Abstract
Background
Severe shock is a life-threatening condition with very high short-term mortality. Whether the long-term outcomes among survivors of severe shock are similar to long-term outcomes of other critical illness survivors is unknown. We therefore sought to assess long-term survival and functional outcomes among 90-day survivors of severe shock and determine whether clinical predictors were associated with outcomes.
Methods
Seventy-six patients who were alive 90 days after severe shock (received ≥1 mcg/kg/min of norepinephrine equivalent) were eligible for the study. We measured three-year survival and long-term functional outcomes using the Medical Outcomes Study 36-Item Short-Form Health Survey, the EuroQOL 5-D-3L, the Hospital Anxiety and Depression Scale, the Impact of Event Scale-Revised and an employment instrument. We also assessed the relationship between in-hospital predictors and long-term outcomes.
Results
The mean long-term survival was 5.1 years: 82% (62/76) of patients survived, of whom 49 were eligible for follow-up. Patients who died were older than patients who survived. Thirty-six patients completed a telephone interview a mean of five years after hospital admission. The patients’ Physical Functioning scores were below US population norms (p<0.001), whereas mental health scores were similar to population norms. Nineteen percent of the patients had symptoms of depression, 39% had symptoms of anxiety and 8% had symptoms of posttraumatic stress disorder. Thirty-six percent were disabled, and 17% were working full time.
Conclusions
Early survivors of severe shock had a high three-year survival rate. Patients’ long term physical and psychological outcomes were similar to those reported for cohorts of less severely ill ICU survivors. Anxiety and depression were relatively common, but only a few patients had symptoms of posttraumatic stress disorder. This study supports the observation that acute illness severity does not determine long-term outcomes. Even extremely critically ill patients have similar outcome to general ICU survivor populations.
Keywords: sepsis, critical care, mortality, organ failure, intensive care
Introduction
Severe shock is a life-threatening condition marked by hypoperfusion and multiple organ dysfunction that requires vasopressor therapy to counteract hypotension through increased vascular tone. Occasionally, patients do not respond to standard doses of vasopressors, and require high-dose vasopressor therapy. Patients with severe shock are critically ill with very high short-term mortality. (1) The high short-term mortality has limited investigations on the long-term outcomes of patients with severe shock. Substantial data now exist regarding functional and psychological outcomes of survivors of less severe, general critical illness, including sepsis, (2, 3) acute respiratory distress syndrome (ARDS) (4–8) and general medical and surgical intensive care unit (ICU) populations. (9–11) These studies demonstrate that many survivors of general critical illness experience long-term functional, physical and cognitive disabilities, depression, anxiety and posttraumatic stress disorder (PTSD). Despite this body of data regarding outcomes following general critical illness, little data exist to characterize the long-term outcomes of patients with severe shock. Prior studies of long-term outcomes suggested that acute illness severity didn’t predict long-term functional outcomes, but there are to our knowledge no specific reports of outcomes among survivors of severe shock. Additionally, this population is particularly prone to withdrawal of care in the ICU, a decision that may be motivated in part by concerns that survival would be associated with intolerable long-term functional outcomes. (12)
Only 76 of 443 (17%) patients who had severe shock survived to 90 days in our initial parent study of patients with severe shock; 58% of non-survivors underwent withdrawal of care before death. (1) In the initial parent study, we did not assess either long-term survival or functional outcomes. The purpose of the present study was to assess long-term survival and functional outcomes among the 76 patients who survived to 90 days. We hypothesized that even years after their critical illness, survivors of severe shock would have a high rate of functional and psychological disability given the severity of their critical illness. We therefore sought to (1) determine how many of the initial 90-day survivors of severe shock survived to three years post-hospital admission, and (2) evaluate long-term outcomes including physical function, psychological symptoms, quality of life and employment status among survivors of severe shock. We also aimed to (3) determine whether clinical predictors were associated with long-term survival and/or quality of life outcomes. Some results of this study were reported in abstract form. (13, 14)
Materials and Methods
Study Population
This study retrospectively assessed three-year survival and long-term (3 to 7 years after admission) outcomes among survivors of severe shock from a previous multi-center study that enrolled patients from 2005–2010 at five Intermountain hospitals. (1) Severe shock in the parent study was categorized as shock plus the requirement for high-dose vasopressor therapy (at least 1 mcg/kg/min of norepinephrine base equivalent) for ≥10 minutes. From the original cohort (n=443), 76 (17%) of the patients survived for 90 days post-hospital admission and were eligible for this long-term outcome study. Details of the parent study were reported previously. (1)
Exclusion criteria for the current, but not the parent study, were non-English speaking and premorbid psychosis or cognitive disability, which were determined by chart review. Three year post-hospital survival was determined by the Intermountain death record and the Social Security Death Master File. From June-December 2013 consenting patients were contacted and interviewed by telephone. The interviews were conducted by a clinical research professional and consisted of standardized questionnaires. The Intermountain Healthcare Institutional Review Board approved this study; participants provided verbal consent at time of telephone interview.
