Abstract
Mandatory folic acid fortification in the United States corresponded with a decline in the prevalence of spina bifida (SB). The aim of this study was to describe the epidemiologic characteristics of isolated versus non-isolated SB cases in both pre- and post-fortification periods. SB cases in the Slone Epidemiology Center Birth Defects Study from 1976 to 2011 without chromosomal anomalies and syndromes were included. A maternal interview, conducted within 6 months of delivery, collected information on demographics, reproductive history, diet, and supplement use. Daily folic acid intake in the periconceptional period was calculated using both dietary and supplement information and categorized as low intake (<400 μg/day) or high intake (≥400 μg/day). SB cases (n=1170) were classified as isolated (80.4%) or non-isolated (19.1%). Non-isolated cases were further divided into subgroups based on accompanying major malformations (midline, renal, genital, heart, laterality). Compared to non-isolated cases, isolated cases were more likely to be white, non-Hispanic and have more than 12 years of education. Cases in the renal, genital, and heart subgroups had the lowest proportions of mothers with a high folic acid intake. The change from pre- to post-fortification was associated with a decrease in the proportion of isolated cases from 83% to 72%, though in both periods isolated cases were more likely to be female and their mothers were more likely to have high folic acid intake. These findings highlight the importance of separating isolated and non-isolated cases in etiologic research of SB.
Keywords: congenital abnormalities, folic acid, spina bifida
INTRODUCTION
Spina bifida (SB) is a type of neural tube defect (NTD) resulting from incomplete closure of the neural tube during embryonic development. SB frequently results in paralysis below the level of the lesion and is associated with long-term physical and cognitive disabilities [Hetherington et al., 2006; Jenkinson et al., 2011]. The prevalence of SB in the United States from 2004 to 2006 was 3.5 per 10,000 live births, affecting an estimated 1,460 infants annually [Parker et al., 2010]. One-year survival of infants with SB is estimated to be 90.8% [Shin et al., 2012].
Established risk factors for SB include family history, pre-gestational diabetes [Correa et al., 2008], maternal obesity [Shaw et al., 1996; Watkins et al., 2003; Waller et al., 2007], and insufficient intake of folic acid [Medical Research Council Vitamin Study Research Group, 1991; Czeizel and Dudas, 1992; Werler et al., 1993]. Studies have investigated several other environmental and dietary exposures as potential risk factors, but findings have been less definitive, leaving the majority of SB cases with unknown causes. It has long been observed that risk factors for neural tube defects differ according to whether the defect is isolated or accompanied by other malformations (“non-isolated”) [Holmes et al., 1976; Khoury et al., 1982], suggesting that isolated SB and non-isolated SB are etiologically heterogeneous. Frequently, epidemiologic studies of risk factors for SB do not distinguish between such classifications, potentially hindering identification of risk factors.
Most of the literature on epidemiologic characteristics of isolated versus non-isolated cases of SB precedes the era of folic acid fortification, which was mandated in the United States in 1998. The transition from pre-fortification to fortification was associated with a 31% decrease in the prevalence of SB in the immediate post-fortification period [Williams et al., 2002]. The prevalence of SB has continued to decrease, but the decline has been more gradual [Boulet et al., 2008]. Further research revealed a significant decline between 1992 and 2009 in the prevalence of isolated NTDs, but not non-isolated NTDs [Collins et al., 2011]. The evidence suggesting that folic acid may be more effective in reducing the occurrence of isolated SB than non-isolated SB raises the possibility that the characteristics of cases have changed over time [Yen et al., 1992]. The objective of this study is to describe characteristics of isolated SB and SB accompanied by other major malformations in a case group that spans both pre- and post-fortification time periods.
MATERIALS AND METHODS
The Slone Epidemiology Center Birth Defects Study (BDS)is an on-going case–control study in the United States and Canada that began in 1976. Cases of birth defects are ascertained from birth hospitals or tertiary care centers in Boston, MA (1976+); Philadelphia, PA (1976+); San Diego, CA (2001+); Toronto, Canada (1976–2005); selected counties in Iowa (1983–1985); and from birth defects registries in Massachusetts (1999+) and parts of New York State (2004+). Cases include primarily livebirths;though fetal deaths and elective terminations were eligible for inclusion beginning in 1990, ascertainment of these cases has not been routine.
A maternal interview is conducted by a trained nurse interviewer within 6 months of delivery. The interview captures information on demographics, pregnancy history, medication use, supplement use, and family history of birth defects.
CASE CLASSIFICATION
The present study includes SB cases ascertained between 1976 and 2011. All cases of SB were classified by a clinical geneticist (LD). Cases were classified as isolated, non-isolated, or unknown. Isolated cases were those with no other major malformations, but may have had minor malformations or deformations (e.g., heart murmurs, patent foramen ovale, patent ductus arteriosus, skin tags, tethered cord, tongue-tie, hip clicks, type B (minimus) post-axial polydactyly, rockerbottom feet, hemangiomas, inguinal hernias, and umbilical hernias), or anomalies considered to be secondary to SB (other NTDs, hydrocephaly, clubfoot, and congenital hip dislocation). Non-isolated cases were those with at least one other major non-NTD malformation. Unknown cases had confirmed SB but lacked sufficient detail on other malformations to warrant classification as either isolated or non-isolated. Cases with a chromosomal anomaly, amniotic band syndrome, a body wall defect, conjoined twins, or selected recognized syndromes (Holt-Oram, Aicardi, Fryns, Meckel-Gruber, Currarino triad, and Seckel) were excluded. Cases without a confirmed medical record diagnosis of SB were also excluded. Information on the source of case confirmation was available beginning in 1988; subsequently 97.4% of cases were confirmed on the basis of information in the medical record.
