasonography, glucagon-like peptide-1 (GLP-1), microvascular recruitment
INTRODUCTION
GLP-1 (glucagon-like peptide-1), a major incretin produced by intestinal L-cells in response to nutrient ingestion, regulates postprandial glucose levels through glucose-dependent insulin secretion, delayed gastric emptying and suppression of glucagon secretion [1,2]. In patients with T2DM (Type 2 diabetes mellitus), the secretion of GLP-1 has been reported to be diminished [3–6], but its biological actions are largely preserved [7]. As a result, GLP-1-based therapies have emerged as major therapeutic options for patients with T2DM.
In addition to its well-characterized glycaemic actions, studies in both animals and humans have repeatedly demonstrated a beneficial action of GLP-1 in improving endothelial function [8–10]. Infusion of GLP-1 into Dahl salt-sensitive rats attenuated the development of hypertension, reduced proteinuria and improved vasodilatory response to Ach (acetylcholine) [11]. In healthy humans, infusion of GLP-1 increased Ach-induced vasodilatation (independent of alterations in blood levels of glucose and insulin) without changing the vasorelaxant response to nitroprusside, probably via the NO pathway involving KATP channels [12]. In patients with T2DM and stable coronary artery disease, infusion of GLP-1 ameliorates endothelial dysfunction as made evident by improved flow-mediated dilatation [10]. The molecular pathway(s) activated by GLP-1 in endothelial cells remains to be defined. Studies using rat arterial rings have shown a direct dose-dependent vasorelaxant effect of GLP-1 which was abolished by removal of the endothelium, confirming the endothelial dependence of GLP-1-mediated vasodilation [13]. Similarly, inhibition of NO production with L-NAME (N G-nitro-L-arginine methyl ester) abolishes the vasorelaxant effect of GLP-1 on rat pulmonary arteries [14]. Multiple in vitro studies using cultured endothelial cells have also shown that GLP-1 can directly activate NOS (NO synthase) [15–18]. Taken together, there is strong evidence that GLP-1 exerts its NO-dependent vasodilatory effect by a direct action on the endothelium. This is not surprising as GLP-1 receptors are abundantly expressed on the vascular endothelium [10].
We have reported recently that GLP-1 infusion acutely relaxes pre-capillary arterioles to recruit skeletal muscle microvasculature and increase muscle perfusion, probably via PKA (protein kinase A)-mediated eNOS (endothelial NOS) activation [15,16]. This was associated with an increase in muscle delivery of insulin and muscle interstitial oxygenation [15,16]. These findings are important in that tissue microvasculature (including pre-capillary arterioles, capillaries and venules) regulates tissue energy metabolism and function as it provides the needed exchange surface area for tissue delivery of oxygen and nutrients as well as hormones, such as insulin, and the removal of metabolic waste [19,20]. Whether GLP-1 increases muscle microvascular perfusion in humans has not been studied.
In the present study we infused GLP-1 systemically to raise the plasma GLP-1 concentration to the level seen postprandially and examined microvascular as well as conduit artery responses in healthy humans. Our results indicate that GLP-1 infusion potently recruits microvasculature in both skeletal and cardiac muscle in addition to increasing brachial artery diameter, flow velocity and total blood flow.
MATERIALS AND METHODS
Human subjects and study protocol
Healthy young volunteers (18–35 years of age) were screened at the University of Virginia Clinical Research Unit. Comprehensive metabolic panels, complete blood counts, lipid profiles and pregnancy tests (if female) were measured, vital signs, weight and height obtained and BMI (body mass index) calculated. Exclusion criteria included BMI <18 or >25 kg/m2, diabetes mellitus, hyperlipidaemia, hypertension or hypotension [BP (blood pressure) <100/60 mmHg], first-degree relative with T2DM, smoking, use of medications or supplements that affect vascular function (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, statins, fibrates, fish oil, vitamins E and C, aspirin, and others), history of congestive heart failure, ischaemic heart disease, severe pulmonary disease, liver disease, kidney disease, presence of intracardiac or intrapulmonary shunt, or known hypersensitivity to perflutren.
The study protocol was carried out in accordance with the Declaration of Helsinki (2013) of the World Medical Association and approved by the University of Virginia Institutional Review Board. Written informed consent was obtained from each subject at the screening visit.
A total of 26 healthy subjects (ten male/16 female; mean years of age, 23.5±0.5; mean BMI, 21.8±0.4 kg/m2) completed the study protocol. After the initial screening, subjects returned for a second outpatient visit where they underwent a treadmill 2 max (maximum oxygen uptake) test using the Bruce protocol as well as measurement of body composition (Bod-PodTM).
