Abstract
Background
Omega-3 fatty acids (FA) supplements lower triglyceride (TG) levels in adults; little pediatric information is available. We evaluated their effect in hypertriglyceridemic adolescents.
Methods
25 patients ages 10–19 years with TG levels 150–1000 mg/dL were randomized to 6 months double-blind trial of Lovaza [∼3360 mg docosahexaenoic acid + eicosapentaenoic acid/day] vs. Placebo.
Results
Baseline mean TG levels were 227 mg/dl (SD 49). TG levels declined at 3 months in the Lovaza group by 54 ± 27 mg/dL [mean ± standard error (SE)] (p=0.02) and by 34 ± 26 mg/dL (p=0.16) in the Placebo group. The difference in TG lowering between groups was not significant (p=0.52). There were no between-group differences in endothelial function, blood pressure, body mass index, C-reactive protein or side effects.
Conclusions
High dose omega-3 FA supplements are well tolerated in adolescents. However, declines in TG levels did not differ significantly from Placebo in this small study.
Keywords: triglycerides, child, adolescent, omega-3 fatty acids, hyperlipidemia, randomized-controlled trial
Introduction
Elevated triglyceride (TG) levels have been shown to be an independent risk factor for coronary heart disease in some studies,1, 2 and severe TG elevations increase the risk for acute pancreatitis.3 Lifestyle modification is the primary therapy, but compliance is generally poor. Cardiovascular benefits have been described from dietary and supplemental omega-3 fatty acids (FA), in particular the marine derived FAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA),4–7 with diet and higher dose omega-3 FA supplements having more benefit.8, 9 The strongest and most consistent cardiometabolic effect of EPA and DHA supplements has been the TG lowering effect,5–7 which can produce declines in plasma TG levels of 25–30% when given in doses of 3–4 grams per day.6 Despite these important declines in TG levels, controversy exists about their role in clinical care, related to the questionable benefits of low dose (1 gm/day) supplements.10, 11
Hypertriglyceridemia is relatively common among youth, affecting 12% of teens,12 and is often related to obesity.13 Pharmacotherapy, including fibrates and niacin is used in adults, but the effect on cardiovascular disease (CVD) events is debated.14, 15 The few studies of fibrates in children have shown them to be reasonably safe and effective in lowering TG levels, but are of short duration, small size and in special populations.16–18 One published report of pediatric patients treated with niacin demonstrated high rates of drug discontinuation related to difficulties with flushing, headaches and rash.19 The role of omega-3 FA supplementation in treating pediatric lipid disorders is less explored. Previous trials in children and adolescents have tested low doses of omega-3 FA supplements and have shown variable results. Engler et al. tested low dose (1.2 gm/day DHA) for 6 weeks and found no statistically significant lowering of TG levels in patients with familial hypercholesterolemia or familial combined hypercholesterolemia;20 however, these children had only mildly elevated TG levels (133 ± 68 mg/dL). Other trials also used lower doses of supplements and found either no effect,21 or minimal effect at low dose.22
This study evaluates the efficacy and safety of omega-3 FA supplements (Lovaza) in youth with moderate to severe hypertriglyceridemia. We also explored biologic mechanisms and therapeutic potential for CVD risk factors, including blood pressure, insulin resistance, vascular function, and inflammation.
Methods
Study Design and Population
We conducted a double-blind placebo controlled randomized trial to evaluate the efficacy and safety of Omega-3 FA in reducing TG levels in healthy youth ages 10–19 years with moderate to severe hypertriglyceridemia. Patients were recruited from subspecialty and primary care clinics at Boston Children’s Hospital and from referrals from community pediatricians. They were eligible to participate if they had a fasting TG level that was 150 to 1000 mg/dL and met all other study inclusion and exclusion criteria. Inclusion criteria included the ability to swallow pills and speak English. Patients were excluded if they reported allergy to fish, corn or other components of the pills, pregnancy or breast feeding, alcohol use, bleeding disorder or coagulopathy, thyroid disorder, diabetes or fasting glucose at or above 126 mg/dL, liver disease or alanine aminotransferase (ALT) greater than 2 times the upper limit of normal, treatment with medications affecting TG levels including oral hypoglycemic agents, insulin, non-statin lipid lowering medications, or omega-3 FA pills.
Eligible subjects who provided informed consent participated in a one month dietary counseling run-in period. Only those subjects whose TG levels remained 150 mg/dL to 1000 mg/dL at the baseline visit were randomized to either four 1 g capsules daily of Lovaza or placebo for 6 months. Randomization was conducted and assigned by the research pharmacy of Boston Children’s Hospital using a list generated by the Clinical Research Center. Randomization was blocked to yield roughly equivalent group assignment over time. Study staff and subjects were blinded to treatment assignment. Follow-up measurements were conducted at 3 and 6 months after randomization. The primary outcome was defined as change in TG levels from baseline at 3 months. Secondary outcomes included TG levels at 6 months, blood pressure, total cholesterol (TC), very low-density lipoprotein (VLDL), low-density lipoprotein (LDL) and high density lipoprotein (HDL) cholesterol at 3 and 6 months. Exploratory outcomes included C-reactive protein, homeostasis model of assessment – insulin resistance (HOMA-IR) as a measure of insulin resistance, and endothelial function measured by pulse amplitude testing (EndoPAT, Itamar Medical). Adult subjects and parents gave written informed consent; all minor subjects gave assent. Subjects received a heart healthy snack post-fast, parking reimbursement, and a gift card upon completing each study visit. The study protocol was approved by the institutional review board of Boston Children’s Hospital.
Intervention
Lovaza (Glaxo-Smith-Kline, Research Triangle Park, NC USA) is a purified preparation of omega-3 long chain polyunsaturated FA. Each 1 gram capsule contains a total of 840 mg of omega-3 FA, 465 mg as EPA and 375 mg as DHA, in 4 mg of carrier vegetable oil. Each placebo capsule contained 1 gram of corn oil as well as <0.05% of trans-FA. Both Lovaza and placebo capsules contained a small amount of iron oxide, used for color blinding, and tocopherol to prevent oxidation.
In addition to the study drug, all participants were advised by a registered dietician to follow a diet low in refined carbohydrate and saturated fat that emphasized increasing intake of fruits and vegetables and consumption of two servings per week of fish rich in omega-3 FA. This is standard of care at our institution and is consistent with National Heart, Lung and Blood Institute (NHLBI) Integrated Guidelines for Cardiovascular Risk Reduction in Children and Adolescents.23
Measurements
All study visits were conducted in the Boston Children’s Hospital Clinical and Translational Research Center by trained staff. Anthropometrics were measured in triplicate while wearing light clothing and no shoes using a stadiometer and digital scale. Waist circumference was measured at the superior-inferior iliac crest using a metal measuring tape. Blood pressure was auscultated three times, one minute apart in a quiet room after a five minute rest period in the right arm using an appropriate sized cuff. Measurements were averaged for the analysis. All patients had a physical exam at the screening visit, and completed self-administered questionnaires with the help of their parent(s) on medical and family history, frequency of fish intake, lifestyle behaviors, and the occurrence of potential side effects (e.g. baseline muscle and gastrointestinal complaints). A 24 hour dietary recall was conducted at each study visit; the results were analyzed by Nutrition Data Services24 and standard dietary characteristics were calculated including macronutrients, omega-3 FA intake, and glycemic load (GL), which was calculated as the product of the foods glycemic index, using a white bread standard, and the amount of carbohydrate divided by 100.25 Lifestyle behaviors, potential side effects, dietary intake by 24 hour recall and fish intake specifically were re-assessed at each study visit.
