Abstract
Purpose
Stromal-derived factor-1 alpha (SDF-1α) is a chemo-attractant that has been investigated for treating various diseases, with the goal of recruiting endogenous stem cells to the site of injury. Biodegradable PLGA microspheres were investigated as a means to deliver SDF-1α in a sustained-release manner.
Methods
We encapsulated SDF-1α into biodegradable poly (lactide-co-glycolide) (PLGA) microspheres using a double-emulsion solvent extraction/evaporation technique. We varied several formulation parameters, characterized the in vitro release profile of SDF-1α and the size and morphology of microspheres, and determined the bioactivity of the released SDF-1α of stimulating migration of mesenchymal stem cells (MSCs).
Results
We found that microspheres fabricated using end-capped PLGA, BSA as an excipient, and low solvent volumes yielded a high encapsulation efficiency (>64%) and released SDF-1α over a >50-day timeframe. The released SDF-1α was bioactive and caused significant migration of MSCs throughout the duration of release from the microspheres.
Conclusions
We have identified several variables that led to successful encapsulation of SDF-1α into PLGA microspheres. We envision that SDF-lα-loaded microspheres may serve as injectable sources of sustained-release chemokine for promoting the recruitment of endogenous stem cells to the site of injury.
Keywords: chemokine, controlled release, microsphere
INTRODUCTION
Stem cell therapy has come to the forefront as a promising method to treat a wide variety of medical conditions, including neural degeneration (1,2), lung diseases (3), chronic wounds (4), and ischemic heart diseases (5). Benefits of stem cell therapy include direct increase in the density of the live cells in the injured area and improved survival and function of existing tissue due to stem cell-secreted factors.
Despite significant progress in the field, stem cell therapy has yet to deliver on its promise as a cure for these diseases. One major reason is that poor cell survival and engraftment after stem cell transplantation have been observed in many cases, such as with embryonic stem cells for Parkinson’s disease (2) and bone marrow mononuclear cells for the treatment of myocardial infarction (MI) (6). Of the stem cells implanted into the heart to treat tissue damage resulting from a heart attack, it is estimated that less than 10% are retained in the area where they are injected (7). Moreover, once in the area of transplant, the surviving cells do not proliferate in sufficient numbers to produce therapeutic effects (2,5). Consequently, clinical studies in patients with cardiovascular diseases have shown that cell-based therapies are generally safe but do not show substantial efficacy in improving heart function (8).
It has been realized that the transplanted cells do not receive adequate signals for proper retention, recruitment, and engraftment in the host tissue. One such signal is stromal-derived factor-1α (SDF-1α). SDF-1α is an 8-kDa protein that can induce mobilization of many cell types, including lymphocytes, monocytes, hematopoietic progenitor cells, and stem cells (9–11). SDF-1α is thought to play a role in neo-angiogenesis and cardiogenesis (11) and is neuroprotective in cases of Parkinson’s disease (12). A recent review suggested that a strategy to improve the homing and engraftment of stem cells—and therefore efficacy of stem cell therapy — is to pre-treat the host tissue with a local injection of SDF-1α before the transplantation of stem cells (6). Indeed, injection of SDF-1α has been shown to recruit stem cells to ischemic myocardium (13–15).
Although direct injection of SDF-1α has shown some benefit in cell therapy, bolus injections of the molecule are transient and the effects short-lived. Consequently, various methods have been developed to prolong the persistence of SDF-1α at the site of injection. Different cell types, including skeletal myoblasts (16), fibroblasts (13), and MSCs (17), have been engineered genetically to over-express SDF-1α and have shown to increase stem cell homing and improve cardiac function in animal models. Methods that enhance the in vivo lifetime of SDF-1α through inhibiting proteolysis of the molecule have also improved cardiac function after infarct (18,19). There may be drawbacks of these biological approaches, however. In particular, the methods involving genetic modification of cells could present a safety concern, since they can result in a permanent increase in SDF-1α expression. Regulating the duration of SDF-1α expression to meet the need of wound healing can also be challenging. Therefore, a biomaterial-based approach would be more desirable because SDF-1α could potentially be delivered in a sustained, transient, and controlled manner.
To our knowledge, only one study has used a biomaterial to deliver SDF-1α in a sustained manner. Zhang et al. (15) designed a PEGylated fibrin patch in which this chemokine was gradually released over a 10-day time-frame and was shown to recruit more stem cells than bolus doses. Because the natural compensatory mechanisms of the infarcted heart result in increased SDF-1α expression for 7 days post-MI (13), the 10-day timeframe would not be long enough to offer long-term improvements in cardiac function. Consequently, we sought to develop a biomaterial-based method that could deliver SDF-1α for longer than 10 days and also be deliverable by minimally invasive means (e.g., injection via a needle). We hypothesized that biodegradable microspheres could be designed for this purpose. While both PEGylated fibrin material (15) and microspheres are able to be delivered through a needle, the PEGylated fibrin must be injected before it sets up as a gel. On the contrary, there is no time constraint on microsphere delivery, which provides much convenience and flexibility to the surgeon.
The goal of our work was to fabricate a robust microsphere formulation that could offer long-term, sustained-release of SDF-1α, whose bioactivity could be preserved after encapsulation and release. We chose poly(lactide-co-glycolide) (PLGA) to fabricate microspheres because this polymer is biodegradable and already in use as a part of FDA-approved medical devices (20,21). We sought to investigate various microsphere fabrication parameters that would affect the release of SDF-1α, and characterized the morphology of the resulting formulations. Lastly, and most importantly, we set out to show that the bioactivity of SDF-1α could be preserved and could stimulate the migration of stem cells throughout the entire duration of release from the microspheres.
MATERIALS AND METHODS
Materials
Recombinant mouse stromal derived factor-1 alpha (SDF-1α) was purchased from R&D Systems (Minneapolis, MN). PLGA with ester end-groups was from Boehringer Ingelheim (Cat No. RG103; Ridgefield, CT). PLGA with free acid end-groups was from Lakeshore Biomaterials (Cat No. DLG4A; Birmingham, AL). All PLGA samples had the composition of 50:50 D,L-lactide:glycolide and Mw of 53 kDa. Bovine serum albumin (BSA) and porcine gelatin (Type A) were purchased from Sigma (St. Louis, MO). TE-lactose consisted of 10 mM Tris–HCl, 1 mM EDTA, 300 mM α-lactose, pH 8.0. Polyvinyl alcohol (PVA) was obtained from Polysciences (MW 25,000; Warrington, PA). RPMI-1640 medium, fetal bovine serum (FBS), and Dulbecco’s phosphate-buffered saline (DPBS) were purchased from Gibco Invitrogen (Carlsbad, CA); FBS was not heat-inactivated before use.
Fabrication of Microspheres
Microencapsulation of proteins was accomplished using the water-in oil-in water (W1/O/W2) solvent extraction/evaporation technique; details of formulations are shown in Table I. PLGA (100 mg) was dissolved in dichloromethane (0.7 mL or 2.0 mL) and constituted the dispersed phase (DP). The internal aqueous phase (66.5 µL or 125 µL) consisted of TE-lactose with SDF-1α (2 µg or 0.002% w/w), BSA (5 mg or 5% w/w), or a combination of the two proteins. This inner aqueous solution was added to the dissolved PLGA and sonicated for 10 s at 6–8 W (Fisher Scientific Sonic Dismembrator Model 100) to produce the first emulsion (W1/O). This first emulsion was then added to 25 mL of 5% PVA in H2O (the continuous phase, CP), and homogenized for 30 s at 4,700 rpm (Silverson L4RT) to produce the second (W1/O/W2) emulsion. In order to increase the extraction of dichloromethane from the microsphere droplets, the W1/O/W2 emulsion was subsequently poured into 50 mL of 1% PVA in H2O and stirred continuously for at least 2 h at room temperature. The solidified microspheres were then centrifuged, rinsed three times with distilled water (pH 8.0), and freeze-dried.
Table I.
