Abstract
Several studies suggest a link between autoimmunity and essential hypertension in humans. However, whether autoimmunity can drive the development of hypertension remains unclear. The autoimmune disease systemic lupus erythematosus is characterized by autoantibody production and the prevalence of hypertension is markedly increased in this patient population compared to normal healthy women. We hypothesized that preventing the development of autoimmunity would prevent the development of hypertension in a mouse model of lupus. Female lupus (NZBWF1) and control mice (NZW) were treated weekly with anti-CD20 or IgG antibodies (both 10 mg/kg, IV) starting at 20 weeks of age for 14 weeks. Anti-CD20 therapy markedly attenuated lupus disease progression as evidenced by reduced CD45R+ B cells and lower double-stranded DNA autoantibody activity. In addition, renal injury in the form of urinary albumin, glomerulosclerosis, and tubulointerstitial fibrosis, as well as tubular injury (indicated by renal cortical expression of neutrophil gelatinase-associated lipocalin) was prevented by anti-CD20 therapy in lupus mice. Finally, lupus mice treated with anti-CD20 antibody did not develop hypertension. The protection against the development of hypertension was associated with lower renal cortical tumor necrosis factor-α expression, a cytokine that has been previously reported by us to contribute to the hypertension in this model, as well as renal cortical monocyte chemoattractant protein -1 expression and circulating T cells. These data suggest that the development of autoimmunity and the resultant increase in renal inflammation is an important underlying factor in the prevalent hypertension that occurs during systemic lupus erythematosus.
Keywords: B cells, autoantibodies, pressure, renal inflammation, T cells, immune
INTRODUCTION
Humoral immune system activation has a central role in the pathogenesis of autoimmune disorders, in part, by promoting antibody-mediated immune complex formation, leading to tissue injury and inflammation1. Several studies show an association between the production of antibodies characteristic of autoimmune disease and human hypertension. For example, work from Kristensen et al. showed that hypertensive patients were more likely to have increased circulating autoantibodies of the IgG and IgM class 2,3. Similarly, Gudbrandsson et al. showed high levels of anti-nuclear antibodies in patients with malignant hypertension 3. More recently, reports of specific activating antibodies that could promote hypertension have been reported 4-6 While there is clear and growing evidence for humoral immune system activation in human hypertension, whether a general loss of immune tolerance leading to systemic autoimmunity can be an underlying causative factor in the pathogenesis of hypertension has not been directly tested.
Systemic lupus erythematosus (SLE), a chronic autoimmune disorder that predominantly affects women of childbearing age, is characterized by circulating anti-nuclear autoantibodies (e.g., anti-dsDNA antibodies), and is associated with a markedly increased prevalence of hypertension 7. Using an established female mouse model of SLE that displays these same characteristics, our laboratory has previously reported on several factors that contribute to the hypertension during SLE including endothelial dysfunction 8, impaired renal hemodynamic function 9,10, inflammatory cytokines 11, and oxidative stress 12. In the present study, we tested the hypothesis that the development of autoimmune disease is the underlying factor that leads to the prevalent hypertension associated with SLE. Our data show that administration of a mouse anti-CD20 antibody (the equivalent of rituximab in humans) to deplete B cells, markedly attenuates autoantibody production and prevents the development of hypertension in a mouse model of SLE.
METHODS
Animals
Twenty-week-old female NZBWF1 mice (Jackson Labs, Bar Harbor, ME), an established model that mimics characteristics of SLE in humans, and NZW/LacJ mice (controls; Jackson Labs) were used in this study because we previously showed that this age precedes the development of autoantibodies, albuminuria and hypertension 8. Mice were maintained on a 12-hour light/dark cycle in temperature-controlled rooms with access to food and water ad libitum. All studies were approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee (IACUC) and were in accordance with National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Antibody Administration
Starting at 20 weeks of age, mice were administered 100 μL of a monoclonal antibody to mouse CD20 (anti-CD20; provided by Genentech, South San Francisco, CA) or IgG (isotype control also provided by Genetech) retro-orbitally at a dose of 10 mg/kg once per week for 14 weeks.
Treatment efficacy was assessed by measuring spleen weight and the percentage of CD45R+ B cells in freshly prepared splenocytes using flow-assisted cell sorting (FACS) as previously described 13.
Blood Pressure
Blood pressure was recorded at the end of the 14-week protocol in conscious freely-moving mice as previously reported by our laboratory 9,10,12,14.
Autoantibodies
Plasma levels of anti-double-stranded DNA (dsDNA) antibodies, a clinical hallmark of SLE, were measured at week 34 as previously described9,11,12,14 and are presented as a positive antibody activity index per the manufacturer's instructions and as previously published 15. An antibody activity less than 1 is considered negative, whereas activity greater than 1 is considered positive.
Renal Inflammation, Oxidative Stress, and Injury
Markers of renal cortical inflammation, including tumor necrosis factor (TNF)-α and monocyte chemoattractant protein (MCP)-1, were measured using Western blot as previously described by our laboratory in control mice and SLE mice with evidence of active disease (autoantibodies and albuminuria) 12,14. Renal cortical expression of catalase, copper-zinc superoxide dismustase (CuZnSOD), and glutathione peroxidase was measured using Western blot as previously described12.
Albuminuria was determined in 24-hour urine samples either weekly using a dipstick assay or at the conclusion of the study by ELISA (presented as excretion rate in μg/day) as previously described 9,10,12,14,16. Paraffin-embedded kidneys were stained with Periodic Acid Schiff (PAS) to assess glomerulosclerosis as previously described 17 and Masson's trichrome staining to assess tubulointerstitial fibrosis. Renal cortical expression of neutrophil gelatinase-associated lipocalin (NGAL) was measured as an index of renal tubular injury as previously reported by our laboratory 14.
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM). Statistical analyses were performed using SigmaPlot 11.0 software (Systat, San Jose, CA). Two-way ANOVA was used followed by the appropriate post-hoc test in order to determine differences between multiple groups. Data were considered statistically different when p < 0.05.
