Abstract
Status epilepticus (SE) is a life threatening condition that often precedes the development of epilepsy. Traditional treatments for epilepsy have been focused on targeting neuronal mechanisms contributing to hyperexcitability, however, approximately 30% of patients with epilepsy do not respond to existing neurocentric pharmacotherapies. A growing body of evidence has demonstrated that profound changes in the morphology and function of astrocytes accompany SE and persist in epilepsy. Astrocytes are increasingly recognized for their diverse roles in modulating neuronal activity, and understanding the changes in astrocytes following SE could provide important clues about the mechanisms underlying seizure generation and termination. By understanding the contributions of astrocytes to the network changes underlying epileptogenesis and the development of epilepsy, we will gain a greater appreciation of the contributions of astrocytes to dynamic circuit changes, which will enable us to develop more successful therapies to prevent and treat epilepsy. This review summarizes changes in astrocytes following SE in animal models of SE and human temporal lobe epilepsy and addresses the functional consequences of those changes that may provide clues to the process of epileptogenesis.
Keywords: Reactive astrocyte, Gliosis, Status Epilepticus, Review, Epileptogenesis, Inflammation, Epilepsy, Seizure
Status Epilepticus Can Result in Temporal Lobe Epilepsy
Status epilepticus (SE) is a medical emergency with a poor prognosis. It is defined as continuous seizure activity lasting greater than 5 minutes or two or more sequential seizures without full recovery between seizures (Lowenstein et al., 1999). While the risk of death following SE has declined somewhat in the industrialized world, older patients experiencing prolonged SE are still at high risk of death. Furthermore, patients who survive a prolonged bout of SE often experience cognitive decline and develop epilepsy (Engel, 1996). Patients with temporal lobe epilepsy (TLE) often first present with SE. TLE is a common seizure disorder characterized by complex partial seizures that can secondarily generalize and are often pharmacoresistant to currently available anti-seizure drugs (ASDs). Because SE can result in difficult to treat forms of epilepsy such as TLE, it is the focus of much research.
Administration of chemoconvulsant agents and electrical stimulation have been used in rodents to induce SE that is then followed by the progressive development of epilepsy. Two of the most well studied rodent models of SE are the systemic kainic acid (KA) and pilocarpine (PILO) models. Following injection of either of these two agents, animals begin a bout of SE that can last hours (Curia et al., 2008; Lehmkuhle et al., 2009). While mortality is lower for the KA model, survival can be enhanced in the PILO model by treatment with a variety of anticonvulsant agents after about an hour of SE. After a latent period of several days to weeks following SE, animals develop recurrent seizures that are limbic in origin. Animals treated with a repeated low dose paradigm of KA have been found to develop spontaneous convulsant seizures on average 18 days following SE (Williams et al., 2009), while animals treated with pilocarpine begin to exhibit spontaneous seizures earlier, with some reported seizures occurring within five days following SE (Raol et al., 2006). Likewise, electrical stimulation of the perforant pathway can induce sustained SE that is followed by the development of recurrent seizures. Depending on the stimulus paradigms used to evoke SE in these models, spontaneous seizures can begin within days (Bumanglag and Sloviter, 2008; Norwood et al., 2010). In all of these models, considerable neuronal cell death is observed throughout the brain and astrocytes begin to exhibit signs of reactive gliosis soon after evoking SE (do Nascimento et al., 2012; Glushakova et al., 2012; Lauritzen et al., 2012a).
Animal models of SE-induced epilepsy provide an unparalleled opportunity to unravel the complex changes that occur in the CNS following SE and during the process of epileptogenesis. In particular, they help identify disease-modifying therapies that can prevent the development of epilepsy following SE or stop the progression of seizure activity once epilepsy is established. While much is known about the complex changes that occur in neurons following SE in these animal models, researchers are now investigating the changes that occur in astrocytes following SE. Astrocytes post-SE may develop functional changes that contribute to, or even prevent, network excitability. By understanding these functional changes in astrocytes, it may be possible to identify novel therapeutic targets or strategies for the prevention of epilepsy. In this review, we will focus our attention on recent findings of the effect of SE or related CNS insults on astrocyte function in limbic and cortical regions known to be associated with the initiation of seizure activity and the development of epilepsy.
Astrocytes Become Reactive Following SE and This Persists in Epilepsy
Since their discovery over 100 years ago, glial cells have traditionally been considered only as passive support cells of the nervous system (for reviews on the history of glial research, see Garcia-Marin et al., 2007; Somjen, 1988; Verkhratsky, 2006). However, recent application of cellular imaging, physiology, and genetics to astrocytes has shown that they are active participants at the neuronal synapse; their diverse roles are entwined with the function of neurons. The structural and functional relationship between astrocytes and the neuronal synapse has led to the use of the term “tripartite synapse” to reflect the important contribution of astrocytes in influencing synaptic transmission (Halassa et al., 2007). The processes of astrocytes are in close proximity to neuronal membranes and express a host of receptors, transporters, and ion channels that enable astrocytes to “listen” to the chemical conversation at the synapse. In return, they are able to “respond” and modify the tone of synaptic transmission by regulating the availability of neurotransmitters and ions in the synaptic cleft, such as glutamate and potassium.
As a consequence of SE and subsequent inflammation, astrocytes change their morphology and protein expression in a process termed reactive astrogliosis. A number of molecules and signaling pathways are known to trigger reactive astrogliosis (for a comprehensive review, see (Sofroniew, 2009)) and many of those receptors/pathways are activated as a consequence of SE. Molecules released from dying neurons, such as reactive oxygen species, ATP and glutamate, are all known to induce astrogliosis. In addition, inflammatory cytokines such as TNF-α and Il1-β are also known triggers and these cytokines can be released by microglia and neurons following SE. Interestingly, cytokines are also released by reactive astrocytes, which can result in a feed-forward system that enhances gliosis (Vezzani et al., 2011). Furthermore, the JAK-STAT pathway is known to be elevated as a consequence of SE and this pathway, when stimulated, can also contribute to the observed changes in astrocytes (Lund et al., 2008; Sofroniew, 2009).
While reactive astrogliosis is one of the most common pathological lesions in epilepsy and other brain insults, it continues to be poorly understood. Dramatic changes in the expression of neurotransmitter receptors, voltage gated ion channels, inflammatory cytokines, and other proteins, have been identified in astrocytes in both animal models and human TLE. However, the time course and the functional significance of such diverse changes that accompany astrogliosis is not clear and it is currently unknown to what extent “reactive astrocytes” either contribute to, or prevent the ultimate development of TLE. Indeed, some components of reactive astrogliosis may be critical compensatory mechanisms following injury, resulting in the dampening of excitability, while other changes, e.g. decreased expression of aquaporin, may in fact contribute to epileptogenesis and/or seizure generation (Lee et al., 2012). Finally, it is possible that some alterations in structure and/or function of astrocytes may even be unrelated to epileptogenesis. We contend that the study of the role of reactive astrogliosis in epileptogenesis is in its infancy and therefore, before disease modifying therapies can be identified for the treatment and/or prevention of SE-induced epilepsy, a mechanistic understanding of structure and function of reactive astrocytes in the disease process must be attained.