NON-ISOLATED DEFECT SUBGROUPS
Non-isolated cases were further classified into subgroups based on the type of other major defects present: midline defects, renal/urinary defects, genital defects, heart defects, and laterality defects. Midline defects included conotruncal heart defects, oral clefts, brain reduction defects, omphalocele, hypospadias, and diaphragmatic hernia [Khoury et al., 1989]. Infants with congenital hydrocephalus were not considered as having a non-isolated midline defect due to the likelihood that it is secondary to SB. Renal/urinary defects included renal agenesis, absent kidney, and polycystic kidney. Hydronephrosis and vesico-ureter reflux were not considered major renal malformations. Genital defects included undescended testes at >37 weeks’ gestation, ambiguous genitalia, and hypospadias. Heart defects included ventricular septal defects, conotruncal heart defects, and coarctation of the aorta. Laterality defects included polysplenia and dextrocardia. Due to the possibility of a case having multiple major malformations, subgroup categories are not mutually exclusive.
COVARIATES
Data on demographics and reproductive history were collected through the maternal interview. Prior to 1983, women who reported Hispanic ethnicity were included in the “other race” category. Folic acid intake in the periconceptional period, defined as the month prior to and after the last menstrual period (LMP), was determined through the information collected on vitamin and supplement use (1976+) and responses to a food frequency questionnaire on natural sources (e.g., spinach) and fortified products (e.g., pasta) (1988+) [Willett et al., 1985]. Folic acid intake was based only on reported consumption of vitamins/supplements prior to 1988 because information on dietary sources was unavailable and the contribution from diet during this period would have been small since fortification had yet to occur. Subsequently, folic acid intake was based on the combination of sources from vitamins/supplements and diet. Subjects were categorized into two groups; those achieving the recommended amount of ≥400 μg/day of folic acid (“high intake”) and those with an intake of <400 μg/day (“low intake”). Food folate was discounted by 30% due to lower bioavailability. Methods to determine folic acid intake from diet and supplements in the BDS have previously been described in detail [Yazdy et al., 2012].
STATISTICAL ANALYSIS
Frequencies and percentages of demographic characteristics were calculated for isolated cases, non-isolated cases overall, and subgroups of non-isolated cases. Demographic characteristics included maternal race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic [1983+], other), age at conception (<20, 20–29, ≥30 years), education (<12, 12, >12 years), infant sex, and study center, along with folic acid intake in the periconceptional period. To assess changes in types of cases over time, the distribution of case types between pre- and post-fortification periods were compared. The pre-fortification period included mothers of cases with an LMP prior to January 1, 1996. The post-fortification period was defined as those with an LMP date on or after January 1, 1998. Cases with an LMP date from 1996 to 1997 were excluded since this was a transitional period in which fortification of the food supply began, but was not yet mandated. Analyses were performed using SAS 9.3 software.
RESULTS
A total of 1,237 cases of SB were ascertained by the BDS from 1976 to 2011. After exclusion of cases with chromosomal anomalies, recognized syndromes, amniotic bands, body wall defects, conjoined twins, and unconfirmed cases, 1,170 cases were included in this analysis (Fig. 1). Of these, 80.4% were classified as isolated, 19.1% were classified as non-isolated, and 0.5% of cases were unknown.
FIG. 1.
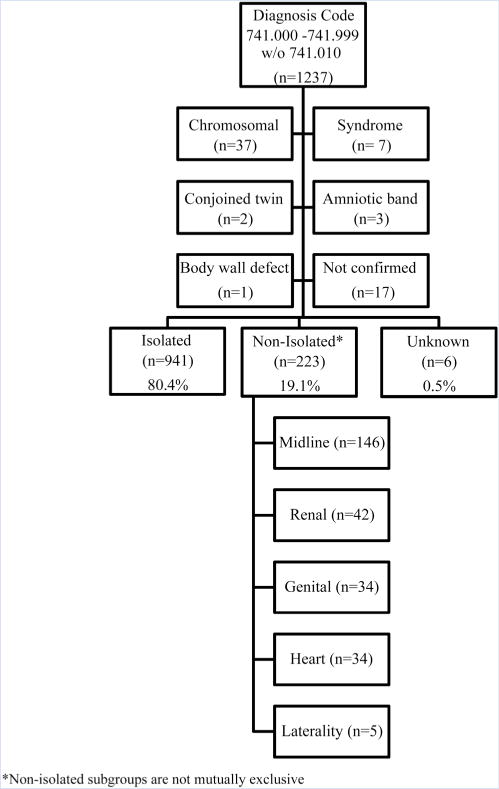
Classification of spina bifida cases and exclusions, Slone Birth Defects Study, 1976–2011.
The 223 non-isolated cases were further divided into the following subgroups based on accompanying major defects; midline defects (n= 146), renal defects (n = 42), genital defects (n = 34), heart defects (n = 34), and laterality defects (n = 5). Among midline defects, present in 65% of non-isolated cases, brain reduction defects were most common, followed by imperforate anus, cloacal exstrophy, omphalocele, and bladder exstrophy (Table I). OEIS complex, which includes all the following defects; omphalocele, exstrophy of the bladder, imperforate anus, and SB [Carey et al., 1978], was observed among four cases. Renal defects were present in 19% of non-isolated cases; the most common defects included unilateral kidney absence/agenesis and horseshoe kidney (14 and 7 cases, respectively). The genital defect group (15%) was largely comprised of undescended testicle at >37 weeks’ gestation and ambiguous genitalia. Among congenital heart defects, (15%), ventricular septal defects were most common, followed by double outlet right ventricle. Laterality defects were observed in only five cases (2%), and thus their epidemiologic characteristics were not tabulated.
TABLE I.
