Abstract
Rationale
Sialylation by α2,3-sialyltransferases has been shown to be a crucial glycosylation step in the generation of functional selectin ligands. Recent evidence suggests that sialylation also affects the binding of chemokines to their corresponding receptor.
Objective
Because the chemokine receptors for Ccl5 and Ccl2 are important in atherogenic recruitment of neutrophils and monocytes, we here investigated the role of α2,3-sialyltransferase IV (ST3Gal-IV) in Ccl5- and Ccl2-mediated myeloid cell arrest and further studied its relevance in a mouse model of atherosclerosis.
Methods and Results
St3Gal4-deficient myeloid cells showed a reduced binding of Ccl5 and an impaired Ccl5-triggered integrin activation. Correspondingly, Ccl5-induced arrest on tumor necrosis factor-α–stimulated endothelium was almost completely abrogated, as observed in flow chamber adhesion assays and during ex vivo perfusion or intravital microscopy of carotid arteries. Moreover, Ccl5-triggered neutrophil and monocyte extravasation into the peritoneal cavity was severely reduced in St3Gal4−/− mice. In contrast, St3Gal4 deficiency did not significantly affect Ccl2 binding and only marginally decreased Ccl2-induced flow arrest of myeloid cells. In agreement with the crucial role of leukocyte accumulation in atherogenesis, and the importance of Ccl5 chemokine receptors mediating myeloid cell recruitment to atherosclerotic vessels, St3Gal4 deficiency drastically reduced the size, stage, and inflammatory cell content of atherosclerotic lesions in Apoe−/− mice on high-fat diet.
Conclusions
In summary, these findings identify ST3Gal-IV as a promising target to reduce inflammatory leukocyte recruitment and arrest.
Keywords: atherosclerosis; beta-galactoside alpha-2, 3-sialyltransferase
Glycosyltransferases are involved in the generation of functional selectin ligands, mediating leukocyte rolling on inflamed endothelium.1 Mice deficient of α2,3-sialyltransferase IV (St3Gal4) displayed a partially impaired E-selectin ligand function and an almost complete lack of L-selectin–dependent leukocyte rolling on tumor necrosis factor-α (Tnfα)-exposed cremaster muscle venules.2–4 More recently, also leukocyte arrest by the chemokine receptor Cxcr2 was shown to depend on ST3Gal-IV–mediated sialylation, because St3Gal4−/− mice displayed decreased leukocyte adhesion to inflamed microvessels on stimulation with the Cxcr2 ligands Cxcl1 or Cxcl8.5 In line, human CCR5 requires the attachment of sialic acid–carrying O-glycans for binding of its chemokine ligands CCL3 and CCL4 and subsequent receptor activation, as shown by a combination of sialidase treatment and CCR5 mutants in which the putative binding sites for sialylated O-glycans were exchanged.6 Because the chemokine receptors Ccr1 and Ccr5 (with high-affinity ligand Ccl5) and Ccr2 (binding Ccl2) were previously shown to be important in myeloid cell recruitment during inflammation,7–9 we investigated the role of ST3Gal-IV in Ccl5- and Ccl2-mediated leukocyte arrest on inflamed endothelium and its effect on atherosclerosis using St3Gal4-deficient mice.
Methods
Detailed methods are provided in the Online Data Supplement. Throughout the article, the letter format of all gene and protein notations was chosen to conform with internationally agreed gene/protein nomenclature guidelines: all letters of human genes/proteins are in uppercase, whereas for mouse genes/proteins, only the first letter is in uppercase and the remaining letters are in lowercase. Gene names are in italics.
Atherosclerosis Study
St3Gal4−/− mice10 were crossed with Apoe−/− mice and received a high-fat diet for 12 weeks. Size and cellular composition of atherosclerotic lesions were assessed by histology and immunofluorescence.
Study of Monocytes and Neutrophils
Primary monocytes and neutrophils were isolated from bone marrow with specific cell separation kits according to the manufacturer’s protocol and were used for chemokine-binding assays, flow chamber adhesion experiments, and ex vivo perfusion of mouse carotid arteries. Integrin activation assays and chemokine-binding assays were performed using whole blood, and neutrophils and monocytes were distinguished using specific fluorescent labeling and flow cytometry.
