Abstract
The provision of iron- fortified foods is a common strategy to prevent iron deficiency; however, ensuring adequate iron absorption is a challenge. Iron bioavailability depends on the choice of iron compound, the presence enhancers and inhibitors of absorption in the food matrix, and the physiological state of the consumer, including iron status, other nutritional deficiencies and inflammatory disorders. Inflammation associated with infections and inflammatory disorders would be expected to decrease iron absorption and reduce the efficacy of iron- fortified foods. The decreased absorption is due to an increase in circulating hepcidin in response to inflammatory cytokines. Hepcidin degrades ferroportin and blocks the passage of iron from the intestinal cell to the plasma. This is the innate immune response to infections and aims to restrict pathogen growth by restricting iron supply. Stable isotope studies have reported women and children with chronic malaria parasitemia or febrile malaria to have increased inflammatory cytokines, increased hepcidin and much decreased iron absorption. No studies have specifically investigated the efficacy of iron- fortified foods in the absence and presence of infections. In contrast, inflammation and increased hepcidin associated with adiposity in overweight have been linked to both lower iron absorption and the decreased efficacy of iron- fortified foods.
Introduction
Approximately 90% of daily iron needs can be met by the reutilization of red cell iron after the breakdown of circulating red cells at the end of their natural life span. Although there is no physiological mechanism for active iron excretion from the body, there are obligatory iron losses from skin, intestine and urinary tract and additional losses during menstruation in women of child- bearing age. To maintain iron balance, obligatory and menstrual iron losses, plus the iron required for growth in infants, children, and adolescents must come from the diet. This represents about 1– 2 mg of absorbed iron per day. Many young women, infants and children fail to meet their iron needs from diet, and these population groups make up the majority of the 2 billion people worldwide estimated to be iron deficient [1]. The situation is worse in the developing world due to low iron bioavailability from plant- based diets, and iron deficiency has more serious negative health and economic consequences including poor pregnancy outcome, poor cognitive development in children, and decreased physical performance and productivity [2].
Four strategies are currently being used to target iron deficiency. These are dietary diversification, supplementation, food fortification and biofortification, although fortification and supplementation are often the only practical options. Improving iron bioavailability by including more animal tissue foods, fruits and vegetables in the diet is usually limited by cost and supply, especially in the developing world, and biofortification of plant staples by breeding or genetic engineering is not yet ready for introduction into the food supply. Iron supplementation can be both therapeutic and preventive and involves the provision of relatively large amounts of medicinal iron as tablets or syrups to population known or expected to be iron deficient. Supplements are recommended to be taken in the absence of foods as food per se decreases iron absorption [3]. Iron- fortified foods contain lower doses of iron than supplements, and are regarded as the best long- term approach for the prevention of iron deficiency. Iron- fortified staple foods and condiments are usually planned and mandated by government, and will reach all population groups including women and older children. Iron- fortified manufactured weaning foods are targeted specifically at infants and young children and, for the lower socioeconomic groups, micronutrient powders and micronutrient- fortified fat- containing pastes have been introduced in recent years. These products, usually in the form of food aid, are designed to be added at time of consumption to the cereal gruels commonly eaten by these infants and young children. The food industry additionally markets a range of iron- fortified foods (milk products, breakfast cereals, chocolate drinks) targeted at infants, children, adolescents and young women.
For the many infants, children and women at risk of iron deficiency, iron-fortified foods can fill the gap between iron intake from the regular diet and iron needs. However, ensuring adequate absorption of the fortification iron is far from easy and iron bioavailability from fortified foods depends on the choice of iron compound [1], the food matrix and the physiological state of the consumer. Iron- fortified foods often contain inhibitors of iron absorption such as phytic acid and polyphenols in cereal and legume foods [4, 5] and calcium in milk products [6]. In order to overcome the negative effects of these inhibitors, the food manufacture usually adds ascorbic acid, although NaFeEDTA and phytase can also be added [7].
