Abstract
Importance
Reward-related disturbances after withdrawal from nicotine are hypothesized to contribute to relapse to tobacco smoking, but mechanisms underlying and linking such processes remain largely unknown.
Objective
To determine whether withdrawal from nicotine affects reward responsiveness (i.e., the propensity to modulate behavior as a function of prior reinforcement experience) across species using translational behavioral assessments in humans and rats.
Design, Setting, Participants, and Main Outcomes and Measures
Analogous reward responsiveness tasks were used in both humans and rats to examine whether reward responsiveness varied in: 1) an ad libitum smoking condition compared to a 24-hour acute nicotine abstinence condition in 31 human smokers with (N=17) or without (N=14) a history of depression; 2) rats 24 hours after withdrawal from chronic nicotine (N=19) or saline (N=20); and 3) rats following acute nicotine exposure after withdrawal from either chronic nicotine or saline administration.
Results
In both human smokers and nicotine-treated rats, reward responsiveness was significantly reduced after 24-hour withdrawal from nicotine. In humans, withdrawal-induced deficits in reward responsiveness were greater in subjects with a history of depression. In rats previously exposed to chronic nicotine, acute nicotine re-exposure long after withdrawal potentiated reward responsiveness
Conclusions and Relevance
These findings across species converge in suggesting that organisms have diminished ability to modulate behavior as a function of reward during withdrawal of nicotine. This blunting may contribute to relapse to tobacco smoking, particularly in depression-vulnerable individuals, in order to re-instate responsiveness to natural rewards, and to experience potentiated nicotine-induced reward responsiveness. Moreover, demonstration of behavioral homology across humans and rodents provides a strong translational framework for the investigation and development of clinical treatments targeting reward responsiveness deficits during early withdrawal of nicotine.
Introduction
Smoking is a leading cause of disease and mortality worldwide1;2, and many smokers experience difficulty quitting and nicotine withdrawal3-8 . While exposure to nicotine is associated with increased responsiveness to rewards9-18 in rodents and humans, less is known about the role of different reward-related processes during nicotine withdrawal. Studies in rodents using the intracranial self-stimulation (ICSS) procedure19-21 have consistently shown decrements in brain reward function during nicotine withdrawal, but assessments of motivation and effort for natural rewards in rodents22-24 and humans14;16;25;26 have produced less consistent results, likely due to the heterogeneity of tests measuring motivation and reward responsiveness between humans and rodents. Thus, it remains unclear which reward-related processes are compromised after withdrawal from nicotine, hindering development of cessation treatments.
Here, we examined the effects of withdrawal of nicotine on reward responsiveness, defined as the propensity to modulate behavior as a function of prior reinforcement experience27-29, using a Response Bias Probabilistic Reward Task (RB-PRT) developed to objectively quantify reward responsiveness in humans27 and rats30. During this task, subjects must distinguish between two ambiguous stimuli, whereby correct identification of either stimulus is partially reinforced (Figure 1). Unbeknownst to the subjects, throughout the test session, correct identification of one stimulus (“rich”) is rewarded three times more frequently than correct identification of the other stimulus (“lean”). Due to the differential reinforcement schedule, healthy subjects develop a response bias in favor of the more frequently rewarded (rich) stimulus. In a placebo-controlled study, acute nicotine administration in current non-smokers was associated with potentiated reward responsiveness18. However, the study included subjects with prior smoking history, which may differentially mediate reward responsiveness during acute nicotine re-exposure relative to subjects without prior smoking experience. Notably, human subjects with elevated depression-related symptoms27,28,31 show blunted reward responsiveness (i.e., reduced response bias) in this task.
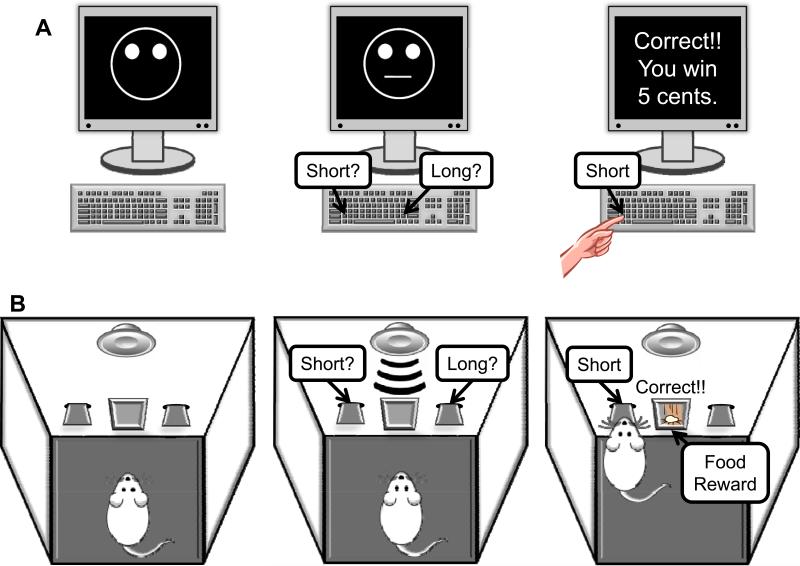
Figure 1. SCHEMATIC REPRESENTATION OF THE HUMAN (A) AND RAT (B) RESPONSE BIAS PROBABILISTIC REWARD TASK.
