Abstract
BACKGROUND & AIMS
Excessive consumption of ethanol is one of the most common causes of acute and chronic pancreatitis. Alterations to the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) also cause pancreatitis. However, little is known about the role of CFTR in the pathogenesis of alcohol-induced pancreatitis.
METHODS
We measured CFTR activity based on chloride concentrations in sweat from patients with cystic fibrosis, patients admitted to the emergency department because of excessive alcohol consumption, and healthy volunteers. We measured CFTR levels and localization in pancreatic tissues and in patients with acute or chronic pancreatitis induced by alcohol. We studied the effects of ethanol, fatty acids, and fatty acid ethyl esters on secretion of pancreatic fluid and HCO3− , levels and function of CFTR, and exchange of Cl− for HCO3− in pancreatic cell lines as well as in tissues from guinea pigs and CFTR knockout mice after administration of alcohol.
RESULTS
Chloride concentrations increased in sweat samples from patients who acutely abused alcohol but not in samples from healthy volunteers, indicating that alcohol affects CFTR function. Pancreatic tissues from patients with acute or chronic pancreatitis had lower levels of CFTR than tissues from healthy volunteers. Alcohol and fatty acids inhibited secretion of fluid and HCO3− , as well as CFTR activity, in pancreatic ductal epithelial cells. These effects were mediated by sustained increases in concentrations of intracellular calcium and adenosine 3’,5’-cyclic monophosphate, depletion of adenosine triphosphate, and depolarization of mitochondrial membranes. In pancreatic cell lines and pancreatic tissues of mice and guinea pigs, administration of ethanol reduced expression of CFTR messenger RNA, reduced the stability of CFTR at the cell surface, and disrupted folding of CFTR at the endoplasmic reticulum. CFTR knockout mice given ethanol or fatty acids developed more severe pancreatitis than mice not given ethanol or fatty acids.
CONCLUSIONS
Based on studies of human, mouse, and guinea pig pancreata, alcohol disrupts expression and localization of the CFTR. This appears to contribute to development of pancreatitis. Strategies to increase CFTR levels or function might be used to treat alcohol-associated pancreatitis.
Keywords: Exocrine Pancreas, Cl− Channel, Alcoholism, Duct
Acute pancreatitis (AP) is the most common cause of hospitalization for nonmalignant gastrointestinal diseases in the United States, with an estimated annual cost of at least $2.5 billion.1 The mortality of the disease is unacceptably high, and no specific pharmaceutical therapy is currently available. Therefore, there is a pressing economic and clinical need to develop new therapies for patients with AP.
Immoderate alcohol consumption is one of the most common causes of AP and chronic pancreatitis (CP),1–3 and therefore the effects of ethanol and ethanol metabolites on the pancreas have been widely investigated.4,5 However, these studies have focused mainly on pancreatic acinar and stellate cells. On the other hand, Sarles et al showed that the initial lesion in the course of pancreatic damage during alcohol-induced chronic calcifying pancreatitis is the formation of mucoprotein plugs in the small pancreatic ducts.6 These changes are very similar to the alterations of the exocrine pancreas in cystic fibrosis (CF), the most common autosomal recessive disease caused by loss-of-function mutations in the CFTR gene. Moreover, Ooi et al showed that patients with CF who have impaired cystic fibrosis transmembrane conductance regulator (CFTR) function are at increased risk for developing pancreatitis.7 These data suggest that changes in the function or expression of the CFTR Cl− channels in pancreatic ductal epithelial cells (PDECs), which alone express CFTR in the exocrine pancreas,8 may play a central role in the pathogenesis of alcohol-induced pancreatitis.
Patients and Methods
Detailed protocols and descriptions of the volunteers, patients, and methods used in this study are provided in Supplementary Methods.
Human Studies
Sweat samples from human subjects were collected by pilocarpine iontophoresis, and sweat chloride concentration was determined by conductance measurement. The messenger RNA (mRNA) and protein expression levels of CFTR and Na+/K+–adenosine triphosphatase (ATPase) of the pancreatic ductal epithelia in human pancreatic tissue were determined.
Cell and Animal Studies
A large variety of human cell lines (Capan-1, MDCK, and HEK) and animal models (mice and guinea pigs) were used to assess the role of CFTR in alcohol-induced AP.
Statistical Analysis
All data are expressed as means ± SEM. Significant differences between groups were determined by analysis of variance. Statistical analysis of the immunohistochemical data was performed using the Mann–Whitney U test. P < .05 was considered statistically significant.
Ethical Approvals
The protocols concerning human subjects or laboratory animals were approved by the relevant agencies.
Results
Alcohol Consumption Decreases CFTR Activity and Expression in Human Subjects
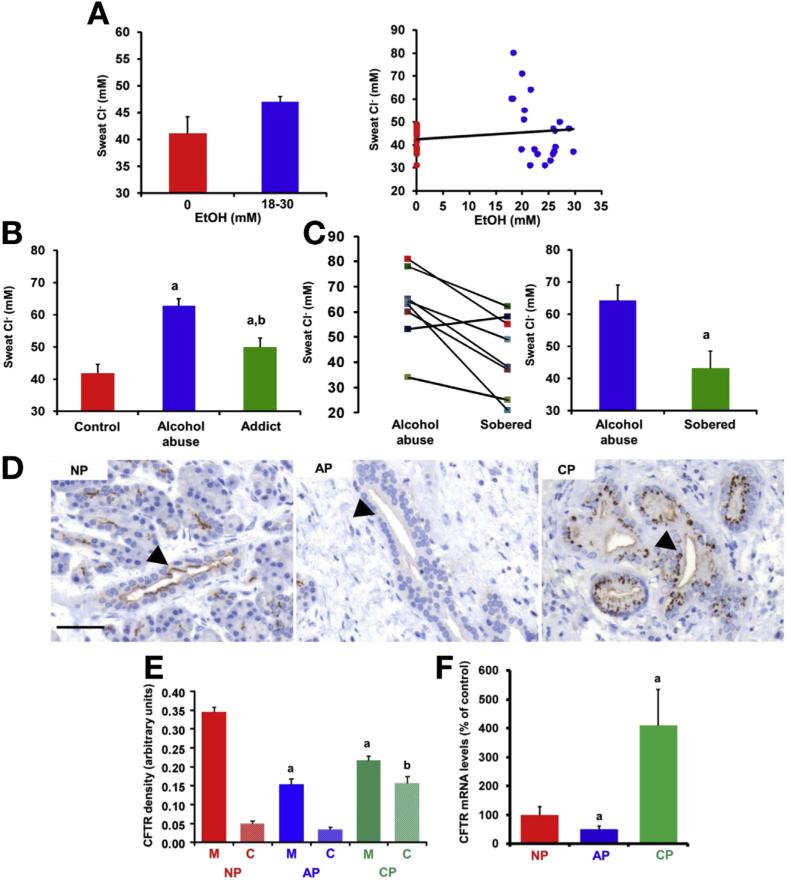
In patients with CF, sweat Cl− concentration (Cl− sw) is elevated due to diminished CFTR absorptive activity.9 In our study, CI− sw at 0 mmol/L blood alcohol concentration (BAC) was 41.08 ± 3.1 mmol/L (Figure 1A). After consuming 1.6 g/kg ethanol within 30 minutes, the average BAC was elevated to 23.3 ± 1.1 mmol/L, with no elevation of CI− sw (47 ± 1 mmol/L). However, to test the effects of higher BAC on CI− sw, we enrolled patients admitted to the emergency department because of excessive alcohol consumption. The average BAC in this group was 74.2 ± 2.6 mmol/L but the CI− sw was 62.7 ± 2.3 mmol/L, suggesting strong inhibition of CFTR (Figure 1B; for patient data, see Supplementary Table 2). Importantly, when the BAC returned to 0, the CI− sw normalized (Figure 1C). To assess the effects of long-term alcohol intake, we also enrolled alcohol-dependent patients from the department of addictology. These patients had a history of alcohol consumption for at least 1 year and did not consume alcohol for at least 1 week before measurement of CI− sw. The mean CI− sw in this group was 49.92 ± 2.8 mmol/L, suggesting that ethanol has long-term effects on CFTR as well (Figure 1B).