Details of Microsphere Formulations
| Formulation IDa | PLGA End-group |
BSA Excipient [w/w] |
Solvent Vols. (W1/O) [µL/mL] |
Sonication Temp. [°C] |
SDF Load [w/w] |
Size, Mean±SD [µm] |
|---|---|---|---|---|---|---|
| A | Acid | 5% | 66.5/0.7 | 21 | 0.002% | 22.5±8.2 |
| B | Acid | 5% | 125/2.0 | 21 | 0.002% | 10.1±3.2 |
| C | Ester | 5% | 125/2.0 | 21 | 0.002% | 5.0±1.4 |
| D | Ester | 0% | 125/2.0 | 21 | 0.002% | 4.6±1.5 |
| E | Acid | 0% | 66.5/0.7 | 21 | 0.002% | 13.0±5.4 |
| F | Acid | 0% | 66.5/0.7 | 21 | 0% | 14.6±6.8 |
| G | Acid | 5% | 66.5/0.7 | 0 | 0.002% | 16.7±8.9 |
All formulations were fabricated with 100 mg of PLGA (Mw 53 kDa, 50:50 D,L-lactide:glycolide), TE-lactose as internal aqueous phase (W1), and dichloromethane as organic solvent (O).
Characterization of Microspheres
Analysis of Microsphere Size
Microsphere size analysis was based on a method described by Xu et al. (22). Lyophilized microspheres were suspended into DPBS and placed onto a glass slide. Using an Olympus IX70 microscope, images were obtained at either 20× (Formulations A, E, F, G) or 40× (Formulations B, C, D) magnification. Particle diameter was determined with ImageJ using the software’s built-in analysis tool. At least 100 microspheres were measured for each formulation.
Determination of Encapsulation Efficiency
Dichloromethane (100 µL) was added to 3 mg of prepared microspheres to dissolve the polymer. The mixture was placed on an end-to-end mixer overnight at 4°C, then 100 µL of DPBS was added, and the samples were vigorously vortexed. The samples were centrifuged at 5,000 × g for 10 min, and the top layer of DPBS was removed. The centrifugation extraction step was repeated with another 100 µL of DPBS, and the DPBS extraction samples were pooled. SDF-1α content of the extraction samples was determined by ELISA (R&D Systems). Encapsulation efficiency (EE) was calculated as the ratio of actual to theoretical SDF-1α content.
Characterization of the Release of SDF-1a from Microspheres
Microspheres (10 mg) were combined with 0.5 mL DPBS, pH 7.4 (Gibco, Carlsbad, CA). Samples were placed in a 37°C incubator with constant shaking (225 rpm). At each time point, samples were centrifuged (10 min at 450×g), and the entire volume of supernatant was removed and replaced with fresh DPBS. Samples were obtained once per day for the first week, then once a week thereafter. Release studies continued until microspheres were completely dissolved. The amount of SDF-1α in release samples was determined by ELISA (R&D Systems) and was expressed as the cumulative percent of the theoretical load of SDF-1α.
Stem Cell Migration Assay
Green fluorescent protein (GFP)-expressing porcine mesenchymal stem cells (MSCs) were provided by Dr. Jianyi Zhang (Department of Medicine, University of Minnesota). In brief, bone marrow of Yorkshire swine were aspirated and subjected to gradient density centrifugation, and the mononuclear layer was allowed to attach to fibronectincoated flasks; cells that were adherent at day 3 after plating were MSCs. Pluripotency of the MSCs was demonstrated by their ability to undergo osteoblast and chondroblast differentiation in vitro (23). The MSCs were geneticallymodified to express GFP according to a method previously described (24).
To maximize the migration capability of the cells, MSCs were incubated with 1 ng/mL TNFα (R&D Systems) in migration medium (MM; 0.25% BSA in RPMI-1640 medium) for 1 day before the start of the assay, according to Ponte et al. (25). Migration assays were performed in 96-well HTS Transwell plates (8.0 µm pore size, polyester membrane; Corning, Lowell, MA); the filters were coated with 0.1% (w/v) porcine gelatin in DPBS for 1 h at 37°C before use. MSCs (7×104 cells) were added to each insert, and 235 µL of migration medium-containing controls, serial dilutions of SDF-1α, or microsphere release samples were placed in the bottom chambers. Positive and negative controls consisted of 30% FBS in MM and MM alone, respectively. Microsphere release samples from the first phase (termed “early release”: generally, samples collected on day 1 or 2) were not deacidified prior to conducting the migration assay. Microsphere release samples from the second release phase (termed “late release”: generally, samples released between days ~35 and 60) were deacidified prior to subjecting to the migration assay using centrifugal filters (Amicon Ultra, 3000 MWCO; Millipore, Cork, Ireland). When possible, samples were diluted with MM to achieve the equivalent SDF-1α concentration as represented in the fresh SDF-1α dose-response. The SDF mass released from Formulation G microspheres at day 1 was fairly low and was concentrated using the Amicon Ultra centrifugal filters; the highest concentration we were able to achieve was 22 ng/mL. The Transwell plate was placed overnight in a humidified incubator at 37°C and 5% CO2, and a cotton swab was used to wipe non-migrated MSCs from the upper side of the filter. The cells that had migrated to the bottom side of the filter were imaged using fluorescence microscopy (20x magnification) and enumerated. Representative images (n≥3) were obtained and extrapolated to represent total number of migrated cells per insert.
Statistical Analysis
Data are shown as mean ± S.D. Statistical analyses were performed using the two-sample equal variance Student’st-test. A probability (p) value of <0.05 was deemed statistically significant.
RESULTS
Microsphere Fabrication and Release Studies
We sought to encapsulate SDF-1α into PLGA microspheres by using a double-emulsion solvent extraction/evaporation technique and investigated the effects of formulation variables of PLGA end-group, solvent volumes, the use of an excipient, and sonication temperature on the subsequent release of SDF-1α.
Seven microsphere formulations were prepared as described in Table I. We chose a low feed mass of SDF-1α in our formulations, partially due to expense of the protein, but also because SDF-1α is a potent chemokine. Concentrations of SDF-1α studied in in vitro chemotaxis assays that can elicit statistically significant cell migration are commonly in the range of 1–200 ng/mL, depending on cell type (25–28).
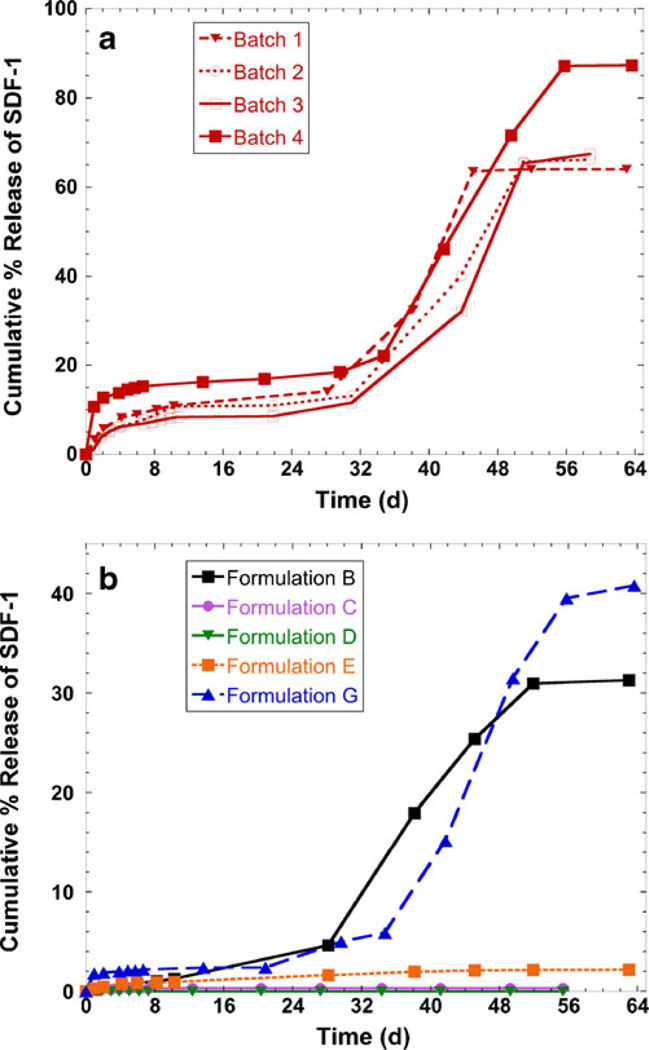
Replicate batches of each formulation were made, and batches were made on different days. The release kinetics of all replicates batches of Formulation A are shown in Fig. 1a, and there is good consistency among the batches. One representative release profile of each of Formulations B-G is shown in Fig. 1b; the other replicates of Formulations B-G had similar inter-batch release profiles. As expected, SDF-1α in release samples from empty microspheres (Formulation F) was not detected by ELISA and is not shown in Fig. 1 for clarity. The release curves exhibited a triphasic shape characteristic of PLGA (29): a high initial burst (days 1–2), followed by a plateau where little protein was released (~days 5–30), followed by second release period that was due to bulk degradation of the polymer (after ~day 35). Of all formulations made, Formulation A released the highest cumulative percent of SDF-1α (64–87%, depending on batch). Formulations B and G released a moderate amount of SDF-1α (~30% and ~40%, respectively), while Formulations C-E released very little SDF-1α (less than 2%). As exemplified by the release curves of Formulation A, the low batch-to-batch variability indicates that our microsphere fabrication process is robust and reproducible. SDF-1α was released over a time period of more than 50 days for Formulations A, B, and G.