(A) In each trial, human subjects were asked to choose whether a short (11.5 mm) or long (13 mm) mouth (briefly flashed for 100 ms) had been presented on a mouthless schematic face by pressing a key (e.g., ‘z’ for short, ‘/’ for long). In each of the 3 blocks (100 trials/block), the mouth stimuli were pseudo-randomly presented in an equal number. For some of the correct trials, the participant received a monetary reinforcement (5 cents). Unbeknownst to the participants, the reinforcement schedule was designed to favor one mouth length (i.e., rich) over the other (i.e., lean) in a 3:1 ratio. Only 40 correct trials were rewarded in each block (30 rich, 10 lean). Participants were instructed that the goal of the task was to win as much money as possible, and that not all correct responses would receive a reward feedback. Response bias, our main variable of interest, was calculated as: log b = ½ log [(RichCorrect * LeanIncorrect)/(RichIncorrect * LeanCorrect)]. As evident from the formula, a high response bias emerges when participants tend to correctly identify the rich stimulus and misclassify the lean stimulus. Discriminability, which is the degree to which the subject can distinguish the two target stimuli and is a measure of task difficulty, was used as a control variable and was calculated as: log d = ½ log [(RichCorrect * LeanCorrect)/(RichIncorrect * LeanIncorrect)]. These formulae include the addition of 0.5 to each cell, to allow for estimation in cases with a zero cell. Accuracy (percentage hit rate) and reaction time in response to the rich and lean stimuli represented additional secondary behavioral variables. (B) Rats were food restricted and trained to discriminate between two tones varying in duration (5 kHz, 60 dB, 0.5 or 2 s) by pressing one of the two levers associated with each tone. Tone durations and lever sides were counterbalanced across subjects and tones were presented in a random order over 100 trials. Each trial was initiated with presentation of a tone, after which levers were extended and rats had a 5 s limited hold period to respond. In each trial, correct identification of tones resulted in a single 45 mg food pellet (Test Diet 5TUM; Richmond, IN, USA). Both levers retracted after a correct, incorrect, or omitted response, followed by a variable intertrial interval (5-8 s). Rats were trained daily until achieving at least 70% accuracy for five consecutive days. Rats that were successful in discriminating the tones were then trained with tone durations of 0.7 and 1.8 s for two days and tone durations of 0.9 and 1.6 s for two days. During a subsequent test session, the ambiguous tone durations (i.e., 0.9 and 1.6 s) were reinforced for 60% and 20% of correct responses (counterbalanced across subjects) over 100 trials, which is identical to the 3:1 reinforcement ratio used in the human Response Bias Probabilistic Reward Task30. Response bias, the primary variable, as well as the three secondary behavioral variables (discriminability, accuracy and reaction time) were computed using identical formulae as for the human experimental data.
Given that nicotine withdrawal is characterized by depression-like symptoms32, these previous findings for depression27,28,31 may suggest that withdrawal of nicotine is also associated with blunted reward responsiveness. Moreover, many smokers have a history of major depression33;34; such individuals are more likely to experience nicotine withdrawal symptoms and continue smoking4;35-37,33;38, and trait anhedonia is associated with relapse to smoking39;40. Such findings promoted the hypothesis that many smokers are self-medicating an underlying depressive vulnerability41;42, which has received varying degrees of support. In smokers with trait anhedonia or history of depression , nicotine use is related to increased positive mood43;44, while abstinence is associated with reduced attentional bias towards positive stimuli45. Similarly, smokers with a history of depression ascribe greater value to cigarettes relative to natural rewards46, which may hinder substitution of healthy rewards for cigarettes during cessation. However, there has been limited consideration of depression history in regards to the effects of withdrawal of nicotine on reward processes. Moreover, the high rate of relapse to smoking during withdrawal from nicotine47-49 may potentially arise from reward responsiveness deficits, with the resumption of nicotine use reversing such deficits.
In light of prior independent lines of evidence, we hypothesized that: 1) withdrawal from chronic nicotine exposure would be associated with blunted reward responsiveness (i.e., reduced response bias) in human smokers and rats; 2) withdrawal-related changes in reward responsiveness would be exacerbated in human smokers with a history of major depressive disorder; and 3) acute nicotine re-exposure after nicotine withdrawal would enhance reward responsiveness in rats.
Methods—humans
Participants
Heavy smokers (smoking >15 cigarettes per day and smoking for >5 years) not planning to quit permanently over the next month participated. Exclusion criteria included: <18 years old, current use of smoking cessation aids, and current or planned pregnancy. Ninety-three percent of ineligible candidates (N=314) did not meet cigarette use criteria or planned to quit cigarettes permanently over the next month. Eligible candidates were scheduled for a screening interview and study overview, read and signed an informed consent, and verified smoking status using an ecolyzer to measure expired carbon monoxide. All procedures were approved by the Human Research Protection Office.
Of the 99 individuals enrolled, 60 completed baseline and two test sessions (see below). The RB-PRT was added halfway through data collection for 37 subjects (see eMethods in Supplement for details). This sample of 37 had the following characteristics: 22.3±Standard Deviation (SD)=6.0 cigarettes smoked/day, 23.3±13.5 years smoked, 41.1±14.2 years old, 54% women, 57% with a lifetime history of major depression, and 89% with a high school education or higher.