Laboratory tests
Blood samples were obtained after a 12-hour fast. Subjects were instructed to re-schedule their visit if they had a febrile illness, due to the effect of acute illness on TG levels. TC, TG, and HDL cholesterol were measured using standard methods. LDL cholesterol was calculated using the Friedewald calculation26 or measured directly in patients with TG levels > 400 mg/dL using the homogenous method (Genzyme Corporation; Cambridge, Massachusetts). Very low density lipoprotein (VLDL) cholesterol levels were calculated by dividing the TG level by 5. Insulin resistance was estimated using HOMA-IR, which is highly correlated with the euglycemic-hyperinsulinemic clamp in obese and non-obese children and adolescents.27 HOMA was calculated as follows: fasting plasma insulin (µU/ml) x fasting plasma glucose (mmol/L)/22.5. High sensitivity C-reactive protein (CRP) was measured by an immunoturbidimetric assay on the Hitachi 911 analyzer (Roche Diagnostics - Indianapolis, Indiana).
Vascular Testing
Trained study staff assessed vascular function non-invasively using EndoPAT (Itamar Medical) after a 12 hour fast. EndoPAT is an FDA approved method of non-invasively assessing endothelial function and has been shown to be reliable in healthy adolescents.28 Post-occlusive volume changes at the fingertip are compared between the occluded and non-occluded arm. EndoPAT has been correlated with brachial artery reactivity testing and validated to detect coronary artery endothelial dysfunction in adults.29, 30
Measures of Compliance
Participants were asked to return their leftover pills and bottles. Red blood cell (RBC) membrane levels of EPA and DHA were measured to assess compliance as well as exposure to and absorption of dietary sources of DHA and EPA. FAs were identified by comparison with a custom-made, weighed, standard mixture consisting of 19 FAs characteristic of RBC (GLC-673b, Nuchek Prep, Elysian, Minnesota). Coefficients of variation (CVs) were reported from the manufacturer as being derived from over 200 runs across 4 instruments. At a low HS-Omega-3 Index (1.7%), the CV of this testing is 6.8%, and at a high HS-Omega-3 Index (11.0%), the CV is 2.5%.
Statistical Methods
The study was designed with 80% power to detect a 20% net decrease in TG levels in the Omega-3 FA group when compared to TG level changes in the placebo group from a sample size of 60 patients. Patients’ characteristics and baseline measures were compared between treatment arms by Student t-test for continuous measures or Fisher’s exact test for discrete traits. The time course of each outcome variable, from baseline to 3 months to 6 months, was compared between treatment arms by repeated-measures analysis of variance (ANOVA), using an autoregressive covariance structure. From parameters of the fitted model, we constructed estimates for the mean value at each time point; the mean change at 3 months and 6 months; the difference between arms in mean change at 3 months and 6 months; and standard errors for all of the above, permitting the changes and differences to be compared to zero by Student t-test. In cases of skewed distribution, we analyzed the log-transformed variable and re-transformed the means and changes for reporting net changes. In cases of extreme skew, we used non-parametric summary statistics and tests, namely, median and range; signed-rank test for zero median change; and Wilcoxon rank-sum test for difference in distribution of changes between treatment arms. The association between TG levels and DHA+EPA levels was assessed using Pearson correlation coefficient. A two-sided p<0.05 was the criterion for statistical significance. Statistical Analysis System software (version 9.2, Cary, NC) was employed for all computations. The primary analyses were performed with the intention to treat principle.
Results
Participants
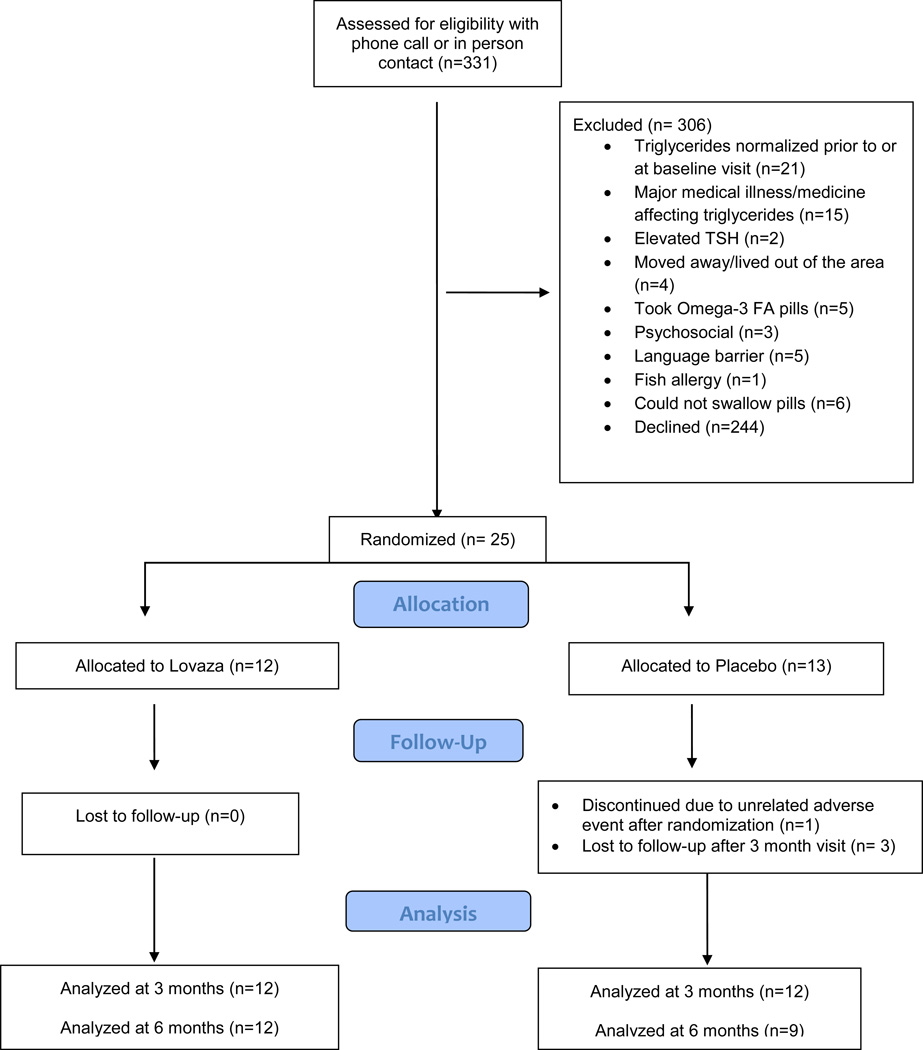
The medical records of 622 patients, ages 10–19 years with TG levels 150 to 1,000 mg/dL were reviewed for eligibility between July 2008 and October 2011, and 331 met initial eligibility criteria for screening. Ultimately 25 participants were randomized for participation (Figure 1). One patient dropped out after randomization before the 3 month visit, and 3 patients dropped out after the 3 month visit; all 4 had been randomized to the placebo group. All randomized participants were recruited from Boston Children’s Hospital subspecialty clinics, either Preventive Cardiology (n=21) or Optimal Weight for Life (n=3). The study was stopped early due to the low recruitment rate.
