Abstract
Viral infection of the CNS can result in encephalitis and acute seizures, increasing the risk for later-life epilepsy. We have previously characterized a novel animal model of temporal lobe epilepsy that recapitulates key sequela in the development of epilepsy following viral infection. C57BL/6J mice inoculated with the Daniel’s strain of Theiler’s Murine Encephalomyelitis Virus (TMEV; 3×105 PFU, i.c.) display acute limbic seizures that secondarily generalize. A majority of acutely seized animals develop spontaneous seizures weeks to months later. As part of our investigation, we sought to assess behavioral comorbidity following TMEV inoculation. Anxiety, depression, cognitive impairment, and certain psychoses are diagnosed in persons with epilepsy at rates far more frequent than in the general population. We used a battery of behavioral tests to assess anxiety, depression, cognitive impairment, and general health in acutely seized animals inoculated with TMEV and compared behavioral outcomes against age-matched controls receiving a sham injection. We determined TMEV-seized animals are less likely to move through the exposed center of an open field and are less likely to enter into the lighted half of a light/dark box; both behaviors may be indicative of anxiety-like behavior. TMEV-seized animals also display early and persistent reductions in novel object exploration during novel object place tasks and do not improve in their ability to find a hidden escape platform in Morris water maze testing, indicative of impairment in episodic and spatial memory, respectively. Cresyl violet staining at 35 and 250 days after injection reveals bilateral reductions in hippocampal area, with extensive sclerosis of CA1 evident bilaterally along the rostral-caudal axis. Early and persistent behavioral changes in the TMEV model provide surrogate markers for assessing disease progression as well as endpoints in screening for the efficacy of novel compounds to manage both seizure burden and comorbid conditions.
Keywords: temporal lobe epilepsy, cognitive impairment, novel object place recognition, viral encephalitis, acute seizures
INTRODUCTION
While most epilepsy etiology is idiopathic in nature, a growing literature describes the prevalence of acquired epilepsy following viral infection. Viral infections of the CNS can result in encephalitis, which has the ability to provoke early acute seizures, increasing the risk for unprovoked, later-life seizures 22-fold (Misra et al., 2008a, Michael and Solomon, 2012). In the United States, encephalitis leads to as many new cases of acquired epilepsy as head trauma (Misra et al., 2008a).
Our group has previously characterized the first infection-based animal model of epilepsy, closely recapitulating temporal lobe epilepsy (TLE). Intracortical injection of Theiler’s Murine Encephalomyelitis Virus (TMEV) into C57BL/6J mice leads to acute encephalitic seizures from 3 to 10 days post injection (DPI)(Libbey et al., 2008, Stewart et al., 2010). TMEV antigens are present bilaterally in limbic and temporal areas during acute infection, including hippocampus (notably CA1 and CA2), periventricular thalamic nuclei, septal nuclei, and piriform, parietal, and entorhinal cortices (Stewart et al., 2009), but are virtually undetectable by 14 DPI (Kirkman et al., 2010, Libbey et al., 2011). During the acute, active infection period, neuronal death is observed preferentially among CA1 and CA2 neurons of the hippocampus (Stewart et al., 2009). After acute infection and viral clearance, a latent period of weeks to months precedes infrequent spontaneous seizures (approximately 2 seizures/animal/week) in a majority of animals that seized during acute infection (Stewart et al., 2010). The TMEV model reflects periods of human epilepsy development following viral infection, and as such, this model may serve as a useful platform in the development and screening of disease modifying therapeutics administered during periods of acute infection.
TLE is the most common form of focal epilepsy and often the most refractory to currently available anti-seizure drugs; like many forms of epilepsy, it often persists with comorbidity. Patients with epilepsy suffer from comorbid psychiatric conditions at rates considerably higher than the general population (Brooks-Kayal et al., 2013), with some of the strongest associations appearing in focal refractory patient populations (Adams et al., 2008, Dalmagro et al., 2012). A recent meta-analysis found approximately 23% of people with epilepsy currently experience depression or have within the past year (Fiest et al., 2013). Often, depressive episodes in persons with epilepsy are associated with anxiety symptoms or an anxiety disorder (Kanner, 2012, Kanner, 2013), but depressive episodes may also manifest independently. Apart from anxiety and mood disorders, cognitive impairment is a particularly prevalent focus in TLE research and is often identified by patients with epilepsy as one of its most debilitating complications (Fisher, 2000, Bell et al., 2011, Murphy, 2013). Cellular and molecular insight is needed to better define relationships between behavioral changes and the neuropathology often observed in epilepsy. In the case of TLE, special emphasis has been placed on hippocampal pathology like sclerosis, mossy fiber sprouting, granule cell dispersion, and gray matter loss (Thom et al., 2012, Kandratavicius et al., 2013, Winston et al., 2013).
In the present investigation, and in keeping with the recent International League Against Epilepsy/American Epilepsy Society workshop on comorbidities (Brooks-Kayal et al., 2013), we have assessed the general health, depression-like and anxiety-like behaviors, cognitive impairment, and hippocampal pathology associated with the TMEV model of infection-induced TLE. We report TMEV-infected animals that initially exhibit acute encephalitic seizures experience early deficits in motor coordination concomitant with significant weight loss. Certain measures of anxiety-like behavior appear elevated in TMEV animals, but do not co-occur with depression-like symptoms. Finally, early cognitive impairment can be detected in parallel with severe hippocampal sclerosis and hippocampal area reduction.
METHODS
Animals
Four- to five-week-old male C57BL/6J (B6) mice (14–20g; Jackson Laboratory, Bar Harbor, ME, U.S.A.) were used in this study. Animals were group housed in a temperature- and light-controlled (12h on/12h off) environment and permitted access to food and water ad libitum throughout the study. All animal care and experimental manipulations were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the University of Utah Institutional Animal Care and Use Committee (IACUC).
TMEV infection and acute period monitoring
Under isoflurane anesthesia, mice were injected intracortically (i.c.) in the right posterior parietal cortex with either 20μl of phosphate-buffered saline (PBS; sham injection; N=30) or 20μl of PBS containing 3×105 plaque forming units (PFUs) of the Daniel’s strain of TMEV (N=45) as previously described (Libbey et al., 2008, Stewart et al., 2009). Following i.c. injections, mice were monitored once daily from 3 to 8 DPI between the hours of 9am and 11am for handling-induced behavioral seizures and changes in body weight (Libbey et al., 2008). Behavioral seizures were scored according to the Racine scale (Racine, 1972), and any TMEV-injected B6 mouse that displayed at least one behavioral seizure during the observation periods was classified as a “TMEV-seized” animal. TMEV-injected mice that did not display a seizure (N=10/45) during the limited observation periods were excluded from study.