RESULTS
Anti-CD20 therapy attenuates the development of hypertension in female mice with SLE
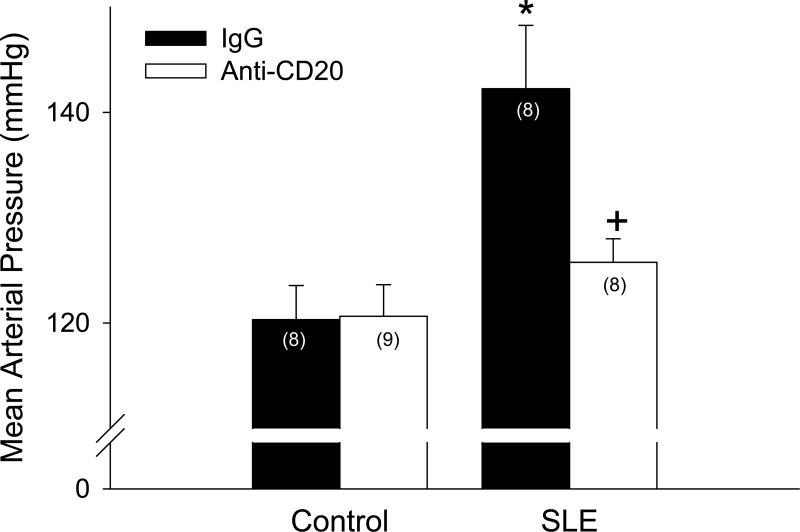
In order to determine whether autoimmunity contributes to the pathogenesis of hypertension during SLE, mean arterial pressure was measured in mice treated with vehicle (IgG) or anti-CD20 antibody. Mean arterial pressure was increased in SLE mice compared to controls (142 ± 6 vs. 120 ± 3 mmHg; p < 0.001; Figure 1). Anti-CD20 treatment starting at 20 weeks of age prevented the increase in blood pressure in SLE mice (126 ± 2 mmHg; p < 0.01), but had no effect in controls (121 ± 3 mmHg). Prior to starting the studies at 20 weeks of age, preliminary experiments were conducted beginning treatment at 26 weeks (8 total weeks of anti-CD20 antibody) and 30 weeks of age (4 total weeks of anti-CD20 antibody). The efficacy of the treatment corresponded with the length and timing of the treatment (please see http://hyper.ahajournals.org).
Figure 1.
Mean arterial pressure measured in control and SLE mice administered IgG or anti-CD20 for 14 weeks (n indicated on graph). * p < 0.001 vs. Control/IgG; + p< 0.01 vs. SLE/IgG
Anti-CD20 therapy depletes B cells and attenuates the development of SLE
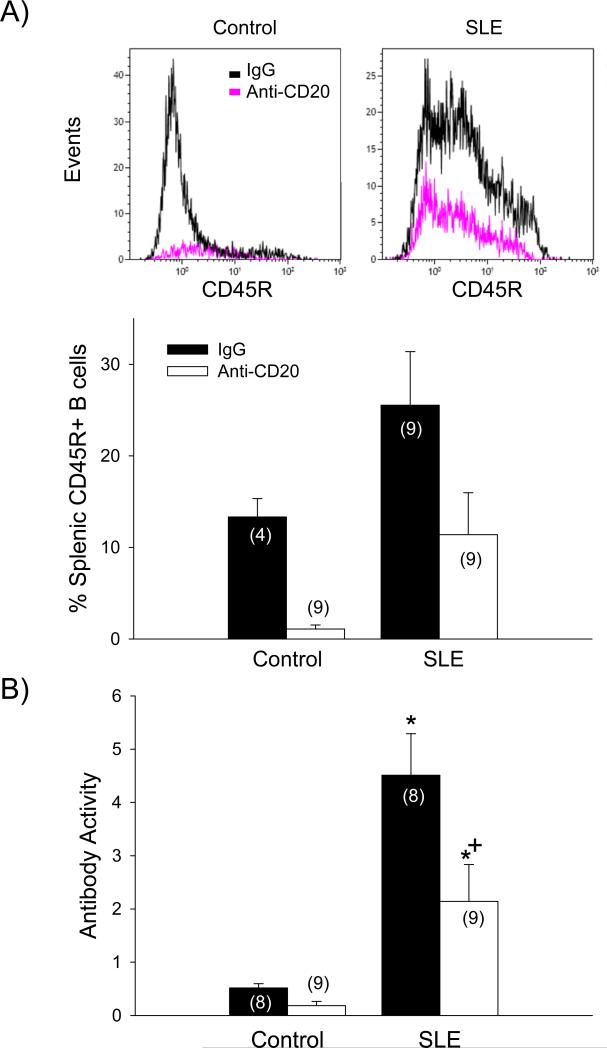
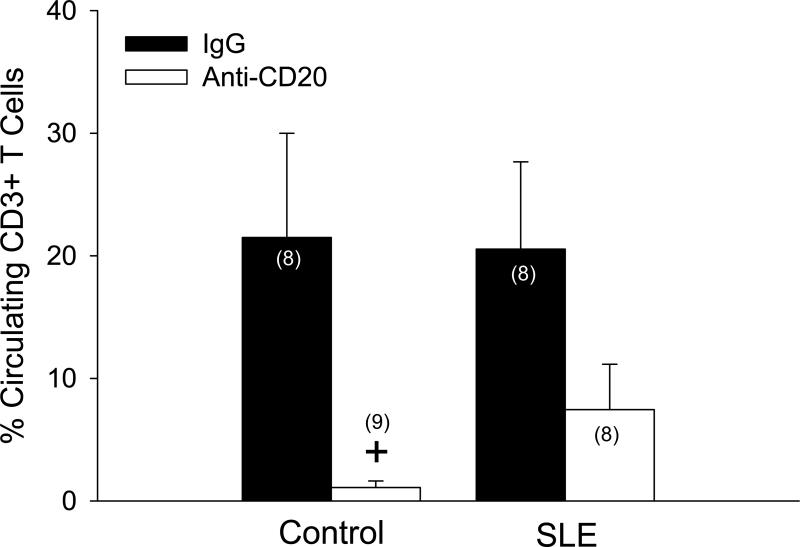
Anti-CD20 therapy reduced spleen weight in control (p < 0.03) and SLE mice (p < 0.001), as well as splenocyte count in control (p < 0.001) and SLE mice (p < 0.001) (data not shown). SLE mice had increased B cells compared to controls, as indicated by a significant group effect on the percentage of splenic CD45R+ B cells (p < 0.03; Figure 2A). Anti-CD20 therapy effectively depleted splenic B cells in control and SLE mice (treatment effect; p < 0.01). Importantly, the percentage of splenic B cells in SLE mice following 14 weeks of anti-CD20 therapy (13.3 ± 2%) was equivalent to control, vehicle-treated animals (11.4 ± 4.6%). Anti-dsDNA autoantibody activity was significantly elevated in SLE mice (4.51 ± 0.78, p<0.05) compared to controls (0.52 ± 0.08), as previously reported by our laboratory (Figure 2B) 12,14,15. B cell depletion did not significantly alter activity of anti-dsDNA autoantibodies in control mice (0.18 ± 0.08); however, autoantibody activity was attenuated by B cell depletion in SLE mice (2.14 ± 0.69; p < 0.005). These data provide important functional evidence supporting the effective depletion of B cells achieved with 14 weeks of anti-CD20 therapy. Although the percentage of circulating CD3+ (pan) T cells was not different between control and SLE mice (21.5 ± 8.5 and 20.5 ± 7.1%, respectively), there was a significant treatment effect of anti-CD20 therapy on CD3+ T cells (Figure 3; p < 0.01).