Procedures and Assessments
i)Baseline Visit
Candidates meeting preliminary inclusion criteria were administered self-report questionnaires and a diagnostic interview - a modified Semi-Structured Assessment for the Genetics of Alcoholism50 with the smoking section modified from the Composite International Diagnostic Interview51, which included lifetime assessments of nicotine withdrawal and major depression32.
ii) Test Sessions (approximately 90 minutes)
During sessions separated by a median number of 7 days and counter-balanced across subjects, participants completed self-report questionnaires and were tested under: 1) ad libitum smoking; and 2) 24-hour nicotine-abstinence conditions. Smoking and abstinence were verified by self-report and a non-invasive breath test ecolyzer measurement of exhaled carbon monoxide (see eMethods in Supplement). The RB-PRT was administered to quantify reward responsiveness. Response bias (the main variable of interest; see Figure 1 for calculation details), discriminability (control variable), accuracy (i.e., correct responses/(correct + incorrect responses)) and reaction time (RT) for each stimulus type (i.e., rich/lean) were calculated. RT shorter than 150 ms or longer than 2500 ms were removed; participants with more than 10% of trials with outlying RTs were removed entirely (N=6), leaving 31 participants with valid data from both test sessions. The six subjects removed were similar in sample characteristics from the remaining 31 subjects (all p's >0.05). Of these 31 smokers, 55% (N=17) had a history of lifetime major depression. The sample was sufficiently remitted at baseline, reflected by the average Profile of Mood States Total Mood Disturbance Scale52 score (POMS TMDS) of participants without (9.9 ±23.1) and with (17.8 ±31.3) a history of depression being lower and within range of the average score published for normative non-psychiatric samples (17-19)53, respectively.
Statistical analyses
For response bias, mixed analysis of variance (ANOVA) with Nicotine Status (smoking, 24-hour abstinence) and Block (1,2,3; 100 trials/block) as repeated measures and History of Depression (present, absent) as the between-subjects factor were performed. Greenhouse-Geisser corrected estimates are reported.
Methods—rats
Subjects
Forty-six adult male Wistar rats (Charles River Laboratories, Raleigh, NC) were pair housed with food and water available ad libitum prior to behavioral training. All procedures were conducted in accordance with guidelines from the National Institutes of Health and the Association for the Assessment and Accreditation of Laboratory Animal Care, and were approved by the university's Institutional Animal Care and Use Committee.
Apparatus
Training and testing were conducted in operant chambers (Med Associates, St. Albans, VT) consisting of two metal retractable levers, a food receptacle located between the levers, and a speaker located above the food receptacle. Tones were generated using a multipurpose sound generator. All programs and data collection were controlled by a computer running MED-PC IV software30.
Procedure
Rats were trained on the RB-PRT and tested under baseline conditions (see Figure 1 and 30 for details). Rats were then surgically prepared with subcutaneous osmotic minipumps (Alzet Osmotic Pumps, Cupertino, CA) delivering either a 6.32 mg/kg/day (base) (−) nicotine hydrogen tartrate solution (Sigma, St. Louis, MO) or vehicle (sterile 0.9% saline) for 28 days.
Rats continued to train during drug administration with the parameters described in Figure 1. Before minipump removal, rats received increasingly ambiguous tones as stimuli while being equally reinforced for all correct responses. Twenty-four hours after minipump removal, rats were tested with the same tone and reinforcement parameters as during the baseline test session.
After the withdrawal test, rats were exposed to the training parameters for two weeks and tested in response to acute nicotine administration. Two days prior to the initial acute nicotine test, all rats received 0.125 mg/kg nicotine (base) subcutaneously after the training session to habituate to the subjective experience of acute nicotine exposure. Rats then received either 0, 0.125, 0.25 or 0.5 mg/kg nicotine (base; 15 min pretreatment) in a within-subjects Latin-square design and reward responsiveness was assessed 2, 4, 6 and 8 weeks after the withdrawal test.
Statistical Analyses
Data were cumulated and analyzed across blocks 1 (trials 1-33), 2 (trials 34-67), and 3 (trials 68-100). Rats were excluded due to insufficient accuracy during discrimination training (i.e.,<70%; N=5) and complications with minipumps (one nicotine, one saline). Thus, data from 39 rats were available for the withdrawal test. Rats with <30% accuracy for either stimulus during testing were excluded because insufficient responding prevents the differential (i.e., 3:1) reward distribution, as in the human task. Five chronic saline-treated rats and two chronic nicotine-treated rats were excluded from the acute nicotine test. Response bias was calculated as described above for humans. For the withdrawal test, response bias was analyzed with a two-way mixed analysis of covariance (ANCOVA) with Chronic Drug Treatment (between-subjects) andBlock (within-subjects) as factors. For the acute nicotine tests, Acute Nicotine Dose was included as a within-subjects factor. Inherent side biases unrelated to the differential reinforcement schedule during testing were controlled as a covariate, defined as the change in response bias from blocks 1 to 3 during the pre-test training session.
For human and rat data analyses, significant main and interaction effects involving ANOVA factors (e.g., nicotine status, block, depression in smokers; acute nicotine dose in rats) were clarified using post hoc t-tests. The significance level was 0.05. Additional detail on samples and procedures for humans and rats are available in eMethods of the Supplement.
