Highlights
* Developmental dyscalculia (DD) probed with univariate and multivariate fMRI analysis. * Arithmetic complexity-related deficits identified in 7–9-year-old children with DD. * Weak task modulation in multiple parietal and prefrontal regions in children with DD. * Children with DD show less differentiated multivoxel activity in intraparietal sulcus. * Weak problem representation and modulation a key feature of DD in young children.
Abbreviations: AG, angular gyrus; BA, Brodmann area; DD, developmental dyscalculia; fMRI, functional magnetic resonance imaging; IFG, inferior frontal gyrus; IPS, intraparietal sulcus; ITG, inferior temporal gyrus; LOC, lateral occipital cortex; MFG, middle frontal gyrus; MTG, middle temporal gyrus; PFC, prefrontal cortex; PMC, premotor cortex; PPC, posterior parietal cortex; ROI, region-of-interest; RSA, representational similarity analysis; SMG, supramarginal gyrus; SPL, superior parietal lobule; TD, typically developing
Keywords: Developmental dyscalculia, Children, fMRI, Intraparietal sulcus, Arithmetic, Prefrontal cortex, Learning disabilities
Abstract
Developmental dyscalculia (DD) is a disability that impacts math learning and skill acquisition in school-age children. Here we investigate arithmetic problem solving deficits in young children with DD using univariate and multivariate analysis of fMRI data. During fMRI scanning, 17 children with DD (ages 7–9, grades 2 and 3) and 17 IQ- and reading ability-matched typically developing (TD) children performed complex and simple addition problems which differed only in arithmetic complexity. While the TD group showed strong modulation of brain responses with increasing arithmetic complexity, children with DD failed to show such modulation. Children with DD showed significantly reduced activation compared to TD children in the intraparietal sulcus, superior parietal lobule, supramarginal gyrus and bilateral dorsolateral prefrontal cortex in relation to arithmetic complexity. Critically, multivariate representational similarity revealed that brain response patterns to complex and simple problems were less differentiated in the DD group in bilateral anterior IPS, independent of overall differences in signal level. Taken together, these results show that children with DD not only under-activate key brain regions implicated in mathematical cognition, but they also fail to generate distinct neural responses and representations for different arithmetic problems. Our findings provide novel insights into the neural basis of DD.
1. Introduction
Developmental dyscalculia (DD) is a disorder of numerical and mathematical abilities in children of normal intelligence who do not have other cognitive impairments (Butterworth et al., 2011, Rubinsten and Henik, 2009, von Aster and Shalev, 2007). Children with DD show poor performance on a broad range of basic numerical tasks including magnitude judgment (Ashkenazi et al., 2009, Geary et al., 2000, Holloway et al., 2010, Mussolin et al., 2010, Piazza et al., 2010, Price et al., 2007) and enumeration (Geary et al., 1992, Geary and Wiley, 1991, Knootz and Berch, 1996, Landerl et al., 2004, Schleifer and Landerl, 2011). Children with DD also lag behind their typically developing (TD) peers in basic arithmetic skills (Geary et al., 1992, Shalev et al., 2000, Shalev et al., 2005). Research over the past two decades has shown that fluent retrieval of arithmetic facts is a key deficit in children with DD (Geary, 1993, Geary, 1994, Geary, 2004, Geary et al., 2007). In TD children, with increased training and practice, typically acquired during the 2nd and 3rd grades, there is a rapid shift in the distribution of strategies towards greater use of direct retrieval in solving simple arithmetic problems (Ashcraft and Fierman, 1982, Carpenter and Moser, 1984, Geary, 1994, Siegler, 1996). In contrast, children with DD demonstrate poorer performance and continue to use less mature strategies such as finger counting to solve arithmetic problems (Geary, 1993, Shalev et al., 2000).
Neuroimaging studies have suggested that DD is associated with core deficits in the representation of numerical information in the posterior parietal cortex (PPC). The intraparietal sulcus (IPS) in dorsal aspects of the PPC has been consistently implicated in numerical information processing (Ansari and Dhital, 2006, Arsalidou and Taylor, 2011, Cohen Kadosh et al., 2008, Delazer et al., 2003, Menon et al., 2002, Pinel et al., 2001, Rosenberg-Lee et al., 2009, Zago et al., 2008) leading to the hypothesis that impaired numerical representation in the IPS is a core feature of DD (Butterworth et al., 2011, Rubinsten and Henik, 2009, Wilson and Dehaene, 2007). However, behavioral studies have suggested that domain general deficits such as lower visuo-spatial working memory capacity and weak central executive processes also contribute to poorer arithmetic skills in children with DD (Geary, 2004, Geary et al., 2007). Consistent with this view, structural brain imaging studies in DD have provided evidence for deficits not only in the IPS but also more extended regions within the PPC, as well as ventral visual stream areas including the bilateral parahippocampal gyrus and fusiform gyrus (Rotzer et al., 2008, Rykhlevskaia et al., 2009).
Only a few studies to date have examined in the neural basis of DD using functional brain imaging; of these, five studies have focused on basic number processing (Kaufmann et al., 2009a, Kaufmann et al., 2009b, Kucian et al., 2011, Mussolin et al., 2010, Price et al., 2007) and two on arithmetic problem solving (Davis et al., 2009, Kucian et al., 2006). In a group of nine 12-year-old children with DD, Price and colleagues observed weaker numerical distance effects in the right IPS, left fusiform and left medial prefrontal cortex (PFC) during a non-symbolic number comparison task (Price et al., 2007). During symbolic number comparison, Mussolin and colleagues observed weaker numerical distance effects in the right IPS, left superior parietal lobule (SPL), right middle frontal gyrus (MFG) and left anterior cingulate cortex in a group of fifteen 10-year-old children with DD (Mussolin et al., 2010). In contrast, Kaufmann et al., 2009a, Kaufmann et al., 2009b found increased bilateral PPC and prefrontal cortex activation during non-symbolic number comparison task in a group of nine children with DD from the 2nd to 4th grade (Kaufmann et al., 2009b). Kucian and colleagues (2011) found reduced activation in the right IPS and SPL, left inferior parietal cortex, and multiple frontal lobe areas during a number ordering task in a group of sixteen 8–10-year-old children with DD (Kucian et al., 2011). Most of these findings support the hypothesis that the right IPS is a common locus of weak numerical representations in children with DD, but also point to the involvement of multiple, but inconsistently identified, PPC and PFC regions.
Findings in arithmetic problem solving tasks have been weaker and even less consistent. Kucian and colleagues examined eighteen children with DD from grades 3 and 6 and found no group differences at the whole-brain level. However, region-of-interest (ROI) analyses revealed reduced brain activity in the right IPS, and inferior and middle frontal gyri. These differences were found for approximate, but not exact, addition problems (Kucian et al., 2006). In contrast, Davis and colleagues (2009) used these same tasks and found increased PPC and PFC activation during both exact and approximation addition in a group of 24 children in the 3rd grade with mathematical disability (Davis et al., 2009). These studies leave unclear the nature of arithmetic processing deficits in children with DD. De Smedt and colleagues compared brain activation to large and small addition and subtraction problems in a group of 10–12-year-old children with typical and low arithmetic achievement (n = 28) (De Smedt et al., 2011). They found that unlike typically achieving children, low achieving children failed to show a problem size effect in the right IPS. Consistent with this result, previous studies of children and adolescents with neurodevelopmental disorders that impair mathematical cognition, such as Fragile-X and Turner syndromes, have reported aberrant task modulation of multiple PPC and PFC regions during arithmetic problem solving (Kesler et al., 2006, Molko et al., 2003, Rivera et al., 2002). Kesler and colleagues (2006), for example, found that while TD children and adolescents had greater PPC and prefrontal activity on more difficult arithmetic problems, age-matched participants with Turner syndrome had comparable levels of activation for both easy and hard problems. However, mathematical difficulties in children with these neurodevelopmental disorders are typically accompanied by profound visuo-spatial and reading disabilities, thereby limiting their generalizability to pure DD (Ross et al., 2002).
In the current study we used both univariate and multivariate approaches to examining brain responses underlying arithmetic processing deficits in well-matched groups of 7–9-year-old children with DD and TD children. We focused on differential modulation of neural responses in relation to arithmetic problem complexity. Specifically, we compared brain responses to two types of two-operand addition problems: Complex problems, where one operand ranged from 2 to 9, the other from 2 to 5 and Simple problems, where one of the operands was always ‘1’. The two problem types have different arithmetic processing demands, but the same sensory and motor response selection demands. We examined whether children with DD exhibit modulation of brain responses in relation to arithmetic problem complexity in the same manner as TD children by using whole-brain analyses, as well as anatomically based ROI analyses. We used cytoarchitectonically defined maps of the IPS (Choi et al., 2006, Scheperjans et al., 2008) to more precisely examine differential brain responses to the Complex and Simple problems in children with DD (Rosenberg-Lee et al., 2011a). In addition to voxel-wise analyses, we also examined multi-voxel activation patterns using representational similarity analysis (RSA). RSA is a multivariate approach for investigating the relationship between stimulus representation and neural activity (Norman et al., 2006). RSA examines the spatial pattern of multi-voxel brain activity in a specific region of interest across task conditions, independent of overall differences in signal level. Here we use RSA to probe the similarity of spatial activation patterns between different types of arithmetic problems, and to provide complementary information about problem representation in children with DD.
Although previous studies of arithmetic (Davis et al., 2009, Kucian et al., 2006) do not point to a clear profile of activation deficits in young children with DD, one common feature of neurodevelopmental disorders which significantly impair mathematical cognition (Kesler et al., 2006, Molko et al., 2003, Rivera et al., 2002) is a lack of arithmetic complexity-related modulation of brain response. We therefore hypothesized that children with DD would show weaker arithmetic complexity-related modulation of brain response in the PPC, fusiform gyrus and PFC than TD children. Given inconsistencies in previous studies which have used only univariate analyses to examine processing deficits in children with DD, we further hypothesized that separate from differences in overall level of activation, they would also show an aberrant pattern of multi-voxel similarity between neural responses to Complex and Simple arithmetic problems. Together, these results would provide convergent evidence for weak problem representations in children with DD.
