Abstract
Objective
Impairment in reward processes has been found in individuals with depression and in the aging population. The purpose of this study was twofold: 1. To use an affective neuroscience probe to identify abnormalities in reward-related decision making in late-life depression. 2. To examine the relationship of reward-related decision making abnormalities in depressed, older adults to the clinical expression of apathy in depression. We hypothesized that relative to elderly, healthy subjects, depressed, elderly patients would exhibit impaired decision making and that apathetic, depressed patients would show greater impairment in decision making than non-apathetic, depressed patients.
Methods
We used the Iowa Gambling Task to examine reward-related decision making in 60 non-demented, elderly patients with non-psychotic major depression and 36 elderly, psychiatrically healthy participants. Apathy was quantified using the Apathy Evaluation Scale. Of those with major depression, 18 individuals reported clinically significant apathy whereas 42 participants did not have apathy.
Results
Older adults with depression and healthy comparison participants did not differ in their performance on the IGT. However, apathetic, depressed older adults adopted an advantageous strategy and selected cards from the conservative decks compared to non-apathetic, depressed older adults. Non-apathetic, depressed patients showed a failure to adopt a conservative strategy and persisted in making risky decisions throughout the task.
Conclusions
This study indicates that apathy in older, depressed adults is associated with a conservative response style on a behavioral probe of the systems involved in reward-related decision making. This conservative response style may be the result of reduced sensitivity to rewards in apathetic individuals.
Keywords: reward, decision making, late-life depression, apathy
INTRODUCTION
Apathy, defined as a state of primary motivational impairment not attributable to diminished level of consciousness, cognitive impairment, or emotional distress, is a common, debilitating symptom of depression (Marin, 1990; Marin et al., 1991; Starkstein and Leentjens, 2008). This deficit in motivation results in diminished goal-directed behavior, lack of intellectual interest and initiative, and indifference or flattening of affect. Apathy affects 1/3 of non-demented, depressed adults and is particularly common in late-life depression (Forsell et al., 1993; Krishnan et al., 1995; Lampe and Heeren, 2004; Mehta et al., 2008). As a barrier to recovery from depression, apathy predicts poor response to antidepressants and chronicity (Chaturvedi and Sarmukaddam, 1986; Lavretsky et al., 1999; Levkovitz et al., 2011; Marin et al., 2003).
Apathy of late-life depression occurs in the context of neurobiological abnormalities related to the positive valence network (Alexopoulos et al., 2013), one of the main domains in the Research Domain Criteria (RDoC) matrix. Impairment in the positive valence system leads to the abandonment of rewarding activities, strengthening the conviction that little meaning or value is left in life and promoting depressive symptoms. Apathy may be related to the approach motivation construct of the positive valence system, specifically the component process of action selection/preference-based decision making. Action selection/preference-based decision making is defined as processes involved in the evaluation of costs and benefits and selection of an action or course of actions from among a set of alternatives. For clarity and brevity, these processes will be referred to as reward-related decision making throughout this manuscript.
Reward-related decision making depends on motivation, evaluation of the rewards and punishments associated with different options, and responsiveness to available rewards. Depressed individuals exhibit impairment in reward-related decision making on both behavioral measures and self-report scales (Elliott et al., 1998; Eshel and Roiser, 2010; Henriques et al., 1994; Vrieze et al., 2013). Further, impairment in reward-related decision making in late-life depression is associated with critical clinical outcomes including functional impairment and suicidality (Dombrovski et al., 2012; Jollant et al., 2005, 2010).
The Iowa Gambling Task has been designated as a well-validated paradigm to study reward-related decision making (Bechara et al., 1994, 1996, 1997). In the classic version of the task, participants are instructed to choose cards from one of four decks in order to win as much money as possible. The four decks have different schedules of reward and loss. Two decks are associated with higher immediate reward and higher long-term punishment, yielding an overall net loss, whereas the other two decks have lower immediate gain and lower long-term punishment, yielding an overall net gain. The task is structured that in order to win the most money, participants need to identify which decks are the advantageous or conservative decks and then select cards only from those decks. Therefore, performance on the IGT provides a measure of reward-related decision making. In this manuscript, we use performance on the IGT to examine how behavioral abnormalities of the positive valence system are expressed clinically in older adults suffering from depression.
Performance on the IGT is influenced by both depression and advancing age. The few studies that have used the IGT to examine reward-related decision making in mid-life depression have yielded conflicting findings, with some finding a disadvantageous or riskier approach to the task in depressed patients and others observing a conservative response bias in depressed patients that results in larger net gains (Cella et al., 2009; Dalgleish et al., 2004; Must et al., 2006; Smoski et al., 2008). In the aging population, approximately 40% of healthy older adults fail to learn to shift their selections from the risky decks to the conservative decks (Bauer et al., 2012; Denburg et al., 2007; Fein et al., 2007; Rogalsky et al., 2012).