Figure 1.
Alcohol consumption decreases activity and expression of the CFTR CI− channel. (A) No significant change in CI− sw was observed in healthy volunteers (n=21) before and after ethanol consumption. (B) CI− sw was significantly higher in patients after excessive alcohol consumption (EAC) compared with age- and sex-matched controls, whereas it was elevated in alcoholic subjects with 0 mmol/L BAC (Addict) compared with the control group but significantly lower than in the alcohol abuse group. Control, n = 26; EAC, n = 49; Addict, n = 15. aP < 0.001 vs control, bP < .001 vs EAC. (C) The CI− sw of patients returned to a normal level when measured several days after EAC at 0 mmol/L BAC. n 8. aP < .001 vs EAC values. (D and E) CFTR expression in human pancreas. Arrows point to the luminal membrane of the intralobular pancreatic ducts. NP, normal pancreas. Scale bar = 50 μm. CFTR staining density at the luminal membrane was decreased in both AP and CP, whereas cytoplasmic density was markedly increased in CP. C, cytoplasm; M, membrane. n = 5/group. aP < .05 vs NP-M, bP < .05 vs NP-C. (F) Quantitative polymerase chain reaction analysis of CFTR mRNA expression in human pancreas. CFTR mRNA levels were decreased in AP and highly increased in CP (normalized to 18 ribosomal RNA; given as percentage of NP mRNA). n = 5/group. aP < .05 vs NP.
Next, we determined the effects of ethanol on CFTR expression and localization in the pancreas using tissue samples from control pancreatic tissue and from patients with acute or chronic alcohol-induced pancreatitis (Figure 1D–F; for a detailed description of tissue samples, see Supplementary Methods). In alcoholic AP, CFTR expression decreased at both mRNA and protein levels. Similarly, in CP, membrane expression of CFTR in PDECs was significantly lower; however, both the mRNA level and cytoplasmic density of CFTR were strongly elevated, suggesting defective endoplasmic reticulum (ER) protein folding and/or translocation of CFTR from the membrane to the cytosol. As a control experiment, we showed that neither mRNA nor protein expression levels of another plasma membrane transporter, namely Na+/K+-ATPase, were changed in AP and CP (Supplementary Figure 1).
Ethanol and Fatty Acid Impair Pancreatic Fluid and Bicarbonate Secretion and Inhibit CFTR Cl− Channel Activity In Vivo and In Vitro
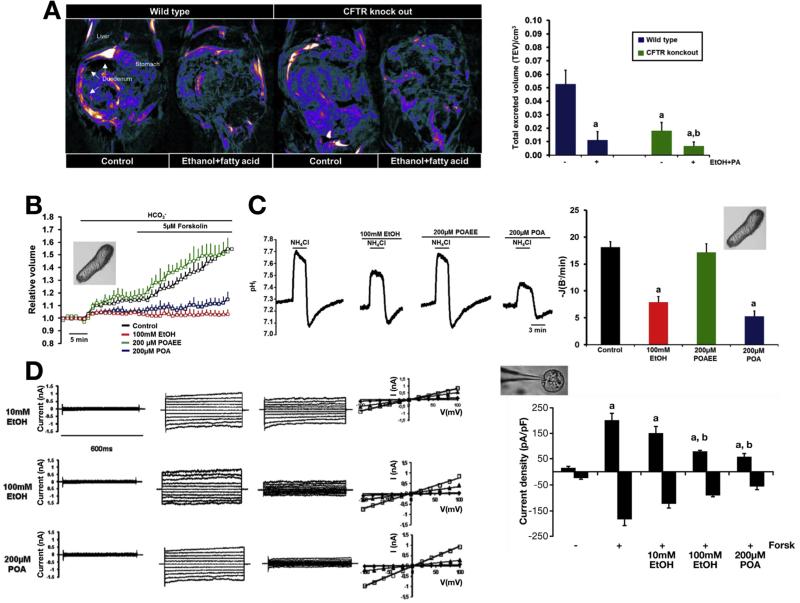
In the next step, we applied different in vivo and in vitro techniques to assess the effects of ethanol and ethanol metabolites on pancreatic fluid and HCO3− secretion in animal models and in a human pancreatic cell line. First, we used magnetic resonance imaging cholangiopancreatography to measure total excreted volume in wild-type (WT) and CFTR knockout (KO) mice. On retro-orbital injection of 10 U/kg body weight secretin, the increase in total excreted volume in WT animals was significantly higher than in CFTR KO animals (Figure 2A). Pancreatic secretion was reassessed 24 hours after intraperitoneal injection of 1.75 g/kg ethanol and 750 mg/kg palmitic acid (PA). The total excreted volume was markedly decreased in WT mice and almost completely abolished in CFTR KO mice. In addition, we showed that intraperitoneal injection of ethanol and PA significantly decreased both basal and secretin-stimulated pancreatic fluid secretion in anesthetized mice in vivo (Supplementary Figure 2).
Figure 2.
Ethanol and fatty acids inhibit pancreatic fluid and HCO3− secretion and CFTR CI− current. (A) Reconstructed images of duodenal filling after secretin stimulation. Compared with WT, duodenal filling was significantly reduced in CFTR KO mice and was abolished after intraperitoneal injection of ethanol plus palmitic acid. n = 6/group. aP < .05 vs WT control, bP < .05 vs KO control. (B) Changes in the relative luminal volume of isolated guinea pigpancreatic ducts show that administration of ethanol and POA but not POAEE for 30 minutes diminished in vitro ductal fluid secretion. n = 3–4 experiments per group. (C) Measurement of luminal CI−/HCO3− exchange activity shows that basolateral administration of 100 mmol/L ethanol and 200 μmol/L POA significantly inhibited activity of the luminal SLC26 CI− /HCO3− exchanger and CFTR and decreased recovery from the alkali load in isolated guinea pig pancreatic ducts. n = 3–5 experiments per group. aP < .05 vs control. (D) Representative fast whole cell CFTR CI− current recordings in guinea pig pancreatic ductal cells. (i) Unstimulated currents, (ii) currents after forskolin stimulation (10 μmol/L; 10 minutes), (iii) stimulated currents after 10 minutes of treatment, and (iv) current-voltage relationships (diamonds, unstimulated; squares, forskolin stimulated; triangles, forskolin-stimulated currents after treatment). The summary of the current densities (pA/pF; measured at Erev: ±60 mV) show that 100 mmol/L ethanol or 200 μmol/L POA blocked the forskolin-stimulated CFTR CI− currents (61.5% ± 5.15% and 73.1% ± 4.46%, respectively). n = 5–6/group. aP < .05 vs basal current, bP < .05 vs forskolin-stimulated current.