Fig. 1.
Cumulative release of SDF-1α from PLGA microspheres in DPBS at 37°C. Replicate batches of Formulation A are shown in panel (a), while one representative release curve of all other formulations is shown in (b). The amount of SDF-1α in release samples was determined by ELISA and is expressed as the cumulative percent of the feed mass of SDF-1α loaded during microsphere fabrication. Details of microsphere formulations are shown in Table I
Encapsulation Efficiency
Encapsulation efficiency (EE) was determined by dichloromethane/ DPBS extraction, then assessment of the extracts by ELISA. When compared to the release curves shown in Fig. 1, the measured EE values were much less than the total cumulative amount of SDF-1α released. For example, although EE analysis determined that only 9% of SDF-1α was loaded into the Formulation A microspheres, the release curve shows that almost 90% of the theoretical loading was released by the time the microspheres were completely degraded.
We attempted to perform complete microsphere digestion by othermeans, both by a mild acid/surfactant method and by a strong acid method, with subsequent protein determination by the TNBSA assay (Pierce, Rockford, IL) for amino groups. We were not able to detect SDF-1α in the microsphere degradation samples using these polymer dissolution methods because the amount of protein released was below the detection limit of the assay. We note that detection of SDF-1α by ELISA after acid digestion would not be possible due to complete degradation of SDF-1α into individual amino acids.
Microsphere Sizing and Morphology
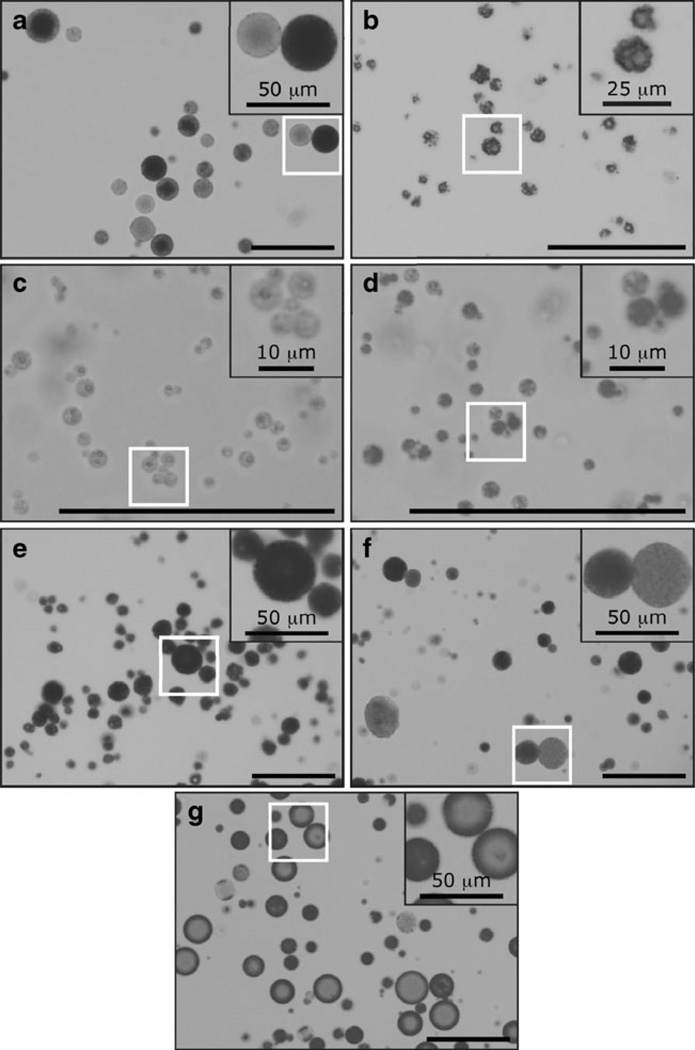
We chose to determine microsphere size and morphology using optical microscopy in order to observe the microspheres in their hydrated state. Brightfield optical images of microspheres from the different fabrication processes are shown in Fig. 2. Formulations A and F yielded microsphere populations that showed heterogeneous opacity, i.e. a mixture of dark microspheres and translucent microspheres. In contrast, Formulations C–E yielded microsphere populations of homogeneous opacity, where the entire population was either dark or translucent. Formulation G produced microspheres that appeared to have a dense “shell” layer at the outer surface and an inner translucent “core.” Formulations B, C, and D appeared noticeably rougher and more porous than the rest of the formulations.
Fig. 2.