Properties of Reactive Gliosis Following SE
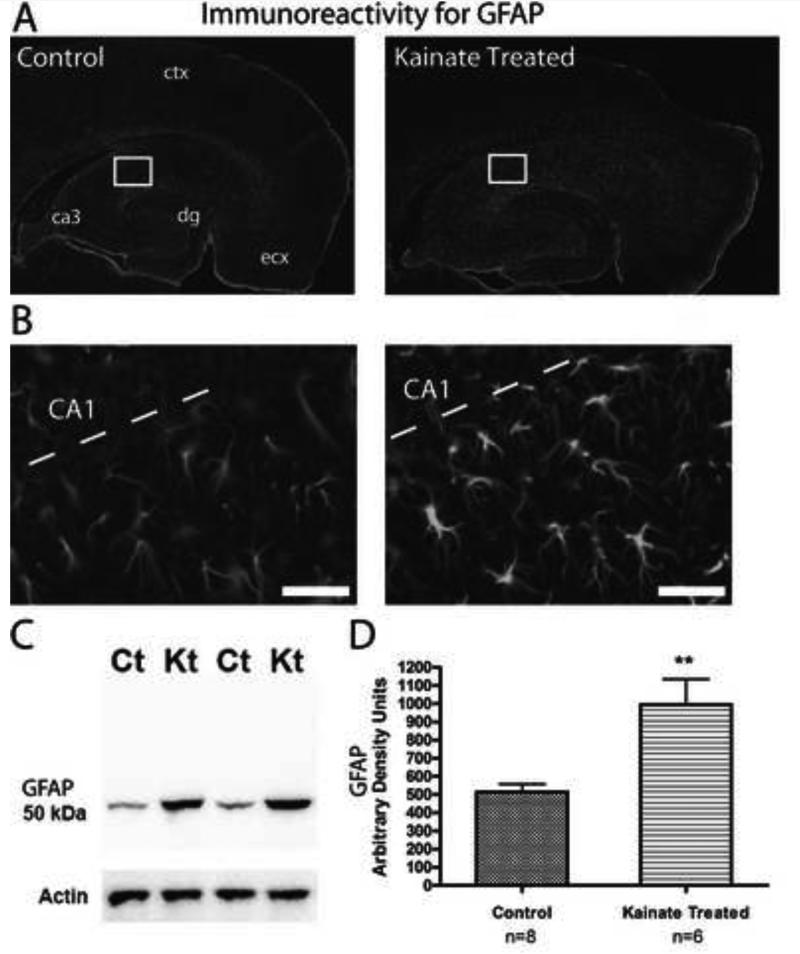
Following SE, reactive astrocytes become hypertrophic, increase expression of intermediate filament proteins (for example, GFAP), and develop longer and thicker processes (Binder and Steinhauser, 2006) (Figure 1). Although the functional consequence of changes in domain organization (see below) and hypertrophied processes with increased expression for GFAP are not exactly clear (Fedele et al., 2005; Gouder et al., 2004), some of the changes in expression of other proteins could have direct consequences on the regulatory mechanisms of gene transcription in reactive astrocytes (Ridet et al., 1997). The term “reactive astrocyte” is rather ambiguous because reactive astrogliosis is not an all-or-none response (Sofroniew, 2009). There can be significant diversity within a reactive astrocyte population (Kálmán, 2003; Ridet et al., 1997). Mild to moderate gliosis, found early on in SE models, typically does not induce the proliferation of new astrocytes (Fernaud-Espinosa et al., 1993; Sofroniew, 2009). There are, however, some studies that have addressed the proliferative ability of astrocytes in the CA fields of the hippocampus following KA-induced SE or lesions (Murabe et al., 1982; Niquet et al., 1994). These studies show that while astrocytes can in fact proliferate after insult, the proliferation accounts for a very small number of the new cells in the area, suggesting that in models of SE, reactive astrocytes are comprised mainly of the resident astrocytes present before the insult.
Figure 1.
Reactive astrocytes are present in the hippocampus one week following KA-induced SE. (A) Immunoreactivity for GFAP is observed throughout the hippocampus and cortex in horizontal brain sections from both control and KA-treated rats. dg = dentate gyrus, ecx = medial entorhinal cortex. (B) Close up of CA1 and stratum radiatum from the area indicated by the white box in (A). Sections from KA-treated rats (right) show characteristics typical of reactive astrocytes including a hypertrophied morphology and an increase in the immunoreactivity for GFAP. Scale bar, 50 μm. (C) Western blot of crude hippocampal membrane fractions from control (Ct) and kainate-treated (Kt) animals probed with an antibody directed against GFAP. Actin served as a lane loading control. (D) Densitometry values of immunoreactivity for GFAP from kainate-treated samples (n = 6) were found to be significantly increased compared to control (n = 8) samples (**p < 0.0001, Student's t test). Figure reprinted from (Takahashi et al., 2010) with permission from Elsevier.
Two of the hallmarks of reactive astrocytes are the increase in expression of intermediate filament proteins (for example, GFAP) in response to injury (Ridet et al., 1997) and hypertrophy of the main cytoskeletal branches. During this process, however, the fine spongiform processes of astrocytes are not immunoreactive for intermediate filaments. GFAP immunolabeling, therefore, does not detect changes in the spatial domains (i.e. cell volume) of reactive astrocytes in the neuropil. Changes in domain organization in reactive astrocytes are important to consider, as they may be indicative of astrocytes that are no longer controlling or modulating the same group of synapses and these changes could indicate pathological astrocyte-astrocyte or astrocyte-neuron relationships.
Two independent groups investigated whether domain organization is preserved in reactive astrocytes. Wilhelmsson et al. (2006) dye filled astrocytes in the hippocampus following disruption of the perforant path from the entorhinal cortex. In this model, astrocytes increased the expression of GFAP and were reactive (presumably due to the axonal degeneration of the perforant path) but did not change their domain volume and did not interdigitate with neighboring astrocytes; thus, reactive astrocytes in this model maintained their spatial domains. In contrast, Oberheim et al. (2008) diolistically labeled astrocytes in the hippocampus of three mouse models of epilepsy: the ferrous chloride model of post traumatic epilepsy, KA-induced SE model, and the SWXL-4 mouse, a genetic model of epilepsy. Hypertrophied astrocytes (observed with GFAP) in these models of epilepsy had increased domain volumes and almost a complete loss of domain organization (observed with diolistic labeling). These changes were observed 1 week post injection of ferrous chloride, and 6 months after, during a time of chronic seizure activity. Interestingly, in ferrous chloride injected animals whose seizures were reduced with the anti-epileptic drug valproate, astrocytes were less hypertrophied, had domain volumes similar to controls, and did not lose their domain organization by interdigitating extensively with neighboring astrocytes. Therefore, changes in domain organization paralleled the severity of seizure activity.
The results from these studies on the changes in spatial domains of reactive astrocytes suggest that a loss of domain organization may be specific to astrocytes in epileptic tissue. In support of this notion, Oberheim et al. (2008) noted that reactive astrocytes in a mouse model of Alzheimer's disease did not lose their domain organization, even though the astrocytes in this model were reactive (i.e. hypertrophied processes and an increase in GFAP expression). The research highlights two salient points. The first point is that reactive astrocytes, as determined by increased immunoreactivity to GFAP and hypertrophied processes, may not be homogeneous. Astrocytes may respond differently according to the type of CNS insult, even though they may appear identical by immunohistochemistry (i.e. reactive, as revealed by GFAP). Indeed, recent genomic analysis of reactive astrocytes revealed a different pattern of gene expression depending on the type of insult (Zamanian et al., 2012). The CNS insults compared in this study were ischemia and LPS injection. Therefore, while it is not clear what patterns of gene expression would be observed following SE, it is possible that an entirely different gene expression pattern would be observed. Second, reactive astrocytes that have lost their domain organization in epileptic tissue may confer unique functional properties that are specific to this neurological disorder. Based on the differences in structural changes, therefore, reactive astrocytes in various models of neuronal injury may have different functional properties. This is particularly important to note because reactive astrocytes are often considered a homogenous population, although the data increasingly indicates that the term reactive gliosis encompasses a very wide range of features with important functional consequences to studying pathologies. An additional point to consider is that the work described above was performed in rodents. However, there exists a dramatic heterogeneity of human astrocytes. It is not currently known how the individual types of human astrocytes differentially respond to either SE or prolonged TLE (Oberheim et al., 2009).
Potassium Buffering Is Not Altered Shortly after SE
Astrocytes play a critical role in maintaining neuronal homeostasis by buffering extracellular potassium (K+) through the highly permeable, inwardly rectifying potassium channel KIR4.1 (Higashi et al., 2001; Poopalasundaram et al., 2000). Neurons extrude K+ with each action potential repolarization and during periods of high activity, K+ concentration in the extracellular space can be rapidly elevated (Dichter et al., 1972; Nicholson and Sykova, 1998). Thus, the tight regulation of [K+]o via astrocytic uptake is critical for maintaining neuronal homeostasis. Given the importance of KIR channels in spatial buffering and the role of potassium in neuronal function, it is no surprise that its role has been heavily investigated in epilepsy. For instance, conditional knock-out of the KIR 4.1 channel in mice results in behavioral ataxia, seizures, and early lethality is observed resulting from deficient K+ uptake in astrocytes (Djukic et al., 2007). Likewise, in the sclerotic hippocampus of patients with TLE, the KIR channel blocker Ba2+ was shown to be ineffective in increasing extracellular potassium levels measured by potassium selective microelectrodes in response to stimulus as was expected, suggesting dysfunction in KIR channels (Kivi et al., 2000). This finding was also observed in some brain slices obtained from epileptic rats that had received pilocarpine to induce SE (Gabriel et al., 1998). These results were corroborated using patch-clamp electrophysiology in acute hippocampal slices obtained from surgical specimens of patients with TLE, which showed reduced inward rectifying potassium conductances in reactive astrocytes from this tissue (Hinterkeuser et al., 2000). Together these findings support the altered ability of astrocytes to effectively buffer potassium in epileptic tissue. However, until recently, changes in KIR and potassium uptake have not been investigated in reactive astrocytes during the latent period in an animal model of SE-induced MTLE. Studies from our laboratory (Takahashi et al. 2010) have demonstrated that potassium conductances were not altered in astrocytes during the latent period one-two weeks following SE in the KA-treated rat. This finding suggests that soon after SE and prior to the development of epilepsy, astrocyte potassium buffering capability remains intact. Further investigation is necessary to determine how and if KIR function contributes to epileptogenesis. Indeed, therapeutic strategies which result in the continued expression and function of specific KIR channels in astrocytes might prove to be useful in delaying the onset of epilepsy following SE.