2. Methods
2.1. Participants
Children in the 2nd and 3rd graders (ages 7–9) were recruited from a wide range of schools in the San Francisco Bay Area using mailings to schools, postings at libraries and community groups. They had no history of psychiatric and neurological illness. All children, except two, were right-handed as determined by the Edinburgh handedness test (Oldfield, 1971). Intelligence was assessed using the Wechsler Abbreviated Scale of Intelligence (WASI, Wechsler, 1999) with an inclusion criterion of full-scale IQ above 80. The Wechsler Individual Achievement Test, Second Edition (WIAT-II, Wechsler, 2001) was used to determine grade specific achievement and dyscalculia status. Seventeen children (11 girls and 6 boys) who scored at or below 90 (i.e. the 25 percentile) on the Numerical Operations subtest of the WIAT-II formed the DD group. The TD group consisted of seventeen children (11 girls, 6 boys) individually matched on age, gender, IQ and reading ability to the DD group. TD children were required to score at or above 95 (i.e. the 37 percentile) on the Numerical Operations subtest (Table 1).
Table 1.
Standardized IQ, math and reading achievement and working memory scores for children in the DD and TD groups.
| Measure | Group |
p | |||
|---|---|---|---|---|---|
| DD (N = 17) |
TD (N = 17) |
||||
| M | SD | M | SD | ||
| Males/females | 6/11 | 6/11 | |||
| Age (months) | 98.05 | 6.72 | 97.41 | 6.59 | .80 |
| WASI | |||||
| Verbal | 106.64 | 12.32 | 112.41 | 16.48 | .26 |
| Performance | 107.58 | 18.2 | 113.23 | 12.76 | .30 |
| Full Scale | 107.7 | 13.36 | 114.58 | 10.73 | .11 |
| WIAT-II | |||||
| Word Reading | 108.47 | 9.23 | 113.29 | 10.65 | .17 |
| Numerical Operations | 85.7 | 3.49 | 115.94 | 15.53 | <.01 |
| Mathematical Reasoning | 102.47 | 14.82 | 115.29 | 12.76 | .01 |
| WMTB-C | |||||
| Backward Digit Recall | 100.17 | 21.1 | 99.1 | 15.89 | .87 |
| Digit Recall | 109.23 | 21.32 | 112.41 | 15.98 | .66 |
| Block Recall | 92.41 | 17.69 | 103.88 | 8.65 | .02 |
| Counting Recall | 93.05 | 18.67 | 97.52 | 20.39 | .51 |
WASI = Wechsler Abbreviated Scales of Intelligence; WIAT-II = Wechsler Individual Achievement Test – Second Edition; WMTB-C = Working Memory Test Battery for Children.
2.2. Standardized cognitive assessments
2.2.1. Mathematical abilities
Mathematical abilities were assessed using the WIAT-II (Wechsler, 2001). This achievement battery includes nationally standardized measures of K-12 academic skills and problem-solving abilities, which are normed by grade and time of the academic year (Fall, Spring, or Summer). The Numerical Operations subtest is a paper-and-pencil test that measures number writing and identification, rote counting, number production, and simple addition, subtraction, multiplication, and division problems. For example, 4 − 2 = ___ and 37 + 54 (presented vertically) are two problems in the 2nd and 3rd grade range. The Mathematical Reasoning subtest is a verbal problem-solving test that measures counting, geometric shape identification, and single- and multi-step word problem-solving involving time, money, and measurement with both verbal and visual prompts. The child is required to solve problems with whole numbers, fractions or decimals, interpret graphs, identify mathematical patterns, and solve problems of statistics and probability. For example, a dime is presented and the child is states: “How many pennies does it take to equal the value of one dime?” A probability problem asks: “If you flipped a coin ten times, how many times would the coin be most likely to land on heads?”
2.2.2. Reading abilities
The WIAT-II was also used to assess reading abilities. The Word Reading subtest involves reading individual words presented visually to the child, and was used for matching the DD and TD groups on reading ability.
2.2.3. Working memory
Four subtests of the Working Memory Test Battery for Children (Pickering and Gathercole, 2001) were used to assess working memory abilities. The Central Executive was assessed using the Counting Recall and Backward Digit Recall subtests. Phonological capacity was assessed using the Digit Recall subtest and visuo-spatial capacity was assessed using the Block Recall test, as described elsewhere (Meyer et al., 2010).
2.3. Brain imaging
2.3.1. Experimental procedures
The functional magnetic resonance imaging (fMRI) experiment was an event-related design with two task conditions: Complex addition (26 trials) and Simple addition (26 trials). In the Complex addition task, participants were presented with an equation involving two addends and asked to indicate, via a button box, whether the answer presented was correct or incorrect (e.g. “3 + 4 = 8”). One operand ranged from 2 to 9, the other from 2 to 5 (tie problems, such as “5 + 5 = 10”, were excluded), and answers were correct in half of the trials. Incorrect answers deviated by ±1 or ±2 from the correct sum (Ashcraft and Battaglia, 1978). The Simple addition task was identical except one of the addends was always ‘1’ (e.g. 3 + 1 = 4). Behavioral research in adults suggests that N + 1 addition is solved by incremental counting (Campbell and Metcalfe, 2007). Our use of this task was based on pilot studies, which suggested that children are consistently faster on these problems compared to the Complex addition problems. Critically, because stimuli in the Simple task have the same format as the Complex task, it provides a high-level control for sensory and number processing, as well as motor response selection. A verification, rather than verbal production, format was used in the scanner because overt verbal responses can result in significant head movement in children resulting in unusable fMRI data. Stimuli were displayed for 5 s with an inter-trial interval of 500 ms and a jitter period which varied between 0 and 3500 ms with an average of 1846 ms. During the inter-trial interval and the jitter period a fixation cross-appeared on the screen. The total length of the experimental run was 6 min and 22 s.
2.3.2. fMRI data acquisition
Images were acquired on a 3T GE Signa scanner (General Electric, Milwaukee, WI) using a custom-built head coil at the Stanford University Lucas Center. Head movement was minimized during the scan by a comfortable custom-built restraint. A total of 29 axial slices (4.0 mm thickness, 0.5 mm skip) parallel to the AC-PC line and covering the whole brain was imaged with a temporal resolution of 2 s using a T2* weighted gradient echo spiral in-out pulse sequence (Glover and Lai, 1998) with the following parameters: TR = 2 s, TE = 30 ms, flip angle = 80°, 1 interleave. The field of view was 20 cm, and the matrix size was 64 × 64, providing an in-plane spatial resolution of 3.125 mm. To reduce blurring and signal loss from field in homogeneity, an automated high-order shimming method based on spiral acquisitions was used before acquiring functional MRI scans (Kim et al., 2002).
2.3.3. fMRI preprocessing
fMRI data were analyzed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). The first 5 volumes were not analyzed to allow for T1 equilibration. A linear shim correction was applied separately for each slice during reconstruction (Glover and Lai, 1998). ArtRepair software was used to correct for excessive participant movement (Mazaika et al., 2007). Images were realigned to correct for movement, smoothed with a 4 mm FWHM Gaussian kernel and motion adjusted. Deviant volumes resulting from sharp movement or spikes in the global signal were then interpolated using the two adjacent scans. No more than 20% of the volumes were interpolated. Participants with head movement exceeding 5 mm in any of the x, y, and z directions were excluded from the study. There were no differences between translational movement parameters in the two groups in the x (DD = 0.64 mm, TD = 0.52 mm; p = .66), y (DD = 1.18 mm, TD = 0.88 mm; p = .54) or z (DD = 1.8 mm, TD = 1.48 mm; p = .45) directions. Finally, images were corrected for errors in slice-timing, spatially transformed for registration to standard MNI space, and smoothed again at 4.5 mm FWHM Gaussian kernel. The two step sequence of first smoothing with a 4 mm FWHM Gaussian kernel and later with 4.5 mm FWHM Gaussian kernel approximates a total smoothing of 6 mm, because total smoothing is equivalent to the square root of the sum of the squares of the individual smoothing steps.
2.3.4. Individual subject and group analyses
Task-related brain activation was identified using the general linear model implemented in SPM8. In the individual subject analyses, interpolated volumes flagged at the preprocessing stage were de-weighted. Each trial was modeled using a boxcar function convolved with the canonical hemodynamic response function and a temporal dispersion derivative to account for voxel-wise latency differences in hemodynamic response. Low-frequency drifts at each voxel were removed using a high-pass filter (0.5 cycles/min). Serial correlations were accounted for by modeling the fMRI time series as a first-degree autoregressive process. Voxel-wise t-statistics maps of the following contrasts were generated for each participant: (1) Complex addition–Simple addition, (2) Complex addition–Rest, and (3) Simple addition–Rest. Two analyses were conducted, one with correct and incorrect trials together, and the second with correct trials only. To examine the neural basis of weaker performance in children with DD both correct and incorrect trials were included in the first analysis. For the second analysis, only correct trials were included in the contrast images.