Results
Response bias (humans)
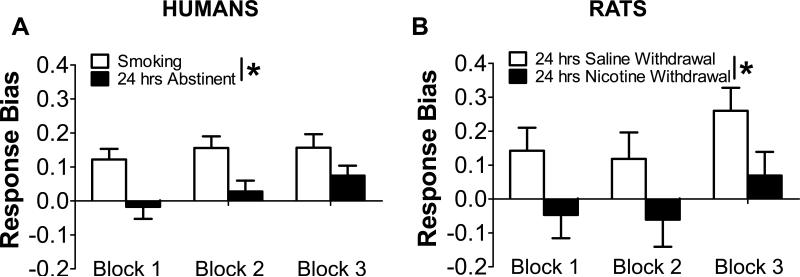
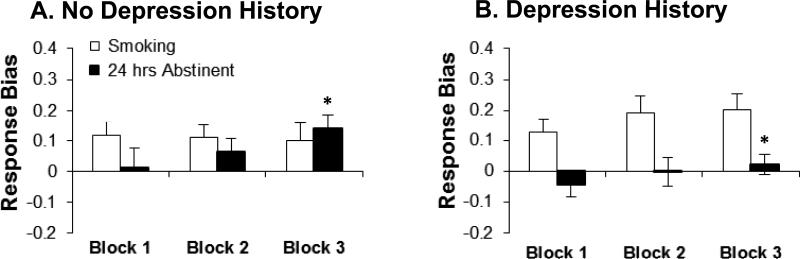
Among adult heavy smoking humans, a three-way ANOVA with Nicotine Status (ad libitum smoking, 24-hour abstinence), Block (1,2,3), and History of Depression (present, absent) as factors revealed that 24-hour nicotine abstinence was associated with a significant reduction in response bias [Nicotine Status: F(1,29)=6.61, p=0.02; partial Eta squared (η 2p)=0.19] (Figure 2A). No other effects emerged. Although the Nicotine Status x History of Depression interaction reached only a statistical trend (p=0.10; ηp2=0.09), a priori subsidiary analyses found that smokers without depression history exhibited significant increases in response bias (i.e., reward learning) across blocks during abstinence (p=0.03; ηp2=0.25; ad libitum: p=0.94; ηp2=0.01); smokers with a history of depression failed to show changes in response bias across blocks (abstinence: p=0.46; ηp2=0.05; ad libitum: p=0.45; ηp2=0.05). This group effect was detectable by block 3, whereby smokers with a history of depression had a smaller response bias during 24-hour abstinence than smokers without such history [t=2.06, p=0.048; ηp2=0.13; ad libitum: t=-1.30 p=0.21; ηp2=0.06] (Figure 3).
Figure 2. WITHDRAWAL OF NICOTINE IS ASSOCIATED WITH BLUNTED REWARD RESPONSIVENESS IN HUMANS (A) AND RATS (B).
(A) Human subjects (N=31) developed a response bias towards the more frequently rewarded (“rich”) stimulus when smoking at their usual rate. By contrast, 24-hour abstinence from chronic tobacco smoking significantly decreased response bias; (B) Control rats administered saline developed a response bias towards the more frequently rewarded (“rich”) stimulus. By contrast, withdrawal from chronic nicotine administration significantly decreased response bias; *p<0.05.
Figure 3. NICOTINE ABSTINENCE AND REWARD RESPONSIVENESS IN HUMANS WITHOUT (N = 14, A) AND WITH (N = 17, B) A HISTORY OF DEPRESSION.
24-hour abstinence from chronic tobacco smoking was associated with decreased response bias in Block 3 for smokers with a history of depression relative to smokers without a history of depression (*p<0.05). Moreover, unlike smokers without a history of depression (A), those with such history failed to develop a response bias towards the more frequently rewarded stimulus (B).
Response bias (rats)
A two-way ANCOVA with Chronic Drug Treatment (nicotine, saline) and Block (1,2,3) as factors and inherent bias as a covariate revealed that withdrawal from chronic nicotine administration significantly reduced response bias relative to saline treatment [Chronic Drug Treatment: F(1,36)=4.18; p=0.048; ηp2=0.10] (Figure 2B). There was also a main effect of Block [F(2,72)=6.05; p=0.004; ηp2=0.14], due to significantly higher response bias in Block 3 relative to Block 1 [t(38)=-2.49, p=0.02] and Block 2 [t(38)=-3.64, p<0.001], indicating that the differential reinforcement schedule was effective.
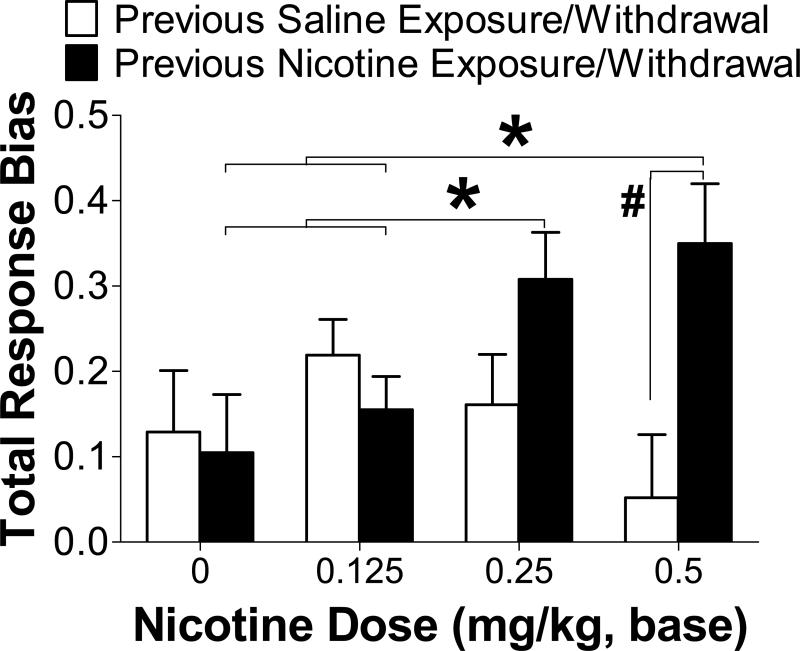
After withdrawal from chronic nicotine or saline administration, a three-way ANCOVA with Chronic Drug Treatment, Block and Acute Nicotine Dose (0, 0.125, 0.25, 0.5 mg/kg nicotine) as factors revealed that acute nicotine treatment differentially altered response bias depending on previous nicotine experience [Chronic Drug Treatment × Acute Nicotine Dose interaction: F(3,87)=4.44; p=0.006; ηp2=0.13]. Specifically, post hoc analyses revealed greater response biases in rats previously treated with chronic nicotine after 0.25 (p=0.079) and 0.5 (p=0.007) mg/kg acute nicotine treatment compared to previously saline-treated rats administered the same doses and compared to chronic nicotine-treated rats administered 0 and 0.125 mg/kg nicotine (all p-values <0.05) (Figure 4). There was also a main effect of Block [F(2,58)=15.10; p<0.01; ηp2=0.34], due to significantly increased response bias from Block 1 to Block 2 to Block 3 (all p-values <0.05).