Representative brightfield optical microscopy images of microspheres. (a–g) Formulations A to G. Images were obtained at 20× (A, E–G) or 40× (B–D) magnification; select areas are enlarged in insets. All scale bars are 100 µm, unless otherwise noted
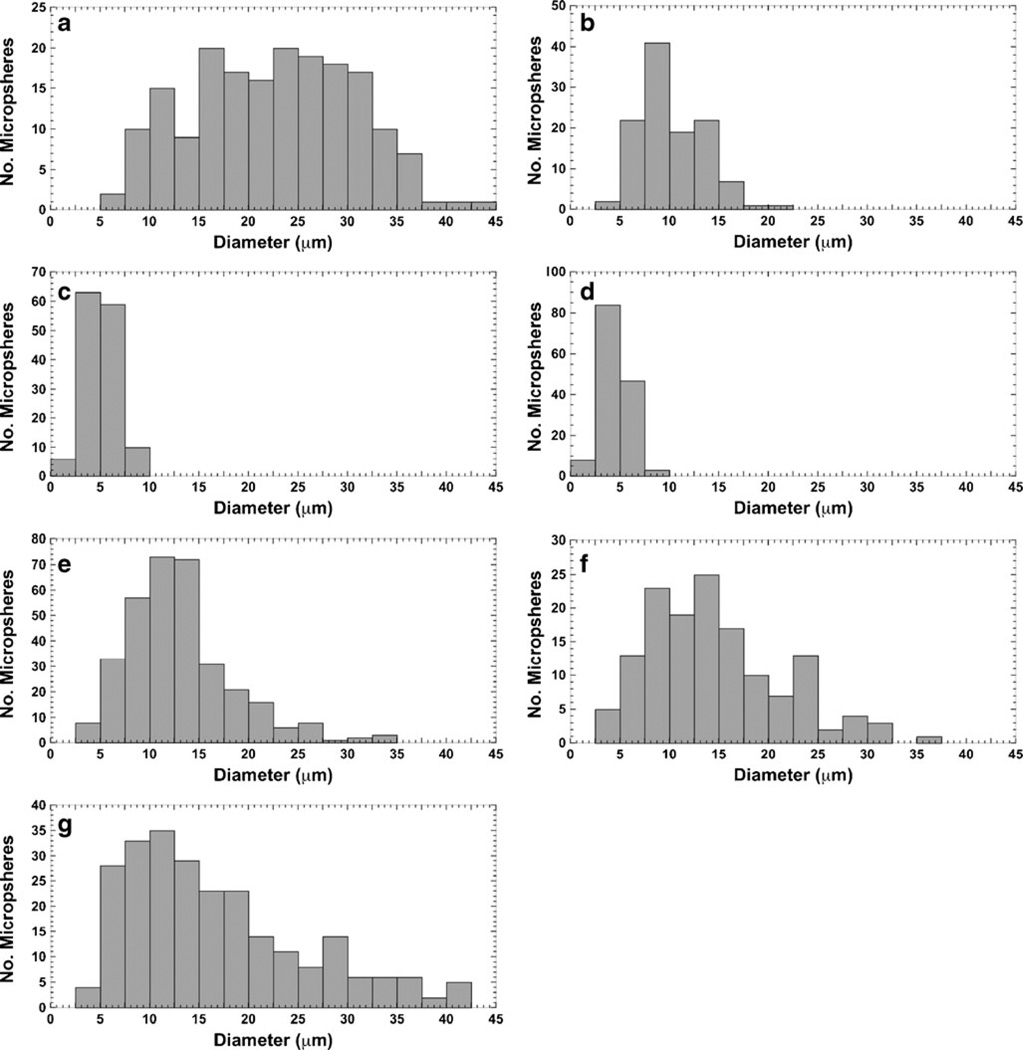
The values of average diameter for all microsphere formulations are listed in Table I. There were wide distributions in particle size within each formulation preparation, as shown visually and graphically in Figs. 2 and 3, respectively. Formulation A yielded the largest microspheres (22.5±8.2 µm); Formulations C and D were the smallest (average of ~5 µm). The rest of the microsphere formulations (Formulations B, E, F, and G) had diameters in the mid-range (~10–17 µm).
Fig. 3.
Histograms of microsphere particle size distribution. (a–g) Formulations A to G. Mean particle sizes are listed in Table I
Bioactivity of Released SDF-1α
The ideal in vitro experiment to test the bioactivity of the chemokine-encapsulating microspheres would be to expose cells to microspheres for the entire time duration of microsphere degradation. However, cells would not be allowed to be in culture undisturbed for the ~70 days that it would take for the microspheres to completely degrade. Therefore, we exposed cells to release samples from different time points, from either early in the degradation process (the initial burst phase, collected at day 1) or late in the degradation process (the second release phase, collected ≥day 35).
Before testing release samples, we first conducted an investigation of the control conditions to ensure that the stem cells were chemoattractant-responsive. Following a method reported in the literature (25), we used a Transwell migration assay to test the bioactivity of the released SDF-1α released from the microspheres. In this assay, cells were placed in the upper chamber of the plate, and test samples were placed into the bottom chamber of the plate. If bioactive, the SDF-1α should cause chemotaxis of MSCs from the upper chamber to the lower chamber. At the end of the migration period, non-migrated cells were removed from the upper chamber by wiping with a cotton swab.
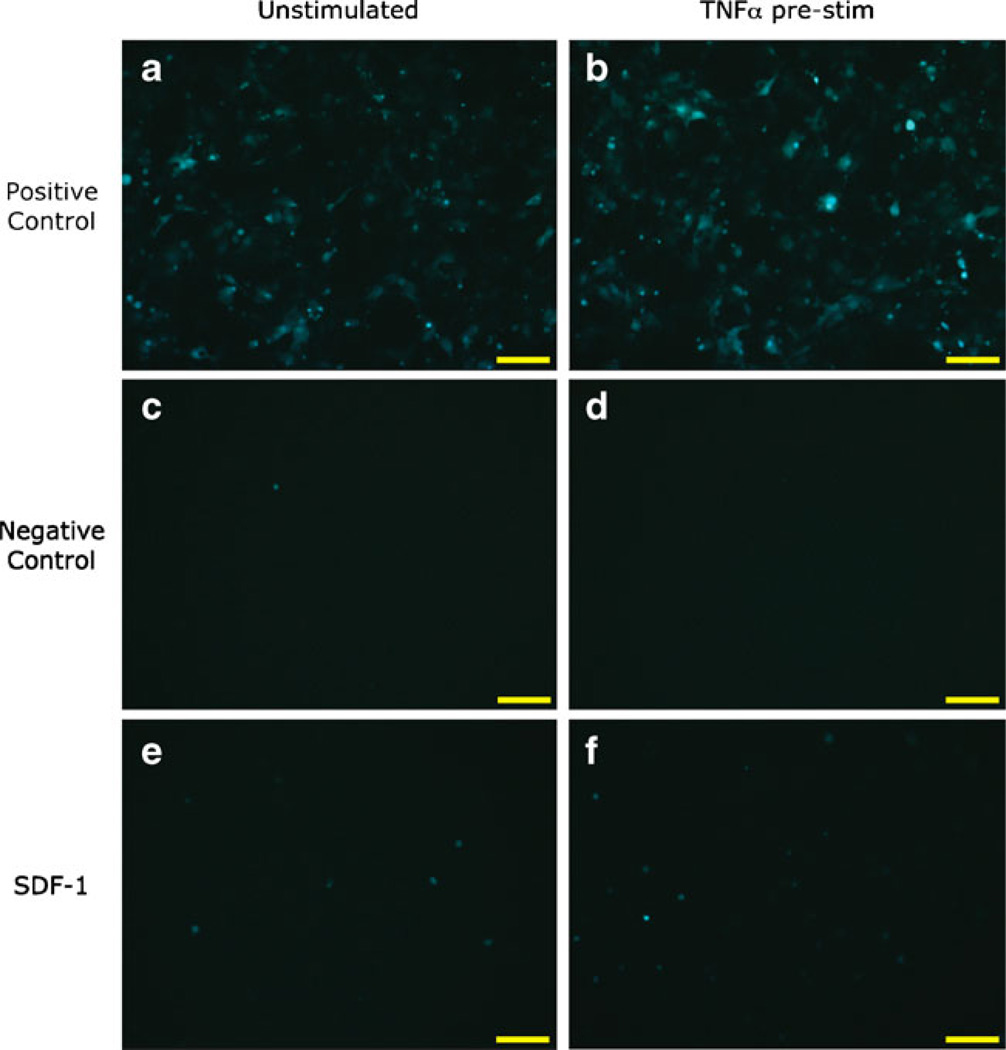
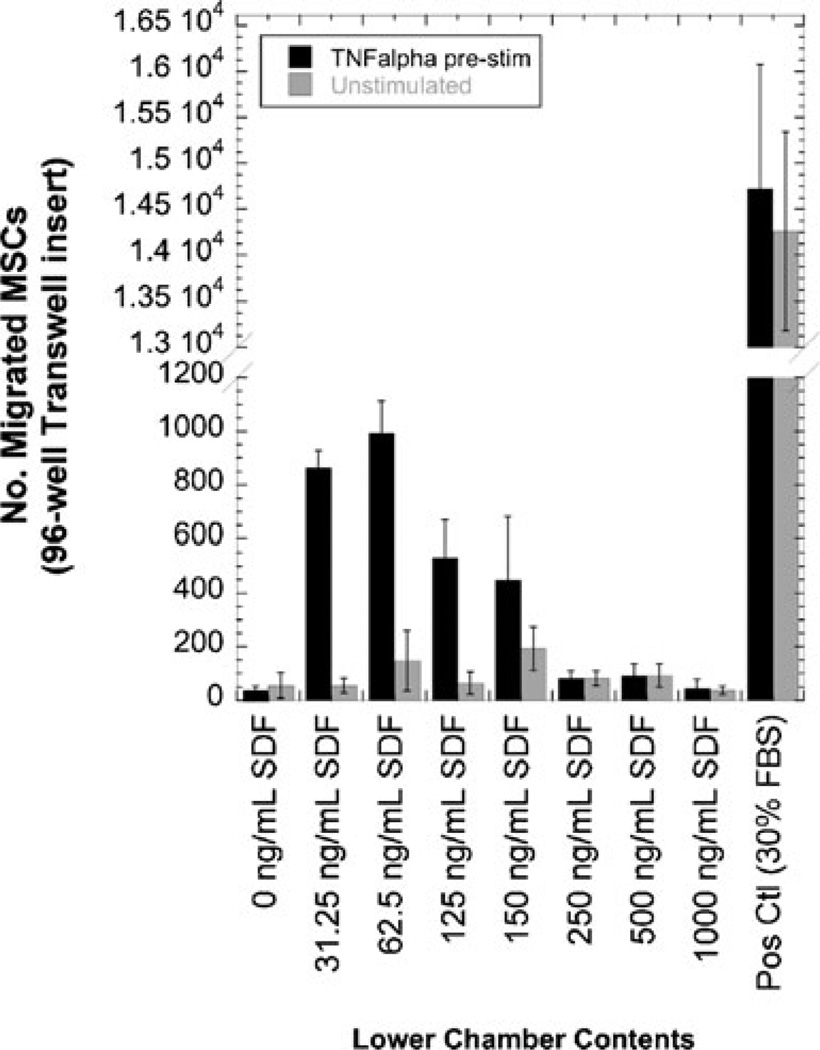
According to Ponte et al., pre-stimulation with a pro-inflammatory molecule, tumor necrosis factor-alpha (TNFα), will elicit a heightened migratory response in MSCs (25). We tested this concept by exposing the MSCs to test samples, with or without pre-treatment with 1 ng/mL TNFα. Test samples consisted of positive and negative controls (30% FBS in MM or MM only, respectively) and different concentrations of SDF-1α. We found that regardless of whether the MSCs were left unstimulated or subjected to TNFα stimulation, many cells migrated in response to the positive control, appeared healthy, and were spindle-like in shape (Figs. 4a and b, respectively); furthermore, there was no difference in cell morphology between unstimulated and TNFα-stimulated cells. Few cells migrated to the negative control, regardless of the absence or presence of TNFα (Fig. 4c and d, respectively). Because SDF-1α samples were diluted into migration medium that did not contain serum, the cells subjected to these conditions were round in shape (Fig. 4e and f); however, the cells were alive since they expressed GFP and fluoresced green. Cells migrated to the source of SDF-1α in a dose-dependent manner (Fig. 5); it can be observed that for each concentration of SDF-1α, MSCs migrated in greater numbers when pre-stimulated with TNFα than when left unstimulated.