To detect pancreatic ductal fluid secretion in vitro, we used isolated guinea pig pancreatic ducts, which is the best in vitro model to mimic the human situation. Administration of 100 mmol/L ethanol or the nonoxidative ethanol metabolite palmitoleic acid (POA; 200 µmol/L) for 30 minutes markedly reduced pancreatic fluid secretion, whereas 200 μmol/L palmitoleic acid ethyl ester (POAEE) had no effect (Figure 2B). Pancreatic ductal HCO3− secretion was measured using NH4Cl pulse, where the initial rate of intracellular pH recovery from an alkali load (base flux; J[B− ]; for details, see Supplementary Methods) reflects the activity of the apical SLC26 CI− /HCO3− exchangers and CFTR (Figure 2C).10 Similarly to ductal fluid secretion, 100 mmol/L ethanol and 200 μmol/L POA significantly diminished ductal HCO3− secretion after 30 minutes of exposure.
We confirmed our results on a human polarized pancreatic cell line (Capan-1) as well. Applying 2 independent methods (luminal CI− removal and NH4Cl pulse) we showed that 15-minute administration of a low concentration of ethanol (10 mmol/L) stimulated whereas a high concentration of ethanol (100 mmol/L) and POA (100, 200 μmol/L) significantly impaired the apical CI− /HCO3− exchange activity (Supplementary Figure 3A). Moreover, 100 mmol/L ethanol and 100 to 200 μmol/L POA significantly inhibited the recovery from acid load during NH4Cl pulse experiments under basal conditions and forskolin stimulation (Supplementary Figure 3B–D), suggesting that activity of the basolateral transporters may be also impaired.
Finally, we directly detected the effects of ethanol and ethanol metabolites on the CFTR CI− current in primary epithelial (Figure 2D) and human Capan-1 cells (Supplementary Figure 3E). Exposure of guinea pig PDECs to 10 mmol/L ethanol had no significant effect on forskolin-stimulated CFTR currents (in Capan-1, significant slight stimulation was observed), whereas 100 mmol/L ethanol or 200 μmol/L POA caused a significant decrease. In both cases, inhibition was voltage independent and irreversible. Administration of 200 μmol/L POAEE had no effect on forskolin-stimulated CFTR currents.
Low Concentration of Ethanol Stimulates Both the Apical SLC26 Cl−/HCO3− Exchanger and CFTR via Inositol Triphosphate Receptor–Mediated Ca2+ Signaling
Apical CI− removal in Capan-1 cells revealed that separate administration of 10 μmol/L CFTR(inh)-172 (CFTR CI− channel inhibitor) or 500 µmol/L dihydro-4,4’-diisothiocyanostilbene-2,2’-disulfonic acid (H2DIDS) (SLC26A6 inhibitor) for 15 minutes could not prevent the stimulatory effect of 10 mmol/L ethanol; however, their combination totally abolished it (Supplementary Figure 4A and C). In case of NH4Cl pulse (where the bicarbonate concentration of the cells is higher), not only coadministration of the 2 inhibitors but also separate administrations alone could prevent the stimulatory effect of ethanol (Supplementary Figure 4B and D). To identify the intracellular mechanisms of stimulation, we showed that 10 mmol/L ethanol induced repetitive Ca2+ spikes in Capan-1 cells (Supplementary Figure 5A). Administration of the inositol triphosphate receptor (IP3R) antagonist caffeine (20 mmol/L) or the phospholipase C inhibitor U73122 (10 μmol/L) completely abolished the Ca2+ response, suggesting that Ca2+ was released from the ER via activation of IP3R. Moreover, 20 mmol/L caffeine totally inhibited the stimulatory effect of ethanol, suggesting that elevation of intracellular Ca2+ concentration ([Ca2+]i) mediates the stimulatory effect of ethanol on HCO3− secretion (Supplementary Figure 5B and C).
High Concentration of Ethanol Inhibits Both the Apical SLC26 Cl−/HCO3−Exchanger and CFTR
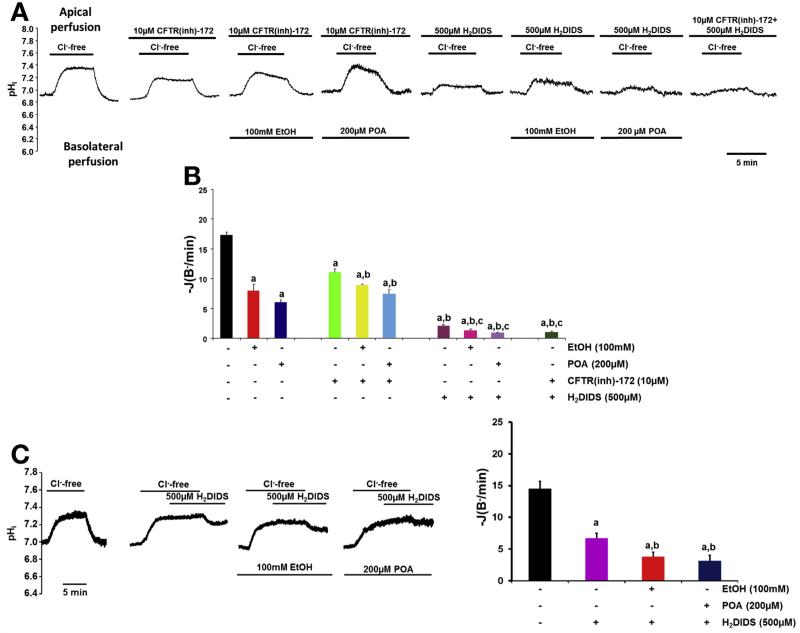
Administration of CFTR(inh)-172 and H2DIDS showed that pretreatment of cells for 15 minutes with either CFTR(inh)-172 or H2DIDS further decreased HCO3− secretion when coadministrated with ethanol or POA, suggesting that both transport mechanisms are involved in inhibitory mechanisms (Figure 3A and B and Supplementary Figure 6A and B). When the SLC26 inhibitor H2DIDS was administered only when the luminal CI− was already removed (Figure 3C), the same effects were observed.
Figure 3.
Ethanol and POA inhibit both the luminal CI− /HCO3− exchanger and CFTR in Capan-1 cells. (A and B) The initial rate of intracellular pH (pHi) recovery after luminal CI− readdition shows the effects of basolateral administration of 100 mmol/L ethanol or 200 μmol/L POA in the presence or absence of 500 μmol/L H2DIDS and/or 10 μmol/L CFTR(inh)-172 (luminal administration). (Labels above the traces, composition of the luminal solution; labels below the traces, composition of the basolateral solution.) A total of 100 mmol/L ethanol and 200 mmol/L POA induced further inhibition after administration of CFTR(inh)-172 and/or H2DIDS, suggesting that high concentrations of ethanol and POA inhibit the activity of CBE and CFTR on the apical membrane of PDECs. aP < .05 vs control, bP < .05 vs 10 mmol/L CFTR(inh)-172, cP < .05 vs 500 μmol/L H2DIDS. (C) Representative pHi traces and summary data of the initial rate of pHi recovery after CI− readdition using a different protocol confirmed our results. aP < .05 vs control . n = 3–5 experiments per group for all groups.