Primary and Secondary Outcomes
The primary functional outcome was the Physical Function domain of the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36). (15) The SF-36 includes eight domain scores and Physical and Mental Health summary scores (range 0 to 100, higher scores indicate better health). Secondary outcomes included the SF-36 Mental Health domain and the Mental and Physical Health summary scores, the EuroQOL 5-D-3L (EQ-5D) (16), the Hospital Anxiety and Depression Scale (HADS) (17), the Impact of Event Scale-Revised (IES-R) (18) and an employment questionnaire. (8) The EQ-5D assesses quality of life and includes a population-weighted utility index that is scored from −0.11 to 1.0 (19, 20) with higher scores indicating better quality of life and a Visual Analog Scale (VAS) that is scored from 0 to 100 with low scores indicating worse perceived health. As per standard practice, we compared the survivors’ SF-36 scores to US population norms (mean 50, SD 10) (21–23), and utilized US-based population weights for the EQ-5D. (20) The HADS is a 14-item scale with scores ranging from 0 to 21 for anxiety and 0 to 21 for depression; scores ≥8 indicate symptoms. (24) The IES-R, which is highly correlated with the gold standard diagnostic Clinician Administered PTSD Scale, (7) evaluates symptoms of posttraumatic stress disorder (PTSD). Items are scored from 0 to 4 (lower scores are better) and a mean score of ≥1.6 indicates PTSD symptoms. The employment instrument assessed patients’ baseline (prior to index hospitalization) and current employment. (8)
Statistical Methods
Descriptive statistics were computed for demographic, medical and outcome data. We analyzed the relationship between survival and clinical predictors using a Cox proportional hazards model of survival. Clinical predictors for all analyses included age, sex, maximum norepinephrine equivalent dose, maximum duration of high-dose vasopressor therapy, APACHE-II (Acute Physiology and Chronic Health Evaluation) (25) scores at onset of high-dose vasopressor therapy, number of days on the ventilator at day 28, and presence of ARDS, as defined by consensus criteria. (26)
For the primary outcome, the relationship between clinical predictors and normalized SF-36 Physical Functioning domain scores were evaluated using linear regression. For secondary outcomes, we used linear regression to evaluate the relationship between clinical predictors and the SF-36 Mental Domain, the Mental and Physical Health summary scores, the EQ-5D utility index and the VAS scores. We used logistic regression to assess relationships between clinical predictors and depression, anxiety and PTSD (HADS scores ≥8 and IES-R mean scores ≥1.6). Chi-square tests were used to compare proportions for baseline and current employment.