Figure 4. ACUTE NICOTINE-INDUCED CHANGES IN REWARD RESPONSIVENESS IN RATS PREVIOUSLY EXPOSED TO CHRONIC NICOTINE (N = 17) OR SALINE (N = 15).
Acute nicotine re-exposure in rats previously treated with chronic nicotine significantly potentiated response bias compared to acute saline exposure and compared to acute nicotine exposure in rats previously treated with chronic saline. Moreover, acute nicotine treatment did not affect reward responsiveness in previously nicotine-naïve rats. * Different from chronic nicotine-treated rats administered 0 and 0.125 mg/kg acute nicotine (p<0.05); # Different from chronic saline-treated rats administered the same acute nicotine dose (p<0.01).
Secondary analyses of discriminability, accuracy and reaction time for humans and rats are detailed in eResults of the Supplement.
Discussion
Capitalizing on a task rooted in signal detection theory previously shown to be sensitive to detecting reward responsiveness deficits in depression and other mood disorders27-29;31;54, the current results provide converging evidence across human smokers and rats chronically administered nicotine that withdrawal from nicotine is associated with reduced reward responsiveness. This compromised ability to modulate behavior as a function of rewarding experiences after withdrawal from chronic nicotine exposure, an effect that was exacerbated in humans with a history of major depression, was reversed with acute nicotine re-exposure in rats. The results suggest that restoring or potentiating responsiveness to natural rewards through nicotine re-exposure may contribute to relapse to tobacco smoking. Furthermore, these findings may help rectify previous inconsistent findings across species19-21,22-24,14;16;25;26, which used heterogeneous measures to assess reward processing during withdrawal from nicotine, generated mixed results, and thus yielded limited translational opportunities. Our findings highlight the value of using a conceptually identical reward task across species to objectively measure withdrawal-related decrements in reward responsiveness and provide a strong translational framework for identifying novel treatment strategies for smoking cessation.
Increased depressive symptoms32 and subjective stress levels55 during withdrawal from chronic nicotine may accompany reward responsiveness deficits, and resuming nicotine use may act to reverse these deficits. Fitting this hypothesis, in the current study, acute nicotine exposure potentiated reward responsiveness in rats previously treated with chronic nicotine without affecting reward responsiveness in nicotine-naïve rats. Interestingly, these acute nicotine effects were observed 2-to-8 weeks after initiation of withdrawal from chronic nicotine. Moreover, human subjects not currently smoking, but some with a history of smoking, showed similar acute nicotine-induced enhancement of reward responsiveness in a previous study18. It is unclear, however, whether subjects without a history of smoking, who were included in that overall analysis18, displayed similar increases in reward responsiveness. By contrast, somatic signs of withdrawal in rats peak within the first 24 hours of and dissipate three days after termination of chronic nicotine exposure19. These results raise the possibility that enhanced reward responsiveness that is produced by acute nicotine re-exposure long after initiation of abstinence, when other symptoms of withdrawal have dissipated, may contribute to relapse that occurs during protracted abstinence. Subsequent studies should consider the extent to which these results relate to putative therapeutic effects of smoking cessation treatment.
We also found suggestive evidence that nicotine abstinence resulted in an exacerbated decrease in reward responsiveness for smokers with a history of depression relative to smokers without such history. This finding extends prior reports that trait anhedonia is associated with reduced attentional bias towards positive stimuli during nicotine abstinence45 and increased risk for relapse to smoking39;40. While there is debate regarding the impact of negative affect on relapse, deficits in reward responsiveness observed here appear to be unrelated to negative affect. Consistent with the literature6, our human sample exhibited increased negative affect after 24 hours of withdrawal from nicotine, as measured by increases in the POMS TMDS52 [F(1,28)=26.2; p<0.001]. Interestingly, however, changes in TMDS were not correlated with changes in reward responsiveness (r=−0.09), suggesting that reward responsiveness deficits observed during withdrawal of nicotine may be distinct from the nicotine withdrawal syndrome characterized by negative mood symptoms6;32.
Blunted reward responsiveness is likely not associated with decrements in discriminability (observed in rats) or cognitive processes such as attention, as accuracy for the lean stimulus was similar during withdrawal and smoking/control conditions in humans and rats, respectively. Furthermore, only accuracy for the rich stimulus was disrupted during withdrawal in both species, suggesting that deficits in responding during the task were selective for the rich stimulus rather than globally for both stimuli, reflecting decreased reward responsiveness and unimpaired cognitive processing (see eResults of the Supplement). Interestingly, although the average response bias during abstinence/withdrawal was lower than levels observed during smoking/saline treatment, response bias slightly increased across blocks. This pattern of results may suggest that reinforcement learning was occurring during withdrawal of nicotine, but at a slower rate than smoking/saline conditions. Indeed, reinforcement learning (i.e., changing behavior based on prior reinforcement) is a key component of reward responsiveness. Future work may further examine how blunted reward responsiveness interrelates with additional cognitive processes across species.