High Concentrations of Ethanol and POA Induce Sustained Elevation of [Ca2+]i, Decreased Mitochondrial Function and Adenosine 3’,5’-Cyclic Monophosphate Level
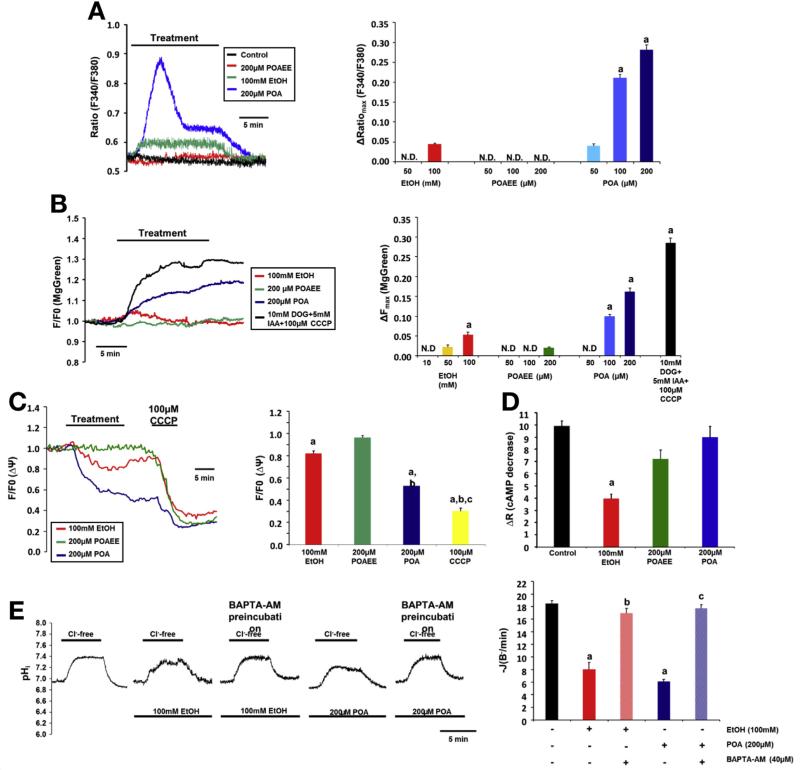
Ethanol (100 mmol/L) induced a moderate but sustained increase in [Ca2+]i in Capan-1 cells, POAEE had no effect, and POA evoked a dose-dependent, sustained increase in [Ca2+]i (Figure 4A and B). The first phase of the Ca2+ signal was inhibited by the ryanodine receptor inhibitor Ruthenium Red, the IP3R inhibitor caffeine, and the phospholipase C inhibitor U73122 (Supplementary Figure 7A), whereas removal of extracellular Ca2+ had no effect on the ΔRatiomax (Supplementary Figure 7B). The plateau phase of the signal was totally dependent on the presence of extracellular Ca2+ and blocked by gadolinium, suggesting the involvement of the store-operated Ca2+ channels. To verify that 200 μmol/L POA completely depletes the ER Ca2+ stores, we administered POA in Ca2+-free media followed by administration of 2 μmol/L thapsigargin (Tg; sarcoplasmic/ER calcium ATPase [SERCA] inhibitor). Under these conditions, Tg was not able to induce further Ca2+ release (Supplementary Figure 8A). For control, we administered Tg before POA, where POA had no effect on [Ca2+]i. These data indicate that POA completely depletes the ER Ca2+ stores and induces extracellular Ca2+ influx.
Figure 4.
High concentrations of ethanol and POA induce sustained elevation of [Ca2+]i, impaired mitochondrial function, and decreased cAMP levels in Capan-1 PDECs. (A) Representative traces and summary data of the ΔRatiomax show the effect of ethanol, POAEE, and POA on [Ca2+]i. Ethanol (100 mmol/L) induced a small, sustained elevation of [Ca2+]i, whereas 100 to 200 μmol/L POA induced a significantly higher increase in [Ca2+]i. aP < .05 vs 100 mmol/L ethanol. (B) Ethanol and POA induced significant and irreversible depletion of (ATP)i. Deoxyglucose/iodoacetic acid (DOG/IAA; glycolysis inhibition) and CCCP (inhibition of mitochondrial ATP production) served as control. (C) Representative traces and summary data of changes in the mitochondrial membrane potential [(ΔΨ)m]. Ethanol (100 mmol/L) induced a moderate decrease in (δΨ)m, whereas 200 μmol/L POA had a more prominent effect. CCCP induced a further decrease in (δΨ)m after treatment with POA. (D) Summary data for cAMP measurements. A total of 100 mmol/L ethanol and 200 μmol/L POAEE significantly decreased forskolin-stimulated cAMP production. (E) Ca2+ chelation abolished the inhibitory effect of ethanol and POA on intracellular pH recovery after luminal CI− readdition. For all conditions, n = 3–5/group. aP < .05 vs control; bP < .05 vs 100 mmol/L ethanol; cP < .05 vs 200 μmol/L POA. N.D., not detected.
To further characterize the effects of POA on extracellular Ca2+ influx, we performed the Tg-Ca2+ readdition protocol11 (Supplementary Figure 8B and C). Treatment with Tg depleted ER Ca2+ and readdition of extracellular Ca2+ evoked store-operated Ca2+ influx, where the steady state is maintained by plasma membrane Ca2+-ATPase activity. POA (200 μmol/L) in Ca2+-free extracellular solution mimicked the effect of Tg (depleted the ER Ca2+ store and induced store-operated Ca2+ entry). However, after the store-operated Ca2+ entry–mediated increase in Ca2+, the decrease in [Ca2+]i was markedly slower than in the case of Tg-treated cells and the plateau was reached on an elevated [Ca2+]i. These results suggest that POA not only depletes ER Ca2+ but also decreases plasma membrane Ca2+-ATPase activity.
Measurement of intracellular adenosine triphosphate [(ATP)i] using Magnesium Green AM revealed that 100 mmol/L ethanol and 100 to 200 μmol/L POA markedly and irreversibly decreased (ATP)i (Figure 4B). (The increase in fluorescent intensity inversely correlates with the cellular adenosine triphosphate [ATP] levels.) The combination of deoxyglucose/iodoacetate/carbonyl cyanide 3-chlorophenylhydrazone was used as control to inhibit cellular glycolysis and mitochondrial ATP production. We also tested the effect of (ATP)i depletion on HCO3− secretion (Supplementary Figure 9C and D) and showed that administration of deoxyglucose/iodoacetate/carbonyl cyanide 3-chlorophenylhydrazone significantly decreased HCO3− secretion, similarly to the effects of 200 mmol/L POA. To further characterize the effects of ethanol and ethanol metabolites on mitochondrial function, we showed that 100 mmol/L ethanol and 100 to 200 μmol/L POA markedly and irreversibly decreased mitochondrial membrane potential [(ΔΨ)m] (Figure 4C). FRET-based adenosine 3’,5’-cyclic monophosphate (cAMP) measurements using Epac1-camps sensor revealed that 100 mmol/L ethanol and 200 μmol/L POAEE significantly decreased forskolin-stimulated cAMP production in HEK cells; however, interestingly, 100 μmol/L POA had no inhibitory effect (Figure 4D). Finally, we showed that chelation of intracellular Ca2+ (with 40 μmol/L 1,2-bis(o-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid [BAPTA-AM]) completely abolished the inhibitory effect of 100 mmol/L ethanol and 200 μmol/L POA on pancreatic ductal HCO3− secretion, suggesting that it was mediated by the sustained elevation of [Ca2+]i (Figure 4E and Supplementary Figure 9A and B).