Subjects were then admitted to the Clinical Research Unit after refraining from exercise and caffeine for 24 h and undergoing a fast that started at 20:00 the night before and continued until the end of the study session the next day. Female subjects had a negative urine pregnancy test before the study. On the day of the study, venous access was obtained at the antecubital fossa for GLP-1 and microbubble infusion and distal forearm for blood sampling. After obtaining baseline blood samples for glucose, insulin, GLP-1 and glucagon measurements, brachial artery diameter and blood flow velocity were obtained as described previously [21]. Colour Doppler imaging was then performed by a certified cardiac sonographer to evaluate for cardiac shunts. Once a cardiac shunt was ruled out, skeletal and cardiac muscle MBV (microvascular blood volume), MFV (microvascular flow velocity) and MBF (microvascular blood flow) were determined using CEU (contrast-enhanced ultrasound)/MCE (myocardial contrast echocardiography) as described previously [21–24]. A systemic infusion of GLP-1 was started at 1.2 pmol/kg of body mass per min and continued for a total of 150 min. At the rate selected, plasma GLP-1 concentrations have been shown to improve endothelial function in healthy humans [12] and normalize blood glucose in patients with T2DM [25], without inducing a significant increase in insulin secretion [26]. CEU and MCE measurements were repeated after 30 and 150 min. Brachial artery diameter and blood flow velocity were measured again at 150 min. Blood samples for glucose and insulin were measured every 30 min and for glucagon and GLP-1 after 30 and 150 min. We did not infuse saline as previous studies have confirmed that saline infusion does not significantly alter muscle microvascular perfusion [27,28].
Determination of brachial artery diameter, blood flow velocity and blood flow
Brachial artery diameter and blood flow velocity were determined using a SONOS 7500 ultrasound system and an L11-3 linear array transducer (Philips Medical Systems) with a transmit frequency of 7.5 MHz to allow 2D imaging of the brachial artery in the long axis as described previously [21,22]. Brachial artery blood flow () was calculated from the averages of three diameter (d) and velocity (v) measurements using the equation = vπ ×(d/2)2.
Determination of microvascular parameters in the skeletal and cardiac muscle
CEU and MCE were performed using a SONOS 7500 ultrasound system and an S3 phased array transducer (Philips Medical Systems) with the subject lying in the left decubitus position as described previously [21,22]. The contrast agent, octafluoropropane gas-filled lipid microbubbles (Definity®; Lantheus Medical Imaging), was infused systemically to trace the microvasculature. Once the microbubble concentrations reached steady-state in the circulation, usually within 2–3 min, three images were captured at each pulsing interval ranging from one to eight cardiac cycles in the caroiac muscle microvasculature (mechanic index, 0.8) and one to 20 cardiac cycles in the skeletal muscle microvasculature (mechanic index, 1.5). Cardiac imaging included the two-, three- and four-chamber views and the fcrearm muscle imaging was performed in a transaxial plane 5 cm distal to the antecubital fossa. Data was recorded digitally and analysed using the QLAB software (Philips Medical Systems) as described previously [21,22]. MBF was derived from the product of MBV and MFV (i.e. MBF=MBV×MFV).
Biochemical analysis
Compre nsive metabolic panels, complete blood counts, lipid profiles and pregnancy tests were assayed at the University of Virginia Clinical Chemistry Laboratory. Plasma glucose was measured using a YSI glucose analyser (Yellow Spring Instruments). Plasma total GLP-1 concentrations were measured using a Millipore ELISA kit. Plasma insulin levels were determined using a Siemens Healthcare Diagnostics Immulite 2000 random access analyser and glucagon levels were quantified using a Millipore Glucagcn RIA kit (catalogue number GL-32K) at the University of Viiginia Center for Research in Reproduction Ligand Assay and Analysis Core Laboratory. The QUICKI (quantitative insulin sensitivity check index) was calculated as 1/[log(fasting insulin micro-units/ml) + log(fasting glucose mgdl)] (29).
Statistical analysis
The principal end points were the changes in myocardial and skeletal muscle microvascular parameters and the brachial artery parameters. All results are means±S.E.M. Comparisons were made between baseline and after 30 and 150 min using ANOVA or paired Student's t test where appropriate and Pearson's correlation testing was performed (SPSS Statistics for Windows, Version 21.0). P < 0.05 was considered statistically significant.
RESULTS
Subject characteristics and biochemical profiles
Table 1 shows the clinical characteristics of all 26 study participants. All subjects had a normal BJlvfI and were normotensive with normal plasma lipid levels. GLP-1 infusion did not change either systolic BP (110±2 compared with 111±2 mmHg, 0 min compared with 150 min; P = 0.83) or oxygenation (determined using pulse oximetry; 98.9 ±0.3% compared with 98.5 ±0.3%, 0 min compared with 150 min; P = 0.08) throughout the present study. It did slightly increase the heart rate (57 ±2 compared with 61±2 beats/min, 0 min compared with 150 min), which, although small, was statistically significant (P = 0.003). It also decreased diastolic BP slightly although at statistical significance (68 ±1compared with 64 ±1 mmHg, 0 min compared with 150 min; P = 0.03).