The primary objectives of this present study were twofold: 1. to investigate IGT performance in older, depressed adults relative to elderly non-psychiatric comparison subjects and 2. to examine the relationship of IGT performance to the presence of apathy in elderly depressed patients. We hypothesized that relative to elderly, non-psychiatric comparison subjects, depressed, elderly patients would exhibit impaired reward-related decision making on the IGT and that apathetic, depressed patients would show greater impairment in decision making on the IGT than non-apathetic depressed patients.
METHODS
Participants
The study included 60 non-demented, elderly (>60 years) patients with non-psychotic major depression and 36 elderly, psychiatrically healthy participants. Subjects were recruited for an escitalopram trial through radio and print advertisements. Data was collected after the 2-week single-blind, placebo lead in and drug wash out period. The Weill Cornell Medical College Institutional Review Board approved all procedures. After a complete description of the study to the participants, written informed consent was obtained.
The depressed group met the DSM-IV-TR criteria and Research Diagnostic Criteria for unipolar major depression and had a >18 on the 24-item Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960). Exclusion criteria for the depressed group were: (1) major depression with psychotic features (according to the DSM-IV-TR); (2) history of other Axis I psychiatric disorders prior to the onset of depression; (3) severe medical illness (i.e., metastatic cancer, brain tumors, unstable cardiac, hepatic or renal disease, myocardial infarction) within the 3 months preceding the study; (4) neurological disorders (i.e., dementia or delirium according to the DSM-IV criteria, history of stroke, head trauma, multiple sclerosis, and brain degenerative diseases, including Parkinson’s disease); (5) drugs causing depression (i.e., steroids, α-methyl-dopa, clonidine, reserpine, tamoxifen, and cimetidine); (6) conditions often associated with depression (i.e., endocrinopathies other than diabetes, lymphoma, and pancreatic cancer); (7) Mini-Mental State Examination (Folstein et al., 1975) score <25; and (8) current psychotherapy. Exclusion criteria for non-depressed participants were the same as above as well as no history of any psychiatric illness.
Assessment
DSM-IV diagnosis was based on the SCID-R (First et al., 2002), administered at entry to the study. Depressive symptoms were assessed using the 24-item Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960). Overall cognitive impairment was rated with the Mini-Mental State Examination (MMSE) (Folstein et al., 1975) and the Dementia Rating Scale (Mattis, 1988). Apathy was quantified using the self-rated Apathy Evaluation Scale (AES), a psychometrically validated instrument in older normal individuals and psychiatric patients (Clarke et al., 2007; Marin et al., 1991). An AES score greater than 38 was considered to be clinically significant apathy (Clarke et al., 2007).
The computerized Iowa Gambling Task (IGT) (Bechara et al., 1997) was administered in the standard fashion, involving 100 card selections from four decks (A, B, C, and D). Participants were instructed to choose cards from one of the four decks presented on the screen and that the aim of the task was to win as much money as possible. Some card selections were followed by reward only (monetary gain) whereas others were followed by a reward and a punishment (monetary loss). The task was manipulated such that decks with lower immediate gain have lower long-term punishment, yielding an overall net gain (decks C and D, referred to as conservative or advantageous decks); decks with higher immediate reward have higher long-term punishment, yielding an overall net loss (decks A and B, referred to as risky or disadvantageous decks). Reward amounts ranged from $40 to $80 for decks C and D and $80 to $170 in decks A and B. Losses ranged from $25 to $375 for decks C and D and $150 to $2500 for decks A and B. Participants were not informed about the number of trials or the reward/punishment schedules, and the schedules could not be deduced mathematically.
To quantify performance on the IGT, the 100 card selections were divided into five discrete blocks of 20 cards each, and for each Trial Block, a performance score was calculated by subtracting the number of risky deck choices (A and B) from the number of conservative deck choices (C and D), ([C+D]−[A+B]). Average response patterns over the five blocks of the task were also calculated and provided an overall index of reward-related decision making performance. The number of cards selected from each deck was computed to determine deck preferences. Furthermore, the contingencies for the frequencies of losses differ between decks, allowing examination of the sensitivity to punishment frequency. The tendency to avoid decks with frequent losses (decks A and C) versus decks with infrequent losses (decks B and D) was examined by subtracting the total number of choices from decks B and D (infrequent larger losses) from decks A and C (frequent smaller losses), ([A+C]−[B+D]) (Stocco et al., 2009).
Statistical Analyses
Statistical analysis was performed with SPSS 19.0 (SPSS, Inc.). Univariate analysis of variance (ANOVA) assessed for differences in demographic and clinical characteristics (age, years of education, HDRS, AES) between depressed subjects and non-depressed, healthy controls and then among apathetic, non-apathetic, and healthy control groups. A repeated measures analysis of variance, with group (depressed vs. non-depressed and apathetic vs. non-apathetic vs. healthy controls) as the between-subjects factor and trial block as the within-subjects factor, was performed to analyze IGT performance among groups over time. Post-hoc Tukey tests for honestly significant differences (HSD) were performed to determine significant group differences and to control for Type 1 errors. Post-hoc independent samples t-tests and follow-up univariate ANOVAs were then used to determine significant differences in trial block between groups. Independent samples t-tests were used to calculate deck preferences and sensitivity to punishment frequency among apathetic and non-apathetic groups.