Fig. 4.
Representative fluorescence microscopy images of the migration of GFP-expressing porcine MSCs to control samples. Cells remained in growth media (a, c, e; “unstimulated”) or were incubated with 1 ng/mL TNFα (b, d, f; “pre-stim”) overnight prior to testing in the migration assay. Positive and negative controls were 30% FBS in migration medium (MM) and MM only, respectively; SDF-1α concentration was 62.5 ng/mL. Images were captured after wiping the non-migrated cells from the top surface of the insert with a cotton swab. Scale bar=100 µm. Magnification, 20×
Fig. 5.
The effect of TNFα pre-incubation on porcine MSC migration in response to SDF-1α. MSCs were incubated overnight either in the presence or absence of 1 ng/mL TNFα before exposure to a various doses of fresh SDF-1α. Data shown as mean±S.D. (n≥3 fields)
Since we observed increased migration of the MSCs after TNFα stimulation, we chose to pre-treat the cells with this molecule for all subsequent studies. Due to inherent differences in biological activity with every experiment, we included the testing of different concentrations of SDF-1α alongside the negative and positive controls for every assay. We chose to focus on testing the release samples from Formulations A, G, and F in order to determine if sonication on ice would show differences in the protection of SDF-1α bioactivity.
MSCs that were exposed to SDF-1α release samples from Formulation A microspheres are shown in Fig. 6; representative fluorescence images are shown before removing the non-migrated cells from the top of the Transwell insert. “Early release” samples did not appear to cause cell death, as evidenced by bright, green cells shown in Fig. 6a. However, when MSCs were exposed to samples from the second release phase (“late release”), no green cells were observed, suggesting that the samples were cytotoxic (Fig. 6b). When PLGA degrades, it breaks down into its monomeric or oligomeric components of lactic and glycolic acids, so we attempted to deacidify the microsphere release samples prior to exposure to the cells. As can be observed in Fig. 6c, the MSCs exposed to deacidified “late release” SDF-1α microsphere samples were bright green, showing that the samples were properly neutralized.
Fig. 6.
Representative fluorescence microscopy images of the migration of GFP-expressing porcine MSCs to release samples from Formulation A microspheres. Cells are shown at day 1 after adding cells to the top of the Transwell inserts. Images were captured before wiping the non-migrated cells from the top surface of the insert with a cotton swab. Microsphere release samples were collected from either the “early release” phase (a) or the “late release” phase (b, c). Cells were subjected to “late release” samples that were either not de-acidified (b) or de-acidified (c). Scale bar=100 µm. Magnification, 20×
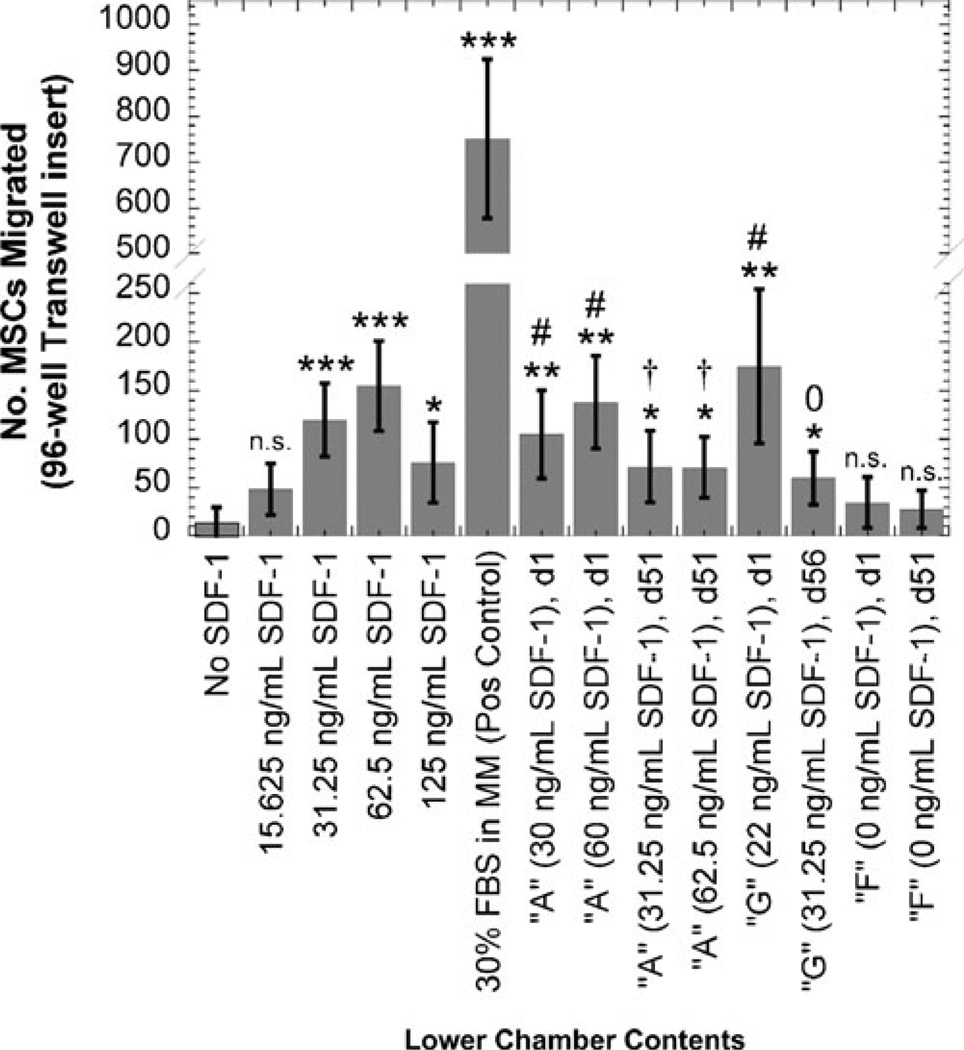
The quantitative results of a representative migration experiment are shown in Fig. 7. The positive control induced the migration of a great number of MSCs, which showed that the cells were responsive to chemoattractant agents. Because of the acidic nature of the “late release” microsphere degradation samples, all samples from that timeframe were deacidified prior to placing into the migration assay.