Co-Occurring Defects Among Cases With Spina Bifida, Slone Birth Defects Study, 1976–2011
| n | % | n | % | ||
|---|---|---|---|---|---|
| Midline defects (n = 146) | Genital defects (n = 34) | ||||
| Brain reduction defects | 73 | 50.0 | Undescended testicles >37 weeks gestation | 11 | 32.4 |
| Imperforate anus | 22 | 15.1 | Ambiguous genitalia | 10 | 29.4 |
| Cloacal exstrophy | 13 | 8.9 | Cervix/vagina/clitoris anomalies | 5 | 14.7 |
| Orofacial clefts | 12 | 8.2 | Hypospadias ± chordee | 5 | 14.7 |
| Omphalocele | 12 | 8.2 | Uterus/ovary anomalies | 3 | 8.8 |
| Bladder exstrophy | 12 | 8.2 | Rectovaginal fistula | 2 | 5.9 |
| Microcephaly | 9 | 6.2 | Epispadias | 2 | 5.9 |
| Hypospadias/epispadias | 7 | 4.8 | Micropenis | 1 | 2.9 |
| Diaphragmatic hernia | 7 | 4.8 | Heart defects (n = 34) | ||
| Double outlet right venticle | 4 | 2.7 | VSD—other/NOS | 15 | 44.1 |
| Tracheoesophageal fistula ± esophageal atresia | 4 | 2.7 | VSD—muscular | 5 | 14.7 |
| OEIS | 4 | 2.7 | Double outlet right ventricle | 4 | 11.8 |
| Caudal regression | 3 | 2.1 | VSD—perimembranous | 3 | 8.8 |
| Truncus arteriosus | 2 | 1.4 | Other heart anomalies | 3 | 8.8 |
| Tetralogy of fallot | 2 | 1.4 | Truncus arteriosus | 2 | 5.9 |
| Ectopia cordis | 1 | 0.7 | Tetralogy of fallot | 2 | 5.9 |
| Renal defects (n = 42) | Pulmonary valve atresia/stenosis | 2 | 5.9 | ||
| Kidney agenesis (unilat.) | 14 | 33.3 | Dextrocardia | 2 | 5.9 |
| Horseshoe kidney | 7 | 16.7 | Other valve anomalies | 2 | 5.9 |
| Double collecting system | 5 | 11.9 | Double outlet left ventricle | 1 | 2.9 |
| Ectopic kidney | 4 | 9.5 | Ostiumprimum ASD | 1 | 2.9 |
| Multicystic kidney type II | 3 | 7.1 | Common AV canal | 1 | 2.9 |
| Kidney agenesis (NOS) | 2 | 4.8 | Aortic valve stenosis | 1 | 2.9 |
| Kidney dysplasia (unilat.) | 1 | 2.4 | Hypoplastic L ventricle | 1 | 2.9 |
| Kidney agenesis (bilat.) | 1 | 2.4 | R ventricular hypertrophy | 1 | 2.9 |
| Polycystic kidneys | 1 | 2.4 | Coarctation of aorta | 1 | 2.9 |
| Accessory kidney | 1 | 2.4 | Right aortic arch | 1 | 2.9 |
| Absent ureter | 1 | 2.4 | Persistent L superior vena cava | 1 | 2.9 |
| Absent bladder/urethra | 1 | 2.4 | Anomalous pulmonary venous return | 1 | 2.9 |
| Prune belly syndrome | 1 | 2.4 | |||
| Kidney dysplasia (bilat.) | 1 | 2.4 |
OEIS, omphalocele, exstrophy, imperforate anus, and spina bifida.
Descriptive characteristics of isolated cases and non-isolated cases and their subgroups are presented in Table II. In all, mothers were more likely to be white, non-Hispanic and 20–29 years old at conception. However, compared to all other groups, cases with genital defects or heart defects had greater proportions of mothers aged less than 20 years and with less than 12 years of education. Cases with heart defects had the highest proportion of mothers whose race was either Hispanic or “other” (17.6% and 29.4%, respectively). Female predominance was evident in all case subgroups with the exception of midline and genital defects, both of which include hypospadias, a defect occurring only among males. Cases with renal defects had the highest proportion of females (61.9%).
TABLE II.
Demographic Characteristics of Spina Bifida Cases, Slone Birth Defects Study, 1976–2011
| Isolated SB (n=941)
|
Non-isolated SB (n=223)
|
Midline defects (n=146)
|
Renal defects (n=42)
|
Genital defects (n=34)
|
Heart defects (n=34)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Infant sex | ||||||||||||
| Male | 414 | 44.0 | 107 | 48.0 | 75 | 51.4 | 15 | 35.7 | 19 | 55.9 | 16 | 47.1 |
| Female | 493 | 52.4 | 110 | 49.3 | 68 | 46.6 | 26 | 61.9 | 15 | 44.1 | 18 | 52.9 |
| Unknown | 34 | 3.6 | 5 | 2.2 | 3 | 2.1 | 1 | 2.4 | 0 | 0.0 | 0 | 0.0 |
| Maternal race/ethnicity | ||||||||||||
| White, non-Hispanic | 812 | 86.3 | 180 | 80.7 | 120 | 82.2 | 39 | 92.9 | 30 | 88.2 | 22 | 64.7 |
| Black, non-Hispanic | 48 | 5.1 | 13 | 5.8 | 8 | 5.5 | 1 | 2.4 | 2 | 5.9 | 2 | 5.9 |
| Other racesa | 81 | 8.6 | 29 | 13.0 | 18 | 12.3 | 2 | 4.8 | 2 | 5.9 | 10 | 29.4 |
| Hispanic (1983+) | 54 | 5.7 | 23 | 10.3 | 16 | 11.0 | 2 | 4.8 | 2 | 5.9 | 6 | 17.6 |
| Maternal age at conception | ||||||||||||
| <20 years | 73 | 7.8 | 22 | 9.9 | 17 | 11.6 | 4 | 9.5 | 6 | 17.6 | 5 | 14.7 |
| 20–29 years | 526 | 55.9 | 129 | 57.8 | 78 | 53.4 | 26 | 61.9 | 20 | 58.8 | 22 | 64.7 |
| ≥30 years | 342 | 36.3 | 72 | 32.3 | 51 | 34.9 | 12 | 28.6 | 8 | 23.5 | 7 | 20.6 |
| Maternal education | ||||||||||||
| <12 years | 142 | 15.1 | 37 | 16.6 | 25 | 17.1 | 6 | 14.3 | 8 | 23.5 | 9 | 26.5 |
| 12 years | 331 | 35.2 | 89 | 39.9 | 55 | 37.7 | 19 | 45.2 | 13 | 38.2 | 14 | 41.2 |
| >12 years | 466 | 49.5 | 96 | 43.0 | 66 | 45.2 | 17 | 40.5 | 13 | 38.2 | 11 | 32.4 |
| Missing data | 2 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Folic acid intake | ||||||||||||
| ≥400 μg | 230 | 24.4 | 57 | 25.6 | 38 | 26.0 | 8 | 19.0 | 7 | 20.6 | 6 | 17.6 |
| <400 μg | 665 | 70.7 | 156 | 70.0 | 102 | 69.9 | 33 | 78.6 | 25 | 73.5 | 26 | 76.5 |
| Missing data | 46 | 4.9 | 10 | 4.5 | 6 | 4.1 | 1 | 2.4 | 2 | 5.9 | 2 | 5.9 |
Other races category includes Hispanic race/ethnicity.