RESULTS
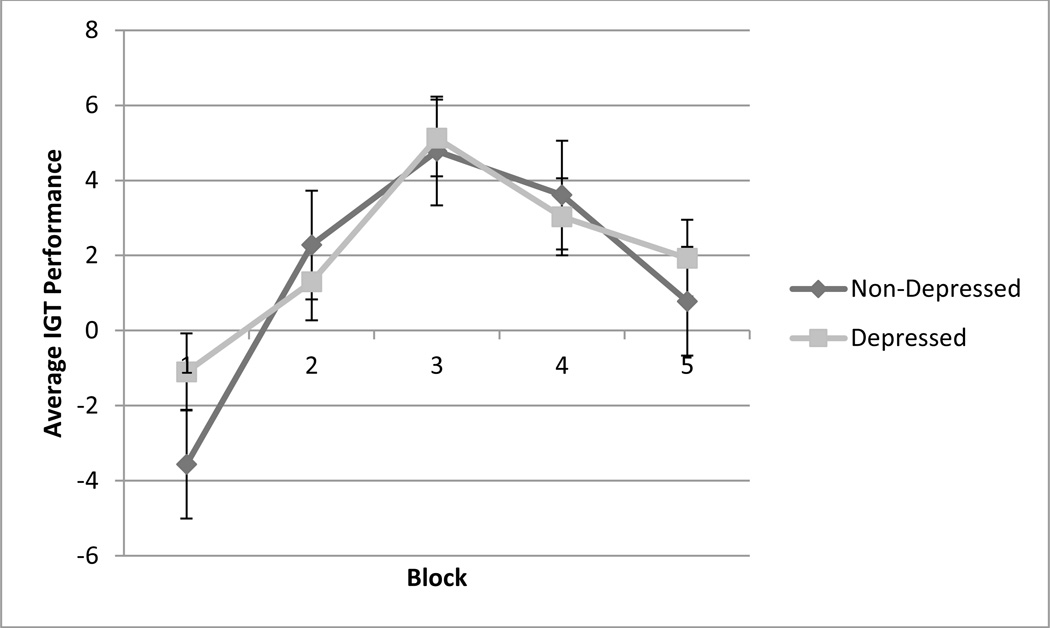
Depressed and non-depressed comparison participants were similar in age, years of education, and overall cognitive function, as measured by the MMSE and DRS. As expected, depression severity, as measured by the HDRS, significantly differed between the groups (t(94) = −33.21, p< 0.001). Furthermore, to assess the differences in IGT performance over time between the two groups, a repeated measures analysis of variance, with group as the between-subjects factor and trial block as the within-subjects factor, was performed. The results showed no significant effect of trial block between groups (F(3.57, 335.36) = 0.49, p = 0.73), indicating that no differences in IGT performance over time exists between depressed subjects and non-depressed controls (Figure 1). Furthermore, depressed and non-depressed comparison participants did not differ significantly in deck preferences (t(94) = 0.40, p = 0.69) or in sensitivity to punishment frequency (t(94) = −0.43, p = 0.67).
Figure 1.
Average IGT performance over time for healthy controls and depressed older adults. The x-axis represents the five trial blocks of the task and the y-axis shows the average IGT performance score, which is calculated by subtracting the total number of cards chosen from the risky decks from the total number of cards chosen from the conservative decks.
Apathetic, non-apathetic, and control participants also were similar in age, years of education, and overall cognitive functioning (Table 1). However, baseline AES scores were significantly greater for apathetic participants than for non-apathetic and control participants (F(2, 93) = 192.30, p< 0.001). Depression severity also significantly differed among the groups (F(2, 93) = 606.12, p< 0.001).
Table 1.
Demographic and clinical characteristics of healthy controls, and non-apathetic and apathetic participants.