Figure 1.
Patient flow diagram describing recruitment, enrollment, randomization, allocation, follow-up and analysis.
Patient characteristics were comparable between the two groups at baseline (Table 1). Patients were generally overweight or obese, with low HDL cholesterol and moderately elevated TG levels. LDL cholesterol was not significantly elevated in most patients. No patients reported alcohol consumption or smoking. Second hand smoke exposure was reported in 1 child in the Omega-3 FA group and 4 children in the Placebo group. None of the randomized participants took a statin or oral contraceptive pill at any point during the study.
Table 1.
Patient characteristics at baseline.
| All (24) | Omega-3 FA (12) | Placebo (12) | |||
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | p* | ||
| Gender | Female | 10 (42) | 7 (58) | 3 (25) | 0.21 |
| Male | 14 (58) | 5 (42) | 9 (75) | ||
| Race | African American | 1 (4) | 1 (8) | 0 (0) | 0.64 |
| Other | 5 (21) | 3 (25) | 2 (17) | ||
| Caucasian | 18 (75) | 8 (67) | 10 (83) | ||
| Ethnicity | Hispanic | 5 (21) | 3 (25) | 2 (17) | >0.99 |
| Non Hispanic | 19 (79) | 9 (75) | 10 (83) | ||
| Family history | |||||
| Heart Disease | Yes | 10 (42) | 3 (25) | 7 (58) | 0.21 |
| Hypercholesterolemia | Yes | 20 (83) | 10 (83) | 10 (83) | >0.99 |
| Hypertriglyceridemia | Yes | 15 (63) | 8 (67) | 7 (58) | >0.99 |
| Household income | Below $40,000 | 4 (17) | 2 (17) | 2 (17) | |
| $40,000-$79,999 | 10 (42) | 6 (50) | 4 (33) | 0.36 | |
| $80,0000 or above | 7 (29) | 4 (33) | 3 (25) | ||
| Declined to answer | 3 (13) | 0 (0) | 3 (25) | ||
| Parental education | Some high school, high school graduate, trade school | 5 (21) | 3 (25) | 2 (17) | >0.99 |
| Some college | 6 (25) | 3 (25) | 3 (25) | ||
| College graduate | 9 (38) | 4 (33) | 5 (42) | ||
| Post-graduate degree | 4 (17) | 2 (17) | 2 (17) | ||
| Mean ± SD | Mean ± SD | Mean ± SD | p† | ||
| Age, yr | 14.0 ± 2.6 | 13.3 ± 2.4 | 14.7 ± 2.7 | 0.21 | |
| BMI, kg/m2 | 31.3 ± 4.4 | 31.3 ± 5.4 | 31.2 ± 3.4 | 0.96 | |
| BMI z-score | 2.09 ± 0.39 | 2.08 ± 0.47 | 2.11 ± 0.31 | 0.82 | |
| Waist circumference, cm | 101.4 ± 12.7 | 99.0 ± 12.2 | 103.8 ± 13.2 | 0.36 | |
| SBP percentile | 52 ± 33 | 49 ± 35 | 56 ± 32 | 0.68 | |
| DBP percentile | 66 ± 23 | 57 ± 25 | 76 ± 16 | 0.06 | |
| TG, mg/dL | 227 ± 49 | 230 ± 44 | 225 ± 55 | 0.84 | |
| TC, mg/dL | 183 ± 38 | 181 ± 36 | 186 ± 42 | 0.79 | |
| HDL, mg/dL | 34.1 ± 4.6 | 34.0 ± 4.4 | 34.1 ± 5.0 | 0.95 | |
| LDL, mg/dL | 104 ± 34 | 102 ± 34 | 107 ± 35 | 0.72 | |
Fisher exact test comparing Omega-3 FA vs. placebo.
Student t-test comparing Omega-3 FA vs. placebo.
BMI – body mass index; DBP – diastolic blood pressure HDL – high density lipoprotein; LDL – low density lipoprotein; SBP – systolic blood pressure; SD – standard deviation; TC – total cholesterol; TG – triglycerides; Yr – year.