For group analysis, contrast images corresponding to the Complex addition–Simple addition were analyzed using a random effects analysis. Two group-level analyses were conducted: (i) one-way t-tests were first used to identify areas of significant activation (Complex addition–Simple addition) and deactivation (Simple addition–Complex addition) for each group separately, and (ii) between group t-tests were used to directly compare activation between TD children and children with DD. Significant activation clusters were determined, after applying a gray matter mask, using a height threshold of p < .01, with family-wise error (FWE) correction for multiple comparisons at p < .01 determined using Monte Carlo simulations (Nichols and Hayasaka, 2003). Monte Carlo simulations were implemented in MatLab using methods similar to AFNI's AlphaSim program (Forman et al., 1995, Ward, 2000). In each iteration of the Monte Carlo procedure, a 3D image with the same resolution and dimensions as the fMRI scan was randomly generated and smoothed with a 6 mm FWHM Gaussian kernel. For consistency with the inclusive mask used to report the results of the general linear model analysis, a gray matter mask was also applied to this image. The maximum cluster size at a given height threshold was recorded for each interaction, and 10,000 iterations were performed. The distribution of maximum cluster size across these 10,000 iterations was used to determine the FWE corrected extent threshold. At a height threshold of p < .01, less than 1% of the iterations had maximum cluster size greater than 128 voxels.
2.3.5. ROI analyses
Anatomically based ROI analyses were conducted to further examine the profile of activation differences between the DD and TD groups. We used the observer-independent cytoarchitectonically defined maps of the IPS, which provide an anatomically precise and consistent basis for examining fMRI responses across participant groups. The IPS consists of two anterior subdivisions, hIP1 and hIP2 (Choi et al., 2006) and a posterior subdivision, hIP3 (Scheperjans et al., 2008). Additional control analyses were performed using the primary visual cortex areas V1 and V2 (Amunts et al., 2000).
2.3.6. Representational similarity analysis
RSA assesses the similarity of spatial activity patterns produced by two experimental conditions within an ROI (Kriegeskorte et al., 2008). RSA were conducted by computing the cross-correlation between individual's t-maps for the Complex and Simple tasks within the cytoarchitectonically defined ROIs. The correlation coefficients between conditions were transformed to a normal distribution using the Fisher's r-to-Z transform.
3. Results
3.1. Neuropsychological measures
Table 1 shows the neuropsychological profiles of the DD and TD groups. The two groups were individually matched on age, gender, IQ and reading ability and so did not differ in any of these measures. All statistical tests were two-tailed. The groups differed significantly on the Numerical Operation subset of the WIAT-II, with the DD group scoring lower than the TD group (t(32) = −7.8, p < .01). The DD group also scored significantly lower than the TDs on the Mathematical Reasoning subtest (t(32) = −2.7, p < .01). DD children had lower scores on the Block Recall subtest of the WMTBC (t(32) = −2.1, p < .05) but not on any of the other three working memory subtests: Digit Recall, Counting Recall and Backward Digit Recall.
3.2. Brain imaging
3.2.1. Behavioral differences between TD and DD groups
3.2.1.1. Accuracy
Mean accuracy was calculated for each participant and entered into a two-way analysis of variance (ANOVA), with Group (DD, TD) as a between-participants factor and Complexity (Complex, Simple) as a within-participants factor. A significant main effect of Complexity was observed (F(1, 32) = 51.3, p < .01), participants had higher accuracy in the Simple compared to the Complex task. There was no main effect of Group (p = .56) but the interaction between Group and Complexity was marginally significant (F(1, 32) = 3.7, p = .06).
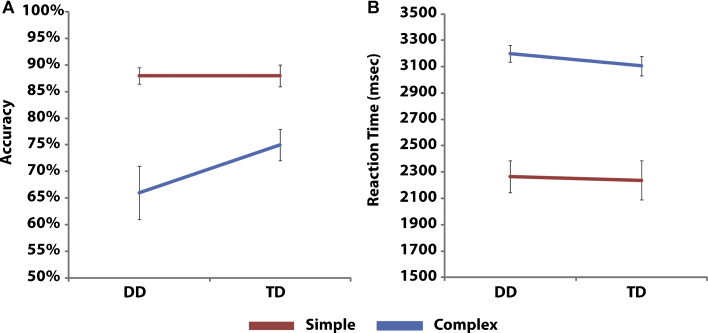
Differences between accuracy for Complex and Simple problems was greater in the DD group (67% vs. 88%), compared to the TD group (75% vs. 88%) (t(32) = −1.43, p < .16) (Fig. 1A).
Fig. 1.
(A and B) Behavioral performance in the DD and TD groups. Both groups showed strong differences between addition problem type, with Simple problems being performed faster and more accurately than Complex problems. There were no group differences in either accuracy or RT. However, the interaction between problem type and group was marginally significant (p = .06) indicating weaker performacne for Complex problems in the DD group.
3.2.1.2. RT
Median reaction time (RT) on correct trials was calculated for each participant and a two-way ANOVA with factors Group and Complexity was conducted. The analysis revealed a significant main effect of Complexity (F(1, 32) = 157.2, p < .01), such that participants were faster for Simple problems (2080 ± 571 ms) compared to Complex problems (3160 ± 365 ms). There was no main effect of Group (F(1, 32) = 0.1, p = .71) or interaction between Group and Complexity (F(1, 32) = 0.2, p = .64) (Fig. 1B).
3.2.2. Brain activation in DD and TD groups
3.2.2.1. TD
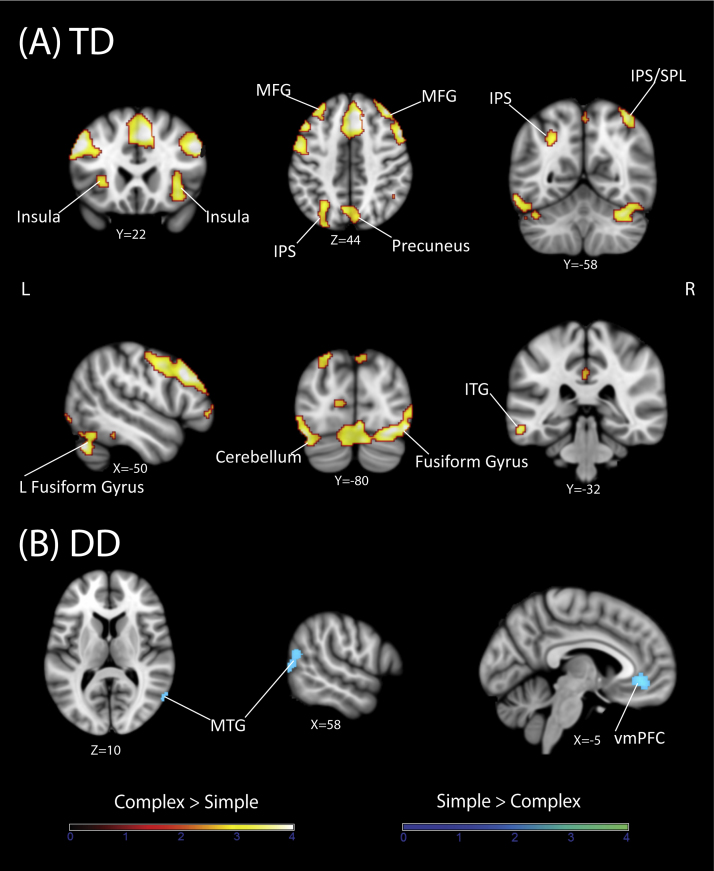
Complex > Simple. We first compared brain responses to Complex and Simple problems separately in each group. In the TD group, Complex problems elicited significantly greater activation in the PPC, specifically, in the bilateral IPS (BA 7), bilateral SPL (BA 7), bilateral precuneus (BA 7/19), and right supramarginal gyrus (SMG, BA 40). The TD group also showed greater activity in the ventral visual stream, specifically, bilateral fusiform gyrus (BA 37), lateral occipital cortex (LOC), visual cortex (BA 17/18), and in the left inferior temporal gyrus (ITG, BA 37) and middle temporal gyrus (MTG, BA 37). Furthermore, Complex problems elicited significantly greater activation in the frontal cortex, specifically, in the bilateral MFG (BA 8/9/10), left inferior frontal gyrus (IFG, BA 47), bilateral premotor cortex (PMC, BA 6) and right frontopolar cortex (BA 11). The TD group also showed greater activity in the anterior, mid and posterior cingulate (BA 23/24/31), left insula (BA 13) (Fig. 2A and Table 2).
Fig. 2.
Arithmetic complexity effects in the TD and DD groups. Brain response related to arithmetic complexity obtained by contrasting Complex and Simple addition problems. Coronal slices show significant activation (Complex > Simple, red scale) and significant deactivation (Simple > Complex, blue scale) in each group. (A) TD children showed greater complexity-related activation in multiple dorsal and ventral stream areas as well as the prefrontal cortex, including left intraparietal sulcus (IPS), right IPS and superior parietal lobule (SPL), bilateral precuneus, bilateral dorsolateral prefrontal cortex (middle frontal gyrus), bilateral insula, left inferior and middle temporal gyrus (MTG) and bilateral fusiform gyrus. (B) In contrast, no brain areas showed greater complexity-related activation in children with DD. Instead, they showed greater activation to Simple problems in left MTG and in the ventromedial prefrontal cortex (vmPFC).
Table 2.