Gap Junctions Between Astrocytes Increase Following SE
Astrocytes are by far the most extensively gap junction coupled cells in the central nervous system and are critical in maintaining homeostatic balance of ions and molecules (for reviews, see Kielian, 2008; Nagy and Rash, 2000; Theis et al., 2005). A complete gap junction is formed by the direct contact of two opposing hemichannels, or connexons, on two different cells. Each connexon consists of a hexameric structure consisting of six connexin (Cx) proteins with a hydrophilic pore at its center. This pore allows transfer of ions, second messengers, metabolites, and other small molecules (Nakase and Naus, 2004), allowing not only electrical coupling between cells but also chemical and metabolic coupling. Astrocytes express predominately Cx43 and Cx30 and form a metabolically coupled syncytium in CNS tissue (Rash et al., 2001).
Our laboratory recently showed that the number of coupled astrocytes in brain slices was significantly increased in KA-treated rats early in the process of epileptogenesis, and consistent with that observation, CX43 expression was greatly elevated (Takahashi et al., 2010). Interestingly, in brain slices obtained from normal rats, epileptiform burst-like activity has been shown to enhance coupling between astrocytes in a rapid activity dependent manner (Rouach et al., 2008). The extent of coupling in astrocytes is thought to underlie spatial buffering and the ability to redistribute potassium and glutamate (Theis et al., 2005). Gap junction coupling has also been implicated in spreading depression, a pathophysiological phenomenon in the CNS characterized by a combined reaction of neurons and glia in the form of a propagating wave of neuronal depolarization followed by neuronal inactivation. Interestingly, a conditional knock out of the main gap junction protein in astrocytes, connexin (Cx)43, results in an animal with decreased intracellular coupling among astrocytes and enhanced velocity in the wave of spreading depression (Theis et al., 2003), suggesting that the redistribution of ions and intracellular molecules in astrocytes is critical for minimizing spreading depression and hyperexcitability. Therefore, in animals experiencing SE, an increase in gap junctionally-coupled astrocytes could be viewed as compensatory, with an enhanced capacity for buffering both glutamate and potassium.
The data concerning the expression of Cx43 and Cx30 in other models of chronic epilepsy and in human resected tissue are conflicting. In the kindled rat and tetanus toxin-induced model of epilepsy, no change in mRNA of Cx43 was observed four weeks following injury (Elisevich et al., 1997a). Likewise, no change in mRNA or protein expression of Cx43 or Cx30 was detected in kindled rats and in the kainate-treated rat four weeks following injury (Sohl et al., 2000). However, in human resected tissue from patients with intractable epilepsy, Cx43 mRNA and protein are generally shown to be increased (Collignon et al., 2006; Fonseca et al., 2002; Naus et al., 1991) except for (Elisevich et al., 1997b). There is evidence of increased gap junction coupling by measuring calcium signaling induced by glutamate exposure using fluorescence recovery after photo bleaching (FRAP) assays in surgically resected tissue (Lee et al., 1995). These findings suggest that gap junction coupling, along with increased Cx43 and Cx30 expression, might be a common feature in human epilepsy, something not always recapitulated in animal models of chronic epilepsy. Some of the discrepancies between studies could be due to the dynamic nature of the connexin protein. Connexins have a rapid turnover rate of ~1 hour and the antigens that have been used to label Cx43 in the past were difficult to detect and were sensitive to fixation (Hossain et al., 1994; Laird et al., 1991).
Glutamate Transporter Function is Enhanced Following SE
Unlike some neurotransmitters that can be enzymatically degraded after being released into the synaptic cleft, glutamate must be actively removed via Na+-dependent glutamate transporters. Fast efficient clearance of glutamate is essential for high signal-to-noise ratios in neurotransmission and for the prevention of excitotoxicity (for review see Danbolt, 2001; Marcaggi and Attwell, 2004; Tzingounis and Wadiche, 2007). The main glutamate transporters, GLAST and GLT-1 are predominately expressed in astrocytes (Lehre et al., 1995), and are responsible for >90% of the total glutamate uptake (Tanaka et al., 1997). The alterations that occur to this system, as well as the closely linked processes governing glutamate and glutamine cycling, during epilepsy are complex and the literature is often conflicting in regards to changes in glutamate transporter expression and function following SE.
In animal models, as would be expected, knock down of GLT-1 expression in the mouse leads to neuronal loss and seizure activity that is caused by the build up of extracellular glutamate (Tanaka et al., 1997). Similarly, eight weeks following stereotaxically injected KA into the amygdala of rats, spontaneous motor seizure activity is observed and hippocampal tissue homogenates show a reduced expression of GLT-1 and GLAST (Ueda et al., 2001). In human tissue from patients with TLE, immunohistochemistry consistently demonstrates reductions in GLT-1 and GLAST expression (Mathern et al., 1999; Proper et al., 2002; Sarac et al., 2009). However, similar studies in human tissue using western blots on hippocampal homogenates do not show a change in GLT-1 or GLAST expression (Bjornsen et al., 2007; Tessler et al., 1999). However, the changes in transporter protein expression described above do not provide a direct assessment of the changes in transporter dependent glutamate uptake in epilepsy. A more quantitative measurement of transporter function, and hence glutamate clearance, in epileptic tissue is to directly record synaptically evoked glutamate transporter dependent currents in reactive hippocampal astrocytes.
In a recent study, we recorded from astrocytes in hippocampal brain slices obtained from control animals and those that had been subjected to KA-induced SE. We demonstrated that there is no effect of SE on the amplitude of glutamate transport currents, nor any concomitant change in the expression of GLT-1 expressed in astrocytes within 1-2 weeks after SE. However, we did determine that there is a significant decrease in the decay kinetics of glutamate transport currents recorded in reactive astrocytes, suggesting that following SE, astrocytes have an enhanced capacity to more quickly take up extracellular glutamate (Takahashi et al., 2010). Previous work by other investigators demonstrated that the time course of glutamate transport currents is not affected by changes in stimulus strength, the probability of release of glutamate, or the diffusion kinetics of glutamate. Instead, the rate of decay of the current is likely due to a variety of mechanisms, including the number and type of glutamate transporters present at the synapse and the extracellular volume (Diamond, 2005; Diamond and Jahr, 2000). Therefore, there are several hypotheses that could explain the faster kinetics of the glutamate transport currents in reactive astrocytes. For example, it has recently been demonstrated that the GLT-1 transporter is rapidly trafficked to activated synapses. (Benediktsson et al., 2012). Therefore, it is possible that following the intense neural activity that occurs during SE, existing transporters may be trafficked to activated synapses. It is also known that several astrocyte signaling pathways can regulate glutamate transporter trafficking (Robinson, 2002) and while we did not detect a change in GLT-1 expression in our studies, it is possible that trafficking of existing transporters could contribute to the changes we observed. Alternatively, decreased extracellular volume contributes to the observed decrease in glutamate transporter current decay kinetics observed during development (Thomas et al., 2011) and thus may also play a role in epilepsy, where the extracellular space is reduced and known to contribute to hyperexcitability (Hochman, 2012). Finally, the rapid buffering of glutamate observed following SE may also be facilitated by the increase in gap junctions between astrocytes (Takahashi et al., 2010). Indeed, recent work in animals with Cx43 and Cx30 deleted from astrocytes, and consequently no gap junctions between astrocytes, demonstrated that the decay time constant of the glutamate transport current was significantly slowed, consistent with the notion that extensive networks are essential for proper buffering of glutamate (Pannasch et al., 2011). Thus, the maintenance of extensive networks of coupled astrocytes could very well be viewed as an essential mechanism for dampening excitability in epileptic networks.
Glutamate Receptor Expression
Astrocyte function depends in part on cell surface receptor expression. Astrocytes in juvenile animals and in cell culture have been shown to express nearly every transmitter receptor as their neuronal counterparts, including metabotropic and ionotropic glutamate receptors; for review see (D'Antoni et al., 2008; Kimelberg, 1995; Lalo et al., 2010). In brain slices obtained from juvenile animals, the functional expression of a-amino-3-hdroxy-5-methyl-4-isoxazolepropionic acid (AMPA) (Jabs et al., 1994; Schroder et al., 2002; Seifert et al., 2004; Seifert et al., 1997) and N-methyl-D-aspartate receptors (NMDA) (Serrano et al., 2008; Steinhauser et al., 1994) have been described. Expression for group I mGluRs is increased in reactive astrocytes 1 week after SE (Aronica et al., 2000), in human MTLE (Notenboom et al., 2006), and in reactive astrocytes following neuronal injury (Ferraguti et al., 2001; Ulas et al., 2000), however, the electrically passive, GFAP-expressing, protoplasmic astrocyte of the adult hippocampus does not express ionotropic glutamate receptors under basal conditions. Interestingly, kainate receptors (KARs) and NMDA receptors are expressed in reactive astrocytes following ischemia in adult animals (Gottlieb and Matute, 1997; Krebs et al., 2003). Furthermore, two recent reports have demonstrated that the protoplasmic GFAP+ astrocyte of the cortex acquires electrophysiological responses to kainate application following ischemic insult or within an Alzheimer's disease model, again suggesting possible KAR expression in response to injury (Peters et al., 2009; Wang et al., 2008). Interestingly, preliminary immunohistochemical studies from our laboratory suggests that following SE, astrocytes begin to express KAR subunits (Vargas et al., 2006).
