SUMMARY
Autoregulatory domains found within kinases may provide more unique targets for chemical inhibitors than the conserved ATP-binding pocket targeted by most inhibitors. The kinase Pak1 contains an autoinhibitory domain that suppresses the catalytic activity of its kinase domain. Pak1 activators relieve this autoinhibition and initiate conformational rearrangements and autophosphorylation events leading to kinase activation. We developed a screen for allosteric inhibitors targeting Pak1 activation and identified the inhibitor IPA-3. Remarkably, pre-activated Pak1 is resistant to IPA-3. IPA-3 also inhibits activation of related Pak isoforms regulated by autoinhibition, but not more distantly related Paks, nor >200 other kinases tested. Pak1 inhibition by IPA-3 in live cells supports a critical role for Pak in PDGF-stimulated Erk activation. These studies illustrate a novel strategy for kinase inhibition and introduce a highly selective, cell-permeable chemical inhibitor of Pak.
INTRODUCTION
Protein kinases are important therapeutic targets and are considered highly druggable due to their conserved ATP binding pocket that can accommodate small molecules. However, because of the evolutionary conservation of this pocket across kinases, ATP-competitive inhibitors can inhibit large numbers of other kinases in addition to their intended targets (Bain et al., 2007; Karaman et al., 2008). Recently, it was demonstrated that ATP-competitive inhibitors such as imatinib (Gleevec) can achieve unusually high kinase selectivity by binding a less conserved region adjacent to the ATP-binding pocket (Nagar et al., 2002; Schindler et al., 2000), thus underscoring the idea that inhibitor interactions with less conserved regions of a kinase can provide opportunities for greater kinase selectivity. Indeed, many kinases contain non-conserved sequence elements outside the kinase domain that mediate important facets of their function such as localization, substrate recruitment, or the regulation of catalytic activity. Several kinases contain autoinhibitory domains that bind and inhibit the activity of the catalytic domain (Cheetham, 2004). We, and others, have proposed that proteins regulated by autoinhibition may be susceptible to inhibition by small molecules that perturb the conformational changes that accompany relief of autoinhibition (Cheetham, 2004; Liu and Gray, 2006; Peterson et al., 2004; Peterson and Golemis, 2004). The additional domains and conformational changes that mediate kinase autoregulation may, therefore, provide novel opportunities for more specific small-molecule inhibition than ATP-competitive compounds.
Members of the p21-activated kinases (Paks) are one such family that is subject to autoregulation. Group I Paks (Paks 1–3) are regulated by autoinhibition that is relieved by binding to the 21 kDa GTP-binding proteins Rac and Cdc42. This distinct regulatory mechanism is not observed, however, in the more distantly related Group II Paks (Paks 4–6). However, Pak5 may undergo autoinhibition mediated by an unrelated domain (Ching et al., 2003). Autoinhibition of Pak1 is mediated by the formation of an inactive homodimer in which the autoregulatory region of one monomer binds and inhibits the catalytic domain of its partner and visa versa (Lei et al., 2000; Parrini et al., 2002). One critical element of the autoregulatory region is the kinase-inhibitory segment, which binds in the active site cleft and sequesters the kinase activation loop in an inactive conformation (Lei et al., 2000). Pak1 activation involves the local unfolding of the autoinhibitory domain caused by binding of Rac/Cdc42 to a partially overlapping region, resulting in Pak1 monomer dissociation and displacement of the inhibitory segment. Subsequent autophosphorylation events at multiple sites along Pak1 stabilize the catalytically competent, monomeric conformation (Chong et al., 2001; Lei et al., 2000; Parrini et al., 2002). This multi-step activation cascade may offer additional opportunities for small-molecule binding that could selectively inhibit Group I Paks.
Increasing data implicates Pak1 in tumorigenesis and metastasis (reviewed in (Kumar et al., 2006)). Thus, inhibitors of Pak1 have been suggested as a novel oncologic therapy (Kumar et al., 2006; Nheu et al., 2002). Though no highly selective inhibitors of Pak1 have been reported, several compounds originally identified for their ability to target other kinases also inhibit Pak family members (Eswaran et al., 2007; Nheu et al., 2002; Porchia et al., 2007). Here we report the identification and characterization of a highly selective, non-ATP competitive inhibitor that targets the autoregulatory mechanism of Group I Paks. This work illustrates how conformational rearrangements accompanying kinase activation can be exploited by compounds to achieve greater target specificity, and introduces a selective reagent for Pak inhibition.
RESULTS
A chemical screen identifies IPA-3 as an inhibitor of Pak1
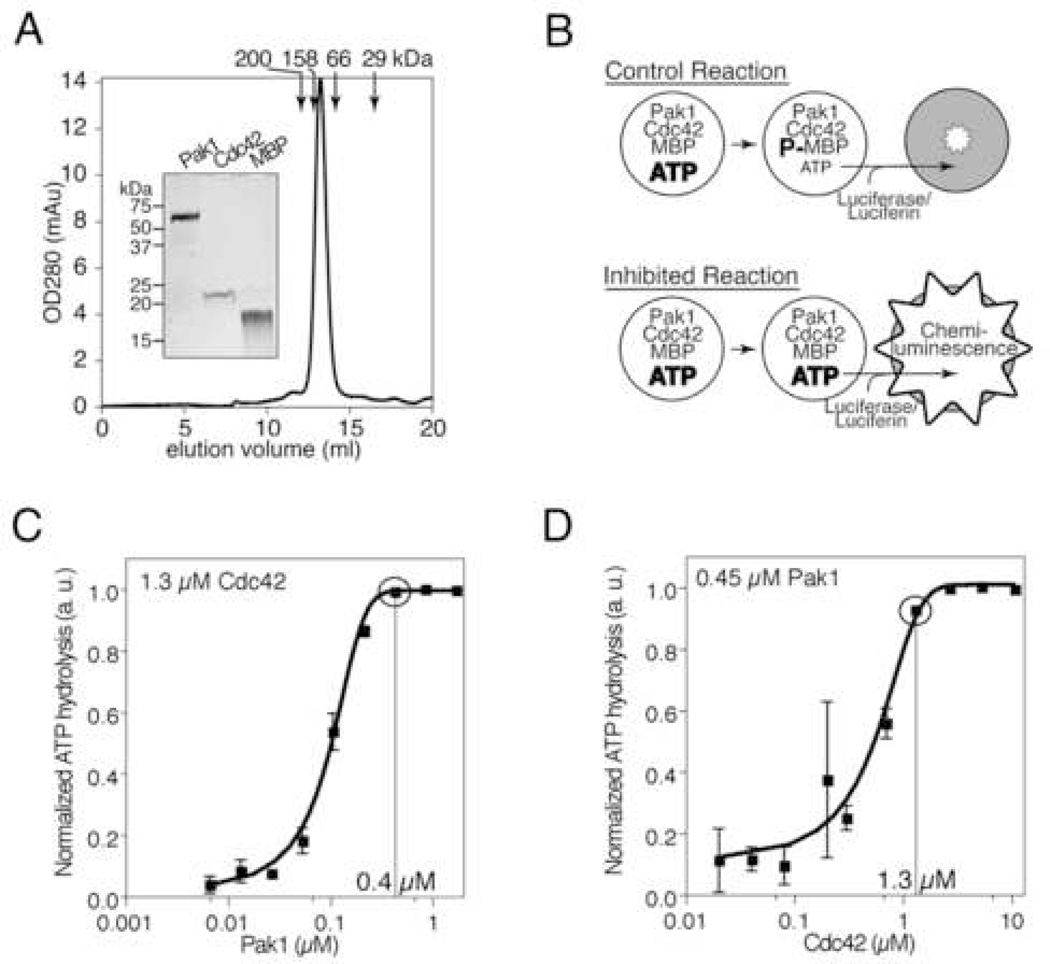
To identify inhibitors of Pak1 activation, we developed a high-throughput assay measuring ATP hydrolysis as an indicator of Pak1 catalytic activity. Recombinant, fulllength Pak1 exhibited an apparent molecular weight of ~130 kDa by gel filtration chromatography (Figure 1A). SDS-PAGE analysis demonstrated the appropriate monomer molecular weight of ~ 60 kDa (Figure 1A, inset) as expected for the inactive Pak1 homodimer (Lei et al., 2000). Pak1 was incubated with individual compounds followed by addition of recombinant Cdc42-GTPγS (hereafter simply “Cdc42”) and myelin basic protein (MBP) as substrate (Figure 1A, inset) in the presence of 10 µM ATP (Figure 1B). Following incubation, nonhydrolyzed ATP was quantified using Kinase-Glo (Koresawa and Okabe, 2004). Titrations of both Pak1 (Figure 1C) and Cdc42 (Figure 1D) demonstrated that ATP hydrolysis was strictly dependent on both Pak1 and Cdc42. These results demonstrate that ATP hydrolysis measured in this assay is due to Pak1 kinase activity and confirm that the recombinant Pak1 utilized in the screen is autoinhibited yet activatable by Cdc42 as expected.
Figure 1. An ATP depletion assay reports Cdc42-dependent Pak1 activation.