Figure 2.
A) The percentage of splenic CD45R+ B cells measured using flow cytometry in freshly prepared splenocytes from control and SLE mice administered IgG or anti-CD20 (n indicated on graph) with representative overlays of flow cytometry data. p (effect of group; SLE vs. controls) < 0.03; p (effect of treatment; Anti-CD20 vs. IgG) < 0.01; B) Circulating double-stranded DNA autoantibodies (presented as antibody activity index) measured in control and SLE mice administered IgG or anti-CD20 (n indicated on graph). * p <0.05 vs. corresponding control; + p<0.0.005 vs. SLE/IgG
Figure 3.
The percentage of circulating CD3+ T cells measured using flow cytometry in freshly prepared peripheral blood mononuclear cells from control and SLE mice administered IgG or anti-CD20 (n indicated on graph). p (effect of treatment) < 0.001. + p < 0.01 vs. control vehicle.
Anti-CD20 therapy attenuates the development of renal inflammation in SLE mice
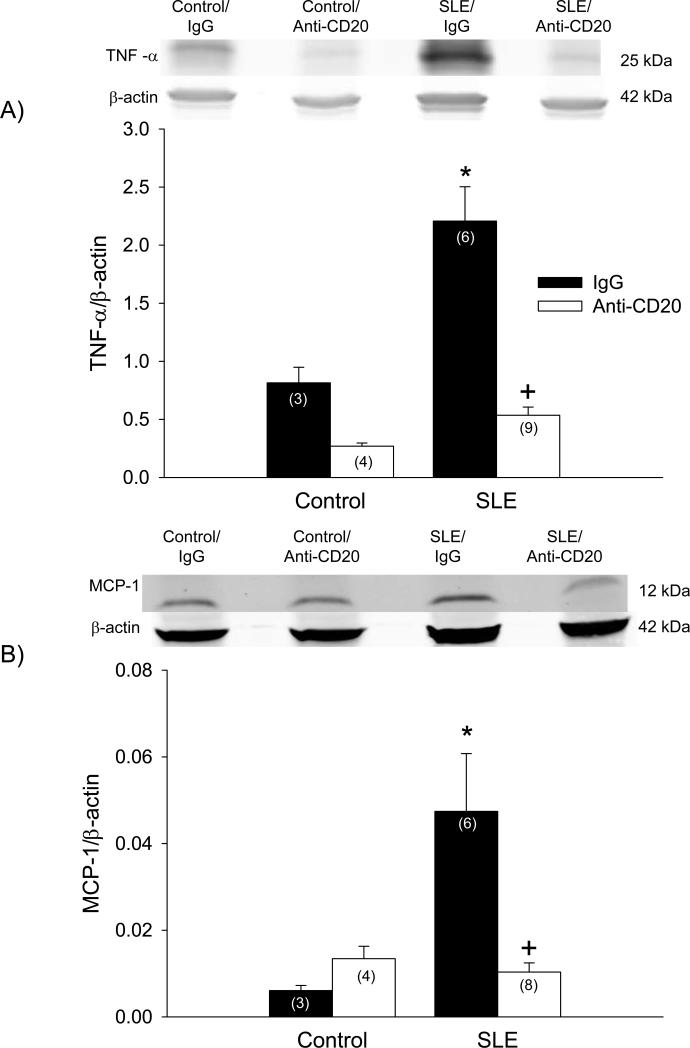
Previous work from our laboratory demonstrated that TNF-α mechanistically contributes to the hypertension during SLE 11. In the present study, the impact of attenuating the development of autoimmunity on renal TNF-α protein expression was examined. Renal cortical expression of TNF-α was increased in vehicle-treated SLE mice compared to vehicle-treated controls (2.2 ± 0.30 vs. 0.81 ± 0.13; p < 0.005; Figure 4A). SLE mice treated with anti-CD20 antibody had significantly lower TNF-α (0.54 ± 0.07; p < 0.01) compared to vehicle-treated SLE mice. The treatment did not impact expression in control mice (0.27 ± 0.03; p = 0.103). Similarly, renal cortical expression of MCP-1 was assessed, and was increased in SLE mice compared to controls (0.047 ± 0.033 vs. 0.006 ± 0.002; p < 0.005; Figure 4B). SLE mice treated with anti-CD20 antibody had lower MCP-1 (0.010 ± 0.006; p < 0.01), but expression was not altered in controls (0.013 ± .006).
Figure 4.
Protein expression of A) tumor necrosis factor (TNF)-alpha and B) monocyte chemoattractant protein (MCP)-1 assessed by Western blot (with representative blot shown) in kidneys of control and SLE mice administered IgG or anti-CD20 (n indicated on graph). * p < 0.005 vs. Control/IgG; + p < 0.01 vs. SLE/IgG
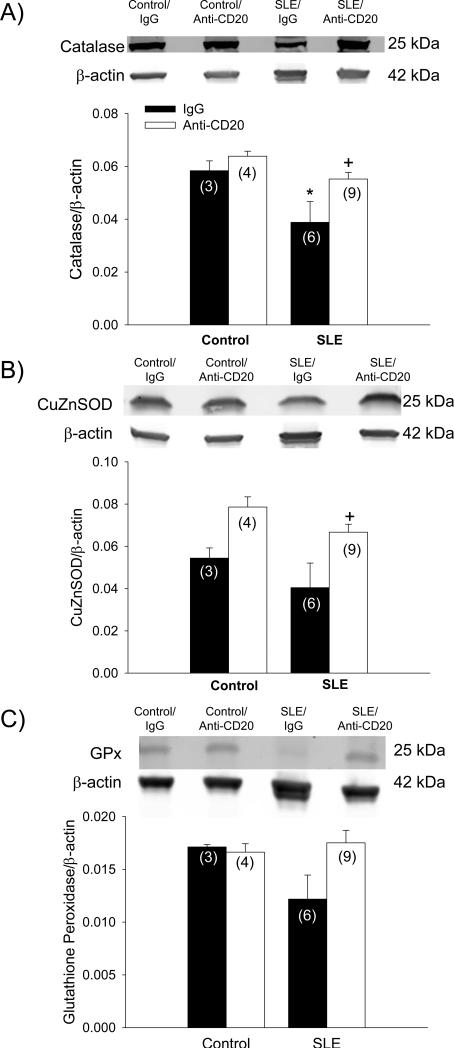
The expression of antioxidant enzymes are increased in Anti-CD20 treated SLE mice
We previously reported that oxidative stress contributes to hypertension during SLE 12. Therefore, renal cortical expression of antioxidant enzymes was assessed. Catalase was lower in SLE mice compared to controls (0.039 ± 0.008 vs. 0.058 ± 0.004; p < 0.03; Figure 5A). Catalase expression was increased in the kidneys of SLE mice treated with anti-CD20 antibodies (0.055 ± 0.003; p < 0.02). Renal CuZnSOD was similar in control and SLE mice (0.054 ± 0.005 and 0.040 ± 0.012, respectively; Figure 5B); however expression was increased in SLE mice treated with anti-CD20 antibodies (0.079 ± 0.005; p < 0.02). Protein expression of glutathione peroxidase in the renal cortex was not statistically different amongst all groups (Figure 5C).