Due to the nature of human and rodent research, it remains challenging to implement completely homologous cross-species procedures. One strength of the RB-PRT used here is its complete objectivity that allows for assessment across species and comparable statistical analyses and data interpretation. The experimental manipulations, while analogous, have some noted dissimilarities. For example, humans intermittently smoke cigarettes throughout the day while ingesting numerous chemicals in addition to nicotine, whereas rats were administered only nicotine continuously via osmotic pumps. While not identical, continuous nicotine infusion is preferred over repeated, intermittent nicotine administration because it more effectively upregulates neuronal nicotinic receptors56, as observed in human heavy smokers57. Moreover, strictly controlling for administration of nicotine in the present rat study suggests that indeed nicotine, and not necessarily other components of cigarette smoke, contribute to deficits in reward responsiveness observed in humans during withdrawal. Lastly, spontaneous withdrawal signs have not been observed after chronic exposure to tobacco smoke vapor in rats58, whereas signs of withdrawal have been well characterized using the same continuous nicotine exposure procedure as presented here59. Thus, the continuous nicotine infusion procedure used in rats is the most appropriate method for replicating the effects of spontaneous withdrawal of chronic nicotine in heavy smoking humans. The extent to which our findings generalize to lighter smokers should be examined in future investigations.
In summary, using an analogous reward responsiveness task in humans and rats, we found that reward responsiveness was significantly reduced after withdrawal from nicotine. Our strong phenotypic alignment is directed at circumventing the typical translational “bottleneck”, which continues to impede progress in psychiatric treatments60;61. The fact that humans and rats showed similar deficits in reward responsiveness using conceptually and procedurally identical versions of the RB-PRT reflects the strong convergent validity of this objective measure. Importantly, our cross-species behavioral paradigm developed and validated in this study may facilitate the identification of novel neurobiological substrates mediating nicotine withdrawal and the testing of new smoking cessation treatments.
Supplementary Material
Acknowledgments
Funding Support: The study was supported by NIH grants: DA019951 (M.L.P.), MH078979 (D.A.P. and A.M.), DA011946 (A.M.), DA012854 (P.A.F.M.), and AA017688 (A.C.H). Funding sources played no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Author Contributions:
The study was designed by M.L.P., A.D., S.S., A.M., and D.A.P. The data were acquired by M.L.P., A.D., A.M., and D.A.P., who had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses. The analyses were conducted and/or interpreted by M.L.P., A.D., M.S.D., P.A.F.M., A.C.H., S.S., A.M., and D.A.P. The manuscript was drafted by M.L.P., A.D., A.M., and D.A.P. All authors contributed to critical revisions and approval of the final version of the manuscript. Funding was obtained by M.L.P., D.A.P., A.M., P.A.F.M., and A.C.H. Supervision of the studies was done by M.L.P., A.D., D.A.P., and A.M.
Disclosures:
Over the last three years, Dr. Pizzagalli has received consulting fees from Advanced Neuro Technology, AstraZeneca, Ono Pharma USA, Pfizer, Servier, and Shire for studies unrelated to this project. Over the last three years, Dr. Markou has received consulting fees from AbbVie and contract research support from Bristol-Myers-Squibb, Forest Laboratories and Astra-Zeneca for studies unrelated to this project. Dr. Markou has a patent on the use of metabotropic glutamate receptor compounds for the treatment of drug dependence. Over the last three years, Dr. Shiffman has received consulting fees from GlaxoSmithKline and has been a partner in a venture to develop nicotine medications.
References
- 1.Centers for Disease Control (CDC). Cigarette Smoking-Attributable Morbidity-United Staes, 2000. Morbidity and Mortality Weekly. 2003;52:842–844. [PubMed] [Google Scholar]
- 2.US Burden of Disease Collaborators The State of US Health, 1990-2010, Burden of Diseases, Injuries, and Risk Factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferguson SG, Shiffman S, Gwaltney CJ. Does reducing withdrawal severity mediate nicotine patch efficacy? A randomized clinical trial. J Consult Clin Psychol. 2006;74:1153–1161. doi: 10.1037/0022-006X.74.6.1153. [DOI] [PubMed] [Google Scholar]
- 4.Pergadia ML, Agrawal A, Heath AC, Martin NG, Bucholz KK, Madden PA. Nicotine withdrawal symptoms in adolescent and adult twins. Twin Res Hum Genet. 2010;13:359–369. doi: 10.1375/twin.13.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings KM, Jaen CR, Giovino G. Circumstances surrounding relapse in a group of recent exsmokers. Prev Med. 1985;14:195–202. doi: 10.1016/0091-7435(85)90035-0. [DOI] [PubMed] [Google Scholar]
- 6.Hughes JR. Clinical significance of tobacco withdrawal. Nicotine Tob Res. 2006;8:153–156. doi: 10.1080/14622200500494856. [DOI] [PubMed] [Google Scholar]
- 7.Piasecki TM, Fiore MC, Baker TB. Profiles in discouragement: two studies of variability in the time course of smoking withdrawal symptoms. J Abnorm Psychol. 1998;107:238–251. doi: 10.1037//0021-843x.107.2.238. [DOI] [PubMed] [Google Scholar]