(A) Recombinant Pak1 is a homodimer. The gel filtration elution profile of Pak1 is shown. Elution volumes of standards are indicated. Inset: Coomassie-stained SDS-PAGE analysis of Pak1 and other proteins used in the screen. (B) Pak1 assay scheme. Pak1 is incubated with its activator, GTPγS-charged Cdc42, and a substrate, myelin basic protein (MBP), in the presence of 10 µM ATP. Control kinase reactions (top) hydrolyze a substantial fraction of the starting ATP resulting in low final ATP concentrations, whereas inhibited reactions (bottom) do not. Residual ATP is enzymatically converted to chemi-luminescence proportional to residual ATP. (C) ATP hydrolysis is strictly Pak1 dependent. Cdc42 was incubated with MBP, ATP, and the indicated concentrations of Pak1. Residual ATP levels were measured as in (B). Results are expressed as ATP hydrolyzed in arbitrary units (normalized to the maximum ATP hydrolyzed) as a function of Pak1 concentration. Data points and error bars show mean and SEM of triplicate wells. Circled point indicates Pak1 concentration used in the screen. (D) ATP hydrolysis by Pak1 is Cdc42 dependent. ATP depletion was monitored in reactions as in (C) except that the indicated concentrations of Cdc42 were used. Circled point indicates Cdc42 concentration used in the screen.
This assay was used to screen 33,000 structurally diverse small molecules in duplicate. Compounds inhibiting ATP hydrolysis by greater than three standard deviations below the mean of control reactions in both replicates were considered for further analysis. Approximately 1% of the compounds tested met this criterion. A secondary screen was then conducted on active compounds to identify those that were potentially non-ATP competitive. Individual compounds (10 µM) were incubated with Pak1, Cdc42 and MBP in the presence of [32P]-γ-ATP and were assayed for their ability to inhibit incorporation of 32P-phosphate into MBP. To reduce the detection of undesired ATP-competitive inhibitors, reactions were conducted in the presence of 1 mM of unlabeled ATP. Of the 342 compounds identified in the primary screen, 32 compounds continued to exhibit robust inhibition at 1 mM ATP. These compounds were ranked according to their relative potency and reproducibility of inhibition in subsequent assays, as well as their commercial availability. The cumulative results of these secondary assays led us to focus on one particular compound, 2,2’-dihydroxy-1,1’-dinapthyldisulfide (Figure 2A; hereafter called “IPA-3”), that at 10 µM inhibited Pak1 activity by 95% ± 3%.
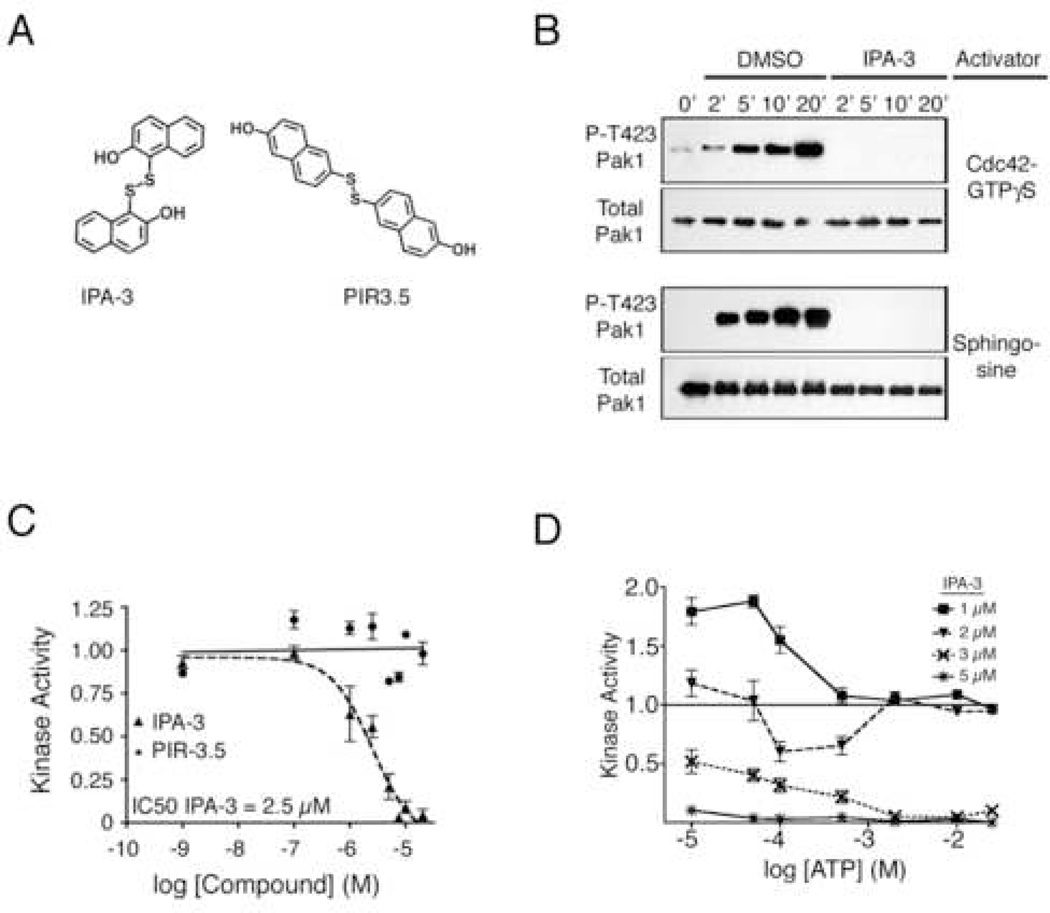
Figure 2. IPA-3 is a direct, non-ATP-competitive inhibitor of Pak1.
(A) Structure of IPA-3 and an inactive relative, PIR-3.5. (B) IPA-3 directly inhibits Pak1 autophosphorylation. Pak1 was incubated with ATP and either Cdc42 (top panels) or sphingosine (bottom panels) in the presence of DMSO or 10 µM IPA-3. Samples were probed using phospho-specific antibodies against Pak1 Thr423. Data is representative of three experiments. (C) IPA-3 inhibits Pak1 kinase activity with low micromolar potency. Pak1 was pre-incubated with the indicated concentrations of IPA-3 or PIR-3.5. Kinase reactions were started by addition of Cdc42, MBP, and a mixture of 1 mM ATP and [32P]-γ-ATP. Kinase activity is reported as phosphate incorporation onto MBP expressed as a ratio to MBP phosphorylated in reactions in the presence of solvent alone (1% DMSO). Data are represented as the mean ± SEM. n ≥ 3. (D) IPA-3 is non-competitive with ATP. Kinase assays were performed as in (C) at the indicated concentrations of ATP and IPA-3. n = 3.
IPA-3 is a direct, non-competitive inhibitor of Pak1
To determine the protein target of IPA-3, we performed Pak1 kinase assays in which MBP was omitted. Pak1 kinase activity was measured by monitoring Pak1 autophosphorylation using phospho-specific antibodies against threonine 423 within the activation loop (Thr423; (Zenke et al., 1999)). 10 µM IPA-3 prevented Cdc42-stimulated Pak1 autophosphorylation on Thr423 (Figure 2B, top), demonstrating that IPA-3 must target either Cdc42 or Pak1. We next substituted Cdc42 with a distinct Pak1 activator, the sphingolipid sphingosine (Bokoch et al., 1998). IPA-3 also prevented sphingosine-dependent Pak1 autophosphorylation (Figure 2B, bottom panel). Together, these assays demonstrate that Pak1 is the protein target of IPA-3. Furthermore, the inhibition of Thr423 phosphorylation, which is required for full activation of Pak1 (Zenke et al., 1999), indicates that IPA-3 may inhibit a step in the Pak1 activation process.
To confirm the chemical identity of the active compound, we developed a novel chemical synthesis of IPA-3 (see Figure S1 in the Supplemental Data). The inhibitory activity of re-synthesized IPA-3 was confirmed using in vitro kinase assays (data not shown), demonstrating that 2,2’-dihydroxy-1, 1’-dinapthyldisulfide was indeed the agent responsible for inhibition of Pak1 activity.
To determine the potency of IPA-3, kinase assays were performed at a range of compound concentrations, yielding an IC50 for IPA-3 of 2.5 µM (Figure 2C). A number of structurally related compounds were also tested (Figure S2), yet none inhibited Pak1 kinase activity as potently as IPA-3. A structural isomer of IPA-3, 2-naphthalenol, 6,6’-dithiobis (Figure 2A, right), termed PIR-3.5 (Pak1 inhibitor relative 3.5), displayed no inhibitory activity towards Pak1 (Figure 2C), and was therefore chosen as a negative control compound for subsequent analyses.
The ability of IPA-3 to inhibit Pak1 activity at 1 mM ATP (Figure 2C) suggested that this compound might be non-competitive with ATP. Indeed, when tested at a range of ATP concentrations, the inhibitory activity of IPA-3 was not decreased by increasing concentrations of ATP (Figure 2D). These results clearly demonstrate that Pak1 inhibition by IPA-3 is not competitive with ATP.
IPA-3 does not target exposed cysteine residues on Pak1
Cysteine-modifying agents that form mixed disulfides with protein targets have been shown to perturb the biological activity of proteins (Rice et al., 1995). Therefore, initial experiments to determine the mechanism of Pak1 inhibition by IPA-3 focused on the role of the disulfide bond in IPA-3 (Figure 2A). We found that IPA-3 inhibitory activity was dramatically reduced in the presence of >1 mM of the reducing agent dithiothreitol (DTT) (Figure 3A). We also found that addition of DTT could relieve inhibition of Pak1 when added after IPA-3 (Figure 3B). Thus, IPA-3 inhibition of Pak1 could be both prevented and reversed by 1 mM DTT.