Table 1.
Mean subject characteristics Results are means ± S.E.M. (n = 26). HDL, high-density lipoprotein; LDL, low-density lipoprotein.
| Characteristic | Value |
|---|---|
| Age (years) | 23.5 ± 0.5 |
| Sex (male/female) | 10/16 |
| Mass (kg) | 63.8 ± 2.0 |
| Height (cm) | 170.3 ± 1.8 |
| BMi (kg/m2) | 21.8 ± 0.4 |
| Body fat (%) | 21.2 ± 1.5 |
| Waist (cm) | 73.7 ± 1.1 |
| Systolic blood pressure (mmHg) | 114.7 ± 2.2 |
| Diastolic blood pressure (mmHg) | 65.8 ± 2.1 |
| Total cholesterol (mg/dl) | 164.8 ± 6.1 |
| LDL-cholesterol (mg/dl) | 92.8 ± 5.0 |
| HDL-cholesterol (mg/dl) | 60.1 ± 2.4 |
| Triacylglycerol (mg/dl) | 74.7 ± 5.6 |
| o2 max (ml/kg of body mass per min) | 45.1 ± 1.8 |
The GLP-1 infusion-induced changes in plasma insulin, glucose, glucagon and GLP-1 concentrations are shown in Table 2. GLP-1 infusion increased total GLP-1 3-fold to the levels seen postprandially after carbohydrate ingestion [30]. This was associated with a statistically significant decrease in plasma glucose concentrations at 30 min, which remained low during the entire study. When plasma insulin levels for the 26 subjects were compared, there was a small, but statistically significant, increase from baseline after 30 (P < 0.005), 120 (P < 0.05) and 150 (P < 0.05) min. We also measured plasma insulin levels in six subjects after 10 minand found them tobe 4-foldthe baseline valres (P < 0.05); however, they returned promptly back to close to baseline after 30 min. Plasma glucagon levels decreased significantly at both 30 (P < 0.005) and 150 (P < 0.05) min when compared with the baseline.
Table 2.
Plasma concentrations of insulin, glucagon, glucose and GLP-1
| Time (min) | Insulin (pM) | Glucagon (pg/ml) | Glucose (mM) | GLP-1 (pM) |
|---|---|---|---|---|
| 0 | 17.5 ± 2.1 | 66.2 ± 2.7 | 4.8 ± 0.1 | 24.3 ± 2.0 |
| 10 | 67.8 ± 17.9# | – | – | – |
| 30 | 24.7 ± 3.1* | 60.0 ± 2.6* | 4.1 ± 0.1* | 69.7 ± 3.3* |
| 60 | 21.8 ± 2.7 | – | 4.3 ± 0.1* | – |
| 90 | 20.9 ± 3.0 | – | 4.4 ± 0.1* | – |
| 120 | 23.6 ± 3.9# | – | 4.5 ± 0.1* | – |
| 150 | 22.1 ± 3.0# | 61.8 ± 3.0# | 4.2 ± 0.2 | 75.1 ± 2.5* |
Results are means ± S.E.M.
P < 0.05
P < 0.005 compared with zero time.
GLP-1 infusion significantly increased brachial artery blood flow
A previous study has shown that GLP-1 at the same infusion rate as used in the present study increased fcrearm total blood flow by 30% [12]. To examine whether this increase in total blood flow was secondary to changes in brachial artery diameter and/or flow velocity, we quantified these parameters before and after GLP-1 infusion. Systemic infusion of GLP-1 significantly increased both brachial artery diameter (3.62±0.10 mm compared with 3.84 ±0.12mm, baseline canpared with 150 min, P < 0.005) and brachial artery flow velocity (8.64 ±0.62 compared with 9.96 ±0.50 emfs, baseline canpared with 150 min, P = 0.05. As a result, brachial artery blood flow increased by ~30% (55.1 ±5.4 ml/min compared with 70.5 ±6.0 ml/min, baseline compared with 150 min, P < 0.005).
GLP-1 infusion significantly recruited skeletal muscle microvasculature
Figure 1 shows the changes in skeletal muscle microvascular parameters during GLP-1 infusicn. Two subjects were excluded from the analysis due to poor image quality. GLP-1 infusion promptly increased skeletal muscle MBV (~36%; P < 0.001) after 30 min, which remained elevated at 150 min (~43%; P < 0.001). There was no significant chan in muscle MFV during GLP-1 infusion (0.42±0.01 compared with 0.40 ±0.01 1/s, 0 min compared with 150 min; P = 0.15). As a result, GLP-1 infusion led to a significant increase in muscle IBF at both 30 min (~26 %; P < 0.001) and 150 min (~32%; P < 0.001).