- 8.Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: II. Improved tests of withdrawal-relapse relations. J Abnorm Psychol. 2003;112:14–27. [PubMed] [Google Scholar]
- 9.Donny EC, Chaudhri N, Caggiula AR, et al. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- 10.Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asgaard GL, Gilbert DG, Malpass D, Sugai C, Dillon A. Nicotine primes attention to competing affective stimuli in the context of salient alternatives. Exp Clin Psychopharmacol. 2010;18:51–60. doi: 10.1037/a0018516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert DG, Carlson JM, Riise H, Rabinovich NE, Sugai C, Froeliger B. Effects of nicotine and depressive traits on affective priming of lateralized emotional word identification. Exp Clin Psychopharmacol. 2008;16:293–300. doi: 10.1037/a0012871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert DG, Rabinovich NE, Malpass D, et al. Effects of nicotine on affect are moderated by stressor proximity and frequency, positive alternatives, and smoker status. Nicotine Tob Res. 2008;10:1171–1183. doi: 10.1080/14622200802163092. [DOI] [PubMed] [Google Scholar]
- 14.al-Adawi S, Powell J. The influence of smoking on reward responsiveness and cognitive functions: a natural experiment. Addiction. 1997;92:1773–1782. [PubMed] [Google Scholar]
- 15.Dawkins L, Powell J. Effects of nicotine and alcohol on affective responses to emotionally toned film clips. Psychopharmacology (Berl) 2011;216:197–205. doi: 10.1007/s00213-011-2197-4. [DOI] [PubMed] [Google Scholar]
- 16.Powell J, Dawkins L, Davis RE. Smoking, reward responsiveness, and response inhibition: tests of an incentive motivational model. Biol Psychiatry. 2002;51:151–163. doi: 10.1016/s0006-3223(01)01208-2. [DOI] [PubMed] [Google Scholar]
- 17.Powell JH, Pickering AD, Dawkins L, West R, Powell JF. Cognitive and psychological correlates of smoking abstinence, and predictors of successful cessation. Addict Behav. 2004;29:1407–1426. doi: 10.1016/j.addbeh.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Barr RS, Pizzagalli DA, Culhane MA, Goff DC, Evins AE. A single dose of nicotine enhances reward responsiveness in nonsmokers: implications for development of dependence. Biol Psychiatry. 2008;63:1061–1065. doi: 10.1016/j.biopsych.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- 20.Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther. 2000;292:1053–1064. [PubMed] [Google Scholar]
- 21.Kenny PJ, Gasparini F, Markou A. Group II metabotropic and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. J Pharmacol Exp Ther. 2003;306:1068–1076. doi: 10.1124/jpet.103.052027. [DOI] [PubMed] [Google Scholar]
- 22.Der-Avakian A, Markou A. Withdrawal from chronic exposure to amphetamine, but not nicotine, leads to an immediate and enduring deficit in motivated behavior without affecting social interaction in rats. Behav Pharmacol. 2010;21:359–368. doi: 10.1097/FBP.0b013e32833c7cc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lesage MG, Burroughs D, Pentel PR. Effects of nicotine withdrawal on performance under a progressive-ratio schedule of sucrose pellet delivery in rats. Pharmacol Biochem Behav. 2006;83:585–591. doi: 10.1016/j.pbb.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 24.Weaver MT, Sweitzer M, Coddington S, et al. Precipitated Withdrawal From Nicotine Reduces Reinforcing Effects of a Visual Stimulus for Rats. Nicotine Tob Res. 2012;14:824–832. doi: 10.1093/ntr/ntr293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawkins L, Powell JH, Pickering A, Powell J, West R. Patterns of change in withdrawal symptoms, desire to smoke, reward motivation and response inhibition across 3 months of smoking abstinence. Addiction. 2009;104:850–858. doi: 10.1111/j.1360-0443.2009.02522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalamboka N, Remington B, Glautier S. Nicotine withdrawal and reward responsivity in a card-sorting task. Psychopharmacology (Berl) 2009;204:155–163. doi: 10.1007/s00213-008-1449-4. [DOI] [PubMed] [Google Scholar]
- 27.Pizzagalli DA, Jahn AL, O'Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pizzagalli DA, Bogdan R, Ratner KG, Jahn AL. Increased perceived stress is associated with blunted hedonic capacity: potential implications for depression research. Behav Res Ther. 2007;45:2742–2753. doi: 10.1016/j.brat.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Der-Avakian A, D'Souza M, Pizzagalli D, Markou A. Assessment of Reward Responsiveness in the Response Bias Probabilistic Reward Task in Rats: Implications for Cross-Species Translational Research. Translational Psychiatry. 2013 doi: 10.1038/tp.2013.74. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vrieze E, Pizzagalli DA, Demyttenaere K, et al. Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry. 2013;73:639–645. doi: 10.1016/j.biopsych.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th edition, Revised ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 33.Glassman AH, Stetner F, Walsh BT, et al. Heavy smokers, smoking cessation, and clonidine. Results of a double-blind, randomized trial. JAMA. 1988;259:2863–2866. [PubMed] [Google Scholar]
- 34.Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- 35.Covey LS, Glassman AH, Stetner F. Depression and depressive symptoms in smoking cessation. Compr Psychiatry. 1990;31:350–354. doi: 10.1016/0010-440x(90)90042-q. [DOI] [PubMed] [Google Scholar]