Cohort selection and behavioral testing
Following the acute period, control (N=30) and TMEV-seized (N=35) animals were separated into three age-matched cohorts (PBS, N=10; TMEV, N=10 to 12) for use in behavioral tests. Most behavioral tests were conducted once within two epochs (10-60 DPI and >60 DPI). With exception to the rotarod and novel object place recognition tasks, no cohort performed the same behavioral task twice to preserve novelty and mitigate stress. Animals were allowed 1 h to acclimate to the testing room on any day experiments were conducted. All tests were conducted during the light cycle. Arenas were cleaned between animals and phases (novel object place recognition) with a 4% Spartan HDQ-basic solution.
Tests of motor performance
Open Field Test (OFT)
Mice were tested for locomotive behaviors by singly placing an animal in a 40L × 40W × 30H cm open field under ambient room light for 30min. Fusion software (OmniTech Electronics, Columbus, OH, USA) scored animals for aspects of horizontal and vertical movement as well as stereotypy.
Rotarod test
We tested mice for motor coordination by assessing their ability to maintain balance on a knurled rod (2.5cm diameter) rotating at 6 rpm. Mice were manually timed for latency to fall off the rotarod during each of three successive 1 min trials separated by 15s. Individual latencies were averaged over three trials and computed into group averages.
Tests of anxiety-like and depression-like behavior
Open field zones (OFZ)
Open field data was analyzed for duration and movement within the periphery of the apparatus (5cm from the edge of all walls) or the center (30L × 30W cm) using Fusion software (OmniTech Electronics).
Light/dark box test (LD)
Employed as an ethological model of anxiety-like behavior, LD testing assessed innate aversion to a white, lighted region (Bourin and Hascoët, 2003). Fusion software (OmniTech Electronics) determined the percentage of time an animal spent in the lighted region of an open field (20L × 40W cm; 425 lux) or the dark, enclosed region (20L × 40W × 14H cm) separated by a small opening (10L cm × 2.5W cm) during a 10m trial.
Saccharin preference test
Mice were singly housed in wire top cages and allowed to acclimate for 24h. Volumes consumed of 1.6mM saccharin (Reed et al., 2004) or tap water solutions were determined by the mass of solution remaining in separate bottles originally containing 200 g of solution (densities: 0.998±0.003 g/ml tap water; 0.997±0.002 g/ml 1.6mM saccharin) after a 48h period. Saccharin preference (%) was calculated as the volume of saccharin solution consumed divided by the total volume of fluid consumed per animal, multiplied by 100. Averages were obtained by group, and anhedonia was defined as a saccharin preference that did not significantly differ from 50% preference.
Tests of cognitive impairment
Novel object place recognition (NOPR)
Animals were habituated to the clear, plexiglass NOPR arena (40L cm × 40W cm × 60H cm) two consecutive days prior to testing for 15min/d. Spatial cues of distinct shape remained 15cm from each wall throughout habituation and experimental trials. Experimentation, conducted on day 3, encompassed three distinct phases. During phase 1, the familiarization phase, animals were exposed to two identical objects placed in two of three possible object locations over a 15min span. Phase 2, the delay phase, involved sequestering the animal within a small opaque enclosure for a 5min period. During the delay phase, both objects were removed, and the arena, familiarization phase objects, and a novel object were cleaned with a 4% HDQ solution. A familiarization phase object was placed back in its original location while a novel object was placed in a new location not used during the familiarization phase. During phase 3, the test phase, the opaque enclosure was removed and the animal was scored for the percentage of time spent exploring the novel object as a function of the total time spent exploring either object, multiplied by 100 (novel object exploration) during this 5min phase. Similarly, during the familiarization phase, animals were scored for the percentage of time spent exploring one familiar object over another (location 1 compared to 2 or 3; location 2 compared to 3) (familiar object exploration). EthoVision software (Noldus, Leesburg, VA, USA) was used to track animals while object-exploration was manually scored in EthoVision as the time an animal spent facing, smelling, or touching an object with its snout at a distance of less than 2cm from the object. We excluded climbing on objects as an exploratory behavior.
Morris water maze test (MWM)
Animals were tested for their ability to find a 5cm escape platform submerged in a 1.8m diameter pool with a distinct spatial cues placed within the inner rim of each quadrant. Water temperature was maintained at 24±1°C. Days 1–5 constituted the learning phase of MWM testing in which an animal was placed in a different starting quadrant of the pool in each of four daily trials. We manually scored animals for their latency to find the escape platform before a 60s trial elapsed. Animals that did not find the platform by the end of a trial were guided to the platform location and retained there for 10s. The daily order of quadrant starting positions was determined at random by Water 2020 software (HVS Systems, Hampton, UK) and was consistent for both treatment groups. Following 5 days of learning, a 2-day delay period elapsed before retention trials on day 8. Day 8 procedure was consistent with the learning phase. Time-to-platform calculations were averaged over four trials per day per animal and this value was used to calculate group averages by day. A lack of contrast prevented tracking software from accurately assessing animal velocity, distance traveled, and path efficiency.
Histology
Following all behavioral experiments, animals were anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and transcardially perfused with PBS followed by a 4% paraformaldehyde (PFA) solution. Perfused brains were extracted and post-fixed in a 4% PFA solution for 24h then transferred to a 15/30% sucrose gradient for cryoprotection. Brains were sectioned coronally on a freezing stage microtome at a thickness of 40μm. Sections containing the hippocampus were stored in PBS for subsequent cresyl violet or Fluoro Jade-B staining.
Ventricular and hippocampal areas were evaluated (Stewart et al., 2010) near the dorsal hippocampus by cresyl violet staining. Every other section was processed immediately caudal to the septum (4–5 sections/animal; 5 animals per treatment and time point). Areas were quantified by drawing a region of interest around the hippocampus or ventricle using Image-J software (NIH, Bethesda, MD, USA). For Fluoro Jade-B staining, every 5th slice was stained beginning with dorsal hippocampus (4 sections/animal; 3–4 animals per treatment and time point), according to Stewart et al., 2009.