Statistical analyses were carried out using SAS 9.3 (27) and R Statistical Package 3.0.1. (28)
Results
Survival and Quality of Life Outcomes
Figure 1 displays the flow of patient enrolment for the present study. Of the 76 patients from the parent study who initially survived severe shock, 62 (82%) survived to three years. The time from hospital discharge to assessment of long-term outcomes ranged from 3 to 7 years (mean 5, SD 1.3 years). Table 1 displays patient population characteristics. On average patients received a maximum norepinephrine equivalent dose of 1.4 mcg/kg/min and an admission APACHE II score of 35. The etiology of the severe shock was heterogeneous, with 62% due to sepsis. Sixty-two percent of patients had ARDS. Table 2 summarizes the clinical predictors comparing patients alive at three years with those who died. Patients who died within three years were older than patients that survived (mean age 64.8 and 53.1, respectively). Younger age was the only clinical predictor that was associated with three-year survival.
Figure 1.
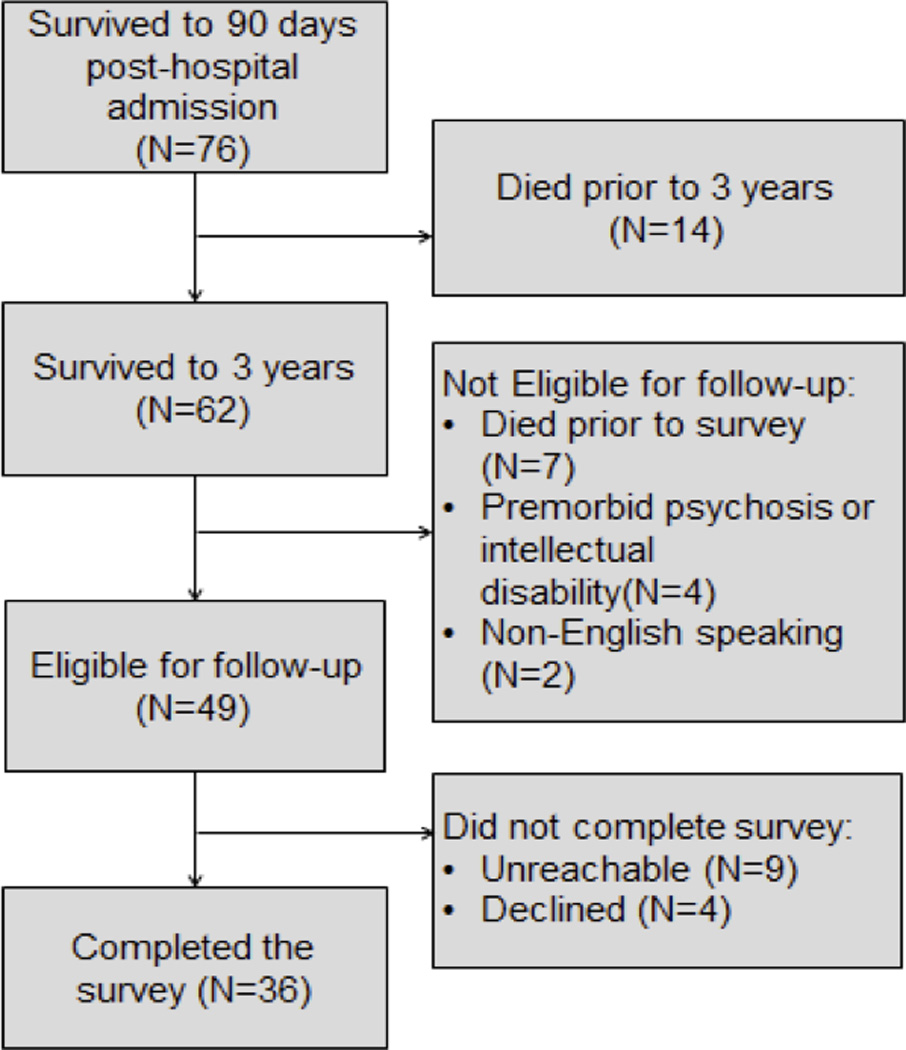
Figure 1 shows study enrollment and 3-year survival.
Table 1.