Figure 1. GLP-1 infusion significantly increased skeletal muscle MBVand MBF.
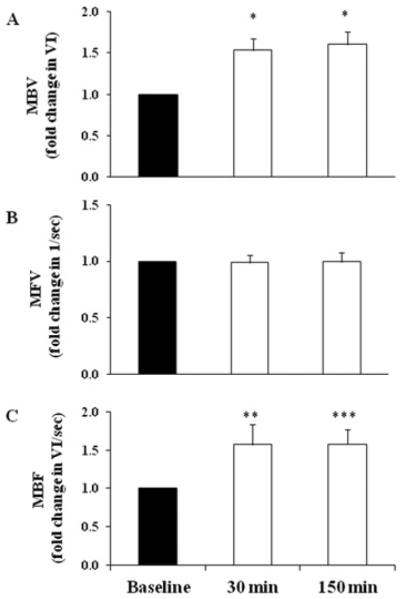
Each subject received a systemic infusion of GLP-1 at 1.2 pmol/kg of body mass per min. (A) MBV. (B) MFV. (C) MBF. Results are means±S.E.M. *P<0.001, **P<0.04 and ***P<0.008 compared with baseline.
GLP-1 infusion significantly increased cardiac muscle MBV and MBF
Figure 2 shows GLP-1-induced changes in cardiac microvascular parameters. One subject was excluded from the analysis due to poor image quality. GLP-1 infusion significantly increased cardiac muscle MBV by ~53% (P < 0.001) after 30 min and by ~57% (P < 0.001) after 150 min when compared with the baseline. These changes were associated with a small, but significant, decrease in cardiac MFV (P < 0.001). Overall, cardiac MBF increased by ~48%(P < 0.001) after 30 min and by ~47% (P < 0.001) after 150 min of GLP-1 infusion.
Figure 2. GLP-1 infusion significantly increased cardiac muscle MBVand MBF.
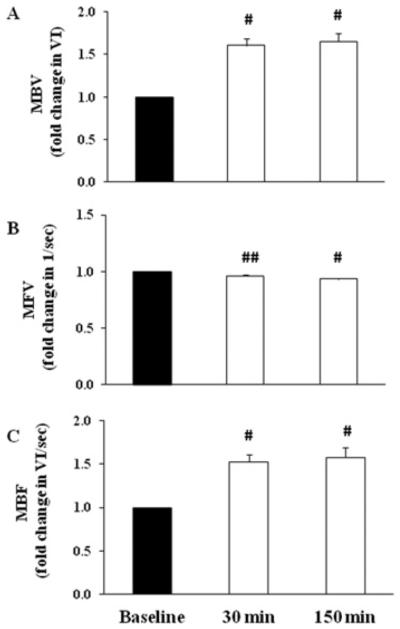
Each subject received a systemic infusion of GLP-1 at 1.2 pmol/kg of body mass per min. (A) MBV. (B) MFV. (C) MBF. Results are means±S.E.M. #P < 0.001 and ##P< 0.003 compared with baseline.
Correlation analysis
We finally conducted multiple regression analyses to assess whether the patient characteristic sand the conduit artery response to GLP-1 were related to the responses to GLP-1 in the skeletal or cardiac muscle microvasculature. As shown in Table 3, the percentage body fat correlated negatively and lean body mass positively with skeletal muscle MBV, whereas age, 2 max and QUICKI had nocorrelationwith skeletal muscle MBV. As for cardiac muscle MBV, only QUICKI correlated positively at baseline and after 150 min. Changes in brachial artery diameter did not correlate with either skeletal muscle or cardiac muscle MBV at any time points. The absolute decrease in plasma glucose levels after 30 min correlated positively with lean body mass (r = 0.38; P = 0.03) and changes in brachial artery diameters (r = 0.39; P = 0.03), but not with the MBV values in either the skeletal or cardiac microvasculature.
Table 3.
Correlation analysis
| MBV | Age (years) | Body fat (%) | Lean mass (kg) |
O2max (ml/kg of body mass per min) |
QUICKI | ΔGIU(0–30) |
|---|---|---|---|---|---|---|
| Skeletal muscle | ||||||
| 0 min | −0.08 | −0.47* | 0.38# | 0.36 | 0.24 | 0.24 |
| 30 min | 0.18 | −0.48* | 0.41## | 0.25 | −0.04 | 0.31 |
| 150 min | −0.08 | −0.6** | 0.67** | 0.12 | −0.07 | 0.32 |
| Cardiac muscle | ||||||
| 0 min | 0.06 | −0.16 | 0.26 | −0.02 | 0.45Δ | −0.18 |
| 30 min | 0.11 | −0.06 | 0.08 | −0.18 | 0.11 | −0.12 |
| 150 min | 0.28 | −0.10 | −0.18 | −0.10 | 0.41## | −0.33 |
ΔGlu(0-30), absolute difference between glucose after 30 min and baseline.