Figure 5.
Protein expression of A) catalase, B) copper-zinc superoxide dismutase (CuZnSOD), and C) glutathione peroxidase assessed by Western blot (with representative blot shown) in kidneys of control and SLE mice administered IgG or anti-CD20 (n indicated on graph). *p < 0.03 vs. Control/IgG; +p < 0.02 vs. SLE/IgG
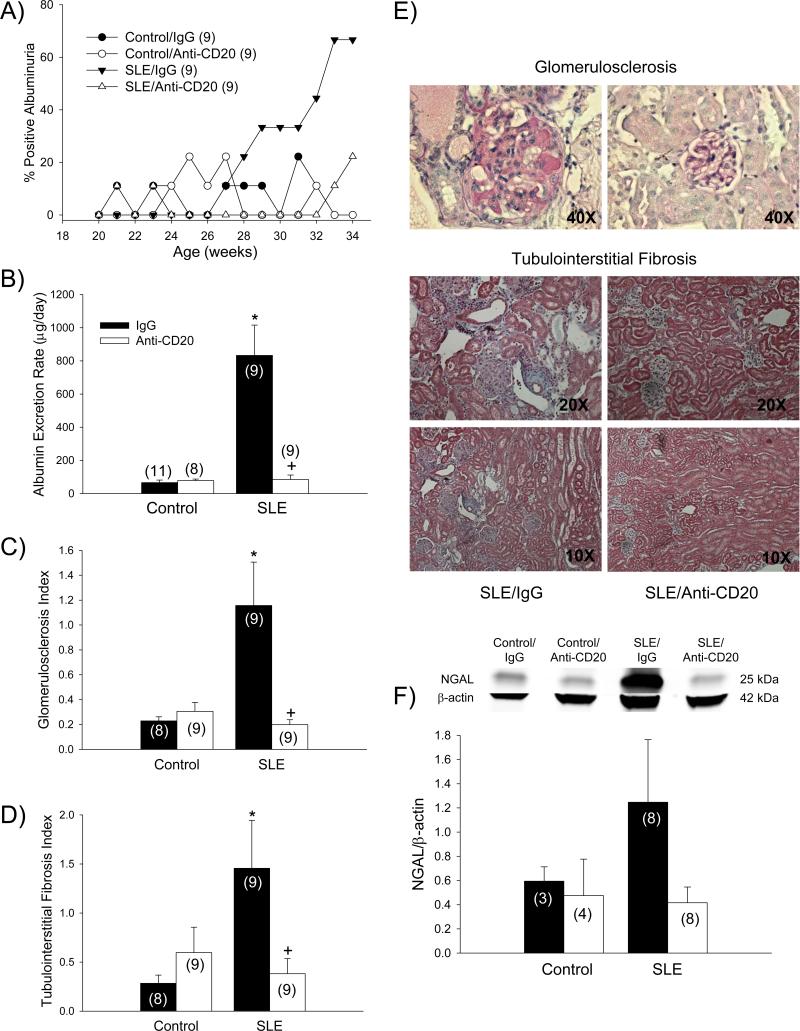
Anti-CD20 therapy protects the kidney in mice with SLE
In order to test whether B cell depletion and the associated reduction in auto-antibodies protected against the development of renal disease common to this model, several indices of renal injury were assessed. The prevalence of albuminuria in vehicle treated SLE mice reached nearly 67% by 34 weeks, whereas the prevalence of albuminuria in SLE mice treated with anti-CD20, as well as treated and untreated controls, remained below 23% throughout the entire course of the 14-week study (Figure 6A). Urinary albumin excretion rate, measured by ELISA at the conclusion of the study, was increased in SLE mice compared to controls (833 ± 183 vs. 70 ± 15 μg/day; p < 0.001; Figure 6B) as previously shown 12,14 and was lower in anti-CD20-treated SLE mice (85 ± 27; p < 0.001). Glomerulosclerosis, defined as mesangial expansion and extracellular matrix deposition, was increased in SLE mice compared to controls (1.16 ± 0.35 vs. 0.23 ± 0.03; p < 0.001; Figure 6C). Anti-CD20 therapy prevented the development of glomerulosclerosis in SLE mice (0.20 ± 0.04; p < 0.001). Similarly tubulointerstitial fibrosis, evident in both the renal cortex and outer medulla, was increased in SLE mice compared to controls (1.46 ± 0.49 vs. 0.28 ± 0.08; p < 0.01; Figure 6D) as indicated by increased collagen deposition and tubular dilatation and atrophy, and was prevented in anti-CD20 treated SLE mice (0.38 ± 0.49; p < 0.02). Representative histological sections for both glomerulosclerosis and tubulointerstitial fibrosis in SLE mice treated with IgG or anti-CD20 are shown in Figure 6E. Renal cortical NGAL, a marker of tubular injury, was increased in mice with SLE (Figure 6F), and this was prevented in anti-CD20-treated animals.
Figure 6.
