Abstract
The P300 is a known ERP component assessing stimulus value, including the value of a monetary reward. In parallel, the incentive value of reinforcers relies on the PFC, a major cortical projection region of the mesocortical reward pathway. Here we show a significant positive correlation between P300 response to money (vs. no money) with PFC gray matter volume in the OFC, ACC, and dorsolateral and ventrolateral PFC in healthy control participants. In contrast, individuals with cocaine use disorders showed compromises in both P300 sensitivity to money and PFC gray matter volume in the ventrolateral PFC and OFC and their interdependence. These results document for the first time the importance of gray matter structural integrity of subregions of PFC to the reward-modulated P300 response.
INTRODUCTION
Decades of work have anatomically outlined the mesocortical dopamine “reward” pathway of the brain, with the nucleus accumbens and mesencephalic ventral tegmental area/substantia nigra at its center, responding to salient reinforcers including monetary reward. Within this pathway, the PFC is a major cortical projection region interfacing reward processing with higher-order cognitive and emotional functions (Haber & Knutson, 2010). In this context, the OFC has been proposed to play an important role in the evaluation of appetitive stimuli (Grabenhorst & Rolls, 2011; Rolls, 2004), whereas the ACC and dorsolateral PFC (DLPFC) have both been proposed to integrate cognitive and motivational information related to value, pleasure, and cost during reward-guided action selection (Grabenhorst & Rolls, 2011; Hornak et al., 2004; Hikosaka & Watanabe, 2000). Indeed, functional neuro-imaging studies have implicated PFC in the processing of the emotional and motivational properties of rewarding stimuli (including money; Elliott, Newman, Longe, & Deakin, 2003; Kringelbach, O’Doherty, Rolls, & Andrews, 2003; Breiter, Aharon, Kahneman, Dale, & Shizgal, 2001; O’Doherty, Kringelbach, Rolls, Hornak, & Andrews, 2001), and its compromised responding to these stimuli in psycho-pathologies affecting motivation and self-control (e.g., drug addiction) has also been reported (Goldstein et al., 2007).
A reliable electrophysiological measure of sensitivity to reward (including money) in healthy participants is the P300, a positive ERP that reaches its maximum amplitude between 250 and 600 msec following a target stimulus (Goldstein et al., 2006, 2008; Hajcak, Holroyd, Moser, & Simons, 2005; Sato et al., 2005; Yeung & Sanfey, 2004). The P300 has a general role in the processing of salient, motivationally significant, stimuli (Polich, 2007; Begleiter, Porjesz, Chou, & Aunon, 1983), further invoking cognitive functions such as selective attention and the updating of working memory (Donchin, Miller, & Farwell, 1986), all at least in part mediated by PFC (Rossi, Pessoa, Desimone, & Ungerleider, 2009; Taylor et al., 2004). In paradigms specifically targeting monetary reward sensitivity, these cognitive functions (attention, working memory) are modulated by the associated monetary reward magnitude, as reflected in graded P300 responses to varying amounts of money across a range of experimental paradigms in healthy participants (Bellebaum, Polezzi, & Daum, 2010; De Pascalis, Varriale, & D’Antuono, 2010; Wu & Zhou, 2009; Bellebaum & Daum, 2008; Goldstein et al., 2006, 2008; Hajcak et al., 2005; Yeung & Sanfey, 2004).
In line with this general role in attention, working memory, and motivation and the widespread network of regions underlying each of these processes, a norepinephrine-induced phasic enhancement of neural activity in the locus coeruleus has been suggested to underlie the P300 generation (including the P3b, associated with familiar, non-distractor, task-relevant stimuli; Nieuwenhuis, De Geus, & Aston-Jones, 2011; Nieuwenhuis, Aston-Jones, & Cohen, 2005). Given that the most prominent descending cortical projections to the locus coeruleus come from the OFC and ACC (Aston-Jones & Cohen, 2005b) and that adaptive gain in reward-related activity in these regions could in turn be modulated by locus coeruleus norepinephrine phasic release (Aston-Jones & Cohen, 2005a), it is conceivable that both the functional and structural integrity of these PFC regions may be vital especially to the modulation of the scalp-recorded EEG and specifically the P300 by reward contingencies. However, to date, a direct link between the structural integrity of these PFC subregions and adaptive modulation of the P300 response to reward has not been investigated.
Our objective in this study was therefore to evaluate whether the neural mechanisms indexed by the P300, as modulated by the preparation of a reward-contingent response, were associated with PFC gray matter (GM) volume in a healthy participant group. Here we hypothesized that the reward-sensitive P300 responses will be positively correlated with PFC GM volume. Demographically matched individuals with cocaine use disorders (CUD) were also included for comparison and to facilitate better understanding of the implications of such a relationship to a pathology known to impact both reward processing (e.g., showing compromised sensitivity to monetary gradients in PFC [Goldstein et al., 2007] and the P300 [Goldstein et al., 2008]) and PFC GM volume (Tanabe et al., 2009; Matochik, London, Eldreth, Cadet, & Bolla, 2003; Franklin et al., 2002; Liu, Matochik, Cadet, & London, 1998).
METHODS
Participants
Full written informed consent was obtained from 39 participants (17 controls [7 women] and 22 CUD [4 women]) in accordance with the local institutional review board. Of these 39 participants, 15 participants (7 controls and 8 CUD) were part of a cohort of 36 participants included in our previous report (Goldstein et al., 2008). Attesting to the novelty of the current study, the prior report did not incorporate MRI for PFC morphometric measures and the main ERP analysis (PCA as described below) is also reported here for the first time. Participants received physical, neurological, and psychiatric examinations, including a clinical interview for DSM-IV Axis I Disorders (research version; Ventura, Liberman, Green, Shaner, & Mintz, 1998) before participation. Exclusion criteria were (i) history of head trauma with loss of consciousness (>30 min) or other neurological disorders; (ii) abnormal vital signs at time of screening and history of major medical conditions, such as cardiovascular, endocrinological, oncological or autoimmune diseases; (iii) history of a major psychiatric disorder (other than cocaine dependence for the CUD group and/or nicotine dependence for both groups; note that participants in the control group were also excluded for alcohol related diagnoses); (iv) more than minimal levels of self-reported state depression (Beck depression inventory score > 15); (v) history of gambling as assessed with the South Oaks Gambling Questionnaire (Lesieur & Blume, 1987; cutoff score > 5); (vi) urine positive (Biopsych, Califon, NJ) for psychoactive drugs or their metabolites (phencyclidine, benzodiazepines, amphetamines, cannabis, opiates, barbiturates, and inhalants) on any study day, except for cocaine in the CUD group; and (vii) contraindications to the MRI environment (e.g., metal in the body or claustrophobia).
All CUD reported cocaine use within the last 30 days, with at least 1 year of cocaine use, and met DSM-IV criteria for current cocaine dependence (n = 18) or abuse (n = 4; all meeting criteria for cocaine dependence in remission). Participants were not seeking treatment at the time of the study. Urine was positive for cocaine in 13 of the 22 CUD; urine was negative for all other drugs in all other participants. Urine positive and negative CUD differed only in duration of current abstinence (mean days abstinent for CUD drug positive: 2.23 ± 1.36 days; CUD drug negative: 7.78 ± 6.26 days, t8.53 = −2.62, p = .03). There were no significant differences noted between urine positive and negative CUD in any of the other inspected variables including clinical severity (e.g., withdrawal symptoms, craving, severity of dependence), reported state depression scores, or alcohol and nicotine use (all ps > .12).