Population Characteristics (N=76)
| Age (mean ±SD) | 55.2 (± 17.2) |
| Female (%) | 47 |
| Etiology of Shock (N [%]) | |
| Sepsis | 47 (61.8) |
| Cardiogenic | 10 (13.2) |
| Cardiac Arrest | 5 (6.6) |
| Post Cardiac Surgery | 4 (5.3) |
| Overdose | 4 (5.3) |
| Hemorrhage | 2 (2.6) |
| Neurogenic | 1 (1.3) |
| Uncertain | 1 (1.3) |
| Pericardial | 1 (1.3) |
| Pulmonary Embolism | 1 (1.3) |
| Acute Respiratory Distress Syndrome (%) | 62 |
| Clinical Characteristics (median, IQR) | |
| APACHE II on admission | 35 (25–40) |
| APACHE II at onset of HDV | 32 (26–38) |
| Charlson Comorbidity Index | 4 (3–7) |
| SOFA Score on admission | 11 (10–13) |
| SOFA Score at onset of HDV | 12 (10–14) |
| Max NE equivalent dosea | 1.4 (1.1–1.7) |
APACHE: Acute Physiology and Chronic Health Evaluation, HDV: High-dose vasopressor therapy, SOFA: Sequential Organ Failure Assessment, NE: Norepinephrine
mcg/kg/minute
Table 2.
Clinical Predictors: Comparing Survivors vs. Deceaseda
| Predictor | Alive at three years |
Deceased at three years |
p value |
|---|---|---|---|
| Patients (n) | 62 | 14 | |
| Age (years ± SD) | 53.1 ± 17.3 | 64.8 ± 13.1 | 0.03 |
| Female (%) | 45.2 | 50.0 | 0.98 |
| Maximum NE equivalentb (median, IQR) | 1.4 (1.1–1.7) | 1.5 (1.1–2.1) | 0.39 |
| Maximum duration HDVc (median, IQR) | 117 (32–375) | 194 (40–590) | 0.62 |
| APACHE II at onset of HDV(median, IQR) | 31.0 (25.3–38.0) | 34.5 (27.5–40.5) | 0.27 |
| Received stress-dose steroids (%) | 61.3 | 57.1 | 0.77 |
| Ventilator days at day 28 (median, IQR) | 7.5 (3.0–13.8) | 6.0 (4.0–16.8) | 0.89 |
NE: Norepinephrine; APACHE: Acute Physiology and Chronic Health Evaluation, HDV: High-dose vasopressor therapy
Differences were tested using Wilcoxon rank sum tests for continuous covariates and chisquare tests for equality of proportions
mcg/kg/min
minutes
Forty-nine of the 62 patients were alive at a mean of 5.1 years and were eligible for follow-up (7 died after three years but prior to follow-up, 2 were non-English speaking and 4 had pre-morbid psychosis or cognitive disability). Of the 49 eligible patients, 36 (73%) completed the interview (4 declined participation and 9 were unreachable, despite multiple efforts to contact them).
Table 3 summarizes the patients’ quality of life and psychological outcomes. For the primary outcome, the patients’ mean Physical Functioning domain score was low compared to US population norms (p<0.001: 1.2 SD lower than US population norms). The mean Physical Health summary score was also lower than US population norms (p<0.001: 1.1 SD lower than the norm). The mean Mental Health domain score and the mean Mental Health summary score were similar to US population norms (p=0.61 and 0.53, respectively). After adjusting for age and sex, the Physical Health summary score remained significantly lower (p<0.001), while the Mental Health summary score still did not differ from US population norms (p=0.42). The EQ-5D utility index mean score (0.72) and the VAS mean score (71.3) were lower than US population norms.
Table 3.