A) Albuminuria assessed by dipstick throughout the course of the 34 week study in control and SLE mice administered IgG or anti-CD20 (n indicated on graph); B) Urinary albumin excretion rate at 34 weeks measured by ELISA in control and SLE mice administered IgG or anti-CD20 (n indicated on graph). * p < 0.001 vs. Control/IgG; +p < 0.001 vs. SLE/IgG; C) Glomerulosclerosis index (GSI) assessed in control and SLE mice administered ant-IgG or anti-CD20 (n indicated on graph). * p < 0.001 vs. Control/IgG; +p < 0.001 vs. SLE/IgG. D) Renal tubulointerstitial fibrosis index assessed in control and SLE mice administered IgG or anti-CD20 (n indicated on graph). * p < 0.01 vs. Control/IgG; +p < 0.02 vs. SLE/IgG. E) Representative pictures of glomerulosclerosis (40X) and tubulointerstitial fibrosis (20X and 10X) from paraffin-embedded kidneys stained with periodic acid Schiff (PAS) or Masson trichrome, respectively. F) Protein expression of neutrophil gelatinase-associated lipocalin (NGAL) assessed by Western blot (with representative blot shown) in kidneys of control and SLE mice administered IgG or anti-CD20 (n indicated on graph);
DISCUSSION
Based on human studies showing a positive correlation between essential hypertension and serum levels of autoantibodies characteristic of systemic autoimmune disease, the present study was designed to test whether the development of autoimmune disease can be an underlying factor in the pathogenesis of hypertension. In order to test this, a mouse anti-CD20 antibody, the equivalent of rituximab used in humans, was administered to an established female mouse model that develops SLE and hypertension. The major new findings of this study are that (1) treatment with an anti-CD20 antibody prevents the development of hypertension during SLE; (2) renal TNF-α, a cytokine that contributes to the development of hypertension in this model, is attenuated in anti-CD20-treated animals, as is the expression of MCP-1; and (3) the expression of protective antioxidant enzymes is increased in the kidney of anti-CD20-treated SLE mice. In addition, the findings of this study support previous work showing that anti-CD20 therapy prevents the development of renal injury (albuminuria, glomerulosclerosis, and tubulointerstitial fibrosis) that is commonly associated with SLE disease progression. Therefore, these data provide an important proof-of-concept that autoimmunity may be an important factor underlying the development of hypertension, in part through mechanisms that promote local renal inflammation and oxidative stress.
Autoimmunity is associated with hypertension
Autoimmunity has long been implicated in the pathogenesis of hypertension. For example, an early study by White and Grollman demonstrated that vascular and renal antigens were associated with experimental hypertension in a renal infarction model, leading them to postulate that autoimmunity was an underlying factor 18. More recently, the idea that autoantigens perpetuate hypertension has been significantly advanced. Harrison and colleagues have proposed the concept that physiological stressors, including high angiotensin II, lead to the release of local antigens that ultimately promote adaptive immune system activation to sustain the hypertension 19,20. In addition, Rodriguez-Iturbe and colleagues provided compelling evidence that heat shock protein 70 is an important antigen that promotes adaptive immune system activation and contributes to the development of salt-sensitive hypertension 21-23. The present study makes an important advance as it directly tests whether preventing autoimmunity can prevent the development of hypertension.
The association between circulating autoantibodies and hypertension has been recognized for many years with studies showing that IgG and anti-IgM antibodies, anti-nuclear, and antiphospholipid antibodies are increased in patients with essential and malignant hypertension 2-6,24,25. The role of specific activating antibodies has also been reported in hypertensive patients. For example, activating AT1 receptor antibodies are linked with preeclampsia 4,26 while alpha 1-adrenergic receptor antibodies have been reported in a number of studies of human hypertension 27. When hypertensive patients with alpha 1-adrenergic autoantibodies were subjected to immunoadsorption therapy to remove those antibodies, blood pressure was reduced 27. As a whole, these data suggest that humoral immune system activation can promote the pathogenesis of hypertension either through specific activating antibodies or more generalized antibodies that characterize systemic autoimmune disease; however, whether autoimmunity per se is an underlying cause of hypertension remains uncertain. The current study provides evidence that humoral immune system activation associated with systemic autoimmune disease is an important factor in the pathogenesis of hypertension and thus addresses this previously unanswered question.
B cell depletion during SLE
It is widely recognized that humoral immune system activation plays a central role in the pathogenesis of SLE. This is based on evidence that B cells, which differentiate into antibody producing plasma cells, are increased during SLE 28. The production and release of autoantibodies contribute to immune complex formation, and their deposition into tissues initiates an inflammatory response that can lead to tissue injury and inflammation. The data from the current study show that anti-CD20 treatment in female SLE mice prevented the increase in B cells so that they remained similar to the vehicle-treated control animals that do not develop disease. Evidence of functional inhibition is also provided given that antibody activity is significantly attenuated in SLE mice treated with anti-CD20 antibody. Therefore, these data confirm the effectiveness of systemic delivery of the antibody to suppress B cell activity, and provides a compelling case that we effectively prevented or delayed the onset of autoimmunity.
Because of their role in the development of SLE, B cells have been targeted for the treatment of SLE with therapeutics like rituximab (anti-CD20 antibody). However, the efficacy of rituximab to achieve the clinically desired outcome (i.e., remission, antibody reduction, reduced renal injury, etc.) has been variable 29,30. The variable efficacy could be related to the fact that CD20 is not expressed on the antibody-producing plasma cells 31, or attributed to differing severity or stages of disease in SLE patients and their medications (i.e., mycophenolate mofetil, azathiaprine, prednisolone) at the time of the studies 32,33. In pilot studies, we administered anti-CD20 antibody to control and SLE mice for 4 and 8 weeks, starting at 30 and 26 weeks of age, respectively, an age in which autoantibodies are already present and active in SLE mice. The results show that 4 weeks of treatment starting at a later age did not affect blood pressure, and 8 weeks of treatment only modestly reduced blood pressure (please see http://hyper.ahajournals.org). This suggests that once the inflammatory process has begun and antibodies are being produced, the efficacy of B cell depletion by anti-CD20 antibody may be limited. Importantly, these results are consistent with previous work showing a greater variability in the efficacy of anti-CD20 treatment as mice with SLE age 34 and suggest that beginning treatment earlier may be more effective in controlling blood pressure and renal injury. In future studies, it will be important to determine whether specific B cell populations are essential for autoimmune mediated hypertension to occur.