Results
St3Gal4−/− Monocytes and Neutrophils Show a Reduced Integrin Activation and Flow Arrest Upon Ccl5 Stimulation
Integrin activation is crucial for leukocyte arrest, enabling an efficient interaction with integrin ligands exposed on the endothelium. Interestingly, Ccl5-induced binding of the integrin ligands Icam1 and Vcam1 was significantly reduced in St3Gal4−/− classical monocytes and neutrophils (Figure 1A and 1B), which was associated with a significantly decreased ability of Ccl5 to trigger the arrest of St3Gal4−/− monocytes and neutrophils on Tnfα-activated, SV40-transformed mouse endothelial cells under flow (Figure 1C). Also, St3Gal4-deficient mice showed a significant decrease in the accumulation of monocytes and neutrophils in the peritoneal cavity 4 hours after intraperitoneal injection of Ccl5 (Figure 1D). Binding of Ccl5 to St3Gal4−/− myeloid cells was reduced by 30% to 40% (Online Figure IA), despite comparable surface expression of the high-affinity Ccl5 receptors Ccr1 and Ccr5 and of the intermediate affinity receptor Ccr3 (Online Figure II). Furthermore, enzymatic removal of sialic acids using sialidase treatment decreased Ccl5 binding to monocytes and neutrophils by 55% to 80% (Online Figure IB) and seemed associated with reduced interaction of Ccl5 with both Ccr5 and Ccr1 as shown by sialidase treatment of Ccr1−/− and Ccr5−/− myeloid cells, respectively (Online Figure III). Together, these data indicate that sialylation by ST3Gal-IV and probably other sialyltransferases improve Ccl5 binding, with ST3Gal-IV–mediated sialylation enabling efficient Ccl5-induced integrin activation and myeloid cell arrest.
Figure 1. St3Gal4-deficient leukocytes display reduced Ccl5-induced integrin activation and flow arrest.
A and B, Ccl5- and Ccl2-induced (2 μg/mL; 5 min) integrin activation in monocytes (A) and neutrophils (B) from St3Gal4+/+ and St3Gal4−/− mice, as quantified by the binding of Icam1 and Vcam1. n=5 to 6. MFI indicates mean fluorescence intensity. C, Adhesion of perfused monocytes (left) and neutrophils (right) on tumor necrosis factor-α–activated (10 ng/mL; 4 h), SV40-transformed mouse endothelial cells (SVECs) after pretreatment of leukocytes or SVECs with Ccl5 (2 μg/mL; 10 min). n=6 to 12. D, Intraperitoneal recruitment of monocytes (left) and neutrophils (right) 4 h after intraperitoneal injection of 5 μg Ccl5. n=11 to 16. A to D, Graphs represent mean±SD; Mann–Whitney test (A and B) or 1-way ANOVA with Tukey multiple comparison test (C and D). *P<0.05; **P<0.01; ***P<0.001.
In contrast, St3Gal4 deficiency did not affect Ccl2-induced integrin activation or flow arrest of neutrophils and could only significantly reduce the binding of Vcam1 but not Icam1 to Ccl2-triggered monocytes. Correspondingly, Ccl2-induced arrest of St3Gal4−/− monocytes was only marginally reduced compared with wild-type monocytes, which showed a significantly increased binding upon Ccl2 treatment (Figure 1A and 1B; Online Figure IVA). Also, no differences were observed in Ccl2 binding to St3Gal4−/− versus St3Gal4+/+ myeloid cells or in the expression of Ccl2 chemokine receptor Ccr2 (Online Figures IVB and II). Thus, these data imply that not all chemokines are equally influenced by ST3Gal-IV.