Statistics
Unless otherwise noted, all behavioral outcomes were compared between treatment groups (PBS-injected or TMEV-seized) using Student’s t-tests for each testing time point. Body weight, rotarod performance, and latency-to-platform calculations in MWM were compared using two-way ANOVA. Treatment was the between subject factor and time was the within-subject factor (DPI). If appropriate, a Bonferroni post hoc test was used to compare mean outcomes by time point. Group trends in daily MWM performance were analyzed using a linear-fit-to-sample test. Histological comparisons between groups utilized a Welch’s t-test for each region analyzed, or a paired t-tests to compare left and right ventricles or hippocampi within a group. Tests of object or solution preference (NOPR, saccharin preference test) compared to chance (designated as 50%) utilized a one-sample t-test comparison to 50%. All parametric data is represented as the mean ± S.E.M. Graphpad Prism was used for all statistical comparisons; p < 0.05 was considered significant.
RESULTS
Acute monitoring
Animals were agitated by lightly shaking their home cage once daily from 3 to 8 DPI and observed for motor seizures as previously described (Libbey et al., 2008, Stewart et al., 2009). During the 6 day monitoring period, approximately 75% (35/45) of B6 mice injected with TMEV displayed at least one behavioral seizure (TMEV-seized), consistent with previous findings using a lower titer of virus (2·104 PFU; Stewart et al., 2010). The majority (>90%; 80/88) of observed handling-induced seizures were severe (Figure 1) with animals exhibiting forelimb and hindlimb clonus progressing to loss of postural control (corresponding to Racine level 4 and 5 criteria, respectively; Racine, 1972). The number of observed behavioral seizures increased between 3 and 6 DPI (from 15 to 24 seizures/monitoring period) with a dramatic cessation in seizure number at 7 and 8 DPI. Most seized animals (26/35, 74%) displayed more than one behavioral seizure (average frequency: 2.6±0.2 seizures/animal between 3-8 DPI). Consistent with our previously studies, no seizures were ever observed in PBS-injected animals during the study. One TMEV-seized animal died during the acute infection period.
Figure 1. TMEV inoculation leads to generalized seizures.
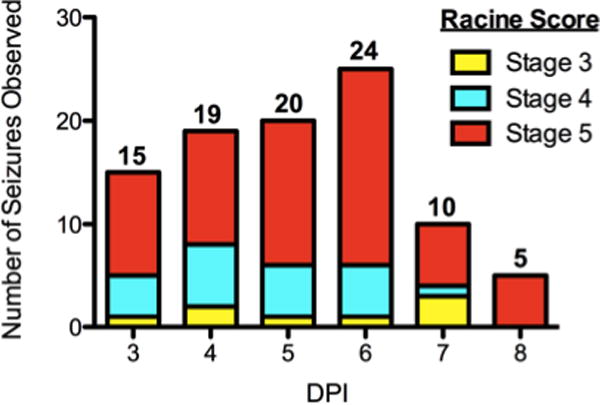
Over a six-day monitoring period following injection of TMEV (3×105 PFU, i.c.), 75% of TMEV animals (N=35/45) displayed at least one Racine stage 3 to 5 seizure, with generalized seizures (stage 4 or 5) being the most common. The number of animals displaying a motor seizure during an observation period (conducted once daily between 9 and 11 am) is indicated above each corresponding day post injection (DPI). After 6 DPI, the number of observed seizures decreases. No control animal ever displayed a seizure during the study.
TMEV animals recover after early signs of illness
Loss of body weight is not uncommon following TMEV encephalitis (Libbey et al., 2008, Stewart et al., 2009) and serves as an overt sign of active infection. On the day of injection (0 DPI), control and TMEV animals do not differ in body weight. However, between 3-8 DPI, control animals (N=30) consistently gain weight while TMEV-seized animals (N=35) display reduced body weight all six days (F1,448=187.11, p<0.0001; Bonferroni post hoc test; Figure 2a). A significant DPI x treatment interaction (F6,444=9.27, p<0.0001) indicates treatment differentially impacts changes in body weight during the monitoring period. Notably, TMEV animals gradually regain lost body weight over a one-month period after infection, and do not differ in body weight from control animals by 35 DPI.
Figure 2. TMEV animals lose body weight during infection and are initially impaired in motor performance.
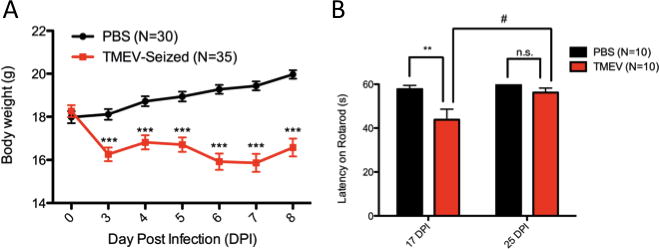
(A) Weight loss is a common occurrence with TMEV infection in B6 animals. Differences in body weight between control and TMEV-seized animals can be observed from 3-8 DPI, while no differences exists before i.c. injection (0 DPI). (B) Repeated rotarod testing at 17 and 25 DPI using a single cohort indicates TMEV-treated animals are initially impaired in their ability to maintain balance on the rotarod at 17 DPI. TMEV performance improves over 8 days and does not differ from control animals at 25 DPI. **(p<0.01) and ***(p<0.001), two-way RM ANOVA with Bonferroni post tests. #(p<0.05), paired t-test. Data are displayed as mean ± S.E.M.
Motor coordination is impaired at 17 DPI shortly after viral antigens can no longer be detected (14 DPI), as demonstrated by rotarod tests of motor coordination (Figure 2b). Both treatment and time of testing were significant covariates in motor performance (treatment: F1,18=10.55, p=0.0045; DPI: F1,18=6.94, p=0.0169) while subject matching and interaction were not significant covariates (matching: F18,18=0.96, p=0.5327; interaction: F1,18=3.35, p=0.0839) in the repeated measures analysis. At 17 DPI, sham-injected animals maintained balance on the rotarod longer than TMEV-injected animals over three consecutive one-minute trials (p<0.01, Bonferroni post hoc test). However, motor coordination improves in TMEV animals over a one-week period (p=0.0219, paired t-test) and does not differ from PBS-control performance at 25 DPI (p>0.05, Bonferroni post hoc test).
TMEV animals display anxiety-like behavior but no overt signs of depression-like behavior
During a 30 min OFT, measures of total distance and horizontal activity are similar at 10 DPI (cohorts 1 and 3; pooled data) and 70 DPI (cohort 2)(Table 1, t-test). Further, no intergroup differences in rest time, rest episodes, movement time, or movement episodes could be detected at 10 or 70 DPI. However, TMEV animals display reduced vertical episodes and vertical activity time (at 10 and 70 DPI) along with increased stereotypy episodes (at 10 and 70 DPI) and stereotypy time (at 10 DPI). Reduced vertical activity may be indicative of anxiety-like behavior (Prut and Belzung, 2003).