Potential mechanisms for attenuated hypertension following prevention of autoimmunity
The data herein suggest that the protection from developing hypertension afforded by preventing autoimmunity is dependent upon reductions in renal inflammation, renal oxidative stress, and/or renal injury. Our laboratory previously reported that expression of the pro-inflammatory cytokine TNF-α is increased in the renal cortex from mice with SLE and that blockade of TNF-α biological activity with etanercept attenuates the hypertension in this model 11,35. The present study confirms that renal cortical TNF-α protein expression is increased in vehicle-treated SLE mice and demonstrates that this is completely prevented in the SLE mice treated with anti-CD20 antibody. Potential mechanisms by which TNF-α could regulate blood pressure are likely dependent on the balance between pro- and anti-hypertensive actions of TNF-α. For example, TNF-α promotes renal vasoconstriction 36, a pro-hypertensive effect, whereas it has also been reported to have natriuretic effects through inhibition of the Na+-K+-2Cl-cotransporter 37.
Although the source of the renal TNF-α in this model of SLE was not directly determined, it is likely that it comes from infiltrating immune cells such as macrophage and T cells which have been reported by us and others to be elevated in the kidneys of these mice 11,17. We previously reported that renal MCP-1 expression, a chemokine that attracts monocytes and T cells, is increased in mice with SLE 14,17. Therefore, renal cortical protein expression of MCP-1 was assessed in the present study. The results show that MCP-1 expression is attenuated in SLE mice treated with anti-CD20 antibody consistent with the concept that infiltrating immune cells in the kidney likely contribute to the increased TNF-α.
Inflammatory cytokines, including TNF-α, are associated with promoting oxidative stress. We previously demonstrated that TNF-α contributes to renal oxidative stress in this model 11 and that oxidative stress has a mechanistic role in the development of hypertension during SLE 12. In the current study, SLE mice treated with anti-CD20 antibodies exhibited increased expression of antioxidants enzymes suggesting that improved regulation of reactive oxygen species may be an important protective mechanism as well.
By preventing the development of autoimmunity, several indices of renal injury (albuminuria, NGAL, glomerulosclerosis, and fibrosis) were markedly blunted in the SLE animals. This is consistent with the renal protective actions of anti-CD20 antibody therapy in the NZBWF1 model reported previously by others 34. While it cannot be completely ruled out that the prevention of renal injury results from the prevention of the hypertension, we believe that it is unlikely to be the case. The NZBWF1 model is a widely established experimental model of immune complex-mediated glomerulonephritis. Among the most commonly studied models of lupus nephritis in mice (i.e., MRL/Lpr and BXSB), only the NZBWF1 mouse develops hypertension, whereas all three strains develop lupus nephritis. In addition, we recently reported that the hypertension and renal injury in the NZBWF1 model mice are unrelated 14,35, thus supporting the concept that the renal injury is not merely pressure dependent.
Finally, it is important to recognize the possibility that T cells may have a mechanistic role in the pathogenesis of hypertension during SLE. T cells not only play a critical role in promoting antibody production through T cell help, but also mechanistically contribute to the progression of angiotensin II and salt-sensitive hypertension 38-42. Therefore, the data showing that circulating T cells are depleted in anti-CD20 treated mice suggests that this may have a mechanistic role in preventing the autoimmunity and the associated hypertension. Interestingly, similar effects of rituximab therapy on T cells have been demonstrated in humans and experimental models. In one study, peripheral blood mononuclear cells were isolated from the blood of newly diagnosed non-Hodgkin lymphoma patients treated with rituximab as part of their therapy. In the absence of B cells, T cell activation was reduced, most likely resulting from reduced antigen presentation following depletion of the antigen-presenting B cells 43. In a mouse model of experimental autoimmune encephalomyelitis with a humanized immune system, rituximab decreased CD4+ T cells 44. Consistent with our findings, mouse anti-CD20 (the mouse equivalent to rituximab) decreased activated T cells in the NZBWF1 mouse 34. These data support the overall hypothesis that autoimmunity contributes to the development of hypertension by promoting tissue inflammation and that humoral immune system activation is an essential component.
PERSPECTIVES
The work presented here makes significant advances on two important clinically-relevant topics. Over the past several years, it has become clear that adaptive immune system activation plays an important role in the development and maintenance of hypertension. Much of that work has focused on T cells in experimental hypertension and has implicated a role for autoimmunity in the pathogenesis of hypertension. In addition, studies in humans suggest that systemic autoimmunity is associated with essential hypertension. Therefore, the current study provides evidence that autoimmunity mechanistically contributes to the development of hypertension. The second advance is more directly related to SLE, a chronic autoimmune inflammatory disorder with prevalent hypertension for reasons that continue to be elucidated. The data provide important evidence that the hypertension associated with SLE is driven by humoral immune system activation that ultimately leads to renal inflammation and injury. The direct impact of inflammatory mediators on renal hemodynamic function has not been widely studied in SLE and will be an important consideration in future experiments. Overall, the current study may have important clinical implications as well. For example, immunosuppressive therapy would be ill-advised for patients with uncomplicated hypertension; however, our data suggest that immunosuppression may have an added benefit of helping to control blood pressure in patients with chronic inflammatory diseases. Indeed, this idea is supported by a small clinical study showing that mycophenolate mofetil treatment reduces blood pressure in hypertensive patients with psoriasis or rheumatoid arthritis 45.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is New?
Preventing the development of systemic autoimmune disease attenuates the development of hypertension.
What Is Relevant?
These studies demonstrate the potentially important underlying role that autoimmunity can have in the pathogenesis of hypertension.
These studies advance the concept that immunosuppressive therapies may contribute to blood pressure control in hypertensive patients with chronic inflammatory disease.
Because hypertension is a multifactorial disease with growing evidence of a role for autoimmunity and adaptive immune system activation, the use of experimental models with autoimmunity and hypertension may be particularly informative for better understanding the pathogenesis of the disease.
Summary.
There is clear evidence supporting an association between autoimmunity and hypertension; however, whether systemic autoimmunity is mechanistically important remains unclear. Systemic lupus erythematosus (SLE) is an autoimmune disease driven by loss of host tolerance leading to humoral immune system activation and is associated with prevalent hypertension and renal disease. The current study set out to determine whether preventing autoimmune disease protects against the development of hypertension in an established female mouse model of SLE. The results show that chronic B cell depletion attenuated the development of hypertension, renal injury, and renal inflammation and support the concept that autoimmune mechanisms can underlie the pathogenesis of hypertension.
ACKNOWLEGMENTS
The authors would like to thank Katie W. Corkern, Stephanie P. Evans, Emily Gilbert, and C. Warren Masterson for their assistance with this study. Flow cytometry experiments were performed at the UMMC Cancer Institute Flow Cytometry Core. We would also like to thank Dr. Eva M. Bengten for her expertise with the interpretation of FACS data.