To unravel whether endothelial ST3Gal-IV also affects Ccl5-induced myeloid cell arrest, we performed ex vivo perfusion assays with mounted and pressurized Tnfα-activated carotid arteries from St3Gal4+/+ and St3Gal4−/− mice. Pretreatment of wild-type leukocytes with Ccl5 before perfusion increased their adhesion on the endothelium of both wild-type as St3Gal4−/− arteries (Figure 2A and 2B, left panels). In contrast, Ccl5 pre-treatment of St3Gal4−/− leukocytes did not enhance their arrest on either St3Gal4+/+ or St3Gal4−/− carotid arteries (Figure 2A and 2B, right), indicating that ST3Gal-IV on leukocytes, but not endothelial cells, enables Ccl5-triggered leukocyte arrest on inflamed endothelium. A role for ST3Gal-IV in inflammatory cell arrest was further confirmed in vivo by the use of intravital fluorescence microscopy of the carotid artery. St3Gal4−/− mice showed a dramatic reduction in adherent rhodamine 6G–labeled leukocytes (Online Figure V), despite comparable WBC counts (4.3±2.3 versus 3.5±2.2×103 leukocytes/μL blood for St3Gal4+/+ and St3Gal4−/−, respectively). A decreased arrest was observed for both neutrophils (CD11b+, Ly6G+) and classical monocytes (CD11b+, Ly6C+) based on intravenous labeling with antibodies to CD11b, Ly6G, and Ly6C (Figure 2C and 2D).
Figure 2. Reduced Ccl5-induced adhesion of St3Gal4−/− leukocytes on mouse carotid arteries.
A and B, Ex vivo flow adhesion of Ccl5-pretreated (2.5 μg/mL; 10 min) leukocytes on tumor necrosis factor-α (Tnfα)-stimulated (20 ng/mL; 4 h) carotids from St3Gal4+/+ (A) and St3Gal4−/− (B) mice. n=5 to 7; Mann–Whitney test. C and D, Intravital microscopy of leukocyte adhesion to Tnfα–stimulated carotid arteries of St3Gal4+/+ and St3Gal4−/− mice, after leukocyte labeling for CD11b (top, labels all myeloid cells), Ly6G (middle, labels neutrophils), or Ly6C (bottom, labels monocytes). n=6 to 14; t test. A to D, Graphs represent mean±SD. *P<0.05; ***P<0.001.
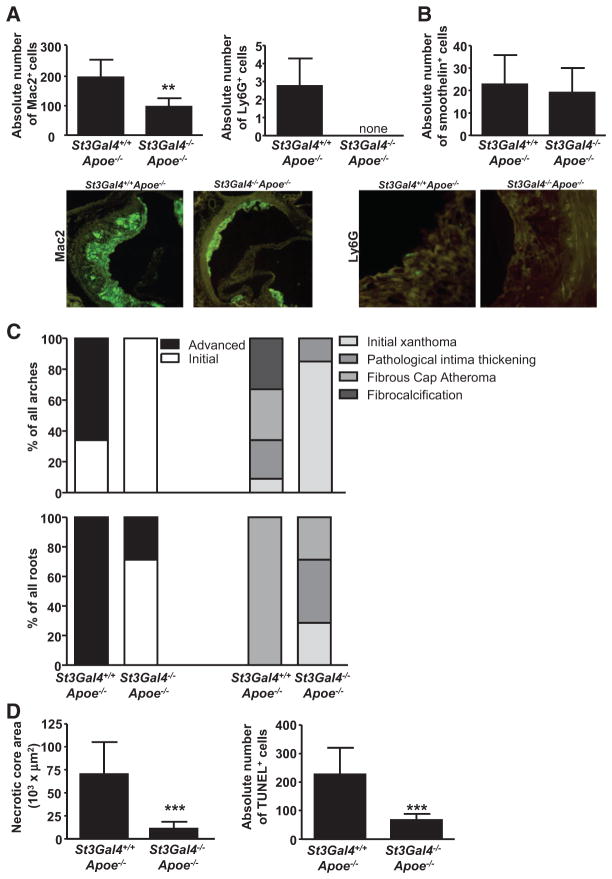
St3Gal4 Deficiency Reduces Atherosclerotic Lesion Size and Myeloid Cell Influx in Mice
As continuous leukocyte adhesion and influx drive atherosclerotic lesion development,11 we examined a potential role of ST3Gal-IV in atherosclerosis using St3Gal4−/−Apoe−/− and St3Gal4+/+Apoe−/− mice on high-fat diet for 12 weeks. Despite comparable leukocyte subpopulations and only small differences in lipid levels between knock-out and wild-type mice (Online Table), aortic arches, roots, and thoracoabdominal aortas of St3Gal4−/−Apoe−/− mice displayed a dramatic reduction in plaque development (Figure 3A and 3B; Online Figure VI). Macrophage and neutrophil numbers in St3Gal4−/−Apoe−/− aortic root lesions were significantly reduced (Figure 4A), whereas the number of smooth muscle cells was not altered (Figure 4B). Furthermore, plaque staging of aortic arches and roots according to Virmani et al12 displayed an initial lesion phenotype in St3Gal4−/−Apoe−/− mice, characterized by 90% initial xanthomas in arches and 70% initial xanthomas and pathological intima thickening in the roots of these mice. In contrast, plaques in wild-type mice showed a very advanced lesion type, mainly represented by fibrous cap atheromas and fibrocalcification in both arches and roots (Figure 4C). In line, the necrotic core area and the accumulation of TUNEL+ cells were significantly diminished in atherosclerotic plaques of St3Gal4−/−Apoe−/− mice (Figure 4D).