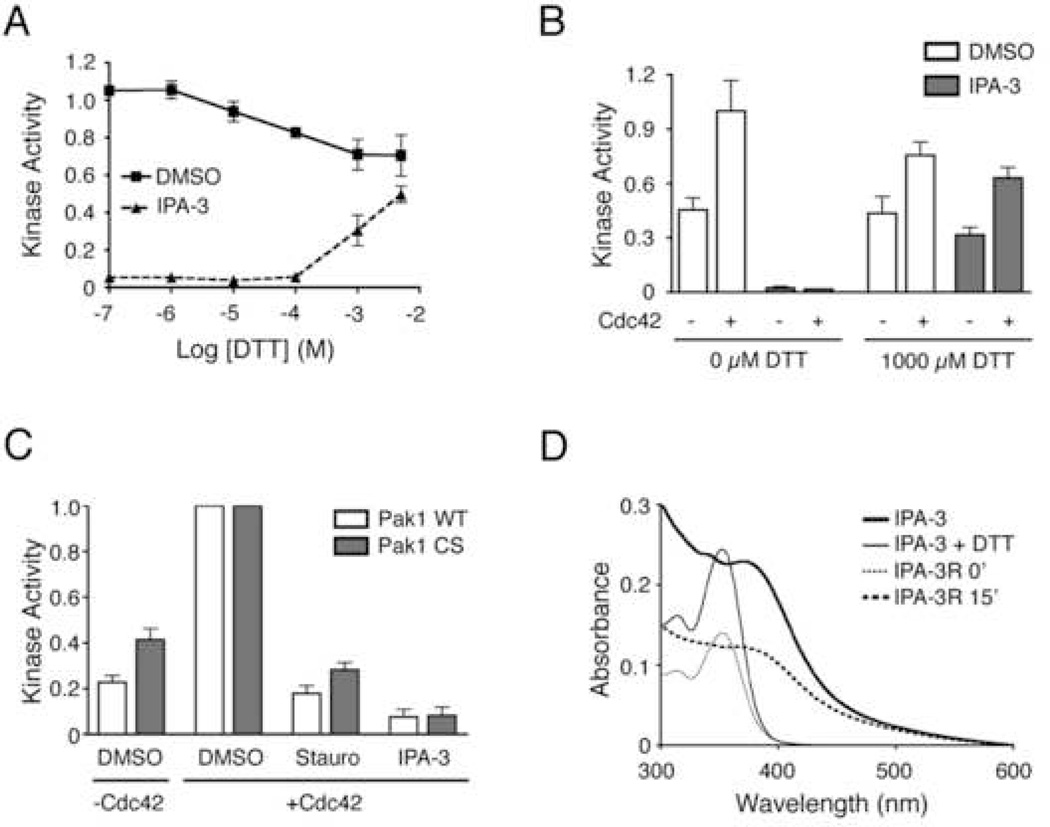
Figure 3. The role of disulfides in IPA-3-mediated Pak1 inhibition.
(A) IPA-3 activity is diminished in the presence of the reducing agent DTT. 10 µM IPA-3 was incubated with the indicated concentrations of DTT, followed by the addition of Pak1. Kinase activity was determined as in Figure 2. n = 6 (B) DTT relieves IPA-3-dependent inhibition of Pak1 and restores Cdc42-mediated activation. Pak1 was incubated with IPA-3 and divided into vessels containing 0 or 1000 µM DTT, followed by addition of Cdc42 where indicated. Samples were subjected to kinase assays as in Figure 2. Data are represented as the mean ± SEM. n = 3. (C) Mutation of solvent accessible cysteine residues in Pak1 to serine does not prevent inhibition by IPA-3. Pak1-CS (Cys360 mutated to serine, Cys411 mutated to serine) and wild-type Pak1 were pre-incubated with either DMSO or the indicated compound. Kinase activity was determined as in Figure 2, and was normalized to the maximum activity in the presence of solvent alone (1% DMSO) and Cdc42. n = 5. (D) Absorption spectroscopy suggests that the disulfide bond in IPA-3 is sensitive to reduction by DTT. Absorption spectra were recorded for 20 µM IPA-3 in the presence of 0 (thick trace) or 1000 µM (thin trace) DTT. A freshly prepared solution (20 µM) of the reduced form of IPA-3, IPA-3R (1-mercapto-2-hydroxynapthalene), was also analyzed either immediately (thin dotted trace) or following a 15-minute incubation on the benchtop (thick dotted trace). Data is representative of multiple experiments.
The sensitivity of IPA-3 inhibition to DTT could be due to reduction of IPA-3 itself or the reduction of an IPA-3-Pak1 adduct. We first considered the possibility that IPA-3 formed mixed disulfides with Pak1. Pak1 contains five cysteine residues, all of which are found in the kinase domain. Computational analysis of crystal structures of Pak1 kinase domain in both active (Lei et al., 2005) and autoinhibited conformations (Lei et al., 2000) using UCSF Chimera demonstrated that only two of those residues, Cys360 and Cys411, are surface exposed. To determine if inhibition by IPA-3 could be due to the formation of mixed disulfides with these cysteines, we mutated both residues to serine. Like wild-type Pak1, the mutated Pak1 (Pak1-CS), exhibited a basal level of kinase activity that was stimulated by addition of Cdc42 (Figure 3C). Importantly, Pak1-CS was similarly inhibited by 10 µM IPA-3 compared to wild-type Pak1 (Figure 3C), demonstrating that the mechanism of IPA-3 inhibition is not through the formation of mixed disulfides with Cys360 or Cys411. In addition, Pak1 migration in non-reducing SDS-PAGE and size exclusion chromatography was not affected by IPA-3, ruling out IPA-3-mediated formation of Pak1 multimers or aggregates (not shown).
To test whether IPA-3 itself was sensitive to DTT, we analyzed a solution of IPA-3 by absorption spectroscopy in the presence of a range of DTT concentrations. We observed a dramatic change in the absorption spectrum of IPA-3 at ≥1 mM DTT (Figure 3D). Interestingly, chemically synthesized 1-mercapto-2-hydroxynapthalene (IPA-3R), the expected product of the reduction of the disulfide in IPA-3, exhibited an absorption spectrum indistinguishable from that of IPA-3 in the presence of 1 mM DTT (Figure 3D). Furthermore, IPA-3R rapidly re-oxidized into IPA-3 under our buffer conditions (Figure 3D). Thus, the sensitivity of IPA-3 inhibition to DTT is likely due to direct reduction of the IPA-3 disulfide, although we cannot formally rule out the possibility that IPA-3 forms a DTT-sensitive adduct with residues other than surface-exposed cysteines.
IPA-3 targets the Pak1 activation mechanism
Pak1 activation is a multi-step process involving the relief of autoinhibitory interactions and autophosphorylation at key residues (Buchwald et al., 2001; Chong et al., 2001; Zenke et al., 1999). IPA-3 inhibition of Thr423 autophosphorylation suggests that IPA-3 may inhibit steps in the Pak1 activation mechanism. To determine the point in Pak1 activation at which IPA-3 may be acting, a series of kinase assays were performed in which the order of addition of reaction components were systematically varied. Consistent with the results of the initial screen, when IPA-3 was added prior to Cdc42 and ATP, it significantly inhibited Pak1 kinase activity (Figure 4A, condition A). When added to Pak1 after incubation with Cdc42, but prior to the addition of ATP, IPA-3 similarly inhibited Pak1 (Figure 4A, condition B). Finally, we tested the ability of IPA-3 to inhibit Pak1 when added after both Cdc42 and ATP, when Pak1 is pre-activated (Figure 2B, 20’). Strikingly, IPA-3 was much less effective at inhibiting this preactivated Pak1 (Figure 4A; IPA-3, condition C). In contrast, the ATP-competitive inhibitor staurosporine inhibited Pak1 kinase activity equally regardless of when it was added (Figure 4A; Stauro). The requirement that IPA-3 be present prior to Pak1 activation to be effective was also observed for Pak1 activated by sphingosine (data not shown). These results demonstrate that IPA-3 is ineffective at inhibiting pre-activated Pak1. Moreover, the ability of IPA-3 to dramatically inhibit Pak1 only when added prior to Pak1 autophosphorylation suggests that IPA-3 inhibits a step in the Pak1 activation process.