The CUD and control groups did not significantly differ in any demographic variables including age, race, gender, socioeconomic status, years of education, and on measures of general intellectual functioning (Table 1). Although we excluded participants with more than minimal levels of self-reported state depression scores, the groups differed in this measure (p = .03; note, however, that consistent with inclusion/exclusion criteria, all scores were within the mild range). Years of lifetime alcohol use (p = .022) and history of cigarette smoking (p = .004) also differed between the groups. To control for these three potential confounds, correlations were conducted to inspect their influence on our dependent measures. If significant across all study participants, these three variables were entered as separate covariates in the relevant ANOVA.
Table 1.
Demographic and Drug Use Variables for Healthy Controls and Individuals with CUDs
| Test | Control, n = 17 | CUD, n =22 | |
|---|---|---|---|
| Sex: male/female | χ22 = 2.5 | 10/7 | 18/4 |
| Race: Black/Other | χ22 = 2.6 | 9/8 | 17/5 |
| Laterality quotient | Z = −1.1 | 0.96 ± 0.07 | 0.92 ± 0.09 |
| Age (years) | t = 1.3 | 40.3 ± 6.7 | 42.9 ± 6.2 |
| Education (years) | t = −1.4 | 14.1 ± 2.1 | 13.2 ± 1.9 |
| Verbal IQ: WRAT-3 Reading | t = −1.6 | 98.8 ± 10.4 | 92.4 ± 13.3 |
| Nonverbal IQ: WASI-Matrix Reasoning Scale | t = −0.5 | 10.8 ± 2.6 | 10.3 ± 3.2 |
| Depression: Beck Depression Inventory II | Z = −2.3* | 2.0 ± 2.6 | 4.2 ± 3.1 |
| Socioeconomic status: Hollingshead Index | Z = −1.4 | 35.1 ± 15.5 | 27.9 ± 11.4 |
| Cigarette smokers (current or past/nonsmokers) | χ22 = 11.1** | 4/13 | 17/5 |
| Cigarette use (lifetime, years; current or past smokers) | t = 0.7 | 17.2 ± 11.3 | 20.6 ± 9.0 |
| Daily cigarettes (current smokers: n = 3/16) | t = 0.2 | 10.0 ± 7.0 | 10.7 ± 6.1 |
| Age of onset of cocaine use (years) | – | – | 24.1 ± 6.5 |
| Duration of current abstinence (days) | – | – | 4.5 ± 4.9 |
| Severity of Dependence Scalea | – | – | 6.1 ± 3.9 |
| Withdrawal symptoms: 18-item CSSA | – | – | 15.9 ± 9.1 |
| Cocaine craving: 5-item questionnaireb | – | – | 15.3 ± 10.6 |
| Cocaine use (past 30 days) | – | – | 15.1 ± 8.3 |
| Cocaine use (lifetime, years) | – | – | 17.8 ± 6.9 |
| Alcohol use (past 30 days; n = 7/22) | t = 2.4* | 1.9 ± 2.0 | 5.9 ± 6.9 |
| Alcohol use (lifetime, years; n = 7/22) | t = 1.0 | 9.9 ± 10.8 | 15.0 ± 12.0 |
χ2 tests were used for categorical variables; t tests (or the nonparametric Mann–Whitney U in cases of skewed distributions) for all other comparisons between the two groups. Values are frequencies or means ± standard deviation (SD). Race: Other = White/Hispanic/Asian; WRAT-3 = Wide Range Achievement Test (3rd edition); WASI = Wechsler Abbreviated Scale of Intelligence; CSSA = Cocaine Selective Severity Assessment Scale. The laterality quotient was computed with the Edinburgh Handedness Inventory (Oldfield, 1971); values closer to 1 indicate right hand dominance.
p < .05.
p < .01.
Missing data for one participant.
Missing data for two participants.
Task Paradigm
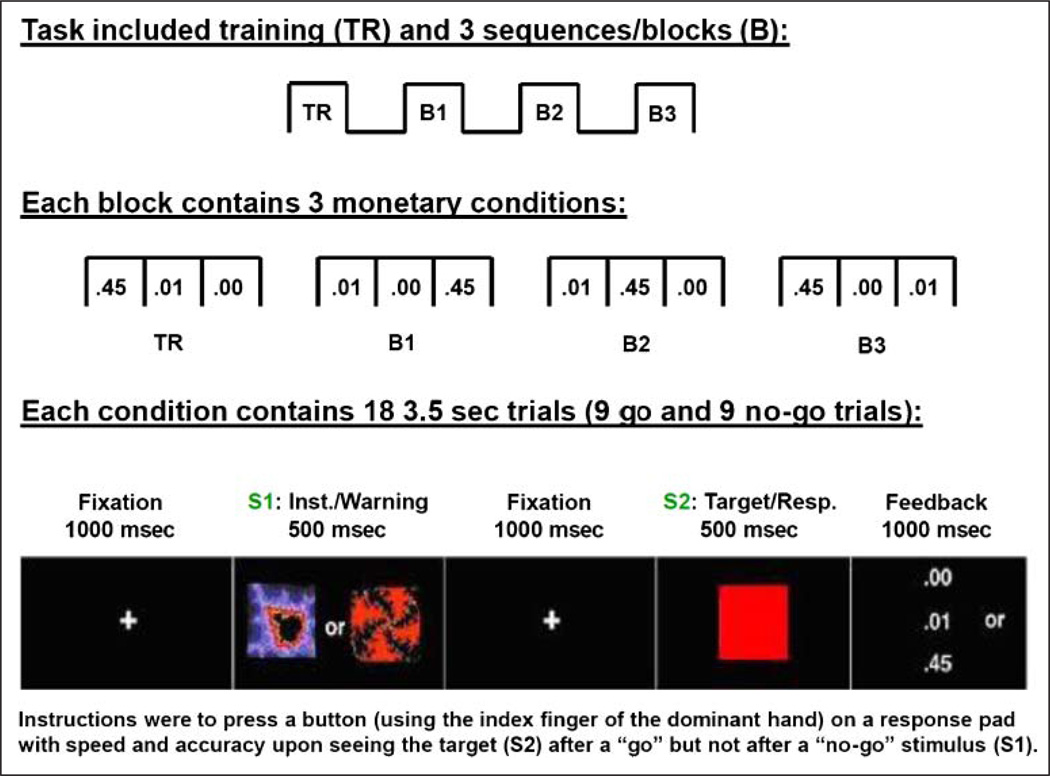
Participants completed a monetary reward paradigm as previously described (Goldstein et al., 2006, 2008). In brief, the task included three blocks, each consisting of three pseudorandomized monetary reward conditions: 45¢, l¢, or 0¢, separated by a 35-sec fixation cross to prevent carryover effects. During each monetary condition, there were 9 go and 9 no-go trials, pseudorandomized across all trials (no more than three consecutive trials of the same type), for a total of 54 go and 54 no-go trials per monetary condition. Two distinct abstract (fractal) images (Thut et al., 1997) served as the go and no-go warning stimuli (S1: this expectation stimulus elicited the P300; see Figure 1).