However, even if the food matrix is optimized, iron absorption ultimately depends on the physiological state of the consumer. There is an inverse correlation between iron status and iron absorption [8] with a 50% decrease in iron stores doubling of iron absorption, although the magnitude of the inhibitory or enhancing effects are independent of iron status. Genetic disorders, nutritional deficiencies, infection and inflammation can also influence iron absorption [9]. Homozygotes for thalassemia and hemochromatosis may have increased iron absorption and must be under medical surveillance, although dietary iron absorption by single gene carriers of these traits is little affected. Vitamin A deficiency can influence several stages of iron metabolism, and riboflavin deficiencies may decrease iron incorporation into hemoglobin. Chronic inflammation [10] and obesity [11] increase hepcidin expression and would be expected to decrease iron absorption [12]. This review evaluates the potential influence of inflammatory disorders and infection on iron absorption and efficacy of iron- fortified foods.
Iron Metabolism during Infection and Inflammation
Iron needed for new red cell synthesis and for replenishing iron enzymes and myoglobin is transported to the bone marrow or body tissues via transferrin in the plasma. Most of the iron supply enters the plasma via macrophages after the destruction of senescent red cells. The remainder enters as absorbed iron via the mucosal cells and, in times of need, from the iron stored as ferritin mainly in hepatocytes. The passage into the plasma is mediated via the transport protein ferroportin situated on the cell membrane [13], and its entry is strictly controlled by the regulatory hormone, hepcidin. This protein is secreted by the liver when iron status is adequate and inhibits the transport of iron into the plasma from both the macrophages and the intestinal cells [14]. Hepcidin binds ferroportin at the cell membrane, causing internalization and degradation [15], and when iron status is low, hepcidin release from the liver is decreased and iron absorption is maximized.
The innate immune response to microbial infection is to increase hepcidin via an inflammatory response and to restrict microbial growth by restricting the entry of iron into the plasma [16]. The anemia of infection results from an interruption in the recycling of red cell iron. Macrophage iron is not released resulting in insufficient iron for erythropoiesis. The outcome of many infectious diseases depends on preventing the invading pathogen from obtaining its iron supply, and provision of high iron doses to an infected patient can worsen the infection. In addition to preventing iron release from the macrophage, the inflammatory response would also be expected to prevent iron release from the mucosal cell and thus restrict iron absorption. Recent evidence indicates that the inflammatory response to malarial parasitemia decreased iron absorption from an iron- fortified sorghum gruel [12], and that the inflammation associated with obesity and overweight decreases iron absorption and reduces the efficacy of iron- fortified foods [17].
Influence of Infection on Iron Absorption and on the Efficacy of Iron- Fortified Foods
Recent studies provide good evidence that the acute- phase response to malarial infection [18] increases cytokines such as IL- 6 which upregulate hepcidin synthesis [19] and decrease iron absorption. Doherty et al. [20] investigated iron absorption from an iron- fortified orange juice in young Gambian children 1 day and 15 days after the treatment of malaria. The acute- phase response to the infection, as indicated by a raised serum ferritin in anemic children, was still present on day 1 after treatment but was much decreased at day 15 (table 1). In this study, thirty-seven 8- to 36- month- old, anemic (Hb <110 g/l) children with uncomplicated malaria followed a 3- day treatment with chloroquine and fansidar. On day 1 after the treatment, they consumed an orange juice fortified with iron and ascorbic acid and labeled with 57 iron stable isotope as ferrous sulfate. Iron absorption was quantified by measuring the incorporation of the stable isotope into erythrocytes at 14 days. On day 15 after treatment, a second iron absorption measurement was made on the same children who again consumed an iron- and ascorbic acid- fortified orange juice, but this time labeled with 58 ferrous sulfate. Fourteen days later, erythrocyte incorporation of the isotope was similarly quantified. The control group consisted of anemic children in the same age range but with no malaria and no malarial treatment. From day 1 to 30, all children received iron supplementation with liquid iron glycine sulfate at 2 mg Fe/kg bodyweight, and the hemoglobin and serum ferritin response of the postmalarial and nonmalarial anemic children to iron supplementation was compared.