P = 0.02,
P = 0.001,
P = 0.07,
P = 0.04
P <0.03.
DISCUSSION
The results of the present study demonstrate that GLP-1, when infused acutely at levels seen postprandially, exerts a potent vasodilatory action on both conduit artery and pre-capillary arterioles in healthy humans as indicated by increased brachial artery diameter and blood flow and skeletal and cardiac muscle microvascular recruitment. Although this observation was made in healthy humans, we speculate that GLP-1 is able to act on the microvasculature in subjects with obesity and insulin resistance, with or without diabetes, as the biological actions of GLP-1 are laigely preserved in patients with diabetes [7]. Our findings are physiologically and clinically important and suggest that GLP-1 not only contributes to glycaemic control, but also plays a significant role in maintaining endothelial, and thus, vascular health and tissue function; both aspects are particularly important in patients with T2DM as they exhibit hyperglycaemia and are prone to cardiovascular complications.
The positive effects of GLP-1 on the cardiovascular system have been observed frequently in both animals and humans [11,18,31–33]. Our observaticn of increased brachialarterydiameter and total brachial blood flow during GLP-1 infusion clearly indicates a vasodilatory effect on the conduit artery. This is consistentwith aprevious report which showed that systemic infusion of GLP-1 at the same infusion rate as the present study increased total forearm blood flow by 30% (measured by plethysmography) and flow responses toacetylcholire [12]. Feeding healthy humans a mixed meal similarly increased brachial artery diameter and blood flow [34]. As dilation of the conduit arteries increases total blood flow to tissues/organs and coronary MBF increased significantly in the present study, it is very probable that total coronary blood flow may have also increased during GLP-1 infusion in the present study.
Similar to our previous reports that GLP-1 acutely and potently recruits muscle microvasculature in rats [15,16], we observed in the present study that GLP-1 recruited microvasculature in both skeletal and cardiac muscle. This is particularly physiologically relevant as the GLP-1 infusicn rate (1.2 pmol/kg of body mass per min) used in humans is much lower than that used in animal studies (30 pmol/kg of body mass per min). Whether at these concentrations GLP-1 also exerts its microvascular action via the PKAJNO-mediated pathway in humans, similar to our rat studies [15,16], remains to be determined. However, the present study does suggest strongly that in humans GLP-1 regulates prandial glycaemia probably via at least three distinct, yet inter-related, actions: stimulation of glucose-dependent insulin secretion, inhibition of glucose-dependent glucagon secretion, and microvascular recruitment with increased insulin delivery to and thus action in muscle.
In addition to increasing tissue delivery of insulin, a major function of the microvasculature is to regulate the oxygen supply of tissues. Thus our findings that GLP-1 infusion potently recruits skeletal and cardiac muscle microvasculature are of great clinical importance. Patients with T2DM tend to have atherosclerotic complications such as coronary atherosclerosis and peripheral arterial diseases, two conditions that cause significant morbidity and mortality in this patient population by limiting the tissue supply of nutrients, oxygen and hormones. We have shown in skeletal muscle that expansion of the muscle microvascular endothelial area by microvascular recruitment markedly increases tissue glucose use and interstitial oxygen saturation [15,35]. In the setting of limited tiss total blood flow, as seen in patients with atherosclerotic diseases, a small increase in tissue MBV could significantly improve tissue oxygen supply and thus function. Indeed, during low-flow ischaemia, GLP-1 enhanced functional recovery and increased ccronary blood flow and myocardial uptake of glucose in a Langendorff-perfused rat heart preparation [36]. However, this remains to be examined in humans.
The acute decrease (small but statistically significant) in plasma glucose concentrations at 30 min is rather intriguing. This was probably secondary to a combination of transient increase in insulin secretion, inhibition of glucagon secretion and increased tiss use of glucose due to microvascular recruitment. As insulin has been repeatedly shown to acutely recruit muscle microvasculature in healthy humans (21,23,27,37,38), we cannot entirely rule out the possibility that insulin has contributed to our observed microvascular recruitment. However, this small and brief increase in plasma insulin concentration may have only minimally contributed to our observation as insulin concentrations promptly returned almost back to the baseline and previous evidence has demonstrated that insulin-mediated microvascular recruitment persisted for only 15–30 min after insulin concentrations returned back to basal levels [39]. In addition, in humans raising plasma insulin concentrations to ~ 200 pM only increases muscle MBV by ~ 30 % [22]. Furthermore, a previous study has suggested that GLP-1 probably increases muscle glucose uptake independent of its ability to enhance insulin secretion [40]. Basu et al. [12] clearly demonstrated a salutary vasorelaxant effect on brachial artery during acute GLP-1 infusion when endogenous secretions of insulin and glucagon were inhibited by continuous infusion of somatostatin. Taken together, the results of the present study strongly suggest that the increase in skeletal and cardiac muscle microvascular perfusion observed during GLP-1 infusion resulted from an effect of GLP-1 at least partially after 30 min and entirely at 150 min.