Quality of Life and Psychological Outcomes Among 36 Patients
| Construct | Normal valuesa |
Value (mean ± SD) or percent with symptoms |
95% CI |
|---|---|---|---|
| Quality of Life: SF-36b | |||
| Physical Functioning domain | 50 (14–58) | 37.6 ± 13.0 | (33.2, 42.0) |
| Mental Health domain | 50 (7–65) | 49.1 ± 13.4 | (44.5, 53.6) |
| Physical Health Summary | 50 (4–71) | 39.1 ± 11.7 | (35.2, 43.1) |
| Mental Health Summary | 50 (2–74) | 49.5 ± 13.6 | (44.8, 54.1) |
| Quality of Life: EQ-5D | |||
| Utility score (range: −0.11–1.00) | 0.86 | 0.72 ± 0.22 | (0.64, 0.79) |
| VAS (range: 0–100) | 79.8 | 71.3 ± 16.1 | (65.8, 76.7) |
| Psychological Symptoms | |||
| HADS Anxiety | <8 | 39% | (24%, 57%) |
| HADS Depression | <8 | 19% | (9%, 37%) |
| IES-R Mean Score (range: 0–4) | <1.6 | 8% | (2%, 24%) |
CI: Confidence Interval
For the SF-36 we specify 1998 US general population norm means (range) (23)
Norm-based scores (mean 50, SD 10)
Of the 36 patients, 7 (19%) had symptoms of depression, 14 (39%) had symptoms of anxiety (scores ≥8), and 3 (8%) had symptoms of PTSD (scores ≥1.6). None of the clinical predictors were associated with quality of life or psychological outcomes (depression, anxiety and PTSD).
Table 4 displays patients’ baseline and current employment status. Of the 17 patients who were employed prior to their critical illness with severe shock, only 9 (53%) returned to work. Ten patients reported new disability. Compared to their baseline, patients were more likely to be disabled (36% vs. 8%, p=0.01) and were less likely to work full-time (17% vs. 39%, p=0.07).
Table 4.
Employment Characteristics (N=36)
| Variable | Baseline | Current | p value |
|---|---|---|---|
| Full-Time | 14 | 6 | 0.07 |
| Part-time | 3 | 3 | 1.00 |
| Homemaker | 2 | 2 | 1.00 |
| Retired | 10 | 12 | 0.80 |
| Disability | 3 | 13 | 0.01 |
| Unemployed | 3 | 0 | 0.24 |
| Student | 1 | 0 | 1.00 |
Discussion
This study, the first to our knowledge to investigate long-term survival and functional outcomes after severe shock with mixed etiology, demonstrates that despite the high 90-day mortality associated with severe shock, 82% of patients who survive to 90 days also survived three or more years. Our study population was more severely ill, judging by APACHE scores and 90-day mortality, than populations in prior long-term outcome studies after critical illness. While rigorous comparisons with previous studies are difficult due to differences in study methodology, the APACHE II scores in our cohort (median 35, range 25–40) are substantially higher than the APACHE II scores reported in prior studies (range 15 to 23). (3, 5, 6, 29, 30) The cumulative (short-term plus long-term) mortality at three years in our cohort (86%) is substantially higher than the 58% cumulative mortality among septic patients reported by Cuthbertson et al. and the 28% cumulative mortality among ARDS patients reported by Herridge et al. (3, 6)
Importantly, while our patient population was more severely ill than those in prior studies; their long-term functional outcomes do not appear to differ substantially from less severely ill ICU survivors. Our data suggest that regardless of the acute severity of illness, survivors of severe shock have similar long-term functional outcomes as other populations of ICU survivors.