Table 1.
Iron absorption and iron status following malaria treatment of anemic 8- to 36-month-old Gambian children and subsequent iron supplementation [20]
| Percent iron absorption | Hb, g/l | Serum ferritin, μg/l | |
|---|---|---|---|
| Children with malaria | |||
| Day 1 after treatment | 8.7 | 84 | 74 |
| Day 15 after treatment | 15.5 | 100 | 22 |
| Day 30 after treatment | – | 111 | 20 |
| Children free of malaria, no treatment | |||
| Day 1 | 26.6 | 91 | 6 |
| Day 15 | 24.0 | 97 | 13 |
| Day 30 | – | 103 | 16 |
Children were supplemented with 2 mg Fe/kg bodyweight per day from day 1 to day 30.
Selected results from this study are summarized in table 1. The serum ferritin values have been recalculated from the published graphics. Iron absorption in the children who had been treated for malaria increased from 8.7% on day 1 after treatment to 15.5% on day 15, but on both days iron absorption was still significantly lower than in the noninfected control children, whose absorption was ca. 25% (p < 0.001). Serum ferritin in the children treated for malaria decreased from 74 μg/l on day 1 to 22 μg/l on day 15 after treatment, even though the children received iron supplements over this period. Thus, the malarial treatment appeared to have decreased the acute- phase response, although not completely. Providing iron supplements to the control children over the same time period increased their serum ferritin from 6 to 16 μg/l. Although the children treated for malaria absorbed less iron than the controls over the supplementation period, they showed a much greater increase in hemoglobin concentration between days 1– 15 (p < 0.001) and between days 16– 30 (p < 0.001). This can be explained by an increased store of macrophage iron from the destruction of senescent red cells being prevented by hepcidin from entering the serum during the malarial infection, but being released following malarial treatment.
In contrast to the Gambian study which investigated iron absorption in children with uncomplicated febrile malaria, a more recent study in Benin investigated iron absorption in women with malarial parasitemia but no fever or other symptoms. While acute febrile malaria affects individuals only a few days a year, in areas of perennial transmission, asymptomatic parasitemia affects much of the population for most of the year giving rise to protracted low- level inflammation [21]. In the Benin study [12], women with relatively high loads of Plasmodium falciparum (>500 parasites/μl blood) but no fever or other infections, consumed a sorghum porridge labeled with 57 ferrous sulfate before and after a 10- day treatment with MalaroneTM. Iron absorption was measured as incorporation of the stable isotopes into hemoglobin 14 days after consumption of the test meals. Hepcidin and other inflammation indices were measured at baseline and on day 25 after clearance of the infection and immediately prior to the second test meal. The results are shown in table 2. Treatment of the malarial parasitemia increased mean iron absorption from 10.2 to 17.6% (p < 0.01), which could be explained by a decrease in inflammation as demonstrated by a decrease in IL- 6, - 8 and - 10 and an almost 50% fall in plasma hepcidin.
Table 2.
Influence of malarial parasitemia on fractional iron absorption and indicators of inflammation in young Beninese women consuming an iron-fortified sorghum porridge [12]
| With malarial parasitemia | After treatment: no parasitemia | p value | |
|---|---|---|---|
| Parasitemia, parasites/μl | 880 (123-2,760) | 0 (0) | <0.01 |
| Percent iron absorption | 10.2 (4.42; 23.5) | 17.6 (9.17; 33.8) | <0.01 |
| Serum ferritin, μg/l | 71 (29–99) | 37 (21–57) | <0.001 |
| Hepcidin, nmol/l | 2.7 (1.0–4.6) | 1.4 (0.7–2.4) | <0.005 |
| IL-6, pg/ml | 1.32 (0.96–1.92) | 1.27 (0.7–1.32) | <0.05 |
| IL-8, pg/ml | 7.31 (4.58–10.0) | 4.18 (2.60–5.67) | <0.001 |
| IL-10, pg/ml | 7.38 (4.44–13.9) | 2.9 (1.91–2.94) | <0.001 |
All values are given as median with 25–75 percentiles in parentheses, except for iron absorption, which is a geometric mean (±SD).