GRANTS
This work was supported by the National Institutes of Health (5T32HL105324 and F32HL114272 to KWM, and P01HL051971, P20GM104357 to UMMC-Physiology), the American Heart Association (GIA2060203 to MJR).
Footnotes
DISCLOSURES
None
REFERENCES
- 1.Tsokos G. Systemic lupus erythematosus. New England Journal of Medicine. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Kristensen B, Andersen P, Wiik A. Autoantibodies and vascular events in essential hypertension: a five-year longitudinal study. J Hypertension. 1984;2:19–24. doi: 10.1097/00004872-198402000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Gudbrandsson T, Hansson L, Herlitz H, Lindholm L, Nilsson LA. Immunological changes in patients with previous malignant essential hypertension. Lancet. 1981;1:406–408. doi: 10.1016/s0140-6736(81)91790-6. [DOI] [PubMed] [Google Scholar]
- 4.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen J, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. Journal of Clinical Investigation. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu MLX, Herlitz H, Schulze W, Wallukate G, Micke P, Eftekhari P, Sjogren K-G, Hjalamarson A, Muller-Esterl W, Hoebeke J. Autoantibodies against the angiotensin receptor in patients with hypertension. Journal of Hypertension. 2000;18:953. doi: 10.1097/00004872-200018070-00017. [DOI] [PubMed] [Google Scholar]
- 6.Luther H, Homuth V, Wallukat G. Alpha 1-adrenergic receptor antibodies in patients with primary hypertension. Hypertension. 1997;29:678–682. doi: 10.1161/01.hyp.29.2.678. [DOI] [PubMed] [Google Scholar]
- 7.Sabio J, Vargas-Hitos J, Navarrete-Navarrete N, Mediavilla J, Jimenez-Jaimez J, Diaz-Chamarro A, Jimenez-Alonso J. Prevalence of and factors associated with hypertension in young and old women with systemic lupus erythematosus. Journal of Rheumatology. 2011;38:1026–1032. doi: 10.3899/jrheum.101132. [DOI] [PubMed] [Google Scholar]
- 8.Ryan MJ, McLemore GR, Hendrix ST. Insulin resistance and obesity in a mouse model of systemic lupus erythematosus. Hypertension. 2006;48:988–993. doi: 10.1161/01.HYP.0000243612.02929.df. [DOI] [PubMed] [Google Scholar]
- 9.Mathis KW, Venegas-Pont M, Masterson C, Wasson K, Ryan MJ. Blood pressure in a hypertensive mouse model of SLE is not salt-sensitive. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2011;301:1281–1285. doi: 10.1152/ajpregu.00386.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venegas-Pont M, Mathis KW, Iliescu R, Ray WH, Glover P, Ryan M. Blood pressure and renal hemodynamic responses to acute angiotensin II infusion are enhanced in a female mouse model of systemic lupus erythematosus. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2011;301:1286–1292. doi: 10.1152/ajpregu.00079.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venegas-Pont M, Manigrasso MB, Grifoni SC, LaMarca BB, Maric C, Racusen LC, Glover PH, Jones AV, Drummond HA, Ryan MJ. Tumor necrosis factor-alpha antagonist etanercept decreases blood pressure and protects the kidney in a mouse model of systemic lupus erythematosus. Hypertension. 2010;56:643–649. doi: 10.1161/HYPERTENSIONAHA.110.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathis KW, Venegas-Pont M, Masterson C, Stewart N, Wasson K, Ryan MJ. Oxidative stress promotes hypertension and albuminuria during the autoimmune disease systemic lupus erythematosus. Hypertension. 2012;59:673–679. doi: 10.1161/HYPERTENSIONAHA.111.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaMarca BB, Wallace K, Herse F, Wallukat G, Martin JN, Weimer A, Dechend R. Hypertension in response to placental ischemia during pregnancy: Role of B lymphocytes. Hypertension. 2011;57:865–871. doi: 10.1161/HYPERTENSIONAHA.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathis KW, Venegas-Pont M, Flynn E, Williams J, Maric-Bilkan C, Dwyer T, Ryan M. Hypertension in an experimental model of systemic lupus erythematosus occures independently of the renal nerves. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2013;301:R711–719. doi: 10.1152/ajpregu.00602.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan MJ, McLemore GR. Hypertension and impaired vascular function in a female mouse model of systemic lupus erythematosus. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2007;292:736–742. doi: 10.1152/ajpregu.00168.2006. [DOI] [PubMed] [Google Scholar]
- 16.Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. Angiotensin II-induced vascular dysfunction is mediated by the AT1a receptor in mice. Hypertension. 2004;43:1074–1079. doi: 10.1161/01.HYP.0000123074.89717.3d. [DOI] [PubMed] [Google Scholar]
- 17.Venegas-Pont M, Sartori-Valinotti JC, Maric C, Racusen LC, Glover PH, McLemore GR, Jones AV, Reckelhoff JF, Ryan MJ. Rosiglitazone decreases blood pressure and renal injury in a female mouse model of systemic lupus erythematosus. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2009;296:1282–1289. doi: 10.1152/ajpregu.90992.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White F, Grollman A. Autoimmune factors associated with infarction of the kidney. Nephron. 1964;1:93–102. doi: 10.1159/000179322. [DOI] [PubMed] [Google Scholar]
- 19.Vinh A, Chen W, BLinder Y, Weiss D, Taylor W, Goronzy J, Weyand C, Harrison D, Guzik T. Inhibition and genetic ablation of the B7/CD28 T cell costimulation axis prevents experimental hypertension. Circulation. 2010;122:2529–2537. doi: 10.1161/CIRCULATIONAHA.109.930446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison D, Guzik T, Lob HE, Madhur MS, Marvar P, Thabet S, Vinh A, Weyand C. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parra G, Quiroz Y, Salazar J, Bravo Y, Pons H, Chavez M, Johnson R, Rodriguez-Iturbe B. Experimental induction of salt-sensitive hypertension is associated iwth lymphocyte proliferative response to HSP70. Kidney International Supplement. 2008:S55–S59. doi: 10.1038/ki.2008.513. [DOI] [PubMed] [Google Scholar]