Survivors had a low quality of life for the Physical Functioning domain and Physical Health summary scores; alternatively the Mental Health domain and summary scores were similar to population norms. Physical and mental functioning scores on the SF-36 among our survivors of severe shock are remarkably similar to the scores of previous survivors of sepsis and septic shock. In survivors of severe sepsis, Cuthbertson et al reported substantially lower physical health scores and slightly lower mental health scores compared to the general population at 5 years. (3) In a 1-year follow-up, Poulsen et al. also reported a low physical component summary score in patients with septic shock. (31)
The long-term outcomes among our patients are similar to established outcomes among ARDS survivors. Patients with ARDS physical scores were 1 SD below the mean population scores five years after discharge. (6) In a 6 and 12-month follow-up, Needham et al. reported substantially lower physical scores in survivors of acute lung injury. (8) Our findings are similar to those of a meta-analysis of survivors of ARDS that found decrements in quality of life persist for years, but seem to be more pronounced in the physical health domains as opposed to mental health domains. (4) Similarly, our patients had lower EQ-5D utility index (0.72) and VAS (71.3) scores than US population norms (utility index 0.860 and VAS 79.8), results (32) similar to ARDS survivors (utility index 0.71, VAS 69). (8)
We found in survivors of severe shock, 19% had symptoms of depression and 39% had symptoms of anxiety, which are consistent with reported rates of depression and anxiety in ICU populations. In a systematic review of survivors of ARDS, the prevalence of depressive symptoms ranged from 17% to 43% and the prevalence of anxiety ranged from 23% to 48%. (33) A systematic review of general ICU survivors reported a median 28% (range 8% to 57%) of depressive symptoms. (11) In our patients, the rate of anxiety was higher than depression, which is similar to the findings of Needham et al. who reported a 42% prevalence of anxiety and a 37% prevalence of depression in survivors of acute lung injury. (8) These findings suggest that survivors of severe shock have similar rates of depression and anxiety years after hospital discharge as found in ICU patients with less severe illness.
The prevalence of PTSD (8%) in our population was at the low end of estimates from other studies of ICU survivors, with range from 7% to as high as 39%. (10, 34–37) The reason for the inconsistency in the rates of PTSD in critically ill populations is unclear and requires further investigation; our longer follow-up could suggest the possibility that post-ICU PTSD abates somewhat with time. We acknowledge that very small numbers limit confident inference in terms of PTSD in our cohort. Given the severity of illness in our population, one may expect greater long-term physical and psychiatric morbidity than less critically ill populations; however our patients’ psychological outcomes are not different from other ICU populations. Although the very high mortality rate of severe shock left few survivors to study, our findings suggest that higher acute severity of illness does not portend worse long-term morbidity among critically ill patients. Our study thus corroborates and extends prior observations that admission APACHE scores were not associated with long-term functional outcomes.
A somewhat higher percent of our cohort did not return to work after their illness. At follow-up 48% of survivors of severe shock who were employed prior to their critical illness did not return to work after hospital discharge, which is consistent with previous studies. (5, 8, 31) Herridge et al. reported that 52% of ARDS patients did not return to work at one year, however, by five years, only 23% had not returned to work. The difference between our findings and those of Herridge and colleagues may be attributed to age and severity of illness; our patients were older on average (median age, 59 vs. 44 years) and had a greater severity of illness than patients in the Herridge study (median APACHE II scores 35 vs. 23). (6) Ten of the patients (28%) in our study were receiving new disability payments, which is consistent with the findings of Hopkins et al. (34%) among survivors of ARDS. (5)
Reliable data about outcomes among the most severely ill patients are lacking. The findings in our study suggest that long-term functional outcomes after critical illness don’t necessarily depend upon initial disease severity. Even extremely critically ill patients, if they survive, have similar long-term outcomes to less severely ill ICU patients. Although it may be logical to assume that the sickest ICU patients will have proportionally worse long-term outcomes, our data question this logic.
This study has several limitations. Due to the low (17%) 90 day survival seen in the initial patient population, our sample size was relatively small despite being a multi-center study spanning five years. As a result, our findings should be validated in a larger population. Similar to most previous ICU studies, we were unable to assess pre-illness quality of life data and health status prior to the index hospitalization due to the emergent nature of critical illness. Without pre-illness baseline data, it is difficult to determine whether or what percentage of the decrements in quality of life may be due to pre-existing problems. Nevertheless, the extent of psychological and functional disability and lack of return to work after severe shock in our study suggests that a substantial component of disability may arise from the critical illness itself. In addition, we exclusively evaluated outcomes by telephone however, this is a widely used method for assessing quality of life and psychological outcomes in survivors of critical illness. (37) Research should assess physical outcomes (i.e. 6-minute walk test, muscle mass), functional outcomes such as instrumental activities of daily living, as well as cognitive function.