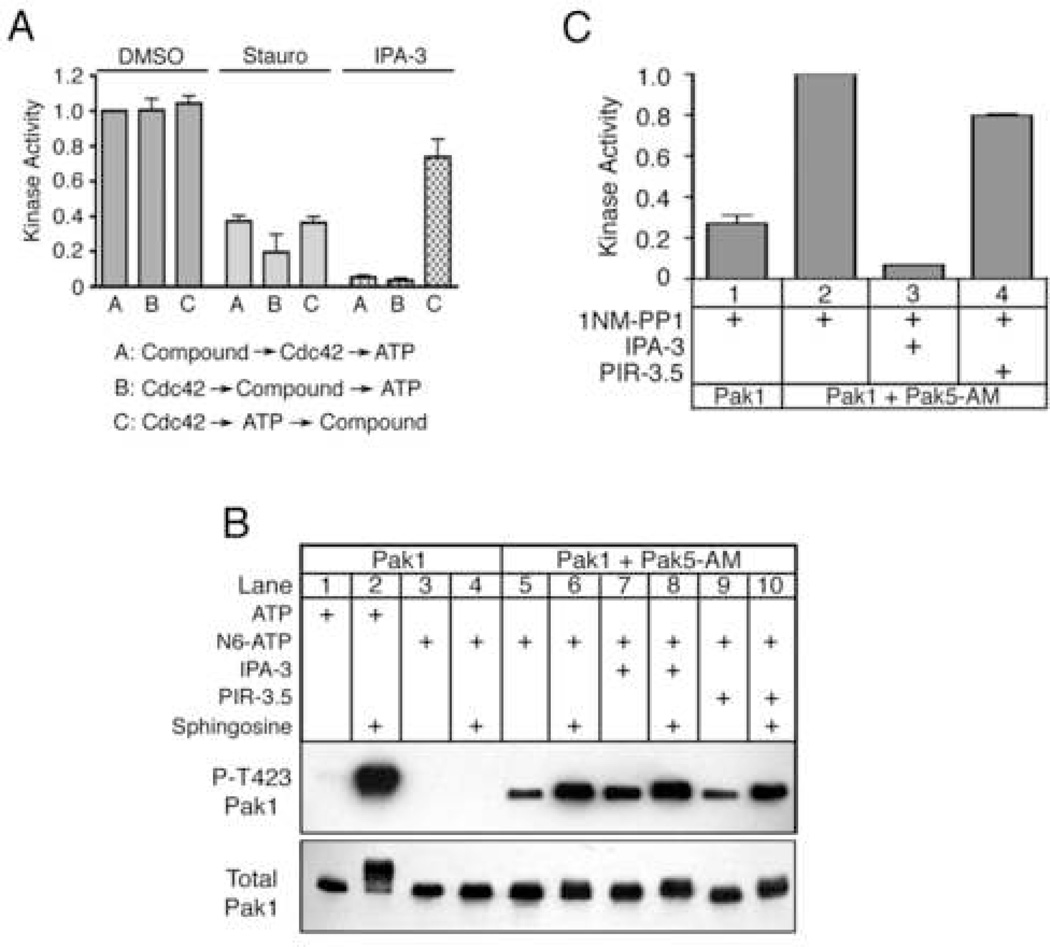
Figure 4. IPA-3 targets the Pak1 autoregulatory mechanism.
(A) IPA-3 inhibits Pak1 activation but does not inhibit pre-activated Pak1. Pak1 was incubated with components in the following order; condition A: 10 µM compound, Cdc42 and ATP. Condition B: Cdc42, compound, ATP. Condition C: Cdc42 and ATP, compound. After all incubations, kinase reactions were initiated by addition of MBP and [32P]-γ-ATP. Kinase activity is reported as in Figure 2 and was normalized to reactions in the presence of 1% DMSO and Cdc42 under condition A. n ≥ 4. (B) IPA-3 promotes the accessibility of the Thr423 to phosphorylation by exogenous kinases. Pak1 was incubated with the indicated compound followed by addition of the ATP-binding pocket mutant Pak5 (Pak5-AM), sphingosine, and ATP or N6-benzyl-ATP (N6-ATP). Reactions were analyzed using phospho-specific antibodies against Pak1 Thr423 or total Pak1. Data is representative of three experiments. (C) Exogenous phosphorylation of Thr423 does not restore the kinase activity of IPA-3-inhibited Pak1. Pak1 was incubated as in (B) in the presence of 125 µM sphingosine and N6-ATP. Post-incubation, 2 µM 1NM-PP1 was added to inhibit Pak5-AM, and kinase assays were conducted. Kinase activity is reported as in Figure 2 and normalized to the reaction in lane 2. Data are represented as the mean ± SEM. n = 4.
The conformation of inhibitor-bound Pak1
We hypothesized that IPA-3 might inhibit Pak1 activation by stabilizing the autoinhibited Pak1 conformation. Indeed, conformational stabilization of the autoinhibitory domain of N-WASP, which is structurally related to the autoinhibitory domain of Pak1 (Kim et al., 2000; Lei et al., 2000), has been reported for a small-molecule N-WASP inhibitor (Peterson et al., 2004). In addition, the symmetrical dimeric structure of IPA-3 suggests that this compound might contact both Pak1 monomers within the autoinhibited dimer. One important feature of the Pak1 autoinhibited conformation is the folding of the activation loop (residues 408–428) into the catalytic site of the kinase (Lei et al., 2000), thereby masking the activation loop and rendering Thr423 inaccessible to phosphorylation. On relief of autoinhibition, a large conformational change in the activation loop exposes Thr423 to phosphorylation by other Pak1 monomers as well as other kinases (King et al., 2000; Parrini et al., 2002; Zenke et al., 1999). Thus, accessibility of Thr423 to phosphorylation can be used to reveal whether the activation loop is in the “open” (active) or “closed” (inactive) conformation. We therefore tested the ability of exogenous kinases to phosphorylate Thr423 in the presence of IPA-3. We have found that Pak5 can phosphorylate Thr423 of Pak1 in vitro but is itself insensitive to IPA-3 (see below). For this reason, Pak5 was chosen as the exogenous kinase for this assay.
To ensure that at all Thr423 phosphorylation was mediated by Pak5, we mutated the gatekeeper residue of Pak5 (methionine 523) to glycine according to the strategy pioneered by Shokat and colleagues (Liu et al., 1998). This Pak5 ATP-binding pocket mutant (Pak5-AM) can utilize the bulky ATP derivative N6-benzyl-ATP (N6-ATP) whereas wild-type Pak1 cannot (Figure 4B; compare lanes 3 and 5). In vitro kinase assays using N6-ATP revealed that Pak5-AM phosphorylates Pak1 on Thr423, and that these phosphorylation events were enhanced in the presence of sphingosine (Figure 4B, lanes 5 and 6). Importantly, IPA-3 did not prevent Pak1 phosphorylation by Pak5-AM and, indeed, promoted Thr423 phosphorylation in the absence of sphingosine (Figure 4B, compare lanes 5 and 7). Thus, IPA-3 does not stabilize the native autoinhibited conformation of Pak1 but instead promotes a conformation of Pak1 in which Thr423 is exposed. Similar results were obtained using 3-phosphoinositide-dependent kinase-1 (PDK1), another Thr423-directed kinase (King et al., 2000) (Figure S3A).
That Thr423 can be phosphorylated by exogenous kinases but not by Pak1 itself in the presence of IPA-3 suggested that phosphorylation of this residue by exogenous kinases might overcome Pak1 inhibition by IPA-3. To test this, we took advantage of the observation that the gatekeeper residue mutation in Pak5-AM renders Pak5-AM sensitive to inhibition by the ATP-competitive inhibitor 1NM-PP1, whereas wild-type Pak1 kinase activity was unaffected (Figure S3B). We first incubated Pak1 with Pak5-AM and N6-ATP in the presence of sphingosine and either DMSO or IPA-3. Next we added 1NM-PP1, MBP, and a mixture of ATP and [32P]-γ-ATP to specifically measure Pak1 catalytic activity. Pre-phosphorylation of Pak1 with Pak5-AM and N6-ATP led to a significant increase in Pak1 catalytic activity (Figure 4C, lanes 1 vs. 2). However, Pak5-AM phosphorylation of Pak1 did not overcome inhibition by IPA-3 (Figure 4C, lane 3). Inhibition of MBP phosphorylation by IPA-3 is due to direct inhibition of Pak1, as the Pak5-AM phosphorylation of Pak1 was not affected by IPA-3 (Figure 4B, lanes 6 and 8). Together, these results demonstrate that in the presence of IPA-3, Pak1 adopts a conformation in which Thr423 is exposed and therefore must be distinct from the autoinhibited conformation. Nevertheless, this conformation is catalytically inactive and cannot be reactivated by phosphorylation by exogenous kinases. In addition, these results demonstrate that a Group II Pak (Pak5) can phosphorylate the activation loop of a Group I Pak (Pak1) in vitro, suggesting the possibility of regulatory crosstalk between the two Pak groups.