Brain areas that showed significant differences between Complex and Simple arithmetic problems in the DD and TD groups.
| Region | BA | # of voxels | Peak Z-score | Peak MNI coordinates |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| TD group | ||||||
| Complex > Simple | ||||||
| L IPS | 7 | 440 | 3.22 | −24 | −82 | 48 |
| L Precuneus | 19 | 3.04 | −20 | −84 | 48 | |
| L SPL | 7 | 2.79 | −28 | −70 | 50 | |
| R Precuneus | 7 | 573 | 3.22 | 2 | −68 | 56 |
| L Precuneus | 7 | 2.87 | −4 | −66 | 44 | |
| R IPS/SPL | 7 | 481 | 3.15 | 36 | −62 | 58 |
| R SMG | 40 | 3.08 | 44 | −50 | 58 | |
| L/R Fusiform gyrus, L | 17, 18 | 5426 | 4.76 | −22 | −94 | −18 |
| Cerebellum, L ITG | 37 | |||||
| L Visual cortex | 18 | 4.66 | −22 | −102 | −4 | |
| L LOC | 19 | 3.81 | −36 | −92 | −14 | |
| R MFG | 9 | 7331 | 4.46 | 50 | 28 | 26 |
| R Frontopolar cortex | 11 | 3.98 | 26 | 60 | −4 | |
| R MFG | 8 | 3.81 | 8 | 32 | 44 | |
| R PMC | 6 | 3.81 | 54 | 12 | 44 | |
| L MFG | 9 | 3061 | 3.77 | −54 | 22 | 32 |
| L MFG | 10 | 3.68 | −46 | 52 | 4 | |
| L MFG | 47 | 3.47 | −36 | 42 | 4 | |
| L PMC | 6 | 134 | 3.08 | −28 | 2 | 60 |
| L Insula | 13 | 176 | 2.90 | −32 | 18 | 2 |
| L Mid-cingulate gyrus | 23 | 402 | 2.90 | 2 | −10 | 34 |
| L Posterior-cingulate gyrus | 31 | 2.74 | −2 | −30 | 32 | |
| R Anterior-cingulate | 24 | 2.58 | 4 | 6 | 30 | |
| Brain Stem | 182 | 3.44 | 0 | −14 | −8 | |
| Simple > Complex | ||||||
| None | ||||||
| DD group | ||||||
| Complex > Simple | ||||||
| None | ||||||
| Simple > Complex | ||||||
| L vmPFC | 11 | 350 | 3.01 | −8 | 38 | −6 |
| R vmPFC | 10 | 2.66 | 6 | 44 | −6 | |
| R Caudate | 143 | 3.28 | 2 | 12 | −6 | |
| R MTG | 37 | 132 | 2.72 | 60 | −60 | 18 |
IPS = intraparietal sulcus; ITG = inferior temporal gyrus; LOC = lateral occipital cortex; MFG = middle frontal gyrus; MTG = middle temporal gyrus; PMC = premotor cortex; SMG = supramarginal gyrus; SPL = superior parietal lobule; vmPFC = ventromedial prefrontal cortex.
Simple > Complex. In the TD group, there were no areas where activation was greater for Simple problems compared to Complex problems (Fig. 2A and Table 2).
3.2.2.2. DD
Complex > Simple. In the DD group, there were no brain areas that showed greater activation for Complex problems compared to Simple problems (Fig. 2B and Table 2).
Simple > Complex. The DD group showed greater activation in bilateral ventromedial prefrontal cortex (BA 11/10), caudate nucleus and right MTG (BA 37) (Fig. 2B and Table 2).
3.2.3. Differences in brain activation between DD and TD groups
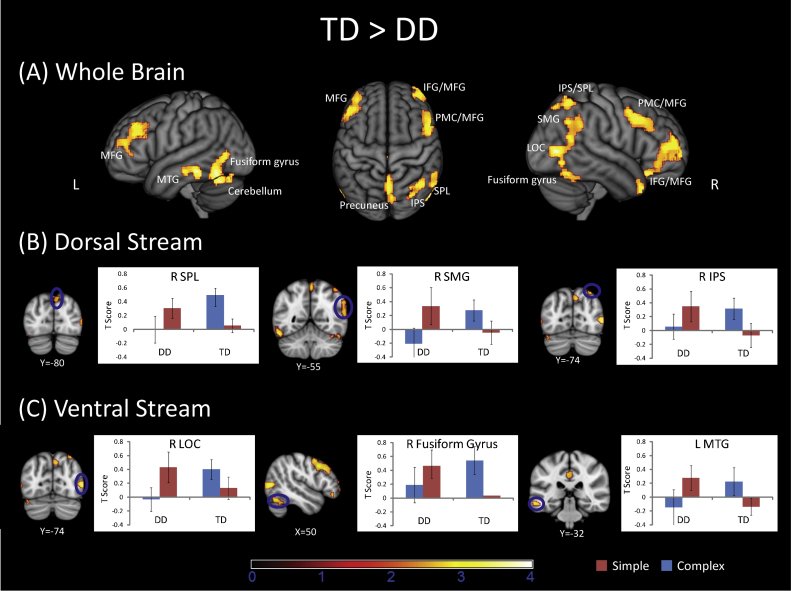
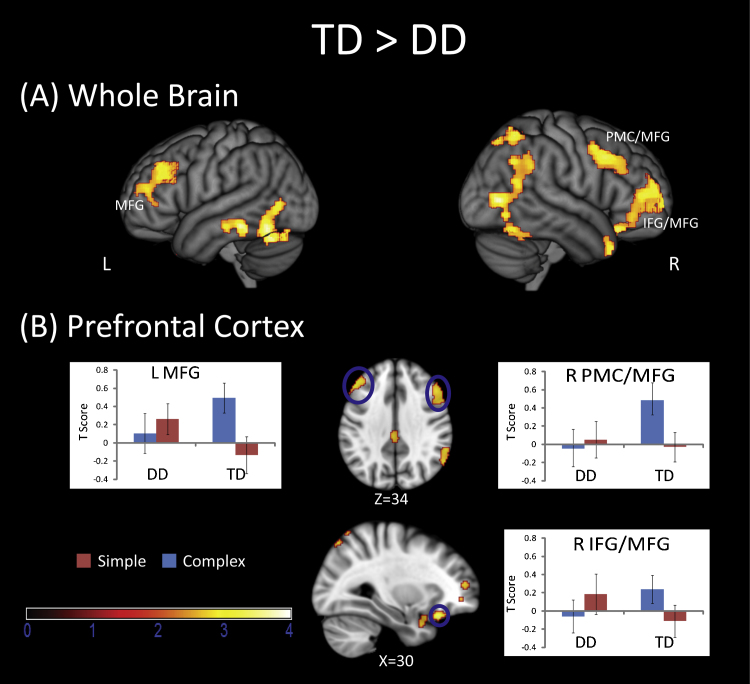
Brain responses to Complex and Simple problems were contrasted in each child and then compared between the two groups. Relative to the TD group, the DD group showed significantly reduced activation in several right PPC areas including IPS and SPL (BA 7), angular gyrus (AG, BA 39), SMG (BA 40) and bilateral precuneus (BA 7). The DD group also showed reduced activation in several ventral and visual areas, including left and right fusiform gyrus, LOC (BA 37), left MTG and ITG and superior temporal gyrus (BA 20/21/22/37) (Fig. 3 and Table 3). The DD group also had less activation in bilateral PFC including MFG (BA 8/9/10), right premotor cortex (PMC, BA 6/8), right IFG (BA 47) and mid-cingulate cortex (BA 23). Analysis of the profile of responses in these regions revealed that TD children had strong activation on Complex problems but near baseline activation on Simple problems. On the other hand, children with DD either had low activation levels for both problem types or greater activity for Simple problems (Fig. 4 and Table 3). Similar results were obtained when contrast images for Complex-rest and Simple-rest conditions were entered into a two-way ANOVA with Group (DD, TD) as a between-participants factor, and Complexity (Complex, Simple) as a within-participants factor (Table S1 in the Supplementary Materials).
Fig. 3.
Comparison of dorsal and ventral visual stream responses in the DD and TD groups. (A) Surface rendering of brain areas that showed reduced complexity-related responses in the DD, compared to the TD, group. (B) The DD group showed reduced activation in the right superior parietal lobule (SPL, 44, −60 58), right supramarginal gyrus (SMG, 60, −52, 34), and the right intraparietal sulcus (IPS, 32, −74, 54). (C) The DD group also had reduced responses in the lateral occipital (LOC, 54, −74, 6), right fusiform gyrus (50, −62, −20), and left middle temporal gyrus (MTG, −60, −28, 14). Plots of signal levels in regional peaks shows that the TD group showed incresed activtity on Complex, compared to Simple, problems. In contrast, children with DD had simlar activation levels for Simple and Complex problems.
Table 3.
Brain areas that showed significant differences in activation between the DD and TD groups.
| Region | BA | # of voxels | Peak Z-score | Peak MNI coordinates |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| TD > DD | ||||||
| R SPL | 7 | 174 | 3.19 | 44 | −60 | 58 |
| R IPS | 7 | 2.95 | 32 | −74 | 54 | |
| L/R Precuneus | 7 | 417 | 3.12 | 2 | −64 | 62 |
| R LOC | 37 | 623 | 3.45 | 56 | −72 | 4 |
| R SMG | 40 | 3.01 | 60 | −60 | 36 | |
| R AG | 39 | 2.94 | 58 | −62 | 38 | |
| R STG | 22 | 2.83 | 60 | −58 | 18 | |
| L Cerebellum | 368 | 3.5 | −50 | −64 | −26 | |
| L Fusiform gyrus | 37 | 3.47 | −58 | −60 | −18 | |
| L MTG | 37 | 2.99 | −58 | −70 | −2 | |
| L MTG | 21 | 222 | 3.40 | −58 | −36 | −16 |
| L ITG | 20 | 2.86 | −62 | −24 | −18 | |
| R Fusiform gyrus | 37 | 169 | 2.9 | 50 | −62 | −20 |
| ITG | 20 | 52 | −48 | −26 | ||
| R MFG, R PMC | 6, 8 | 505 | 3.17 | 56 | 16 | 40 |
| R IFG | 47 | 943 | 3.16 | 30 | 26 | −20 |
| R Superior Temporal gyrus | 38 | 3.04 | 36 | 20 | −38 | |
| R Frontopolar cortex | 10 | 3.03 | 36 | 52 | 10 | |
| L MFG | 9 | 554 | 2.99 | −52 | 30 | 30 |
| L MFG | 8 | 2.94 | −42 | 36 | 38 | |
| L MFG | 10 | 2.76 | −38 | 48 | 18 | |
| L/R Mid-cingulate gyrus | 23 | 134 | 2.69 | 0 | −32 | 38 |
| Brain Stem | 146 | 3.02 | 2 | −16 | −12 | |
| DD > TD | ||||||
| None | ||||||
AG = angular gyrus; IFG = inferior frontal gyrus; IPS = intraparietal sulcus; ITG = inferior temporal gyrus; LOC = lateral occipital cortex; MFG = middle frontal gyrus; MTG = middle temporal gyrus; PMC = premotor cortex; SMG = supramarginal gyrus; SPL = superior parietal lobule; STG = superior temporal gyrus.