Figure 1.
Experimental paradigm for the monetary incentive task. Overall design and experimental blocks are depicted at the top; at each condition onset (conditions were separated by 35 sec), a 5-sec screen (not depicted) displayed the monetary reward to be earned (45¢, 1¢, 0¢) on the following set of trials. Together with the feedback delivered at the end of each trial, this 5-sec screen (similar in appearance to the feedback screen) guaranteed the participants were continuously aware of the reward contingencies. Inst. = instruction: Resp. = response.
Participants were instructed to press a button on a response pad with speed and accuracy upon seeing the target stimulus (S2; a red square) after a go S1 stimulus and to refrain from pressing the button upon seeing S2 after a no-go S1 stimulus. Feedback was presented immediately after responses following the offset of S2 by displaying the amount of money earned for each correct trial (45¢, 1¢, or 0¢) and an “X” for an incorrect response. A short training session preceded the task, where no money could be earned. At the end of the experiment, participants provided task ratings of interest, excitement, and frustration and were informed of their total gain. Compensation of up to $50 was given. There were no group differences in the amount of money earned (p = .25).
EEG Recordings and Data Reduction
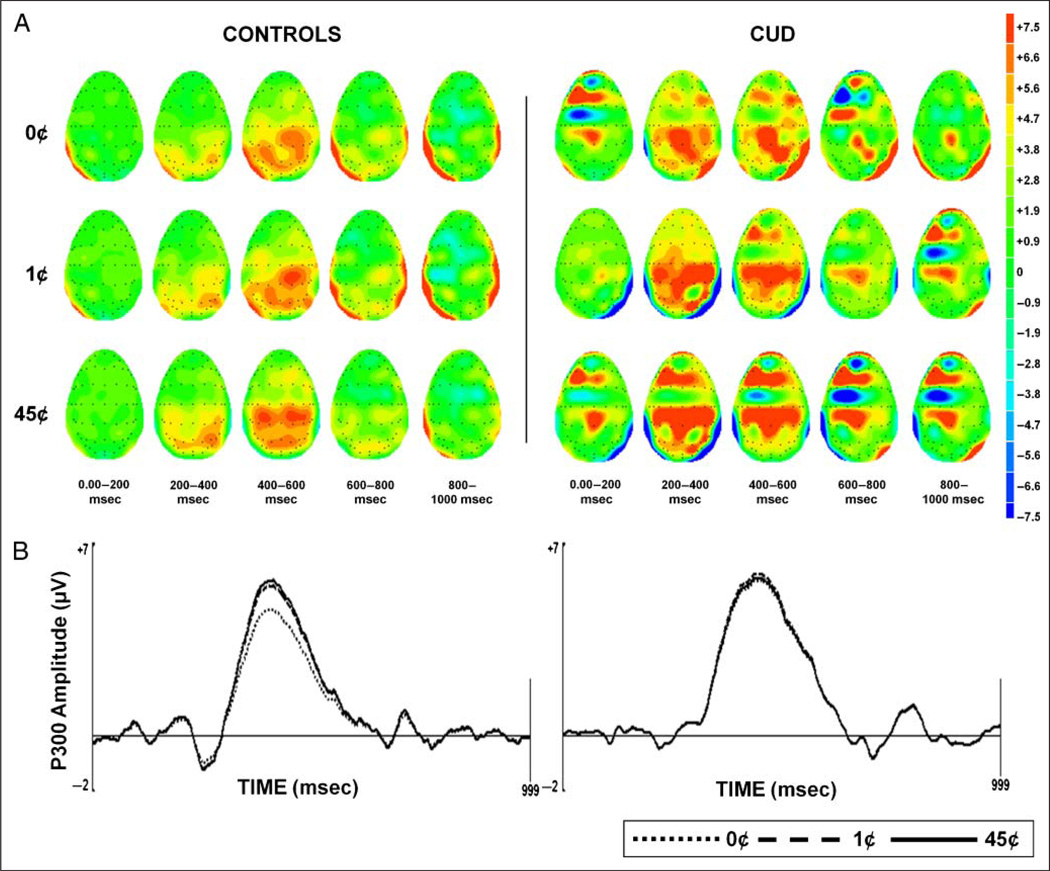
Continuous EEG (Neuroscan, Inc., Sterling, VA) recordings were obtained in all task conditions using a 64-channel electrode cap. The digitized, continuous EEG was rereferenced to electronically linked mastoid electrodes, transformed using a DC offset algorithm, and divided into epochs extending from 200 msec before the onset of S1 to 1800 msec after. A baseline correction algorithm was applied to the epoched EEG with respect to the 200 msec prestimulus baseline. All epochs were then subjected to a band pass filter (0.1–30 Hz). An artifact rejection procedure followed: (1) an amplitude threshold of ±75 µV was applied automatically to remove EOG and movement artifacts and (2) all trials that appeared contaminated by technical artifacts such as global drifts in EEG were manually rejected. A minimum of 30 epochs per task condition remained after artifact rejection. Grand averages were composed for each monetary condition during go trials on the task (Figure 2A). No-go trials were excluded from this study because of lack of significant reward effects in both our prior studies (Goldstein et al., 2006, 2008).
Figure 2.
(A) Scalp topographies for the control participants (left; n = 17) and individuals with CUDs (right; n = 22) reflecting 0–1000 msec after the onset of the expectation stimulus (S1) for each monetary reward condition (45¢, 1¢, and 0¢) during go trials on the task. (B) P300 factor isolated by PCA for the two study groups for each monetary reward condition at Pz.
Temporal ROIs in the averaged waveforms were then chosen quantitatively using temporal PCA (with the Matlab ERP PCA Toolbox, version 1.35). This temporal PCA captures variance across time and maximizes the separation of overlapping ERP components (Dien, Beal, & Berg, 2005). Kaiser normalization and Promax rotation (Dien, Khoe, & Mangun, 2007) were applied to the resulting factors. The first of these factors (explaining 17.9% of the total variance) was labeled the P300 waveform based on its time course (occurring 280–600 msec after S1; Picton, 1992; Pritchard, 1981) and scalp distribution (lowest amplitude in frontal electrodes, e.g., FZ, and highest in parietal electrodes, e.g., Pz; Sutton, Braren, Zubin, & John, 1965; Figure 2). Note that, similar to the current temporal PCA results, the Pz electrode has consistently been regarded as the source of the most pronounced P300 response to money (Goldstein et al., 2006, 2008; Hajcak et al., 2005; Yeung & Sanfey, 2004) as confirmed by quantitative methods (e.g., spatial PCA; Foti & Hajcak, 2009; Spencer, Dien, & Donchin, 2001). Therefore, similar to our past analyses (Goldstein et al., 2008), our current analyses were restricted to Pz. Consistent with previous studies using ERP PCA factor loadings, only the peak amplitude of the P300 factor was used for further analysis (Dien, 2010; Dien, Michelson, & Franklin, 2010; Foti & Hajcak, 2009). Specifically, all subsequent analyses used Pz P300 peak amplitude averages that were composed for the 45¢ (high reward), 1¢ (low reward), and 0¢ (nonreward) task conditions during go trials on our EEG paradigm.
