Abstract
The female reproductive cycle is gated by the circadian timing system and may be vulnerable to disruptions in the circadian system. Prior work suggests that vasoactive intestinal peptide (VIP) expressing neurons in the suprachiasmatic nucleus (SCN) are one pathway by which the circadian clock can influence the estrous cycle but the impact of the loss of this peptide on reproduction has not been assessed. In the present study, we first examine the impact of the genetic loss of the neuropeptide VIP on the reproductive success of female mice. Significantly, mutant females produced about half the offspring of their wild type sisters even when mated to the same males. We also find that VIP-deficient females exhibit a disrupted estrous cycle i.e. ovulation occurs less frequently and results in the release of fewer oocytes compared to controls. Circadian rhythms of wheel running activity are disrupted in the female mutant mice as are the spontaneous electrical activity of dorsal SCN neurons. On a molecular level, the VIP-deficient SCN tissue exhibit lower amplitude oscillations with altered phase relationships between the SCN and peripheral oscillators as measured by PER2-driven bioluminescence. The simplest explanation of our data is that the loss of VIP results in a weakened SCN oscillator which reduces the synchronization of the female circadian system. These results clarify one of the mechanisms by which disruption of the circadian system reduces female reproductive success.
Keywords: vasoactive intestinal peptide, reproduction, estrous cycle, circadian rhythms, female, mouse, ovulation
Introduction
Many people in our society experience chronic disruptions of their sleep/wake cycle. While not explicitly tested, there is every reason to suspect that many of the people suffering from disrupted sleep are also experiencing the disruption and misalignment of their circadian system. There are a number of lines of evidence that link circadian disruption and declining reproductive success. For example, several studies have demonstrated reduced fecundity in mice with mutations in their core clock genes (Kennaway et al., 2004; Miller et al., 2004; Dolatshad et al., 2006; Alvarez et al., 2008; Ratajczak et al., 2009). Likewise, in Drosophila, clock mutants show reduced reproductive fitness (Beaver et al., 2002). Environmental disruptions of circadian timing also reduce reproductive success (Endo and Wanatabe, 1989; Summa et al., 2012), although this phenomenon is understudied. Finally, epidemiological studies have linked shift work with decreases in human reproductive success (Mahoney, 2010; Mong et al., 2011).
The female reproductive cycle is controlled by a finely-tuned multi-organ feedback loop, involving hypothalamic control of pituitary hormone secretion and corresponding steroid release from the ovaries. In female rodents, ovulation occurs spontaneously in regular 4 to 5 day cycles, and is gated by the circadian system (Everett and Sawyer, 1950; Turek et al., 1984; Barbacka-Surowiak et al., 2003). With the isolation or ablation of the master pacemaker of the circadian timing system, the suprachiasmatic nuclei (SCN), pre-ovulatory hormone surges are lost in female rats (Nunez and Stephan, 1977; Gray et al., 1978; Mosko and Moore, 1979; Wiegand et al., 1980). These experimental paradigms in rodents, along with the observed deficits in clock mutants, deliberate circadian disruption models, and epidemiological studies in humans, demonstrate that circadian disruption is negatively correlated with fertility and inspire examination of the links between the female circadian and reproductive cycles.
Previous anatomical studies have established that the connectivity from the SCN to gonadotrophin-releasing hormone (GnRH) neurons in the medial preoptic area consists of both a direct connection with neurons expressing vasoactive intestinal peptide (VIP) (Van Der Beek et al., 1997; Kriegsfeld et al, 2002; Ward et al., 2009) and an indirect connection with kisspeptin- and arginine vasopressin (AVP)-positive neurons via the periventricular nucleus (De la Iglesia and Schwartz, 2006; Robertson et al., 2009; Vida et al., 2010; Williams et al., 2011). Physiologically, exogenous VIP increases the electrical activity of GnRH neurons during proestrus (Christian et al., 2005; Christian and Moenter, 2008) suggesting that VIP provides an excitatory signal from the circadian clock that helps time the GnRH surge. Transient reduction of VIP by infusing anti-sense to Vip mRNA or antibodies against VIP leads to a dampening of the magnitude of the proestrus surge in luteinizing hormone (LH) in female rats (Harney et al., 1996; Van Der Beek et al., 1999; Gerhold et al., 2005). Nonetheless, the impact of the loss of VIP on the female circadian and reproductive cycles has not been explored.
We postulate that VIP-deficiency may be particularly detrimental to the SCN-GnRH circuit and affect downstream measures of reproductive function in female mice, including fertility, regularity of the estrous cycle, and LH-induced ovulation. Additionally, the loss of VIP (VIP KO) in male mice results in severe disruption in behavioural rhythms (Colwell et al., 2003), which stems from the loss of neuronal synchrony (Aton et al., 2005; Ciarleglio et al., 2009), and results in altered phasing of peripheral oscillators (Loh et al., 2011). The impact of VIP-deficiency on female rhythms has been understudied, and we address this shortfall in our present study.
Materials and Methods
Mice
The experimental protocols used in this study were approved by the UCLA Animal Research Committee, and all recommendations for animal use and welfare, as dictated by the UCLA Division of Laboratory Animal Medicine and the guidelines from the National Institutes of Health, were followed. Vip−/− mutants (VIP−/− ; also deficient in histidine isoleucine [PHI] encoded by the same locus) backcrossed to the C57BL/6 strain (Colwell et al., 2003) were crossed to PERIOD2::LUCIFERASE (PER2::LUC, Per2Luc) mice also on a C57BL/6 background (Yoo et al., 2004) as previously described (Loh et al., 2011). All wild-type (WT) and VIP−/− mice described in this study were maintained as homozygotes for the PER2::LUC knocked-in fusion gene. All mice were housed under 12:12 light-dark (LD) conditions for a minimum of 2 weeks prior to any experimentation.
Measurement of fertility
Female WT (n = 8) and VIP−/− (n = 8) littermates at 2 months of age (mo) were paired with male WT mice (2 mo) to determine fecundity in terms of number of litters, number of pups born, and number of pups weaned. Breeders were checked daily for pregnancy and births, and dams were left undisturbed to avoid maternal stress leading to litter loss. As such, the number of pups born could only be accurately determined from P7.
Determination of estrous cycles by vaginal lavage
WT (n = 24) and VIP−/− (n = 24) female mice (2 mo) housed under a 12:12 LD cycle were monitored using vaginal lavage at zeitgeber time (ZT) 0–1 (within 1 hr of lights on). Care was taken to avoid insertion of the lavage pipet, and the lavage samples were spotted in small (~100 µl) drops onto glass microscope slides. Cell types contained within each vaginal smear were determined using light microscopy (10X power) and scored for estrus state (Caligioni, 2009), where proestrus is indicated by the predominance of nucleated epithelial cells, estrus is denoted by the absence of nucleated cells and the presence of cornified squamous epithelial cells, and lavages from mice in metestrus and diestrus contain leukocytes. Mice were scored as having regular estrous cycles if they exhibited 4–5 day cycles throughout the monitoring period (14–21 days). Conversely, if mice exhibited more than 3 days in metestrus or diestrus, more than 2 days in proestrus, more than 1 day in estrus, or did not follow the order of progression of estrus states, they were scored as having irregular estrous cycles. The percentage of proestrus or estrus states were determined over the number of days sampled, and the percentage of proestrus smears followed by estrous were determined by visually scoring the number of proestrus smears that were immediately followed by an estrus-positive smear and dividing that by the total number of proestrus smears within each mouse.
Measurement of serum hormones
Serum was collected from 3 month old WT (n = 10) and VIP−/− (n = 10) female mice at ZT 6 (mid-day) on the day of a proestrus smear using methods previously described (Loh et al., 2008). Serum was assayed for estradiol using radioimmunoassay by the National Peptide and Hormone Program laboratory at Harbour-UCLA (Torrance, CA). For measurement of luteinizing hormone (LH) concentrations, serum was collected from a separate cohort of age-matched WT (n = 4) and VIP−/− (n = 4) females at hourly intervals between ZT 7 (7 h after lights on) and ZT 15 (3 h after lights off) housed under a 12:12 LD cycle on the day of a proestrus smear. The serum samples were assayed for LH using the Milliplex MAP mouse pituitary magnetic bead assay (MPTMAG-49K, EMD Millipore, Billerica, MA). Assay sensitivity was reported as 1.7 pg/ml, with intra- and inter- assay %CVs reported as <15 and <20 respectively for LH. No other analytes were run in this panel, thus ruling out cross-reactivity with other antibodies. Pituitary standard samples run in this assay contained LH ranging from 4.9 pg/ml to 20 ng/ml.
Determination of spontaneous oocyte release during estrus
Vaginal smears were monitored for 2 estrous cycles for WT (n = 12) females or at least 7 days until a proestrus smear for VIP−/− (n = 12) females. During the estrous stage, mice were sacrificed at ZT 0–1, and the ovaries with oviducts attached were removed by cutting the ovary fat pad and the uterus. The ovary and oviduct were further dissected in Hank’s Balanced Salt Solution (HBSS; Gibco, Invitrogen, Carlsbad, CA) to remove the fat and uterus, and transferred to fresh solution in an indented microscopic slide for analysis under a dissecting microscope. Each oviduct was carefully examined for released oocytes by making a small incision in the oviduct and teasing out the contents of the entire oviduct. The cumulus mass was dissociated by treatment in HBSS containing 300 µg/ml hyaluronidase (Sigma-Aldrich, St Louis, MO) and the oocytes were distinguished from other detritus by their spherical nature. Sampling at earlier time points (ZT 12 – 23 on the day of a pro-estrous smear) revealed an absence of oocytes in both WT (n = 5) and VIP−/− (n = 3) oviducts.