The modulating role of KARs and their unique ability to couple to G-proteins makes this family of glutamate receptor an important component of CNS function. It is currently unknown what functional role KARs expressed in astrocytes following KA-induced SE play in the disease process of epileptogenesis. However, it is enticing to hypothesize that increased calcium signaling and subsequent gliotransmission mediated in part by the pathological expression of ionotropic KARs and increased expression of mGluRs in reactive astrocytes could cause the synchronization of neurons thus contributing to hyperexcitability.
Blood-Brain Barrier Disruption Following SE
Astrocytes form close associations with the endothelial cells of capillaries and help maintain proper function of the blood brain barrier (BBB). Disruption of the BBB is often observed in epileptic brain regions, but has generally been thought to result from seizure activity, rather than contribute to the generation of seizures. A body of clinical and experimental evidence now suggests that opening of the BBB triggers a chain of events that may contribute to the processes underlying epileptogenesis. Van Vliet and coworkers have demonstrated that pronounced levels of BBB leakage occur during the acute and latent phases following SE in humans and rats (van Vliet et al., 2007). In addition, following BBB disruption, uptake of the serum protein albumin by astrocytes through TGF-β receptors has been associated with reactive gliosis (Ivens, Kaufer et al. 2007). Studies in cultured astrocytes from rat brains show that albumin induces calcium signaling (Nadal et al., 1995) and that TGF-β receptor activation causes a down regulation of KIR channels (Perillan et al., 2002), suggesting that albumin can directly modulate astrocyte functions and enhance excitability. Together these data suggest that disruptions of the blood brain barrier may directly contribute to processes underlying epileptogenesis through astrocyte-mediated mechanisms (Ivens et al., 2007).
It is also important to note that changes in BBB permeability following SE and other insults may also have significant consequences for delivery of pharmacotherapies in drug-resistant epilepsies (Loscher and Potschka, 2005). For instance, increased expression of multidrug-resistance-associated proteins (MDPs) in endothelial cells of blood vessels in the brain following seizures has been implicated as a possible mechanism in drug-resistant epilepsies. In chronic epilepsy, increased levels of MDPs are thought to facilitate the efflux of antiepileptic drugs such as carbamazepine and valproate, thereby preventing the accumulation of therapeutic levels of antiepileptic drugs in brain tissue (Loscher, 2007). One study demonstrated that increased expression of MDPs in reactive astrocytes and blood vessels in the hippocampus occurs immediately following electrically evoked status epilepticus (SE) in the rat, indicating that changes in MDP expression may occur earlier than initially thought (van Vliet et al., 2005). Furthermore, increased expression of MDPs during the acute and chronic phase was also accompanied by decreased levels of an administered ASD, phenytoin (PHT). These results suggest that the development of pharmacoresistant epilepsy via the increased expression of MDPs may be initiated after a single bout of SE and should be taken into account when considering potential therapeutic interventions aimed at disrupting the process of epileptogenesis.
Additionally, reductions in MCT1, a key BBB carrier molecule for monocarboxylates and amino acids, have been observed on the endothelial cell membrane of microvessels in the hippocampus of human patients with TLE (Lauritzen et al., 2011) as well as two rodent models of SE (Lauritzen et al., 2012b). Reduced levels of MCT1 in the epileptogenic hippocampus may impair the uptake of monocarboxylates resulting in decreased concentrations of blood-derived fuels, such as ketone bodies, which could contribute to enhanced seizure susceptibility. Intriguingly, the transport of drugs such as valproic acid across the blood brain barrier is also facilitated by MCT1. Thus, concentrations of anti-seizure drugs in the MCT1-deficient epileptogenic hippocampus may be severely reduced, representing a possible explanation for pharmacoresistance in TLE (Lauritzen et al., 2012b).
Brain inflammation occurs as a consequence of seizures and is intimately linked to changes in BBB permeability. Reactive astrocytes have been identified as key players in the mechanisms involved in CNS inflammation (Aronica et al., 2012). Indeed, mRNA of inflammatory mediators are induced within 30 minutes of SE onset (De Simoni et al., 2000) and upregulation of astrocyte genes related to immune and inflammatory responses persists for up to 1 week following SE (Gorter et al., 2006). It is now recognized that a myriad of immune responses, many of which are mediated by astrocytes, can occur as a consequence of seizure activity and may contribute to the development of epilepsy. A more complete discussion of these topics are included in this special issue (see Carson and Fabene's reviews).
Increased Expression of Adenosine Kinase Following SE
In addition to the list of the changes in astrocytes following SE, alterations in the astrocyte-mediated adenosine/adenosine kinase system have shown intriguing potential as a target in the prevention and treatment of epilepsy. For over 40 years, adenosine has been recognized as an endogenous anticonvulsant. Adenosine levels are elevated in patients following seizures, suggesting that adenosine is released during a seizure and may contribute to seizure termination (Boison, 2005). Astrocytes regulate adenosine levels via the production of the enzyme adenosine kinase (ADK) and during epilepsy, reactive astrocytes produce increased levels of ADK. Studies from transgenic animals have shown that ADK overexpression leads to the development of spontaneous seizures and results in mortality following KA-induced SE, presumably due to a reduction in adenosine (Li et al., 2008). Furthermore, SE may cause an imbalance of adenosine/ADK that leads to epileptogenesis. Gouder et al. (2004) demonstrated that ADK is increased in reactive astrocytes around 1 week post SE, prior to the development of spontaneous seizures. Intriguingly, reduction of ADK in the brain can prevent the development of spontaneous seizures following KA-induced SE and prevents cell death, indicating that ADK reduction confers a neuroprotective effect following status (Li et al., 2008). Recently Theofilas et al. demonstrated that an antisense viral approach to modulate astrocytic ADK expression was able to trigger or prevent seizures in mice, providing the first line of evidence that gene therapy may be successfully applied to this system in order to control epilepsy (Theofilas et al., 2011).
Changes in Astrocyte Calcium Signaling After SE
While astrocytes do not exhibit prominent electrical excitability as do neurons, they are able to dynamically regulate calcium using internal stores (Charles et al., 1991; Cornell-Bell et al., 1990; Finkbeiner, 1992). Calcium transients in astrocytes occur in response to the activation of g-protein coupled receptors (e.g. mGluRs) and are thought to modulate the release of a number of gliotransmitters that could influence synaptic function (Aguado et al., 2002; Araque et al., 1998; Fellin et al., 2004; Halassa and Haydon, 2010; Parpura and Haydon, 2000; Pasti et al., 1997; Shigetomi et al., 2008). Consistent with effective synaptic modulation, astrocytic calcium transients have been shown to correlate with structured neuronal network activity (Aguado et al., 2002; Kuga et al., 2011; Winship et al., 2007). These observations naturally lead to the question of potential changes in astrocytic calcium dynamics during and following SE and how any changes might affect neural network function. Astrocyte calcium dynamics and SE have been studied in only a couple of papers to date (Ding et al., 2007) (Smeal et al., In press). Ding et al. used two-photon microscopy to study cortical astrocytic calcium dynamics in vivo using a pilocarpine model of SE (Ding et al., 2007). They found that astrocytic calcium activity increased during and after SE. Interfering with the increase in calcium activity in astrocytes that followed SE was neuroprotective, suggesting that glutamate release from astrocytes was contributing to excitotoxic cell death following SE (Ding et al., 2007).
Recent work from our laboratory using two photon microscopy and a fast scanning technique showed that calcium waves mediated by the astrocytic syncytium in CA3 region of the hippocampus can be very fast and widespread in vitro (Smeal et al., In press). This fast wave activity was much reduced in brain slices prepared from juvenile rats that experienced SE induced with kainic acid. Therefore, based on these two studies, calcium dynamics in reactive astrocytes following SE appear to be altered. The functional consequences of these changes in the context of epileptogenesis however, remains to be determined.
One of the primary difficulties in studying astrocyte calcium dynamics related to SE is the inability to bulk load calcium indicating dyes in brain slices obtained from the older animals that are used in the models of SE. The labeling of cells in vitro using the acetoxymethyl (AM) ester calcium indicator dyes becomes much more difficult as the animals age past a few weeks (Peterlin et al., 2000; Reeves et al., 2011). Transfection of protein calcium indicators using viruses is a potential solution, but has the drawback of causing astrocytes to become reactive independent of the insult that causes the SE (Ortinski et al., 2010). In order to bypass these loading problems, we have recently used the in utero electroporation technique (Chen and LoTurco, 2012; Gee et al., 2011; LoTurco et al., 2009) to introduce genetic calcium indicators into animals that are subsequently used in the standard SE animal models. Using plasmids that contain modifications to introduce the genetic calcium indicator (GCaMP-3) into the genome and subsequently target it to the cell membrane (Shigetomi et al., 2011) has resulted in well-labeled astrocytes in a number of brain regions including the hippocampus (Figure 2). Calcium transients can be detected even in the fine processes (Figure 2). Future experiments using this technique will study both the potential changes of calcium activity within the fine structure of individual astrocytes and in the calcium dynamics of large networks of astrocytes following SE and during the process of epileptogenesis.