Structural MRI
MRI acquisition was performed on a 4-T Varian/Siemens scanner, with a self-shielded whole-body SONATA gradient set. A T1-weighted anatomical MRI scan was obtained from all participants using a 3-D modified driven equilibrium Fourier transform (MDEFT) sequence (Lee et al., 1995; echo time/repetition time = 7/15 msec, spatial resolution = 0.94 × 0.94 × 1.00 mm3, axial orientation, 256 readout and 192 × 96 phase-encoding steps, scan time = 16 min). The MDEFT is particularly effective for tissue differentiation producing the most precise characterization of GM tissue compared with other sequences (Tardif, Collins, & Pike, 2009). A T2-weighted hyperecho scan was also obtained to rule out any gross morphological abnormalities. Structural scans were obtained from all participants within 1 week (1.79 ± 2.88 days) of completing the psycho-physiological recordings and clinical interviews, with no differences between the groups in this time gap (p > .41).
Image Preprocessing
Data preprocessing and analyses were performed using the SPM5 suite (Wellcome Department of Cognitive Neurology, London, United Kingdom; www.fil.ion.ucl.ac.uk/spm) running on Matlab version 7.0 (Mathworks, Inc., Natick, MA). Voxel-based morphometry, a whole-brain, fully automated, unbiased, and operator-independent MRI analysis technique commonly used to detect regionally specific differences in brain tissue composition using a voxel-wise comparison across participants (Ashburner & Friston, 2000), was conducted with the voxel-based morphometry toolbox (VBM5.1; C. Gaser, Department of Psychiatry, University of Jena, Jena, Germany; dbm.neuro.uni-jena.de/vbm/) implemented in SPM5, which combines spatial normalization, tissue segmentation, and bias correction. The MDEFT scans were first spatially normalized to a standard proportional stereotaxic space and segmented into GM, white matter, and cerebrospinal fluid tissue classes according to a priori tissue probability maps (Ashburner & Friston, 2000, 2005). A hidden Markov random field (Cuadra, Cammoun, Butz, Cuisenaire, & Thiran, 2005) was applied to minimize the noise level by “removing” isolated voxels of one tissue class that are unlikely to be members of that tissue class, thereby increasing the accuracy of the segmentation. Jacobian modulation was also applied to compensate for the effect of spatial normalization and to restore the original absolute GM volume in the segmented GM images. Total brain volume (TBV) was computed as the sum of the extracted total GM and white matter volumes for each participant, calculated as an adjustment factor to account for the effect of overall head size on regional GM volume. TBV did not differ between the groups (p > .21). Statistical analysis of regional GM volume was performed after smoothing the normalized and modulated segments with a 10 mm3 FWHM Gaussian kernel.
Statistical Analyses
ERP Analysis
Repeated measures ANOVAs with Money (45¢, 1¢, and 0¢) as the within-subject factor and Group (controls, CUD) as the between-subject factor were conducted for the task-related measures (accuracy, RT, and post-task ratings) and the PCA peak P300 amplitudes at Pz. Given our prior results where only controls but not CUD showed modulation of the P300 response to money (Goldstein et al., 2008), the current P300 analyses were conducted with an a priori focus on reward sensitivity in controls.
Morphometry Analyses
Whole-brain regression analyses were performed in SPM5 with the peak P300 amplitudes as seed variables regressed against participants’ regional GM volumes, across all participants, and separately in healthy controls and CUD. That is, the P300 component amplitudes (computed using PCA) in response to 45¢, 1¢, and 0¢ trials separately (Figure 2B) and the differentials 45¢ minus 0¢, 45¢ minus 1¢, and 1¢ minus 0¢ that served as the reward-modulated P300 seed variables were regressed—one at a time—against participants’ GM maps. Age and TBV were included as covariates in all analyses. Statistical maps were thresholded at p < .001 voxel-level uncorrected; clusters that contained at least 50 contiguous voxels meeting the p < .05 cluster-level family-wise error (FWE) correction for multiple comparisons using random field theory (Friston, Holmes, Poline, Price, & Frith, 1996) are reported. For completeness, we also report results of a whole-brain ANCOVA conducted to assess regional differences in PFC GM volume between the groups. Here we used an exploratory voxel-level threshold of p < .005 uncorrected and 50 voxels. Significance for this analysis is reported at p < .05 voxel-level FWE-corrected after small volume correction. Anatomical specificity for all analyses was corroborated with the Anatomy toolbox (Eickhoff et al., 2005), which provides probabilistic cytoarchitectonic neuroanatomical localization maps. Participants’ individual cluster volume measures were extracted using the EasyROI toolbox in Matlab (www.sbirc.ed.ac.uk/cyril/cp_download.html) and assessed for outliers.
RESULTS
Task Behavior and Ratings
The 3 (Money: 45¢, 1¢, and 0¢) × 2 (Group: control, CUD) ANOVA for RT revealed a main effect of Money (F2, 74 = 3.14, p = .049), such that RTs were significantly faster for 45¢ than 0¢ across all 39 participants (p = .02; all other effects on RT, p > .75). As expected (given our prior results and the low level of task difficulty), there were no significant Money, Group, or interaction effects on accuracy (F < 0.76, p > .39). In addition, all participants reported being fully engaged in the experiment, with significantly higher interest and excitement ratings and significantly lower frustration ratings reported for the high (45¢) than either of the two lower (0¢ or 1¢) money conditions (main effect of money for all three rating scales, F > 6.83, p < .01; all other effects, p > .17).
Modulation of the P300 Response to Money
The 3 (Money: 45¢, 1¢, and 0¢) × 2 (Group: controls and CUD) ANOVA for peak P300 amplitude revealed a main effect of Money (F2, 74 = 5.87, p = .004), such that P300 amplitudes were significantly higher for 45¢ than 0¢ across all 39 participants (p = .003; all other effects, p > .64). Following our a priori hypothesis (of P300 amplitude response to reward magnitude within controls but not CUD), we also tested this Money effect separately for each group. Compared with testing an interaction effect, this potentially less rigorous statistical approach indicated that, in controls only, peak P300 amplitudes increased linearly with money value (45¢ > 1¢ > 0¢, linear contrast for money, p = .003, maximum effect for 45¢ vs. 0¢); this effect was not significant in CUD (45¢ = 1¢ = 0¢, p = .13; quadric contrast, p = ns for both groups; Figure 2B). Similarity of these results to our previous findings (where we applied a different method to isolate the P300 component amplitudes; Goldstein et al., 2008) argues against a Type I error.
There were no significant differences between the groups in absolute P300 amplitudes for any of the three money conditions (45¢, 1¢, or 0¢) even in follow-up independent t tests (t < |0.54|, p > .59). Thus, only the expected graded sensitivity to monetary reward magnitude was compromised in CUD and not the ability to generate a P300 response on the task. Furthermore, given lack of group differences in task performance and ratings, this compromised sensitivity in CUD is not explained by lack of task engagement or impaired task performance.