Folic acid intake was similar between isolated and non-isolated cases, with at least 70% not achieving the current recommendation of ≥400 μg. Renal and heart defect subgroups had the highest proportion of mothers with <400 μg of daily folic acid (78.6% and 76.5%, respectively). Sufficient data to categorize folic acid intake were lacking in 2.4–5.9% of each defect grouping.
As expected, the proportion of women with intake of at least 400 μg of folic acid was higher in the post-fortification period. Following mandated folic acid fortification of the foods in 1998, the percentage of case mothers achieving a folic acid intake ≥400 μg increased from 17.5% to 49.4%. During those periods, the proportion of isolated cases decreased from 83% to 72% (Table III).
TABLE III.
Distribution of Spina Bifida Case Types, Pre- and Post-Fortification
| Pre-fortificationa (n=880)
|
Post-fortificationb (n =256)
|
|||
|---|---|---|---|---|
| n | % | n | % | |
| Isolated | 733 | 83.3 | 185 | 72.3 |
| Non-isolated | 147 | 16.7 | 71 | 27.7 |
| Midline | 100 | 68.0 | 44 | 62.0 |
| Renal | 32 | 21.8 | 9 | 12.7 |
| Genital | 22 | 15.0 | 12 | 16.9 |
| Heart | 17 | 11.6 | 16 | 22.5 |
LMP dates prior to January 1, 1996.
LMP dates on or after January 1, 1998.
The demographic characteristics among isolated and nonisolated cases also changed from pre- to post-fortification periods. In the pre-fortification time period, mothers of isolated cases were less likely to be black, <20 years old, or have <12 years of education compared to non-isolated cases, whereas the opposite was observed in the post-fortification period (Table IV). The female predominance was most apparent in pre-fortification isolated cases. Despite changing demographics, in both periods, mothers of non-isolated cases were less likely to achieve a folic acid intake ≥400 μg. Characteristics of non-isolated sub-groups in pre- and post-fortification periods are presented in Table V. The heart subgroup had the highest proportion of mothers not achieving the recommended amount of folic acid in both the pre- and post-fortification periods.
TABLE IV.
Distribution of Demographic Characteristics by Spina Bifida Classification, Pre- and Post-Fortification
| Pre-fortificationa
|
Post-fortificationb
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Isolated SB (n = 733)
|
Non-isolated SB (n = 147)
|
Isolated SB (n = 185)
|
Non-isolated SB (n = 71)
|
|||||
| n | % | n | % | n | % | n | % | |
| Infant sex | ||||||||
| Male | 315 | 43.0 | 70 | 47.6 | 87 | 47.0 | 36 | 50.7 |
| Female | 390 | 53.2 | 73 | 49.7 | 93 | 50.3 | 34 | 47.9 |
| Unknown | 28 | 3.8 | 4 | 2.7 | 5 | 2.7 | 1 | 1.4 |
| Maternal race/ethnicity | ||||||||
| White, non-Hispanic | 681 | 92.9 | 130 | 88.4 | 109 | 58.9 | 46 | 64.8 |
| Black, non-Hispanic | 24 | 3.3 | 10 | 6.8 | 24 | 13.0 | 3 | 4.2 |
| Other racesc | 28 | 3.8 | 7 | 4.8 | 52 | 28.1 | 22 | 31.0 |
| Hispanic (1983+) | 15 | 2.0 | 5 | 3.4 | 38 | 20.5 | 18 | 25.4 |
| Maternal age at conception | ||||||||
| <20 years | 57 | 7.8 | 18 | 12.2 | 16 | 8.6 | 4 | 5.6 |
| 20–29 years | 430 | 58.7 | 82 | 55.8 | 81 | 43.8 | 44 | 62.0 |
| ≥30 years | 246 | 33.6 | 47 | 32.0 | 88 | 47.6 | 23 | 32.4 |
| Maternal education | ||||||||
| <12 years | 109 | 14.9 | 28 | 19.0 | 30 | 16.2 | 9 | 12.7 |
| 12 years | 289 | 39.4 | 62 | 42.2 | 37 | 20.0 | 25 | 35.2 |
| >12 years | 334 | 45.6 | 57 | 38.8 | 117 | 63.2 | 37 | 52.1 |
| Missing data | 1 | 0.1 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 |
| Folic acid intake | ||||||||
| ≥400 μg | 129 | 17.6 | 22 | 15.0 | 94 | 50.8 | 33 | 46.5 |
| <400 μg | 567 | 77.4 | 117 | 79.6 | 82 | 44.3 | 36 | 50.7 |
| Missing data | 37 | 5.0 | 8 | 5.4 | 9 | 4.9 | 2 | 2.8 |
LMP dates prior to January 1, 1996.
LMP dates on or after January 1, 1998.
Other races includes Hispanic race/ethnicity.
TABLE V.