IPA-3 targets the Group I Paks
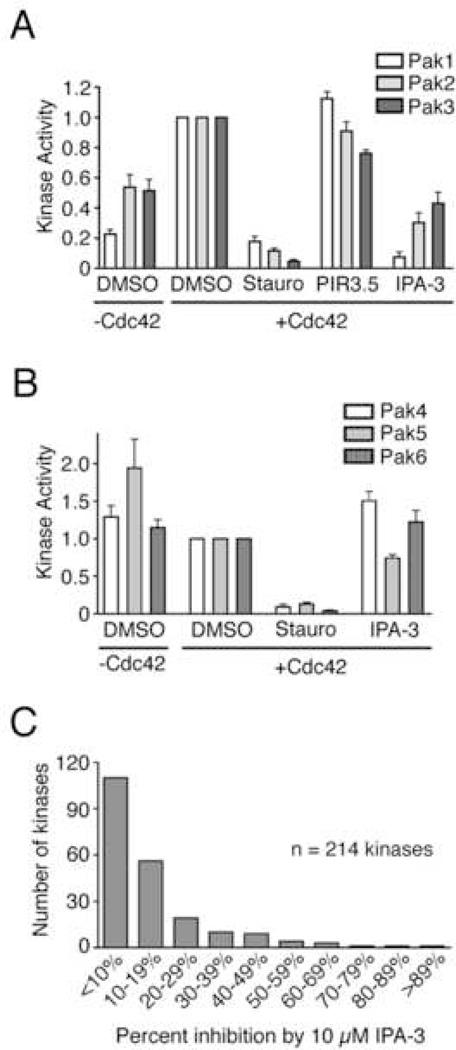
The Pak1 autoregulatory strategy is conserved in all Group I Paks (Paks 1–3). By contrast, the Group II Paks (Paks 4–6) do not contain the inhibitory domain found in Group I Paks, and are not thought to undergo autoinhibition by the same mechanism. We therefore tested IPA-3 on all members of the Pak family. As expected, full-length Paks 1–3 displayed basal levels of kinase activity that were stimulated by the addition of Cdc42 (Figure 5A). Dramatic inhibition of kinase activity in the presence 10 µM IPA-3 but not PIR-3.5 was observed for all three Group I Paks, with the strongest inhibition observed for Pak1 (Figure 5A).
Figure 5. IPA-3 kinase selectivity.
(A) IPA-3 inhibits all Group I Paks. Paks were incubated with 10 µM of the indicated compound followed by addition of buffer or Cdc42, MBP, and a mixture of 1 mM ATP and [32P]-γ-ATP. Kinase activity is reported as in Figure 2. n ≥ 3. (B) IPA-3 is not an effective inhibitor of the Group II Paks. Purified Pak4 kinase domain or full-length Pak5 and Pak6 were tested in kinase reactions as described in (A). n ≥ 4. (C) Distribution of IPA-3 inhibitory activity against recombinant kinases. 214 full-length human kinases were treated with 10 µM IPA-3 and subjected to kinase assays. The histogram represents the number of kinases that displayed the indicated mean percent inhibition compared to control reactions without inhibitor in two independent experiments. Data are represented as the mean ± SEM.
We next tested the ability of IPA-3 to inhibit full-length Pak5 and Pak6, and a catalytic fragment of Pak4. Consistent with reports demonstrating that these kinases do not undergo autoinhibition or Rac / Cdc42-mediated activation (Abo et al., 1998; Cau et al., 2001; Lee et al., 2002), the basal kinase activity of the Group II Paks was not stimulated by Cdc42 (Figure 5B). Importantly, the kinase activity of Paks 4–6 was not dramatically inhibited by 10 µM IPA-3 (Figure 5B, IPA-3). Taken together, these results demonstrate that IPA-3 exhibits selectivity for the Group I versus Group II Paks.
Kinase specificity of IPA-3
To determine the specificity of IPA-3 across a larger fraction of the kinome, we tested the ability of this compound to inhibit 214 full-length human kinases using an assay that monitors phosphorylation of a peptide substrate (Invitrogen Z’-Lyte™). 10 µM IPA-3 significantly inhibited (≥50% inhibition) only 9 of the kinases tested (4% of total) (Figure 5C and Table S1). By comparison, the broad-spectrum kinase inhibitor staurosporine, the highly selective Bcr-Abl inhibitor imatinib, and the receptor tyrosine kinase inhibitor gefitinib inhibited (by ≥ 50%) 93%, 12%, and 21% of the kinases tested, respectively (data not shown). Although Pak2 and Pak3 were represented in the collection of kinases tested, they were not significantly inhibited by IPA-3. This can be explained by the fact that the recombinant Paks used in the Z’-Lyte assay are preactivated and no longer responsive to Cdc42 (Figure S4). It should be also be noted that 34 of 214 kinases were tested in the presence of 1 mM DTT (see Table S1), conditions sufficient to reduce the IPA-3 disulfide. Nevertheless, this data demonstrates the remarkable kinase specificity of IPA-3, and further supports the notion that this inhibitor acts on the unique regulatory cycle of Group I Paks.
IPA-3 inhibits Pak1-mediated signaling in vivo
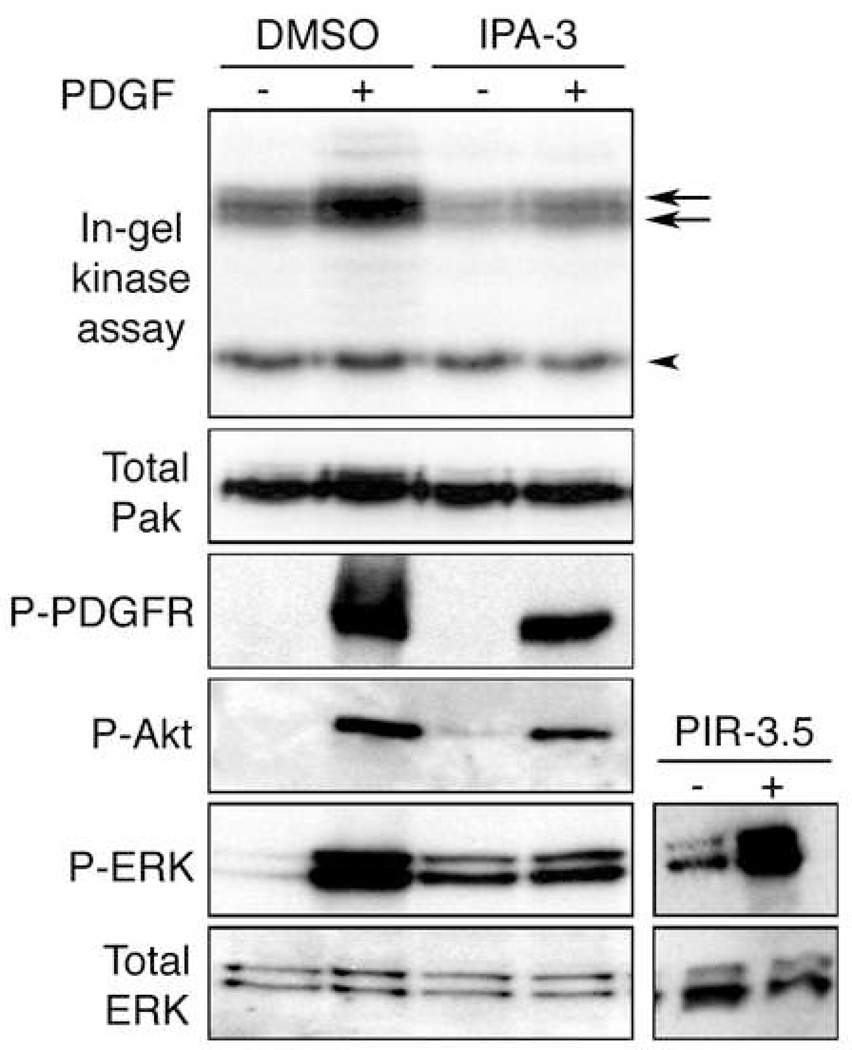
Platelet-derived growth factor (PDGF) promotes Pak activation in fibroblasts (Beeser et al., 2005). We therefore tested the ability of IPA-3 to inhibit PDGF-stimulated Pak activation in mouse embryonic fibroblasts. Both basal and PDGF-stimulated Pak activities were impeded by 30 µM IPA-3 as assessed by in-gel kinase assay (Figure 6, upper panel, arrows). In contrast, the activity of an unidentified ~40 kDa kinase was unaffected (upper panel, arrowhead). Western blotting of the same lysates with Group I-specific Pak antibodies showed that the increase in Pak apparent molecular weight in control PDGF-stimulated cells, likely due to Pak autophosphorylation, was also inhibited by IPA-3 (Figure 6, “Total Pak” panel). The inhibition of Pak’s apparent molecular weight increase by IPA-3 in cells was also observed in vitro (Figure 2B, bottom panel), supporting a common inhibitory mechanism. These data show that IPA-3 can inhibit activation of Group I Paks in cells. The need for higher concentrations of IPA-3 for Pak inhibition in cells compared to in vitro is likely due to reduction of the IPA-3 disulfide bond in the reducing cytoplasmic environment. This would be expected to be counteracted by the large, exchangeable reservoir of IPA-3 present in the non-reducing cell culture medium, thus maintaining a sufficient intracellular concentration of active compound.
Figure 6. IPA-3 selectively inhibits Pak1 activation in mammalian cells.
Mouse embryonic fibroblasts were serum starved for 18 hours, followed by treatment with 30 µM IPA-3, PIR-3.5 (right panels) or DMSO for 10 min. Cells were stimulated with 10 µg/ml PDGF-BB for 5 min and lysed. Cell lysates were normalized by protein concentration and probed with antibodies as described in Materials and Methods. Endogenous Pak kinase activity was observed by in-gel kinase assay.
To assess the affect of IPA-3 on signaling components upstream of Pak, the same cell lysates were analyzed using phospho-specific antibodies against activated forms of the PDGF receptor and Akt. IPA-3 had little effect on these signaling events upstream of Pak. By contrast, Erk activation by PDGF, which we and others have shown is dependent on Pak (Beeser et al., 2005; Tang et al., 1997), was strongly inhibited by IPA-3 (Figure 6). Interestingly, IPA-3 caused a slight elevation in basal Erk activity even in the absence of PDGF, although this effect was cell-type dependent (data not shown). As expected, the control compound PIR-3.5 did not inhibit Erk activation (Figure 6, right panels). Thus, IPA-3 specifically and selectively inhibits signaling events mediated by Group I Paks.