GM Correlates of the Reward-modulated P300 Response
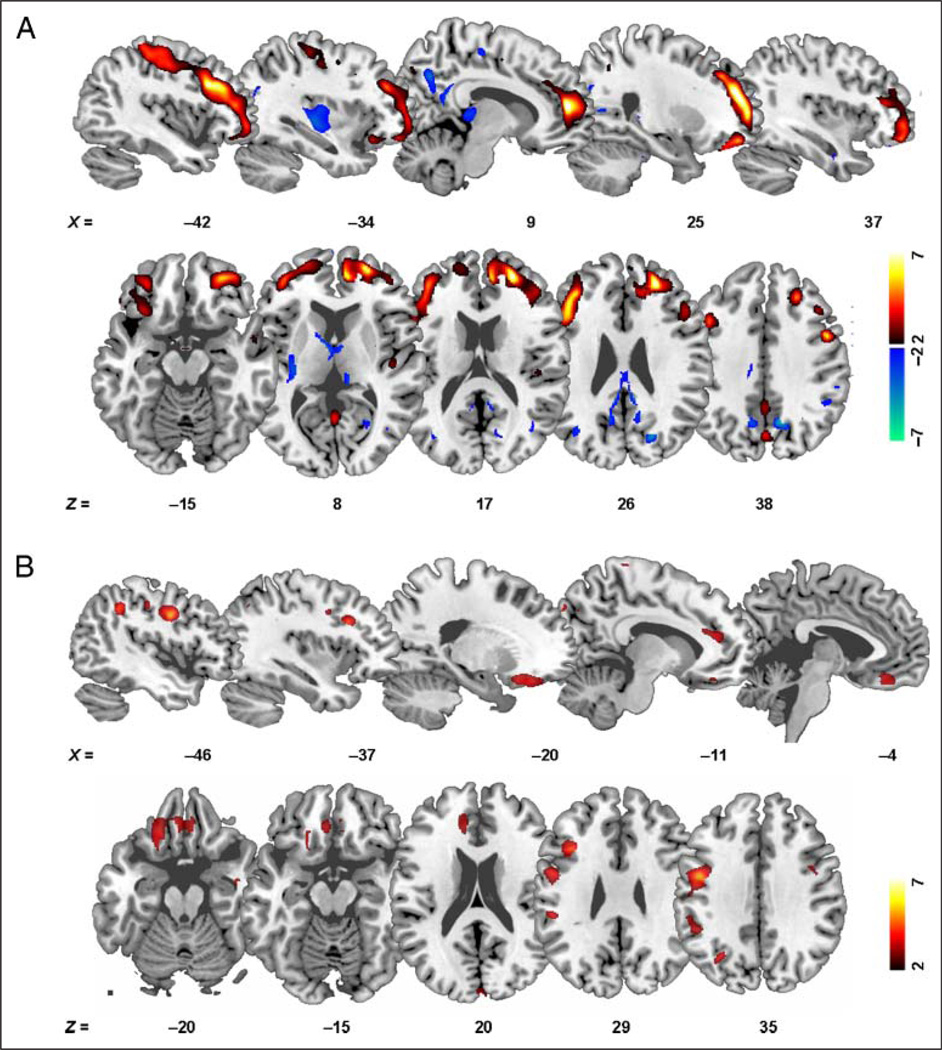
To evaluate whether the reward-modulated P300 was associated with PFC GM volume, we performed unbiased whole-brain regression analyses, controlling for the effects of age and TBV, using the P300 amplitude responses as seed values. There were no significant correlations at the set significance threshold level or at a reduced threshold of p < .01, uncorrected when considering the whole sample of 39 participants. When performing the analyses only within controls, results revealed that the psychophysiological sensitivity to money (P300 amplitude at 45¢ minus 0¢) was significantly positively correlated with GM volume in four distinct clusters encompassing the right DLPFC (BA 46), right ACC (BA 32, after small volume correction), left ventrolateral PFC (VLPFC; BA 44), and right lateral OFC (BA 47) (cluster level p < .05, FWE-corrected for multiple comparisons; Table 2). No other regions survived this whole-brain correction even at the reduced set threshold level of p < .01, uncorrected. There were also no significant correlations at the set significance threshold level (or at the reduced p < .01 level) in the CUD group or within either study group between regional GM volume and P300 responses to 45¢, 1¢, and 0¢ (absolute amplitudes) or the differentials 45¢ minus 1¢ and 1¢ minus 0¢ as separately inspected. Thus, in controls only, increased PFC GM volume was associated with increased P300 amplitude modulation by reward (high monetary reward versus nonreward). Figure 3A shows a subtraction image comparing the whole-brain regression maps between the groups (relationship of the maximal differential P300 response, 45¢ minus 0¢, to regional GM volume for controls > CUD and CUD > controls). This map shows that the groups’ t maps did not significantly overlap, providing support for our conclusion that the reported correlations were observed only in controls but not CUD.
Table 2.
Regression Results in 17 Healthy Controls between Regional GM Volume and Psychophysiological Sensitivity to Reward
| Region | MNI Coordinates |
Peak t | Peak Z | Voxels | p, Corrected | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| R DLPFC, BA 46 | 25 | 44 | 26 | 6.86 | 4.39 | 4433 | <.0001 |
| R ACC, BA 32 | 9 | 49 | 13 | 6.24 | 4.17 | 629 | .022, svc |
| L VLPFC, BA 44 | −42 | 19 | 30 | 6.12 | 4.13 | 4861 | <.0001 |
| R Lateral OFC, BA 47 | 37 | 49 | −15 | 5.69 | 3.96 | 1595 | .032 |
P300 amplitude differential responses to money (45¢ minus 0¢) were regressed against regional GM volume in whole-brain analyses. Statistical maps were thresholded at cluster-level p < .05, FWE-corrected for multiple comparisons (voxel-level p < .001, uncorrected) with a minimum cluster extent of 50 contiguous voxels. Age and TBV were used as covariates in all analyses. R = right; L = left; svc = small volume correction; BA, Brodmann’s area.
Figure 3.
(A) Subtraction image showing the neuroanatomical correlates of reward-sensitive P300 amplitudes in 17 healthy controls compared with 22 individuals with CUDs. Differential P300 responses to 45¢ (reward) versus 0¢ (nonreward) trials on the forced-choice sustained attention monetary reward paradigm were significantly positively correlated with GM volume in the PFC in control participants, but not in CUD (see Table 2). There were no significant correlations with the differential P300 response at the set significance threshold level in CUD. Color bars represent tdifference values (hot colors: controls > CUD: cold colors: CUD > controls). (B) A direct whole-brain group comparison of GM volume indicated that healthy controls also had increased GM volume in the left VLPFC (BA 44) and the left OFC (BA 11), a cluster encompassing the bilateral rectal gyri, compared with CUD. Color bar represents t values. Age and TBV were used as covariates in all analyses. Color maps are overlaid on a single participant T1-weighted template, and images are presented in neurological view (right is right).