Figure 3. St3Gal4 deficiency reduces atherosclerosis.
Quantification of atherosclerotic lesions in the aortic arch (A) and aortic root (B) of St3Gal4+/+Apoe−/− and St3Gal4−/−Apoe−/− mice after 12 wk of high-fat diet. HE indicates hematoxylin and eosin. Graphs represent mean±SD; n=6 to 13; Mann–Whitney test. ***P<0.001.
Figure 4. St3Gal4−/−Apoe−/− lesions contain fewer leukocytes and display an initial plaque phenotype.
A and B, Absolute number of macrophages (Mac2+), neutrophils (Ly6G+; A), and smooth muscle cells (smoothelin+; B) in the aortic root lesions of St3Gal4+/+Apoe−/− and St3Gal4−/−A poe−/− mice. Representative figures are shown. C, Lesion staging in arches (top) and roots (bottom) according to Virmani et al.12 D, Necrotic core area (left) and absolute number of apoptotic (TUNEL+) cells in the root lesions (right). A to D, Graphs represent mean±SD; n=5 to 10; Mann–Whitney or t test as appropriate. **P<0.01; ***P<0.001.
Interestingly, immunofluorescent staining of initial versus advanced human lesions revealed increased CCL5 levels in advanced compared with initial plaques (Online Figure VII). In line, the smaller root lesions of St3Gal4−/−Apoe−/− mice displayed reduced Ccl5 staining compared with controls (Online Figure VIII), although Ccl5 serum levels were not significantly changed (Online Figure IX).
Discussion
This study reveals that the sialyltransferase ST3Gal-IV enables Ccl5-triggered arrest of monocytes and neutrophils on inflamed endothelium. St3Gal4−/− leukocytes showed a significant reduction in Ccl5 binding and Ccl5-induced integrin activation despite comparable expression of Ccl5 chemokine receptors. This suggests that sialylation facilitates efficient Ccl5 binding through favorable conformational changes in Ccl5 receptors, or through enforced electrostatic interactions of basic chemokine residues with negatively charged sialic acids attached to the chemokine receptors.6 The contribution of sialylation to efficient Ccl5 binding is supported by the reduction in Ccl5 binding on sialidase treatment, which we observed for both Ccr1−/− and Ccr5−/− leukocytes and by a previous report demonstrating human CCR5 to require N-terminal sialylation for efficient chemokine binding.6 Comparably, St3Gal4 deficiency in leukocytes was previously shown to reduce Cxcl8 binding to Cxcr2 and to impair Cxcl1/Cxcr2-triggered neutrophil arrest.5 Nonetheless, ST3Gal-IV–mediated sialylation does not seem to be a general requirement for efficient chemokine functioning, because Ccl2-triggered leukocyte arrest was not significantly affected by St3Gal4 deficiency.