From these studies, it would be expected that inflammatory disorders and infections that lead to an inflammatory response would lead to a hepcidin-modulated decrease in iron absorption. Any long- term or chronic condition would then be expected to blunt the absorption of iron- fortified foods and the efficacy of iron fortification programs. To date, the only infection investigated in relation to iron absorption is malaria, although other common infections such as tuberculosis [22] and Schistosoma haematobium [23] also lead to an inflammatory response and increase hepcidin concentrations. There is no direct evidence however that the efficacy of iron fortification programs is blunted in malaria- endemic areas or regions of widespread infections. Lack of efficacy of electrolytic iron- fortified cereal products in Ivory Coast [24] and Kenya [25] has been blamed on widespread infections, but lack of gastric acid in malnourished populations could be an alternative explanation. Electrolytic iron- fortified wheat flour products were efficacious in China and Thailand [26]. While no studies have directly compared efficacy in infected and noninfected subjects from the same populations, there are efficacy studies with similarly fortified salt or cereals which have been made in populations with little or no infection or with widespread infections. The salt studies with ferric pyrophosphate in Morocco (no infections) [27] and Ivory Coast [28] reported similar improvements in serum ferritin and transferrin receptor as did the NaFeEDTA- fortified cereal studies in India (no infections) [Muthayya et al., unpubl.] and Kenya [25]. This failure to demonstrate reduced efficacy in the presence of infection is probably due to differences in baseline iron status and other physiological differences in the subjects, as well as differences in food vehicle, diet, and fortification levels.
Obesity and Overweight in Relation to Iron Absorption and the Efficacy of Iron- Fortified Foods
American national surveys have consistently shown that overweight toddlers, children, adolescents and adults are more likely to be iron deficient than their normal weight counterparts, and recent evidence suggests that adiposity- related inflammation may play a central role through its regulation of hepcidin [11]. In a recent Swiss study, overweight children were reported to consume similar amounts of bioavailable iron as normal weight children but have a lower iron status, higher serum hepcidin and higher subclinical inflammation as measured by IL- 6 and C- reactive protein [29]. The adipokine leptin was also upregulated, and both IL- 6 and leptin can increase hepcidin expression [11].
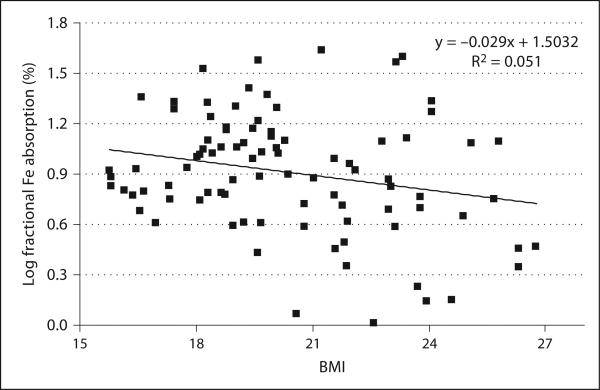
We have earlier reported that adiposity in women and children in transition countries predicts decreased iron absorption, iron deficiency and a decreased response to iron fortification [17]. The stable isotope iron absorption studies were made in 92 premenopausal Thai women of which some 20% were iron deficient and 22% overweight. They all consumed a reference meal of vegetable soup and white rice labeled with 57 or 58 ferrous sulfate. Iron absorption was quantified from erythrocyte incorporation of the isotope after 14 days. Independent of iron status, a higher BMI was associated with a lower iron absorption (p < 0.03; fig. 1). After adjustment for the differences in iron status (serum ferritin), fractional iron absorption was negatively correlated with C- reactive protein (p < 0.001) and BMI (p < 0.05).