DISCUSSION
The work presented here describes the identification of a highly selective inhibitor of Group I Pak kinases that achieves this selectivity by targeting the distinct autoregulatory mechanism conserved in Group I Paks. That IPA-3 targets this regulatory mechanism and not the kinase active site directly is supported by the following findings. First, the inhibition of Pak1 is non-competitive with respect to ATP. Second, IPA-3 targets the Group I but not Group II Pak kinases, which share a highly conserved C-terminal kinase domain but differ in their N-terminal regulatory sequence elements. Thirdly, IPA-3 inhibits activation of Pak1 by diverse activators, but does not inhibit pre-activated Pak1. Finally, IPA-3 does not prevent rearrangements of the activation loop that result in phosphorylation by exogenous kinases. One interpretation of these findings is that IPA-3 prevents Pak1 activation by stabilizing an activation intermediate that is semi-open yet catalytically inactive. A precise understanding of the molecular basis of Pak1 inhibition by IPA-3, however, awaits detailed structural studies.
A number of endogenous protein regulators of Pak1 kinase activity have been reported that appear to target the Pak1 autoregulatory strategy. hPIP1 (Xia et al., 2001), CRIPak (Talukder et al., 2006), and the tumor suppressor merlin (Kissil et al., 2003) have all been shown independently to bind the Pak1 autoregulatory region and inhibit kinase activity. CIB1 also binds this region but, like Rac and Cdc42, promotes Pak1 activity rather than inhibiting it (Leisner et al., 2005). These observations suggest that modulation of the Pak1 autoregulatory domain may be a physiologically relevant strategy for regulating catalytic activity. Therefore, it is conceivable that IPA-3 may bind and/or inhibit Pak1 in a manner that mimics endogenous protein inhibitors.
As expected for an allosteric kinase inhibitor, kinase-profiling experiments revealed a high degree of kinase selectivity to IPA-3 inhibition (Table S1). The lack of kinase domain sequence similarity or functional overlap in IPA-3 kinase targets is not unexpected for a compound targeting a non-conserved regulatory mechanism. Indeed, specificity profiling of clinical kinase inhibitors has shown that many selective compounds also target seemingly unrelated kinases (Karaman et al., 2008). These observations emphasize the importance of broad kinase profiling experiments in the characterization of kinase inhibitor selectivity. The profiling studies of IPA-3 reported here (Table S1) describe the first chemical inhibitor of Pak1 that achieves documented specificity comparable to that of the clinically relevant kinase inhibitors imatinib and gefitinib.
Although IPA-3 represents a novel experimental reagent to inhibit Pak kinase activity, other groups have reported the use of small molecules derived from known ATP-competitive kinase inhibitors to target Pak1 (Nheu et al., 2002; Porchia et al., 2007). However, these inhibitors target a number of other kinases, and their broader kinase selectivity has not been reported. An alternative approach to inhibiting Pak kinase activity has been the expression of recombinant fragments such as the Pak inhibitory domain (PID) or catalytically inactive mutants of Pak1 that inhibit the activity of endogenous Pak protein, although these may cause unintended Pak-independent side effects (Thullberg et al., 2007). By contrast, we show here that IPA-3 is Pak isoform selective, cell permeable, and rapidly acting. As such, this compound can serve as a distinct tool to elucidate Pak function in cells and may facilitate the validation of Pak inhibitors as a therapeutic strategy.
The screening strategy used to identify IPA-3 relied on the use of full-length Pak1 prepared under conditions that preserve the native autoregulatory mechanism. This is in contrast to the dominant paradigm in kinase-targeted screens of utilizing constitutively active kinase or isolated kinase domains (Sebolt-Leopold and English, 2006). These screens are biased toward inhibitors targeting the active site and are restricted in their ability to exploit sequence differences outside the catalytic domain. The results presented here support the possibility that screens using full-length kinase may provide unexpected opportunities for allosteric inhibition. This strategy need not be restricted to kinases, however, and could be utilized for other enzymes or non-catalytic proteins that undergo conformational regulation (Peterson and Golemis, 2004). Indeed for scaffolding proteins, conformational inhibitors of this type may provide the only route to achieving inhibition by small molecules.
SIGNIFICANCE
Lack of target specificity is a major limitation of many widely used kinase inhibitors. This results in part from the high degree of evolutionary conservation in the ATP-binding pocket targeted by these compounds across the human kinome. Nonconserved regulatory elements found in some kinases may offer unique targets for more selective kinase inhibition. Here we test this hypothesis using the p21-activated kinase 1 and report the screening for and identification of a highly selective, cell permeable inhibitor that targets the Pak1 autoregulatory mechanism. Biochemical studies suggest that IPA-3 may trap a transient intermediate step in Pak activation, since pre-activated Pak1 is insensitive to IPA-3. In addition, IPA-3-bound Pak1 exhibits some but not all structural features of the active conformation, and is catalytically inactive. Critically, kinase specificity profiling studies reveal an exceptional degree of kinase selectivity by IPA-3, consistent with its targeting the unique Pak1 regulatory domain. Furthermore, cell-based experiments using IPA-3 provide evidence that Pak promotes mitogen activated protein kinase activation and illustrate selective inhibition of Pak in live cells. Thus, kinase autoregulatory mechanisms provide an alternative target for kinase inhibition by small molecules. The widespread use of constitutively active, recombinant kinase forms in kinase inhibitor screening programs likely limits the identification of compounds acting by such novel allosteric mechanisms. Our results suggest that screening assays that recapitulate biologically important regulatory mechanisms may reveal additional opportunities for selective kinase inhibition.
EXPERIMENTAL PROCEDURES
Protein purification and characterization
The following proteins were purified as described: Cdc42-GTPγS (Peterson et al., 2001), Pak2 (Rennefahrt et al., 2007), Pak4 and Pak5 catalytic domains (Eswaran et al., 2007), and full-length Pak1 (Rennefahrt et al., 2007). Full-length Pak3 (Bagrodia et al., 1995) and Pak5 (Cotteret et al., 2003) were expressed in HEK-293 cells and immunopurified (see below). Full-length Pak6 (PV3502) was purchased from Invitrogen. MBP was purchased (Sigma) or purified from bovine brain acetone powder (Sigma) as described (Prowse et al., 2000).
Recombinant Pak1 was characterized by analytical gel filtration chromatography on a calibrated Superdex 200 10/300 GL column. For the identification of surface-exposed cysteine residues within Pak1, computational analysis of crystal structures of Pak1 kinase domain in both active (Lei et al., 2005) and autoinhibited conformations (Lei et al., 2000) was performed using the MSMS solvent accessibility module of UCSF Chimera (Pettersen et al., 2004).
Immunopurification of Pak3 and Pak5
HEK-293 cells were transfected with 0.5 µg Pak3 or myc-Pak5 DNA using liposome-mediated transfection (Lipofectamine 2000; Invitrogen). 48 h post-transfection, cells were washed in ice cold phosphate-buffered saline (PBS) and lysed into Lysis Buffer (20 mM Tris pH 7.5; 150 mM NaCl; 1 mM EDTA; 1 mM EGTA; 1% Triton X-100; 1 mM phenylmethylsulfonyl fluoride; 10 µg/ml each of chymostatin, leupeptin and pepstatin). Insoluble material was removed by centrifugation at 12,000 × g for 10 min at 4°C. Lysates were incubated with protein Aagarose (Pierce) and either anti-Pak3 (Cell Signaling Technology) or anti-myc (Santa Cruz Biotechnology) for at least 2 h at 4°C. Precipitates were washed twice in Lysis buffer and once in Assay buffer (50 mM Hepes pH 7.5, 25 mM NaCl, 1.25 mM MgCl2, 1.25 mM MnCl2).
Chemical compounds
The compound library consisted of 30,000 diverse compounds, largely conforming to Lipinski’s rules, obtained from ChemDiv Inc. (San Diego) and the “Challenge Set”, “Mechanistic Diversity Set”, “Natural Products Set”, and “Structural Diversity Set” provided by the Developmental Therapeutic Program (DTP) of the National Cancer Institute. Compounds were stored as 5 mM DMSO stock solutions (except for the Mechanistic Diversity Set compounds which were stored at 0.5 mM) at −80°C and were thawed immediately prior to use in a dessicator. IPA-3 was re-synthesized and characterized as described in Supplemental Experimental Procedures. PIR (Pak inhibitor related) compounds were provided as dry powder by the DTP.
High-throughput luminescence assay for inhibitors of Pak1 activation
Recombinant Pak1 (8 µl of a 50 µg/ml solution) in Assay Buffer (50 mM Hepes pH 7.5, 25 mM NaCl, 1.25 mM MgCl2, 1.25 mM MnCl2) was aliquotted using a BioTek MicroFill into wells of columns 2–23 of a 384-well plate (Corning #3704). Individual compounds were then added using disposable 384-pin polypropylene pin replicators (Genetix) to transfer ~ 20 nl from compound stock plates. Reactions were started by the addition of a solution (7 µl per well) containing MBP (0.32 mg/ml), GTPγS-charged Cdc42 (2.9 mg/ml), and ATP (21 µM) in Assay Buffer. Final reaction composition: 450 nM Pak1, 1.3 µM Cdc42, 0.15 mg/ml MBP, 10 µM ATP, 0.1% DMSO, library compounds at ~ 7 µM. Plates were incubated for 2 h at 30°C. Reactions were stopped by the addition of an equal volume of Kinase-Glo reagent (Promega) and incubated 10 min at room temperature. Chemiluminescence was measured at 555 nm (20 nm emission slit) using a Cary Eclipse (Varian) equipped with a microplate carrier.