| Healthy Controls (N = 36) |
Non-Apathetic (N = 42) |
Apathetic (N = 18) |
F | p | |
|---|---|---|---|---|---|
| Age (years) | 71.6 (6.8) | 72.2 (7.7) | 71.1 (7.3) | 0.156 | 0.856 |
| Education (years) | 16.4 (2.2) | 16.0 (3.4) | 16.6 (1.8) | 0.351 | 0.705 |
| MMSE | 28.5 (0.9) | 28.2 (2.1) | 28.2 (1.9) | 0.289 | 0.750 |
| DRS | 137.2 (4.0) | 136.1 (4.9) | 135.9 (5.1) | 0.727 | 0.486 |
| HDRS | 1.14 (2.1) | 22.4 (3.3) | 25.0 (3.7) | 606.109 | 0.000 |
| AES | 22.9 (3.2) | 31.4 (4.3) | 46.1 (5.2) | 192.297 | 0.000 |
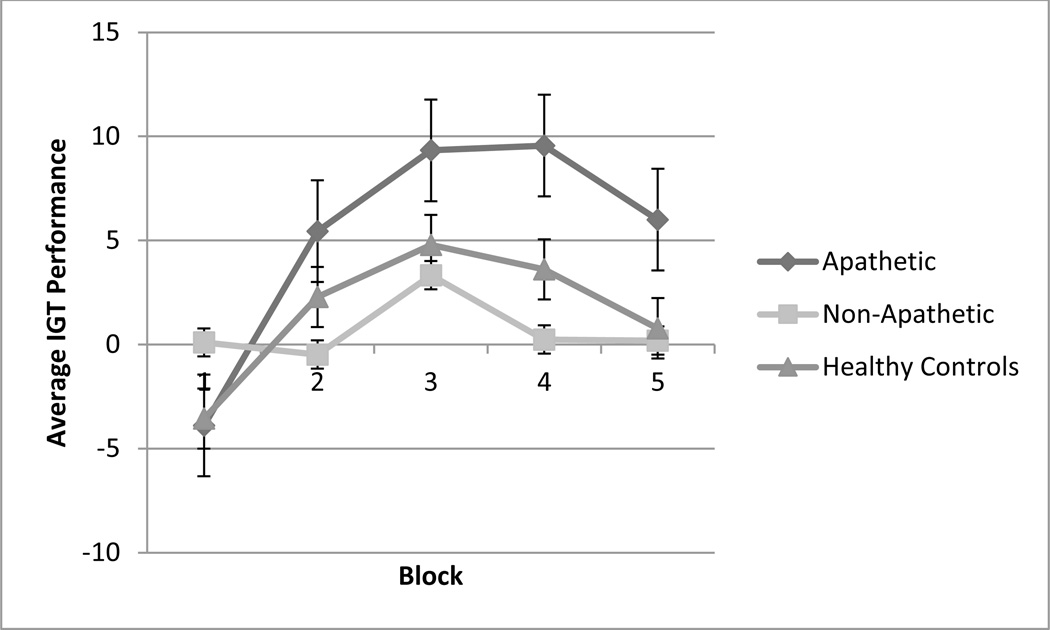
To assess differences in IGT performance over time among the apathetic, non-apathetic, and control groups, a repeated measures analysis of variance with a Greenhouse-Geisser correction determined a significant effect of trial block (F(3.55, 329.82) = 8.69, p< 0.001) and group (F(2, 93) = 4.43, p< 0.05). The interaction between group and trial blocks (F(7.09, 329.82) = 2.15, p < 0.05) was also significant, indicating that non-apathetic and apathetic, depressed patients and healthy controls displayed different decision making patterns during the task (Figure 2). Follow-up univariate ANOVAs specifically showed a marginally significant difference among groups on Trial Block 2 (F(2, 93) = 2.67, p < 0.1) and a significant difference among groups on Trial Block 4 (F(2, 93) = 5.08, p < 0.05). Post-hoc Tukey tests of HSD revealed that the apathetic group selected significantly more cards from the conservative decks than the non-apathetic group in Trial Block 4, with no significant difference between the apathetic and healthy control groups.
Figure 2.
Average IGT performance over time for healthy controls, non-apathetic, and apathetic participants. The x-axis represents the five trial blocks of the task and the y-axis shows the average IGT performance score, which is calculated by subtracting the total number of cards chosen from the risky decks from the total number of cards chosen from the conservative decks.