Figure 2.
Ca2+ transients recorded at high resolution in the soma and throughout the processes in an astrocyte expressing Lck-GCaMP3 are observed in a series of images extracted from a movie using a standard x-y raster. Changes in fluorescence as a consequence of increased Ca2+ are highlighted in pseudo-color and superimposed on the mean image of the movie. At time 0.0 second, there is activity throughout the astrocyte. Time 66.9 sec to 74.3 sec shows a slow wave moving from the main processes to the soma. The final two panels demonstrate isolated Ca2+ transients in the astrocytic processes. Scale bar = 100 μM.
Disease Modifying Therapies Targeting Astrocytes
The vast majority of anti-seizure (ASD) drugs target ion channels and receptors that directly modulate neuronal function (Bialer and White, 2010; White et al., 2007). While this approach can confer seizure control to a majority of patients with epilepsy, it is estimated that between 25-35% of patients do not have their seizures adequately controlled with existing ASDs and the side effects of these drugs also negatively impact the quality of life of patients with epilepsy. Thus, novel ASDs that target inflammation and as a consequence, reactive astrocytes, may provide viable approaches to seizure control. Indeed, there is currently a clinical trial investigating the ability of VX-765, a caspase-1 inhibitor that prevents the production of IL-1β, to reduce seizures in patients with refractory partial seizures. This is the first clinical trial designed to target inflammatory processes in patients with epilepsy and a positive outcome will set the stage for novel therapeutic approaches for the treatment of existing seizure disorders.
There is currently no strategy to prevent the development of epilepsy in patients at risk following a CNS insult such as SE. However, initial work with VX-765 in rodents suggested that this compound may possess disease modifying properties that could alter the development of epilepsy (Ravizza et al., 2008). Furthermore, the increase in experiments that study the function of astrocytes following SE has opened the door for the development of novel compounds or approaches for targeting astrocyte function in an effort to interfere with the process of epileptogenesis. In addition to the VX-765 studies already discussed, the manipulation of the adenosine kinase system as described in this issue by Boison and colleagues may prove to be an important new therapeutic direction. Likewise, as more information is gleaned about the role of reactive astrocytes in the progression of epilepsy, the more opportunities we will have for interfering with the process of epileptogenesis.
Conclusions
SE results in rapid structural and functional changes to not only neurons, but astrocytes as well. Numerous studies performed in resected epileptic tissue and in chronic models of epilepsy have noted many changes in astrocyte function that might contribute to seizure activity. However, less is known about early changes in function in astrocytes following SE and to what extent astrocytes prevent or contribute to the development of epilepsy following SE. Ideally, understanding the wide array of astrocyte functions will give us clues as to how to exploit those processes that dampen excitability, such as glutamate uptake, gap junctions, and potassium buffering, and how to minimize those processes that contribute to network excitability, such as decreased expression of aquaporins and secretion of cytokines and gliotransmitters. The development of new tools for wide scale imaging of networks of neurons and astrocytes with genetically encoded calcium indicators will undoubtedly contribute to our quest for disease modification and prevention of acquired epilepsy.
Review of astrocyte structure and function after status epilepticus (SE)
Pro-convulsant and anti-convulsant changes in astrocytes are described
Genetically encoded calcium indicators will detect network changes in SE
Astrocytes may prove to be an important therapeutic target following SE
Acknowledgements
The authors wish to thank Dr. John A. White (JAW) for use of the two-photon microscope for calcium imaging, Mr. J.M.Gee and Dr. P. Tvrdik for plasmids, and Ms. Katherine Flood for technical assistance with in utero electroporation experiments. This work was supported by an Epilepsy Foundation Predoctoral Fellowship (DKT), NIH NS066774 (JRV), NIH NS062419 (KSW), NIH RC1 NS069033 (KSW & JAW) and NIH T32 NS076067 (MG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Aguado F, Espinosa-Parrilla JF, Carmona MA, Soriano E. Neuronal activity regulates correlated network properties of spontaneous calcium transients in astrocytes in situ. J Neurosci. 2002;22:9430–9444. doi: 10.1523/JNEUROSCI.22-21-09430.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Sanzgiri RP, Parpura V, Haydon PG. Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J Neurosci. 1998;18:6822–6829. doi: 10.1523/JNEUROSCI.18-17-06822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, Ravizza T, Zurolo E, Vezzani A. Astrocyte immune responses in epilepsy. Glia. 2012;60:1258–1268. doi: 10.1002/glia.22312. [DOI] [PubMed] [Google Scholar]
- Aronica E, van Vliet EA, Mayboroda OA, Troost D, da Silva FH, Gorter JA. Upregulation of metabotropic glutamate receptor subtype mGluR3 and mGluR5 in reactive astrocytes in a rat model of mesial temporal lobe epilepsy. Eur J Neurosci. 2000;12:2333–2344. doi: 10.1046/j.1460-9568.2000.00131.x. [DOI] [PubMed] [Google Scholar]
- Benediktsson AM, Marrs GS, Tu JC, Worley PF, Rothstein JD, Bergles DE, Dailey ME. Neuronal activity regulates glutamate transporter dynamics in developing astrocytes. Glia. 2012;60:175–188. doi: 10.1002/glia.21249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialer M, White HS. Key factors in the discovery and development of new antiepileptic drugs. Nat Rev Drug Discov. 2010;9:68–82. doi: 10.1038/nrd2997. [DOI] [PubMed] [Google Scholar]
- Binder DK, Steinhauser C. Functional changes in astroglial cells in epilepsy. Glia. 2006;54:358–368. doi: 10.1002/glia.20394. [DOI] [PubMed] [Google Scholar]
- Bjornsen LP, Eid T, Holmseth S, Danbolt NC, Spencer DD, de Lanerolle NC. Changes in glial glutamate transporters in human epileptogenic hippocampus: inadequate explanation for high extracellular glutamate during seizures. Neurobiol Dis. 2007;25:319–330. doi: 10.1016/j.nbd.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Boison D. Adenosine and epilepsy: from therapeutic rationale to new therapeutic strategies. Neuroscientist. 2005;11:25–36. doi: 10.1177/1073858404269112. [DOI] [PubMed] [Google Scholar]
- Bumanglag AV, Sloviter RS. Minimal latency to hippocampal epileptogenesis and clinical epilepsy after perforant pathway stimulation-induced status epilepticus in awake rats. J Comp Neurol. 2008;510:561–580. doi: 10.1002/cne.21801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles AC, Merrill JE, Dirksen ER, Sanderson MJ. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991;6:983–992. doi: 10.1016/0896-6273(91)90238-u. [DOI] [PubMed] [Google Scholar]
- Chen F, LoTurco J. A method for stable transgenesis of radial glia lineage in rat neocortex by piggyBac mediated transposition. J Neurosci Methods. 2012;207:172–180. doi: 10.1016/j.jneumeth.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collignon F, Wetjen NM, Cohen-Gadol AA, Cascino GD, Parisi J, Meyer FB, Marsh WR, Roche P, Weigand SD. Altered expression of connexin subtypes in mesial temporal lobe epilepsy in humans. J Neurosurg. 2006;105:77–87. doi: 10.3171/jns.2006.105.1.77. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Curia G, Longo D, Biagini G, Jones RS, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172:143–157. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Antoni S, Berretta A, Bonaccorso CM, Bruno V, Aronica E, Nicoletti F, Catania MV. Metabotropic glutamate receptors in glial cells. Neurochem Res. 2008;33:2436–2443. doi: 10.1007/s11064-008-9694-9. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Progress in Neurobiology. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- De Simoni MG, Perego C, Ravizza T, Moneta D, Conti M, Marchesi F, De Luigi A, Garattini S, Vezzani A. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur J Neurosci. 2000;12:2623–2633. doi: 10.1046/j.1460-9568.2000.00140.x. [DOI] [PubMed] [Google Scholar]