Oocyte release in response to ovarian stimulation
In a separate cohort, ovulation was induced using a combination of pregnant mare serum gonadotropin (PMSG) and human chorionic gonadotropin (hCG). Female WT (n=6) and VIP−/− (n=6) mice (2 mo) were hormone primed with an intraperitoneal (ip) injection of 5 IU of PMSG (G4877, Sigma-Aldrich) applied at ZT 11 on the day of metestrus, and ovulation was induced 48 hr later by ip injection of 5 IU of hCG (Novarel, Ferring Pharmaceuticals, Parsippany, NJ). On the morning following the hCG injection, mice were sacrificed between ZT 0 and 1, and the number of oocytes released into the oviducts were counted.
Wheel running activity
Adult female mice (2–4 mo; WT n = 10, VIP−/− n = 10) were singly housed in cages containing wheels, and wheel running activity was recorded as previously described (Colwell et al., 2003). Mice were entrained to a 12:12 hr LD cycle for a minimum of 2 weeks prior to collection of 10–14 days of data under LD conditions, followed by 10–14 days in constant darkness (DD) to obtain free-running activity. Data was recorded using Mini Mitter (Bend, OR) data loggers in 3 min bins, and 10 days of data under each condition were averaged for analysis. Free-running period (tau) was determined using the χ2 periodogram and the power of the rhythm was calculated by multiplying the Qp by 100/n, where n = number of data points examined using the El Temps program (Barcelona, Spain). Activity duration (alpha) was determined by the duration of activity over the threshold of the mean using an average waveform of 10 days of activity. The phase of activity onset was also defined as the time at which activity crossed the threshold of the mean of total activity. To calculate the phase angle of entrainment revealed by release into DD, the difference on the day of lighting change was determined between best-fit regression lines drawn through activity onset prior to and after release into DD. Precision was determined by calculating the daily variation in onset from a best-fit regression line drawn through 10 days of activity in both LD and DD conditions using the Clocklab (Actimetrics, Wilmette, IL) program. Fragmentation was defined by bouts/day with a max-gap of 21 min.
Measurement of spontaneous firing rate in SCN
Whole cell patch-clamp electrophysiology methods used are similar to those described previously (Kudo et al., 2013). Young female mice (2–3 mo) were anaesthetised in an isoflurane chamber in the day (ZT 2.5) or in the night (ZT 12.5). 300 µm coronal sections were prepared and superfused continuously with room temperature artificial cerebral spinal fluid (ACSF) aerated with 95% O2/5% CO2 in a recording chamber (PH-1, Warner Instruments, Hamden, CT) attached to the stage of a fixed stage upright DIC microscope (OLYMPUS, Tokyo, Japan). Whole cell patch clamp SCN recordings were made using electrode micropipettes (3–7MΩ) and recording electrodes were filled with standard internal solution (in mM): K-gluconate, 112.5; EGTA, 1; Hepes, 10; MgATP, 5; and MgCl2, 1. Internal solution pH was adjusted to 7.25-7.3 and osmolarity adjusted to 290–300mOsm. Recordings were obtained with the AXOPATCH 200B amplifier (Molecular Devices, Sunnyvale, CA) and monitored on-line with pCLAMP (Ver 10, Molecular Devices).
For these experiments, recordings were made from cells located in the dorsal SCN (dSCN) as defined by being dorsal to the tip of the 3rd ventricle. The access resistance of these cells ranged from 15 to 35 MΩ and cell capacitance typically ranged between 6–18pF. Data were not collected if access resistance values changed significantly (>20%) during the course of the experiment and/or if a neurone was not able to decrease firing rate following excitatory treatment with N-methyl-D-aspartate (25 µM; NMDA). Drug treatments were performed by dissolving gabazine (10µM; GabZ) in ACSF and delivered via rapid gravity feed system into the slice bath during recording. Spontaneous firing rates (SFR) were recorded with pCLAMP for 1 min using current-clamp mode in the whole cell patch configuration. No current was injected during recording. SFR for each cell was determined using the total number of action potentials recorded in the 1 min time window. SFR for WT and VIP−/− females at two time points was determined by averaging data from at least 10 neurons collected from a minimum of 3 animals.
Monitoring of PER2::LUC bioluminescence
Female WT (n = 7) and VIP−/− (n = 10) mice (2–3 mo) were housed under a 12:12 LD cycle for at least 2 weeks prior to sampling. Vaginal smears were performed during the second week to determine estrous state, and mice in proestrus (nucleated cells) were sampled at ZT 10–11. SCN explants were dissected from 300 µm coronal sections, dissected ovaries were further halved, and 1–2 mm3 explants were dissected from the pituitary and mid-point of the uterus. PER2::LUC bioluminescence recordings using a Lumicycle photometer (Actimetrics, Wilmette, IL) were conducted and analyzed as previously described (Loh et al., 2011; Kuljis et al., 2013). Briefly, bioluminescence readings were taken every 10 min, and for analysis, were baseline-subtracted, de-trended (24 hr window) and smoothed (2 hr window). Due to this process, we do not report the first recorded peak but instead the first calculated peak for phase of PER2::LUC expression. Amplitude of each cycle was determined by the sum of the peak and subsequent trough values, and period was determined from a minimum of 4 consecutive cycles.
Statistical Analysis
For comparison of female WT and VIP−/− measures, that passed normality and equal variance tests, we applied Student’s t-tests and deemed differences as significant if P < 0.05. For parameters that failed either normality or equal variance tests, rank sum t-tests were applied. Two-factor repeated measures analysis of variance (ANOVA) was applied to examine the relative contributions of genotype and time of day of pro-estrous serum LH concentration. Post hoc Bonferroni comparisons were performed to compare the effect of genotype at each sampling time. Values are reported as mean ± standard error mean (SEM).
Results
Female reproduction is greatly reduced in the VIP-deficient mice
To determine if VIP is critical for reproduction, we paired naïve WT and VIP −/− females littermates with WT males. VIP −/− females produced dramatically fewer pups (10.6 ± 1.5) than their sisters (21.5 ± 3.0, P < 0.01) during the 6 m of breeding, demonstrating reduced fecundity. The mean number of pups per litter was not significantly different between the genotypes (WT 4.8 ± 1.7, VIP−/− 4.0 ± 1.6 pups/litter; P = 0.32); however, reduced fecundity is accounted for by the fewer litters born to VIP−/− dams (2.9 ± 0.3) compared to WT females (4.5 ± 0.3, P < 0.01). Furthermore, VIP KO females spent a smaller percentage of time either pregnant or nursing (46.8 ± 3.1 %) versus WT females (76.9 ± 5.2 %, P < 0.001). We can conclude from these findings that although VIP−/− females are capable of reproduction, they are sub-fertile, spending less time pregnant or nursing, and producing half the number of offspring compared to their sisters held under the same conditions.
The regularity of the estrous cycle is greatly reduced in the VIP-deficient mice
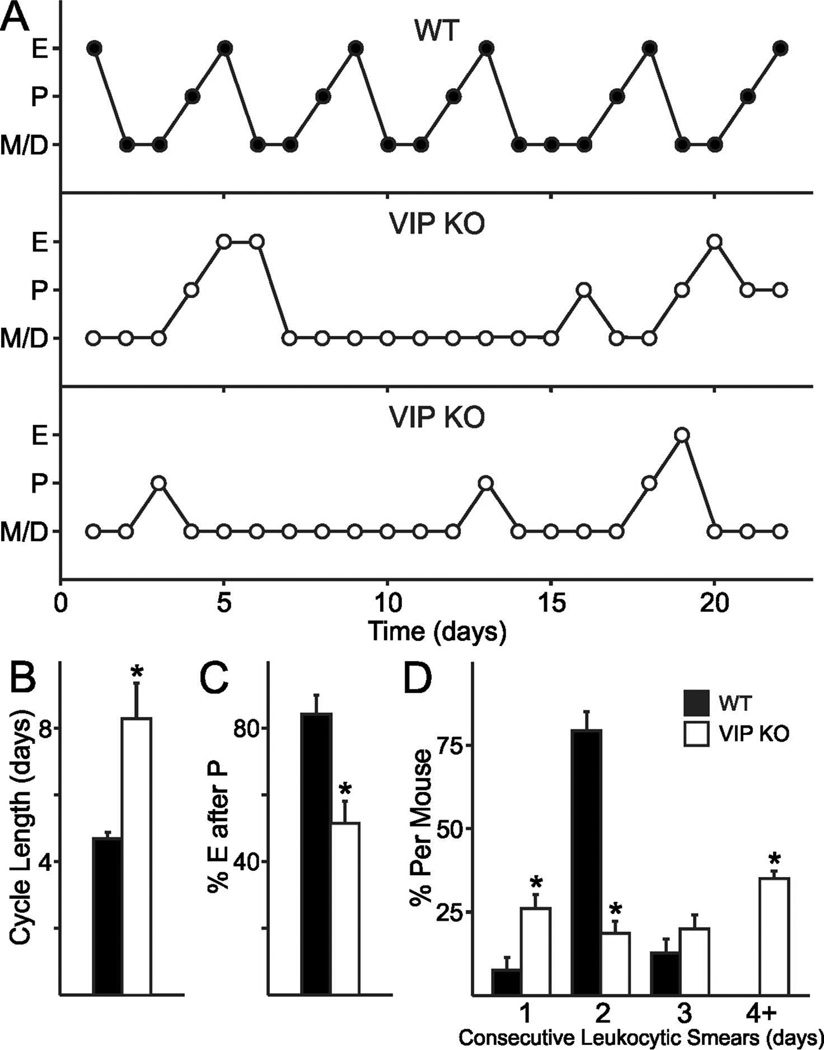
To determine if the basic reproductive biology underlying ovulation is affected by VIP-deficiency, we examined the estrous cycle by daily vaginal lavage in WT and VIP−/− female littermates (representative examples shown in Fig. 1A). We found irregular estrous cycles in the majority of VIP−/− females (20/24), while the majority of WT females exhibited regular cycling through proestrus, estrus, metestrus and diestrus (19/24). Cycle duration as determined by the time between estrus was found to be longer in VIP−/− females (8.18 ± 1.05 days) compared to typical WT cycles (4.64 ± 0.17 days; P < 0.001; Fig. 1B). A common finding in VIP−/− females was that not every proestrus smear was followed by an estrus smear (51.6 ± 6.5 %; Fig. 1A,C), whereas almost every proestrus smear in WT females was immediately followed by estrus (84.4 ± 5.5 %, P < 0.001; typical example depicted in Fig. 1A, top). Additionally, VIP−/− females exhibited elongated duration in diestrus/metestrus as indicated by the presence of leukocytic cells (57.69 ± 3.88 %) compared to WT females (44.68 ± 2.06 %, P = 0.008; Fig. 1D). Over the entire period in which estrous cycles were assessed, VIP−/− females had significantly fewer percentages of days in proestrus (15.9 ± 1.8 %) compared to WT females (20.7 ± 1.2 %, P < 0.01), and also had fewer days in estrus (14.7 ± 2.6 % vs. WT 20.5 ± 1.6 %, P < 0.05). Our data indicates that VIP is essential for regular estrous cycles, and VIP-deficiency is characterised by long spells in the diestrus and metestrus states. Furthermore, estrus is not guaranteed after proestrus, which further adds to the longer duration between estrous states in mutant females.