Fig. 4.
Comparison of prefrontal cortex responses in the DD and TD groups. (A) Surface rendering of prefrotnal areas that showed reduced complexity-related brain responses in the DD, compared to the TD, group. (B) The DD group showed lower activation in the left middle frontal gyrus (MFG, −52, 30, 30), right premotor cortex (PMC, 56, 16, 40) and adjoining MFG, and in the right inferior frontal gyrus (IFG, 30, 28, −20) and adjoining MFG. Plots of signal levels in regional peaks shows that the TD group had increased activtity on the Complex, compared to Simple, problems. In contrast, children with DD had similar activation levels for Simple and Complex problems.
In order to examine whether these activation deficits arose from lower visuo-spatial working memory in the TD group, we conducted additional analyses using an ANOCVA model with block recall scores as a covariate. This analysis replicated our previous findings without the covariate, except for the mid-cingulate cortex (BA 31) which was no longer significant (Table S2 in the Supplementary Materials).
The above analyses were conducted using both correct and incorrect trials, in order to examine the neural basis of weaker performance in children with DD. Because brain and cognitive processes for incorrect trials may differ from those involved in processing correct trials, we conducted additional analyses using only correct trials. The results of this analysis largely replicated findings from our previous analysis. At the stringent threshold used in this study (p < .01, with FWE correction for multiple comparisons at p < .01) significant group differences were found in the left fusiform gyrus (BA 37), left IFG (BA 47) and right MFG (BA6/8/9) (Table S3 in the Supplementary Materials). At a threshold of p < .05, with FWE correction for multiple comparisons at p < .05, all the areas reported above were found to be significant, except for the left MTG.
3.2.4. Group differences in cytoarchitectonically defined IPS ROIs
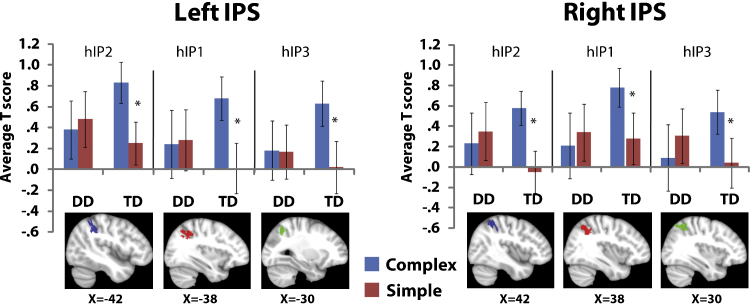
To further investigate potential PPC deficits in children with DD, we examined task-related responses in anatomically defined ROIs in the IPS. Mean t-scores for each participant were calculated for each problem type, in each subdivision of the IPS, and entered into a four-way ANOVA with between-participants factor Group (DD, TD), and within-subject factors of Laterality (Left, Right), Subdivision (hIP1, hIP2, hIP3), and Complexity (Complex, Simple). There was a significant interaction between Group and Complexity (F(1, 32) = 5.2, p < .05). In children with DD, activation was comparable for Complex and Simple problems in all the IPS subdivisions whereas the TD group showed greater activation to the Complex problems in all the subdivisions (Fig. 5). There was no main effect of Group or other interactions with Group. To examine the specificity of our findings additional control analyses were performed using the primary visual cortex areas V1 and V2. There were no group differences or interactions with group in V1 or V2 (p > .22) (Supplementary Materials).
Fig. 5.
ROI analysis in bilateral cytoarchitectonically defined subdivisions of the IPS. In all three subdivisions (hIP2, hIP1, hIP3) of the bilateral IPS, TD children showed greater activation for Complex, compared to Simple, problems. In contrast, children with DD showed similar activation levels for Complex and Simple problems in all three subdivisions of the left and right IPS; *p < .05.
3.2.5. Representational similarity
RSA was used to examine the similarity between multi-voxel activation patterns elicited by Complex and Simple problems. Anatomically defined subdivisions of the IPS were examined separately. We used only responses to correct trials and excluded one child with DD who had overall accuracy of less than 55%.
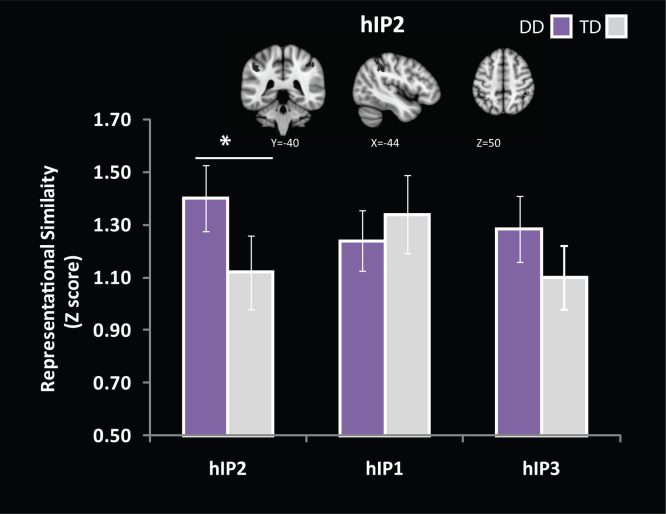
Mean similarity between Complex and Simple problems was computed for each IPS subdivision, and for each participant, and normalized using Fisher's r-to-Z transform. The resulting Z-scores were subjected to a three-way ANOVA with Group as a between-participants factor, and Laterality (Left, Right), and Subdivision (hIP1, hIP2, hIP3) as within-participants factors. A significant interaction between Group and Subdivision was observed (F(2, 62) = 6.1, p < .01), with the DD group having higher representational similarity (r = .89; Z = 1.41) than the TD group (r = .81; Z = 1.09) in hIP2 (F(1, 31) = 4.5, p < .05) between Complex and Simple problems. No group differences were observed in hIP1 (F(1, 31) = 0.7, p = .66) and hIP3 (F(1, 31) = 1.8, p = .18). There were no other significant interactions or main effects (Fig. 6).
Fig. 6.
Representational similarity analysis in cytoarchitectonically defined subdivisions of the IPS. Children with DD showed greater similarity of multi-voxel brain responses to Complex and Simple problems in the anterior most subdivision (hIP2: −44, −40, 48) of the left and right IPS; *p < .05.
4. Discussion
In this study we examined brain activation and neural representations during arithmetic problem solving in children with DD using both univariate and multivariate approaches. Importantly, IQ, reading ability and working memory capacity of the DD group were all within the normal range; visuo-spatial working memory capacity was the only general cognitive measure where the TD group had greater abilities. This well-matched group of children therefore allowed us to investigate the neural correlates of DD without comorbid cognitive deficits. Using this sample, we examined behavioral and brain responses to Complex and Simple problems which differed in arithmetic complexity but not in sensory processing, decision making or motor responses. Children with DD, like TD children, were significantly faster on Simple, compared to Complex, arithmetic problems. There were no group differences in RT, but for accuracy the interaction between problem type and group was marginally significant, such that children with DD performed worse on Complex problems. In spite of modest differences in performance on two-operand addition problems during the fMRI session, children with DD showed significant deficits in activation in multiple brain systems that are known to be important for mathematical cognition. Furthermore, independent of overall deficits in brain activation, RSA showed poor differentiation between Complex and Simple problems in children with DD. Thus, both univariate and multivariate analyses revealed that children with DD have weak modulation and problem representation associated with arithmetic complexity.
4.1. Children with DD show weaker activation in multiple cortical regions
Children with DD had weaker activity in multiple cortical regions. The loci of deficits in the DD group can be categorized into three major brain systems that are important for numerical cognition – the dorsal visual stream, the ventral visual stream and the PFC (Arsalidou and Taylor, 2011, Rivera et al., 2005, Rosenberg-Lee et al., 2011a). In the dorsal visual stream, DD children had deficits in both lateral PPC, including the right IPS, SPL and AG, and medial PPC, including the precuneus. In the ventral visual stream deficits were observed bilaterally in the LOC and fusiform gyri, in the left MTG and ITG. The distributed nature of these task-related reductions suggests that impairments during arithmetic problem solving in the DD group extend beyond the IPS. These deficits were observed for both correct and incorrect trials taken together and when we restricted the analysis to just the correct trials, providing multiple converging evidence for aberrant processing of arithmetic problems in children with DD.
Although the IPS has been the focus of most neuroimaging studies of DD our findings point to multiple foci of PPC deficits in young children with DD. Specifically, we found differences not only in the IPS, but also the SPL, AG and the SMG. Consistent with stronger gray matter deficits in the right hemisphere (Rykhlevskaia et al., 2009), functional differences were all localized to the right PPC. The IPS, SPL and SMG are known to be involved in different cognitive processes during numerical problem solving (Cohen Kadosh et al., 2008, Dehaene et al., 2003, Menon, 2010). While the IPS is sensitive to quantity representation and the semantic aspects of number processing (Ansari and Dhital, 2006, Cohen Kadosh et al., 2008, Delazer et al., 2003, Menon et al., 2002, Pinel et al., 2001, Rosenberg-Lee et al., 2009, Zago et al., 2008), adjoining areas of the SPL are thought to be involved in covert visuo-spatial attention processes (Andres et al., 2011, Wu et al., 2009) and attentional deficits have been reported in individuals with DD (Ashkenazi and Henik, 2010a, Ashkenazi and Henik, 2010b, Ashkenazi et al., 2009). The right IPS, SPL and SMG are all also known to be involved in visuo-spatial working memory in children (Kwon et al., 2002). Consistent with this observation, cognitive testing in our DD sample revealed significant deficits in visuo-spatial working memory capacity even though the central executive, phonological capacity and other cognitive abilities were well matched between the groups.