Ethanol and Nonoxidative Ethanol Metabolites Cause Translocation and Expression Defect of CFTR
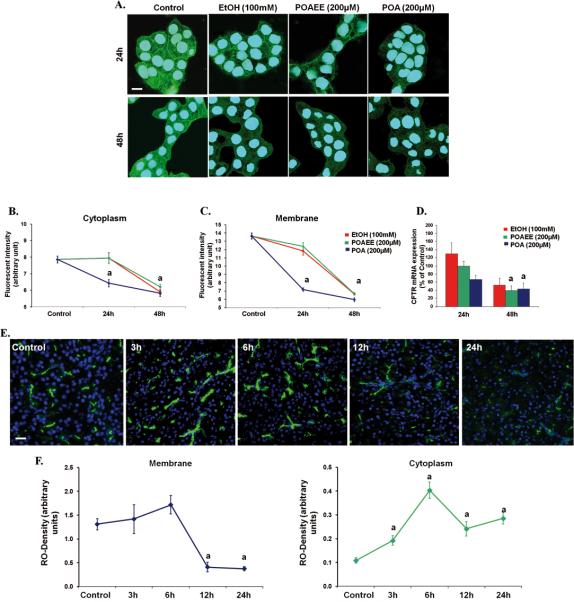
Our experiments showed that high concentrations of ethanol, POAEE, and POA time- and dose-dependently decreased both mRNA and protein expression of CFTR in human pancreatic epithelial cells in vitro (Figure 5A–C). To reproduce these observations in vivo, an appropriate animal model was used. Guinea pigs were injected intraperitoneally with 0.8 g/kg ethanol and 300 mg/kg PA. Importantly, apical CFTR expression in the pancreatic ducts was not changed at 3 and 6 hours; however, it was significantly decreased 12 and 24 hours after treatment (Figure 5E and F). Moreover, cytoplasmic CFTR levels were elevated after 3 hours, suggesting a membrane trafficking defect of CFTR. As a control experiment, expression of Na+/K+-ATPase was also measured and no changes were observed (Supplementary Figure 10).
Figure 5.
Ethanol, POAEE, and POA decrease CFTR expression in Capan-1 cells and in guinea pig pancreatic ducts. (A–C) High concentrations of ethanol, POAEE, and POA induced a significant decrease in CFTR membrane and cytoplasmic expression. Scale bar = 10 μm. (D) Ethanol, POAEE, and POA decreased CFTR mRNA expression after 48 hours of Data were normalized to HPRT mRNA levels and expressed as percentage of untreated control mRNA levels. (E and F) CFTR expression in guinea pig pancreas. Expression of CFTR on the luminal membrane of guinea pig pancreatic was significantly decreased 12 hours after a single intraperitoneal injection of 0.8 g/kg ethanol and 300 mg/kg PA. Scale bar = 100 μm. n = 5/group. aP < vs control.
Ethanol and Its Metabolites Decrease CFTR Expression and Plasma Membrane Density via Accelerated Channel Plasma Membrane Turnover and Damaged Protein Folding
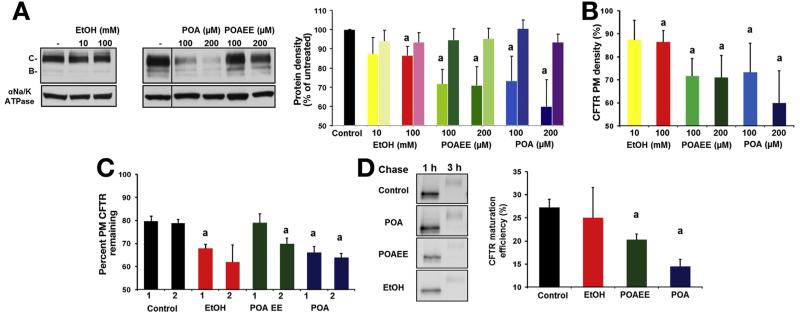
To dissect the mechanism of CFTR expression defect on ethanol, POA, or POAEE exposure, we exposed monolayers of MDCK-II cells expressing WT human CFTR containing a 3HA epitope in the fourth extracellular loop to ethanol, POAEE, or POA for 48 hours. Quantitative immunoblot analysis by anti-HA antibody revealed that in contrast to the modest effect of 100 mmol/L ethanol, 100 to 200 μmol/L POAEE and POA significantly decreased mature, complex glycosylated CFTR expression as compared with control (Figure 6A). Importantly, protein expression of Na+/K+-ATPase did not change during treatment. Loss of cellular CFTR expression coincided with reduction of apical CFTR plasma membrane density, monitored by the cell surface enzyme-linked immunosorbent assay taking advantage of the extracellular 3HA epitope (Figure 6μ). CFTR apical plasma membrane density was reduced by ~40% in the presence of 200 μmol/L POA, while only ~15% and ~30% was evident after ethanol and POAEE exposure, respectively (Figure 6C). Accelerated channel turnover at the plasma membrane and/or impaired biosynthetic secretion can account for the pronounced apical expression defect of CFTR in treated cells. To assess the first possibility, apical plasma membrane stability of CFTR was measured by enzyme-linked immunosorbent assay, which revealed that ethanol, POAEE, and POA provoked increased removal of CFTR from the plasma membrane during a 2-hour chase, suggesting that channel turnover was accelerated (Figure 6C). The conformational maturation efficiency of CFTR was measured by the conversion efficiency of the metabolically labeled core glycosylated form into the complex glycosylated CFTR (Figure 6D). CFTR folding efficiency was diminished from 24% ± 3% to 17% ± 2% and 20% ± 1% by POA and POAEE, respectively (Figure 6D), indicating that nonoxidative ethanol metabolites compromise both the biosynthetic processing and peripheral stability of the channel.
Figure 6.
Effect of ethanol and its metabolites on CFTR and Na+/K+-ATPase expression. (A) Immunoblotting and densitometry of CFTR and Na+/K+-ATPase expression levels in transfected MDCK monolayers after 48 hours of treatment with ethanol, POA, or POAEE (right panel). Results are expressed as percentage of the complex glycosylated CFTR (band C) or Na+/K+-ATPase expression in untreated cells (control). (First column, CFTR; second column, Na+/K+-ATPase for each condition.) (B) Enzyme-linked immunosorbent assay measurement of the apical plasma membrane (PM) density of CFTR revealed that ethanol, POA, and POAEE decreased this parameter after 48 hours of incubation. Results are presented as percentage of CFTR cell surface density of the untreated cells. (C) Ethanol, POAEE, and POA reduce the PM stability of CFTR determined by cell surface enzyme-linked immunosorbent assay. Cell surface resident CFTR was labeled with anti-HA antibody and chased for 1 or 2 hours in the presence of the indicated compounds at 37− C. Results are presented as percentage of the initial CFTR surface density (1 and 2 indicate 1-hour and 2-hour chase, respectively). (D) CFTR folding efficiency was reduced by 100 mmol/L ethanol and diminished by 200 μmol/L POA or POAEE after 48 hours. CFTR folding efficiency was calculated as the percentage of the pulse-labeled, core glycosylated form converted into the mature complex glycosylated form during 3-hour chase. n = 3 for each condition. aP < .05 vs control.
Genetic Deletion of CFTR Increases the Severity of Alcohol-Induced Pancreatitis
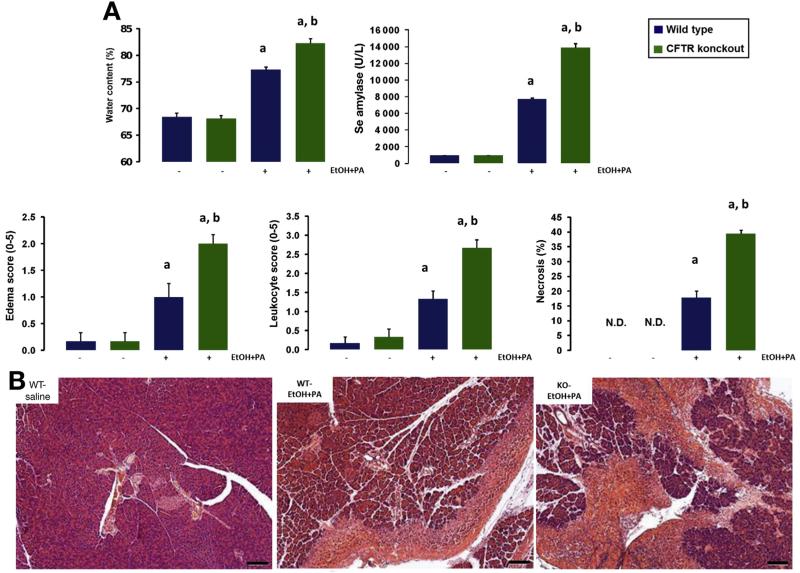
To further confirm the central role of CFTR in alcohol-induced pancreatic damage, we compared the severity of alcohol- and PA-induced pancreatitis in WT and CFTR KO mice. Intraperitoneal administration of 1.75 g/kg ethanol and 750 mg/kg PA induced significant elevation of all investigated parameters of severity of pancreatitis (pancreatic water content, serum amylase activity, edema score, leukocyte score, and necrosis) (Figure 7A and B) in WT animals. Importantly, these alterations were significantly higher in CFTR KO animals, showing that when expression and activity of CFTR is impaired by alcohol abuse, alcohol-induced AP worsens.