Fig. 1.
Impact of VIP-deficiency on estrous cycles. A. VIP-deficiency results in disrupted estrous cycles (middle and bottom) compared to the 4–5 day estrous cycles exhibited by WT females (top). P: proestrus, majority nucleated epithelial cells. E: estrus, cornified cells. D/M: diestrus or metestrus, presence of leukocytic cells. B. The average length of an estrous-to-estrous cycles in VIP-deficient mice is longer than in WT females (* P < 0.05). C. VIP-deficiency reduces the number of proestrus to estrus transitions. D. The number of consecutive days in di- and met-estrus increase with the loss of VIP (* P < 0.05).
Serum estradiol levels were not altered by the loss of VIP
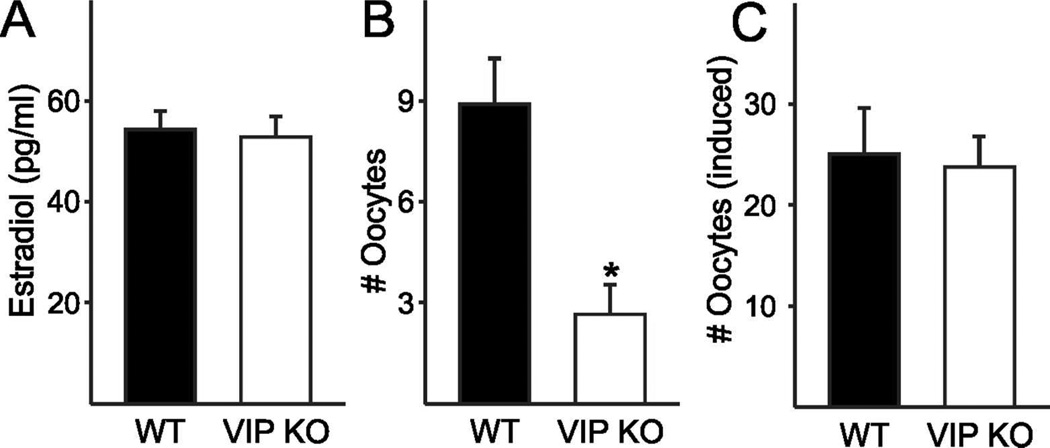
To determine if the steroidal signals that define the ovulatory process are affected by the VIP mutation, we measured serum concentrations of estradiol during proestrus. The rise in circulating estradiol during the day of proestrus precedes the activation of GnRH neurons, which initiates the LH surge and in turn stimulates ovulation (Legan et al., 1975). The mid-day (ZT 6) serum concentration of estradiol was no different in VIP−/− females (52.9 ± 4.2 pg/µl) from WT females (54.4 ± 3.8 pg/µl, P = 0.77; Fig. 2A). We also performed hourly measurements of LH from ZT 7 to ZT 15 to determine if surge time and/or concentration were altered in VIP−/− females. The maximum LH measured in VIP−/− females (0.2 ± 0.04 pg/ml) was not significantly different from WT females (1.1 ± 0.8 ng/ml; rank sum test, P = 0.11; Fig. S1). The maximum LH concentration in WT females was found to be lower than surge-like concentrations of LH (>5 ng/ml) despite the hourly sampling on the day of proestrus. Coupled with the variability in LH concentrations within WT females, we are unable to draw conclusions about genotypic differences in LH surge concentration. We ascertained that the temporal window of peak LH concentration within animals was not significantly altered by the loss of VIP (WT ZT 11.3 ± 1.4, VIP−/− ZT 11.4 ± 1.3; rank sum test P = 0.73; Fig. S1). Our findings indicate that mid-day rise in estradiol concentration during proestrus is not altered in the VIP−/− mice, which do not exhibit any measurable changes in LH concentration or peak timing.
Fig. 2.
Impact of VIP-deficiency on circulating estradiol and ovulation in adult females. A. The proestrus mid-day increase in serum estradiol is not affected by the loss of VIP in female mice. B. The number of oocytes spontaneously released on the morning of estrus is reduced in VIP−/− oviducts compared to WT (* P = 0.002) C. The number of oocytes released in response to PMSG-hCG ovarian stimulation technique in hormone-primed female mice was not different between the genotypes.
Spontaneous ovulation is dependent on VIP
Mice ovulate spontaneously following estrus, and we examined the number of oocytes released in both WT and VIP−/− mice on the day following a proestrus smear. We found that 6 out of 12 VIP−/− females did not release any oocytes after a proestrus smear compared to 2 out of 12 WT females. Of the mice that released oocytes, significantly fewer oocytes were found in VIP−/− oviducts (5.3 ± 0.7 vs. WT 10.7 ± 0.7 oocytes/pair of oviducts, P < 0.001; Fig. 2B). To determine if the reduced number of oocytes in VIP−/− females during ovulation is due to an inability of the ovaries to release the oocytes, we hormone-primed female mice using the PMSG-hCG ovarian stimulation technique commonly used to increase fertility in the production of transgenic mice. Following ovarian stimulation, we found that VIP−/− mice release 26.7 ± 1.7 oocytes, which is not significantly different from the 24.7 ± 2.8 oocytes released by WT females (P = 0.51; Fig. 2C). In contrast to our finding that 50% of VIP−/− females do not spontaneously ovulate on the day after proestrus, the entire cohort of hormone primed VIP−/− females released oocytes, demonstrating that the VIP−/− ovaries are able to respond to gonadotropin stimulation.