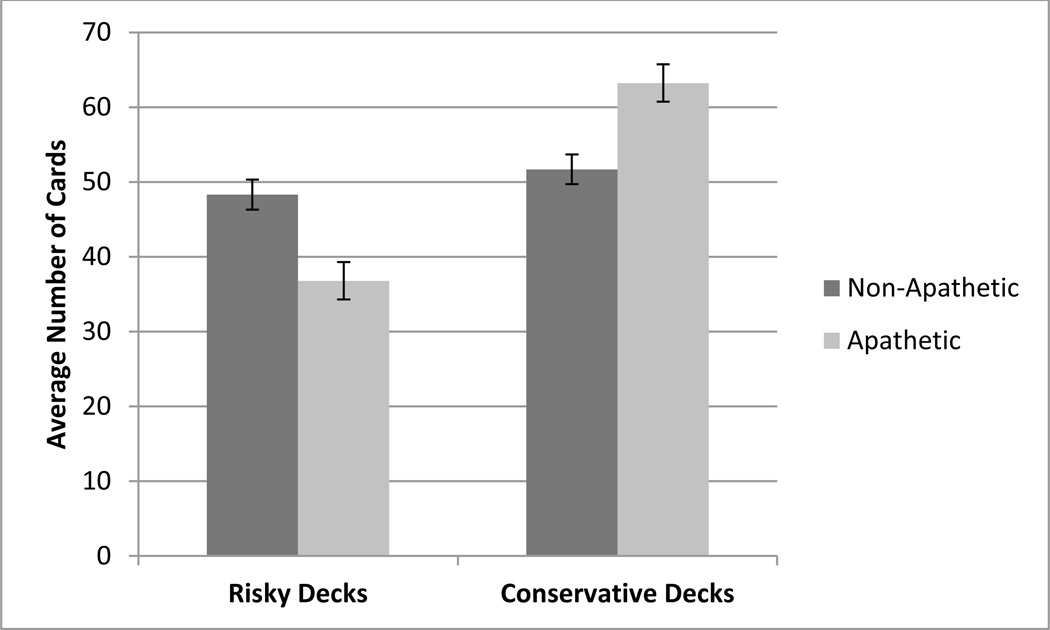
To specifically investigate the deck preferences between the apathetic and non-apathetic participants, independent samples t-tests were conducted between the apathetic and non-apathetic groups. Apathetic participants selected significantly fewer cards (t(58) = 3.0, p< 0.05) from risky decks (A and B) than the non-apathetic participants. Furthermore, apathetic participants selected significantly more cards (t(58) = −3.0, p< 0.05) from the conservative decks (C and D) compared to non-apathetic participants (Figure 3). As a measure of overall performance of the entire task, the apathetic group made significantly more advantageous decisions (t(58) = −3.0, p< 0.05) and earned significantly more money than the non-apathetic group.
Figure 3.
Average number of cards selected from risky and conservative decks by non-apathetic and apathetic patients.
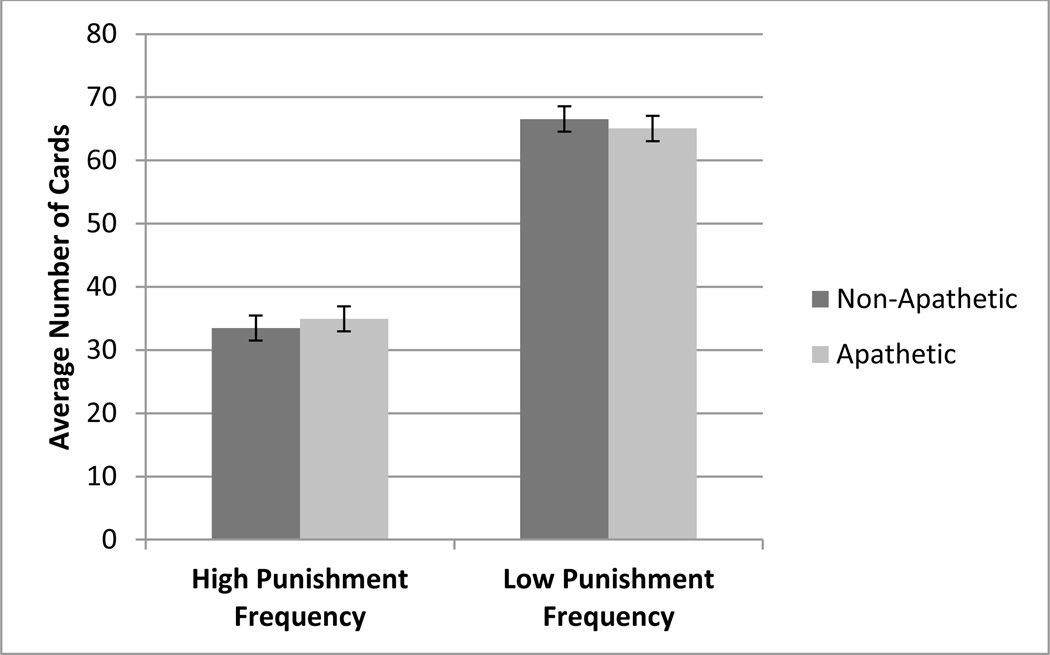
Lastly, to assess participants’ sensitivity to punishment frequency, the difference between the number of card selections from the low (B and D) and the high (A and C) loss-frequency decks was calculated. The apathetic and non-apathetic groups both preferred decks offering low-frequency penalties (B and D) over those with high-frequency penalties (t(58) = 0.46, p = 0.65). Therefore, there was no significant difference in sensitivity to punishment frequency between the apathetic and non-apathetic groups (Figure 4).
Figure 4.
Average number of cards selected from high punishment frequency and low punishment frequency punishment decks by non-apathetic and apathetic patients.
DISCUSSION
The principal finding of this study is that apathy in depressed, older adults is expressed as a conservative response style on a behavioral probe of the positive valence system. Apathetic, depressed older adults more effectively evaluated costs and benefits and shifted their selections to the conservative decks. In contrast, non-apathetic, depressed older adults did not adopt an advantageous strategy and continued to make risky decisions on the task. This is the first study, to our knowledge, to examine how apathy impacts reward-related decision making abilities in elderly, depressed adults.