Fig. 1.
Relationship between log fractional iron absorption and body mass index in healthy premenopausal Thai women (n = 92) who consumed meals of rice and vegetables labeled with ≈4 mg of [57Fe/58Fe]- ferrous sulfate [17].
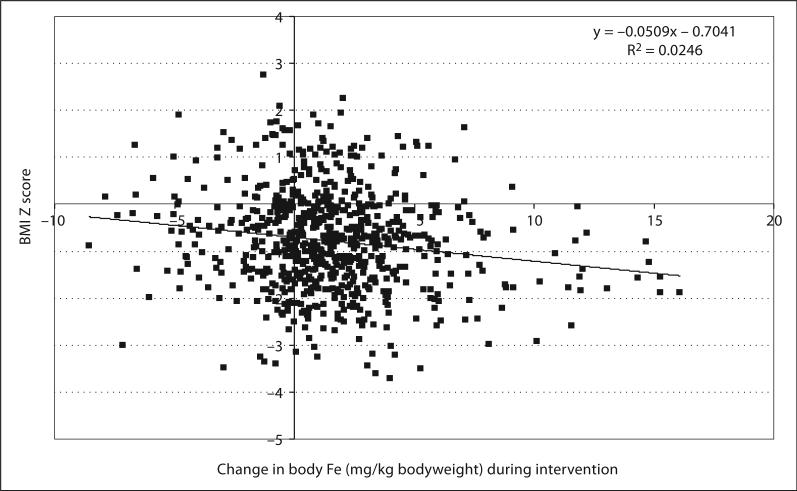
In order to evaluate the influence of adiposity on the efficacy of iron fortification programs, we analyzed baseline (n = 1,688) and intervention (n = 727) data from previously published efficacy studies in schoolchildren in Morocco and India to look for associations between BMI z scores and iron status measures. In this cohort of children, 42% were iron deficient but only 6.3% overweight; however, a lower BMI z score predicted poorer iron status at baseline (p < 0.001) and less improvement in iron status on consumption of the iron- fortified food (fig. 2). Iron status was measured as the serum ferritin- to- transferrin receptor ratio. This influence of adiposity on iron intervention has been confirmed in a recent Italian study in 15 obese children which reported that weight loss was associated with a significant decrease in circulating hepcidin and an increased response to iron supplements [30]. Overweight is increasing in some low-income countries at 2– 4 times the rate in the developing world, and the high levels of overweight in women and school children in transition countries such as Thailand and India [11] could reduce the impact of iron interventions.
Fig. 2.
BMI z score and the change in body iron stores (calculated from the ratio of SF/TfR) in iron fortification intervention studies in Moroccan and Indian children (n = 727) [17].
Conclusions
Inflammatory disorders and inflammation associated with infections would be expected to decrease iron absorption and reduce the efficacy of iron- fortified foods. The likely explanation is that the passage of iron from the intestinal cell to the plasma is prevented by the degradation of the transport protein ferroportin by higher hepcidin levels produced as a response to the inflammatory cytokines. While iron absorption has been reported to be much reduced in response to chronic malarial parasitemia and acute malarial infection, there is no evidence to confirm a reduced efficacy of iron- fortified foods in areas of widespread malaria and other infections. In contrast, increased inflammation and circulating hepcidin levels as a result of adiposity have been linked to both decreased iron absorption and a reduced efficacy of iron- fortified foods. While this might be due to the additional influence of the adipokine leptin in further increasing hepcidin levels in overweight individuals, it seems likely that inflammation associated with infections will decrease the impact of iron- fortified foods, but well- planned studies have yet to be made.