Circadian behaviour is disrupted in female VIP-deficient mice
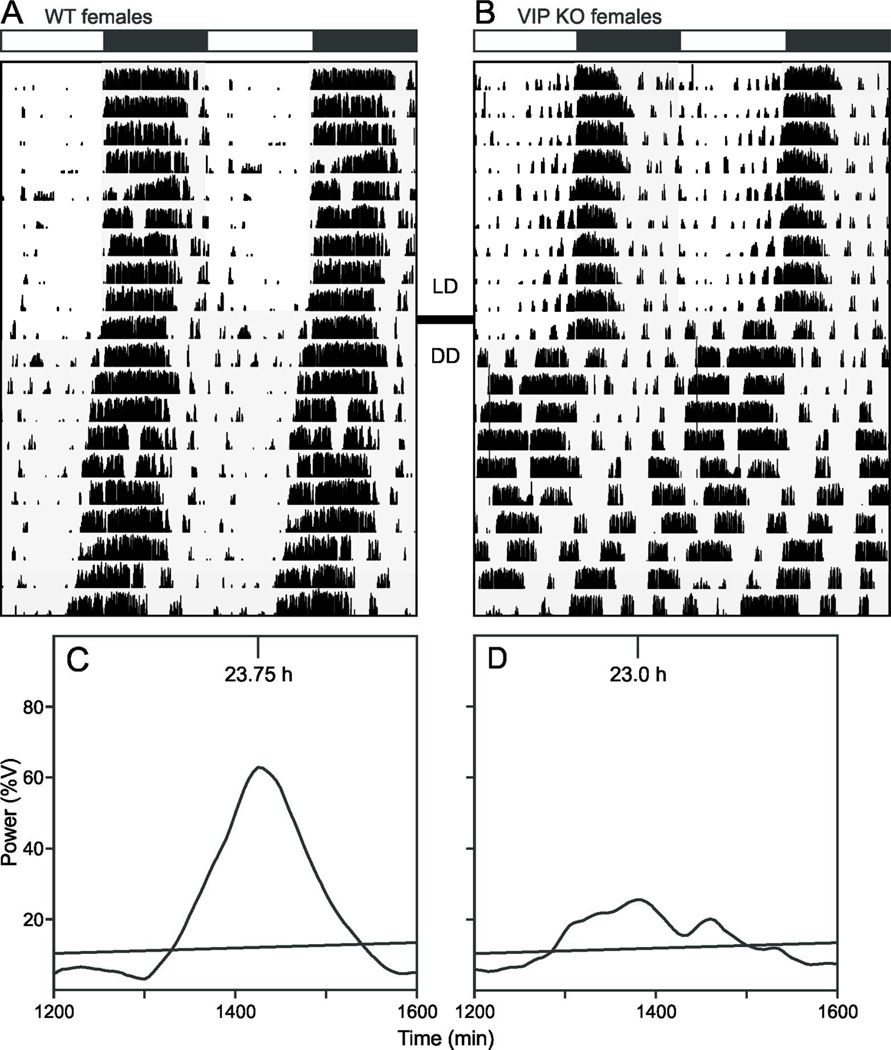
Several studies have characterized the circadian phenotype in male VIP-deficient mice (Colwell et al., 2003; Aton et al., 2005; Ciarleglio et al., 2009) while surprisingly neglecting to describe the behavior of the female mutants. We used wheel-running activity to determine the impact of the loss of VIP on diurnal and circadian rhythms of behavior in female mice (Fig. 3). In LD conditions, the diurnal rhythms are largely similar between the genotypes with the only significant change seen in the extent of fragmentation of the nocturnal activity bout (Table 1). It is in DD that the full extent of the circadian phenotype in these mutant animals emerge. We observed significant decreases in the power and precision of the wheel running activity. Additionally, there is a significant decrease in the free-running period and a dramatic decrease in the magnitude of light-induced phase shifts to light exposure to CT 16 (Table 1). Overall activity levels are not altered highlighting the general conclusion that the loss of VIP does not produce a gross motor phenotype. A characteristic feature of the behavior of VIP−/− mice is the vastly advanced angle of entrainment to the LD cycle, as revealed when the mice are placed in DD. Like their male counterparts, VIP−/− females are abnormally entrained to the LD cycle, but at a significantly smaller magnitude, suggesting a sex-dependent effect of the mutation (Table 2). It is worth noting that there was no problem measuring key circadian parameters in WT female mice despite their likely estrous cycling. Overall, the female VIP-deficient mice showed clear and dramatic disruption of their circadian rhythms in wheel running activity.
Fig. 3.
Representative actograms and periodograms of female WT (A, C) and VIP−/− (B, D) mice under LD and DD conditions. A, C: Wheel-running activity records are double plotted and gray shading on the actograms denotes lights off. B, D: Plot of power of activity as a function of period (%V) under DD. The diagonal line represents 0.1% significance level.
Table 1.
Parameters of wheel running activity in female WT and VIP−/− mice.
| WT | VIP−/− | |
|---|---|---|
| LD | ||
| LD activity, rev/hr | 331.96 ± 27.95 | 280.08 ± 25.18 |
| LD phase angle, min | −7.98 ± 4.02 | −8.70 ± 8.55 |
| LD precision, min | −17.79 ± 4.25 | −23.25 ± 10.14 |
| LD fragmentation, bouts/day | 4.95 ± 0.33 | 6.83 ± 0.63* |
| DD | ||
| LD to DD phase angle, hr | −0.35 ± 0.11 | −3.85 ± 1.40** |
| Tau, hr (DD) | 23.69 ± 0.06 | 23.15 ± 0.15** |
| DD power, %V | 44.75 ± 4.67 | 29.68 ± 3.00** |
| DD activity, rev/hr | 311.64 ± 33.88 | 364.28 ± 47.62 |
| DD precision, min | −38.82 ± 12.10 | −302.98 ± 71.08*** |
| DD fragmentation, bouts/day | 6.19 ± 0.49 | 5.72 ± 0.49 |
| Phase shift to light at CT 16 | 162.8 ± 18.4 | −7.14 ± 12.63*** |
Values are reported as mean ± S.E.M. n = 10 for WT and n = 10 for VIP−/− females. LD, light dark cycle; DD, constant darkness; rev, wheel revolutions, %V, normalized Qp.
Significant differences between WT and VIP−/− mice are indicated with for P < 0.05
for P < 0.01
for P < 0.001.
Table 2.
Sex-dependent effects of VIP-deficiency on wheel running activity.
| LD | Male VIP−/− | Female VIP−/− |
|---|---|---|
| LD activity, rev/hr | 234.05 ± 35.17** | 280.08 ± 25.18 |
| LD phase angle, min | −63.63 ± 44.20 | −8.70 ± 8.55 |
| LD precision, min | −53.84 ± 30.63 | −23.25 ± 10.14 |
| LD fragmentation, bouts/day | 6.00 ± 0.84 | 6.83 ± 0.63* |
| LD alpha, min | 474.25 ± 64.78 | 518.20 ± 56.06 |
| %activity in light | 20.43 ± 10.27 | 8.89 ± 3.51 |
| DD | Male VIP−/− | Female VIP−/− |
| LD-DD Phase angle, hr | −9.00 ± 0.39*** | −3.85 ± 1.40**# |
| DD tau, hr | 23.10 ± 0.27* | 23.15 ± 0.15** |
| DD power, hr | 26.88 ± 3.25** | 29.68 ± 3.00** |
| DD activity, rev/hr | 297.19 ± 42.30 | 364.28 ± 47.62 |
| DD precision, min | −131.62 ± 17.53*** | −302.98 ± 71.08***# |
| DD fragmentation, bouts/day | 7.24 ± 0.73 | 5.72 ± 0.49 |
| DD alpha, min | 599.43 ± 35.13* | 693.70 ± 44.44 |
Wheel running activity was monitored in male VIP−/− littermates from the same colony of VIP;PER2::LUC double transgenics. As previously described (13), male VIP−/− ;PER2::LUC mice have a shorter free-running period, vastly altered angle of entrainment as indicated by the large phase advance upon release to constant darkness, greatly reduced power of rhythm, and poor precision of daily activity onset. The same differences are observed in female VIP−/−;PER2::LUC mice. However, the angle of entrainment is less affected by the loss of VIP in females than males (2 way ANOVA sex x genotype interaction F = 11.22, P = 0.002; post-hoc Holm-Sidak t = 7.69, P < 0.001). In contrast, female VIP−/− mice have more imprecise activity onset than males (2 way ANOVA sex x genotype interaction F = 4.11, P = 0.05; post-hoc Holm-Sidak t = 3.19, P = 0.003).
indicates significant differences between WT and VIP−/− mice within sex as determined by Student’s t-test
indicates significant differences between male and female VIP−/− mice as determined by ANOVA post-hoc tests.
SCN neural activity is compromised in female VIP-deficient mice
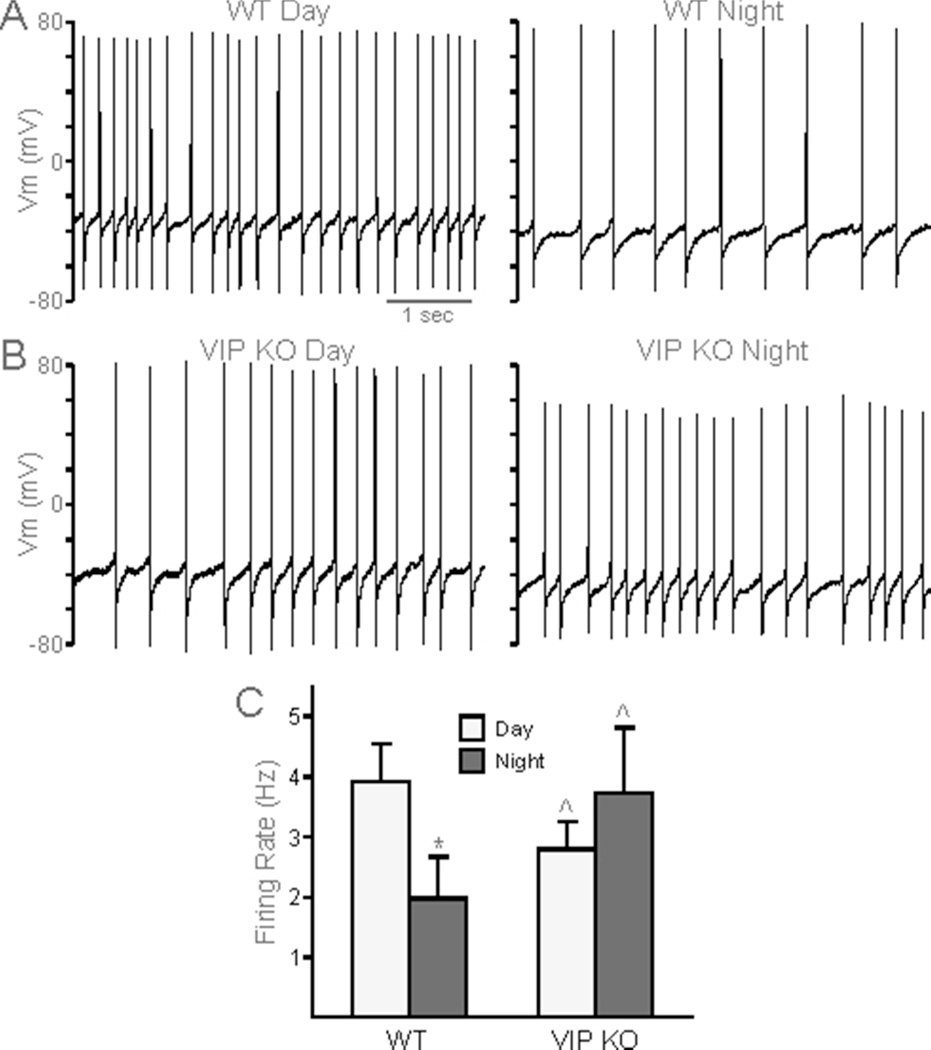
SCN neurons are spontaneously electrically active cells that generate rhythms in action potential frequency with peak activity during the day (Colwell, 2011). We examined day-time (ZT 4 – 6) and night-time (ZT 14 – 16) neural activity of dSCN neurons from WT and VIP−/− mice using whole cell patch clamp electrophysiology. As expected, female WT mice exhibit a day-night difference in SFR, with higher frequency of firing during the day (3.57 ± 0.56 Hz) than during the night (1.25 ± 0.44, P = 0.011; Fig. 4A). In comparison, female VIP−/− dSCN neurons exhibited lower firing rates during the day (1.89 ± 0.33 Hz, vs WT, P = 0.019) and there was no longer a significant day-night difference in SFR (night SFR 3.74 ± 1.08 Hz, vs day SFR, P = 0.054; Fig. 4B). When the cells were synaptically isolated using a GABA-blocker (GabZ), VIP−/− dSCN neurons were again found to have lost the day-night differences in SFR (day 2.61 ± 0.56 Hz vs. night 3.29 ± 1.10 Hz; P = 0.54 Fig. 4C). The reduced daytime SFR in the dSCN in female VIP −/− mice demonstrates that the mutants exhibit a weakened output from the SCN circuit in vitro and suggests that the rest of the circadian system is getting a low amplitude signal from the SCN in vivo.