In the ventral visual stream we found differences in the right and left fusiform gyrus and the LOC. Structural deficits have been found in the right fusiform gyrus in children with DD (Rykhlevskaia et al., 2009). Moreover, white matter projections linking the right fusiform gyrus to the right PPC are also weaker in children with DD (Rykhlevskaia et al., 2009). Taken together, these results suggest that weak functional interactions along this and other ventral-dorsal pathways may contribute to DD.
Children with DD also showed prominent deficits in three distinct clusters within the PFC. These three clusters were localized to the right PMC, the right dorsolateral PFC extending to the ventrolateral PFC, and the left dorsolateral PFC. Reduced right dorsolateral PFC activation in children with DD has been reported during both symbolic number comparison (Mussolin et al., 2010) and approximate arithmetic tasks (Kucian et al., 2006), but not during non-symbolic magnitude comparison (Price et al., 2007). Our findings suggest that multiple dorsolateral and ventrolateral PFC regions in both hemispheres contribute to arithmetic processing deficits in children with DD. Central executive and working memory deficits are known to be important factors contributing to poor problem solving and fact retrieval in children with DD (Geary, 2004, Geary et al., 2007, Rotzer et al., 2009). Although, children in the DD group in our study were well matched on a number of cognitive measures, including central executive measures, they showed significant deficits in visuo-spatial working memory. However, additional analysis showed that deficits in PFC activation were prominent even after controlling for differences in visuo-spatial working memory. These results suggest that children with DD do not adequately engage PFC regions known to be important for domain general functions, such as working memory, even when their cognitive and intellectual capacities are not deficient (Kwon et al., 2002, Rivera et al., 2005).
Finally, a surprising finding of this study were the processing deficits in the left MTG. No such deficits were found in the homologous right hemisphere regions. The MTG has generally not been reported as a source of functional or structural deficits in DD. Yet there is a growing recognition of the role of the left MTG in the verbal retrieval of semantic information (Booth et al., 2002, Fiebach et al., 2002). In the context of numerical cogntion, it is noteworthy, that the left MTG shows both numerical distance (Pinel et al., 2001) and developmental changes in size congruity effects (Wood et al., 2009). Recently, it was reported that a left MTG region identified by comparing words to symbols strings also displayed greater activation for multiplication problems than subtraction (Prado et al., 2011). The authors attributed these differences to the greater use of verbally mediated retrieval for multiplication than subtraction. These left MTG regions implicated in numerical and arithmetic cogntion tend to be more dorsal to the area identified in the current study. However, developmental increases in activitation for an arithmetic task were found in regions spanning the left inferior and middle temporal gyrus (Rivera et al., 2005). Further research is needed to examine whether the left lateralized temporal deficits observed in children with DD are directly related to poor arithmetic fact retrieval in this population.
4.2. Children with DD show weak differentiation between problem types
A striking novel finding of our current study is that children with DD show less differentiated neural responses to Complex and Simple problems. In the PPC and the PFC, for example, TD children showed the expected pattern of increases in activation for Complex problems compared to Simple problems. While children with DD showed significant activation in the same fronto-parietal regions as TD children, they did not demonstrate greater activation to Complex, compared to the Simple problems. Furthermore, in contrast to TD children, who showed greater activation for Complex versus Simple problems, children with DD showed greater activation for Simple versus Complex problems in several regions. This reversed pattern of the expected complexity-related modulation of responses was observed in all three systems highlighted above: the dorsal and ventral visual streams and the PFC (Fig. 3, Fig. 4). For Complex problems, children with DD also showed weaker activation than TD children. This pattern of brain response suggests that children with DD overcompensate when solving Simple problems and fail to modulate neural activity in response to increasing arithmetic complexity.
4.3. IPS as a locus of weak neural representations in children with DD
The IPS has been an area of particular interest in DD, since it plays an important role in both basic number processing and arithmetic problem solving (Ansari and Dhital, 2006, Cohen Kadosh et al., 2008, Dehaene et al., 2003, Delazer et al., 2003, Menon et al., 2002, Pinel et al., 2001, Rosenberg-Lee et al., 2009, Zago et al., 2008). Neuroanatomical studies have found that children with DD show reduced gray matter volume in the IPS and adjoining PPC (Rotzer et al., 2008, Rykhlevskaia et al., 2009). However, as previously noted, functional imaging studies of arithmetic processing in children with DD have yielded mixed findings, with some studies reporting increased and others decreased IPS responses in comparison to the TD group. To resolve this discrepancy we conducted both univariate and multivariate RSA analyses to examine task-related modulation of responses in the IPS.
At the whole-brain level, significant differences were observed in the right IPS and multiple adjoining regions of the right PPC. Convergent results were obtained from a more refined neuroanatomical analysis of IPS responses using the three cytoarchitectonically defined subdivisions (hIP2, hIP1 and hIP3) that span its anterior–posterior axis (Caspers et al., 2006, Choi et al., 2006, Rosenberg-Lee et al., 2011b, Wu et al., 2009). Children with DD showed significant task-related differences in the right IPS; additionally, we found that all three subdivisions of both the right and left IPS showed a significant group by complexity interaction such that children with DD failed to up-regulate brain responses to the Complex problems. In contrast, in each of these regions, TD children showed the expected pattern of significant complexity-related modulation. These three IPS regions are not only cytoarchitectonically distinct, but they also differ in their patterns of intrinsic functional connectivity and structural connectivity with other brain areas (Uddin et al., 2010), pointing to multiple circuits and functional processes that are dysfunctional in children with DD.
To further examine the underlying neural representations, we used RSA to compare spatial patterns of IPS activity to Complex and Simple problems in the two groups. We found that children with DD have less distinct neural representations for the two problem types than TD children in both the left and right IPS. Interestingly, this effect was localized to the anterior most subdivision of the IPS, specifically hIP2. Connectivity analysis has shown that hIP2 has strong connections with the dorsolateral PFC, premotor cortex and the frontal eye field (Uddin et al., 2010), suggesting that processing deficits in this region may result in weaker task-dependent top-down influences in children with DD. Consistent with this view, PFC responses were significantly undifferentiated in these children. Because RSA examines spatial correlations in brain activity, these results are independent of overall differences in magnitude of signal level and provide new information about the lack of distinct problem representations in children with DD. Taken together, both univariate and multivariate analyses suggest that weak problem representations and task-related modulation of neural activity in the IPS is an important hallmark of DD.
4.4. Limitations
Multiple naming conventions and diagnostic criteria have been used by researchers in recent years to characterize and classify children with mathematical disability (Rubinsten and Henik, 2009), including DD (Ashkenazi et al., 2009, Kaufmann et al., 2009b, Rykhlevskaia et al., 2009), mathematical learning disabilities (Geary et al., 2007, Mazzocco et al., 2011, Rousselle and Noël, 2008) with cutoffs at 46th percentile and below (Geary et al., 1992), 25th percentile and below (Davis et al., 2009), 15th percentile and below (Rousselle and Noël, 2008), 10th percentile and below (Mazzocco et al., 2011), and 7th percentile and below (Price et al., 2007). In the present study, we used a 25th percentile cutoff for mathematical disability, a more liberal criterion than those recommended in previous behavioral studies (Mazzocco et al., 2011). An important issue here is that at the low end of math abilities, high rates of co-morbidity with dyslexia or attention deficit/hyperactivity disorder have been reported (Hanich, 2001, Rubinsten and Henik, 2009, von Aster and Shalev, 2007). Using a stricter definition of DD therefore runs the risk of increasing co-morbid disabilities, and failing to match on non-numerical abilities, such as IQ, reading and working memory (Rubinsten and Henik, 2009). Importantly, in the present study, we used an extensive battery of neuropsychological measures to not only demonstrate that the two groups are matched on IQ, reading abilities, attention and working memory, but also to ensure that all measures of non-numerical abilities are in the normal range, and therefore unimpaired, in the DD group. The tradeoff here is clearly that as one imposes stricter selection criteria based on numerical measures, comorbid disabilities in other domains, such as reading disabilities, also become more prominent – a situation we tried to avoid. Nevertheless, given the current lack of consensus in defining DD, the interpretation of the present results and other related neuroimaging studies of children with math disabilities, should still be tempered by the disparate nature of the selection and exclusion criteria employed.
5. Conclusions
DD is a disability that impacts mathematical learning and skill acquisition in school-age children, and arithmetic fact retrieval is one of the most pronounced deficits in children with DD. Our study overcomes limitations of previous neuroimaging studies by employing a narrow age range, using a standardized test to identify children with DD, and excluding children with comorbid cognitive disabilities. With this sample, we were able to assess univariate and multivariate differences in brain response independent of cognitive and performance differences. We found that children with DD not only exhibit aberrant activity in key brain regions implicated in mathematical cognition but they also fail to modulate task-relevant neural responses and representations of distinct arithmetic problems. Both univariate and multivariate analysis suggest that the IPS is a major locus of poor problem representation and arithmetic complexity-related modulation in children with DD. Our study offers novel insights into the neural correlates of DD by providing for the first time, evidence of representational differences in key brain areas, and highlights the utility of multivariate approaches to understanding aberrant stimulus representations underlying complex learning disabilities. Our approach is likely to be applicable to other developmental learning disorders and has important practical implications for members of the educational neuroscience community.
Acknowledgments
This research was supported by grants from the National Institutes of Health (HD047520, HD059205, MH084164) and the National Science Foundation (BCS/DRL 0750340).