References
- 1.World Health Organization/Food and Agricultural Organization . Guidelines on Food Fortification with Micronutrients. World Health Organization; Geneva: 2006. [Google Scholar]
- 2.Zimmermann MB. Hurrell RF: Nutritional iron deficiency. Lancet. 2007;370:511–520. doi: 10.1016/S0140-6736(07)61235-5. [DOI] [PubMed] [Google Scholar]
- 3.Cook JD. Diagnosis and management of iron-deficiency anaemia. Best Pract Res Clin Haematol. 2005;18:319–332. doi: 10.1016/j.beha.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Petry N, Egli I, Zeder C, et al. Polyphenols and phytic acid contribute to the low iron bioavailability from common beans in young women. J Nutr. 2010;140:1977–1982. doi: 10.3945/jn.110.125369. [DOI] [PubMed] [Google Scholar]
- 5.Hallberg L, Brune M, Rossander L. Iron absorption in man: ascorbic acid and dose-dependent inhibition by phytate. Am J Clin Nutr. 1989;49:140–144. doi: 10.1093/ajcn/49.1.140. [DOI] [PubMed] [Google Scholar]
- 6.Hallberg L, Brune M, Erlandsson M, et al. Am J Clin Nutr. 1991;Calcium: effect of different amounts on nonheme- and heme-iron absorption in humans.53:112–119. doi: 10.1093/ajcn/53.1.112. [DOI] [PubMed] [Google Scholar]
- 7.Troesch B, Egli I, Zeder C, et al. Optimization of a phytase-containing micronutrient powder with low amounts of highly bio-available iron for in-home fortification of complementary foods. Am J Clin Nutr. 2009;89:539–544. doi: 10.3945/ajcn.2008.27026. [DOI] [PubMed] [Google Scholar]
- 8.Cook JD, Dassenko SA, Lynch SR. Assessment of the role of nonheme-iron availability in iron balance. Am J Clin Nutr. 1991;54:717–722. doi: 10.1093/ajcn/54.4.717. [DOI] [PubMed] [Google Scholar]
- 9.Hurrell R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr. 2010;91:1461s–1467s. doi: 10.3945/ajcn.2010.28674F. [DOI] [PubMed] [Google Scholar]
- 10.Nemeth E, Valore EV, Territo M, et al. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 11.Cepeda-Lopez AC, Aeberli I, Zimmermann MB. Does obesity increase risk for iron deficiency? A review of the literature and the potential mechanisms. Int J Vitam Nutr Res. 2010;80:263–270. doi: 10.1024/0300-9831/a000033. [DOI] [PubMed] [Google Scholar]
- 12.Cercamondi CI, Egli IM, Ahouandjinou E, et al. Afebrile Plasmodium falciparum parasitemia decreases absorption of fortification iron but does not affect systemic iron utilization a double stable-isotope study in young Beninese women. Am J Clin Nutr. 2010;92:1385–1392. doi: 10.3945/ajcn.2010.30051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donovan A, Lima CA, Pinkus JL, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Ganz T. Hepcidin – a regulator of intestinal iron absorption and iron recycling by macrophages. Best Pract Res Clin Haematol. 2005;18:171–182. doi: 10.1016/j.beha.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 16.Wander K, Shell-Duncan B, McDade TW. Evaluation of iron deficiency as a nutritional adaptation to infectious disease: an evolutionary medicine perspective. Am J Hum Biol. 2009;21:172–179. doi: 10.1002/ajhb.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmermann MB, Zeder C, Muthayya S, et al. Adiposity in women and children from transition countries predicts decreased iron absorption, iron deficiency and a reduced response to iron fortification. Int J Obes (Lond) 2008;32:1098–1104. doi: 10.1038/ijo.2008.43. [DOI] [PubMed] [Google Scholar]