Fig. 4.
Reduced amplitude in rhythm of spontaneous firing in VIP−/− female SCN neurons. A, B. Representative traces of spontaneous action potentials in day (left) and night (right) in WT (A) and VIP−/− (B) dorsal SCN neurons. C. Population averages of firing rate (Hz) across WT (day n = 11, night n = 9) and VIP−/− (day n = 14, night n = 9) SCN neurons synaptically isolated by treatment with GabZ. Student’s t-tests determined a significant difference between day-time and night-time firing rates in WT (*P < 0.05) but not in VIP−/− SCN. VIP−/− daytime firing rates were also found to be significantly different from WT (^P < 0.05).
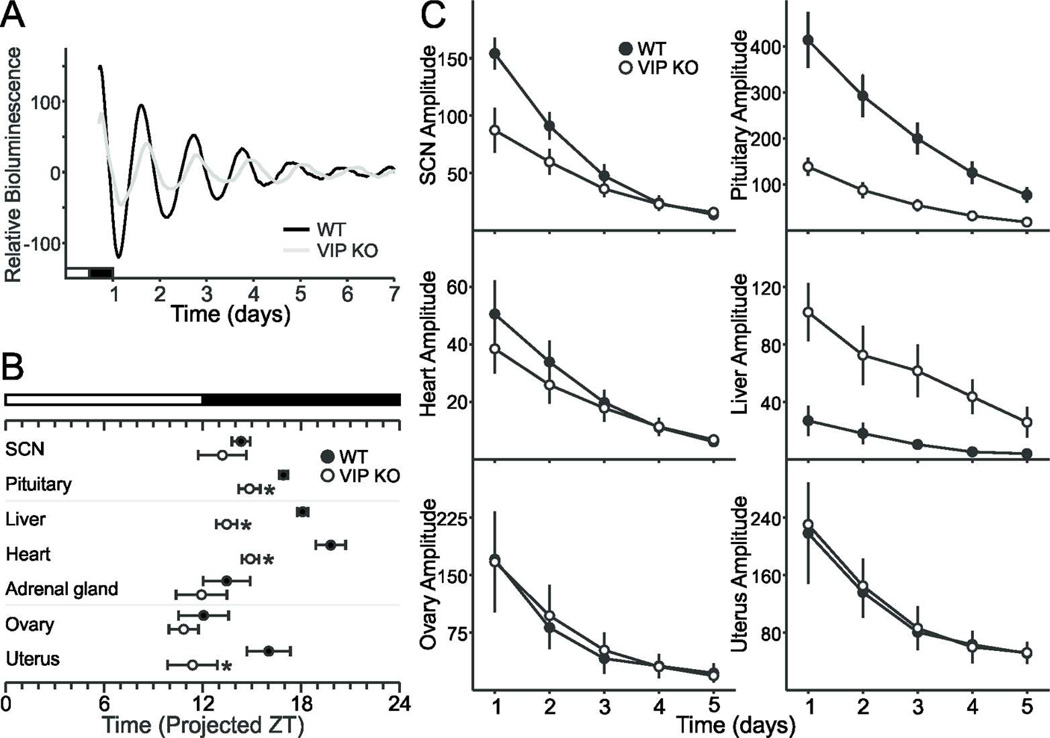
PER2 rhythms in the SCN, pituitary and reproductive organs are differentially affected in female VIP-deficient mice
The circadian timing system consists of a central clock located in the SCN and peripheral clocks found throughout the body (Albrecht, 2012; Mohawk et al., 2012). To determine the impact of the loss of VIP on the molecular clockwork, we measured the phase of the expression of a critical circadian gene, Period2, using the PER2::LUC reporter which drives expression of luciferase fused to the PERIOD2 protein in double transgenics for the VIP mutation and the reporter (Loh et al., 2011). To control for the effects of estrous cycle-driven hormones, we only used proestrus female mice. While the period and phase of peak PER2::LUC bioluminescence in the SCN was not affected by loss of VIP (Fig. 5A; Table 3), the phasing of the pituitary and uterus were advanced in VIP−/− PER2::LUC explants (Fig. 5B). Conversely, the amplitude of the oscillations in the peripheral explants is unaffected by loss of VIP (Fig. 5D), whereas the amplitude of PER2::LUC bioluminescence in the SCN and pituitary are blunted in the mutant mice (Fig. 5A, C). The loss of VIP did not affect the period of PER2::LUC rhythms in any of the explants (Table 3). We can thus conclude that VIP is not needed for intrinsic rhythmicity of peripheral oscillators in the ovary or uterus, since the period and amplitude of these rhythms are not different from WT controls. In contrast, VIP is important for the amplitude of PER2::LUC bioluminescence in the SCN and the pituitary and is also critical for the normal phasing of the pituitary and uterus.
Fig. 5.
Impact of the loss of VIP on the amplitude and phasing of PER2::LUC bioluminescence in female SCN and peripheral organs. A. Representative bioluminescence rhythms from WT and VIP−/− SCN ex vivo culture. B. Phases of peak expression of PER2::LUC of the pituitary and the uterus are altered in the VIP-deficient mice when compared to WT controls (* P < 0.05). C. Amplitude of PER2::LUC bioluminescence in SCN and pituitary are dependent on VIP (* P < 0.05), while the amplitude of the ovary and uterus are unaltered.
Table 3.
PER2::LUC bioluminescence parameters of WT and VIP−/− ex vivo explants.
| WT | VIP−/− | |
|---|---|---|
| SCN | ||
| Period (hr) | 25.84 ± 0.54 | 25.62 ± 0.60 |
| Phase (first calculated peak) | 14.38 ± 0.56 | 13.24 ± 1.47 |
| Amplitude | 154.10 ± 13.82 | 87.40 ± 19.57* |
| Damping Rate | −0.61 ± 0.05 | −0.42 ± 0.04** |
| R2 | 0.972 ± 0.015 | 0.968 ± 0.006 |
| Pituitary | ||
| Period (hr) | 23.54 ± 0.23 | 23.98 ± 0.68 |
| Phase (first calculated peak) | 16.96 ± 0.27 | 14.86 ± 0.66* |
| Amplitude | 414.01 ± 60.65 | 138.67 ± 19.95** |
| Damping Rate | −0.45 ± 0.03 | −0.59 ± 0.05* |
| R2 | 0.992 ± 0.002 | 0.967 ± 0.009* |
| Liver | ||
| Period (hr) | 25.73 ± 1.24 | 25.32 ± 0.62 |
| Phase (first calculated peak) | 18.12 ± 0.33 | 13.47 ± 0.63*** |
| Amplitude | 27.02 ± 10.40 | 102.54 ± 20.25** |
| Damping Rate | −0.40 ± 0.04 | −0.38 ± 0.09 |
| R2 | 0.824 ± 0.037 | 0.797 ± 0.087 |
| Heart | ||
| Period (hr) | 24.90 ± 0.51 | 24.45 ± 0.41 |
| Phase (first calculated peak) | 19.81 ± 0.91 | 14.91 ± 0.52*** |
| Amplitude | 50.62 ± 11.68 | 38.52 ± 8.53 |
| Damping Rate | −0.53 ± 0.03 | −0.42 ± 0.04 |
| R2 | 0.985 ± 0.005 | 0.943 ± 0.014 |
| Adrenal Glands | ||
| Period (hr) | 24.40 ± 0.53 | 23.70 ± 0.90 |
| Phase (first calculated peak) | 13.47 ± 1.43 | 11.94 ± 1.55 |
| Amplitude | 24.31 ± 3.63 | 26.84 ± 4.41 |
| Damping Rate | −0.57 ± 0.05 | −0.58 ± 0.05 |
| R2 | 0.678 ± 0.069 | 0.692 ± 0.028 |
| Ovary | ||
| Period (hr) | 22.93 ± 0.53 | 24.25 ± 0.55 |
| Phase (first calculated peak) | 12.09 ± 1.52 | 10.85 ± 0.90 |
| Amplitude | 170.71 ± 49.90 | 167.29 ± 65.56 |
| Damping Rate | −0.57 ± 0.08 | −0.53 ± 0.04 |
| R2 | 0.923 ± 0.043 | 0.899 ± 0.039 |
| Uterus | ||
| Period (hr) | 24.00 ± 0.40 | 24.05 ± 0.49 |
| Phase (first calculated peak) | 16.06 ± 1.32 | 11.39 ± 1.52* |
| Amplitude | 218.35 ± 70.77 | 230.47 ± 53.74 |
| Damping Rate | −0.47 ± 0.15 | −0.45 ± 0.06 |
| R2 | 0.867 ± 0.053 | 0.815 ± 0.072 |
Values are reported as mean ± S.E.M. n = 7 for WT and n = 10 for VIP−/−. Significant differences determined by Student’s t-tests between WT and VIP−/− mice are indicated with
for P < 0.05
for P < 0.01
for P < 0.001.