Lipid Outcomes
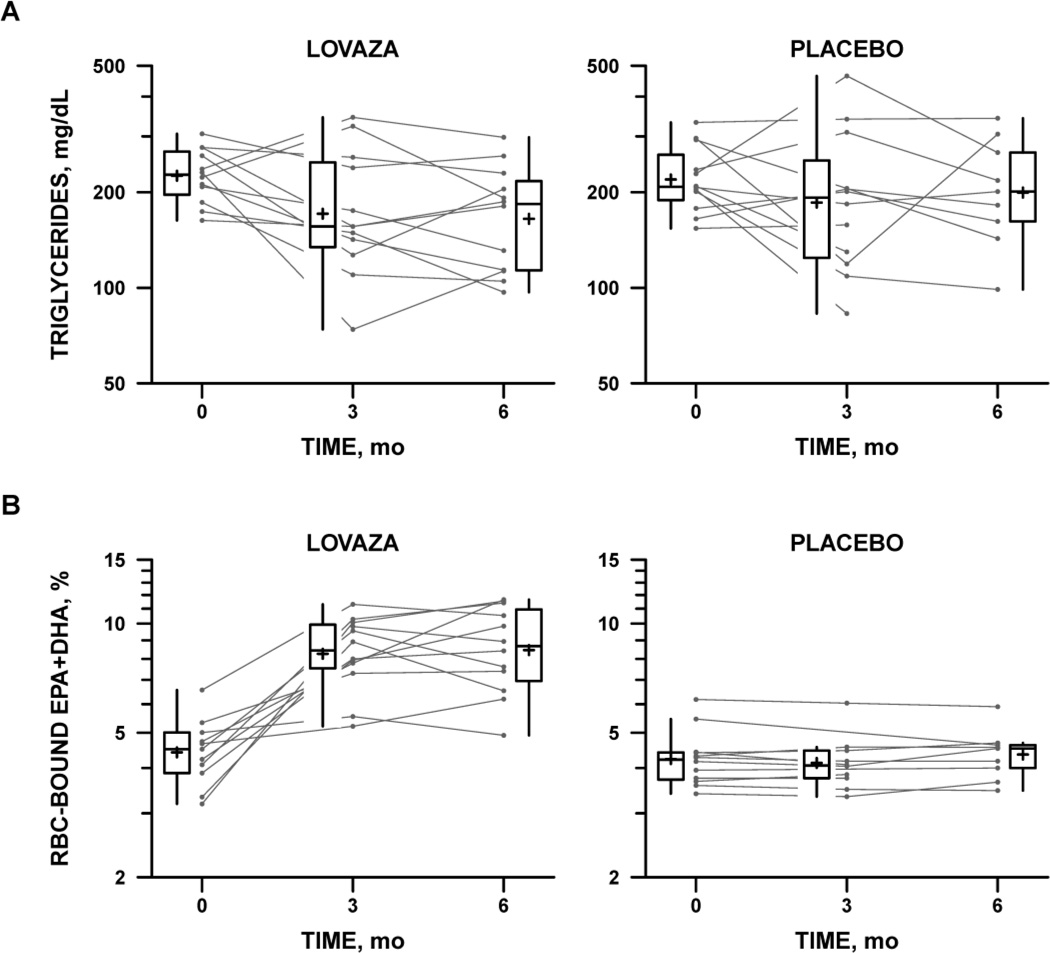
TG levels declined significantly in the Omega-3 FA group at 3 months and 6 months after baseline (−54 ± 27 mg/dL; p=0.02 and −61 ± 31 mg/dL; p=0.03, respectively) Figure 2, panel A and Table 2. TG levels also declined in the Placebo group at each timepoint (−34 ± 26 mg/dL; p=0.16 and −31 ± 33 mg/dL; p=0.32, respectively); however, this decline did not reach statistical significance. When the change in TG level was examined, there was no statistically significant difference between the two groups at either timepoint.
Figure 2.
Serum triglycerides (Panel A) and percentage of eicosapentaenoic acid (EPA) + docosahexaenoic acid (DHA) found in red cell membranes (Panel B) by treatment allocation and study time point. A: Serum triglyceride levels in the Omega-3 FA treatment arm (left) and placebo arm (right). Box plots summarize the distribution of triglyceride levels at each time point. Cross (+) indicates the mean, center line the median, top and bottom the interquartile range (IQR). Vertical lines extend to the farthest data point within 1.5×IQR above or below the box. Individual subjects’ levels are represented by a line connecting baseline (time 0) with 3-month and 6-month follow-up levels. B: Percentage of EPA and DHA bound to red blood cell (RBC), a marker of compliance with Omega-3 FA treatment. Format as in A
Table 2.
Lipid parameters, mean level and change over time by treatment group.
| Mean ± SE | Change, 3 mo | Change, 6 mo | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | BL | 3 mo | 6 mo | Mean ± SE |
P (change)* | P (diff)† | Mean ± SE | P (change)* | P (diff)† | |
| Triglycerides, mg/dL‡ | Omega-3 FA | 226 ± 24 | 171 ± 18 | 165 ± 18 | −54 ± 27 | 0.02 | 0.52 | −61 ± 31 | 0.03 | 0.43 |
| Placebo | 219 ± 23 | 186 ± 20 | 189 ± 23 | −34 ± 26 | 0.16 | −31 ± 33 | 0.32 | |||
| Total cholesterol, mg/dL | Omega-3 FA | 181 ± 12 | 189 ± 12 | 178 ± 12 | 8 ± 7 | 0.27 | 0.19 | −3 ± 10 | 0.75 | 0.69 |
| Placebo | 186 ± 12 | 180 ± 12 | 177 ± 12 | −5 ± 7 | 0.45 | −9 ± 10 | 0.41 | |||
| HDL cholesterol, mg/dL | Omega-3 FA | 34.0 ± 1.7 | 35.8 ± 1.7 | 34.2 ± 1.7 | 1.8 ± 1.4 | 0.22 | 0.27 | 0.2 ± 1.8 | 0.92 | 0.81 |
| Placebo | 34.1 ± 1.7 | 33.6 ± 1.7 | 35.0 ± 1.9 | −0.5 ± 1.4 | 0.74 | 0.8 ± 2.0 | 0.67 | |||
| LDL cholesterol, mg/dL | Omega-3 FA | 102 ± 11 | 116 ± 11 | 109 ± 11 | 14 ± 6 | 0.02 | 0.06 | 7 ± 8 | 0.37 | 0.41 |
| Placebo | 107 ± 11 | 104 ± 11 | 104 ± 11 | −2 ± 6 | 0.70 | −2 ± 9 | 0.78 | |||
| VLDL cholesterol, mg/dL | Omega-3 FA | 46 ± 4 | 38 ± 4 | 35 ± 4 | −8 ± 4 | 0.06 | 0.92 | −11 ± 5 | 0.04 | 0.40 |
| Placebo | 45 ± 4 | 37 ± 4 | 41 ± 4 | −8 ± 4 | 0.10 | −4 ± 5 | 0.44 | |||
| Non-HDL cholesterol, mg/dL | Omega-3 FA | 149 ± 11 | 153 ± 11 | 144 ± 11 | 5 ± 7 | 0.50 | 0.31 | −5 ± 9 | 0.60 | 0.72 |
| Placebo | 151 ± 11 | 147 ± 11 | 142 ± 11 | −5 ± 6 | 0.45 | −10 ± 9 | 0.32 | |||
Testing for zero mean change within treatment group, by t-test constructed from parameters of repeated-measures analysis of variance.
Testing for equal mean change between treatment groups.
Log-transformed for analysis; results re-transformed to natural units.
BL – baseline; Diff – difference; HDL – high density lipoprotein; LDL – low density lipoprotein; SE – standard error; mo – months; VLDL – very low density lipoprotein.
Compliance with study medication was assessed by RBC membrane EPA and DHA levels and by pill counts. Figure 2, panel B shows RBC EPA+DHA levels and TG levels in the study groups over time. EPA + DHA levels were low at baseline in both groups (median 4%) and increased significantly in the Omega-3 FA group over the course of the study (1.9-fold at 3 months and 6 months; p<0.0001) but did not change in the Placebo group. When the relationship between TG levels and EPA+DHA was evaluated in the Omega-3 FA group, TG levels were significantly and inversely related to DHA+EPA levels (r=-0.46, p=0.02). There were no differences in levels by age or sex. Pill counts suggested good compliance at about 80% in both groups.
Table 2 shows changes in lipid parameters over time. LDL levels increased significantly in the Omega-3 FA group by 14 ± 6 mg/dL (p=0.02) at 3 months but not at 6 months (7 ± 8 mg/dl; p=0.37) and were unchanged in the Placebo group at both timepoints. Again, when the change from baseline to 3 months or 6 months was compared between groups, there was no significant difference in LDL change. VLDL cholesterol decreased significantly in the treatment group at 6 months (p=0.04). No significant changes were seen in any of the other lipid parameters within or between groups at any other time points.