Circulating monocytes and neutrophils adhere to and accumulate in atherosclerotic vessels, where they crucially contribute to atherogenesis.11 The recruitment of classical monocytes into atherosclerotic lesions requires Ccr19 and Ccr5,7,9 whereas the precise role of Ccr27,9,13 and Cx3cr17,9 in monocyte incorporation into lesions has recently been debated. The observed reduction in lesion size in Cx3cr1−/−Apoe−/− mice14–16 may depend on the role of Cx3cr1 in monocyte and macrophage survival,16 rather than on a direct role of Cx3cr1 in monocyte recruitment into atherosclerotic plaques.9 Similarly, although the reduced atherosclerotic lesion size in Ccr2−/−Apoe−/− mice14,17 and Ccr2−/− bone marrow chimeras18 clearly indicates an important role for Ccr2 in atherosclerosis, the specific role of Ccr2 in the incorporation of circulating monocytes into atherosclerotic arteries requires further investigation. Contradictory findings have been reported on such direct involvement of Ccr2 in lesional monocyte accumulation,9,13 suggesting that the proatherogenic role of Ccr2 may rather be related to a crucial role for the Ccl2/Ccr2 axis in the mobilization of monocytes from the bone marrow in inflammatory and atherosclerotic conditions.9,15,19–22 Compared with monocytes, neutrophils infiltrate atherosclerotic arteries primarily through Cxcr2, Ccr1, Ccr2, and Ccr5.8 Thus, the importance of ST3Gal-IV in mediating myeloid cell arrest in response to Ccl5 and Cxcl1/Cxcl8,5 representing high-affinity ligands for Ccr1 and Ccr5, and for Cxcr2, respectively, could explain why St3Gal4-deficient mice display a severely reduced leukocyte arrest on inflamed endothelium and an associated decrease in accumulating macrophages and neutrophils in atherosclerotic vessels. The previous finding that blocking only Ccl5 reduces the arrest of perfused monocytes on atherosclerotic endothelium by ≈50%23 further supports the importance of ST3Gal-IV in atherogenic myeloid cell accumulation. Furthermore, the requirement of leukocytic ST3Gal-IV for the generation of functional selectin ligands2–4 may additionally contribute to reduced leukocyte rolling and arrest in St3Gal4−/− mice.
Interestingly, atherosclerotic lesion size was previously shown to be strongly correlated with the number of circulating monocytes, displaying ≈90% reduction in atherosclerosis when circulating monocyte numbers were reduced with comparable extent.22 Thus, the arrest and infiltration of circulating monocytes are crucial in determining the lesion size, implying that the severely reduced leukocyte arrest in St3Gal4−/− mice could, to a great extent, explain their drastic reduction in atherosclerosis. In addition, the less advanced plaque phenotype combined with a lower platelet count on St3Gal4 deficiency10 could underlie the decreased Ccl5 levels in atherosclerotic vessels of St3Gal4−/ −Apoe−/− mice, which may further add to the reduction in leukocyte recruitment and atherosclerosis progression. Also, it is possible that reduced Ccl5-induced activation of myeloid cells, as displayed by decreased integrin activation, further contributes to reduced atherogenesis in St3Gal4−/−Apoe−/− mice. However, further studies are required to pinpoint the exact role of Ccl5 in the atherogenic functions of monocytes and neutrophils, before being able to address the effect of ST3Gal-IV in this context. In addition, the role of endothelial St3Gal4 in inflammation remains unclear. Although our in vitro data revealed a comparable Ccl5-triggered leukocyte adhesion to St3Gal4−/− versus St3Gal+/+ carotids after 4 hours of Tnfα stimulation, further studies are required to address the specific role of St3Gal4 in endothelial activation and in leukocyte adhesion to chronically inflamed endothelium in more detail in vivo. It is not excluded that St3Gal4 deficiency in vascular cells further contributes to the drastic reduction in atherosclerosis observed in this study.
Altogether, our data point toward an important contribution of ST3Gal-IV in efficient leukocyte recruitment and arrest under inflammatory conditions. Hence, targeting sialylation in atherosclerosis, for example by specific inhibitors of ST3Gal-IV, might be a new promising therapeutic approach.
Supplementary Material
Novelty and Significance.
What Is Known?
Chemokine receptors and their ligands play a crucial role in the adhesion of leukocytes on the endothelium during inflammation.
Receptors for the chemokine Ccl5 are important in mediating inflammatory leukocyte arrest, particularly in the context of atherosclerosis.
α2,3-Sialyltransferase IV (ST3Gal-IV) is known to be involved in Cxcr2-mediated leukocyte arrest on inflamed endothelium, but it remains unknown whether ST3Gal-IV also affects the binding of other chemokine ligand–receptor pairs.