Discussion
We wished to address the impact of VIP-deficiency on the reproductive success of female mice, due to the importance of both VIP and the circadian timing system on reproduction. Anecdotal evidence from our colony indicated difficulty maintaining the homozygote VIP-deficient line. Careful measurements over 6 months confirmed that the VIP−/− females are sub-fertile, spending less time pregnant or nursing, and producing half the number of offspring compared to their sisters held under the same conditions. Low fecundity has also been reported in VIPR2 knock-out mice (Dolatshad et al., 2006). These studies indicate that the loss of VIP signalling can result in the decline of reproduction and raise questions about the underlying mechanisms.
Previous studies have established that VIP expressing neurons provide one of the connections between the SCN and GnRH neurons in the medial preoptic area (Van Der Beek et al., 1997; Horvath et al., 1998; Kriegsfeld et al., 2002; Ward et al., 2009; Vida et al., 2010). One of the receptors for VIP (VIPR2) is expressed on GnRH neurons (Smith et al., 2000) and, physiologically, the exogenous treatment of VIP increases the electrical activity of GnRH neurons while blocking the receptor decreases firing (Christian and Moenter, 2008). Therefore, we examined the impact of the loss of VIP on the estrous cycle. We found that VIP-deficient female mice have irregular and lengthened estrous cycles, due to a failure to progress from proestrus to estrus (Fig. 1). The VIPR2 KO mice also show a disrupted estrous cycle but these mice spent more time in estrus (Dolatshad et al., 2006). We cannot explain the differences between the mutant lines but point out that VIPR2 also binds PACAP. Both lines of mutants are able to reproduce and the AVP and kisspeptin pathway most likely allows for continued, albeit lower, fertility. Current thinking places greater importance on the indirect multi-synaptic connection between the SCN and GnRH neurons via AVP and kisspeptin (Robertson et al., 2009), which may be timed or gated by the direct VIPergic connection (Williams et al., 2011). In rats, a sophisticated model of internal desynchrony within the SCN provides evidence that the AVP and VIP expressing subdivisions need to have coupled cross-talk to achieve maximal GnRH-dependent LH surge activity (Smarr et al., 2012). Ovulation involves the coincidence between high estrogen levels sensitizing the gonadotrophs with a gating signal from the SCN controlling the time of day of ovulation (Legan and Karsch, 1975; Christian and Moenter, 2010; Williams and Krigsfeld, 2012). We predicted a blunted LH surge in the mutant females as has been seen in rats treated with anti-sense against Vip (Harney et al., 1996; Gerhold et al., 2005) but were unable to successfully measure the surge with hourly sampling. We were able to determine that the necessary rise in serum estradiol is not affected by the loss of VIP (Fig. 2). The end measure of the estrous cycle in females is ultimately ovulation, which we found to be deficient in VIP−/− females, where oocytes were released less frequently and in fewer numbers than WT controls. VIP has been shown to mediate the development and survival of female rat ovarian follicles (Flaws et al., 1995; Cecconi et al., 2004; Chen et al., 2013), and has a steroidogenic role in the ovaries (Ahmed et al, 1986; Kowalewski et al., 2010). In our study, ovarian stimulation in response to exogenous treatment with gonadotropins did not vary between the genotypes (Fig. 2) demonstrating that the deficit in the mutants is not in the oocyte content of the ovaries but rather in the luteinizing signal. Additionally, the comparable proestrus concentration of serum estradiol in VIP-competent and VIP−/− females suggests that VIP is not critically necessary for ovarian steroidogenesis. It is worth noting that prior studies examining the effect of VIP on ovarian development, survival and steroidogenesis used in vitro manipulations, supplementing cultures with exogenous VIP. In contrast, development in our VIP−/− model may have used redundant pathways, e.g. PACAP (Cecconi et al., 2004), allowing for reproduction to continue, albeit at sub-optimal levels. The disruption in the estrous cycle provides a proximate explanation for the observed decline in reproductive success and is likely a common consequence of circadian disruption.
The circadian timing system of the female VIP-deficient mice is compromised at several levels. Behaviorally, the female mutant mice have severe deficits in circadian rhythms of locomotor activity, characterized by a shortened free-running period, low power of rhythms, and poor precision of cycle-to-cycle activity onset (Fig. 3; Table 1). A further characteristic observed in both male and female VIP−/− mice is the abnormally advanced angle of entrainment revealed when mice are released from LD to free-running DD conditions. This suggests the mice are abnormally entrained to the prior LD cycle, and we and others have reported phase-advanced physiology and behavior in VIP−/− and VIPR2−/− males under an LD cycle (Colwell et al., 2003; Aton et al., 2005; Ciarleglio et al., 2009). Although the VIP−/− female mice also exhibit an advanced phase angle of entrainment, its magnitude is half that of their male counterparts (Table 2), suggesting there is a sex-dependent effect of the mutation. Additional sex-dependent effects include the reduced amount of activity specific to the VIP−/− males, which may be mediated by testosterone, also reduced in VIP−/− males (Lacombe et al., 2007). Physiologically, our data indicates that the VIP−/− females have severe deficits in the expression of daily rhythms in spontaneous electrical activity (Fig. 4). VIP is critical for coupling SCN neurons, as mice deficient for the peptide or its receptor lose coherence and synchrony at the level of electrical firing (Aton et al., 2005; Brown et al., 2007) and gene expression (Harmar et al., 2002; Maywood et al., 2006; Hughes et al., 2008; Loh et al., 2008; Ciarleglio et al., 2009; Dragich et al., 2010; Loh et al., 2011). Our finding that daytime SFR of SCN neurons is reduced in VIP-deficient females even in the presence of the GABA-blocker, GabZ, suggests that even when GABA-dependent neuronal cross-talk is disabled acutely, the deficits remain. This is consistent with earlier work indicating that VIP signalling is a critical regulator of intrinsic membrane events in SCN neurons (Pakhotin et al., 2006; Kudo et al., 2013). This in vitro electrophysiological data coupled with the behavioral analysis indicates a weakly rhythmic SCN in the female mutant mice.
Finally, we assessed the molecular circadian clockwork measuring PER2-driven bioluminescence in female VIP-deficient mice (Fig. 5; Table 3). At the level of the SCN, the PER2::LUC rhythms showed reduced amplitude but a normal phase relationship to the prior LD cycle. With the exception of the pituitary, the peripheral clocks mostly showed no change in amplitude but exhibited changes in phasing relative to each other and to the prior LD cycle. These alterations in the timing of gene expression in the peripheral oscillators were similar to those previously found in male VIP-deficient mice (Loh et al., 2011). Curiously, although the loss of VIP led to a phase advance of most of the peripheral oscillators (Table 3), the phasing of VIP−/− ovaries was not different from WT (Fig. 5), suggesting that VIP is not necessary for the circadian rhythm of PER2 expression in the ovary (Sellix et al., 2010), and that the temporal gating of ovarian response to gonadotropins is unlikely to underlie the ovulation deficits. The circadian phase of ovaries can be altered by gonadotropins (Yoshikawa et al., 2009), and is also dependent on estrous state (Nakamura et al., 2008). By fixing our collection of PER2::LUC organs to proestrus, we may have picked an estrous state in which the phase of the circadian oscillator in the ovary is governed by circulating steroids. It is also important to note that the phase advance of the other peripheral oscillators is not due to a change in the intrinsic period of PER2 expression, which remains unaltered between the WT and VIP−/− tissues. Again, the gene expression data is best explained by a weak SCN clock whose output is less able to synchronize the network of circadian oscillators. These three lines of evidence for a weakened SCN clock suggest a severe impact on the strength of SCN output signals. These include a dampening of the indirect AVP-kisspeptin route to the GnRH neurons, and the loss of the direct VIP-GnRH relay, both of which ultimately result in a gross decrease in reproductive success.