Table 1.
Difference in Open Field Measures of Motor Activity
| Measure | PBS (N=20) | 10 DPI TMEV (N=22) | p value | PBS (N=10) | 70 DPI TMEV (N=12) | p value |
|---|---|---|---|---|---|---|
| Total Distance (cm) | 4969 ± 254.9 | 5920 ± 951.0 | 0.3440 a | 6839 ± 312.2 | 6879 ± 869.9 | 0.9665 a |
| Horizontal Activity Count | 6058 ± 328.6 | 5449 ± 780.7 | 0.4782 a | 8278 ± 341.6 | 7748 ± 804.0 | 0.5540 a |
| Rest Time (s) | 601.9 ±31.81 | 788.6 ± 94.31 | 0.7240 a | 375.7 ± 20.56 | 410.7 ± 69.26 | 0.6365 a |
| Rest Episodes | 314.5 ± 10.66 | 283.0 ± 19.53 | 0.1672 a | 217.4 ± 11.73 | 220.8 ± 28.08 | 0.9139 a |
| Movement Time (s) | 1198 ± 31.83 | 1011 ± 94.32 | 0.0727 a | 1424 ± 20.56 | 1389 ± 69.25 | 0.6360 a |
| Movement Episodes | 315.3 ± 10.72 | 283.2 ± 19.43 | 0.1574 a | 218.3 ± 11.70 | 221.4 ± 28.00 | 0.9197 a |
| Stereotypy Time (s) | 37.87 ± 1.929 | 26.52 ± 1.952 | 0.0002 b | 52.66 ± 3.112 | 40.46 ± 5.017 | 0.0630 b |
| Stereotypy Episodes | 114.4 ± 4.140 | 77.64 ± 8.014 | 0.0003 b | 175.1 ± 13.34 | 127.4 ± 14.09 | 0.0250 b |
| Vertical Activity Time (s) | 186.0 ± 14.52 | 96.07 ± 33.56 | 0.0203 a | 286.2 ± 17.29 | 127.2 ± 19.83 | <0.0001 b |
| Vertical Activity Episodes | 231.5 ± 13.52 | 120.4 ± 32.35 | 0.0037 a | 307.7 ± 10.85 | 181.6 ± 22.42 | 0.0001 a |
Data are displayed as Mean ± S.E.M. a=unequal variance in data sets; analyzed by Welch’s t-test. b=equal variance in data sets; analyzed by Student’s t-test. 10 DPI (cohorts 1 and 3; pooled data); 70 DPI (cohort 2). Significant differences (p<0.05) highlighted in bold
To analyze anxiety-like behavior in the open field, we tracked animal movement and duration through central and peripheral regions of the arena throughout a 30 min trial. TMEV animals (cohort 1) were less likely to spend time in the exposed center of the open field at 10 DPI (p=0.0080, Welch’s correction) or at 70 DPI (p<0.0001; cohort 2) as a function of the total time spent in either zone (Figure 3a). Further, TMEV animals travelled less total distance through the exposed center of the field than controls at both 10 DPI (p=0.0130) and 70 DPI (p=0.0007, Welch’s correction) (Figure 3b–c). It should be noted that overall differences in horizontal movement were not significant at either time point (Table 1).
Figure 3. TMEV animals display signs of anxiety-like behavior.
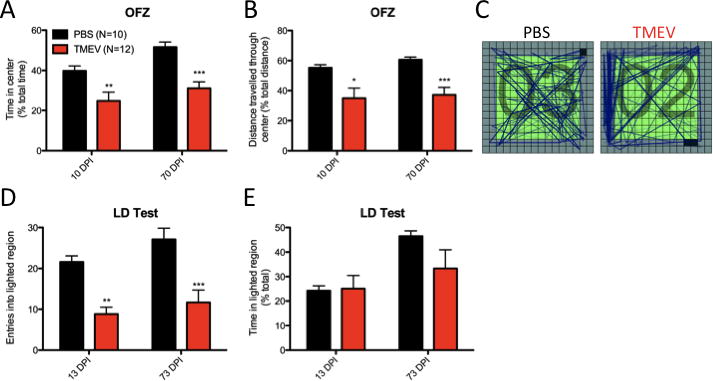
(A–C) Open field zone analysis (OFZ) during a 30 min trial indicates (A) TMEV animals spend less total time in the exposed center of the field compared to controls at 10 (cohort 1) and 70 DPI (cohort 2). Further, (B) TMEV animals travel less total distance through the center of the field at 10 and 70 DPI. No differences in total distance are observed between groups at either time point (see Table 1). (C) Representative traces from PBS and TMEV animals through the demarcated zones (D–E) Light/dark box testing demonstrates (D) TMEV animals entered the lighted region of the LD apparatus less often than controls at both 13 (cohort 1) and 73 DPI (cohort 2); however, (E) no differences were observed in the percentage of time a TMEV or control animal spent in the lighted region during a 10 min trial. *(p<0.05) **(p<0.01) ***(p<0.001), t-test. Data are displayed as mean ± S.E.M.
We also assessed anxiety-like behavior using an LD box test to assess aversion to a lighted region compared to a dark enclosed region. TMEV animals (cohort 1) entered the lighted region less frequently than controls (Figure 3d) at both 13 DPI (p<0.0001) and 73 DPI (p=0.0015; cohort 2). However, as a function of time, no intergroup differences were observed in the percentage of time spent in the lighted region at either 10 DPI (p=0.8879, Welch’s correction) or 73 DPI (p=0.1206, Welch’s correction)(Figure 3e). Measures of total distance between groups in the LD box were similar at each time point suggesting differences in spontaneous activity were not a confounding factor (13 DPI: 1742.0 ± 131.4 cm PBS vs. 1447.5 ± 278.8 cm TMEV, p=0.3544, Welch’s correction; 73 DPI: 1928.5 ± 173.4 cm PBS vs. 1688.2 ± 180.1 cm TMEV, p=0.3537).