Non–Lipid Outcomes and Safety Measures
Changes in non-lipid parameters are shown in Table 3. Weight increased significantly in both groups at both time points but the difference between groups was not significant. There was also no significant difference between groups in change in body mass index (BMI), BMI z-score, waist circumference and systolic blood pressure (BP). Diastolic BP decreased significantly more in the Placebo group than the Omega-3 FA group at 3 months (p=0.02) but not at 6 months (p=0.25). Fasting glucose, fasting insulin and HOMA-IR did not change significantly in the Omega-3 FA group, and there were no significant differences between groups at either timepoint. The majority of CRP levels were at the lower limit of reporting (0.1 mg/dL), and the prevalence of detectable CRP showed no change (p=0.28) or difference in time course between treatment groups (p=0.62) (data not shown). Vascular function by EndoPAT was consistent with values reported in healthy normal weight adolescents28 and did not change over time (data not shown). Safety labs were normal on average throughout the study, and there was no significant difference between groups over time (data not shown).
Table 3.
Non-lipid parameters, mean level and change over time by treatment group.
| Mean ± SE | Change, 3 mo | Change, 6 mo | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | BL | 3 mo | 6 mo | Mean ± SE | P (change)* | P (diff)† |
Mean ± SE | P (change)* | P (diff)† |
|
| Glucose, mg/dL | Omega-3 FA | 88.5 ± 1.8 | 90.2 ± 1.8 | 92.1 ± 1.8 | 1.7 ± 1.7 | 0.34 | 0.86 | 3.6 ± 2.1 | 0.10 | 0.30 |
| Placebo | 89.5 ± 1.8 | 90.8 ± 1.8 | 89.8 ± 2.0 | 1.3 ± 1.7 | 0.47 | 0.3 ± 2.3 | 0.91 | |||
| Insulin, mcIU/mL‡ | Omega-3 FA | 18.1 ± 2.5 | 20.8 ± 2.9 | 22.7 ± 3.1 | 2.7 ± 1.8 | 0.16 | 0.61 | 4.6 ± 2.4 | 0.09 | 0.81 |
| Placebo | 22.6 ± 3.1 | 28.0 ± 3.8 | 27.0 ± 4.0 | 5.4 ± 2.2 | 0.04 | 4.4 ± 3.2 | 0.21 | |||
|
HOMA- IR mg/dL×mcIU/mL‡ |
Omega-3 FA | 4.1 ± 0.6 | 4.6 ± 0.7 | 5.1 ± 0.7 | 0.5 ± 0.4 | 0.27 | 0.47 | 1.0 ± 0.6 | 0.11 | 0.82 |
| Placebo | 5.0 ± 0.7 | 6.3 ± 0.9 | 6.0 ± 0.9 | 1.3 ± 0.5 | 0.03 | 1.0 ± 0.7 | 0.23 | |||
| EndoPAT index‡ | Omega-3 FA | 1.71 ± 0.14 | 1.58 ± 0.14 | 1.58 ± 0.13 | −0.13 ± 0.13 | 0.32 | 0.67 | −0.14 ± 0.15 | 0.36 | 0.33 |
| Placebo | 1.59 ± 0.13 | 1.53 ± 0.13 | 1.66 ± 0.15 | −0.05 ± 0.12 | 0.66 | −0.08 ± 0.15 | 0.63 | |||
| BMI, kg/m2 | Omega-3 FA | 31.3 ± 1.3 | 31.7 ± 1.3 | 31.8 ± 1.3 | 0.40 ± 0.23 | 0.09 | 0.33 | 0.51 ± 0.33 | 0.13 | 0.91 |
| Placebo | 31.2 ± 1.3 | 31.9 ± 1.3 | 31.7 ± 1.3 | 0.72 ± 0.23 | 0.003 | 0.46 ± 0.36 | 0.21 | |||
| BMI z-score | Omega-3 FA | 2.08 ± 0.12 | 2.07 ± 0.12 | 2.06 ± 0.12 | −0.01 ± 0.02 | 0.62 | 0.08 | −0.02 ± 0.03 | 0.58 | 0.74 |
| Placebo | 2.11 ± 0.12 | 2.16 ± 0.12 | 2.11 ± 0.12 | 0.04 ± 0.02 | 0.05 | −0.00 ± 0.03 | 0.95 | |||
| Weight, kg | Omega-3 FA | 81.4 ± 5.9 | 83.3 ± 5.9 | 84.9 ± 5.9 | 1.9 ± 0.7 | 0.006 | 0.26 | 3.5 ± 0.9 | 0.0006 | 0.82 |
| Placebo | 82.9 ± 5.9 | 85.9 ± 5.9 | 86.1 ± 5.9 | 3.0 ± 0.7 | <0.0001 | 3.2 ± 1.0 | 0.003 | |||
| Weight z-score | Omega-3 FA | 2.22 ± 0.20 | 2.22 ± 0.20 | 2.23 ± 0.20 | −0.00 ± 0.02 | 0.93 | 0.09 | 0.01 ± 0.03 | 0.81 | 0.65 |
| Placebo | 1.97 ± 0.20 | 2.03 ± 0.20 | 2.00 ± 0.20 | 0.06 ± 0.02 | 0.02 | 0.03 ± 0.04 | 0.41 | |||
|
Waist circumference cm |
Omega-3 FA | 99.0 ± 3.7 | 102.3 ± 3.7 | 103.2 ± 3.7 | 3.3 ± 1.0 | 0.002 | 0.24 | 4.2 ± 1.4 | 0.004 | 0.32 |
| Placebo | 103.8 ± 3.7 | 105.4 ± 3.7 | 106.0 ± 3.7 | 1.6 ± 1.0 | 0.11 | 2.1 ± 1.5 | 0.16 | |||
| SBP z-score | Omega-3 FA | 0.01 ± 0.27 | −0.42 ± 0.27 | −0.51 ± 0.27 | −0.44 ± 0.23 | 0.06 | 0.38 | −0.53 ± 0.29 | 0.08 | 0.72 |
| Placebo | 0.23 ± 0.28 | 0.09 ± 0.28 | −0.13 ± 0.31 | −0.14 ± 0.24 | 0.56 | −0.36 ± 0.34 | 0.29 | |||
| DBP z-score | Omega-3 FA | 0.30 ± 0.24 | 0.56 ± 0.24 | 0.31 ± 0.24 | 0.27 ± 0.21 | 0.22 | 0.018 | 0.01 ± 0.27 | 0.96 | 0.25 |
| Placebo | 0.86 ± 0.25 | 0.35 ± 0.25 | 0.39 ± 0.28 | −0.50 ± 0.22 | 0.03 | −0.46 ± 0.31 | 0.15 | |||
Diff – difference; BMI – body mass index; DBP - diastolic blood pressure; HOMA-IR homeostasis model of assessment – insulin resistance; mo – months; SBP - systolic blood pressure; SE – standard error.