One limitation of our study that we freely acknowledge is that using a whole-body knockout of VIP does not allow us to determine which of these deficits has the strongest impact on the observed sub-fertility of VIP KO females. The tripartite functions of VIP as a synchronizer between SCN neurons, its role as an SCN output signal to GnRH neurons, and its local role in ovarian follicular development cannot be easily distinguished in our model. Ideally, to distinguish the degree to which these varying functions of VIP affect reproduction, one would employ gene ablation targeted to specific organs or cell types, but a floxed-VIP mutation does not exist at present. Alternatively, one could use targeted rescue of VIP loss, for instance using viral constructs to restore VIP expression in SCN neurons. Nevertheless, our study reinforces the importance of VIP in female reproduction.
For the purposes of this study, the VIP-deficient female mice served as a model for circadian disruption caused by deficits in cellular communication rather than direct disruption of the molecular clockwork. In this sense, the present study serves to bring the females into the story being generated by a number of laboratories working with VIP and VIPR2 deficient mice. While, in general, the circadian phenotype observed with female mutants was similar to that gathered from males, there were also some interesting differences that highlight the importance of including both sexes in our preclinical work (Clayton and Collins, 2014). In addition, there is good reason to be specifically interested in the role of VIP in the circadian regulation of female reproduction. VIP expressing neurons couple the SCN with GnRH neurons and alter their physiology. In particular, as has been previously described (Duncan, 2006; Downs and Wise, 2009; Christian and Moenter, 2010), a decline in VIP has particular relevance to aging in women, where the issue of menopause and its corresponding index of symptoms have negative consequences on the quality of life for middle-aged women. In female rats, the beginnings of reproductive senescence are heralded by a decline in Vip expression in middle-age (Krajnak et al., 1998) that is correlated with a decline in the sensitivity of VIP-innervated GnRH neurons (Krajnak et al., 2001). When middle-aged female rats are treated with intracerebroventricular injections of VIP, the LH surge is restored to levels similar to young females (Sun et al., 2012). These findings suggest that reproductive aging may be due in part to hypothalamic changes as well as gonadal decline. Our findings that the absence of VIP has a greater impact on the SCN than the ovaries supports the view that reproductive aging has a hypothalamic cause, and provides at least one mechanism (see Sellix, 2013 for discussion of other mechanisms) by which circadian disruption can cause a decline in reproductive function.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the contributions of the following people. Dr. Analyne Schroeder, Tamara Cutler, Dr. Takashi Kudo, and Dr. Takahiro Nakamura helped with dissections for bioluminescence organotypic cultures. Donna Crandall prepared the figures for submission. Dr. A. Parlow’s laboratory at UCLA Harbour, Long Beach, CA, performed the radioimmunoassay for estradiol.
Research support
DHL and CSC received funding support from the Iris-Cantor Women’s Health Center at UCLA. DAK was supported by a NIH training grant from the Laboratory of Neuroendocrinology (T32 HD 07228-26).
Footnotes
Author Contributions
Designed study: DHL, DAK, and CSC. Draft of manuscript: DHL, DAK, and CSC. Behaviour: DHL, LA and DT. Electrophysiology: DAK. PER2::LUC bioluminescence: DHL. Estrous and ooctye counts: DHL, LA and YW. Hormones: HBW, DHL.
Disclosure statement
The authors have nothing to disclose.
References
- Ahmed CE, Dees WL, Ojeda SR. The immature rat ovary is innervated by vasoactive intestinal peptide (VIP)-containing fibers and responds to VIP with steroid secretion. Endocrinology. 1986;118:1682–1689. doi: 10.1210/endo-118-4-1682. [DOI] [PubMed] [Google Scholar]
- Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron. 2012 Apr 26;74(2):246–260. doi: 10.1016/j.neuron.2012.04.006. 2012. [DOI] [PubMed] [Google Scholar]
- Alvarez JD1, Hansen A, Ord T, Bebas P, Chappell PE, Giebultowicz JM, Williams C, Moss S, Sehgal A. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J Biol Rhythms. 2008;23:26–36. doi: 10.1177/0748730407311254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacka-Surowiak G, Surowiak J, Stoklosowa S. The involvement of suprachiasmatic nuclei in the regulation of estrous cycles in rodents. Reprod Biol. 2003;3:99–129. [PubMed] [Google Scholar]
- Beaver L, Gvakharia B, Vollintine T, Hege D, Stanewsky R, Giebultowicz J. Loss of circadian clock function decreases reproductive fitness in males of Drosophila melanogaster. Proc Nat Acad Sci USA. 2002;99:2134–2139. doi: 10.1073/pnas.032426699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TM, Colwell CS, Waschek JA, Piggins HD. Disrupted neuronal activity rhythms in the suprachiasmatic nuclei of vasoactive intestinal polypeptide-deficient mice. J Neurophysiol. 2007;97:2553–2558. doi: 10.1152/jn.01206.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligioni CS. Assessing reproductive status/stages in mice. Curr Protocol Neurosci Appendix. 2009;4 doi: 10.1002/0471142301.nsa04is48. Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecconi S, Rossi G, Barberi M, Scaldaferri L, Canipari R. Effect of pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal polypeptide on mouse preantral follicle development in vitro. Endocrinology. 2004;145:2071–2079. doi: 10.1210/en.2003-1004. [DOI] [PubMed] [Google Scholar]
- Chen N, Li Y, Wang W, Ma Y, Yang D, Zhang Q. Vasoactive intestinal peptide can promote the development of neonatal rat primordial follicles during in vitro culture. Biol Reprod. 2013;88:12. doi: 10.1095/biolreprod.111.098335. [DOI] [PubMed] [Google Scholar]
- Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci U S A. 2005;102:15682–15687. doi: 10.1073/pnas.0504270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev. 2010;31:544–577. doi: 10.1210/er.2009-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Moenter SM. Vasoactive intestinal polypeptide can excite gonadotropin-releasing hormone neurons in a manner dependent on estradiol and gated by time of day. Endocrinology. 2008;149:3130–3136. doi: 10.1210/en.2007-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarleglio CM, Gamble KL, Axley JC, Strauss BR, Cohen JY, Colwell CS, McMahon DG. Population encoding by circadian clock neurons organizes circadian behavior. J Neurosci. 2009;29:1670–1676. doi: 10.1523/JNEUROSCI.3801-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JA, Collins FS. NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R939–R949. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- De la Iglesia HO, Schwartz WJ. Minireview: timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology. 2006;147:1148–1153. doi: 10.1210/en.2005-1311. [DOI] [PubMed] [Google Scholar]
- Dolatshad H, Campbell EA, O’Hara L, Maywood ES, Hastings MH, Johnson MH. Developmental and reproductive performance in circadian mutant mice. Hum Reprod. 2006;21:68–79. doi: 10.1093/humrep/dei313. [DOI] [PubMed] [Google Scholar]
- Downs JL, Wise PM. The role of the brain in female reproductive aging. Mol Cell Endocrinol. 2009;299:32–38. doi: 10.1016/j.mce.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragich JM, Loh DH, Wang LM, Vosko AM, Kudo T, Nakamura TJ, Odom IH, Tateyama S, Hagopian A, Waschek JA, Colwell CS. The role of the neuropeptides PACAP and VIP in the photic regulation of gene expression in the suprachiasmatic nucleus. Eur J Neurosci. 2010;31:864–875. doi: 10.1111/j.1460-9568.2010.07119.x. [DOI] [PubMed] [Google Scholar]
- Duncan MJ. Aging of the mammalian circadian timing system: Changes in the central pacemaker and its regulation by photic and nonphotic signals. Neuroembryol Aging. 2006;4:85–101. [Google Scholar]
- Endo A, Wanatabe T. Effects of non-24-hour days on reproductive efficiency and embryonic development in mice. Gamete Res. 1989;22:435–441. doi: 10.1002/mrd.1120220409. [DOI] [PubMed] [Google Scholar]
- Everett JW, Sawyer CH. A 24-hour periodicity in the “LH-release apparatus” of female rats, disclosed by barbiturate sedation. Endocrinology. 1950;47:198–218. doi: 10.1210/endo-47-3-198. [DOI] [PubMed] [Google Scholar]
- Flaws JA, DeSanti A, Tilly KI, Javid RO, Kugu K, Johnson AL, Hirshfield AN, Tilly JL. Vasoactive intestinal peptide-mediated suppression of apoptosis in the ovary: potential mechanisms of action and evidence of a conserved antiatretogenic role through evolution. Endocrinology. 1995;136:4351–4359. doi: 10.1210/endo.136.10.7664654. [DOI] [PubMed] [Google Scholar]