What New Information Does This Article Contribute?
ST3Gal-IV enables efficient binding of Ccl5 to neutrophils and classical monocytes.
ST3Gal-IV mediates Ccl5-triggered integrin activation and leukocyte arrest on inflamed endothelium.
St3Gal4 deficiency reduces atherosclerosis in mice, suggesting that the prevention or reduction of sialylation may be a promising therapeutical approach.
A crucial step in the formation of atherosclerotic lesions is the recruitment and adhesion of neutrophils and monocytes to the inflamed vascular endothelium, driven by the interaction of chemokines with their corresponding receptors on leukocyte cell surface. Whereas the chemokine receptors Ccr1 and Ccr5 are important for the atherogenic recruitment of classical monocytes, neutrophil mobilization and recruitment is mediated through Cxcr2, Ccr1, Ccr2, and Ccr5. Interestingly, sialylation by sialyltransferase ST3Gal-IV has been shown to be required for Cxcr2-dependent leukocyte arrest and efficient binding of Cxcl1 and Cxcl8 to Cxcr2. However, it remains unknown whether ST3Gal-IV also affects other chemokine receptor–ligand interactions. The results of this study suggest that ST3Gal-IV in myeloid cells enables efficient binding of Ccl5 (a ligand for the chemokine receptors Ccr1 and Ccr5) and mediates Ccl5-triggered integrin activation and leukocyte arrest on inflamed endothelium. In contrast, St3Gal4 deficiency did not significantly affect the binding of Ccl2 (a ligand for Ccr2), or Ccl2-induced flow arrest of myeloid cells, suggesting that ST3Gal-IV–mediated sialylation is not a general requirement for efficient chemokine functioning. Corresponding with the important role of the Ccl5 chemokine receptors in the recruitment of both monocytes and neutrophils to atherosclerotic lesions, inflammatory cell accumulation and atherosclerosis were severely reduced in St3Gal4−/−Apoe−/− mice. These findings reveal a potentially important role of sialylation in Ccl5-mediated leukocyte recruitment and arrest under chronic inflammatory conditions and suggest that targeting sialylation in atherosclerosis, for example by specific inhibitors of ST3Gal-IV, might be a new promising therapeutical approach.
Acknowledgments
We thank Yvonne Jansen, Patricia Lemnitzer, Susanne Bierschenk, Melanie Garbe, Stephanie Elbin, and Leon Decker for excellent technical assistance.
Sources of Funding
This work was supported by the European Research Council (ERC AdG 249929 to C.W.), the Netherlands Organisation for Scientific Research (NWO; VIDI project 91712303 to O.S.), the German Research Foundation (DFG; SO876/3-1, SO876/6-1, FOR809, SFB914-B08 to O.S. and C.W.; SFB914-B01 to M.S.), the Else Kröner Fresenius Stiftung (to O.S.), the Mizutani Foundation (090063/2009 to M.S.), and the LMU excellent initiative.
Nonstandard Abbreviations and Acronyms
- ST3Gal-IV
α2,3-sialyltransferase IV
- TNFα
tumor necrosis factor-α
Footnotes
Disclosures
C. Weber is a shareholder of Carolus Therapeutics Inc, a company developing chemokine-based anti-inflammatory strategies.
The online-only Data Supplement is available with this article at http://circres.ahajournals.org/lookup/suppl/doi:10.1161/CIRCRESAHA.114.302426/-/DC1.