Testing for zero mean change within treatment group, by t-test constructed from parameters of repeated-measures analysis of variance.
Testing for equal mean change between treatment groups.
Log-transformed for analysis; results re-transformed to natural units.
Tolerability
Potential adverse and side effects were specifically assessed by questionnaire at each timepoint, including at baseline. We asked specifically about burping, upset stomach, abdominal gas, bloated feeling, unusual taste, stomach pains/cramps, loose stools, chest pain, back pain, muscle or joint aches, skin rash, fever and chills, and infection. Musculoskeletal and gastrointestinal complaints were commonly reported in all participants at baseline prior to randomization (28% and 80%, respectively) and at all follow-up study time points (36% and 28% for musculoskeletal, 44% and 44% for gastrointestinal at 3 and 6 months, respectively). There were no significant increases in the frequency of reported symptoms within groups or differences between groups at baseline, 3 months and 6 months in reported musculoskeletal, gastrointestinal, infectious or rash (p=0.3–1.0). One female participant receiving Omega-3 FA who had recently experienced menarche developed prolonged menstrual bleeding; laboratory testing showed no defects in coagulation (including a normal bleeding time), and her symptoms were thought by study physicians and her primary care provider to be un-related to study drug. Safety laboratory tests, including transaminases, blood urea nitrogen, creatinine, platelets, prothrombin and partial thromboplastin time were normal and did not differ between groups (data not shown).
Nutrition and Physical Activity
There were no consistent changes in dietary intakes over time (data not shown). Although statistical significance was not met, the Placebo group showed a trend towards a decline in glycemic load, percent of total calories from carbohydrate, and added sugars. Those in the Placebo group also had a non-significant increase in fish intake and in dietary intake of omega-3 FA. Reported physical activity was similar in the Omega-3 FA and Placebo groups (data not shown).
Discussion
In this randomized double blind placebo controlled trial of high dose omega-3 FA supplements TG levels declined in both the Omega-3 FA and the Placebo group (24% vs. 16% at 3 months, sustained at 6 months), although the difference in the decline between the groups did not reach statistical significance. We demonstrated good safety and tolerability of Omega-3 FA, despite reports of gastrointestinal side effects in patients taking high doses of fish oil.8
Studies of omega-3 FA supplements in adults show a dose-dependent TG lowering effect in healthy volunteers, patients with CVD, and patients with dyslipidemia.5–7 A review of 65 well-controlled clinical trials using 3–4 grams per day of combined EPA and DHA doses of 4 grams per day produced a 25–30% reduction in TG levels, with stronger TG lowering effect seen among patients with higher baseline TG.7 In patients receiving omega-3 FA, LDL cholesterol increased 5–10% and HDL cholesterol increased 1–3%.6 Although our results did not meet statistical significance, changes in lipid parameters were similar to those reported in adults.
Very few studies have examined the use of omega-3 FA supplements to treat elevated TG in children. Engler et al conducted a randomized placebo controlled crossover trial of diet and DHA supplementation (1.2 grams /day, 6 capsules of oil per day) in 20 children with familial hypercholesterolemia or familial combined hyperlipidemia.20 They showed an 11% decrease in fasting TGs from 133 mg/dL to 119 mg/dL in the treated group and essentially no change in the placebo group; the findings were not statistically significant. In that study, baseline TG levels were only mildly elevated, [mean ± standard deviation (SD), 139 ± 82 mg/dL], and baseline LDL was extremely high (275 ± 89 mg /dL), suggesting many of these patients might have met criteria for statin therapy. Several other small trials have tested the effect of omega-3FA in pediatric patients with hypertriglyceridemia, but used lower doses and found either no,21 or minimal (−14 mg/dL) triglyceride lowering effect at low doses.22
DHA and EPA supplements have been shown to be generally safe in adults;4, 31 our trial also demonstrates safety in children and adolescents. Worsening glycemic control has been reported in diabetic patients,31 although other reports suggest omega-3 FA may improve glucose homeostasis.32,33 None of our participants had diabetes, but many had insulin resistance and we observed no change in HOMA-IR or fasting insulin levels within or between groups.
The underlying mechanism by which omega-3 FA affects lipids is not fully understood. Omega-3 FAs may lower plasma TGs by decreasing hepatic secretion of VLDL cholesterol and by increasing chylomicron metabolism.34 Increased intake reduces the synthesis of VLDL by inhibiting enzymes in TG synthesis.35 Omega-3 FA may have other beneficial effects including inhibiting adverse inflammatory responses, decreasing platelet aggregation, preventing thrombosis and cardiac arrhythmias, and decreasing cholesterol accumulation in the arterial wall.36, 37 Omega-3 FAs may directly improve endothelial vasomotor function, including brachial artery reactivity38, 39 and vascular biomarkers.40–42 We did not demonstrate any improvements in vascular reactivity, which could be related to low statistical power or a lack of effect of omega-3 FA on vasculature.
Strengths of our study include randomization and blinding, low drop-out rates, rigorous data collection, and the assessment of a range of relevant physiologic variables. Weaknesses of the study include diminished power due to low enrollment rates, despite the fact that we used many different recruitment strategies. Recruitment was likely difficult in part because our patient population did not feel ill, did not perceive any acute risk to hypertriglyceridemia, and could obtain omega-3FA supplements over the counter. The attained sample size was 24, and the observed net decline in mean TG was 20 mg/dL, or 10% of baseline levels. Post-hoc power calculations indicate that to demonstrate statistical significance for an effect of that magnitude, the present study carried less than 10% power. More than 200 participants would be needed for each group in order to have 80% power to show a significant TG lowering effect by omega-3 FA, if indeed there is an effect of the observed magnitude. Our results also may have been diluted by differential changes in diet, since patients in the placebo group tended to report more favorable changes in glycemic load and added sugar, and increased ingestion of fish/dietary omega-3 FA. It is notable that our patients responded nicely to our nutritional counseling; TG levels declined 28 mg/dL between screening and baseline visits; 11 patients were excluded from the trial because their TG levels normalized. TG declined a further −34 ± 26 mg/dL over 3 months in the placebo group, demonstrating the continued benefit of lifestyle counseling in pediatric hypertriglyceridemia.
Conclusion
The treatment of hypertriglyceridemia during childhood relies primarily on dietary modification. For those children who are unable to adequately implement lifestyle modification, or for whom lifestyle modification is insufficient to achieve substantial TG lowering, few pharmacologic options exist and data on their use during childhood are sparse. Omega-3 FA supplements may represent a tolerable and safe option for these children. Because pre-clinical atherosclerotic vascular disease is found in youth, CVD risk factor levels track into adulthood and predict adult vascular changes,23, 43–45 childhood may be a critical period during which omega-3 FAs can have important health benefits.