- Diamond JS. Deriving the glutamate clearance time course from transporter currents in CA1 hippocampal astrocytes: transmitter uptake gets faster during development. J Neurosci. 2005;25:2906–2916. doi: 10.1523/JNEUROSCI.5125-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Synaptically released glutamate does not overwhelm transporters on hippocampal astrocytes during high-frequency stimulation. J Neurophysiol. 2000;83:2835–2843. doi: 10.1152/jn.2000.83.5.2835. [DOI] [PubMed] [Google Scholar]
- Dichter MA, Herman CJ, Selzer M. Silent cells during interictal discharges and seizures in hippocampal penicillin foci. Evidence for the role of extracellular K+ in the transition from the interictal state to seizures. Brain Res. 1972;48:173–183. doi: 10.1016/0006-8993(72)90177-1. [DOI] [PubMed] [Google Scholar]
- Ding S, Fellin T, Zhu Y, Lee SY, Auberson YP, Meaney DF, Coulter DA, Carmignoto G, Haydon PG. Enhanced astrocytic Ca2+ signals contribute to neuronal excitotoxicity after status epilepticus. J Neurosci. 2007;27:10674–10684. doi: 10.1523/JNEUROSCI.2001-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:11354–11365. doi: 10.1523/JNEUROSCI.0723-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Nascimento AL, Dos Santos NF, Campos Pelagio F, Aparecida Teixeira S, de Moraes Ferrari EA, Langone F. Neuronal degeneration and gliosis time-course in the mouse hippocampal formation after pilocarpine-induced status epilepticus. Brain Res. 2012;1470:98–110. doi: 10.1016/j.brainres.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Elisevich K, Rempel SA, Smith B, Allar N. Connexin 43 mRNA expression in two experimental models of epilepsy. Molecular and chemical neuropathology / sponsored by the International Society for Neurochemistry and the World Federation of Neurology and research groups on neurochemistry and cerebrospinal fluid. 1997a;32:75–88. doi: 10.1007/BF02815168. [DOI] [PubMed] [Google Scholar]
- Elisevich K, Rempel SA, Smith BJ, Edvardsen K. Hippocampal connexin 43 expression in human complex partial seizure disorder. Experimental Neurology. 1997b;145:154–164. doi: 10.1006/exnr.1997.6467. [DOI] [PubMed] [Google Scholar]
- Engel J., Jr. Clinical evidence for the progressive nature of epilepsy. Epilepsy Research. 1996;12:9–20. [PubMed] [Google Scholar]
- Fedele DE, Gouder N, Guttinger M, Gabernet L, Scheurer L, Rulicke T, Crestani F, Boison D. Astrogliosis in epilepsy leads to overexpression of adenosine kinase, resulting in seizure aggravation. Brain. 2005;128:2383–2395. doi: 10.1093/brain/awh555. [DOI] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Fernaud-Espinosa I, Nieto-Sampedro M, Bovolenta P. Differential activation of microglia and astrocytes in aniso- and isomorphic gliotic tissue. Glia. 1993;8:277–291. doi: 10.1002/glia.440080408. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Corti C, Valerio E, Mion S, Xuereb J. Activated astrocytes in areas of kainate-induced neuronal injury upregulate the expression of the metabotropic glutamate receptors 2/3 and 5. Exp Brain Res. 2001;137:1–11. doi: 10.1007/s002210000633. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S. Calcium waves in astrocytes-filling in the gaps. Neuron. 1992;8:1101–1108. doi: 10.1016/0896-6273(92)90131-v. [DOI] [PubMed] [Google Scholar]
- Fonseca CG, Green CR, Nicholson LF. Upregulation in astrocytic connexin 43 gap junction levels may exacerbate generalized seizures in mesial temporal lobe epilepsy. Brain Res Mol Brain Res. 2002;929:105–116. doi: 10.1016/s0006-8993(01)03289-9. [DOI] [PubMed] [Google Scholar]
- Gabriel S, Eilers A, Kivi A, Kovacs R, Schulze K, Lehmann TN, Heinemann U. Effects of barium on stimulus induced changes in extracellular potassium concentration in area CA1 of hippocampal slices from normal and pilocarpine-treated epileptic rats. Neurosci Lett. 1998;242:9–12. doi: 10.1016/s0304-3940(98)00012-3. [DOI] [PubMed] [Google Scholar]
- Garcia-Marin V, Garcia-Lopez P, Freire M. Cajal's contributions to glia research. Trends Neurosci. 2007;30:479–487. doi: 10.1016/j.tins.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Gee JM, Flood KP, Morris SC, Smeal RM, Economo MN, Capecchi MR, Tvrdik P, Wilcox KS, White JA. A toolset for targeting GCaMP3 to cytosol, membrane or mitochondria with In utero electroporation.. Society for Neuroscience Annual Meeting; Washington, D. C.. 2011. [Google Scholar]
- Glushakova O, Jeromin A, Martinez J, Johnson D, Denslow ND, Streeter J, Hayes R, Mondello S. CSF Protein Biomarker Panel for Assessment of Neurotoxicity Induced by Kainic Acid in Rats. Toxicol Sci. 2012 doi: 10.1093/toxsci/kfs224. [DOI] [PubMed] [Google Scholar]
- Gorter JA, van Vliet EA, Aronica E, Breit T, Rauwerda H, Lopes da Silva FH, Wadman WJ. Potential new antiepileptogenic targets indicated by microarray analysis in a rat model for temporal lobe epilepsy. J Neurosci. 2006;26:11083–11110. doi: 10.1523/JNEUROSCI.2766-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb M, Matute C. Expression of ionotropic glutamate receptor subunits in glial cells of the hippocampal CA1 area following transient forebrain ischemia. J Cereb Blood Flow Metab. 1997;17:290–300. doi: 10.1097/00004647-199703000-00006. [DOI] [PubMed] [Google Scholar]
- Gouder N, Scheurer L, Fritschy JM, Boison D. Overexpression of adenosine kinase in epileptic hippocampus contributes to epileptogenesis. J Neurosci. 2004;24:692–701. doi: 10.1523/JNEUROSCI.4781-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi K, Fujita A, Inanobe A, Tanemoto M, Doi K, Kubo T, Kurachi Y. An inwardly rectifying K(+) channel, Kir4.1, expressed in astrocytes surrounds synapses and blood vessels in brain. Am J Physiol Cell Physiol. 2001;281:C922–931. doi: 10.1152/ajpcell.2001.281.3.C922. [DOI] [PubMed] [Google Scholar]
- Hinterkeuser S, Schroder W, Hager G, Seifert G, Blumcke I, Elger CE, Schramm J, Steinhauser C. Astrocytes in the hippocampus of patients with temporal lobe epilepsy display changes in potassium conductances. Eur J Neurosci. 2000;12:2087–2096. doi: 10.1046/j.1460-9568.2000.00104.x. [DOI] [PubMed] [Google Scholar]
- Hochman DW. The extracellular space and epileptic activity in the adult brain: explaining the antiepileptic effects of furosemide and bumetanide. Epilepsia 53 Suppl. 2012;1:18–25. doi: 10.1111/j.1528-1167.2012.03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MZ, Sawchuk MA, Murphy LJ, Hertzberg EL, Nagy JI. Kainic acid induced alterations in antibody recognition of connexin43 and loss of astrocytic gap junctions in rat brain. Glia. 1994;10:250–265. doi: 10.1002/glia.440100404. [DOI] [PubMed] [Google Scholar]
- Ivens S, Kaufer D, Flores LP, Bechmann I, Zumsteg D, Tomkins O, Seiffert E, Heinemann U, Friedman A. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain. 2007;130:535–547. doi: 10.1093/brain/awl317. [DOI] [PubMed] [Google Scholar]
- Jabs R, Kirchhoff F, Kettenmann H, Steinhauser C. Kainate activates Ca(2+)-permeable glutamate receptors and blocks voltage-gated K+ currents in glial cells of mouse hippocampal slices. Pflugers Arch. 1994;426:310–319. doi: 10.1007/BF00374787. [DOI] [PubMed] [Google Scholar]
- Kálmán M. Glia reaction and reactive glia. Adv in Mol and Cell Biol. 2003;31:787–835. [Google Scholar]
- Kielian T. Glial connexins and gap junctions in CNS inflammation and disease. J Neurochem. 2008;106:1000–1016. doi: 10.1111/j.1471-4159.2008.05405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK. Receptors on astrocytes--what possible functions? Neurochem Int. 1995;26:27–40. doi: 10.1016/0197-0186(94)00118-e. [DOI] [PubMed] [Google Scholar]
- Kivi A, Lehmann TN, Kovacs R, Eilers A, Jauch R, Meencke HJ, von Deimling A, Heinemann U, Gabriel S. Effects of barium on stimulus-induced rises of [K+]o in human epileptic non-sclerotic and sclerotic hippocampal area CA1. Eur J Neurosci. 2000;12:2039–2048. doi: 10.1046/j.1460-9568.2000.00103.x. [DOI] [PubMed] [Google Scholar]