References
- 1.Sperandio M, Gleissner CA, Ley K. Glycosylation in immune cell trafficking. Immunol Rev. 2009;230:97–113. doi: 10.1111/j.1600-065X.2009.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellies LG, Sperandio M, Underhill GH, Yousif J, Smith M, Priatel JJ, Kansas GS, Ley K, Marth JD. Sialyltransferase specificity in selectin ligand formation. Blood. 2002;100:3618–3625. doi: 10.1182/blood-2002-04-1007. [DOI] [PubMed] [Google Scholar]
- 3.Sperandio M, Frommhold D, Babushkina I, Ellies LG, Olson TS, Smith ML, Fritzsching B, Pauly E, Smith DF, Nobiling R, Linderkamp O, Marth JD, Ley K. Alpha 2,3-sialyltransferase-IV is essential for L-selectin ligand function in inflammation. Eur J Immunol. 2006;36:3207–3215. doi: 10.1002/eji.200636157. [DOI] [PubMed] [Google Scholar]
- 4.Yang WH, Nussbaum C, Grewal PK, Marth JD, Sperandio M. Coordinated roles of ST3Gal-VI and ST3Gal-IV sialyltransferases in the synthesis of selectin ligands. Blood. 2012;120:1015–1026. doi: 10.1182/blood-2012-04-424366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frommhold D, Ludwig A, Bixel MG, et al. Sialyltransferase ST3Gal-IV controls CXCR2-mediated firm leukocyte arrest during inflammation. J Exp Med. 2008;205:1435–1446. doi: 10.1084/jem.20070846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bannert N, Craig S, Farzan M, Sogah D, Santo NV, Choe H, Sodroski J. Sialylated O-glycans and sulfated tyrosines in the NH2-terminal domain of CC chemokine receptor 5 contribute to high affinity binding of chemokines. J Exp Med. 2001;194:1661–1673. doi: 10.1084/jem.194.11.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122:1837–1845. doi: 10.1161/CIRCULATIONAHA.110.961714. [DOI] [PubMed] [Google Scholar]
- 9.Soehnlein O, Drechsler M, Döring Y, et al. Distinct functions of chemokine receptor axes in the atherogenic mobilization and recruitment of classical monocytes. EMBO Mol Med. 2013;5:471–481. doi: 10.1002/emmm.201201717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellies LG, Ditto D, Levy GG, Wahrenbrock M, Ginsburg D, Varki A, Le DT, Marth JD. Sialyltransferase ST3Gal-IV operates as a dominant modifier of hemostasis by concealing asialoglycoprotein receptor ligands. Proc Natl Acad Sci U S A. 2002;99:10042–10047. doi: 10.1073/pnas.142005099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 12.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 13.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veillard NR, Steffens S, Pelli G, Lu B, Kwak BR, Gerard C, Charo IF, Mach F. Differential influence of chemokine receptors CCR2 and CXCR3 in development of atherosclerosis in vivo. Circulation. 2005;112:870–878. doi: 10.1161/CIRCULATIONAHA.104.520718. [DOI] [PubMed] [Google Scholar]
- 15.Saederup N, Chan L, Lira SA, Charo IF. Fractalkine deficiency markedly reduces macrophage accumulation and atherosclerotic lesion formation in CCR2−/− mice: evidence for independent chemokine functions in atherogenesis. Circulation. 2008;117:1642–1648. doi: 10.1161/CIRCULATIONAHA.107.743872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landsman L, Bar-On L, Zernecke A, Kim KW, Krauthgamer R, Shagdarsuren E, Lira SA, Weissman IL, Weber C, Jung S. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood. 2009;113:963–972. doi: 10.1182/blood-2008-07-170787. [DOI] [PubMed] [Google Scholar]
- 17.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 18.Guo J, Van Eck M, Twisk J, Maeda N, Benson GM, Groot PH, Van Berkel TJ. Transplantation of monocyte CC-chemokine receptor 2-deficient bone marrow into ApoE3-Leiden mice inhibits atherogenesis. Arterioscler Thromb Vasc Biol. 2003;23:447–453. doi: 10.1161/01.ATV.0000058431.78833.F5. [DOI] [PubMed] [Google Scholar]
- 19.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 20.Engel DR, Maurer J, Tittel AP, Weisheit C, Cavlar T, Schumak B, Limmer A, van Rooijen N, Trautwein C, Tacke F, Kurts C. CCR2 mediates homeostatic and inflammatory release of Gr1(high) monocytes from the bone marrow, but is dispensable for bladder infiltration in bacterial urinary tract infection. J Immunol. 2008;181:5579–5586. doi: 10.4049/jimmunol.181.8.5579. [DOI] [PubMed] [Google Scholar]
- 21.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Combadière C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 23.von Hundelshausen P, Weber KS, Huo Y, Proudfoot AE, Nelson PJ, Ley K, Weber C. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation. 2001;103:1772–1777. doi: 10.1161/01.cir.103.13.1772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.