The conservative response style of apathetic, depressed older adults on the IGT may reflect either a reduced motivation to seek rewarding experiences or a reduced sensitivity to rewards. Depression has been shown to be associated with deficits in reward-related decision making (Elliot et al., 1996; Eshel and Roiser, 2010; Henriques et al., 1994), high variability in action selection (Kunisato et al., 2012), and impulsive and inconsistent reward valuation (Takahashi et al., 2008). Neuroimaging studies indicate that apathy in late-life depression is associated with abnormal functional connectivity within reward systems. Apathy in late-life depression is accompanied by low resting functional connectivity (FC) of the nucleus accumbens with the amygdala, caudate, putamen, globus pallidus, and thalamus and increased FC with the dorsomedial prefrontal cortex, superior frontal cortex, and the insula (Alexopoulos et al., 2013).
The RDoC framework proposes that apathy is also a clinical expression of dysfunction of the Arousal and Regulatory System (Alexopoulos and Arean, 2013). The arousal construct is defined as the sensitivity of the individual to both internal and external stimuli and apathy has been conceptualized as a lack of responsiveness to stimuli or self-initiated action (Stuss et al., 2000). Even though arousal is conceptualized as distinct from, but interacting with, motivational value, apathy may represent as a behavioral manifestation of dysfunction in this system. Our findings suggest that networks necessary for arousal may interact with networks critical for approach motivation to produce abnormal decision making.
These findings should be considered in the context of the limitations of this study. All groups demonstrated deteriorating performance in later time blocks of the IGT. Even though this pattern is common in the IGT literature, there is no unimpaired reference group in our study. Furthermore, since the IGT is one of several well-studied decision making tasks, it is important to determine whether other complex decision making tasks reveal similar patterns. Specifically, paradigms that are used to study additional constructs in the positive valence system (e.g., the monetary incentive delay task evaluates reward prediction) should be used to examine other aspects of approach motivation. Identifying distinct, biologically driven behavioral targets will help inform the development of therapeutic interventions for late-life depression.
Despite these limitations, the presence of apathy in older adults with late-life depression may have critical implications for pharmacological treatment. For example, Yuen and colleagues (2014) demonstrated that in geriatric depression, apathy is separable from depression with regards to medication response and neural correlates. That is, apathy in elderly depressed patients persists despite improvement in depression. Further, structural abnormalities of the posterior subgenual cingulate and lower uncinate fasciculus appear to interfere with response of apathy to an SSRI.
Apathy of late-life depression is also resistant to traditional psychotherapy interventions. Apathy may present an obstacle for patients with late-life depression because they have difficulties initiating tasks and may not feel rewarded when even engaging in pleasurable activities. Therefore, treatments that entail increasing exposure to rewarding activities (Alexopoulos and Arean, 2013; Dirmaier et al., 2012; Gawrysiak et al., 2012; Hunnicutt-Ferguson et al., 2012; Moss et al., 2012; Riebe et al., 2012) as well as concrete techniques that reduce apathetic states (e.g., the use of prompts, reminders, token economies) may be more suitable for targeting apathy in late-life depression.
In conclusion, this study indicates that apathy in older, depressed adults is associated with a conservative response style on a behavioral probe of systems involved in reward-related decision making. The clinical findings detailed in this study highlight the impact of apathy on the positive valence system in late-life depression. The implications of these findings are twofold: 1. To explore the role of the interaction of the positive valence system and the arousal regulatory system in the clinical expression of late-life depression; 2. To identify novel treatment targets for apathetic depression.