Acknowledgements
We would like to acknowledge the contributions of Nutrition staff in the Clinical and Translational Services Unit led by Nicolle Quinn with support from her colleagues Dianne Difabio and Elizabeth Cannell. Further thanks go to Dr. Christopher Duggan for his contribution to the Data Safety and Monitoring Board. Most importantly, we would like to acknowledge our participants and their families.
Abbreviations
- ALT
alanine aminotransferase
- ANOVA
analysis of variance
- AST
aspartate aminotransferase
- BMI
body mass index
- BP
blood pressure
- BUN
Blood urea nitrogen
- CRP
C-reactive protein
- CV
Coefficients of variation
- CVD
cardiovascular disease
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FA
fatty acids
- FDA
Federal Drug Administration
- HDL
high density lipoprotein
- HOMA-IR
homeostasis model of assessment,–insulin resistance
- LDL
low density lipoprotein
- NHLBI
National Heart, Lung and Blood Institute
- RBC
red blood cell
- SD
standard deviation
- TG
triglycerides
- TSH
thyroid stimulating hormone
- VLDL
very low density lipoprotein
Footnotes
Contributor’s Statement Page
All authors made substantive contributions to the manuscript.
Sarah de Ferranti and Stavroula Osganian conceptualized and designed the study, developed the study protocol and methods, and examined the participants. Both planned the analysis and drafted the manuscript.
Sarah de Ferranti, Sarah Steltz and Erica Denhoff performed the vascular testing.
Erica Denhoff and Sarah Steltz assisted in developing the study protocol, were responsible for recruiting the participants, the data acquisition and cleaning, and contributed to the manuscript.
Elif Seda Selamet Tierney assisted in design of the vascular protocol and critically reviewed the manuscript.
Henry Feldman and Carly Milliren performed the statistical analysis and contributed to the reporting of the results.
All authors critically reviewed the final version of the manuscript and made edits wherever appropriate, and all are responsible for the content.
Please contact the corresponding author for questions about access to original research materials at sarah.deferranti@cardio.chboston.org
Financial Disclosure: Study drug and additional research support was provided by GlaxoSmithKline. Additional support was supplied by the Boston Children’s Heart Foundation and Harvard Catalyst. Dr. de Ferranti is supported by a National Institutes of Health Grant K23 HL 085308-03, Bethesda, MD and by the Boston Children’s Heart Foundation. Dr. Feldman was supported by Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health.
Conflict of Interest: The authors have no conflict of interest.
References
- 1.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 western prospective studies. Circulation. 2007;115:450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 2.Haim M, Benderly M, Boyko V, Goldenberg I, Tanne D, Battler A, Goldbourt U, Behar S. Decrease in triglyceride level by bezafibrate is related to reduction of recurrent coronary events: A bezafibrate infarction prevention substudy. Coron Artery Dis. 2006;17:455–461. doi: 10.1097/01.mca.0000224406.60573.8e. [DOI] [PubMed] [Google Scholar]
- 3.Toskes PP. Hyperlipidemic pancreatitis. Gastroenterol Clin North Am. 1990;19:783–791. [PubMed] [Google Scholar]
- 4.Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J. N-3 fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: A systematic review. Am J Clin Nutr. 2006;84:5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- 5.Bucher HC, Hengstler P, Schindler C, Meier G. N-3 polyunsaturated fatty acids in coronary heart disease: A meta-analysis of randomized controlled trials. Am J Med. 2002;112:298–304. doi: 10.1016/s0002-9343(01)01114-7. [DOI] [PubMed] [Google Scholar]
- 6.Harris WS, Ginsberg HN, Arunakul N, Shachter NS, Windsor SL, Adams M, Berglund L, Osmundsen K. Safety and efficacy of omacor in severe hypertriglyceridemia. J Cardiovasc Risk. 1997;4:385–391. [PubMed] [Google Scholar]
- 7.Balk E, Chung M, Lichtenstein A, Chew P, Kupelnick B, Lawrence A, DeVine D, Lau J. Effects of omega-3 fatty acids on cardiovascular risk factors and intermediate markers of cardiovascular disease. Evid Rep Technol Assess (Summ) 2004:1–6. [PMC free article] [PubMed] [Google Scholar]
- 8.Kotwal S, Jun M, Sullivan D, Perkovic V, Neal B. Omega 3 fatty acids and cardiovascular outcomes: Systematic review and meta-analysis. Circulation. Cardiovascular quality and outcomes. 2012;5:808–818. doi: 10.1161/CIRCOUTCOMES.112.966168. [DOI] [PubMed] [Google Scholar]
- 9.Mozaffarian D, Lemaitre RN, King IB, Song X, Huang H, Sacks FM, Rimm EB, Wang M, Siscovick DS. Plasma phospholipid long-chain omega-3 fatty acids and total and cause-specific mortality in older adults: A cohort study. Ann Intern Med. 2013;158:515–525. doi: 10.7326/0003-4819-158-7-201304020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Caterina R. N-3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 11.Risk, Prevention Study. Collaborative G, Roncaglioni MC, Tombesi M, Avanzini F, Barlera S, Caimi V, Longoni P, Marzona I, Milani V, Silletta MG, Tognoni G, Marchioli R. N-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368:1800–1808. doi: 10.1056/NEJMoa1205409. [DOI] [PubMed] [Google Scholar]
- 12.Kit BK, Carroll MD, Lacher DA, Sorlie PD, DeJesus JM, Ogden C. Trends in serum lipids among us youths aged 6 to 19 years, 1988–2010. JAMA. 2012;308:591–600. doi: 10.1001/jama.2012.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Ferranti SD, Crean S, Cotter J, Boyd D, Osganian SK. Hypertriglyceridemia in a pediatric referral practice: Experience with 300 patients. Clin Pediatr (Phila) 2011;50:297–307. doi: 10.1177/0009922810379498. [DOI] [PubMed] [Google Scholar]
- 14.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low hdl cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 15.Suh HS, Hay JW, Johnson KA, Doctor JN. Comparative effectiveness of statin plus fibrate combination therapy and statin monotherapy in patients with type 2 diabetes: Use of propensity-score and instrumental variable methods to adjust for treatment-selection bias. Pharmacoepidemiol Drug Saf. 2012;21:470–484. doi: 10.1002/pds.3261. [DOI] [PubMed] [Google Scholar]
- 16.Buyukcelik M, Anarat A, Bayazit AK, Noyan A, Ozel A, Anarat R, Aydingulu H, Dikmen N. The effects of gemfibrozil on hyperlipidemia in children with persistent nephrotic syndrome. Turk J Pediatr. 2002;44:40–44. [PubMed] [Google Scholar]