During GLP-1 infusion, plasma glucose concentrations decreased with a nadir of 4.1 mM. However, it is unlikely that this decrease in glycaemia induced a significant catecholamine response as our subjects remained asymptomatic, heart rates only increased by 4 beats/min and systolic BP did not change. In addition, systemic infusion of adrenaline (epinephrine) does not alter MBV despite increasing femoral artery blood flow in rats [41], and during euglycaemic hyperinsulinaemia in lean healthy humans blockade of β-adrenergic receptors does not alter insulin-induced increase in muscle blood flow [42].
Our observation of a small, but significant, increase in heart rate during GLP-1 infusion is similar to a previous report that infusion of GLP-1 in patients with chronic compensated heart failure resulted in a 2 beats/min increase in heart rate [43]. This may reflect the chronotropic effect of GLP-1 receptor activation [44] and/or feedback from MBV expansion and is unlikely to explain the 30–50 % increase in muscle or cardiac MBV observed in the present study.
In conclusion, acute GLP-1 infusion in healthy humans resulted in skeletal and cardiac muscle microvascular recruitment as well as an increase in brachial artery diameter and blood flow. As increased total blood flow to tissue and larger microvascular endothelial surface area are associated with improved tissue delivery of oxygen, nutrients and insulin, the results of the present study strongly suggest that GLP-1 receptor-mediated vascular actions could contribute to the glycaemic effect of GLP-1 receptor agonists used to treat T2DM and may help decrease the cardiovascular morbidity and mortality associated with T2DM.
CLINICAL PERSPECTIVES.
The vascular endothelium expresses abundant GLP-1 receptors and GLP-1 has been shown to exert beneficial cardiovascular actions in both humans and laboratory animals in addition to its well-known effects on the pancreatic islets.
The present study shows that acute GLP-1 infusion in healthy humans resulted in skeletal and cardiac muscle microvascular recruitment as well as an increase in brachial artery diameter and blood flow.
These findings are of physiological as well as clinical importance as microvasculature controls the delivery of oxygen, nutrients and hormones into tissue and their exchanges between plasma and tissue interstitium by providing endothelial exchange surface area. These GLP-1 receptor-mediated vascular actions could contribute to the glycaemic effect of GLP-1 receptor agonists used to treat T2DM and may help decrease the cardiovascular morbidity and mortality associated with T2DM.
Acknowledgments
FUNDING
This work was supported by the American Diabetes Association [grant numbers 1-11-CT-30 and 9-09-NOVO-11 (to Z.L.)] and the National Institutes of Health [grant number R01HL094722 (to Z.L.)]. S.C.S. and M.A.S. were supported by a T32 training grant to the University of Virginia [grant number DK007320].