Compound stock plates were organized with library compounds in columns 3–22 (20 µl of a 5 mM solution in DMSO). Column 2 contained 3 wells of 2 mM staurosporine as positive controls and the remaining wells of column 2 contained DMSO as negative controls. Column 23 was empty and these wells served as a “no-addition” control. All compound plates were screened in duplicate.
Data analysis was conducted on a plate-by-plate basis in Microsoft Excel. Mean and standard deviation of the luminescence intensity of all wells was calculated. Wells containing compounds that resulted in luminescence intensities greater than three standard deviations above the mean of control samples in both replicate plates were considered hits.
In vitro kinase assays
Pak1 (571 nM final) in Assay buffer was mixed with DMSO or compound for 5 min at room temp, followed by addition of 4 µM Cdc42 and 8.3 µM MBP for an additional 5 min. Kinase reactions were started by addition of unlabeled ATP and 1–10 µCi [32P]-γ-ATP per reaction for 10 min at 30°C. Final DMSO concentration was 1%. Kinase reactions were stopped on dry ice. Under these conditions, MBP phosphorylation was linear with time.
Kinase assays for Figure 4 were carried out as follows. Condition A: Pak1 was incubated for 5 min at room temperature with compound followed by addition of 4 µM Cdc42 and ATP for 20 min at 30°C. Condition B: Pak1 was incubated with Cdc42 for 20 min at 30°C, followed by addition of compound for 5 min. Condition C: Pak1 was incubated with Cdc42 and ATP for 20 min at 30°C, followed by addition of compound for 5 min. These incubations were followed by addition of 8.3 µM MBP and a mixture of ATP and [32P]-γ-ATP for 10 min at 30°C. Final conditions for all reactions were 1% DMSO, 1 mM ATP, and 1–10 µCi [32P]-γ-ATP. Reaction products were analyzed by scintillation counting (Rennefahrt et al., 2007) or by PhosphorImager analysis (Fujifilm). Background counts from no kinase controls were subtracted from all values (scintillation counting assays) and kinase activity was reported as phosphate incorporation onto MBP expressed as a ratio to MBP phosphorylated in the presence of solvent alone. PhosphorImager background values were defined as the signal density of an area surrounding each individual phospho-MBP band and were subtracted from the corresponding phospho-MBP band.
Pak1 autophosphorylation assays
Recombinant Pak1 (571 nM final) in Assay buffer was incubated with either DMSO or the indicated compound in DMSO (1% DMSO final). This mixture was incubated for 5 min at room temperature followed by the addition of either 4 µM Cdc42-GTPγS or 50 µM sphingosine (Avanti Polar Lipids) for 5 min. Kinase reactions were started by the addition of 1 mM ATP. Reactions were stopped by addition of SDS-sample buffer (62.5 mM Tris pH 6.8, 70 mM SDS, 100 mM DTT, 10% glycerol) and boiled at 95°C for 5 min. Samples were subjected to SDS-PAGE and western blotting analysis using phospho-specific antibodies against the activation loop autophosphorylation site threonine 423 (# 2601, Cell Signaling Technology). Total Pak1 was detected with anti-Pak antibodies (C19, Santa Cruz).
Spectral analysis of IPA-3
IPA-3 was diluted to 20 µM in 50 mM Tris (pH 8.0) containing the indicated concentration of DTT. Samples were vortexed and analyzed in a Cary 50 Bio spectrophotometer (Varian). IPA-3 spectral traces were background-subtracted from traces of buffer containing DMSO alone (0.1%). Individual traces were normalized to 0 absorbance units at 600 nm.
The absorption spectrum IPA-3R was obtained by preparing a fresh 20 mM DMSO stock from powder that was immediately diluted into degassed 50mM Tris (pH 7.5)to 20 µM (0.1% DMSO). This sample was vortexed and the spectrum obtained as described above either immediately or after incubation for 15 min on the benchtop.
Thr423 accessibility assay
571 nM Pak1 in Assay buffer was incubated with compound and Pak5-AM (692.5 nM final), ATP or N6-benzyl-ATP (25 µM, Axxora), and 125 µM sphingosine or DMSO for 30°C for 20 min. Samples were analyzed by immunoblot analysis using antibodies against Pak1 phospho-Thr423.
For determination of Pak1 kinase activity, samples prepared as above received DMSO or 2 µM 1NM-PP1 (Calbiochem) (3% final DMSO), 8.3 µM MBP, and a mixture of ATP and [32P]-γ-ATP (25 µM). Samples were incubated for 10 min at 30°C. Pak1 kinase activity was determined via PhosphorImager analysis.
Dephosphorylation of recombinant Pak2
To obtain inactive, dephosphorylated Pak2, 100 nM recombinant full-length Pak2 was treated with 100 U lambda protein phosphatase (New England Biolabs) for 30 min at 30°C in Assay buffer supplemented with 3.25 µM MnCl2. Dephosphorylation of Pak2 was confirmed by western blot using phospho-specific antibodies against Pak T423. Phosphatase activity was inhibited by addition of 50 µM NaVO4 and 1mM β-glycerophosphate.
Z-Lyte™ Assay
The Z-Lyte™ assay was performed by Invitrogen (Carlsbad, CA). Percent inhibition was expressed as a ratio of phosphorylated product formed in the presence of 10 µM IPA-3 compared to phosphorylated product formed in reactions containing 1% DMSO. Inhibition data reflects mean kinase inhibition from two independent trials.
Cell treatment with IPA-3
Mouse embryonic fibroblasts immortalized with SV40 large T antigen were grown in DMEM + 10 % serum. Cells at 60–80% confluency were starved for 18 hours in serum-free DMEM. Cells were pretreated with DMSO or 30 µM compound for 10 min, followed by addition of PDGF-BB (10 ng/ml, Sigma) for 5 min. Cells were washed in ice cold PBS and lysed into Roberts Buffer (50 mM Tris pH 8, 10% glycerol, 1mM EDTA, 137 mM NaCl, 1% NP-40 plus aprotinin, 1 mM PMSF and 1 mM NaVO4). Insoluble material was removed by centrifugation, and protein concentration determined by BCA Assay (Pierce). Equal protein amounts were subjected to SDS-PAGE and Western blotting using: Anti Pak1 (C19, Santa Cruz), anti phospho-PDGFR (3161), anti phospho-Akt (9271), anti phospho-ERK (9102) (Cell Signaling Technologies), and anti ERK (V1141, Promega). In-gel kinase assays were performed as described (Beeser et al., 2005) with MBP as substrate.
Additional methods can be found in Supplemental Experimental Procedures.