Fig. 7.
Migration of porcine MSCs in response to microsphere release samples. The cells were pre-treated overnight with 1 ng/mL TNFα, and then were exposed to microsphere release samples and various doses of SDF-1α. Late time-point release samples (collected at day 51/56) were de-acidified prior to testing, as described in the Materials and Methods section. Data are shown as mean±S.D. (n≥3 fields); statistical significance was are shown by Student’s t-test: *p<0.05, **p<0.005, ***p<0.001, n.s. = not significant compared to negative control (no SDF-1α); #p< 0.05, compared to day 1 empty microspheres (“F”); †p<0.05, °not significant, compared to day 51 empty microspheres (“F”)
When compared to the negative control (no SDF-1α), all SDF-containing release samples yielded statistically significant MSC migration (Fig. 7). Because the PLGA degradation products might affect cell migration, we also compared the migration results to release samples from empty microspheres and showed that when matched for collection time point (i.e., day 1 release samples compared to each other, and day 51/56 release samples compared to each other), we show that all Formulation A (sonicated at room temperature) release samples caused significant cell migration compared to empty microsphere samples (Formulation F). For the ice-sonicated microspheres (Formulation G), the release sample collected at day 1 yielded a significant increase in MSC migration versus the release sample from empty microspheres, but this significance was not observed when comparing release samples from late time points. As a whole, it appeared that the release samples collected early in the microsphere degradation process (at day 1) were more potent chemoattractants than late time point (day 51/56) release samples.
DISCUSSION
Biodegradable microspheres are able to deliver bioactive molecules in a sustained-release fashion. SDF-1α is a chemokine that has been shown to recruit endogenous stem cells and has not yet been encapsulated into microspheres prior to this work. We investigated the use of PLGA to fabricate microspheres and to encapsulate SDF-1α using the double-emulsion solvent extraction/evaporation technique. This method is generally safe for proteins, although the maintenance of protein stability is dependent on the interplay of the processing parameters and the chosen protein (30).
Our microspheres (Formulations A, B, G) released SDF-1α for more than 50 days, which was much longer than the release duration previously reported for this molecule. Only one other biomaterial-mediated delivery of SDF-1α has been reported in the literature, which showed that a PEGylated fibrin gel was able to deliver this molecule over a 10-day period (15). It is quite certain that ten days of SDF-1 release will not be long enough to elicit a lasting and beneficial degree of cell migration in a disease such as myocardial infarction, because the infarcted heart itself over-expresses SDF-1α for 1 week after injury (13). The ideal duration of SDF-1α signaling is not known at this point but is likely dependent on the healing process of the injured heart, which will take at least weeks and months in animals and humans. Therefore, our microsphere formulations capable of releasing bioactive SDF-1α for over 50 days may provide clinical benefit to heart attack patients by prolonging the duration of SDF-1α at the site of injection.
Several microsphere fabrication variables were investigated and were observed to have profound effects on the release of SDF-1α. Formulations C and D were made with PLGA that had ester end-groups (“capped”); all other formulations (A–B, E–G) were made with PLGA that had carboxylic acid end-groups (“uncapped”). The capped PLGA is more hydrophobic than the uncapped PLGA and is therefore more resistant to degradation by hydrolysis. It has also been proposed that the carboxylic acid group of uncapped PLGA may interact with proteins; therefore, the protein is better entrapped within the microspheres (31). Lastly, because the upcapped PLGA is more hydrophilic, it is less soluble in organic solvent than capped PLGA and will precipitate more quickly during solvent extraction. As a result, the microspheres made from uncapped PLGA might have solidified more quickly than the capped PLGA, trapping the protein within a less porous polymer shell (32). Because release studies continued until microspheres were completely degraded, the lack of measurable release from Formulations C and D (less than 1% of feed; Fig. 1b) suggest that SDF-1α was not efficiently encapsulated during microsphere fabrication.
The effect of solvent volume on protein release rate can be seen by comparing Formulations A and B. We dissolved 100 mg of PLGA into either 0.7 or 2.0 mL of dichloromethane, which produced an oil phase (O) of two different polymer concentrations. When highly concentrated, the polymer precipitates quickly upon contact with the water phase (W1), yielding a dense polymer barrier that prevents protein from diffusing out of the microspheres before they are fully formed. This increases the amount of protein retained inside of the microspheres (that is, it should improve encapsulation efficiency). Highly concentrated polymer solutions are also more viscous, which also prevents protein diffusion out of the microspheres before they are fully solidified. Lastly, the dispersed phase/ continuous phase (DP/CP) ratio of Formulation A (1/36) is lower than that for Formulation B (1/13), which induces fast solidification of the microspheres. All of these aspects have been shown to improve encapsulation efficiency (32), which therefore can translate to more protein available for release.
Although both Formulations A and E were fabricated with the same feed mass of SDF-1α (2 µg per 100 mg PLGA), Formulation A included BSA as an excipient, and it can be seen in Fig. 1 that there was dramatically more SDF-1α released from this set of microspheres than that released from Formulation E, which were fabricated without BSA. BSA has frequently been used as a carrier protein to provide stabilization to the payload protein (29). Co-encapsulation with BSA may also prevent exposure of SDF-1α to organic solvent which helps preserve the integrity of the protein (33). A third possible explanation is due to charge-charge interaction between the two proteins: BSA has a net negative charge at physiological pH (34) which may interact with the cluster of positive charges in the central β-sheet region of the SDF-1α molecule (35) and may thereby provide protection for SDF-1α. Lastly, the incorporation of BSA increases the concentration of hydrophilic components within the microsphere; when more water can gain access into the microsphere, more protein can diffuse out.
We investigated the sonication temperature of the first W1/O emulsion on the release of SDF-1α (Formulations A versus G). As can be seen in Fig. 1, reducing the sonication temperature reduced the total cumulative release of SDF-1α. We were not able to find any extensive investigations of this variable in the literature; in fact, a recent review plainly states that not much is known about how this parameter would affect the microspheres or the encapsulated proteins (29). One report was found in which a so-called “cryopreparation” method has been described for encapsulating plasmid DNA (36). In the cryopreparation method, the first emulsion (W1/O) was frozen in liquid nitrogen before homogenization with the outer aqueous phase (W2). The freezing step was shown to drastically improve encapsulation efficiency, most likely by preventing the diffusion of the DNA contents from the inner aqueous phase. In our preparation of Formulation G microspheres, the W1/O emulsion was performed on ice; this should also increase viscosity and decrease protein diffusion. However, we did not observe any increase in SDF-1α encapsulation and release.