Distribution of Demographic Characteristics by Spina Bifida Non-Isolated Sub-Groups, Pre- and Post-Fortification
| Pre-fortificationa
|
Post-fortificationb
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Midline (n = 100)
|
Renal (n = 32)
|
Genital (n = 22)
|
Heart (n = 17)
|
Midline (n = 44)
|
Renal (n = 9)
|
Genital (n = 12)
|
Heart (n = 16)
|
|||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Infant sex | ||||||||||||||||
| Male | 50 | 50.0 | 11 | 34.4 | 12 | 54.5 | 9 | 52.9 | 25 | 56.8 | 4 | 44.4 | 7 | 58.3 | 7 | 43.8 |
| Female | 47 | 47.0 | 20 | 62.5 | 10 | 45.5 | 8 | 47.1 | 19 | 43.2 | 5 | 55.6 | 5 | 41.7 | 9 | 56.3 |
| Unknown | 3 | 3.0 | 1 | 3.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Maternal race/ethnicity | ||||||||||||||||
| White, non-Hispanic | 88 | 88.0 | 31 | 96.9 | 21 | 95.5 | 14 | 82.4 | 30 | 68.2 | 7 | 77.8 | 9 | 75.0 | 7 | 43.8 |
| Black, non-Hispanic | 7 | 7.0 | 0 | 0.0 | 1 | 4.5 | 1 | 5.9 | 1 | 2.3 | 1 | 11.1 | 1 | 8.3 | 1 | 6.3 |
| Other racesc | 5 | 5.0 | 1 | 3.1 | 0 | 0.0 | 2 | 11.8 | 13 | 29.5 | 1 | 11.1 | 2 | 16.7 | 8 | 50.0 |
| Hispanic (1983+) | 4 | 4.0 | 1 | 3.1 | 0 | 0.0 | 0 | 0.0 | 12 | 27.3 | 1 | 11.1 | 2 | 16.7 | 6 | 37.5 |
| Maternal age at conception | ||||||||||||||||
| <20 years | 13 | 13.0 | 3 | 9.4 | 4 | 18.2 | 4 | 23.5 | 4 | 9.1 | 1 | 11.1 | 2 | 16.7 | 1 | 6.3 |
| 20–29 years | 50 | 50.0 | 21 | 65.6 | 15 | 68.2 | 10 | 58.8 | 27 | 61.4 | 4 | 44.4 | 5 | 41.7 | 12 | 75.0 |
| ≥30 years | 37 | 37.0 | 8 | 25.0 | 3 | 13.6 | 3 | 17.6 | 13 | 29.5 | 4 | 44.4 | 5 | 41.7 | 3 | 18.8 |
| Maternal education | ||||||||||||||||
| <12 years | 19 | 19.0 | 5 | 15.6 | 6 | 27.3 | 5 | 29.4 | 6 | 13.6 | 1 | 11.1 | 2 | 16.7 | 4 | 25.0 |
| 12 years | 41 | 41.0 | 14 | 43.8 | 9 | 40.9 | 7 | 41.2 | 14 | 31.8 | 5 | 55.6 | 4 | 33.3 | 6 | 37.5 |
| >12 years | 40 | 40.0 | 13 | 40.6 | 7 | 31.8 | 5 | 29.4 | 24 | 54.5 | 3 | 33.3 | 6 | 50.0 | 6 | 37.5 |
| Missing data | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Folic acid intake | ||||||||||||||||
| ≥400μg | 15 | 15.0 | 3 | 9.4 | 1 | 4.5 | 1 | 5.9 | 22 | 50.0 | 5 | 55.6 | 6 | 50.0 | 4 | 25.0 |
| <400μg | 80 | 80.0 | 28 | 87.5 | 19 | 86.4 | 16 | 94.1 | 21 | 47.7 | 4 | 44.4 | 6 | 50.0 | 10 | 62.5 |
| Missing data | 5 | 5.0 | 1 | 3.1 | 2 | 9.1 | 0 | 0.0 | 1 | 2.3 | 0 | 0.0 | 0 | 0.0 | 2 | 12.5 |
LMP dates prior to January 1, 1996.
LMP dates on or after January 1, 1998.
Other races includes Hispanic race/ethnicity.
DISCUSSION
This large sample of SB cases with detailed medical record information and maternal interview data, permitted description of the epidemiologic characteristics of SB cases with and without accompanying malformations. Approximately one-fifth of all SB cases in the present study were accompanied by other major malformations, a proportion similar to the 20.2% reported among a hospital-based population in France from 1979 through 2008 [Stoll et al., 2011] and the 21.0% reported in the population-based Metropolitan Atlanta Congenital Defects Program from 1968 to 1979 [Khoury et al., 1982]. A much lower rate of non-isolated SB (12%) was reported from the South Carolina Birth Defects Surveillance Program (1992–2002), which might be due to under-ascertainment of accompanying malformations in the large number of cases that were identified by prenatal screening [Stevenson et al., 2004].
In the present study, the most common subgroup of accompanying defects was midline defects, which were present in 65% of non-isolated cases. Previous studies focusing on all types of NTDs reported midline defects among 41–48% of all non-isolated NTDs [Stevenson et al., 2000; Stoll et al., 2011]. The concept of a primary midline developmental field was first introduced by Opitz and Gilbert [1982] who postulated that insults during early blastogenesis would affect the whole embryo and produce associated defects of blastogenic origin [Martinez-Frias, 1995]. Among our cases, brain reduction defects, such as agenesis of the corpus callosum and holoproscencephaly, constituted the majority of midline defects. Development of midline brain structures, including the corpus callosum, is known to be compromised in embryos affected by SB [Juranek and Salman, 2010] and may therefore be secondary to SB. However, after exclusion of brain reduction defects from the midline category, the midline subgroup was still the largest, with 76 cases, or 34% of all non-isolated cases. It has been suggested that other midline defects, such as omphalocele, may in fact be secondary to SB [Calzolari et al., 1997]. In the event that some of these cases are truly isolated, misclassification would be introduced by including them in the midline sub-grouping. Other large subgroups of accompanying defects were renal, genital, and heart defects. This is the first study that describes epidemiologic characteristics of not only isolated and non-isolated SB cases, but also these large subgroups of defects co-occurring with SB.
The consistently observed female predominance of SB [Shaw et al., 2003] was evident in isolated cases, but not in non-isolated cases overall. This predominance diminished in the post-fortification period. In the pre-fortification era, Stevenson et al. [2000] also observed a female predominance in isolated, but not non-isolated, cases of SB. The renal defect subgroup had the greatest proportion of females (62%). There is no clear explanation for this observation given that renal defects are not known to occur more frequently in female offspring [Shaw et al., 2003; Rittler et al., 2004]. Unilateral renal agenesis can be asymptomatic and while the presence of SB may have prompted the diagnosis, such diagnostic bias would not explain the female predominance. The genital defect group showed a male predominance which was driven by the inclusion of cases of undescended testicles at >37 weeks gestation and hypospadias, birth defects occurring only in males.