Figure 7.
AP induced by ethanol and fatty acids is more severe in CFTR KO mice. (A) Induction of AP by a single intraperitoneal injection of ethanol and PA induced significant elevation of pancreatic water content as measured by 100 * (wet weight dry – weight)/wet weight), serum amylase activity, edema and leukocyte scores, and necrosis. The severity of pancreatitis was significantly higher in CFTR KO mice. (B) Representative H&E-stained light micrographs of pancreas sections from WT control and ethanol + PA–treated WT or CFTR KO mice. Note the massive necrosis in the treated animals. Scale bars = 100 μm. Data are shown as means ± SEM. aP < .05 vs control, bP < .05 vs WT ethanol + PA–treated group. n = 6/group. N.D., not detected.
Discussion
In this study, we showed that ethanol and its nonoxidative metabolites cause impairment of CFTR function and expression, which exacerbate alcohol-induced pancreatitis (Supplementary Figure 11). Although a single binge of alcohol in healthy volunteers did not impair CFTR function as determined by sweat chloride absorption, excessive alcohol consumption in habitual drinkers markedly reduced the function of CFTR, as evidenced by a rise in CI− sw, which returned to the normal range when the measurement was repeated on sobered patients.
Pancreatic tissue metabolizes ethanol mainly via the nonoxidative pathway mediated by FAEE synthases, which combine ethanol and FA and produce FAEE.12 A clinical study showed that blood FAEE concentration was elevated in parallel with ethanol concentration during alcohol consumption, but FAEE remained increased longer in serum compared with ethanol.13 Moreover, compared with the liver, pancreatic FAEE synthases activity is higher, which creates the possibility of local accumulation of nonoxidative ethanol metabolites.14 Werner et al15 showed that infusion of FAEE induced pancreatic edema, intrapancreatic trypsinogen activation, and vacuolization of acinar cells. Recently, Huang et al16 developed a novel model of alcohol-induced pancreatitis using combined intraperitoneal injection of ethanol and FA, in which the pharmacological inhibition of nonoxidative ethanol metabolism decreased pancreatic damage.
We showed that pancreatic ductal HCO3− secretion plays a central role in the physiology of the exocrine pancreas, maintaining intraductal pH17,18; therefore, in our experiments, we used different in vivo and in vitro techniques to clarify the short-term effects of ethanol and ethanol metabolites on fluid and HCO3− secretion and CFTR CI− current in PDECs. Importantly, our magnetic resonance imaging cholangiopancreatography experiments showed that ductal secretion is remarkably diminished in CFTR KO mice compared with WT; moreover, ethanol and PA strongly impaired ductal secretion in both groups. The inhibitory effect of ethanol and PA on pancreatic fluid secretion was confirmed in vivo using pancreatic duct cannulation in anesthetized mice and in vitro using isolated sealed guinea pig pancreatic ducts as well. Besides fluid transport, we characterized the effects of ethanol and its metabolites on HCO3− secretion. Our results showed that ethanol in low concentrations stimulates and in high concentrations inhibits HCO3− secretion and decreases CFTR activity. Similar dual effects of ethanol on fluid secretion were highlighted earlier.19 In our study, the stimulatory effect of 10 mmol/L ethanol on HCO3− secretion was mediated by IP3R-dependent Ca2+ release from the ER. In contrast, high concentrations of ethanol and POA induced sustained [Ca2+]i elevation mediated by both IP3R and ryanodine receptor as well as extracellular Ca2+ influx (Figure 7C). Notably, similar toxic Ca2+ elevation was found in pancreatic acinar cells and other cell types, leading to premature protease activation and cell death.20–23 It is well documented that sustained [Ca2+]i elevation causes mitochondrial Ca2+ overload,24 which impairs (ΔΨ)m and ATP production.20,25 Very recently ethanol was shown to sensitize pancreatic mitochondria to activate the mitochondrial permeability transition pore, leading to mitochondrial failure.26 In this study, high concentrations of ethanol and POA also induced depletion of intracellular ATP and decreased (ΔΨ)m. Although the toxic effects of ethanol and POA were similar to those of a high concentration of bile acids,27,28 in this study chelation of [Ca2+]i abolished the inhibitory effect of ethanol and POA on HCO3− secretion. This observation indicates that ethanol and POA, in a similar manner to trypsin,29 inhibit HCO3− secretion via a sustained increase in [Ca2+]i. Importantly, CFTR single channel parameters do not change as a result of use of ethanol (personal communication, Aleksandrov Andrei and John R. Riordan), suggesting that the effects of a high dose of ethanol do not alter the biophysical characteristics of CFTR.
One of the crucial observations of this study is that expression of CFTR is decreased on the luminal membrane of human PDECs during alcohol-induced pancreatitis. Because decreased CFTR expression during alcoholic pancreatitis is very similar to the CFTR mislocalization found in autoimmune pancreatitis,30 we wanted to confirm that decreased CFTR expression is caused by alcohol and not by cellular damage during the inflammatory process. The in vitro experiments in human PDECs and the in vivo experiments in guinea pigs clearly showed that alcohol and its nonoxidative metabolites indeed strongly decrease CFTR expression without pancreatitis. The pronounced apical expression defect of CFTR was caused by accelerated channel turnover at the plasma membrane and impaired biosynthetic secretion (Figure 7C). The latter effect may be attributed, at least in part, to chronic cytoplasmic ATP depletion, considering that CFTR conformation maturation is an ATP-sensitive process at the ER.31 These results indicate that long-term exposure of PDECs to ethanol or ethanol metabolites compromise both the biosynthetic processing and peripheral stability of the channel. It is known that the phosphorylation and dephosphorylation of CFTR mediated by WNK/SPAK and IRBIT/PP1 regulates the plasma membrane trafficking of CFTR and other transporters in epithelial cells32,33; however, the effect of ethanol or ethanol metabolites on these systems is not known. Long-term alcohol consumption dose-dependently increases the risk of developing malignancies, diabetes, hypertension, and cardiovascular diseases.34 Interestingly, Guo et al35 showed that CFTR activity plays a crucial role in insulin secretion of pancreatic beta cells, whereas diabetes is a well-known complication of alcoholism.34 The decreased CFTR activity induced by alcohol consumption might play an important role in disease development.