Between-group Comparison of Regional GM Volume
To directly assess differences in PFC GM volume between the groups, we performed a follow-up whole-brain group ANCOVA (controlling for the effects of age and TBV). Results indicated that healthy controls had significantly increased GM volume in the left VLPFC (BA 44, x = −37, y = 18, z = 29, peak t = 4.36, peak Z = 3.87, 864 voxels) and the left OFC, a cluster encompassing the bilateral rectal gyri (BA 11, x = −20, y = 28, z = −20, peak t = 3.75, peak Z = 3.42, 2259 voxels), compared with CUD (voxel level p < .05, FWE-corrected for multiple comparisons after small volume correction; Figure 3B). There were no voxels of increased GM volume in CUD compared with controls in any of our a priori ROIs. These results are consistent with previously reported drug-related PFC GM volume reductions in CUD (Matochik et al., 2003; Franklin et al., 2002) as we recently observed in a larger sample of CUD (Alia-Klein et al., 2011). Taken together with the regression results in controls, where increased PFC GM was associated with increased P300 differential amplitudes (45¢ > 0¢), these results further support a role for the structural integrity of PFC in the reward-modulated P300.
Consideration of Potential Confounds
State depression scores and years of lifetime alcohol use were not significantly correlated with P300 amplitudes in the entire sample or separately in either study group (all rs < |0.67|, p > .10; note Spearman’s r was used for state depression scores). Similarly, as inspected with independent t tests separately for each study group, these amplitude measures did not differ by history of cigarette smoking (past or current smokers vs. nonsmokers; for both groups, t < |1.08|, p > .29; this analysis was not conducted across the entire sample given the almost parallel distribution with study group). Furthermore, for current smokers (3 controls/16 CUD), the differential P300 response was not associated with number of cigarettes smoked per day or years of nicotine use (r < | 0.05|, p > .84). Cocaine urine status in CUD also did not significantly impact P300 modulation (t < |0.28|, p > .78). Therefore, these variables were not entered as covariates in the relevant ANOVAs.
State depression scores and years of lifetime alcohol use were also not significantly correlated with regional GM volume (VLPFC and OFC regions from the group ANCOVA on GM; all r < |0.33| , p > .13). Similarly, GM volume did not differ by history of cigarette smoking (past or current smokers vs. nonsmokers; for both groups as separately inspected, t< |1.45|, p > .17) and across all current smokers, GM volume was not significantly associated with cigarettes smoked per day or years of nicotine use (r < |0.27|, p > .26). Finally, GM volume did not differ by cocaine urine status in CUD (t < |1.47 |, p > .15). Therefore, these variables were also not entered as additional covariates in the group ANCOVA on GM.
DISCUSSION
To the best of our knowledge, this study is the first to specifically explore the neuroanatomical correlates of reward sensitive P300 amplitudes. We found a robust positive correlation between P300 differential (but not absolute) amplitude responses to the expectation of monetary reward and GM volume in brain regions functionally involved in reward processing, namely the DLPFC and VLPFC, ACC, and lateral OFC, in healthy controls. In contrast, cocaine-addicted individuals demonstrated—in addition to the expected compromised psychophysiological sensitivity to money and reduced PFC GM volume (specifically in the VLPFC and the OFC)—lack of interdependence between these two measures. Taken together, these results suggest that structural integrity of PFC modulates electrocortical sensitivity to monetary reward (but not P300 generation per se). Note that correlation analyses are inconclusive about direction, causality, or predisposition.
The P300 amplitude is proposed to primarily reflect brain mechanisms facilitating the focal attention needed to process the motivational significance of stimuli (Polich, 2007; Nieuwenhuis et al., 2005). Subregions of PFC comprise such a neural network where attention and higher-order executive functions (Asplund, Todd, Snyder, & Marois, 2010) interface reward processing (Elliott et al., 2003; Kringelbach et al., 2003; O’Doherty et al., 2001). Within this network, the complementary functions of the OFC, ACC, VLPFC, and DLPFC (Grabenhorst & Rolls, 2011; Hornak et al., 2004; Rolls, 2004; Hikosaka & Watanabe, 2000) make these regions likely candidates for reward-related modulation of the P300 response, as indeed supported by correlations in the controls in the current study. These results also extend our previous findings where, using functional MRI with the same task reported in the current EEG study, we showed lateral OFC sensitivity to money in healthy controls (Goldstein et al., 2007). That is, across our studies and using the same task paradigm, healthy individuals showed lateral OFC sensitivity to money, P300 sensitivity to money, and a positive association between P300 sensitivity to money and lateral OFC GM volume. Nevertheless, although the observed correlations were confined within subregions of PFC (even when substantially reducing our statistical threshold), previous studies have identified a more widely distributed network of neural sources of the P300 (P3b, reported in this study). In addition to sources in PFC (e.g., ACC), these studies have also identified sources of the reward-modulated P300 in the posterior cingulate cortex (Kamarajan et al., 2010; Zhou, Yu, & Zhou, 2010). In addition, the nonreward-modulated P300, elicited by a range of task demands, has been localized to PFC, including ACC, OFC, VLPFC, and the middle and inferior frontal gyri (Volpe et al., 2007; Neuhaus et al., 2006; Mulert et al., 2004; Yamazaki et al., 2000; Halgren, Marinkovic, & Chauvel, 1998), the inferior temporal gyrus (Bledowski et al., 2004), the parietal lobe (Moores et al., 2003), and the TPJ (Mulert et al., 2004). Therefore, it is likely that the structural integrity of PFC may contribute directly but also indirectly, via PFC interactions with more posterior brain regions, to adaptive modulation of the P300 response to both reward and nonreward contingencies.
Interestingly, the comparison group of individuals with CUD showed both lack of P300 amplitude modulation to money and reduced GM volume in the VLPFC and OFC. Although in the current sample of CUD these two findings were not statistically related, these results are generally in line with lesion studies, where, compared with controls, patients with frontal lesions manifest deficits in P300 (P3b) amplitudes in response to predictive contextual processing cues (Fogelson, Shah, Scabini, & Knight, 2009; Barcelo & Knight, 2007; although see Knight, 1984, an earlier study that showed null effects of lateral PFC lesions on the P300 [P3b]; here, effects were in the expected direction [smaller P3b amplitudes compared with controls] but significant differences were primarily confined to the P3a, which peaks earlier than the P3b [described in this study] and primarily reflects an orienting response to unexpected, novel, or infrequent stimuli). Similarly, albeit at a trend level, ACC GM density reductions correlated with irregularities in auditory P300 amplitudes in patients with posttraumatic stress disorder (Araki et al., 2005). Nevertheless, our results should be interpreted with caution given the lack of a significant group main effect or group by money interaction on the P300 and the null correlations between these responses and GM volume in CUD. Given the heterogeneous nature of clinical populations such as CUD, studies employing larger samples may be needed to clearly establish the significance of the proposed effects. Furthermore, although tempting, causality for P300 deficits may not be solely attributed to GM decrements in CUD, as other factors may be as crucially implicated (e.g., neural sensitivity to reward gradients in drug addiction has been shown to also correlate with baseline levels of dopamine neurotransmission [Asensio et al., 2010] and impulsivity [Bjork, Smith, & Hommer, 2008], factors potentially predisposing to the development of addiction in susceptible individuals).