The reduced quality of life in the physical domain, psychiatric morbidity and low rates of return to work in survivors of severe shock are similar to previous reports in survivors of critical illness. Judging by the short-term mortality rate and APACHE II scores, our patient cohort was more severely ill than prior cohorts. Despite the burden of severe critical illness, the subsequent burdens of post-ICU morbidity of survivors of severe shock are similar to long-term outcomes in other ICU populations. This information may help survivors, care-givers and clinicians to not only understand the patterns of long-term recovery, but also to guide informed decisions regarding rehabilitation and participation in follow-up clinics and outreach services.
Acknowledgments
We would like to acknowledge the contributions of Mardee Merrill, CCRP (Intermountain Medical Center) for recruiting patients and conducting interviews and Daniel Knox, MD (University of Utah Health Sciences Center) for database queries for this study.
Source of funding: This study was funded by K23GM094465 to Samuel M. Brown.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.Brown SM, Lanspa MJ, Jones JP, Kuttler KG, Li Y, Carlson R, Miller RR, Hirshberg EL, Grissom CK, Morris AH. Survival after shock requiring high-dose vasopressor therapy. Chest. 2013;143(3):664–671. doi: 10.1378/chest.12-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuthbertson BH, Elders A, Hall S, Taylor J, Maclennan G, Mackirdy F, Mackenzie SJ. Mortality and quality of life in the five years after severe sepsis. Crit Care. 2013;17(2):R70. doi: 10.1186/cc12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dowdy DW, Eid MP, Dennison CR, Mendez-Tellez PA, Herridge MS, Guallar E, Pronovost PJ, Needham DM. Quality of life after acute respiratory distress syndrome: a meta-analysis. Intensive Care Med. 2006;32(8):1115–1124. doi: 10.1007/s00134-006-0217-3. [DOI] [PubMed] [Google Scholar]
- 5.Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF., Jr Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171(4):340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 6.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 7.Bienvenu OJ, Williams JB, Yang A, Hopkins RO, Needham DM. Posttraumatic stress disorder in survivors of acute lung injury: evaluating the Impact of Event Scale-Revised. Chest. 2013;144(1):24–31. doi: 10.1378/chest.12-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Needham DM, Dinglas VD, Bienvenu OJ, Colantuoni E, Wozniak AW, Rice TW, Hopkins RO, Network NNA. One year outcomes in patients with acute lung injury randomised to initial trophic or full enteral feeding: prospective follow-up of EDEN randomised trial. BMJ. 2013;346:f1532. doi: 10.1136/bmj.f1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuthbertson BH, Roughton S, Jenkinson D, Maclennan G, Vale L. Quality of life in the five years after intensive care: a cohort study. Crit Care. 2010;14(1):R6. doi: 10.1186/cc8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med. 2011;39(2):371–379. doi: 10.1097/CCM.0b013e3181fd66e5. [DOI] [PubMed] [Google Scholar]
- 11.Davydow DS, Gifford JM, Desai SV, Bienvenu OJ, Needham DM. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med. 2009;35(5):796–809. doi: 10.1007/s00134-009-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turnbull AE, Krall JR, Ruhl AP, Curtis JR, Halpern SD, Lau BM, Needham DM. A scenario-based, randomized trial of patient values and functional prognosis on intensivist intent to discuss withdrawing life support. Crit Care Med. 2014;42(6):1455–1462. doi: 10.1097/CCM.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pratt CM, Lanspa MJ, Jones JP, Hirshberg EL, Kuttler KG, Wilson EL, Hopkins RO, Brown SM. Long-term Functional Outcomes after Severe Shock [abstract] Am J Respir Crit Care Med. 2014:A3126. [Google Scholar]
- 14.Pratt C, Lanspa MJ, Jones JP, Hirshberg EL, Kuttler KG, Dickerson J, Grissom C, Brown SM. Long-term Survival after Severe Shock [abstract] Am J Respir Crit Care Med. 2013:A1560. [Google Scholar]