The association between CFTR gene mutations and the risk of development of recurrent AP36 or CP37 provides strong evidence that mutations in CFTR and/or insufficiency of electrolyte and fluid secretion by pancreatic ductal cells lead to an increased risk of pancreatitis.38 Heterozygous carriers of CFTR mutations are at increased risk for CP39; moreover, Ooi et al7 showed that the risk of developing pancreatitis was much higher in patients with CF, who had milder CFTR mutations (type IV and V) and were pancreatic sufficient compared with those who had severe mutations and were pancreatic insufficient. In the pathogenetic model proposed in this study, the risk of developing pancreatitis inversely correlates with CFTR function. However, in other studies, the association between CFTR gene mutation and alcoholic pancreatitis was inconsistent.40,41 Very recently, LaRusch et al elegantly showed that CFTR gene mutations that do not cause typical CF but disrupt the WNK1-SPAK–mediated HCO3− permeability of the channel are associated with pancreatic disorders.42 In an animal model of pancreatitis, DiMagno et al earlier showed that CFTR KO mice developed more severe AP after cerulein hyperstimulation than WT mice.43 Pallagi et al recently had the same observation in mice with genetic deletion of Na+/H+ exchanger regulatory factor (NHERF1), which regulates CFTR expression.44 Here we have shown with CFTR KO mice that genetic deletion of CFTR leads to more severe pancreatitis after ethanol and fatty acid administration, confirming the crucial role of CFTR in the pathogenesis of alcohol-induced pancreatitis.
Taken together, our observations provide evidence that loss of CFTR function not only plays a crucial role in CFTR mutation–related pancreatitis but also contributes to the pathogenesis of alcohol-induced pancreatitis. These data indicate that correcting CFTR function should offer therapeutic benefit in AP.
Supplementary Material
Acknowledgments
The authors thank John Riordan (University of North Carolina at Chapel Hill) and Cystic Fibrosis Foundation Therapeutics for providing the CFTR antibody, Ursula Seidler (Department of Gastroenterology, Hannover Medical School) for the kind gift of the CFTR knockout mice, Éva Kereszthy (Department of Forensic Medicine, University of Szeged) for legal advice concerning investigation of alcoholic patients, Erzsébet Schneider and coworkers in the emergency unit of the Second Department of Medicine (University of Szeged) for help with measurements in alcohol-intoxicated patients and the volunteers participating in our study, and the 1st Department of Surgery (Semmelweis University) for providing surgical resection samples.
Funding
Supported by MTA-SZTE Momentum Grant (LP2014-10/2014), by the Hungarian National Development Agency (TÁMOP-4.2.2.A-11/1/KONV-2012-0035, TÁMOP-4.2.2-A-11/1/KONV-2012-0052, TÁMOP-4.2.2.A-11/1/KONV-2012-0073, TÁMOP-4.2.4.A/2-11-1-2012-0001, TÁMOP-4.2.4.A2-SZJÖ-TOK-13-0017) and the Hungarian Scientific Research Fund (NF100677). M.M.L. was supported by grants from the Alfried Krupp von Bohlen und Halbach Foundation (Graduate Schools of Tumour Biology and Free Radical Biology), the Deutsche Krebshilfe/Dr Mildred Scheel Stiftung (109102), the Deutsche Forschungsgemeinschaft (DFG GRK840-E3/E4, DFG GRK1947, MA 4115/1-2/3), the Federal Ministry of Education and Research (BMBF GANI-MED 03152061A and BMBF 0314107, 01ZZ9603, 01ZZ0103, 01ZZ0403, 03ZIK012), and the European Union (EUFP-7: EPC-TM and EU-FP7-REGPOT-2010-1, EPC-TM-Net). MS-T was supported by the National Institutes of Health (NIH) grant R01 DK058088.
Abbreviations used in this paper
- AP
acute pancreatitis
- ATP
adenosine triphosphate
- ATPase
adenosine triphosphatase
- (ATP)i
intracellular adenosine triphosphate
- BAC
blood alcohol concentration
- [Ca2+]i
intracellular Ca2+ concentration
- cAMP
adenosine 3’,5’-cyclic monophosphate
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- Cl−sw
sweat chloride concentration
- CP
chronic pancreatitis
- ER
endoplasmic reticulum
- H2DIDS
dihydro-4,4’-diisothiocyanostilbene- 2,2’-disulfonic acid
- IP3R
inositol triphosphate receptor
- KO
knockout
- mRNA
messenger RNA
- PDEC
pancreatic ductal epithelial cell
- PA
palmitic acid
- POA
palmitoleic acid
- POAEE
palmitoleic acid ethyl ester
- Tg
thapsigargin
- WT
wild-type
Footnotes
Conflicts of interest The authors disclose no conflicts.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org and at http://dx.doi.org/10.1053/j.gastro.2014.11.002.
References
- 1.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagar AB, Gorelick FS. Acute pancreatitis. Curr Opin Gastroenterol. 2002;18:552–557. doi: 10.1097/00001574-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Braganza JM, Lee SH, McCloy RF, et al. Chronic pancreatitis. Lancet. 2011;377:1184–1197. doi: 10.1016/S0140-6736(10)61852-1. [DOI] [PubMed] [Google Scholar]
- 4.Pandol SJ, Lugea A, Mareninova OA, et al. Investigating the pathobiology of alcoholic pancreatitis. Alcohol Clin Exp Res. 2011;35:830–837. doi: 10.1111/j.1530-0277.2010.01408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen OH, Tepikin AV, Gerasimenko JV, et al. Fatty acids, alcohol and fatty acid ethyl esters: toxic Ca2+ signal generation and pancreatitis. Cell Calcium. 2009;45:634–642. doi: 10.1016/j.ceca.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Sarles H, Sarles JC, Camatte R, et al. Observations on 205 confirmed cases of acute pancreatitis, recurring pancreatitis, and chronic pancreatitis. Gut. 1965;6:545–559. doi: 10.1136/gut.6.6.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ooi CY, Dorfman R, Cipolli M, et al. Type of CFTR mutation determines risk of pancreatitis in patients with cystic fibrosis. Gastroenterology. 2011;140:153–161. doi: 10.1053/j.gastro.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 8.Trezise AE, Buchwald M. In vivo cell-specific expression of the cystic fibrosis transmembrane conductance regulator. Nature. 1991;353:434–437. doi: 10.1038/353434a0. [DOI] [PubMed] [Google Scholar]
- 9.Siegenthaler P, Dehaller R, Dubach UC. Salt excretion in sweat in cystic fibrosis. JAMA. 1963;186:1178. doi: 10.1001/jama.1963.03710130074023. [DOI] [PubMed] [Google Scholar]
- 10.Hegyi P, Gray MA, Argent BE. Substance P inhibits bicarbonate secretion from guinea pig pancreatic ducts by modulating an anion exchanger. Am J Physiol Cell Physiol. 2003;285:C268–C276. doi: 10.1152/ajpcell.00574.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bird GS, DeHaven WI, Smyth JT, et al. Methods for studying store-operated calcium entry. Methods. 2008;46:204–212. doi: 10.1016/j.ymeth.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laposata EA, Lange LG. Presence of nonoxidative ethanol metabolism in human organs commonly damaged by ethanol abuse. Science. 1986;231:497–499. doi: 10.1126/science.3941913. [DOI] [PubMed] [Google Scholar]
- 13.Doyle KM, Cluette-Brown JE, Dube DM, et al. Fatty acid ethyl esters in the blood as markers for ethanol intake. JAMA. 1996;276:1152–1156. [PubMed] [Google Scholar]
- 14.Gukovskaya AS, Mouria M, Gukovsky I, et al. Ethanol metabolism and transcription factor activation in pancreatic acinar cells in rats. Gastroenterology. 2002;122:106–118. doi: 10.1053/gast.2002.30302. [DOI] [PubMed] [Google Scholar]