Differences in socio-demographic factors and folic acid intake were observed for various phenotypic subgroups, suggesting possible etiologic heterogeneity. Specifically, while relatively little differences in maternal race/ethnicity, age, education, and folic acid intake were observed for SB cases occurring alone compared to those accompanied by other major malformations overall, for cases with a genital or heart defect, mothers were younger and less educated than other case subgroups. Cases with SB and a heart defect were also more likely to be Hispanic and least likely to ingest ≥400 μg of folic acid daily. Lower socioeconomic status is correlated with lower folic acid intake [Centers for Disease Control and Prevention, 1999; Carmichael et al., 2006] and many other nutritional and behavioral factors, raising the possibility that our observations could be explained by any number of such correlated exposures. However, when the effects of folic acid intake and indicators of socioeconomic status(maternal race/ethnicity, age, or education) were considered simultaneously, the proportion of heart defect cases remained greater among those with folic acid intake <400 μg/day (data not shown). We were unable to assess known risk factors for SB such as pre-gestational diabetes and valproate exposure due to small numbers.
Folic acid fortification was associated with a decrease in the proportion of isolated cases and thereby an increase in the proportion of non-isolated cases. The higher proportion of non-isolated SB cases overall after fortification is consistent with previous reports, indicating that folic acid is most effective in reducing the occurrence of isolated SB [Stevenson et al., 2004;Lopez-Camelo et al., 2010]. This finding was most striking for SB cases with heart defects. If folic acid intake of <400 μg/day is more strongly associated with SB when it occurs with a heart defect, then we might expect there to be more such cases during the pre-fortification years. However, we observed the opposite pattern, with a higher proportion of heart defects in the post-fortification years. Explanations for this observation include improved ascertainment of heart defects relative to the other defect categories or the role of folic acid in reducing the other defect sub-groups disproportionately to the heart defect group. Thus, this study provides new evidence that folic acid may be more effective in reducing the occurrence of particular subgroups of non-isolated SB.
A study by De Wals et al. [2008] observed that the transition from pre-to post-fortification in Canada was associated with a reduction in upper SB, defined as cranial, cervical, and thoracic lesions, but not lower SB, defined as lumbar and sacral lesions. Information on lesion was not available for all subjects in the present analysis, but a sub-analysis similarly showed a decline in upper SB, but not lower SB. The decline in upper SB occurred in both isolated and non-isolated SB, but was larger for non-isolated SB (data not shown).
The cases in this descriptive analysis are part of a larger case–control study that was designed to study potential risk factors for a spectrum of birth defects, therefore, the prevalence of SB cannot be estimated. A challenge in studying NTDs is the ascertainment of electively terminated cases. Though terminated cases were included in this study, they were not routinely collected. Compared to previous studies which found that elective terminations accounted for 35–44% of SB cases [Stevenson et al., 2000; Cragan and Gilboa, 2009], 18.2% of SB cases included in this study were electively terminated, that proportion was 11.3% in another large multi-site case–control study of birth defects in the United States [Agopian et al., 2012]. A previous study reported that terminated fetuses with SB were more likely to have body wall defects, diaphragmatic hernia, and cystic kidney compared to their liveborn counterparts [Kalien et al., 1998], and a recent meta-analysis showed that the frequency of termination for all cases was higher than for isolated cases alone [Johnson et al., 2012]. Thus, it is likely that our study underestimated the proportion of non-isolated defects due to incomplete ascertainment of terminations. However, ascertainment of terminations was higher during the pre-fortification period, 19.5% compared to 15.7% in the post-fortification period, and would not explain the greater proportion of non-isolated cases during the post-fortification period.
The present study supports previous findings that folic acid is more effective in reducing the occurrence of isolated SB compared to non-isolated SB; this effect was observed in both the pre- and post-fortification periods, and was most striking for heart defects. Further, our study extends previous findings in highlighting the importance of distinguishing between isolated and non-isolated cases in the etiologic research of SB. Differentiating non-isolated cases of SB into groupings based on their accompanying defects may provide clearer insights into their underlying etiology.
Acknowledgments