The results of this study need to be considered in light of its main limitations. First, because the precise GM histo-pathological characteristics that influence MRI segmentation are not yet known, results of this study remain to be validated with postmortem or lesion studies. Future studies should also consider the influence of GM volume on neural source waveforms or other reward-sensitive ERP components. Importantly, although the effects of depression, cigarette smoking, alcohol use, and urine status for cocaine were statistically inspected, their potential impact on results remains to be separately investigated. For example, given that it is not practical to exclude cigarette smoking CUD (where concomitant use of nicotine and comorbid nicotine dependence are much higher than in the general population: 70–80% for nicotine use and 50% for nicotine dependence as compared with 22% and 13% in controls, respectively; Weinberger & Sofuoglu, 2009; Kalman, Morissette, & George, 2005; Grant, Hasin, Chou, Stinson, & Dawson, 2004; Lasser et al., 2000; Budney, Higgins, Hughes, & Bickel, 1993), a future study would need to recruit more cigarette smoking controls. Similarly, studies employing longitudinal within-subject designs and larger, more heterogeneous samples of CUD would also be necessary to clearly establish the potential impact of abstinence on psychophysiological sensitivity to reward or GM volume. Generalizability of results to other cognitive tasks also remains to be established.
In summary, we showed that reward-modulated P300 amplitudes were significantly positively correlated with GM volume in prefrontal brain regions centrally involved in reward processing in healthy controls, but not CUD, who instead showed compromises in both P300 sensitivity to money and PFC GM volume. This is an important finding as it extends the study of reward processing, commonly accomplished with a single imaging modality, to a multi-modal functional–structural investigation. Establishing a direct relationship between function and structure has methodological implications for numerous future studies, spanning healthy development to monitoring disease course or impact of treatment in psychopathologies affecting both PFC and reward processing/motivation.
Acknowledgments
We would like to thank Thomas Maloney, Nelly Alia-Klein, Patricia A. Woicik, and Frank Telang for their assistance in participant recruitment, assessment, and study conduct and Ruiliang Wang for the technical support in MRI acquisition and data reconstruction. This work was supported by grants from the National Institute on Drug Abuse (Grant 1R01DA023579 to R. Z. G.) and General Clinical Research Center (Grant 5-MO1-RR-10710). This manuscript has been authored by Brookhaven Science Associates, LLC, under contract DE-AC02-98CHI-886 with the U.S. Department of Energy. The U.S. Government retains and, by accepting the article for publication, the publisher acknowledges a worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for the U.S. Government purposes.
REFERENCES
- Alia-Klein N, Parvaz MA, Woicik PA, Konova AB, Maloney T, Shumay E, et al. Gene × Disease interaction on orbito-frontal gray matter in cocaine addiction. Archives of General Psychiatry. 2011;68:283–294. doi: 10.1001/archgenpsychiatry.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Kasai K, Yamasue H, Kato N, Kudo N, Ohtani T, et al. Association between lower P300 amplitude and smaller anterior cingulate cortex volume in patients with posttraumatic stress disorder: A study of victims of Tokyo subway sarin attack. Neuroimage. 2005;25:43–50. doi: 10.1016/j.neuroimage.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Asensio S, Romero MJ, Romero FJ, Wong C, Alia-Klein N, Tomasi D, et al. Striatal dopamine D2 receptor availability predicts the thalamic and medial prefrontal responses to reward in cocaine abusers three years later. Synapse. 2010;64:397–402. doi: 10.1002/syn.20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—The methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Asplund CL, Todd JJ, Snyder AP, Marois R. A central role for the lateral prefrontal cortex in goal-directed and stimulus-driven attention. Nature Neuroscience. 2010;13:507–512. doi: 10.1038/nn.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. Journal of Comparative Neurology. 2005a;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annual Review of Neuroscience. 2005b;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Barcelo F, Knight RT. An information-theoretical approach to contextual processing in the human brain: Evidence from prefrontal lesions. Cerebral Cortex. 2007;17(Suppl 1):51–i60. doi: 10.1093/cercor/bhm111. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Chou CL, Aunon JI. P3 and stimulus incentive value. Psychophysiology. 1983;20:95–101. doi: 10.1111/j.1469-8986.1983.tb00909.x. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Daum I. Learning-related changes in reward expectancy are reflected in the feedback-related negativity. European Journal of Neuroscience. 2008;27:1823–1835. doi: 10.1111/j.1460-9568.2008.06138.x. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Polezzi D, Daum I. It is less than you expected: The feedback-related negativity reflects violations of reward magnitude expectations. Neuropsychologia. 2010;48:3343–3350. doi: 10.1016/j.neuropsychologia.2010.07.023. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Hommer DW. Striatal sensitivity to reward deliveries and omissions in substance dependent patients. Neuroimage. 2008;42:1609–1621. doi: 10.1016/j.neuroimage.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledowski C, Prvulovic D, Hoechstetter K, Scherg M, Wibral M, Goebel R, et al. Localizing P300 generators in visual target and distractor processing: A combined event-related potential and functional magnetic resonance imaging study. Journal of Neuroscience. 2004;24:9353–9360. doi: 10.1523/JNEUROSCI.1897-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Hughes JR, Bickel WK. Nicotine and caffeine use in cocaine-dependent individuals. Journal of Substance Abuse. 1993;5:117–130. doi: 10.1016/0899-3289(93)90056-h. [DOI] [PubMed] [Google Scholar]
- Cuadra MB, Cammoun L, Butz T, Cuisenaire O, Thiran JP. Comparison and validation of tissue modelization and statistical classification methods in T1-weighted MR brain images. IEEE Transactions on Medical Imaging. 2005;24:1548–1565. doi: 10.1109/TMI.2005.857652. [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Varriale V, D’Antuono L. Event-related components of the punishment and reward sensitivity. Clinical Neurophysiology. 2010;121:60–76. doi: 10.1016/j.clinph.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Dien J. The ERP PCA Toolkit: An open source program for advanced statistical analysis of event-related potential data. Journal of Neuroscience Methods. 2010;187:138–145. doi: 10.1016/j.jneumeth.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Dien J, Beal DJ, Berg P. Optimizing principal components analysis of event-related potentials: Matrix type, factor loading weighting, extraction, and rotations. Clinical Neurophysiology. 2005;116:1808–1825. doi: 10.1016/j.clinph.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Dien J, Khoe W, Mangun GR. Evaluation of PCA and ICA of simulated ERPs: Promax vs. Infomax rotations. Human Brain Mapping. 2007;28:742–763. doi: 10.1002/hbm.20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien J, Michelson CA, Franklin MS. Separating the visual sentence N400 effect from the P400 sequential expectancy effect: Cognitive and neuroanatomical implications. Brain Research. 2010;1355:126–140. doi: 10.1016/j.brainres.2010.07.099. [DOI] [PubMed] [Google Scholar]