- Gerhold L, Rosewell K, Wise P. Suppression of vasoactive intestinal polypeptide in the suprachiasmatic nucleus leads to aging-like alterations in cAMP rhythms and activation of gonadotropin-releasing hormone neurons. J Neurosci. 2005;25:62–67. doi: 10.1523/JNEUROSCI.3598-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray GD, Söderstein P, Tallentire D, Davidson JM. Effects of lesions in various structures of the suprachiasmatic-preoptic region on LH regulation and sexual behavior in female rats. Neuroendocrinology. 1978;25:174–91. doi: 10.1159/000122739. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JB, Kelly JS, Maywood ES, Hastings MH. The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109:497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- Harney JP, Scarbrough K, Rosewell KL, Wise PM. In vivo antisense antagonism of vasoactive intestinal peptide in the suprachiasmatic nuclei causes aging-like changes in the estradiol-induced luteinizing hormone and prolactin surges. Endocrinology. 1996;137:3696–3701. doi: 10.1210/endo.137.9.8756535. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Cela V, Van Der Beek EM. Gender-specific apposition between vasoactive intestinal peptide-containing axons and gonadotrophin-releasing hormone-producing neurons in the rat. Brain Res. 1998;795:277–281. doi: 10.1016/s0006-8993(98)00208-x. [DOI] [PubMed] [Google Scholar]
- Hughes ATL, Guilding C, Lennox L, Samuels RE, McMahon DG, Piggins HD. Live imaging of altered period1 expression in the suprachiasmatic nuclei of Vipr2−/− mice. J Neurochem. 2008;106:1646–57. doi: 10.1111/j.1471-4159.2008.05520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennaway DJ, Boden MJ, Voultsios A. Reproductive performance in female Clock Delta19 mutant mice. Reprod Fertil Dev. 2004;16:801–810. doi: 10.1071/rd04023. [DOI] [PubMed] [Google Scholar]
- Kowalewski MP, Dyson MT, Boos A, Stocco DM. Vasoactive intestinal peptide (VIP)-mediated expression of steroidogenic acute regulatory protein (StAR) in granulosa cells. Mol Cell Endocrinol. 2010;328:93–103. doi: 10.1016/j.mce.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnak K, Kashon ML, Rosewell KL, Wise PM. Aging alters the rhythmic expression of vasoactive intestinal polypeptide mRNA but not arginine vasopressin mRNA in the suprachiasmatic nuclei of female rats. J Neurosci. 1998;18:4767–4774. doi: 10.1523/JNEUROSCI.18-12-04767.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnak K, Rosewell KL, Wise PM. Fos-induction in gonadotropin-releasing hormone neurons receiving vasoactive intestinal polypeptide innervation is reduced in middle-aged female rats. Biol Reprod. 2001;64:1160–1164. doi: 10.1095/biolreprod64.4.1160. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Silver R, Gore AC, Crews D. Vasoactive intestinal polypeptide contacts on gonadotropin-releasing hormone neurones increase following puberty in female rats. J Neuroendocrinol. 2002;14:685–690. doi: 10.1046/j.1365-2826.2002.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Tahara Y, Gamble KL, McMahon DG, Block GD, Colwell CS. Vasoactive intestinal peptide produces long-lasting changes in neural activity in the suprachiasmatic nucleus. J Neurophysiol. 2013;110(5):1097–1106. doi: 10.1152/jn.00114.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuljis DA1, Loh DH, Truong D, Vosko AM, Ong ML, McClusky R, Arnold AP, Colwell CS. Gonadal- and sex-chromosome-dependent sex differences in the circadian system. Endocrinology. 2013;154:1501–1512. doi: 10.1210/en.2012-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe A, Lelievre V, Roselli CE, Muller JM, Waschek JA, Vilain E. Lack of vasoactive intestinal peptide reduces testosterone levels and reproductive aging in mouse testis. J Endocrinol. 2007;194(1):153–160. doi: 10.1677/JOE-07-0102. [DOI] [PubMed] [Google Scholar]
- Legan SJ, Coon GA, Karsch FJ. Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology. 1975;96:50–56. doi: 10.1210/endo-96-1-50. [DOI] [PubMed] [Google Scholar]
- Loh DH, Abad C, Colwell CS, Waschek JA. Vasoactive intestinal peptide is critical for circadian regulation of glucocorticoids. Neuroendocrinology. 2008;88:246–255. doi: 10.1159/000140676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh DH, Dragich JM, Kudo T, Schroeder AM, Nakamura TJ, Waschek JA, Block GD, Colwell CS. Effects of vasoactive intestinal peptide genotype on circadian gene expression in the suprachiasmatic nucleus and peripheral organs. J Biol Rhythms. 2011;26:200–209. doi: 10.1177/0748730411401740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney MM. Shift work, jet lag, and female reproduction. Int J Endocrinol. 2010:813764. doi: 10.1155/2010/813764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Reddy AB, Wong GK, O'Neill JS, O'Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol. 2004;14:1367–1373. doi: 10.1016/j.cub.2004.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong JA, Baker FC, Mahoney MM, Paul KN, Schwartz MD, Semba K, Silver R. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J Neurosci. 2011;31(45):16107–16116. doi: 10.1523/JNEUROSCI.4175-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosko SS, Moore RY. Neonatal ablation of the suprachiasmatic nucleus. Effects on the development of the pituitary-gonadal axis in the female rat. Neuroendocrinology. 1979;29:350–361. doi: 10.1159/000122944. [DOI] [PubMed] [Google Scholar]
- Nakamura TJ, Sellix MT, Menaker M, Block GD. Estrogen directly modulates circadian rhythms of PER2 expression in the uterus. Am J Physiol Endocrinol Metab. 2008;295:E1025–E1031. doi: 10.1152/ajpendo.90392.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez AA, Stephan FK. The effects of hypothalamic knife cuts on drinking rhythms and the estrus cycle of the rat. Behav. Biol. 1977;20:224–234. doi: 10.1016/s0091-6773(77)90786-6. [DOI] [PubMed] [Google Scholar]
- Pakhotin P, Harmar AJ, Verkhratsky A, Piggins H. VIP receptors control excitability of suprachiasmatic nuclei neurones. Pflugers Arch. 2006;452:457–15. doi: 10.1007/s00424-005-0003-z. [DOI] [PubMed] [Google Scholar]
- Ratajczak CK, Boehle KL, Muglia LJ. Impaired steroidogenesis and implantation failure in Bmal1−/− mice. Endocrinology. 2009;150:1879–1885. doi: 10.1210/en.2008-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JL, Clifton DK, De la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology. 2009;150:3664–3671. doi: 10.1210/en.2009-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellix MT, Yoshikawa T, Menaker M. A circadian egg timer gates ovulation. Curr Biol. 2010;20:R266–R267. doi: 10.1016/j.cub.2010.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellix MT. Clocks underneath: the role of peripheral clocks in the timing of female reproductive physiology. Front Endocrinol (Lausanne) 2013;4:91. doi: 10.3389/fendo.2013.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smarr BL, Morris E, De la Iglesia HO. The dorsomedial suprachiasmatic nucleus times circadian expression of Kiss1 and the luteinizing hormone surge. Endocrinology. 2012;153:2839–2850. doi: 10.1210/en.2011-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Jiennes L, Wise PM. Localization of the VIP2 receptor protein on GnRH neurons in the female rat. Endocrinology. 2000;141:4317–4320. doi: 10.1210/endo.141.11.7876. [DOI] [PubMed] [Google Scholar]
- Summa KC, Vitaterna MH, Turek FW. Environmental perturbation of the circadian clock disrupts pregnancy in the mouse. PLoS One. 2012;7:e37668. doi: 10.1371/journal.pone.0037668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Shu J, Kyei K, Neal-Perry GS. Intracerebroventricular infusion of vasoactive intestinal Peptide rescues the luteinizing hormone surge in middle-aged female rats. Front Endocrinol. 2012;3:24. doi: 10.3389/fendo.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek F, Swann J, Earnest D. Role of the circadian system in reproductive phenomena. Recent Prog Horm Res. 1984;40:143–183. doi: 10.1016/b978-0-12-571140-1.50009-8. [DOI] [PubMed] [Google Scholar]
- Van Der Beek EM, Horvath TL, Wiegant VM, Van den Hurk R, Buijs RM. Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. J Comp Neurol. 1997;384:569–579. doi: 10.1002/(sici)1096-9861(19970811)384:4<569::aid-cne6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Van Der Beek EM, Swarts HJ, Wiegant VM. Central administration of antiserum to vasoactive intestinal peptide delays and reduces luteinizing hormone and prolactin surges in ovariectomized, estrogen-treated rats. Neuroendocrinology. 1999;69:227–237. doi: 10.1159/000054423. [DOI] [PubMed] [Google Scholar]
- Vida B, Deli L, Hrabovszky E, Kalamatianos T, Caraty A, Coen CW, Liposits Z, Kalló I. Evidence for suprachiasmatic vasopressin neurones innervating kisspeptin neurones in the rostral periventricular area of the mouse brain: regulation by oestrogen. J Neuroendocrinol. 2010;22:1032–1039. doi: 10.1111/j.1365-2826.2010.02045.x. [DOI] [PubMed] [Google Scholar]
- Ward DR, Dear FM, Ward IA, Anderson SI, Spergel DJ, Smith PA, Ebling FJ. Innervation of gonadotropin-releasing hormone neurons by peptidergic neurons conveying circadian or energy balance information in the mouse. PLoS One. 2009;4:e5322. doi: 10.1371/journal.pone.0005322. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology. 1980;31:147–157. doi: 10.1159/000123066. 1980. [DOI] [PubMed] [Google Scholar]
- Williams WP, III, Kriegsfeld LJ. Circadian control of neuroendocrine circuits regulating female reproductive function. Front Endocrinol. 2012;3:60. doi: 10.3389/fendo.2012.00060. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams WP, III, Jarjisian SG, Mikkelsen JD, Kriegsfeld LJ. Circadian control of kisspeptin and a gated GnRH response mediate the preovulatory luteinizing hormone surge. Endocrinology. 2011;152:595–606. doi: 10.1210/en.2010-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Sellix M, Pezuk P, Menaker M. Timing of the ovarian circadian clock is regulated by gonadotropins. Endocrinology. 2009;150:4338–4347. doi: 10.1210/en.2008-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.