This work was supported by the Centers for Disease Control and Prevention (DD000697). Samantha Parker was a pre-doctoral trainee supported by NIH T32 HD052458 (Boston University Reproductive, Perinatal, and Pediatric Epidemiology training program). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. We thank Dawn Jacobs, RN, MPH, Fiona Rice, MPH, Rita Krolak, RN, Kathleen Sheehan, RN, Moira Quinn, RN, Clare Coughlin, RN, Laurie Cincotta, RN, Nancy Rodriquez-Sheridan, Ileana Gatica, Laine Catlin Fletcher, Joan Shander, Mark Abcede, Judy Jean, RN, and Julia Venanzi for their assistance in data collection; Nastia Dynkin for computer programming; the staff of the Massachusetts Department of Public Health Center for Birth Defects Research and Prevention and the Massachusetts Registry of Vital Records, Dr. Charlotte Druschel and the New York State Health Department, and Drs. Christina Chambers and Kenneth Jones of the University of California, San Diego, as well as the medical and nursing staff at all participating hospitals for assistance with case ascertainment: Baystate Medical Center, Beth Israel Deaconess Medical Center, Boston Medical Center, Brigham & Women’s Hospital, Brockton Hospital, Cambridge Hospital, Caritas Good Samaritan Medical Center, Charlton Memorial Hospital, Children’s Hospital, Emerson Hospital, Falmouth Hospital, Haverhill-Hale Hospital, Jordan Hospital, Kent Hospital, Lawrence General Hospital, Lowell General Hospital, Melrose-Wakefield Hospital, Metro West Medical Center—Framingham, Mt. Auburn Hospital, New England Medical Center, Newton-Wellesley Hospital, North Shore Medical Center, Rhode Island Hospital, Saints Memorial Medical Center, South Shore Hospital, Southern New Hampshire Medical Center, St. Elizabeth’s Medical Center, St. Luke’s Hospital, St. Vincent Hospital, UMASS Memorial Health Care, Women & Infants’ Hospital, Abington Memorial Hospital, Albert Einstein Medical Center, Alfred I. duPont Hospital for Children, Bryn Mawr Hospital, Chester County Hospital, Children’s Hospital of Philadelphia, Christiana Care Health Services, Community Hospital, Crozer-Chester Medical Center, Doylestown Hospital, Frankford Hospital, Hahnemann University Hospital, The Hospital of the University of Pennsylvania, Lankenau Hospital, Lancaster General Hospital, Lehigh Valley Hospital, Nanticoke Memorial Hospital, Pennsylvania Hospital, Sacred Heart Hospital, St. Christopher’s Hospital for Children, St. Mary Medical Center, Temple University Health Sciences Center, Reading Hospital & Medical Center, Thomas Jefferson University Hospital, Grand River Hospital, Guelph General Hospital, Hamilton Health Sciences Corporation, The Hospital for Sick Children, Humber River Regional Hospital—Church Site, Humber River Regional Hospital—Finch Site, Joseph Brant Memorial Hospital, Lakeridge Health Corporation, London Health Sciences Center, Mt. Sinai Hospital, North York General Hospital, Oakville Trafalgar Memorial Hospital, Scarborough Hospital—General Division, Scarborough Hospital—Grace Division, St. Joseph’s Health Centre—London, St. Joseph’s Health Centre—Toronto, St. Joseph’s Healthcare—Hamilton, St. Michael’s Hospital, Sunnybrook & Women’s College Health Sciences Center, Toronto East General Hospital, Toronto General Hospital, Trillium Health Center, William Osler Heath Centre, York Central Hospital, York County Hospital, Alvarado Hospital, Balboa Naval Medical Center, Camp Pendleton Naval Hospital, Children’s Hospital and Health Center, Kaiser Zion Medical Center, Palomar Medical Center, Pomerado Hospital, Scripps Mercy Hospital, Scripps Memorial Hospital—Chula Vista, Scripps Memorial Hospital—Encinitas, Scripps Memorial Hospital—La Jolla, Sharp Chula Vista Hospital, Sharp Coronado Hospital, Sharp Grossmont Hospital, Sharp Mary Birch Hospital, Tri-City Medical Center, and UCSD Medical Center; we particularly thank all the mothers who participated in the study.
Grant sponsor: Centers for Disease Control and Prevention; Grant number: DD000697; Grant sponsor: NIH; Grant number: T32 HD052458.
Footnotes
Conflict of Interest: Dr. Mitchell reports a potential conflict of interest. Until August 2012, Dr. Mitchell owned J&J stock valued at under $20,000. He serves on Biogen-Idec Pregnancy Exposure Registry Advisory Committees for biologic products used in the treatment of multiple sclerosis.
References
- Agopian AJ, Canfield MA, Olney RS, Lupo PJ, Ramadhani T, Mitchell LE, Shaw GM, Moore CA. Spina bifida subtypes and sub-phenotypes by maternal race/ethnicity in the National Birth Defects Prevention Study. Am J Med Genet Part A. 2012;158A:109–115. doi: 10.1002/ajmg.a.34383. [DOI] [PubMed] [Google Scholar]
- Boulet SL, Yang Q, Mai C, Kirby RS, Collins JS, Robbins JM, Meyer R, Canfield MA, Mulinare J. Trends in the postfortification prevalence of spina bifida and anencephaly in the United States. Birth Defects Res A Clin Mol Teratol. 2008;82:527–532. doi: 10.1002/bdra.20468. [DOI] [PubMed] [Google Scholar]
- Calzolari E, Bianchi B, Dolk H, Stone D, Milan M, EUROCAT Working Group Are omphalocele and neural tube defects related congenital anomalies?: Data from 21 registries in Europe (EUROCAT) Am J Med Genet. 1997;72:79–84. [PubMed] [Google Scholar]
- Carey JC, Greenbaum B, Hall BD. The OEIS complex (omphalocele, exstrophy, imperforate anus, spinal defects) Birth Defects Orig Artic Ser. 1978;14:253–263. [PubMed] [Google Scholar]
- Carmichael SL, Shaw GM, Yang W, Laurent C, Herring A, Royle MH, Canfield M. Correlates of intake of folic acid-containing supplements among pregnant women. Am J Obstet Gynecol. 2006;194:203–210. doi: 10.1016/j.ajog.2005.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Knowledge and use of folic acid by women of childbearing age—United States, 1995 and 1998. MMWR Morb Mortal Wkly Rep. 1999;48:325–327. [PubMed] [Google Scholar]
- Collins JS, Atkinson KK, Dean JH, Best RG, Stevenson RE. Long term maintenance of neural tube defects prevention in a high prevalence state. J Pediatr. 2011;159:143–149.e2. doi: 10.1016/j.jpeds.2010.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa A, Gilboa SM, Besser LM, Botto LD, Moore CA, Hobbs CA, Cleves MA, Riehle-Colarusso TJ, Waller DK, Reece EA. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199:237.e1–9. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragan JD, Gilboa SM. Including prenatal diagnoses in birth defects monitoring: Experience of the Metropolitan Atlanta Congenital Defects Program. Birth Defects Res A Clin Mol Teratol. 2009;85:20–29. doi: 10.1002/bdra.20508. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- De Wals P, Tairou F, Van Allen MI, Lowry RB, Evans JA, Van den Hof MC, Crowley M, Uh S, Zimmer P, Sibbald B, Fernandez B, Lee NS, Niyonsenga T. Spina bifida before and after folic acid fortification in Canada. Birth Defects Res A Clin Mol Teratol. 2008;82:622–628. doi: 10.1002/bdra.20485. [DOI] [PubMed] [Google Scholar]