We found it difficult to obtain an accurate determination of the encapsulation efficiency of SDF-1α into our formulations of microspheres. We used the dichloromethane/ DPBS extraction method: the polymer was dissolved in organic solvent, and when mixed with DPBS, the water-soluble protein should partition into the aqueous DPBS phase. Detection of the extracted SDF-1α by ELISA is very sensitive and can detect analyte in concentrations as low as ~150 pg/mL. But despite the high sensitivity of the ELISA, we measured very low amounts of encapsulated SDF-1α in the microsphere digest samples. There are several reasons that may explain this discrepancy. First, there may have been incomplete dissolution of the PLGA in the mixed solvent, so the SDF-1α protein was trapped within the polymer and could not be assayed. Uncapped PLGA is more hydrophilic than capped PLGA due to the presence of carboxylic acid groups and may not have been completely dissolved in the solvent. Second, the protein may have been trapped at the dichloromethane/DPBS interface and was not able to be retrieved. The three-dimensional structure of SDF-1α reveals that the C-terminus is an amphiphilic α-helix (35); therefore, it seems plausible that this molecule could be preferentially found at the hydrophobic/hydrophilic dichloromethane/DPBS interface. Lastly, the SDF-1α protein may have been denatured by the solvent used in the extraction procedure and, therefore, was not recognized by the anti-SDF-1α antibody of the ELISA. For these reasons, we believe that the results of the cumulative release study (Fig. 1) reflect the true encapsulation efficiency more accurately than the assay of the samples from the solvent extraction method.
We observed a wide size distribution of microspheres, which has also been observed by others that have used the double emulsion technique to fabricate PLGA microspheres (37,38). Varying the solvent volume used in the manufacturing process appeared to have caused the largest difference in porosity and size (Figs. 2 and 3). Formulation B, C, and D microspheres were noticeably more porous and smaller than the other microsphere formulations. As already mentioned above, microspheres made with a high DP/CP ratio (such as Formulation B) will solidify slower than those at low DP/CP ratio. Therefore, in addition to lowering encapsulation efficiency, slower solidification typically results in more porous, irregular microsphere structures and smaller particles (32), just as observed with Formulations B, C, and D.
Furthermore, if the variable of solvent volumes was kept constant, we observed that microspheres made with the capped PLGA (Formulations C and D) were much smaller (roughly half the diameter) than microspheres made with uncapped PLGA (Formulation B). The ester end-groups of PLGA are more hydrophobic, and the polymer is more soluble in dichloromethane than the uncapped polymer, so solvent extraction is slower. As a result, nascent microspheres made of PLGA with ester end-groups have more time to solidify and will produce smaller particles (32).
Pair-wise comparisons of the fabrication parameters do not easily explain which ones were responsible for the differences in opacity of the microspheres. For example, a comparison of Formulation A (which contain both SDF-1α and BSA) and Formulation E (which contain only SDF-1α) may cause one to draw the conclusion that the encapsulation of both proteins would yield a heterogeneously opaque population of microspheres, whereas the encapsulation of only one protein produces a homogeneous batch of microspheres. However, a further comparison to the heterogeneous population of Formulation F microspheres (which contained neither protein) makes this conclusion invalid. It has been reported that microspheres fabricated with a higher W1/O ratio are uniformly dark, while those fabricated with a low W1/O ratio are uniformly translucent (39), but we did not observe this correlation from our samples. Furthermore, it does not appear that the opacity of the microspheres correlates with the SDF-1α release kinetics, which agrees with findings in the literature (39).
When cells were pre-treated with TNFα, an increase in cell migration was measured. Ponte et al. (25) state that exposing the cells to chemokine alone may not be sufficient to cause cell migration and that inflammatory cues (such as TNFα) are important in recruiting cells to the area of injury. In fact, these results by us and others support the findings of in vivo studies where stem cell migration was enhanced in models of both heart (40) and brain injury (41). It is also possible that low amounts of PLGA degradation products could be beneficial; the acidic conditions may stimulate inflammation, which, in turn, may recruit stem cells to the injured site.
The parabolic dose-response shape of the dose-response curve is characteristic of cell responses to chemoattractant (42). Migration of the cells in response to chemokine is dependent on two factors: the absolute concentration of the molecule in the vicinity of the cell and the gradient of the chemokine across the length of the cell. At low concentrations, a sufficient concentration gradient does not exist along a cell’s length, and the cell will therefore not move in response to the molecule. At high concentrations, however, a cell’s chemokine receptors will be saturated so it will be unable to detect differences in chemokine levels, even if a sufficient gradient exists (43).
Our concern that thermal and shear stresses might damage the SDF-1α protein is what had prompted the fabrication of Formulation G microspheres where sonication was performed on ice. In the cryopreparation method described earlier (36), the first emulsion (W1/O) was frozen to a solid so shear forces were completely eliminated, and it was found that the structure of the contents (DNA) remained intact after encapsulation into the microspheres. In our case, however, it is not clear if the lower sonication temperature provided protection to the SDF-1α protein: release samples from Formulation G microspheres collected early in the degradation study yielded statistically significant MSC migration compared to empty (Formulation F) microspheres, but this observation did not hold with the release samples collected late in the degradation process. In summary, sonication of the primary emulsion on ice improved the bioactivity of SDF-1α released at early time-points but without much benefit at late time-points.
Most importantly, our results show that the SDF-1α released from microsphere Formulations A and G remained bioactive, regardless of when the molecule was released from the microspheres. In myocardial infarction, for example, it is important to recruit stem cells beyond the first week of injury. Therefore, it is crucial that the SDF-1α released later in the degradation timeframe remains bioactive and is able to induce the migration of stem cells to the site of injury.
CONCLUSIONS
We have shown that SDF-1α can be successfully encapsulated into PLGA microspheres. By varying manufacturing parameters, a variety of different formulations have been prepared that release the protein at different rates, including a formulation that can release SDF-1α over a period of at least 50 days. We have identified parameters that are important factors in the encapsulation and release of SDF-1α, namely, the use of PLGA with acid end-groups, low solvent volumes, BSA as an excipient, and sonication at room temperature. Most importantly, we have found that the SDF-1α released from the microspheres can stimulate the migration of stem cells, which shows that the encapsulation method preserves the bioactivity of the protein. Furthermore, the released SDF-1α has intact bioactivity whether it is released at an early or late time point in the microsphere degradation process. With further tuning of the release profile and in vivo evaluation, this approach has the potential of making clinically relevant impact on stem cell-mediated cardiac repair.
ACKNOWLEDGMENTS
This work is supported in part by the University of Minnesota’s Institute for Engineering in Medicine (IEM) and an NIH Biotechnology Training Grant (Grant Number T32 GM008347). Special thanks to Dr. Jianyi Zhang (Department ofMedicine,University ofMinnesota) for kindly providing the GFP-expressing porcine MSCs. We are also grateful to Dr. Nathan Lockwood for his careful review and critique of the manuscript.