- 18.O'Donnell A, Fowkes FJI, Allen SJ, et al. The acute phase response in children with mild and severe malaria in Papua New Guinea. Trans R Soc Trop Med Hyg. 2009;103:679–686. doi: 10.1016/j.trstmh.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 19.de Mast Q, van Dongen-Lases EC, Swinkels DW, et al. Mild increases in serum hepcidin and interleukin-6 concentrations impair iron incorporation in haemoglobin during an experimental human malaria infection. Br J Haematol. 2009;145:657–664. doi: 10.1111/j.1365-2141.2009.07664.x. [DOI] [PubMed] [Google Scholar]
- 20.Doherty CP, Cox SE, Fulford AJ, et al. Iron incorporation and post-malaria anaemia. PLoS One. 2008;3:e2133. doi: 10.1371/journal.pone.0002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imrie H, Fowkes FJ, Michon P, et al. Low prevalence of an acute phase response in asymptomatic children from a malaria-endemic area of Papua New Guinea. Am J Trop Med Hyg. 2007;76:280–284. [PubMed] [Google Scholar]
- 22.Vander Beken S, Al Dulayymi JR, Naessens T, et al. Molecular structure of the Myco-bacterium tuberculosis virulence factor, mycolic acid, determines the elicited inflammatory pattern. Eur J Immunol. 2011;41:450–460. doi: 10.1002/eji.201040719. [DOI] [PubMed] [Google Scholar]
- 23.Ayoya MA, Spiekermann-Brouwer GM, Stoltzfus RJ, et al. Alpha 1-acid glycoprotein, hepcidin, C-reactive protein, and serum ferritin are correlated in anemic schoolchildren with Schistosoma haematobium. Am J Clin Nutr. 2010;91:1784–1790. doi: 10.3945/ajcn.2010.29353. [DOI] [PubMed] [Google Scholar]
- 24.Rohner F, Zimmermann MB, Amon RJ, et al. In a randomized controlled trial of iron fortification, anthelmintic treatment, and intermittent preventive treatment of malaria for anemia control in Ivorian children, only anthelmintic treatment shows modest benefit. J Nutr. 2010;140:635–641. doi: 10.3945/jn.109.114256. [DOI] [PubMed] [Google Scholar]
- 25.Andang'o PEA, Osendarp SJM, Ayah R, et al. Efficacy of iron-fortified whole maize flour on iron status of schoolchildren in Kenya: a randomised controlled trial. Lancet. 2007;369:1799–1806. doi: 10.1016/S0140-6736(07)60817-4. [DOI] [PubMed] [Google Scholar]
- 26.Hurrell R, Ranum P, de Pee S, et al. Revised recommendations for iron fortification of wheat flour and an evaluation of the expected impact of current national wheat flour fortification programs. Food Nutr Bull. 2010;31:S7–S21. doi: 10.1177/15648265100311S102. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann MB, Wegmueller R, Zeder C, et al. Dual fortification of salt with iodine and micronized ferric pyrophosphate: a randomized, double-blind, controlled trial. Am J Clin Nutr. 2004;80:952–959. doi: 10.1093/ajcn/80.4.952. [DOI] [PubMed] [Google Scholar]
- 28.Wegmueller R, Fatoumata C, Zimmermann MB, et al. Salt dual fortified with iodine and micronized ground ferric pyrophosphate affects iron status but not hemoglobin in children in Côte d'Ivoire. J Nutr. 2006;136:1–7. doi: 10.1093/jn/136.7.1814. [DOI] [PubMed] [Google Scholar]
- 29.Aeberli I, Beljean N, Lehmann R, I'Allemand D, Spinas GA, Zimmermann MB. The increase of fatty acid-binding protein aP2 in overweight and obese children: interactions with dietary fat and impact on measures of subclinical inflammation. Int J Obes (Lond) 2008;32:1513–1520. doi: 10.1038/ijo.2008.128. [DOI] [PubMed] [Google Scholar]
- 30.Amato A, Santoro N, Calabro P, et al. Effect of body mass index reduction on serum hepcidin levels and iron status in obese children. Int J Obes (Lond) 2010;34:1772–1774. doi: 10.1038/ijo.2010.204. [DOI] [PubMed] [Google Scholar]