- Hetherington R, Dennis M, Barnes M, Drake J, Gentili F. Functional outcome in young adults with spina bifida and hydrocephalus. Childs Nerv Syst. 2006;22:117–124. doi: 10.1007/s00381-005-1231-4. [DOI] [PubMed] [Google Scholar]
- Holmes LB, Driscoll SG, Atkins L. Etiologic heterogeneity of neural-tube defects. N Engl J Med. 1976;294:365–369. doi: 10.1056/NEJM197602122940704. [DOI] [PubMed] [Google Scholar]
- Jenkinson MD, Campbell S, Hayhurst C, Clark S, Kandasamy J, Lee MK, Flynn A, Murphy P, Mallucci CL. Cognitive and functional outcome in spina bifida-Chiari II malformation. Childs Nerv Syst. 2011;27:967–974. doi: 10.1007/s00381-010-1368-7. [DOI] [PubMed] [Google Scholar]
- Johnson CY, Honein MA, Dana Flanders W, Howards PP, Oakley GP, Jr, Rasmussen SA. Pregnancy termination following prenatal diagnosis of anencephaly or spina bifida: A systematic review of the literature. Birth Defects Res A Clin Mol Teratol. 2012;94:857–863. doi: 10.1002/bdra.23086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juranek J, Salman MS. Anomalous development of brain structure and function in spina bifida myelomeningocele. Dev Disabil Res Rev. 2010;16:23–30. doi: 10.1002/ddrr.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalien B, Robert E, Harris J. Associated malformations in infants and fetuses with upper or lower neural tube defects. Teratology. 1998;57:56–63. doi: 10.1002/(SICI)1096-9926(199802)57:2<56::AID-TERA3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Khoury MJ, Erickson JD, James LM. Etiologic heterogeneity of neural tube defects: Clues from epidemiology. Am J Epidemiol. 1982;115:538–548. doi: 10.1093/oxfordjournals.aje.a113335. [DOI] [PubMed] [Google Scholar]
- Khoury MJ, Cordero JF, Mulinare J, Opitz JM. Selected midline defect associations: A population study. Pediatrics. 1989;84:266–272. [PubMed] [Google Scholar]
- Lopez-Camelo JS, Castilla EE, Orioli IM. Folic acid flour fortification: Impact on the frequencies of 52 congenital anomaly types in three South American countries. Am J Med Genet Part A. 2010;152A:2444–2458. doi: 10.1002/ajmg.a.33479. [DOI] [PubMed] [Google Scholar]
- Martinez-Frias ML. Primary midline developmental field. I. Clinical and epidemiological characteristics. Am J Med Genet. 1995;56:374–381. doi: 10.1002/ajmg.1320560406. [DOI] [PubMed] [Google Scholar]
- Medical Research Council Vitamin Study Research Group. Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- Opitz JM, Gilbert EF. CNS anomalies and the midline as a “developmental field”. Am J Med Genet. 1982;12:443–455. doi: 10.1002/ajmg.1320120408. [DOI] [PubMed] [Google Scholar]
- Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, Anderson P, Mason CA, Collins JS, Kirby RS, Correa A. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–1016. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- Rittler M, Lopez-Camelo J, Castilla EE. Sex ratio and associated risk factors for 50 congenital anomaly types: Clues for causal heterogeneity. Birth Defects Res A Clin Mol Teratol. 2004;70:13–19. doi: 10.1002/bdra.10131. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Velie EM, Schaffer D. Risk of neural tube defect-affected pregnancies among obese women. JAMA. 1996;275:1093–1096. doi: 10.1001/jama.1996.03530380035028. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Carmichael SL, Kaidarova Z, Harris JA. Differential risks to males and females for congenital malformations among 2.5 million California births, 1989–1997. Birth Defects Res A Clin Mol Teratol. 2003;67:953–958. doi: 10.1002/bdra.10129. [DOI] [PubMed] [Google Scholar]
- Shin M, Kucik JE, Siffel C, Lu C, Shaw GM, Canfield MA, Correa A. Improved Survival Among Children with Spina Bifida in the United States. J Pediatr. 2012;161:1132–1137. doi: 10.1016/j.jpeds.2012.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RE, Allen WP, Pai GS, Best R, Seaver LH, Dean J, Thompson S. Decline in prevalence of neural tube defects in a high-risk region of the United States. Pediatrics. 2000;106:677–683. doi: 10.1542/peds.106.4.677. [DOI] [PubMed] [Google Scholar]
- Stevenson RE, Seaver LH, Collins JS, Dean JH. Neural tube defects and associated anomalies in South Carolina. Birth Defects ResA Clin Mol Teratol. 2004;70:554–558. doi: 10.1002/bdra.20062. [DOI] [PubMed] [Google Scholar]
- Stoll C, Dott B, Alembik Y, Roth MP. Associated malformations among infants with neural tube defects. Am J Med Genet Part A. 2011;155A:565–568. doi: 10.1002/ajmg.a.33886. [DOI] [PubMed] [Google Scholar]
- Waller DK, Shaw GM, Rasmussen SA, Hobbs CA, Canfield MA, Siega-Riz A-M, Gallaway MS, Correa A. Prepregnancy obesity as a risk factor for structural birth defects. Arch Pediat Adol Med. 2007;161:745–750. doi: 10.1001/archpedi.161.8.745. [DOI] [PubMed] [Google Scholar]
- Watkins ML, Rasmussen SA, Honein MA, Botto LD, Moore CA. Maternal obesity and risk for birth defects. Pediatrics. 2003;111:1152–1158. [PubMed] [Google Scholar]
- Werler MM, Shapiro S, Mitchell AA. Periconceptional folic acid exposure and risk of occurrent neural tube defects. JAMA. 1993;269:1257–1261. [PubMed] [Google Scholar]
- Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- Williams LJ, Mai CT, Edmonds LD, Shaw GM, Kirby RS, Hobbs CA, Sever LE, Miller LA, Meaney FJ, Levitt M. Prevalence of spina bifida and anencephaly during the transition to mandatory folic acid fortification in the United States. Teratology. 2002;66:33–39. doi: 10.1002/tera.10060. [DOI] [PubMed] [Google Scholar]
- Yazdy MM, Tinker SC, Mitchell AA, Demmer LA, Werler MM. Maternal tea consumption during early pregnancy and the risk of spina bifida. Birth Defects Res A Clin Mol Teratol. 2012;94:756–761. doi: 10.1002/bdra.23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen IH, Khoury MJ, Erickson JD, James LM, Waters GD, Berry RJ. The changing epidemiology of neural tube defects. United States, 1968–1989. Am J Dis Child. 1992;146:857–861. doi: 10.1001/archpedi.1992.02160190089028. [DOI] [PubMed] [Google Scholar]