REFERENCES
- 1.Kim S, De Vellis J. Stem cell-based cell therapy in neurological diseases: a review. J Neurosci Res. 2009;87:2183–2200. doi: 10.1002/jnr.22054. [DOI] [PubMed] [Google Scholar]
- 2.Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 3.Siniscalco D, Sullo N, Maione S, Rossi F, D’Agostino B. Stem cell therapy: the great promise in lung disease. Ther Adv Respir Dis. 2008;2:173–177. doi: 10.1177/1753465808092340. [DOI] [PubMed] [Google Scholar]
- 4.Branski LK, Gauglitz GG, Herndon DN, Jeschke MG. A review of gene and stem cell therapy in cutaneous wound healing. Burns. 2009;35:171–180. doi: 10.1016/j.burns.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segers VF, Lee R. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 6.Chavakis E, Urbich C, Dimmeler S. Homing and engraftment of progenitor cells: a prerequisite for cell therapy. J Mol Cell Cardiol. 2008;45:514–522. doi: 10.1016/j.yjmcc.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Teng CJ, Luo J, Chiu RC, Shum-Tim D. Massive mechanical loss of microspheres with direct intramyocardial injection in the beating heart: implications for cellular cardiomyoplasty. J Thorac Cardiovasc Surg. 2006;132:628–632. doi: 10.1016/j.jtcvs.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 8.Laflamme MA, Zbinden S, Epstein SE, Murry CE. Cell-based therapy for myocardial ischemia and infarction: pathophysiological mechanisms. Annu Rev Pathol. 2007;2:307–339. doi: 10.1146/annurev.pathol.2.010506.092038. [DOI] [PubMed] [Google Scholar]
- 9.Ghadge SK, Muhlstedt S, Ozcelik C, Bader M. SDF-1alpha as a therapeutic stem cell homing factor in myocardial infarction. Pharmacol Ther. 2011;129:97–108. doi: 10.1016/j.pharmthera.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Imitola J, Raddassi K, Park K, Mueller FJ, Nieto M, Teng Y, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandervelde S, Van Luyn MJ, Tio RA, Harmsen MC. Signaling factors in stem cell-mediated repair of infarcted myocardium. J Mol Cell Cardiol. 2005;39:363–376. doi: 10.1016/j.yjmcc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Wang F, Yasuhara T, Shingo T, Kameda M, Tajiri N, Yuan WJ, et al. Intravenous administration of mesenchymal stem cells exerts therapeutic effects on parkinsonian model of rats: focusing on neuroprotective effects of stromal cell-derived factor-1alpha. BMC Neurosci. 2010;11:52. doi: 10.1186/1471-2202-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 14.Wei YJ, Tang Y, Li J, Cui CJ, Zhang H, Zhang XL, et al. Cloning and expression pattern of dog SDF-1 and the implications of altered expression of SDF-1 in ischemic myocardium. Cytokine. 2007;40:52–59. doi: 10.1016/j.cyto.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Zhang G, Nakamura Y, Wang X, Hu Q, Suggs LJ, Zhang J. Controlled release of stromal cell-derived factor-1 alpha in situ increases c-kit + cell homing to the infarcted heart. Tissue Eng. 2007;13:2063–2071. doi: 10.1089/ten.2006.0013. [DOI] [PubMed] [Google Scholar]
- 16.Elmadbouh I, Haider HK, Jiang S, Idris NM, Lu G, Ashraf M. Ex vivo delivered stromal cell-derived factor-1alpha promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2007;42:792–803. doi: 10.1016/j.yjmcc.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 18.Segers VF, Tokunou T, Higgins LJ, Macgillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–1692. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- 19.Zaruba MM, Theiss HD, Vallaster M, Mehl U, Brunner S, David R, et al. Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell. 2009;4:313–323. doi: 10.1016/j.stem.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Cleland JL. Protein delivery from biodegradable microspheres. Pharm Biotechnol. 1997;10:1–43. doi: 10.1007/0-306-46803-4_1. [DOI] [PubMed] [Google Scholar]
- 21.Shive M, Anderson J. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 22.Xu X, Yu H, Gao S, Ma HQ, Leong KW, Wang S. Polyphosphoester microspheres for sustained release of biologically active nerve growth factor. Biomaterials. 2002;23:3765–3772. doi: 10.1016/s0142-9612(02)00116-3. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura Y, Wang X, Xu C, Asakura A, Yoshiyama M, From A, et al. Xenotransplantation of long-term-cultured swine bone marrow-derived mesenchymal stem cells. Stem Cells. 2007;25:612–620. doi: 10.1634/stemcells.2006-0168. [DOI] [PubMed] [Google Scholar]
- 24.Wilber A, Linehan JL, Tian X, Woll PS, Morris JK, Belur LR, et al. Efficient and stable transgene expression in human embryonic stem cells using transposon-mediated gene transfer. Stem Cells. 2007;25:2919–2927. doi: 10.1634/stemcells.2007-0026. [DOI] [PubMed] [Google Scholar]
- 25.Ponte A, Marais E, Gallay N, Langonne A, Delorme B, Herault O, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 26.Hamada T, Mohle R, Hesselgesser J, Hoxie J, Nachman RL, Moore MA, et al. Transendothelial migration of megakaryocytes in response to stromal cell-derived factor 1 (SDF-1) enhances platelet formation. J Exp Med. 1998;188:539–548. doi: 10.1084/jem.188.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 28.Kitaori T, Ito H, Schwarz E, Tsutsumi R, Yoshitomi H, Oishi S, et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009;60:813–823. doi: 10.1002/art.24330. [DOI] [PubMed] [Google Scholar]
- 29.Mundargi R, Babu V, Rangaswamy V, Patel P, Aminabhavi T. Nano/micro technologies for delivering macromolecular therapeutics using poly(D, L-lactide-co-glycolide) and its derivatives. J Control Release. 2008;125:193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Bilati U, Allemann E, Doelker E. Strategic approaches for overcoming peptide and protein instability within biodegradable nano- and microparticles. Eur J Pharm Biopharm. 2005;59:375–388. doi: 10.1016/j.ejpb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Jaklenec A, Hinckfuss A, Bilgen B, Ciombor DM, Aaron R, Mathiowitz E. Sequential release of bioactive IGF-I and TGF-beta 1 from PLGA microsphere-based scaffolds. Biomaterials. 2008;29:1518–1525. doi: 10.1016/j.biomaterials.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Yeo Y, Park K. Control of encapsulation efficiency and initial burst in polymeric microparticle systems. Arch Pharm Res. 2004;27:1–12. doi: 10.1007/BF02980037. [DOI] [PubMed] [Google Scholar]
- 33.Zhu XH, Wang CH, Tong YW. In vitro characterization of hepatocyte growth factor release from PHBV/PLGA microsphere scaffold. J Biomed Mater Res A. 2009;89:411–423. doi: 10.1002/jbm.a.31978. [DOI] [PubMed] [Google Scholar]
- 34.Böhme U, Scheler U. Effective charge of bovine serum albumin determined by electrophoresis NMR. Chemical Physics Letters. 2007;435:342–345. [Google Scholar]
- 35.Luo J, Luo Z, Zhou N, Hall J, Huang Z. Attachment of C-terminus of SDF-1 enhances the biological activity of its N-terminal peptide. Biochem Biophys Res Commun. 1999;264:42–47. doi: 10.1006/bbrc.1999.1476. [DOI] [PubMed] [Google Scholar]
- 36.Ando S, Putnam D, Pack DW, Langer R. PLGA microspheres containing plasmid DNA: preservation of supercoiled DNA via cryopreparation and carbohydrate stabilization. J Pharm Sci. 1999;88:126–130. doi: 10.1021/js9801687. [DOI] [PubMed] [Google Scholar]
- 37.Crotts G, Park TG. Preparation of porous and nonporous biodegradable polymeric hollow microspheres. Journal of Controlled Release. 1995;35:91–105. [Google Scholar]
- 38.Chung TW, Tsai YL, Hsieh JH, Tsai WJ. Different ratios of lactide and glycolide in PLGA affect the surface property and protein delivery characteristics of the PLGA microspheres with hydrophobic additives. J Microencapsul. 2006;23:15–27. doi: 10.1080/02652040500286110. [DOI] [PubMed] [Google Scholar]
- 39.Chen XQ, Yang YY, Wang L, Chung TS. Effects of inner water volume on the peculiar surface morphology of microspheres fabricated by double emulsion technique. J Microencapsul. 2001;18:637–649. doi: 10.1080/02652040110055234. [DOI] [PubMed] [Google Scholar]
- 40.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 41.Ji J, He B, Dheen S, Tay S. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells. 2004;22:415–427. doi: 10.1634/stemcells.22-3-415. [DOI] [PubMed] [Google Scholar]
- 42.Starr AE, Overall CM. Methods in Enzymology: Chemokines, Part B. San Diego: Academic Press; 2009. p. 298. [Google Scholar]
- 43.Zhao X, Jain S, Larman HB, Gonzalez S, Irvine DJ. Directed cell migration via chemoattractants released from degradable microspheres. Biomaterials. 2005;26:5048–5063. doi: 10.1016/j.biomaterials.2004.12.003. [DOI] [PubMed] [Google Scholar]