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.dcn.2011.09.006.
Contributor Information
Sarit Ashkenazi, Email: sarita1@stanford.edu.
Vinod Menon, Email: menon@stanford.edu.
Appendix A. Supplementary data
References
- Amunts K., Malikovic A., Mohlberg H., Schormann T., Zilles K. Brodmann's areas 17 and 18 brought into stereotaxic space-where and how variable? Neuroimage. 2000;11:66–84. doi: 10.1006/nimg.1999.0516. [DOI] [PubMed] [Google Scholar]
- Andres M., Pelgrims B., Michaux N., Olivier E., Pesenti M. Role of distinct parietal areas in arithmetic: an fMRI-guided TMS study. Neuroimage. 2011;54:3048–3056. doi: 10.1016/j.neuroimage.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Ansari D., Dhital B. Age-related changes in the activation of the intraparietal sulcus during nonsymbolic magnitude processing: an event-related functional magnetic resonance imaging study. J. Cogn. Neurosci. 2006;18:1820–1828. doi: 10.1162/jocn.2006.18.11.1820. [DOI] [PubMed] [Google Scholar]
- Arsalidou M., Taylor M.J. Is 2 + 2 = 4? Meta-analyses of brain areas needed for numbers and calculations. Neuroimage. 2011;54:2382–2393. doi: 10.1016/j.neuroimage.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Ashcraft M.H., Battaglia J. Cognitive arithmetic – evidence for retrieval and decision-processes in mental addition. J. Exp. Psychol.: Hum. Learn. Memory. 1978;4:527–538. [Google Scholar]
- Ashcraft M.H., Fierman B.A. Mental addition in third, fourth, and sixth graders. J. Exp. Child Psychol. 1982;33:18. [Google Scholar]
- Ashkenazi S., Henik A. Attentional networks in developmental dyscalculia. Behav. Brain Funct. 2010;6:1–12. doi: 10.1186/1744-9081-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi S., Henik A. A disassociation between physical and mental number bisection in developmental dyscalculia. Neuropsychologia. 2010;48:2861–2868. doi: 10.1016/j.neuropsychologia.2010.05.028. [DOI] [PubMed] [Google Scholar]
- Ashkenazi S., Rubinsten O., Henik A. Attention, automaticity, and developmental dyscalculia. Neuropsychology. 2009;23:535–540. doi: 10.1037/a0015347. [DOI] [PubMed] [Google Scholar]
- Booth J.R., Burman D.D., Meyer J.R., Gitelman D.R., Parrish T.B., Mesulam M.M. Modality independence of word comprehension. Hum. Brain Mapp. 2002;16:251–261. doi: 10.1002/hbm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B., Varma S., Laurillard D. Dyscalculia: from brain to education. Science. 2011;332:1049–1053. doi: 10.1126/science.1201536. [DOI] [PubMed] [Google Scholar]
- Campbell J.I.D., Metcalfe A.W.S. Arithmetic rules and numeral format. J. Cogn. Psychol. 2007;19:335–355. [Google Scholar]
- Carpenter T.P., Moser J.M. The acquisition of addition and subtraction concepts in grades one through three. J. Res. Math. Educ. 1984;15:179–202. [Google Scholar]
- Caspers S., Geyer S., Schleicher A., Mohlberg H., Amunts K., Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage. 2006;33:430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Choi H.J., Zilles K., Mohlberg H., Schleicher A., Fink G.R., Armstrong E., Amunts K. Cytoarchitectonic identification and probabilistic mapping of two distinct areas within the anterior ventral bank of the human intraparietal sulcus. J. Comp. Neurol. 2006;495:53–69. doi: 10.1002/cne.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh R., Lammertyn J., Izard V. Are numbers special? An overview of chronometric, neuroimaging, developmental and comparative studies of magnitude representation. Prog. Neurobiol. 2008;84:132–147. doi: 10.1016/j.pneurobio.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Davis N., Cannistraci C.J., Rogers B.P., Gatenby J.C., Fuchs L.S., Anderson A.W., Gore J.C. Aberrant functional activation in school age children at-risk for mathematical disability: a functional imaging study of simple arithmetic skill. Neuropsychologia. 2009;47:2470–2479. doi: 10.1016/j.neuropsychologia.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S., Piazza M., Pinel P., Cohen L. Three parietal circuits for number processing. Cogn. Neuropsychol. 2003;20:487–506. doi: 10.1080/02643290244000239. [DOI] [PubMed] [Google Scholar]
- Delazer M., Domahs F., Bartha L., Brenneis C., Lochy A., Trieb T., Benke T. Learning complex arithmetic—an fMRI study. Brain Res. Cogn. Brain Res. 2003;18:76–88. doi: 10.1016/j.cogbrainres.2003.09.005. [DOI] [PubMed] [Google Scholar]
- De Smedt B., Holloway I.D., Ansari D. Effects of problem size and arithmetic operation on brain activation during calculation in children with varying levels of arithmetical fluency. Neuroimage. 2011;57:771–781. doi: 10.1016/j.neuroimage.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Fiebach C.J., Friederici A.D., Muller K., von Cramon D.Y. fMRI evidence for dual routes to the mental lexicon in visual word recognition. J. Cogn. Neurosci. 2002;14:11–23. doi: 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- Forman S.D., Cohen J.D., Fitzgerald M., Eddy W.F., Mintun M.A., Noll D.C. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Geary D.C. Mathematical disabilities: cognitive, neuropsychological, and genetic components. Psychol. Bull. 1993;114:345–362. doi: 10.1037/0033-2909.114.2.345. [DOI] [PubMed] [Google Scholar]
- Geary D.C. American Psychological Association; Washington, DC, US: 1994. Children's Mathematical Development: Research and Practical Applications. [Google Scholar]
- Geary D.C. Mathematics and learning disabilities. J. Learn. Disabil. 2004;37:4–15. doi: 10.1177/00222194040370010201. [DOI] [PubMed] [Google Scholar]
- Geary D.C., Bow-Thomas C.C., Yao Y. Counting knowledge and skill in cognitive addition: a comparison of normal and mathematically disabled children. J. Exp. Child Psychol. 1992;54:372–391. doi: 10.1016/0022-0965(92)90026-3. [DOI] [PubMed] [Google Scholar]
- Geary D.C., Hamson C.O., Hoard M.K. Numerical and arithmetical cognition: a longitudinal study of process and concept deficits in children with learning disability. J. Exp. Child Psychol. 2000;77:236–263. doi: 10.1006/jecp.2000.2561. [DOI] [PubMed] [Google Scholar]
- Geary D.C., Hoard M.K., Byrd-Craven J., Nugent L., Numtee C. Cognitive mechanisms underlying achievement deficits in children with mathematical learning disability. Child Dev. 2007;78:1343–1359. doi: 10.1111/j.1467-8624.2007.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary D.C., Wiley J.G. Cognitive addition: strategy choice and speed-of-processing differences in young and elderly adults. Psychol. Aging. 1991;6:474–483. doi: 10.1037//0882-7974.6.3.474. [DOI] [PubMed] [Google Scholar]
- Glover G.H., Lai S. Self-navigated spiral fMRI: interleaved versus single-shot. Magn. Reson. Med. 1998;39:361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- Hanich L. Performance across different areas of mathematical cognition in children with learning difficulties. J. Educ. Psychol. 2001;93:615–626. [Google Scholar]
- Holloway I.D., Price G.R., Ansari D. Common and segregated neural pathways for the processing of symbolic and nonsymbolic numerical magnitude: an fMRI study. Neuroimage. 2010;49:1006–1017. doi: 10.1016/j.neuroimage.2009.07.071. [DOI] [PubMed] [Google Scholar]
- Kaufmann L., Vogel S.E., Starke M., Kremser C., Schocke M. Numerical and non-numerical ordinality processing in children with and without developmental dyscalculia: evidence from fMRI. Cogn. Dev. 2009;24:486–494. [Google Scholar]
- Kaufmann L., Vogel S.E., Starke M., Kremser C., Schocke M., Wood G. Developmental dyscalculia: compensatory mechanisms in left intraparietal regions in response to nonsymbolic magnitudes. Behav. Brain Funct. 2009;5:35. doi: 10.1186/1744-9081-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S.R., Menon V., Reiss A.L. Neuro-functional differences associated with arithmetic processing in Turner syndrome. Cereb. Cortex. 2006;16:849–856. doi: 10.1093/cercor/bhj028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.H., Adalsteinsson E., Glover G.H., Spielman D.M. Regularized higher-order in vivo shimming. Magn. Reson. Med. 2002;48:715–722. doi: 10.1002/mrm.10267. [DOI] [PubMed] [Google Scholar]
- Knootz K.L., Berch D.B. Mathematical Cognition. 1996. Identifying simple numerical stimuli: processing inefficiencies exhibited by arithmetic learning disabled children. p. 23. [Google Scholar]
- Kriegeskorte N., Mur M., Bandettini P. Representational similarity analysis – connecting the branches of systems neuroscience. Front. Syst. Neurosci. 2008;2:4. doi: 10.3389/neuro.06.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucian K., Grond U., Rotzer S., Henzi B., Schonmann C., Plangger F., Galli M., Martin E., von Aster M. Mental number line training in children with developmental dyscalculia. Neuroimage. 2011;57:782–795. doi: 10.1016/j.neuroimage.2011.01.070. [DOI] [PubMed] [Google Scholar]
- Kucian K., Loenneker T., Dietrich T., Dosch M., Martin E., von Aster M. Impaired neural networks for approximate calculation in dyscalculic children: a functional MRI study. Behav. Brain Funct. 2006;2:31. doi: 10.1186/1744-9081-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H., Reiss A.L., Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proc. Natl. Acad. Sci. U.S.A. 2002;99:13336–13341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landerl K., Bevan A., Butterworth B. Developmental dyscalculia and basic numerical capacities: a study of 8–9-year-old students. Cognition. 2004;93:99–125. doi: 10.1016/j.cognition.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Mazaika P., Whitfield-Gabrieli S., Reiss A., Glover G. Artifact repair for fMRI data from high motion clinical subjects. 13th Annual Meeting of the Organization for Human Brain Mapping; Chicago, IL ; 2007. [Google Scholar]