Abbreviations
- Ach
acetylcholine
- BMI
body mass index
- BP
blood pressure
- CEU
contrast-enhanced ultrasound
- GLP-1
glucagon-like peptide-1
- MBF
microvascular blood flow
- MBV
microvascular blood volume
- MCE
myocardial contrast echocardiography
- MFV
microvascular flow velocity
- NOS
nitric oxide synthase
- PKA
protein kinase A
- QUICKI
quantitative insulin sensitivity check index
- T2DM
Type 2 diabetes mellitus
- o2 max
maximum oxygen uptake
REFERENCES
- 1.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 2.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Vilsboll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609–613. doi: 10.2337/diabetes.50.3.609. [DOI] [PubMed] [Google Scholar]
- 4.Toft-Nielsen M-B, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 2001;86:3717–3723. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 5.Vilsboll T, Krarup T, Sonne J, Madsbad S, Volund A, Juul AG, Holst JJ. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2003;88:2706–2713. doi: 10.1210/jc.2002-021873. [DOI] [PubMed] [Google Scholar]
- 6.Jones I, Owens D, Luzio S, Williams S, Hayes T. The glucose dependent insulinotropic polypeptide response to oral glucose and mixed meals is increased in patients with Type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1989;32:668–677. doi: 10.1007/BF00274255. [DOI] [PubMed] [Google Scholar]
- 7.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Invest. 1993;91:301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nystrom T. The potential beneficial role of glucagon-like peptide-1 in endothelial dysfunction and heart failure associated with insulin resistance. Horm. Metab. Res. 2008;40:593–606. doi: 10.1055/s-0028-1082326. [DOI] [PubMed] [Google Scholar]
- 9.Nystroöm T, Gonon AT, Sjoöholm Å, Pernow J. Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul. Pept. 2005;125:173–177. doi: 10.1016/j.regpep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 10.Nystrom T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahren B, Sjoholm A. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am. J. Physiol. Endocrinol. Metab. 2004;287:E1209–E1215. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- 11.Yu M, Moreno C, Hoagland KM, Dahly A, Ditter K, Mistry M, Roman RJ. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J. Hypertens. 2003;21:1125–1135. doi: 10.1097/00004872-200306000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Basu A, Charkoudian N, Schrage W, Rizza RA, Basu R, Joyner MJ. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am. J. Physiol. Endocrinol. Metab. 2007;293:E1289–E1295. doi: 10.1152/ajpendo.00373.2007. [DOI] [PubMed] [Google Scholar]
- 13.Richter G, Feddersen O, Wagner U, Barth P, Goke R, Goke B. GLP-1 stimulates secretion of macromolecules from airways and relaxes pulmonary artery. Am. J. Physiol. Lung Cell. Mol. Physiol. 1993;265:L374–L381. doi: 10.1152/ajplung.1993.265.4.L374. [DOI] [PubMed] [Google Scholar]
- 14.Golpon HA, Puechner A, Welte T, Wichert PV, Feddersen CO. Vasorelaxant effect of glucagon-like peptide-(7–36)amide and amylin on the pulmonary circulation of the rat. Regul. Pept. 2001;102:81–86. doi: 10.1016/s0167-0115(01)00300-7. [DOI] [PubMed] [Google Scholar]
- 15.Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W, Liu Z. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes. 2012;61:888–896. doi: 10.2337/db11-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong Z, Chai W, Wang W, Zhao L, Fu Z, Cao W, Liu Z. Protein kinase A mediates glucagon-like peptide 1-induced nitric oxide production and muscle microvascular recruitment. Am. J. Physiol. Endocrinol. Metab. 2013;304:E222–E228. doi: 10.1152/ajpendo.00473.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erdogdu O, Nathanson D, Sjoöholm Å, Nystroöm T, Zhang Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol. Cell. Endocrinol. 2010;325:26–35. doi: 10.1016/j.mce.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 18.Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, Stolarski C, Shen Y-T, Shannon RP. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 19.Barrett E, Eggleston E, Inyard A, Wang H, Li G, Chai W, Liu Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia. 2009;52:752–764. doi: 10.1007/s00125-009-1313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrett EJ, Wang H, Upchurch CT, Liu Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am. J. Physiol. Endocrinol. Metab. 2011;301:E252–E263. doi: 10.1152/ajpendo.00186.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z. Insulin at physiological concentrations increases microvascular perfusion in human myocardium. Am. J. Physiol. Endocrinol. Metab. 2007;293:E1250–E1255. doi: 10.1152/ajpendo.00451.2007. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, Liu J, Jahn LA, Fowler DE, Barrett EJ. Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J. Clin. Endocrinol. Metab. 2009;94:3543–3549. doi: 10.1210/jc.2009-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Jahn LA, Fowler DE, Barrett EJ, Cao W, Liu Z. Free fatty acids induce insulin resistance in both cardiac and skeletal muscle microvasculature in humans. J. Clin. Endocrinol. Metab. 2011;96:438–446. doi: 10.1210/jc.2010-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sauder MA, Liu J, Jahn LA, Fowler DE, Chai W, Liu Z. Candesartan acutely recruits skeletal and cardiac muscle microvasculature in healthy humans. J. Clin. Endocrinol. Metab. 2012;97:E1208–E1212. doi: 10.1210/jc.2011-3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meier JJ, Gallwitz B, Salmen S, Goetze O, Holst JJ, Schmidt WE, Nauck MA. Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2003;88:2719–2725. doi: 10.1210/jc.2003-030049. [DOI] [PubMed] [Google Scholar]