REFERENCES
- Alexopoulos GS, Arean P. A model for streamlining psychotherapy in the RDoC era: the example of ‘Engage’. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Hoptman MJ, Yuen G, Kanellopoulos D, Seirup JK, Lim KO, Gunning FM. Functional connectivity in apathy in late-life depression: a preliminary study. J Affect Disord. 2013;149:398–405. doi: 10.1016/j.jad.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer AS, Timpe JC, Edmonds EC, Bechara A, Tranel D, Denburg NL. Myopia for the future or hypersensitivity to reward? Age-related changes in decision making on the Iowa Gambling Task. Emotion. 2013;13:19–24. doi: 10.1037/a0029970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Iowa Gambling Task (IGT) Professional Manual. Lutz, FL: Psychological Assessment Resources; 2007. [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;272:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond automatically to anticipated future outcomes following damage to prefrontal cortex. Cerebral Cortex. 1996;6:215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Cella M, Dymond S, Cooper A. Impaired flexible decision-making in Major Depressive Disorder. J Affect Disord. 2010;124:207–210. doi: 10.1016/j.jad.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Chaturvedi SK, Sarmukaddam SB. Prediction of outcome in depression by negative symptoms. Acta Psychiatr Scand. 1986;74:184–186. doi: 10.1111/j.1600-0447.1986.tb10603.x. [DOI] [PubMed] [Google Scholar]
- Clarke DE, Reekum R, Simard M, Streiner DL, Freedman M, Conn D. Apathy in dementia: An examination of the psychometric properties of the apathy evaluation scale. J Neuropsychiatry Clin Neurosci. 2007;19:57–64. doi: 10.1176/jnp.2007.19.1.57. [DOI] [PubMed] [Google Scholar]
- Dalgleish T, Yiend J, Bramham J, Teasdale JD, Ogilvie AD, Malhi G, Howard R. Neuropsychological processing associated with recovery from depression after stereotactic subcaudatetractotomy. Am J Psychiatry. 2004;161:1913–1916. doi: 10.1176/ajp.161.10.1913. [DOI] [PubMed] [Google Scholar]
- Denburg NL, Cole CA, Hernandez M, Yamada TH, Tranel D, Bechara A, Wallace RB. The orbitofrontal cortex, real-world decision making, and normal aging. Ann N Y Acad Sci. 2007;1121:480–498. doi: 10.1196/annals.1401.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirmaier J, Steinmann M, Krattenmacher T, Watzke B, Barghaan D, Koch U, Schulz H. Non-pharmacological treatment of depressive disorders: a review of evidence-based treatment options. Rev Recent Clin Trials. 2012;7:141–149. doi: 10.2174/157488712800100233. [DOI] [PubMed] [Google Scholar]
- Dombrovski AY, Siegle GJ, Szanto K, Clark L, Reynolds CF, Aizenstein H. The temptation of suicide: striatal gray matter, discounting of delayed rewards, and suicide attempts in late-life depression. Psychol Medicine. 2012;42:1203–1215. doi: 10.1017/S0033291711002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, Michael A, Paykel ES, Dolan RJ. Abnormal neural response to feedback on planning and guessing tasks in patients with unipolar depression. Psychological Medicine. 1998;28:559–571. doi: 10.1017/s0033291798006709. [DOI] [PubMed] [Google Scholar]
- Eshel N, Roiser JP. Reward and punishment processing in depression. Biol Psychiatry. 2010;68:118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Fein G, McGillivray S, Finn P. Older adults make less advantageous decisions than younger adults: Cognitive and psychological correlates. J Int Neuropsychol Soc. 2007;13:480–489. doi: 10.1017/S135561770707052X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, William JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forsell Y, Jorm AF, Fratiglioni L, Grut M, Winblad B. Application of DSM-III-R criteria for major depressive episode to elderly subjects with and without dementia. Am J Psychiatry. 1993;150:1199–1202. doi: 10.1176/ajp.150.8.1199. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques JB, Glowacki JM, Davidson RJ. Reward fails to alter response bias in depression. J Abnorm Psychol. 1994;103:460–466. doi: 10.1037//0021-843x.103.3.460. [DOI] [PubMed] [Google Scholar]
- Hunnicutt-Ferguson K, Hoxha D, Gollan J. Exploring sudden gains in behavioral activation therapy for Major Depressive Disorder. Behav Res Ther. 2012;50:223–230. doi: 10.1016/j.brat.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollant F, Bellivier F, Leboyer M, Astruc B, Torres S, Verdier R, Castelnau D, Malafosse A, Courtet P. Impaired decision making in suicide attempters. Am J Psychiatry. 2005;162:304–310. doi: 10.1176/appi.ajp.162.2.304. [DOI] [PubMed] [Google Scholar]
- Jollant F, Lawrence NS, Olie E, O’Daly O, Malafosse A, Courtet P, Philips ML. Decreased activation of lateral orbitofrontal cortex during risky choices under uncertainty is associated with disadvantageous decision-making and suicidal behavior. Neuroimage. 2010;51:1275–1281. doi: 10.1016/j.neuroimage.2010.03.027. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, Hays JC, Tupler LA, George LK, Blazer DG. Clinical and phenomenological comparisons of late-onset and early-onset depression. Am J Psychiatry. 1995;152:785–788. doi: 10.1176/ajp.152.5.785. [DOI] [PubMed] [Google Scholar]