- Donchin E, Miller GA, Farwell LA. The endogenous components of the event-related potential—A diagnostic tool? Progress in Brain Research. 1986;70:87–102. doi: 10.1016/s0079-6123(08)64299-5. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg FL, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Elliott R, Newman JL, Longe OA, Deakin JF. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: A parametric functional magnetic resonance imaging study. Journal of Neuroscience. 2003;23:303–307. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelson N, Shah M, Scabini D, Knight RT. Prefrontal cortex is critical for contextual processing: Evidence from brain lesions. Brain. 2009;132:3002–3010. doi: 10.1093/brain/awp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Hajcak G. Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biological Psychology. 2009;81:1–8. doi: 10.1016/j.biopsycho.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biological Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRL Levels of inference and power. Neuroimage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, et al. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? American Journal of Psychiatry. 2007;164:43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Cottone LA, Jia Z, Maloney T, Volkow ND, Squires NK. The effect of graded monetary reward on cognitive event-related potentials and behavior in young healthy adults. International Journal of Psychophysiology. 2006;62:272–279. doi: 10.1016/j.ijpsycho.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Parvaz MA, Maloney T, Alia-Klein N, Woicik PA, Telang F, et al. Compromised sensitivity to monetary reward in current cocaine users: An ERP study. Psychophysiology. 2008;45:705–713. doi: 10.1111/j.1469-8986.2008.00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends in Cognitive Sciences. 2011;15:56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: Results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Holroyd CB, Moser JS, Simons RF. Brain potentials associated with expected and unexpected good and bad outcomes. Psychophysiology. 2005;42:161–170. doi: 10.1111/j.1469-8986.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- Halgren E, Marinkovic K, Chauvel P. Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalography and Clinical Neurophysiology. 1998;106:156–164. doi: 10.1016/s0013-4694(97)00119-3. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Watanabe M. Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cerebral Cortex. 2000;10:263–271. doi: 10.1093/cercor/10.3.263. [DOI] [PubMed] [Google Scholar]
- Hornak J, O’Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, et al. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. Journal of Cognitive Neuroscience. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. American Journal on Addictions. 2005;14:106–123. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Rangaswamy M, Tang Y, Chorlian DB, Pandey AK, Roopesh BN, et al. Dysfunctional reward processing in male alcoholics: An ERP study during a gambling task. Journal of Psychiatric Research. 2010;44:576–590. doi: 10.1016/j.jpsychires.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RT. Decreased response to novel stimuli after prefrontal lesions in man. Electroencephalography and Clinical Neurophysiology. 1984;59:9–20. doi: 10.1016/0168-5597(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. Journal of the American Medical Association. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Lee JH, Garwood M, Menon R, Adriany G, Andersen P, Truwit CL, et al. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magnetic Resonance in Medicine. 1995;34:308–312. doi: 10.1002/mrm.1910340305. [DOI] [PubMed] [Google Scholar]
- Lesieur HR, Blume SB. The South Oaks Gambling Screen (SOGS): A new instrument for the identification of pathological gamblers. American Journal of Psychiatry. 1987;144:1184–1188. doi: 10.1176/ajp.144.9.1184. [DOI] [PubMed] [Google Scholar]
- Liu X, Matochik JA, Cadet JL, London ED. Smaller volume of prefrontal lobe in polysubstance abusers: A magnetic resonance imaging study. Neuropsychopharmacology. 1998;18:243–252. doi: 10.1016/S0893-133X(97)00143-7. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: A magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- Moores KA, Clark CR, Hadfield JL, Brown GC, Taylor DJ, Fitzgibbon SP, et al. Investigating the generators of the scalp recorded visuo-verbal P300 using cortically constrained source localization. Human Brain Mapping. 2003;18:53–77. doi: 10.1002/hbm.10073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulert C, Pogarell 0, Juckel G, Rujescu D, Giegling I, Rupp D, et al. The neural basis of the P300 potential. Focus on the time-course of the underlying cortical generators. European Archives of Psychiatry and Clinical Neuroscience. 2004;254:190–198. doi: 10.1007/s00406-004-0469-2. [DOI] [PubMed] [Google Scholar]
- Neuhaus A, Bajbouj M, Kienast T, Kalus P, von Haebler D, Winterer G, et al. Persistent dysfunctional frontal lobe activation in former smokers. Psychopharmacology (Berlin) 2006;186:191–200. doi: 10.1007/s00213-006-0366-7. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychological Bulletin. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, De Geus EJ, Aston-Jones G. The anatomical and functional relationship between the P3 and autonomic components of the orienting response. Psychophysiology. 2011;48:162–175. doi: 10.1111/j.1469-8986.2010.01057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Picton TW. The P300 wave of the human event-related potential. Journal of Clinical Neurophysiology. 1992;9:456–479. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard WS. Psychophysiology of P300. Psychological Bulletin. 1981;89:506–540. [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain and Cognition. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Rossi AF, Pessoa L, Desimone R, Ungerleider LG. The prefrontal cortex and the executive control of attention. Experimental Brain Research. 2009;192:489–497. doi: 10.1007/s00221-008-1642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Yasuda A, Ohira H, Miyawaki K, Nishikawa M, Kumano FL, et al. Effects of value and reward magnitude on feedback negativity and P300. NeuroReport. 2005;16:407–411. doi: 10.1097/00001756-200503150-00020. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Dien J, Donchin E. Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology. 2001;38:343–358. [PubMed] [Google Scholar]
- Sutton S, Braren M, Zubin J, John ER. Evoked-potential correlates of stimulus uncertainty. Science. 1965;150:1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, et al. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biological Psychiatry. 2009;65:160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif CL, Collins DL, Pike GB. Sensitivity of voxel-based morphometry analysis to choice of imaging protocol at 3 T. Neuroimage. 2009;44:827–838. doi: 10.1016/j.neuroimage.2008.09.053. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Welsh RC, Wager TD, Phan KL, Fitzgerald KD, Gehring WJ. A functional neuroimaging study of motivation and executive function. Neuroimage. 2004;21:1045–1054. doi: 10.1016/j.neuroimage.2003.10.032. [DOI] [PubMed] [Google Scholar]
- Thut G, Schultz W, Roelcke U, Nienhusmeier M, Missimer J, Maguire RP, et al. Activation of the human brain by monetary reward. NeuroReport. 1997;8:1225–1228. doi: 10.1097/00001756-199703240-00033. [DOI] [PubMed] [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Research. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Volpe U, Mucci A, Bucci P, Merlotti E, Galderisi S, Maj M. The cortical generators of P3a and P3b: A LORETA study. Brain Research Bulletin. 2007;73:220–230. doi: 10.1016/j.brainresbull.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Weinberger AFL, Sofuoglu M. The impact of cigarette smoking on stimulant addiction. American Journal of Drug and Alcohol Abuse. 2009;35:12–17. doi: 10.1080/00952990802326280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zhou X. The P300 and reward valence, magnitude, and expectancy in outcome evaluation. Brain Research. 2009;1286:114–122. doi: 10.1016/j.brainres.2009.06.032. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Kamijo K, Kenmochi A, Fukuzumi S, Kiyuna T, Takaki Y, et al. Multiple equivalent current dipole source localization of visual event-related potentials during oddball paradigm with motor response. Brain Topography. 2000;12:159–175. doi: 10.1023/a:1023467806268. [DOI] [PubMed] [Google Scholar]
- Yeung N, Sanfey AG. Independent coding of reward magnitude and valence in the human brain. Journal of Neuroscience. 2004;24:6258–6264. doi: 10.1523/JNEUROSCI.4537-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Yu R, Zhou X. To do or not to do? Action enlarges the FRN and P300 effects in outcome evaluation. Neuropsychologia. 2010;48:3606–3613. doi: 10.1016/j.neuropsychologia.2010.08.010. [DOI] [PubMed] [Google Scholar]