- 15.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): Conceptual framework and item selection. Medical Care. 1992:473–483. [PubMed] [Google Scholar]
- 16.EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 17.Zigmond A, Snaith R. The hospital anxiety and depression scale. Acta Anaesthesiol Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 18.Weiss DS, Marmar CR. The impact of event scale-revised. Assessing Psychological Trauma and PTSD. 2004;2:168–189. [Google Scholar]
- 19.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Medical Care. 2005;43(3):203–220. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Lizza JP. Persons, Humanity, and the Definition of Death. Baltimore, MD: Johns Hopkins University Press; 2005. [Google Scholar]
- 21.Hays RD, Sherbourne CD, Spritzer KL, Dixon WJ. A microcomputer program (SF-36.EXE) that generates SAS code for scoring the SF-36 health survey. SAS Users Group International. 1997:1128–1132. [Google Scholar]
- 22.Ware J. SF-36 Health Survey Update, [Internet] [accessed 2013 Aug 30]; Available from: http://wwwsf-36/tools/sf36shtml. [Google Scholar]
- 23.The SF Community. U.S. Population Norms, [Internet] [accessed 2013 Aug 30]; Available from: http://wwwsf-36org/research/sf98normspdf. [Google Scholar]
- 24.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 25.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Critical Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 26.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 27.SAS Institute. SAS/STAT 9.3 user's guide. SAS Institute; 2011. [Google Scholar]
- 28.R Core Team. R: A Language and Environment for Statistical Computing, 2013 [Internet] [accessed 2013 Nov 3];R Foundation for Statistical Computing. Available from: http://wwwR-projectorg. [Google Scholar]
- 29.Cuthbertson BH, Scott J, Strachan M, Kilonzo M, Vale L. Quality of life before and after intensive care. Anaesthesia. 2005;60(4):332–339. doi: 10.1111/j.1365-2044.2004.04109.x. [DOI] [PubMed] [Google Scholar]
- 30.Garcia Lizana F, Peres Bota D, De Cubber M, Vincent JL. Long-term outcome in ICU patients: what about quality of life? Intensive Care Med. 2003;29(8):1286–1293. doi: 10.1007/s00134-003-1875-z. [DOI] [PubMed] [Google Scholar]
- 31.Poulsen JB, Moller K, Kehlet H, Perner A. Long-term physical outcome in patients with septic shock. Acta Anaesthesiol Scand. 2009;53(6):724–730. doi: 10.1111/j.1399-6576.2009.01921.x. [DOI] [PubMed] [Google Scholar]
- 32.Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Making. 2006;26(4):391–400. doi: 10.1177/0272989X06290497. [DOI] [PubMed] [Google Scholar]
- 33.Davydow DS, Desai SV, Needham DM, Bienvenu OJ. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med. 2008;70(4):512–519. doi: 10.1097/PSY.0b013e31816aa0dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samuelson KA, Lundberg D, Fridlund B. Stressful memories and psychological distress in adult mechanically ventilated intensive care patients - a 2-month follow-up study. Acta Anaesthesiol Scand. 2007;51(6):671–678. doi: 10.1111/j.1399-6576.2007.01292.x. [DOI] [PubMed] [Google Scholar]
- 35.Jones C, Backman C, Capuzzo M, Flaatten H, Rylander C, Griffiths RD. Precipitants of post-traumatic stress disorder following intensive care: a hypothesis generating study of diversity in care. Intensive Care Med. 2007;33(6):978–985. doi: 10.1007/s00134-007-0600-8. [DOI] [PubMed] [Google Scholar]
- 36.Jackson JC, Pandharipande PP, Girard TD, Brummel NE, Thompson JL, Hughes CG, Pun BT, Vasilevskis EE, Morandi A, Shintani AK, Hopkins RO, Bernard GR, Dittus RS, Ely EW. Depression, posttraumatic stress disorder and functional disability in survivors of critical illness: Results from the BRAIN ICU (Bringing to light the Risk Factors And Incidence of Neuropsychological dysfunction in ICU Survivors. Lancet Respir Med. 2014 doi: 10.1016/S2213-2600(14)70051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, Localio AR, Demissie E, Hopkins RO, Angus DC. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185(12):1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