- Kunisato Y, Okamoto Y, Ueda K, Onoda K, Okada G, Yoshimura S, Suzuki S, Samejima K, Yamawaki S. Effects of depression on reward-based decision making and variability of action in probabilistic learning. J Behav Ther Exp Psychiatry. 2012;43:1088–1094. doi: 10.1016/j.jbtep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Lampe IK, Heeren TJ. Is apathy in late-life depressive illness related to age-at-onset, cognitive function or vascular risk? Int Psychogeriatr. 2004;16:481–486. doi: 10.1017/s1041610204000766. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Lesser IM, Wohl M, Miller BL, Mehringer CM. Clinical and neuroradiologic features associated with chronicity in late-life depression. Am J Geriatr Psychiatry. 1999;7:309–316. [PubMed] [Google Scholar]
- Levkovitz Y, Sheer A, Harel EV, Katz LN, Most D, Zangen A, Isserles M. Differential effects of deep TMS of the prefrontal cortex on apathy and depression. Brain Stimul. 2011;4:266–274. doi: 10.1016/j.brs.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Marin RS. Differential diagnosis and classification of apathy. Am J Psychiatry. 1990;147:22–30. doi: 10.1176/ajp.147.1.22. [DOI] [PubMed] [Google Scholar]
- Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the apathy evaluation scale. Psychiatry Research. 1991;38:143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- Marin RS, Firinciogullari S, Biedrzycki RC. The sources of convergence between measures of apathy and depression. J Affect Disord. 1993;28:7–14. doi: 10.1016/0165-0327(93)90072-r. [DOI] [PubMed] [Google Scholar]
- Marin RS, Firinciogullari S, Biedrzycki RC. Group differences in the relationship between apathy and depression. J Nerv Ment Dis. 1994;182:235–239. doi: 10.1097/00005053-199404000-00008. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale professional manual. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- Mehta M, Whyte E, Lenze E, Hardy S, Roumani Y, Subashan P, Huang W, Studenski S. Depressive symptoms in late life: associations with apathy, resilience and disability vary between young–old and old–old. Int J Geriatr Psychiatry. 2008;23:238–243. doi: 10.1002/gps.1868. [DOI] [PubMed] [Google Scholar]
- Moss K, Scogin F, Di Napoli E, Presnell A. A self-help behavioral activation treatment for geriatric depressive symptoms. Aging Ment Health. 2012;16:625–635. doi: 10.1080/13607863.2011.651435. [DOI] [PubMed] [Google Scholar]
- Must A, Szabó Z, Bódi N, Szász A, Janka Z, Kéri S. Sensitivity to reward and punishment and the prefrontal cortex in major depression. J Affect Disord. 2006;90:209–215. doi: 10.1016/j.jad.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebe G, Fan MY, Unutzer J, Vannoy S. Activity scheduling as a core component of effective care management for late-life depression. Int J Geriatr Psychiatry. 2012;27:1298–1304. doi: 10.1002/gps.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Cools R, Carlisi CO, Sahakian BJ, Drevets WC. Ventral striatum response during reward and punishment reversal learning in unmedicated major depressive disorder. Am J Psychiatry. 2012;169:152–159. doi: 10.1176/appi.ajp.2011.11010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalsky C, Vidal C, Li X, Damasio H. Risky decision-making in older adults without cognitive deficits: an fMRI study of VMPFC using the Iowa Gambling Task. Soc Neurosci. 2012;7:178–190. doi: 10.1080/17470919.2011.588340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski MJ, Lynch TR, Rosenthal MZ, Cheavens JS, Chapman AL, Krishnan RR. Decision-making and risk aversion among depressive adults. J Behav Ther Exp Psychiatry. 2008;39:567–576. doi: 10.1016/j.jbtep.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein SE, Leentjens AF. The nosological position of apathy in clinical practice. J Neurol Neurosurg Psychiatry. 2008;79:1088–1092. doi: 10.1136/jnnp.2007.136895. [DOI] [PubMed] [Google Scholar]
- Stocco A, Fum D, Napoli A. Dissociable processes underlying decisions in the Iowa Gambling Task: a new integrative framework. Behav Brain Funct. 2009;5:1–12. doi: 10.1186/1744-9081-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Van Reekum R, Murphy KJ. Differentiation of states and causes of apathy. In: Borod JC, editor. The Neuropsychology of Emotion. Oxford: Oxford University Press; 2000. pp. 340–363. [Google Scholar]
- Takahashi T, Oono H, Inoue T, Boku S, Kako Y, Kitaichi Y, Kusumi I, Masui T, Nakagawa S, Suzuki K, Tanaka T, Koyama T, Radford MH. Depressive patients are more impulsive and inconsistent in intertemporal choice behavior for monetary gain and loss than healthy subjects--an analysis based on Tsallis' statistics. Neuro Endocrinol Lett. 2008;29:351–358. [PubMed] [Google Scholar]
- Unutzer J, Katon W, Williams JW, Jr, Callahan CM, Harpole L, Hunkeler EM, Hoffing M, Arean P, Hegel MT, Schoenbaum M, Oishi SM, Langston CA. Improving primary care for depression in late life: the design of a multicenter randomized trial. Med Care. 2001;39:785–799. doi: 10.1097/00005650-200108000-00005. [DOI] [PubMed] [Google Scholar]
- Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, de Boer P, Schmidt M, Claes S. Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry. 2013;73:639–645. doi: 10.1016/j.biopsych.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen GS, Gunning FM, Woods E, Klimstra SA, Hoptman MJ, Alexopoulos GS. Neuroanatomical correlates of apathy in late-life depression and antidepressant treatment response. J Affect Disord. 2014;166:179–186. doi: 10.1016/j.jad.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]