- Krebs C, Fernandes HB, Sheldon C, Raymond LA, Baimbridge KG. Functional NMDA receptor subtype 2B is expressed in astrocytes after ischemia in vivo and anoxia in vitro. J Neurosci. 2003;23:3364–3372. doi: 10.1523/JNEUROSCI.23-08-03364.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuga N, Sasaki T, Takahara Y, Matsuki N, Ikegaya Y. Large-scale calcium waves traveling through astrocytic networks in vivo. J Neurosci. 2011;31:2607–2614. doi: 10.1523/JNEUROSCI.5319-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird DW, Puranam KL, Revel JP. Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. The Biochemical Journal. 1991;273(Pt 1):67–72. doi: 10.1042/bj2730067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Parpura V, Verkhratsky A. Ionotropic receptors in neuronalastroglial signalling: What is the role of “excitable” molecules in non-excitable cells. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbamcr.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Lauritzen F, de Lanerolle NC, Lee TS, Spencer DD, Kim JH, Bergersen LH, Eid T. Monocarboxylate transporter 1 is deficient on microvessels in the human epileptogenic hippocampus. Neurobiol Dis. 2011;41:577–584. doi: 10.1016/j.nbd.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen F, Heuser K, de Lanerolle NC, Lee TS, Spencer DD, Kim JH, Gjedde A, Eid T, Bergersen LH. Redistribution of monocarboxylate transporter 2 on the surface of astrocytes in the human epileptogenic hippocampus. Glia. 2012a;60:1172–1181. doi: 10.1002/glia.22344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen F, Perez EL, Melillo ER, Roh JM, Zaveri HP, Lee TS, Wang Y, Bergersen LH, Eid T. Altered expression of brain monocarboxylate transporter 1 in models of temporal lobe epilepsy. Neurobiol Dis. 2012b;45:165–176. doi: 10.1016/j.nbd.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Hsu MS, Seldin MM, Arellano JL, Binder DK. Decreased expression of the glial water channel aquaporin-4 in the intrahippocampal kainic acid model of epileptogenesis. Exp Neurol. 2012;235:246–255. doi: 10.1016/j.expneurol.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Magge S, Spencer DD, Sontheimer H, Cornell-Bell AH. Human epileptic astrocytes exhibit increased gap junction coupling. Glia. 1995;15:195–202. doi: 10.1002/glia.440150212. [DOI] [PubMed] [Google Scholar]
- Lehmkuhle MJ, Thomson KE, Scheerlinck P, Pouliot W, Greger B, Dudek FE. A simple quantitative method for analyzing electrographic status epilepticus in rats. J Neurophysiol. 2009;101:1660–1670. doi: 10.1152/jn.91062.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, Itohara S, Simon RP, Boison D. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Invest. 2008;118:571–582. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W. Drug transporters in the epileptic brain. Epilepsia. 2007;48(Suppl 1):8–13. doi: 10.1111/j.1528-1167.2007.00993.x. [DOI] [PubMed] [Google Scholar]
- Loscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci. 2005;6:591–602. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- LoTurco J, Manent JB, Sidiqi F. New and improved tools for in utero electroporation studies of developing cerebral cortex. Cereb Cortex 19 Suppl. 2009;1:i120–125. doi: 10.1093/cercor/bhp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein DH, Bleck T, Macdonald RL. It's time to revise the definition of status epilepticus. Epilepsia. 1999;40:120–122. doi: 10.1111/j.1528-1157.1999.tb02000.x. [DOI] [PubMed] [Google Scholar]
- Lund IV, Hu Y, Raol YH, Benham RS, Faris R, Russek SJ, Brooks-Kayal AR. BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway. Sci Signal. 2008;1:ra9. doi: 10.1126/scisignal.1162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcaggi P, Attwell D. Role of glial amino acid transporters in synaptic transmission and brain energetics. Glia. 2004;47:217–225. doi: 10.1002/glia.20027. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Mendoza D, Lozada A, Pretorius JK, Dehnes Y, Danbolt NC, Nelson N, Leite JP, Chimelli L, Born DE, Sakamoto AC, Assirati JA, Fried I, Peacock WJ, Ojemann GA, Adelson PD. Hippocampal GABA and glutamate transporter immunoreactivity in patients with temporal lobe epilepsy. Neurology. 1999;52:453–472. doi: 10.1212/wnl.52.3.453. [DOI] [PubMed] [Google Scholar]
- Murabe Y, Ibata Y, Sano Y. Morphological studies on neuroglia. IV. Proliferative response of non-neuronal elements in the hippocampus of the rat to kainic acid-induced lesions. Cell Tissue Res. 1982;222:223–226. doi: 10.1007/BF00218302. [DOI] [PubMed] [Google Scholar]
- Nadal A, Fuentes E, Pastor J, McNaughton PA. Plasma albumin is a potent trigger of calcium signals and DNA synthesis in astrocytes. Proc Natl Acad Sci U S A. 1995;92:1426–1430. doi: 10.1073/pnas.92.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JI, Rash JE. Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain Res Brain Res Rev. 2000;32:29–44. doi: 10.1016/s0165-0173(99)00066-1. [DOI] [PubMed] [Google Scholar]
- Nakase T, Naus CC. Gap junctions and neurological disorders of the central nervous system. Biochim Biophys Acta. 2004;1662:149–158. doi: 10.1016/j.bbamem.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Naus CC, Bechberger JF, Paul DL. Gap junction gene expression in human seizure disorder. Exp Neurol. 1991;111:198–203. doi: 10.1016/0014-4886(91)90007-y. [DOI] [PubMed] [Google Scholar]
- Nicholson C, Sykova E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 1998;21:207–215. doi: 10.1016/s0166-2236(98)01261-2. [DOI] [PubMed] [Google Scholar]
- Niquet J, Ben-Ari Y, Represa A. Glial reaction after seizure induced hippocampal lesion: immunohistochemical characterization of proliferating glial cells. J Neurocytol. 1994;23:641–656. doi: 10.1007/BF01191558. [DOI] [PubMed] [Google Scholar]
- Norwood BA, Bumanglag AV, Osculati F, Sbarbati A, Marzola P, Nicolato E, Fabene PF, Sloviter RS. Classic hippocampal sclerosis and hippocampal-onset epilepsy produced by a single “cryptic” episode of focal hippocampal excitation in awake rats. J Comp Neurol. 2010;518:3381–3407. doi: 10.1002/cne.22406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notenboom RG, Hampson DR, Jansen GH, van Rijen PC, van Veelen CW, van Nieuwenhuizen O, de Graan PN. Up-regulation of hippocampal metabotropic glutamate receptor 5 in temporal lobe epilepsy patients. Brain. 2006;129:96–107. doi: 10.1093/brain/awh673. [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Tian GF, Han X, Peng W, Takano T, Ransom B, Nedergaard M. Loss of astrocytic domain organization in the epileptic brain. J Neurosci. 2008;28:3264–3276. doi: 10.1523/JNEUROSCI.4980-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, Haydon PG, Coulter DA. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci. 2010;13:584–591. doi: 10.1038/nn.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannasch U, Vargova L, Reingruber J, Ezan P, Holcman D, Giaume C, Sykova E, Rouach N. Astroglial networks scale synaptic activity and plasticity. Proc Natl Acad Sci U S A. 2011;108:8467–8472. doi: 10.1073/pnas.1016650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Haydon PG. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci U S A. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci. 1997;17:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perillan PR, Chen M, Potts EA, Simard JM. Transforming growth factor-beta 1 regulates Kir2.3 inward rectifier K+ channels via phospholipase C and protein kinase C-delta in reactive astrocytes from adult rat brain. J Biol Chem. 2002;277:1974–1980. doi: 10.1074/jbc.M107984200. [DOI] [PubMed] [Google Scholar]
- Peterlin ZA, Kozloski J, Mao BQ, Tsiola A, Yuste R. Optical probing of neuronal circuits with calcium indicators. Proc Natl Acad Sci U S A. 2000;97:3619–3624. doi: 10.1073/pnas.97.7.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters O, Schipke CG, Philipps A, Haas B, Pannasch U, Wang LP, Benedetti B, Kingston AE, Kettenmann H. Astrocyte function is modified by Alzheimer's disease-like pathology in aged mice. J Alzheimers Dis. 2009;18:177–189. doi: 10.3233/JAD-2009-1140. [DOI] [PubMed] [Google Scholar]
- Poopalasundaram S, Knott C, Shamotienko OG, Foran PG, Dolly JO, Ghiani CA, Gallo V, Wilkin GP. Glial heterogeneity in expression of the inwardly rectifying K(+) channel, Kir4.1, in adult rat CNS. Glia. 2000;30:362–372. doi: 10.1002/(sici)1098-1136(200006)30:4<362::aid-glia50>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Proper EA, Hoogland G, Kappen SM, Jansen GH, Rensen MG, Schrama LH, van Veelen CW, van Rijen PC, van Nieuwenhuizen O, Gispen WH, de Graan PN. Distribution of glutamate transporters in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain. 2002;125:32–43. doi: 10.1093/brain/awf001. [DOI] [PubMed] [Google Scholar]