- 22.Pons H, Ferrebuz A, Quiroz Y, Romero-Vasquez F, Parra G, Johnson R, Rodriguez-Iturbe B. Immune reactivity to heat shock protein 70 expressed in the kidney is cause of salt-sensitive hypertension. American Journal of Physiology: Renal Physiology. 2013;304:289–299. doi: 10.1152/ajprenal.00517.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez-Iturbe B, Franco M, Tapia E, Quiroz Y, Johnson R. Renal inflammation, autoimmunity and salt-sensitive hypertension. Clinical and Experimental Pharmacology and Physiology. 2012;39:96–103. doi: 10.1111/j.1440-1681.2011.05482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kristensen B, Andersen P. Autoantibodies in untreated and treated essential hypertension. Acta Med Scand. 1978;203:55–59. doi: 10.1111/j.0954-6820.1978.tb14831.x. [DOI] [PubMed] [Google Scholar]
- 25.Rollino C, Boero R, Elia F, Montaruli B, Massara C, Betrame G, Ferro M, Quattrocchio G, Quarello F. Antiphospholipid antibodies and hypertension. Lupus. 2004;13:769–772. doi: 10.1191/0961203304lu1082oa. [DOI] [PubMed] [Google Scholar]
- 26.Liao Y, Wei Y, Wang M, Wang Z, Yuan H, Cheng L. Autoantibodies against AT1-receptor and alpha1-adrenergic receptor in patients with hypertension. Hypertension Research. 2002;25:641–646. doi: 10.1291/hypres.25.641. [DOI] [PubMed] [Google Scholar]
- 27.Wenzel K, Haase H, Wallukat G, Derer W, Bartel S, Homuth V, Herse F, Hubner N, Schulz H, Janczikowski M, Lindschau C, Schroeder C, Verlohren S, Morano I, Muller D, Luft F, Dietz R, Dechend R, Karczewski P. Potential relevance of alpha (1)-adrenergic receptor autoantibodies in refractory hypertension. PLoS ONE. 2008;3:e3742. doi: 10.1371/journal.pone.0003742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dar O, Salaman M, Seifert M, Isenberg D. B lymphocyte activation in systemic lupus erythematosus-spontaneous production of IgG antibodies to DNA and environmental antigens in cultures of blood mononuclear cells. Clinical and Experimental Immunology. 1988;73:430–435. [PMC free article] [PubMed] [Google Scholar]
- 29.Duxbury B, Combescure C, Chizzolini C. Rituximab in systemic lupus erythematosus: an updated systemic review and meta-analysis. Lupus. 2013;22:1489–1503. doi: 10.1177/0961203313509295. [DOI] [PubMed] [Google Scholar]
- 30.Terrier B, Amoura Z, Ravaud P, Hachulla E, Jouenne R, Combe B, Bonnet C, Cacoub P, Cantagrel A, deBandt M, Fain O, Fautrel B, Gaudin P, Godeau B, Harle J, Hot A, Kahn J, Lambotte O, Larroche C, Le'one J, Meyer O, Pallot-Prades B, Pertuiset E, Quartier P, Schaervereke T, Sibilia J, Somogyi A, Soubrier M, Vignon E, Bader-Meunier B, Mariette X, Gottenberg J. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French AutoImmunity and Rituximab registry. Arthritis and Rheumatism. 2010;62:2458–2466. doi: 10.1002/art.27541. [DOI] [PubMed] [Google Scholar]
- 31.Sanz I, Lee F-H. B cells as therapeutic targets in SLE. Nature Reviews Rheumatology. 2010;6:326–337. doi: 10.1038/nrrheum.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merrill J, Neuwelt C, Wallace D, Shanahan J, Latinis K, Oates J, Utset T, Gordon C, Isenberg D, Hsieh H, Zhang D, Brunetta P. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: The randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62:222–233. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rovin B, Furie R, Latinis K, Looney R, Fervenza F, Sanchez-Guerrero J, Maciuca R, Zhang D, Garg J, Brunetta P, Appel G. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: The Lupus Nephritis Assessment with Rituximab study. Arthritis and Rheumatism. 2012;64:1215–1226. doi: 10.1002/art.34359. [DOI] [PubMed] [Google Scholar]
- 34.Bekar KW, Owen T, Dunn R, Ichikawa T, Wang W, Wang R, Barnard J, Brady S, Nevarez S, Goldman BI, Kehry M, Anolik JH. Prolonged effects of short-term anti-CD20 B cell depletion therapy in murine systemic lupus erythematosus. Arthritis and Rheumatism. 2010;62:2443–2457. doi: 10.1002/art.27515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilbert E, Mathis K, Ryan M. 17β-Estradiol protects against the progression of hypertension during adulthood in a mouse model of systemic lupus erythematosus. Hypertension. 2014;63:616–623. doi: 10.1161/HYPERTENSIONAHA.113.02385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shahid M, Francis J, Majid D. Tumor necrosis factor-alpha induces renal vasoconstriction as well as natriuresis in mice. American Journal of Physiology: Renal Physiology. 2008;295:1836–1844. doi: 10.1152/ajprenal.90297.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Battula S, Hao S, Pedraza P, Stier C, Ferreri N. Tumor necrosis factor-alpha isan endogenous inhibitor of Na+-K+-2Cl-cotransporter (NKCC2) isoform A in the thick ascending limb. American Journal of Physiology: Renal Physiology. 2011;301:94–100. doi: 10.1152/ajprenal.00650.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2010;298:1136–1142. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II-induced hypertension and vascular dysfunction. The Journal of Experimental Medicine. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crowley S, Song Y-S, Lin EE, Griffiths R, Kim H-S, Ruiz P. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2010;298:1089–1097. doi: 10.1152/ajpregu.00373.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chavez M, Herrera0Acosta J, Johnson RJ, Pons HA. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. American Journal of Physiology: Renal Physiology. 2002;282:191–201. doi: 10.1152/ajprenal.0197.2001. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez-Iturbe B, Quiroz Y, Gordon K, Rincon J, Chavez M, Parra G, Herrera-Acosta J, Gomez-Garre D, Largo R, Egido J, Johnson R. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney International. 2001;59:2222–2232. doi: 10.1046/j.1523-1755.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 43.Saville M, Benyunes M, Multani P. No clinical evidence for CD4+ cell depletion caused by Rituximab. Blood. 2003;102:408. doi: 10.1182/blood-2003-03-1005. [DOI] [PubMed] [Google Scholar]
- 44.Monson N, Cravens P, Hussain R, Harp C, Cummings M, dePilar M, Ben L, Do J, Lyons J, Lovette-Racke A, Cross A, Racke M, Stuve O, Shlomchik M, Eagar T. Rituximab therapy reduces organ-specific T cell responses and ameliorates experimental autoimmune encephalomyelitis. PLoS ONE. 2011;6:e17103. doi: 10.1371/journal.pone.0017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. Journal of American Society of Nephrology. 2006;17:218–225. doi: 10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.