- 26.Hvidberg A, Nielsen MT, Hilsted J, Ørskov C, Holst JJ. Effect of glucagon-like peptide-1 (proglucagon 78–107amide) on hepatic glucose production in healthy man. Metabolism. 1994;43:104–108. doi: 10.1016/0026-0495(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 27.Coggins M, Lindner J, Rattigan S, Jahn L, Fasy E, Kaul S, Barrett E. Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes. 2001;50:2682–2690. doi: 10.2337/diabetes.50.12.2682. [DOI] [PubMed] [Google Scholar]
- 28.Meijer RI, De Boer MP, Groen MR, Eringa EC, Rattigan S, Barrett EJ, Smulders YM, Serne EH. Insulin-induced microvascular recruitment in skin and muscle are related and both are associated with whole-body glucose uptake. Microcirculation. 2012;19:494–500. doi: 10.1111/j.1549-8719.2012.00174.x. [DOI] [PubMed] [Google Scholar]
- 29.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 30.Smushkin G, Sathananthan A, Man CD, Zinsmeister AR, Camilleri M, Cobelli C, Rizza RA, Vella A. Defects in GLP-1 response to an oral challenge do not play a significant role in the pathogenesis of prediabetes. J. Clin. Endocrinol. Metab. 2012;97:589–598. doi: 10.1210/jc.2011-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, Shannon RP. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 32.Moberly SP, Berwick ZC, Kohr M, Svendsen M, Mather KJ, Tune JD. Intracoronary glucagon-like peptide 1 preferentially augments glucose uptake in ischemic myocardium independent of changes in coronary flow. Exp. Biol. Med. 2012;237:334–342. doi: 10.1258/ebm.2011.011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sokos GG, Bolukoglu H, German J, Hentosz T, Magovern GJ, Jr, Maher TD, Dean DA, Bailey SH, Marrone G, Benckart DH, et al. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am. J. Cardiol. 2007;100:824–829. doi: 10.1016/j.amjcard.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 34.Keske MA, Clerk LH, Price WJ, Jahn LA, Barrett EJ. Obesity blunts microvascular recruitment in human forearm muscle after a mixed meal. Diabetes Care. 2009;32:1672–1677. doi: 10.2337/dc09-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chai W, Wang W, Liu J, Barrett EJ, Carey RM, Cao W, Liu Z. Angiotensin II type 1 and type 2 receptors regulate basal skeletal muscle microvascular volume and glucose use. Hypertension. 2010;55:523–530. doi: 10.1161/HYPERTENSIONAHA.109.145409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen Y-T, Shannon RP. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J. Pharmacol. Exp. Ther. 2006;317:1106–1113. doi: 10.1124/jpet.106.100982. [DOI] [PubMed] [Google Scholar]
- 37.Chai W, Liu J, Jahn LA, Fowler DE, Barrett EJ, Liu Z. Salsalate attenuates free fatty acid-induced microvascular and metabolic insulin resistance in humans. Diabetes Care. 2011;34:1634–1638. doi: 10.2337/dc10-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes. 2006;55:1436–1442. doi: 10.2337/db05-1373. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Vincent MA, Richards SM, Clerk LH, Rattigan S, Clark MG, Barrett EJ. Insulin sensitivity of muscle capillary recruitment in vivo. Diabetes. 2004;53:447–453. doi: 10.2337/diabetes.53.2.447. [DOI] [PubMed] [Google Scholar]
- 40.Ayala JE, Bracy DP, James FD, Julien BM, Wasserman DH, Drucker DJ. The glucagon-like peptide-1 receptor regulates endogenous glucose production and muscle glucose uptake independent of its incretin action. Endocrinology. 2009;150:1155–1164. doi: 10.1210/en.2008-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rattigan S, Clark MG, Barrett EJ. Hemodynamic actions of insulin in rat skeletal muscle: evidence for capillary recruitment. Diabetes. 1997;46:1381–1388. doi: 10.2337/diab.46.9.1381. [DOI] [PubMed] [Google Scholar]
- 42.Randin D, Vollenweider P, Tappy L, Jequier E, Nicod P, Scherrer U. Effects of adrenergic and cholinergic blockade on insulin-induced stimulation of calf blood flow in humans. Am. J. Physiol. Regul. Integrat. Comp. Physiol. 1994;266:R809–R816. doi: 10.1152/ajpregu.1994.266.3.R809. [DOI] [PubMed] [Google Scholar]
- 43.Halbirk M, Norrelund H, Moller N, Holst JJ, Schmitz O, Nielsen R, Nielsen-Kudsk JE, Nielsen SS, Nielsen TT, Eiskjar H, et al. Cardiovascular and metabolic effects of 48-h glucagon-like peptide-1 infusion in compensated chronic patients with heart failure. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H1096–H1102. doi: 10.1152/ajpheart.00930.2009. [DOI] [PubMed] [Google Scholar]
- 44.Nathanson D, Ullman B, Lofstrom U, Hedman A, Frick M, Sjoholm A, Nystrom T. Effects of intravenous exenatide in type 2 diabetic patients with congestive heart failure: a double-blind, randomised controlled clinical trial of efficacy and safety. Diabetologia. 2012;55:926–935. doi: 10.1007/s00125-011-2440-x. [DOI] [PubMed] [Google Scholar]