- 15.Werner J, Laposata M, Fernandez-del Castillo C, et al. Pancreatic injury in rats induced by fatty acid ethyl ester, a nonoxidative metabolite of alcohol. Gastroenterology. 1997;113:286–294. doi: 10.1016/s0016-5085(97)70106-9. [DOI] [PubMed] [Google Scholar]
- 16.Huang W, Booth DM, Cane MC, et al. Fatty acid ethyl ester synthase inhibition ameliorates ethanol-induced Ca2+-dependent mitochondrial dysfunction and acute pancreatitis. Gut. 2014;63:1313–1324. doi: 10.1136/gutjnl-2012-304058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hegyi P, Petersen OH. The exocrine pancreas: the acinar-ductal tango in physiology and pathophysiology. Rev Physiol Biochem Pharmacol. 2013;165:1–30. doi: 10.1007/112_2013_14. [DOI] [PubMed] [Google Scholar]
- 18.Hegyi P, Maleth J, Venglovecz V, et al. Pancreatic ductal bicarbonate secretion: challenge of the acinar acid load. Front Physiol. 2011;2:36. doi: 10.3389/fphys.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto A, Ishiguro H, Ko SB, et al. Ethanol induces fluid hypersecretion from guinea-pig pancreatic duct cells. J Physiol. 2003;551:917–926. doi: 10.1113/jphysiol.2003.048827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Criddle DN, Murphy J, Fistetto G, et al. Fatty acid ethyl esters cause pancreatic calcium toxicity via inositol trisphosphate receptors and loss of ATP synthesis. Gastroenterology. 2006;130:781–793. doi: 10.1053/j.gastro.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 21.Kouzoukas DE, Li G, Takapoo M, et al. Intracellular calcium plays a critical role in the alcohol-mediated death of cerebellar granule neurons. J Neurochem. 2013;124:323–335. doi: 10.1111/jnc.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakayama N, Eichhorst ST, Muller M, et al. Ethanol-induced apoptosis in hepatoma cells proceeds via intracellular Ca(2+) elevation, activation of TLCK-sensitive proteases, and cytochrome c release. Exp Cell Res. 2001;269:202–213. doi: 10.1006/excr.2001.5319. [DOI] [PubMed] [Google Scholar]
- 23.Kruger B, Albrecht E, Lerch MM. The role of intracellular calcium signaling in premature protease activation and the onset of pancreatitis. Am J Pathol. 2000;157:43–50. doi: 10.1016/S0002-9440(10)64515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 25.Walsh C, Barrow S, Voronina S, et al. Modulation of calcium signalling by mitochondria. Biochim Biophys Acta. 2009;1787:1374–1382. doi: 10.1016/j.bbabio.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Shalbueva N, Mareninova OA, Gerloff A, et al. Effects of oxidative alcohol metabolism on the mitochondrial permeability transition pore and necrosis in a mouse model of alcoholic pancreatitis. Gastroenterology. 2013;144:437–446 e6. doi: 10.1053/j.gastro.2012.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maleth J, Venglovecz V, Razga Z, et al. Non-conjugated chenodeoxycholate induces severe mitochondrial damage and inhibits bicarbonate transport in pancreatic duct cells. Gut. 2011;60:136–138. doi: 10.1136/gut.2009.192153. [DOI] [PubMed] [Google Scholar]
- 28.Maleth J, Rakonczay Z, Jr, Venglovecz V, et al. Central role of mitochondrial injury in the pathogenesis of acute pancreatitis. Acta Physiol (Oxf) 2013;207:226–235. doi: 10.1111/apha.12037. [DOI] [PubMed] [Google Scholar]
- 29.Pallagi P, Venglovecz V, Rakonczay Z, Jr, et al. Trypsin reduces pancreatic ductal bicarbonate secretion by inhibiting CFTR Cl(-) channels and luminal anion exchangers. Gastroenterology. 2011;141:2228–2239 e6. doi: 10.1053/j.gastro.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ko SB, Mizuno N, Yatabe Y, et al. Corticosteroids correct aberrant CFTR localization in the duct and regenerate acinar cells in autoimmune pancreatitis. Gastroenterology. 2010;138:1988–1996. doi: 10.1053/j.gastro.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukacs GL, Mohamed A, Kartner N, et al. Conformational maturation of CFTR but not its mutant counterpart (delta F508) occurs in the endoplasmic reticulum and requires ATP. EMBO J. 1994;13:6076–6086. doi: 10.1002/j.1460-2075.1994.tb06954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang D, Li Q, So I, et al. IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway. J Clin Invest. 2011;121:956–965. doi: 10.1172/JCI43475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park S, Hong JH, Ohana E, et al. The WNK/SPAK and IRBIT/PP1 pathways in epithelial fluid and electrolyte transport. Physiology (Bethesda) 2012;27:291–299. doi: 10.1152/physiol.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shield KD, Parry C, Rehm J. Chronic diseases and conditions related to alcohol use. Alcohol Res. 2013;35:155–173. [PMC free article] [PubMed] [Google Scholar]
- 35.Guo JH, Chen H, Ruan YC, et al. Glucose-induced electrical activities and insulin secretion in pancreatic islet beta-cells are modulated by CFTR. Nat Commun. 2014;5:4420. doi: 10.1038/ncomms5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavestro GM, Zuppardo RA, Bertolini S, et al. Connections between genetics and clinical data: Role of MCP-1, CFTR, and SPINK-1 in the setting of acute, acute recurrent, and chronic pancreatitis. Am J Gastroenterol. 2010;105:199–206. doi: 10.1038/ajg.2009.611. [DOI] [PubMed] [Google Scholar]
- 37.Weiss FU, Simon P, Bogdanova N, et al. Complete cystic fibrosis transmembrane conductance regulator gene sequencing in patients with idiopathic chronic pancreatitis and controls. Gut. 2005;54:1456–1460. doi: 10.1136/gut.2005.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hegyi P, Rakonczay Z. Insufficiency of electrolyte and fluid secretion by pancreatic ductal cells leads to increased patient risk for pancreatitis. Am J Gastroenterol. 2010;105:2119–2120. doi: 10.1038/ajg.2010.191. [DOI] [PubMed] [Google Scholar]
- 39.Sharer N, Schwarz M, Malone G, et al. Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. N Engl J Med. 1998;339:645–652. doi: 10.1056/NEJM199809033391001. [DOI] [PubMed] [Google Scholar]
- 40.Maruyama K, Harada S, Yokoyama A, et al. Association analyses of genetic polymorphisms of GSTM1, GSTT1, NQO1, NAT2, LPL, PRSS1, PSTI, and CFTR with chronic alcoholic pancreatitis in Japan. Alcohol Clin Exp Res. 2010;34(suppl 1):S34–S38. doi: 10.1111/j.1530-0277.2008.00757.x. [DOI] [PubMed] [Google Scholar]
- 41.Pezzilli R, Morselli-Labate AM, Mantovani V, et al. Mutations of the CFTR gene in pancreatic disease. Pancreas. 2003;27:332–336. doi: 10.1097/00006676-200311000-00011. [DOI] [PubMed] [Google Scholar]
- 42.LaRusch J, Jung J, General IJ, et al. Mechanisms of CFTR functional variants that impair regulated bicarbonate permeation and increase risk for pancreatitis but not for cystic fibrosis. PLoS Genet. 2014;10:e1004376. doi: 10.1371/journal.pgen.1004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dimagno MJ, Lee SH, Hao Y, et al. A proinflammatory, antiapoptotic phenotype underlies the susceptibility to acute pancreatitis in cystic fibrosis transmembrane regulator (−/−) mice. Gastroenterology. 2005;129:665–681. doi: 10.1016/j.gastro.2005.05.059. [DOI] [PubMed] [Google Scholar]
- 44.Pallagi P, Balla Z, Singh AK, et al. The role of pancreatic ductal secretion in protection against acute pancreatitis in mice. Crit Care Med. 2014;42:e177–e188. doi: 10.1097/CCM.0000000000000101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.