Supplementary Material
ACKNOWLEDGMENTS
We thank B. Turk and H. Roeder for comments on the manuscript, and G. Michaud for his contribution to IPA-3 specificity profiling. This work was supported by the Department of Defense NF Research Program (W81XWH-05-1-0200), by an AACRFCCC Career Development Award, and by a grant from the Pennsylvania Department of Health, to JRP, and by NIH (RO1-CA117884) to JC. The Pennsylvania Department of Health disclaims responsibility for any analyses, interpretations, or conclusions. SWD was supported by NCI funding (CA009035). Further support was provided by the NIH (CA006927) and an appropriation from the Commonwealth of Pennsylvania to Fox Chase Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- Abo A, Qu J, Cammarano MS, Dan C, Fritsch A, Baud V, Belisle B, Minden A. PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. Embo J. 1998;17:6527–6540. doi: 10.1093/emboj/17.22.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagrodia S, Taylor SJ, Creasy CL, Chernoff J, Cerione RA. Identification of a mouse p21Cdc42/Rac activated kinase. J Biol Chem. 1995;270:22731–22737. doi: 10.1074/jbc.270.39.22731. [DOI] [PubMed] [Google Scholar]
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeser A, Jaffer ZM, Hofmann C, Chernoff J. Role of group A p21-activated kinases in activation of extracellular-regulated kinase by growth factors. J Biol Chem. 2005;280:36609–36615. doi: 10.1074/jbc.M502306200. [DOI] [PubMed] [Google Scholar]
- Bokoch GM, Reilly AM, Daniels RH, King CC, Olivera A, Spiegel S, Knaus UG. A GTPase-independent mechanism of p21-activated kinase activation. Regulation by sphingosine and other biologically active lipids. J Biol Chem. 1998;273:8137–8144. doi: 10.1074/jbc.273.14.8137. [DOI] [PubMed] [Google Scholar]
- Buchwald G, Hostinova E, Rudolph MG, Kraemer A, Sickmann A, Meyer HE, Scheffzek K, Wittinghofer A. Conformational switch and role of phosphorylation in PAK activation. Mol Cell Biol. 2001;21:5179–5189. doi: 10.1128/MCB.21.15.5179-5189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cau J, Faure S, Comps M, Delsert C, Morin N. A novel p21-activated kinase binds the actin and microtubule networks and induces microtubule stabilization. J Cell Biol. 2001;155:1029–1042. doi: 10.1083/jcb.200104123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham GM. Novel protein kinases and molecular mechanisms of autoinhibition. Curr Opin Struct Biol. 2004;14:700–705. doi: 10.1016/j.sbi.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Ching YP, Leong VY, Wong CM, Kung HF. Identification of an autoinhibitory domain of p21-activated protein kinase 5. J Biol Chem. 2003;278:33621–33624. doi: 10.1074/jbc.C300234200. [DOI] [PubMed] [Google Scholar]
- Chong C, Tan L, Lim L, Manser E. The mechanism of PAK activation. Autophosphorylation events in both regulatory and kinase domains control activity. J Biol Chem. 2001;276:17347–17353. doi: 10.1074/jbc.M009316200. [DOI] [PubMed] [Google Scholar]
- Cotteret S, Jaffer ZM, Beeser A, Chernoff J. p21-Activated kinase 5 (Pak5) localizes to mitochondria and inhibits apoptosis by phosphorylating BAD. Mol Cell Biol. 2003;23:5526–5539. doi: 10.1128/MCB.23.16.5526-5539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswaran J, Lee WH, Debreczeni JE, Filippakopoulos P, Turnbull A, Fedorov O, Deacon SW, Peterson JR, Knapp S. Crystal Structures of the p21-activated kinases PAK4, PAK5, and PAK6 reveal catalytic domain plasticity of active group II PAKs. Structure. 2007;15:201–213. doi: 10.1016/j.str.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature. 2000;404:151–158. doi: 10.1038/35004513. [DOI] [PubMed] [Google Scholar]
- King CC, Gardiner EM, Zenke FT, Bohl BP, Newton AC, Hemmings BA, Bokoch GM. p21-activated kinase (PAK1) is phosphorylated and activated by 3-phosphoinositide-dependent kinase-1 (PDK1) J Biol Chem. 2000;275:41201–41209. doi: 10.1074/jbc.M006553200. [DOI] [PubMed] [Google Scholar]
- Kissil JL, Wilker EW, Johnson KC, Eckman MS, Yaffe MB, Jacks T. Merlin, the product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol Cell. 2003;12:841–849. doi: 10.1016/s1097-2765(03)00382-4. [DOI] [PubMed] [Google Scholar]
- Koresawa M, Okabe T. High-throughput screening with quantitation of ATP consumption: a universal non-radioisotope, homogeneous assay for protein kinase. Assay Drug Dev Technol. 2004;2:153–160. doi: 10.1089/154065804323056495. [DOI] [PubMed] [Google Scholar]
- Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6:459–471. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- Lee SR, Ramos SM, Ko A, Masiello D, Swanson KD, Lu ML, Balk SP. AR and ER interaction with a p21-activated kinase (PAK6) Mol Endocrinol. 2002;16:85–99. doi: 10.1210/mend.16.1.0753. [DOI] [PubMed] [Google Scholar]
- Lei M, Lu W, Meng W, Parrini MC, Eck MJ, Mayer BJ, Harrison SC. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell. 2000;102:387–397. doi: 10.1016/s0092-8674(00)00043-x. [DOI] [PubMed] [Google Scholar]
- Lei M, Robinson MA, Harrison SC. The active conformation of the PAK1 kinase domain. Structure. 2005;13:769–778. doi: 10.1016/j.str.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Leisner TM, Liu M, Jaffer ZM, Chernoff J, Parise LV. Essential role of CIB1 in regulating PAK1 activation and cell migration. J Cell Biol. 2005;170:465–476. doi: 10.1083/jcb.200502090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Gray NS. Rational design of inhibitors that bind to inactive kinase conformations. Nat Chem Biol. 2006;2:358–364. doi: 10.1038/nchembio799. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shah K, Yang F, Witucki L, Shokat KM. Engineering Src family protein kinases with unnatural nucleotide specificity. Chem Biol. 1998;5:91–101. doi: 10.1016/s1074-5521(98)90143-0. [DOI] [PubMed] [Google Scholar]
- Nagar B, Bornmann WG, Pellicena P, Schindler T, Veach DR, Miller WT, Clarkson B, Kuriyan J. Crystal structures of the kinase domain of c-Abl in complex with the small molecule inhibitors PD173955 and imatinib (STI-571) Cancer Res. 2002;62:4236–4243. [PubMed] [Google Scholar]
- Nheu TV, He H, Hirokawa Y, Tamaki K, Florin L, Schmitz ML, Suzuki-Takahashi I, Jorissen RN, Burgess AW, Nishimura S, et al. The K252a derivatives, inhibitors for the PAK/MLK kinase family selectively block the growth of RAS transformants. Cancer J. 2002;8:328–336. doi: 10.1097/00130404-200207000-00009. [DOI] [PubMed] [Google Scholar]
- Parrini MC, Lei M, Harrison SC, Mayer BJ. Pak1 kinase homodimers are autoinhibited in trans and dissociated upon activation by Cdc42 and Rac1. Mol Cell. 2002;9:73–83. doi: 10.1016/s1097-2765(01)00428-2. [DOI] [PubMed] [Google Scholar]
- Peterson JR, Bickford LC, Morgan D, Kim AS, Ouerfelli O, Kirschner MW, Rosen MK. Chemical inhibition of N-WASP by stabilization of a native autoinhibited conformation. Nat Struct Mol Biol. 2004;11:747–755. doi: 10.1038/nsmb796. [DOI] [PubMed] [Google Scholar]
- Peterson JR, Golemis EA. Autoinhibited proteins as promising drug targets. J Cell Biochem. 2004;93:68–73. doi: 10.1002/jcb.20184. [DOI] [PubMed] [Google Scholar]
- Peterson JR, Lokey RS, Mitchison TJ, Kirschner MW. A chemical inhibitor of N-WASP reveals a new mechanism for targeting protein interactions. Proc Natl Acad Sci U S A. 2001;98:10624–10629. doi: 10.1073/pnas.201393198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Porchia LM, Guerra M, Wang YC, Zhang Y, Espinosa AV, Shinohara M, Kulp SK, Kirschner LS, Saji M, Chen CS, et al. OSU03012, A Celecoxib Derivative, Directly Targets p21 Activated Kinase. Mol Pharmacol. 2007 doi: 10.1124/mol.107.037556. [DOI] [PubMed] [Google Scholar]
- Prowse CN, Hagopian JC, Cobb MH, Ahn NG, Lew J. Catalytic reaction pathway for the mitogen-activated protein kinase ERK2. Biochemistry. 2000;39:14002. doi: 10.1021/bi005116m. [DOI] [PubMed] [Google Scholar]
- Rennefahrt UE, Deacon SW, Parker SA, Devarajan K, Beeser A, Chernoff J, Knapp S, Turk BE, Peterson JR. Specificity profiling of Pak kinases allows identification of novel phosphorylation sites. J Biol Chem. 2007;282:15667–15678. doi: 10.1074/jbc.M700253200. [DOI] [PubMed] [Google Scholar]
- Rice WG, Supko JG, Malspeis L, Buckheit RW, Jr, Clanton D, Bu M, Graham L, Schaeffer CA, Turpin JA, Domagala J, et al. Inhibitors of HIV nucleocapsid protein zinc fingers as candidates for the treatment of AIDS. Science. 1995;270:1194–1197. doi: 10.1126/science.270.5239.1194. [DOI] [PubMed] [Google Scholar]
- Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kuriyan J. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000;289:1938–1942. doi: 10.1126/science.289.5486.1938. [DOI] [PubMed] [Google Scholar]
- Sebolt-Leopold JS, English JM. Mechanisms of drug inhibition of signalling molecules. Nature. 2006;441:457–462. doi: 10.1038/nature04874. [DOI] [PubMed] [Google Scholar]
- Talukder AH, Meng Q, Kumar R. CRIPak, a novel endogenous Pak1 inhibitor. Oncogene. 2006;25:1311–1319. doi: 10.1038/sj.onc.1209172. [DOI] [PubMed] [Google Scholar]
- Tang Y, Chen Z, Ambrose D, Liu J, Gibbs JB, Chernoff J, Field J. Kinase-deficient Pak1 mutants inhibit Ras transformation of Rat-1 fibroblasts. Mol Cell Biol. 1997;17:4454–4464. doi: 10.1128/mcb.17.8.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thullberg M, Gad A, Beeser A, Chernoff J, Stromblad S. The kinaseinhibitory domain of p21-activated kinase 1 (PAK1) inhibits cell cycle progression independent of PAK1 kinase activity. Oncogene. 2007;26:1820–1828. doi: 10.1038/sj.onc.1209983. [DOI] [PubMed] [Google Scholar]
- Xia C, Ma W, Stafford LJ, Marcus S, Xiong WC, Liu M. Regulation of the p21-activated kinase (PAK) by a human Gbeta -like WD-repeat protein, hPIP1. Proc Natl Acad Sci U S A. 2001;98:6174–6179. doi: 10.1073/pnas.101137298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke FT, King CC, Bohl BP, Bokoch GM. Identification of a central phosphorylation site in p21-activated kinase regulating autoinhibition and kinase activity. J Biol Chem. 1999;274:32565–32573. doi: 10.1074/jbc.274.46.32565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.