Anhedonia is a clinical feature of depression that can be modeled using saccharin preference testing in rodents (Overstreet et al., 2007). Chemoconvulsant animal models of TLE are noted to display signs of anhedonia during saccharin preference testing (Pineda et al., 2010, Tchekalarova et al., 2013). However, TMEV animals do not differ from controls in the percentage of saccharin solution consumed as a function of total liquid consumption during a 48 h testing period beginning at 29 DPI (70.62 ± 3.31% PBS vs. 70.10 ± 2.73% TMEV, p=0.9049) or 79 DPI (68.07 ± 1.45% PBS vs. 65.63 ± 3.83 TMEV, p=0.7309) (data not shown). Both treatment groups displayed a preference for saccharin that was significantly greater than chance at both time points (p≤0.0003 for all groups, one-sample t-test comparison to 50%), indicating neither group met the criteria for anhedonia. Neither group differed in total volume consumed at either 29 DPI (21.02 ± 0.67 ml PBS vs. 22.52 ± 1.32 ml TMEV, p=0.2226) or 79 DPI (20.02 ± 0.71 ml PBS vs. 21.05 ± 0.45 ml TMEV, p=0.0549), suggesting differences in consumption were not influencing the observed saccharin preference index.
TMEV infection leads to persistent cognitive impairment
Cognitive impairment is a common complication in TLE that may be largely attributable to hippocampal pathology (Bell et al., 2011). We assessed cognition in our virus model of TLE using two tasks. Our NOPR task is based upon the observation that rodents generally prefer to investigate a novel object rather than an object perceived as familiar (Ennaceur and Delacour, 1988). Different aspects of episodic memory are believed to underlie discernment of object novelty and placement novelty in this task (Ennaceur, 2010). The MWM task (Morris et al., 1982) investigates learning and memory based upon spatial reference.
NOPR testing was conducted using separate cohorts at 23-24 DPI and 170-177 DPI with animals repeating the task at 170-177 DPI using different object pairs. During a 15 minute familiarization phase in which two identical objects were placed in the arena, no group displayed a preference for one identical object over another during either time period (one sample t-test comparison to 50%, p≥0.2805 for all groups, Figure 4a). However, during the novel object place phase, TMEV animals consistently spent less total exploration time investigating the novel object compared to controls (23-24 DPI: p=0.0237; 170-177 DPI: p=0.0382, Figure 4b). At 23-24 DPI, TMEV exploration of the novel object is not statistically different from chance (p=0.1395, one sample-t-test comparison to 50%). Intergroup differences in total distance during the familiarization phase and the novel object place phase were not significant at either time point, suggesting locomotive differences were not preventing exploration.
Figure 4. TMEV animals are impaired in cognitive tasks.
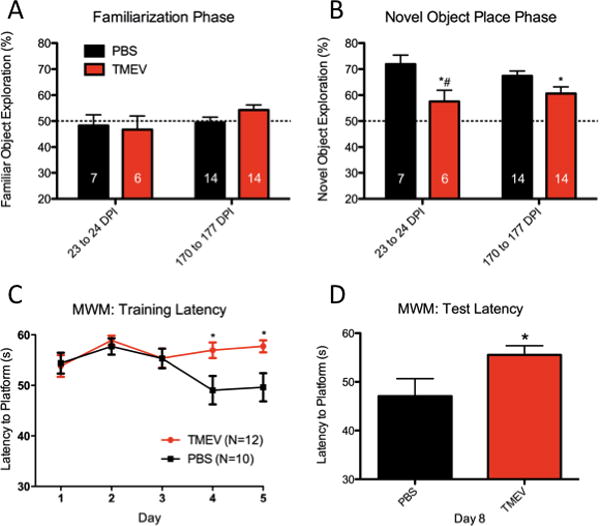
(A–B) Novel object place recognition testing indicates (A) no group differences in identical object preference during the familiarization phase. However, during the novel object place phase (B) control animals explore the novel object location to a greater degree than TMEV animals at both 23-24 DPI and 170-177 DPI. TMEV exploration of the novel object at 23-24 DPI is not greater than chance (#). The dotted line at 50% represents no object preference. (C–D) Morris water maze (MWM) data show TMEV animals do not improve in their ability to find a hidden platform over successive trials, compared to control animals. (C) Differences in latency to platform are observed during training on days 4 and 5 and (D) during retention testing on day 8. *(p<0.05), t-test (NOPR or MWM retention trails) or two-way RM ANOVA with Bonferroni post hoc test (MWM training). Data are represented as mean ± S.E.M.
MWM testing, beginning at 96 DPI, assessed the latency to find a hidden platform over four daily trials using visuospatial cues. The most significant source of variation was attributable to treatment (p=0.0004) with day of testing also providing significant variation (p=0.0155). An interaction between treatment and day of testing was also a significant source of variation (p=0.0409). Latency-to-platform differences did not differ during the first three days of the learning phase. However, during the last two days of learning (days 4 and 5) and the retention day (day 8), TMEV animals displayed an increased latency to find the hidden platform compared to controls (p<0.05, Bonferroni post test, Figure 4c–d). A linear-fit-to-sample analysis for the data displayed in Figure 4c describes an overall decrease in daily latency for control but not TMEV animals (PBS: -1.46±0.47 s/day; TMEV: 0.09±0.32 s/day), with the slope of PBS performance being significantly non-zero (p=0.0022). TMEV latency-to-platform changes over the testing period did not significantly differ from zero (p=0.76), indicating no improvement in task performance over time.
TMEV infection leads to severe hippocampal sclerosis and reduction in hippocampal area
At both early (35 DPI) and late (250 DPI) time points, a cohort of animals was euthanized and coronal slices from dorsal hippocampus were processed for cresyl violet (CV) staining. Lateral ventricles and hippocampi were measured bilaterally and compared between TMEV and control animals by corresponding region.
As early as 35 DPI, coronal slices from TMEV animals reveal a dramatic reduction in the bilateral hippocampal area and a corresponding enlargement of the lateral ventricles compared to age-matched, sham injected controls (Figure 5a–b, representative slices). Left and right hippocampi in TMEV animals are reduced in area by approximately 60% compared to control animals (Left: 1.549 ± 0.1211 mm2 PBS vs. 0.6764 ± 0.05415 mm2 TMEV, p<0.0001; Right: 1.595 ± 0.1107 PBS vs. 0.5845 ± 0.04217 mm2 TMEV, p<0.0001) while ventricles are enlarged approximately 67% (Left: 0.4311 ± 0.02658 mm2 PBS vs. 0.7593 ± 0.05939 mm2 TMEV, p<0.0001; Right: 0.4976 ± 0.05744 mm2 PBS vs. 0.9084 ± 0.08724 mm2 TMEV, p=0.0003; Figure 5e). While TMEV infection leads to substantial damage bilaterally, the right hippocampus (ipsilateral to the injection site) is particularly deteriorated within subjects compared to the left hippocampus (p=0.0037, paired t-test). No other bilateral comparison of hippocampal or ventricle area within a group was significant. Measures of dorsal hippocampus and ventricle areas in 250 DPI animals were not statistically distinguishable from 35 DPI animals.