- 36.Madden PA, Bucholz KK, Dinwiddie SH, et al. Nicotine withdrawal in women. Addiction. 1997;92:889–902. [PubMed] [Google Scholar]
- 37.Xian H, Scherrer JF, Madden PA, et al. Latent class typology of nicotine withdrawal: genetic contributions and association with failed smoking cessation and psychiatric disorders. Psychol Med. 2005;35:409–419. doi: 10.1017/s0033291704003289. [DOI] [PubMed] [Google Scholar]
- 38.Japuntich SJ, Smith SS, Jorenby DE, Piper ME, Fiore MC, Baker TB. Depression predicts smoking early but not late in a quit attempt. Nicotine Tob Res. 2007;9:677–686. doi: 10.1080/14622200701365301. [DOI] [PubMed] [Google Scholar]
- 39.Leventhal AM, Waters AJ, Kahler CW, Ray LA, Sussman S. Relations between anhedonia and smoking motivation. Nicotine Tob Res. 2009;11:1047–1054. doi: 10.1093/ntr/ntp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cook J, Spring B, McChargue D, Doran N. Effects of anhedonia on days to relapse among smokers with a history of depression: a brief report. Nicotine Tob Res. 2010;12:978–982. doi: 10.1093/ntr/ntq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- 42.Gilbert DG. Smoking: individual differences, psychopathology, and emotion. Taylor & Francis; Washington D.C.: 1995. [Google Scholar]
- 43.Cook JW, Spring B, McChargue D. Influence of nicotine on positive affect in anhedonic smokers. Psychopharmacology (Berl) 2007;192:87–95. doi: 10.1007/s00213-006-0688-5. [DOI] [PubMed] [Google Scholar]
- 44.Spring B, Cook JW, Appelhans B, et al. Nicotine effects on affective response in depression-prone smokers. Psychopharmacology (Berl) 2008;196:461–471. doi: 10.1007/s00213-007-0977-7. [DOI] [PubMed] [Google Scholar]
- 45.Leventhal AM, Munafo M, Tidey JW, et al. Anhedonia predicts altered processing of happy faces in abstinent cigarette smokers. Psychopharmacology (Berl) 2012;222:343–351. doi: 10.1007/s00213-012-2649-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spring B, Pingitore R, McChargue DE. Reward value of cigarette smoking for comparably heavy smoking schizophrenic, depressed, and nonpatient smokers. Am J Psychiatry. 2003;160:316–322. doi: 10.1176/appi.ajp.160.2.316. [DOI] [PubMed] [Google Scholar]
- 47.Shiffman S, Ferguson SG, Gwaltney CJ, Balabanis MH, Shadel WG. Reduction of abstinence-induced withdrawal and craving using high-dose nicotine replacement therapy. Psychopharmacology (Berl) 2006;184:637–644. doi: 10.1007/s00213-005-0184-3. [DOI] [PubMed] [Google Scholar]
- 48.Westman EC, Behm FM, Simel DL, Rose JE. Smoking behavior on the first day of a quit attempt predicts long-term abstinence. Arch Intern Med. 1997;157:335–340. [PubMed] [Google Scholar]
- 49.Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 50.Bucholz KK, Cadoret R, Cloninger CR, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- 51.Cottler LB, Robins LN, Grant BF, et al. The CIDI-core substance abuse and dependence questions: cross-cultural and nosological issues. The WHO/ADAMHA Field Trial. Br J Psychiatry. 1991;159:653–658. doi: 10.1192/bjp.159.5.653. [DOI] [PubMed] [Google Scholar]
- 52.McNair, Lorr M, Droppelman LF. Manual for the profile of mood states. Education and Industrial Testing Service; San Diego, CA: 1971. [Google Scholar]
- 53.Nyenhuis DL, Yamamoto C, Luchetta T, Terrien A, Parmentier A. Adult and geriatric normative data and validation of the profile of mood states. J Clin Psychol. 1999;55:79–86. doi: 10.1002/(sici)1097-4679(199901)55:1<79::aid-jclp8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 54.Pizzagalli DA, Goetz E, Ostacher M, Iosifescu DV, Perlis RH. Euthymic patients with bipolar disorder show decreased reward learning in a probabilistic reward task. Biol Psychiatry. 2008;64:162–168. doi: 10.1016/j.biopsych.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parrott AC. Stress modulation over the day in cigarette smokers. Addiction. 1995;90:233–244. doi: 10.1046/j.1360-0443.1995.9022339.x. [DOI] [PubMed] [Google Scholar]
- 56.Ulrich YM, Hargreaves KM, Flores CM. A comparison of multiple injections versus continuous infusion of nicotine for producing up-regulation of neuronal [3H]-epibatidine binding sites. Neuropharmacology. 1997;36:1119–1125. doi: 10.1016/s0028-3908(97)00107-x. [DOI] [PubMed] [Google Scholar]
- 57.Benwell ME, Balfour DJ, Anderson JM. Evidence that tobacco smoking increases the density of (−)-[3H]nicotine binding sites in human brain. J Neurochem. 1988;50:1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- 58.Small E, Shah HP, Davenport JJ, et al. Tobacco smoke exposure induces nicotine dependence in rats. Psychopharmacology (Berl) 2010;208:143–158. doi: 10.1007/s00213-009-1716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Semenova S, Markou A. Clozapine treatment attenuated somatic and affective signs of nicotine and amphetamine withdrawal in subsets of rats exhibiting hyposensitivity to the initial effects of clozapine. Biol Psychiatry. 2003;54:1249–1264. doi: 10.1016/s0006-3223(03)00240-3. [DOI] [PubMed] [Google Scholar]
- 60.Markou A, Chiamulera C, Geyer MA, Tricklebank M, Steckler T. Removing obstacles in neuroscience drug discovery: the future path for animal models. Neuropsychopharmacology. 2009;34:74–89. doi: 10.1038/npp.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hyman SE, Fenton WS. Medicine. What are the right targets for psychopharmacology? Science. 2003;299:350–351. doi: 10.1126/science.1077141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.