- Raol YH, Lund IV, Bandyopadhyay S, Zhang G, Roberts DS, Wolfe JH, Russek SJ, Brooks-Kayal AR. Enhancing GABA(A) receptor alpha 1 subunit levels in hippocampal dentate gyrus inhibits epilepsy development in an animal model of temporal lobe epilepsy. J Neurosci. 2006;26:11342–11346. doi: 10.1523/JNEUROSCI.3329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Davidson KG, Furman CS, Dudek FE, Nagy JI. Identification of cells expressing Cx43, Cx30, Cx26, Cx32 and Cx36 in gap junctions of rat brain and spinal cord. Cell communication & adhesion. 2001;8:315–320. doi: 10.3109/15419060109080745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza T, Noe F, Zardoni D, Vaghi V, Sifringer M, Vezzani A. Interleukin Converting Enzyme inhibition impairs kindling epileptogenesis in rats by blocking astrocytic IL-1beta production. Neurobiol Dis. 2008;31:327–333. doi: 10.1016/j.nbd.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Reeves AM, Shigetomi E, Khakh BS. Bulk loading of calcium indicator dyes to study astrocyte physiology: key limitations and improvements using morphological maps. J Neurosci. 2011;31:9353–9358. doi: 10.1523/JNEUROSCI.0127-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- Robinson MB. Regulated trafficking of neurotransmitter transporters: common notes but different melodies. J Neurochem. 2002;80:1–11. doi: 10.1046/j.0022-3042.2001.00698.x. [DOI] [PubMed] [Google Scholar]
- Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- Sarac S, Afzal S, Broholm H, Madsen FF, Ploug T, Laursen H. Excitatory amino acid transporters EAAT-1 and EAAT-2 in temporal lobe and hippocampus in intractable temporal lobe epilepsy. APMIS : Acta Pathologica, Microbiologica, et Immunologica Scandinavica. 2009;117:291–301. doi: 10.1111/j.1600-0463.2009.02443.x. [DOI] [PubMed] [Google Scholar]
- Schroder W, Seifert G, Huttmann K, Hinterkeuser S, Steinhauser C. AMPA receptor-mediated modulation of inward rectifier K+ channels in astrocytes of mouse hippocampus. Mol Cell Neurosci. 2002;19:447–458. doi: 10.1006/mcne.2001.1080. [DOI] [PubMed] [Google Scholar]
- Seifert G, Huttmann K, Schramm J, Steinhauser C. Enhanced relative expression of glutamate receptor 1 flip AMPA receptor subunits in hippocampal astrocytes of epilepsy patients with Ammon's horn sclerosis. J Neurosci. 2004;24:1996–2003. doi: 10.1523/JNEUROSCI.3904-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert G, Rehn L, Weber M, Steinhauser C. AMPA receptor subunits expressed by single astrocytes in the juvenile mouse hippocampus. Brain Res Mol Brain Res. 1997;47:286–294. doi: 10.1016/s0169-328x(97)00059-4. [DOI] [PubMed] [Google Scholar]
- Serrano A, Robitaille R, Lacaille JC. Differential NMDA-dependent activation of glial cells in mouse hippocampus. Glia. 2008;56:1648–1663. doi: 10.1002/glia.20717. [DOI] [PubMed] [Google Scholar]
- Shigetomi E, Bowser DN, Sofroniew MV, Khakh BS. Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J Neurosci. 2008;28:6659–6663. doi: 10.1523/JNEUROSCI.1717-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Kracun S, Khakh BS. Monitoring astrocyte calcium microdomains with improved membrane targeted GCaMP reporters. Neuron Glia Biol. 2011:1–9. doi: 10.1017/S1740925X10000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeal RM, Economo MN, Lillis KP, Wilcox KS, White JA. Targeted path scanning: an emerging method for recording fast changing network dynamics across large distances. J Bioeng & Biom Sci. In press. [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohl G, Guldenagel M, Beck H, Teubner B, Traub O, Gutierrez R, Heinemann U, Willecke K. Expression of connexin genes in hippocampus of kainate-treated and kindled rats under conditions of experimental epilepsy. Brain Research. Molecular Brain Research. 2000;83:44–51. doi: 10.1016/s0169-328x(00)00195-9. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Nervenkitt: notes on the history of the concept of neuroglia. Glia. 1988;1:2–9. doi: 10.1002/glia.440010103. [DOI] [PubMed] [Google Scholar]
- Steinhauser C, Jabs R, Kettenmann H. Properties of GABA and glutamate responses in identified glial cells of the mouse hippocampal slice. Hippocampus. 1994;4:19–35. doi: 10.1002/hipo.450040105. [DOI] [PubMed] [Google Scholar]
- Takahashi DK, Vargas JR, Wilcox KS. Increased coupling and altered glutamate transport currents in astrocytes following kainic-acid-induced status epilepticus. Neurobiol Dis. 2010;40:573–585. doi: 10.1016/j.nbd.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Tessler S, Danbolt NC, Faull RL, Storm-Mathisen J, Emson PC. Expression of the glutamate transporters in human temporal lobe epilepsy. Neuroscience. 1999;88:1083–1091. doi: 10.1016/s0306-4522(98)00301-7. [DOI] [PubMed] [Google Scholar]
- Theis M, Jauch R, Zhuo L, Speidel D, Wallraff A, Doring B, Frisch C, Sohl G, Teubner B, Euwens C, Huston J, Steinhauser C, Messing A, Heinemann U, Willecke K. Accelerated hippocampal spreading depression and enhanced locomotory activity in mice with astrocyte-directed inactivation of connexin43. J Neurosci. 2003;23:766–776. doi: 10.1523/JNEUROSCI.23-03-00766.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis M, Sohl G, Eiberger J, Willecke K. Emerging complexities in identity and function of glial connexins. Trends Neurosci. 2005;28:188–195. doi: 10.1016/j.tins.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Theofilas P, Brar S, Stewart KA, Shen HY, Sandau US, Poulsen D, Boison D. Adenosine kinase as a target for therapeutic antisense strategies in epilepsy. Epilepsia. 2011;52:589–601. doi: 10.1111/j.1528-1167.2010.02947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CG, Tian H, Diamond JS. The relative roles of diffusion and uptake in clearing synaptically released glutamate change during early postnatal development. J Neurosci. 2011;31:4743–4754. doi: 10.1523/JNEUROSCI.5953-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8:935–947. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Doi T, Tokumaru J, Yokoyama H, Nakajima A, Mitsuyama Y, Ohya-Nishiguchi H, Kamada H, Willmore LJ. Collapse of extracellular glutamate regulation during epileptogenesis: down-regulation and functional failure of glutamate transporter function in rats with chronic seizures induced by kainic acid. J Neurochem. 2001;76:892–900. doi: 10.1046/j.1471-4159.2001.00087.x. [DOI] [PubMed] [Google Scholar]
- Ulas J, Satou T, Ivins KJ, Kesslak JP, Cotman CW, Balazs R. Expression of metabotropic glutamate receptor 5 is increased in astrocytes after kainate-induced epileptic seizures. Glia. 2000;30:352–361. [PubMed] [Google Scholar]
- van Vliet EA, da Costa Araujo S, Redeker S, van Schaik R, Aronica E, Gorter JA. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130:521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Redeker S, Aronica E, Edelbroek PM, Gorter JA. Expression of multidrug transporters MRP1, MRP2, and BCRP shortly after status epilepticus, during the latent period, and in chronic epileptic rats. Epilepsia. 2005;46:1569–1580. doi: 10.1111/j.1528-1167.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- Vargas JR, Takahashi DK, Wilcox KS. Kainate receptor expression in astrocytes in a model of temporal lobe epilepsy. Society for Neuroscience Annual Meeting; Atlanta, GA. 2006. [Google Scholar]
- Verkhratsky A. Patching the glia reveals the functional organisation of the brain. Pflugers Arch. 2006;453:411–420. doi: 10.1007/s00424-006-0099-9. [DOI] [PubMed] [Google Scholar]
- Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LP, Cheung G, Kronenberg G, Gertz K, Ji S, Kempermann G, Endres M, Kettenmann H. Mild brain ischemia induces unique physiological properties in striatal astrocytes. Glia. 2008;56:925–934. doi: 10.1002/glia.20660. [DOI] [PubMed] [Google Scholar]
- White HS, Smith MD, Wilcox KS. Mechanisms of action of antiepileptic drugs. Int Rev Neurobiol. 2007;81:85–110. doi: 10.1016/S0074-7742(06)81006-8. [DOI] [PubMed] [Google Scholar]
- Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, Ellisman MH, Pekny M. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci U S A. 2006;103:17513–17518. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, White AM, Clark S, Ferraro DJ, Swiercz W, Staley KJ, Dudek FE. Development of spontaneous recurrent seizures after kainate-induced status epilepticus. J Neurosci. 2009;29:2103–2112. doi: 10.1523/JNEUROSCI.0980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winship IR, Plaa N, Murphy TH. Rapid astrocyte calcium signals correlate with neuronal activity and onset of the hemodynamic response in vivo. J Neurosci. 2007;27:6268–6272. doi: 10.1523/JNEUROSCI.4801-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]