Figure 5. Hippocampi from TMEV animals display sclerosis with severe tissue atrophy.
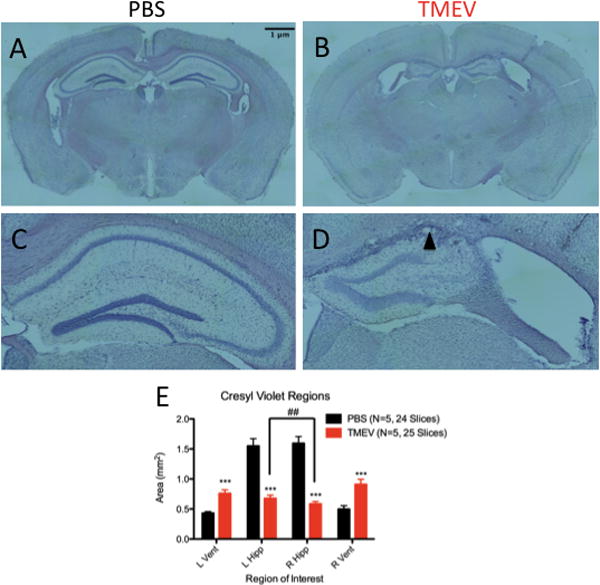
(A–D) Coronal sections (40μm) of dorsal hippocampi from control (A) and TMEV (B) animals. Clear atrophy of the hippocampus and enlargement of ventricles can be seen in slices from TMEV animals. At 10X magnification, clear preservation of cellular organization can be observed in the right hippocampus of a control animal (C); however, loss of CA1 and CA2 is evident in the right hippocampus of TMEV animals. Glial scarring (black arrow) is also observed in sclerosed TMEV hippocampi. (E) Quantification of left and right hippocampal and ventricle areas from control and TMEV animals. Both left and right hippocampal area is reduced in TMEV animals compared to controls, while lateral ventricles are enlarged. The right hippocampus, ipsylateral to the injection site, is more severely atrophied in TMEV animals compared to the left hippocampus. ##(p<0.01), paired t-test within subjects; ***(p<0.001) t-test between treatment groups. L=Left, R=Right, Vent=Ventricle, Hipp=Hippocampus. Data are displayed as mean ± S.E.M.
Hippocampal sclerosis is a common feature of coronal slices from TMEV animals. In order to assess the extent of hippocampal sclerosis, alternate slices from TMEV and PBS animals at 250 DPI were stained with cresyl violet, encompassing an approximate 320μm stretch of the rostral-caudal axis of the hippocampus (N=5 TMEV; N=3 PBS, 5 slices/animal). No age-matched, sham-injected control animal displayed sclerosis (Figure 5c, representative image). However, four out of five TMEV animals displayed complete sclerosis of CA1 bilaterally along the rostral-caudal axis analyzed (Figure 5d, representative image), where complete sclerosis denotes loss of the entire CA1 pyramidal layer along the rostral-caudal axis (Zarow et al., 2012). The remaining TMEV animal displayed complete sclerosis of CA1 ipsilateral to the site of injection and partial sclerosis of CA1 contralateral to the site of injection. Glial scarring is a prominent feature in the coronal sections processed from TMEV animals at the interface between cortex and the former CA1 region.
Of note, Fluoro Jade-B staining at 35 DPI and 250 DPI did not positively label any cell regions found within coronal slices containing the hippocampus, indicating cell death does not persist in TMEV animals as late as one month after i.c. injection (data not shown). However, cell death in hippocampus has been noted during acute infection (Stewart et al., 2009).
DISCUSSION
Using a battery of behavior tests and histological assessment, we have characterized basic and complex features that arise following acute encephalitic seizure in B6 mice.
Although TMEV-seized animals are initially ill during acute infection, we stipulate weight and motor coordination differences between groups are likely not confounding factors in interpreting behavioral outcomes during the first 30 DPI (open field and LD box testing). Notably, behavioral differences between treatments in the open field and LD box at 10-13 DPI (e.g. stereotypy, vertical activity, and entries into the lighted region) are also observed at 70-73 DPI in the absence of acute infection, weight differences, or motor coordination impairments. It is also important to mention that both LD box and OFT performance are influenced by motor activity as well as emotionality/affect (Karl et al., 2003). For both OFT and LD box tests, we did not detect treatment differences in the total distance traveled (cm); however, we could observe affective differences, as discussed below.
Numerous measures indicate TMEV animals display anxiety-like behavior. Vertical activity reduction in TMEV animals could be observed independent of any difference in horizontal locomotion in the open field. While consistent reductions in both horizontal and vertical movement in the open field are often indicative of motor toxicity (Lin et al., 2010), treatment differences in vertical activity alone (i.e. in the absence of any treatment effect on horizontal activity or other measures of locomotion) may provide an important biomarker for anxiety in rodent epilepsy models (Prut and Belzung, 2003). In support of this premise, certain animal models to date have demonstrated a bidirectional correspondence between vertical activity and anxiety (Müller et al., 2009, Tchekalarova et al., 2013). The TMEV model further substantiates this idea by demonstrating vertical activity deficits co-occurring with reduced time and movement within the exposed center of the open field and reduced entry into the lighted region of the LD box.