- 17.Chicaud P, Demange J, Drouin P, Debry G. [action of fenofibrate in hypercholesterolemic children. 18-month follow-up] Presse Med. 1984;13:417–419. [PubMed] [Google Scholar]
- 18.Steinmetz J, Morin C, Panek E, Siest G, Drouin P. Biological variations in hyperlipidemic children and adolescents treated with fenofibrate. Clin Chim Acta. 1981;112:43–53. doi: 10.1016/0009-8981(81)90267-9. [DOI] [PubMed] [Google Scholar]
- 19.Colletti RB, Neufeld EJ, Roff NK, McAuliffe TL, Baker AL, Newburger JW. Niacin treatment of hypercholesterolemia in children. Pediatrics. 1993;92:78–82. [PubMed] [Google Scholar]
- 20.Engler MM, Engler MB, Malloy MJ, Paul SM, Kulkarni KR, Mietus-Snyder ML. Effect of docosahexaenoic acid on lipoprotein subclasses in hyperlipidemic children (the early study) Am J Cardiol. 2005;95:869–871. doi: 10.1016/j.amjcard.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen MH, Molgaard C, Hellgren LI, Matthiessen J, Holst JJ, Lauritzen L. The effect of dietary fish oil in addition to lifestyle counselling on lipid oxidation and body composition in slightly overweight teenage boys. J Nutr Metab. 2011;2011:348368. doi: 10.1155/2011/348368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nobili V, Bedogni G, Alisi A, Pietrobattista A, Rise P, Galli C, Agostoni C. Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: Double-blind randomised controlled clinical trial. Arch Dis Child. 2011;96:350–353. doi: 10.1136/adc.2010.192401. [DOI] [PubMed] [Google Scholar]
- 23.Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics. 2011;128(Suppl 5):S213–S256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol.Metab. 2002;87:4231–4237. doi: 10.1210/jc.2002-020311. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins DJ, Wolever TM, Jenkins AL, Thorne MJ, Lee R, Kalmusky J, Reichert R, Wong GS. The glycaemic index of foods tested in diabetic patients: A new basis for carbohydrate exchange favouring the use of legumes. Diabetologia. 1983;24:257–264. doi: 10.1007/BF00282710. [DOI] [PubMed] [Google Scholar]
- 26.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 27.Schwartz B, Jacobs DR, Jr, Moran A, Steinberger J, Hong CP, Sinaiko AR. Measurement of insulin sensitivity in children: Comparison between the euglycemic-hyperinsulinemic clamp and surrogate measures. Diabetes Care. 2008;31:783–788. doi: 10.2337/dc07-1376. [DOI] [PubMed] [Google Scholar]
- 28.Selamet Tierney ES, Newburger JW, Gauvreau K, Geva J, Coogan E, Colan SD, de Ferranti SD. Endothelial pulse amplitude testing: Feasibility and reproducibility in adolescents. J Pediatr. 2009;154:901–905. doi: 10.1016/j.jpeds.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 29.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH, Udelson JE. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 30.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 31.Fish oil supplements. Med Lett Drugs Ther. 2006;48:59–60. [PubMed] [Google Scholar]
- 32.Carpentier YA, Portois L, Malaisse WJ. N-3 fatty acids and the metabolic syndrome. Am J Clin Nutr. 2006;83:1499S–1504S. doi: 10.1093/ajcn/83.6.1499S. [DOI] [PubMed] [Google Scholar]
- 33.Delarue J, Couet C, Cohen R, Brechot JF, Antoine JM, Lamisse F. Effects of fish oil on metabolic responses to oral fructose and glucose loads in healthy humans. Am J Physiol. 1996;270:E353–E362. doi: 10.1152/ajpendo.1996.270.2.E353. [DOI] [PubMed] [Google Scholar]
- 34.Harris WS, Rothrock DW, Fanning A, Inkeles SB, Goodnight SH, Jr, Illingworth DR, Connor WE. Fish oils in hypertriglyceridemia: A dose-response study. Am J Clin Nutr. 1990;51:399–406. doi: 10.1093/ajcn/51.3.399. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt EB, Kristensen SD, De Caterina R, Illingworth DR. The effects of n-3 fatty acids on plasma lipids and lipoproteins and other cardiovascular risk factors in patients with hyperlipidemia. Atherosclerosis. 1993;103:107–121. doi: 10.1016/0021-9150(93)90254-r. [DOI] [PubMed] [Google Scholar]
- 36.Seo T, Blaner WS, Deckelbaum RJ. Omega-3 fatty acids: Molecular approaches to optimal biological outcomes. Curr Opin Lipidol. 2005;16:11–18. doi: 10.1097/00041433-200502000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among us men and women. Circulation. 2003;108:155–160. doi: 10.1161/01.CIR.0000079224.46084.C2. [DOI] [PubMed] [Google Scholar]
- 38.Boak L, Chin-Dusting JP. Hypercholesterolemia and endothelium dysfunction: Role of dietary supplementation as vascular protective agents. Curr Vasc Pharmacol. 2004;2:45–52. doi: 10.2174/1570161043476546. [DOI] [PubMed] [Google Scholar]
- 39.Goodfellow J, Bellamy MF, Ramsey MW, Jones CJ, Lewis MJ. Dietary supplementation with marine omega-3 fatty acids improve systemic large artery endothelial function in subjects with hypercholesterolemia. J Am Coll Cardiol. 2000;35:265–270. doi: 10.1016/s0735-1097(99)00548-3. [DOI] [PubMed] [Google Scholar]
- 40.Calder PC. N-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 41.Mori TA, Beilin LJ. Omega-3 fatty acids and inflammation. Curr Atheroscler Rep. 2004;6:461–467. doi: 10.1007/s11883-004-0087-5. [DOI] [PubMed] [Google Scholar]
- 42.Satoh N, Shimatsu A, Kotani K, Sakane N, Yamada K, Suganami T, Kuzuya H, Ogawa Y. Purified eicosapentaenoic acid reduces small dense ldl, remnant lipoprotein particles, and c-reactive protein in metabolic syndrome. Diabetes Care. 2007;30:144–146. doi: 10.2337/dc06-1179. [DOI] [PubMed] [Google Scholar]
- 43.Berenson GS. Childhood risk factors predict adult risk associated with subclinical cardiovascular disease. The bogalusa heart study. Am J Cardiol. 2002;90:3L–7L. doi: 10.1016/s0002-9149(02)02953-3. [DOI] [PubMed] [Google Scholar]
- 44.Morrison JA, Glueck CJ, Wang P. Childhood risk factors predict cardiovascular disease, impaired fasting glucose plus type 2 diabetes mellitus, and high blood pressure 26 years later at a mean age of 38 years: The princeton-lipid research clinics follow-up study. Metabolism. 2012;61:531–541. doi: 10.1016/j.metabol.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schrott HG, Clarke WR, Abrahams P, Wiebe DA, Lauer RM. Coronary artery disease mortality in relatives of hypertriglyceridemic school children: The muscatine study. Circulation. 1982;65:300–305. doi: 10.1161/01.cir.65.2.300. [DOI] [PubMed] [Google Scholar]