- Mazzocco M.M.M., Feigenson L., Halberda J. Impaired acuity of the approximate number system underlies mathematical learning disability (Dyscalculia) Child Dev. 2011;82:1224–1237. doi: 10.1111/j.1467-8624.2011.01608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Developmental cognitive neuroscience of arithmetic: implications for learning and education. ZDM. 2010;42:515–525. doi: 10.1007/s11858-010-0242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Mackenzie K., Rivera S.M., Reiss A.L. Prefrontal cortex involvement in processing incorrect arithmetic equations: evidence from event-related fMRI. Hum. Brain Mapp. 2002;16:119–130. doi: 10.1002/hbm.10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M.L., Salimpoor V.N., Wu S.S., Geary D.C., Menon V. Differential contribution of specific working memory components to mathematics achievement in 2nd and 3rd graders. Learn. Individual Differ. 2010;20:101–109. doi: 10.1016/j.lindif.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molko N., Cachia A., Riviere D., Mangin J.F., Bruandet M., Le Bihan D., Cohen L., Dehaene S. Functional and structural alterations of the intraparietal sulcus in a developmental dyscalculia of genetic origin. Neuron. 2003;40:847–858. doi: 10.1016/s0896-6273(03)00670-6. [DOI] [PubMed] [Google Scholar]
- Mussolin C., De Volder A., Grandin C., Schlogel X., Nassogne M.C., Noël M.-P. Neural correlates of symbolic number comparison in developmental dyscalculia. J. Cogn. Neurosci. 2010;22:860–874. doi: 10.1162/jocn.2009.21237. [DOI] [PubMed] [Google Scholar]
- Nichols T., Hayasaka S. Controlling the familywise error rate in functional neuroimaging: a comparative review. Stat. Methods Med. Res. 2003;12:419–446. doi: 10.1191/0962280203sm341ra. [DOI] [PubMed] [Google Scholar]
- Norman K.A., Polyn S.M., Detre G.J., Haxby J.V. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn. Sci. 2006;10:424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Piazza M., Facoetti A., Trussardi A.N., Berteletti I., Conte S., Lucangeli D., Dehaene S., Zorzi M. Developmental trajectory of number acuity reveals a severe impairment in developmental dyscalculia. Cognition. 2010;116:33–41. doi: 10.1016/j.cognition.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Pickering S., Gathercole S. The Psychological Corporation; London: 2001. Working Memory Test Battery for Children. [Google Scholar]
- Pinel P., Dehaene S., Riviere D., LeBihan D. Modulation of parietal activation by semantic distance in a number comparison task. Neuroimage. 2001;14:1013–1026. doi: 10.1006/nimg.2001.0913. [DOI] [PubMed] [Google Scholar]
- Prado J., Mutreja R., Zhang H., Mehta R., Desroches A.S., Minas J.E., Booth J.R. Distinct representations of subtraction and multiplication in the neural systems for numerosity and language. Hum. Brain Mapp. 2011;32:1932–1947. doi: 10.1002/hbm.21159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G.R., Holloway I., Rasanen P., Vesterinen M., Ansari D. Impaired parietal magnitude processing in developmental dyscalculia. Curr. Biol. 2007;17:R1042–R1043. doi: 10.1016/j.cub.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Rivera S.M., Menon V., White C.D., Glaser B., Reiss A.L. Functional brain activation during arithmetic processing in females with fragile X syndrome is related to FMR1 protein expression. Hum. Brain Mapp. 2002;16:206–218. doi: 10.1002/hbm.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera S.M., Reiss A.L., Eckert M.A., Menon V. Developmental changes in mental arithmetic: evidence for increased functional specialization in the left inferior parietal cortex. Cereb. Cortex. 2005;15:1779–1790. doi: 10.1093/cercor/bhi055. [DOI] [PubMed] [Google Scholar]
- Rosenberg-Lee M., Barth M., Menon V. What difference does a year of schooling make? Maturation of brain response and connectivity between 2nd and 3rd grades during arithmetic problem solving. Neuroimage. 2011;57:796–808. doi: 10.1016/j.neuroimage.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg-Lee M., Chang T.T., Young C.B., Wu S., Menon V. Functional dissociations between four basic arithmetic operations in the human posterior parietal cortex: a cytoarchitectonic mapping study. Neuropsychologia. 2011;49:2592–2608. doi: 10.1016/j.neuropsychologia.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg-Lee M., Lovett M.C., Anderson J.R. Neural correlates of arithmetic calculation strategies. Cogn. Affect. Behav. Neurosci. 2009;9:270–285. doi: 10.3758/CABN.9.3.270. [DOI] [PubMed] [Google Scholar]
- Ross J.L., Stefanatos G.A., Kushner H., Zinn A., Bondy C., Roeltgen D. Persistent cognitive deficits in adult women with Turner syndrome. Neurology. 2002;58:218–225. doi: 10.1212/wnl.58.2.218. [DOI] [PubMed] [Google Scholar]
- Rotzer S., Kucian K., Martin E., von Aster M., Klaver P., Loenneker T. Optimized voxel-based morphometry in children with developmental dyscalculia. Neuroimage. 2008;39:417–422. doi: 10.1016/j.neuroimage.2007.08.045. [DOI] [PubMed] [Google Scholar]
- Rotzer S., Loenneker T., Kucian K., Martin E., Klaver P., von Aster M. Dysfunctional neural network of spatial working memory contributes to developmental dyscalculia. Neuropsychologia. 2009;47:2859–2865. doi: 10.1016/j.neuropsychologia.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Rousselle L., Noël M.-P. Mental arithmetic in children with mathematics learning disabilities. J. Learn. Disabilities. 2008;41:498–513. doi: 10.1177/0022219408315638. [DOI] [PubMed] [Google Scholar]
- Rubinsten O., Henik A. Developmental dyscalculia: heterogeneity might not mean different mechanisms. Trends Cogn. Sci. 2009;13:92–99. doi: 10.1016/j.tics.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Rykhlevskaia E., Uddin L.Q., Kondos L., Menon V. Neuroanatomical correlates of developmental dyscalculia: combined evidence from morphometry and tractography. Front. Hum. Neurosci. 2009;3:51. doi: 10.3389/neuro.09.051.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F., Eickhoff S.B., Homke L., Mohlberg H., Hermann K., Amunts K., Zilles K. Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cereb. Cortex. 2008;18:2141–2157. doi: 10.1093/cercor/bhm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer P., Landerl K. Subitizing and counting in typical and atypical development. Dev. Sci. 2011;14:280–291. doi: 10.1111/j.1467-7687.2010.00976.x. [DOI] [PubMed] [Google Scholar]
- Shalev R., Auerbach J., Manor O., Gross-Tsur V. Developmental dyscalculia: prevalence and prognosis. Eur. Child Adolesc. Psychiatry. 2000;9:S58–S64. doi: 10.1007/s007870070009. [DOI] [PubMed] [Google Scholar]
- Shalev R., Manor O., Gross-Tsur V. Developmental dyscalculia: a prospective six-year follow-up. Dev. Med. Child Neurol. 2005;47:121–125. doi: 10.1017/s0012162205000216. [DOI] [PubMed] [Google Scholar]
- Siegler R.S. Oxford University Press; New York, NY, US: 1996. Emerging Minds: The Process of Change in Children's Thinking. [Google Scholar]
- Uddin L.Q., Supekar K., Amin H., Rykhlevskaia E., Nguyen D.A., Greicius M.D., Menon V. Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb. Cortex. 2010;20:2636–2646. doi: 10.1093/cercor/bhq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Aster M., Shalev R. Number development and developmental dyscalculia. Dev. Med. Child Neurol. 2007;49:868–873. doi: 10.1111/j.1469-8749.2007.00868.x. [DOI] [PubMed] [Google Scholar]
- Ward B.D. Medical College of Wisconsin; 2000. Simultaneous Inference for FMRI Data. AFNI 3dDeconvolve Documentation. [Google Scholar]
- Wechsler D. The Psychological Corporation; SanAntonio, TX: 1999. Wechsler Abbreviated Scale of Intelligence. [Google Scholar]
- Wechsler D. The Psychological Corporation; 2001. The Wechsler Individual Achievement Test – Second Edition (WIAT-II) [Google Scholar]
- Wilson A.J., Dehaene S. Number sense and developmental dyscalculia. In: Coch D., Dawson G., Fischer K.W., editors. Human Behavior, Learning, and the Developing Brain: Atypical Development. Guilford Press; New York, NY, US: 2007. pp. 212–238. [Google Scholar]
- Wood G., Ischebeck A., Koppelstaetter F., Gotwald T., Kaufmann L. Developmental trajectories of magnitude processing and interference control: an FMRI study. Cereb. Cortex. 2009;19:2755–2765. doi: 10.1093/cercor/bhp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.S., Chang T.T., Majid A., Caspers S., Eickhoff S.B., Menon V. Functional heterogeneity of inferior parietal cortex during mathematical cognition assessed with cytoarchitectonic probability maps. Cereb. Cortex. 2009;19:2930–2945. doi: 10.1093/cercor/bhp063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zago L., Petit L., Turbelin M.-R., Andersson F., Vigneau M., Tzourio-Mazoyer N. How verbal and spatial manipulation networks contribute to calculation: an fMRI study. Neuropsychologia. 2008;46:2403–2414. doi: 10.1016/j.neuropsychologia.2008.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.