Alongside anxiety, cognitive impairment often presents as a debilitating complication of TLE and likely has strong associations with hippocampal pathology (Bell et al., 2011). In conjunction with previous work (Stewart et al., 2010), a titer-dependent effect of infection on hippocampal atrophy can be observed and provides a strategy to study cognition as an endotype linked to hippocampal pathology. At a lower titer, TMEV inoculation was not associated with complete sclerosis of CA1, or hippocampal atrophy to the extent described herein. The observed pathological consequences of high-titer TMEV infection, namely complete, bilateral sclerosis of CA1, relates to human post-mortem analyses describing bilateral or symmetrical sclerosis in hippocampi from patients with epilepsy (Thom et al., 2012) and in mixed autopsy samples with hippocampal sclerosis (Zarow et al., 2012). Further, a large proportion of autopsies containing hippocampal sclerosis match criteria for complete sclerosis of CA1 (Zarow et al., 2012). While our previous studies did not investigate cognition in association with hippocampal pathology, in the present study we show TMEV inoculation impacts spatial learning and memory in the MWM and object/place recognition in NOPR. In light of affective differences between control and TMEV animals, stress in the MWM may confound learning and memory differences (Murphy, 2013); however, tasks investigating object and/or placement novelty are considered relatively non-aversive (Ennaceur and Delacour, 1988). Previous research using object recognition paradigms has demonstrated the ability to dissociate different aspects of object recognition (e.g. location, features, and context) following discrete lesions of hippocampus or its afferent pathways (Hunsaker et al., 2007, Hunsaker et al., 2008, Hunsaker et al., 2013). Drawing on this research, we will initially examine treatment differences in novel object or novel place recognition tasks, where performance in each task encompasses different aspects of episodic memory (Ennaceur, 2010) and may be subserved by different cognitive structures and networks (Dere et al., 2007, Byun and Lee, 2010). Future studies may provide insight into functional impairment specific to hippocampal subregions, or nonhippocampal structures involved in learning and memory.
With the propensity for encephalitic seizures to develop into epilepsy, the need to determine disease-modifying treatment in this patient population is necessary. In the United States, nearly 19,000 people are hospitalized yearly due to encephalitis (Misra et al., 2008a). While the propensity to display acute symptomatic seizures during encephalitis varies widely, seizures developed following encephalitis are strongly associated with pharmacoresistance to currently available anti-seizure drugs and high mortality rates (Misra et al., 2008b). In the TMEV model, FluoroJade staining indicates cell death in hippocampus is an immediate feature (within a week of infection; Stewart et al., 2009) and does not persist as late as 30 DPI, highlighting the importance of early intervention.
By recapitulating key sequela of disease progression, TMEV inoculation serves as a relevant approach to model viral encephalitic seizures as a precipitating factor for epilepsy. Previous studies (Stewart et al., 2010) have detailed spontaneous recurrent seizures in TMEV animals as early as 2 months and as late as 7 months post infection using video EEG monitoring. Determining behavioral comorbidities in the TMEV model related to human epilepsy (i.e. anxiety and cognitive impairment) provides surrogate markers for testing the impact of anti-seizure drugs (ASDs) on comorbidity as well as seizure burden. Using the TMEV model, we are currently investigating the ability of ASDs to attenuate seizures and early comorbid outcomes if ASDs are administered during the acute encephalitic seizure phase. By providing relevant behavioral, ictal, and pathological endpoints as measures of efficacy, the TMEV model of infection-induced TLE provides a novel platform to test disease-modifying treatments during the disease process.
Highlights.
We investigated behavioral comorbidity in the TMEV model of temporal lobe epilepsy
TMEV animals display anxiety-like behavior in the open field and light/dark box
Acute infection leads to impaired episodic memory in novel object place tasks
TMEV inoculation also leads to impaired spatial memory in the Morris water maze
Hippocampal area reductions and bilateral sclerosis of CA1 is evident
Acknowledgments
We would like to thank Dr. Kristen Keefe for use of her freezing-stage microtome and Dr. Joanna Beachy for use of her Morris water maze apparatus.
Funding: NIH RO1 NS065434 (HSW and KSW)
NIH T32 NS076067 (ADU and GJR)
NIH HHSN 271201100029C (HSW)
Footnotes
Conflicts of Interest
HSW discloses that he is one of two scientific co-founders of NeuroAdjuvants, Salt Lake City, Utah.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams SJ, O’Brien TJ, Lloyd J, Kilpatrick CJ, Salzberg MR, Velakoulis D. Neuropsychiatric morbidity in focal epilepsy. The British journal of psychiatry : the journal of mental science. 2008;192:464–469. doi: 10.1192/bjp.bp.107.046664. [DOI] [PubMed] [Google Scholar]
- Bell B, Lin JJ, Seidenberg M, Hermann B. The neurobiology of cognitive disorders in temporal lobe epilepsy. Vol. 7. Nature Publishing Group; 2011. pp. 154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin M, Hascoët M. The mouse light/dark box test. European Journal of Pharmacology. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Bath KG, Berg AT, Galanopoulou AS, Holmes GL, Jensen FE, Kanner AM, O'Brien TJ, Whittemore VH, Winawer MR, Patel M, Scharfman HE. Issues related to symptomatic and disease-modifying treatments affecting cognitive and neuropsychiatric comorbidities of epilepsy. Epilepsia. 2013;54:44–60. doi: 10.1111/epi.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun J, Lee I. Disambiguation of Similar Object-Place Paired Associations and the Roles of the Brain Structures in the Medial Temporal Lobe. Exp Neurobiol. 2010;19:15–18. doi: 10.5607/en.2010.19.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmagro CL, Velasco TR, Bianchin MM, Martins AP, Guarnieri R, Cescato MP, Carlotti CG, Jr, Assirati JA, Jr, Araujo D, Jr, Santos AC, Hallak JE, Sakamoto AC. Psychiatric comorbidity in refractory focal epilepsy: a study of 490 patients. Epilepsy & behavior : E&B. 2012;25:593–597. doi: 10.1016/j.yebeh.2012.09.026. [DOI] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neuroscience & Biobehavioral Reviews. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. Behavioural brain research. Vol. 215. Elsevier B.V; 2010. One-trial object recognition in rats and mice: Methodological and theoretical issues; pp. 244–254. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behavioural brain research. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Fiest KM, Dykeman J, Patten SB, Wiebe S, Kaplan GG, Maxwell CJ, Bulloch AG, Jette N. Depression in epilepsy: a systematic review and meta-analysis. Neurology. 2013;80:590–599. doi: 10.1212/WNL.0b013e31827b1ae0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS. Epilepsy from the Patient’s Perspective: Review of Results of a Community-Based Survey. Epilepsy & behavior : E&B. 2000;1:S9–S14. doi: 10.1006/ebeh.2000.0107. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Chen V, Tran GT, Kesner RP. The medial and lateral entorhinal cortex both contribute to contextual and item recognition memory: A test of the binding of items and context model. Hippocampus. 2013;23:380–391. doi: 10.1002/hipo.22097. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Mooy GG, Swift JS, Kesner RP. Dissociations of the medial and lateral perforant path projections into dorsal DG, CA3, and CA1 for spatial and nonspatial (visual object) information processing. Behavioral Neuroscience. 2007;121:742–750. doi: 10.1037/0735-7044.121.4.742. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Rosenberg JS, Kesner RP. The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus. 2008;18:1064–1073. doi: 10.1002/hipo.20464. [DOI] [PubMed] [Google Scholar]
- Kandratavicius L, Rosa-Neto P, Monteiro MR, Guiot M-C, Assirati J, Alberto Joao, Carlotti J, Gilberto Carlos, Kobayashi E, Leite JP. Distinct increased metabotropic glutamate receptor type 5 (mGluR5) in temporal lobe epilepsy with and without hippocampal sclerosis. Hippocampus. 2013:n/a–n/a. doi: 10.1002/hipo.22160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner AM. Can neurobiological pathogenic mechanisms of depression facilitate the development of seizure disorders? Lancet neurology. 2012;11:1093–1102. doi: 10.1016/S1474-4422(12)70201-6. [DOI] [PubMed] [Google Scholar]
- Kanner AM. The treatment of depressive disorders in epilepsy: What all neurologists should know. Epilepsia. 2013;54:3–12. doi: 10.1111/epi.12100. [DOI] [PubMed] [Google Scholar]
- Karl T, Pabst R, von Horsten S. Behavioral phenotyping of mice in pharmacological and toxicological research. Experimental and toxicologic pathology : official journal of the Gesellschaft fur Toxikologische Pathologie. 2003;55:69–83. doi: 10.1078/0940-2993-00301. [DOI] [PubMed] [Google Scholar]
- Kirkman NJ, Libbey JE, Wilcox KS, White HS, Fujinami RS. Innate but not adaptive immune responses contribute to behavioral seizures following viral infection. Epilepsia. 2010;51:454–464. doi: 10.1111/j.1528-1167.2009.02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Once initiated, viral encephalitis-induced seizures are consistent no matter the treatment or lack of interleukin-6. J Neurovirol. 2011;17:496–499. doi: 10.1007/s13365-011-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Kirkman NJ, Smith MCP, Tanaka T, Wilcox KS, White HS, Fujinami RS. Seizures following picornavirus infection. Epilepsia. 2008;49:1066–1074. doi: 10.1111/j.1528-1167.2008.01535.x. [DOI] [PubMed] [Google Scholar]
- Lin Z, Dodd CA, Filipov NM. Neurotoxicology and Teratology. Vol. 39. Elsevier Inc; 2010. Short-term atrazine exposure causes behavioral deficits and disrupts monoaminergic systems in male C57BL/6 mice; pp. 1–10. [DOI] [PubMed] [Google Scholar]
- Michael BD, Solomon T. Seizures and encephalitis: Clinical features, management, and potential pathophysiologic mechanisms. Epilepsia. 2012;53:63–71. doi: 10.1111/j.1528-1167.2012.03615.x. [DOI] [PubMed] [Google Scholar]
- Misra UK, Tan CT, Kalita J. Viral encephalitis and epilepsy. Epilepsia. 2008a;49:13–18. doi: 10.1111/j.1528-1167.2008.01751.x. [DOI] [PubMed] [Google Scholar]
- Misra UK, Kalita J, Nair PP. Status Epilepticus in Central Nervous System Infections: An Experience From a Developing Country. The American Journal of Medicine. 2008b;121:618–623. doi: 10.1016/j.amjmed.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Müller CJ, Gröticke I, Bankstahl M, Löscher W. Experimental Neurology. Vol. 219. Elsevier Inc; 2009. Behavioral and cognitive alterations, spontaneous seizures, and neuropathology developing after a pilocarpine-induced status epilepticus in C57BL/6 mice; pp. 284–297. [DOI] [PubMed] [Google Scholar]
- Murphy GG. Spatial Learning and Memory-What's TLE Got To Do With It? Epilepsy Curr. 2013;13:26–29. doi: 10.5698/1535-7511-13.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Rezvani AH, Djouma E, Parsian A, Lawrence AJ. Depressive-like behavior and high alcohol drinking cooccur in the FH/WJD rat but appear to be under independent genetic control. Neuroscience and biobehavioral reviews. 2007;31:103–114. doi: 10.1016/j.neubiorev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Pineda E, Shin D, Sankar R, Mazarati AM. Comorbidity between epilepsy and depression: Experimental evidence for the involvement of serotonergic, glucocorticoid, and neuroinflammatory mechanisms. Epilepsia. 2010;51:110–114. doi: 10.1111/j.1528-1167.2010.02623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European Journal of Pharmacology. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalography and clinical neurophysiology. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, Taniguchi K, West DB, Ohmen JD, Beauchamp GK, Bachmanov AA. Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:938–946. doi: 10.1523/JNEUROSCI.1374-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart K-AA, Wilcox KS, Fujinami RS, White HS. Theiler’s virus infection chronically alters seizure susceptibility. Epilepsia. 2009;51:1418–1428. doi: 10.1111/j.1528-1167.2009.02405.x. [DOI] [PubMed] [Google Scholar]
- Stewart KA, Wilcox KS, Fujinami RS, White HS. Development of postinfection epilepsy after Theiler’s virus infection of C57BL/6 mice. Journal of neuropathology and experimental neurology. 2010;69:1210–1219. doi: 10.1097/NEN.0b013e3181ffc420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchekalarova J, Petkova Z, Pechlivanova D, Moyanova S, Kortenska L, Mitreva R, Lozanov V, Atanasova D, Lazarov N, Stoynev A. Epilepsy and Behavior. Vol. 27. Elsevier Inc; 2013. Prophylactic treatment with melatonin after status epilepticus: Effects on epileptogenesis, neuronal damage, and behavioral changes in a kainate model of temporal lobe epilepsy; pp. 174–187. [DOI] [PubMed] [Google Scholar]
- Thom M, Liagkouras I, Martinian L, Liu J, Catarino CB, Sisodiya SM. Epilepsy Research. Vol. 102. Elsevier B.V; 2012. Variability of sclerosis along the longitudinal hippocampal axis in epilepsy: A post mortem study; pp. 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston GP, Stretton J, Sidhu MK, Symms MR, Thompson PJ, Duncan JS. Structural correlates of impaired working memory in hippocampal sclerosis. Epilepsia. 2013;54:1143–1153. doi: 10.1111/epi.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarow C, Weiner MW, Ellis WG, Chui HC. Prevalence, laterality, and comorbidity of hippocampal sclerosis in an autopsy sample. Brain and Behavior. 2012;2:435–442. doi: 10.1002/brb3.66. [DOI] [PMC free article] [PubMed] [Google Scholar]


