Abstract
Background
Chronic kidney disease is common and associated with increased cardiovascular disease risk. Currently, markers of renal tubular injury are not used routinely to describe kidney health and little is known about risk of cardiovascular events and death associated with these biomarkers independent of glomerular filtration—based markers (such as serum creatinine or albuminuria).
Study Design
Cohort study, Chronic Renal Insufficiency Cohort (CRIC) Study.
Setting & Participants
3386 participants with estimated glomerular filtration rate of 20-70 mL/min/1.73 m2 enrolled from June 2003 through August 2008.
Predictor
Urine neutrophil gelatinase-associated lipocalin (NGAL) concentration.
Outcomes
Adjudicated heart failure event, ischemic atherosclerotic event (myocardial infarction, ischemic stroke or peripheral artery disease) and death through March 2011.
Measurements
Urine NGAL concentration measured at baseline with a two-step assay using chemiluminescent microparticle immunoassay technology on an ARCHITECT i2000SR (Abbott Laboratories).
Results
There were 428 heart failure events (during 16383 person-years of follow-up), 361 ischemic atherosclerotic events (during 16584 person-years of follow-up) and 522 deaths (during 18214 person-years of follow-up). In Cox regression models adjusted for estimated glomerular filtration rate, albuminuria, demographics, traditional cardiovascular disease risk factors and cardiac medications, higher urine NGAL levels remained independently associated with ischemic atherosclerotic events (adjusted HR for the highest [>49.5 ng/ml] vs. lowest [≤6.9 ng/ml] quintile, 1.83 [95% CI, 1.20-2.81]; HR, per 0.1-unit increase in log urine NGAL, 1.012 [95% CI, 1.001-1.023]), but not heart failure events or deaths.
Limitations
Urine NGAL was measured only once.
Conclusions
Among patients with chronic kidney disease, urine levels of NGAL, a marker of renal tubular injury, were independently associated with future ischemic atherosclerotic events but not with heart failure events or deaths.
Keywords: neutrophil gelatinase-associated lipocalin (NGAL), renal tubular injury, renal tubular dysfunction, biomarker, chronic kidney disease (CKD), Chronic Renal Insufficiency Cohort (CRIC) Study, cardiovascular disease, ischemic atherosclerotic event
Chronic kidney disease (CKD) is common, estimated to affect over 10% of the US general population and imposes substantial morbidity and cost.1 Patients with CKD, as reflected by reduced glomerular filtration rate and/or proteinuria, not only are at risk for acute kidney injury or end-stage renal disease, but also have heightened risk of cardiovascular events and premature death.2-4 Reasons behind this association are not completely understood but may include shared risk factors leading to both kidney and heart disease (such as inflammation) and metabolic abnormalities caused by kidney disease which are detrimental to the cardiovascular system (such as anemia or abnormalities in calcium and phosphorus regulation).
The most common methods to assess kidney function—measurement of serum creatinine with formulas to estimate glomerular filtration rate and quantification of urine protein (albuminuria)—are primarily indicators of glomerular dysfunction.5 Newer biomarkers such as serum cystatin C are also measures of glomerular filtration rate.5 Currently, markers of renal tubular injury are not used routinely to describe kidney health; little is known about risk of cardiovascular events and death associated with these biomarkers independent of filtration-based markers.
Urine neutrophil gelatinase-associated lipocalin (NGAL), a novel biomarker of renal tubular injury and a ubiquitous lipocalin iron-carrying protein, has become increasingly recognized as an indicator of both acute and chronic kidney disease.6-11 Based on reporter expression studies in transgenic mice, NGAL is upregulated in the distal renal epithelium and excreted into the urine during periods of renal injury.12 Elevations in urine NGAL levels have been shown to be an early and sensitive marker of acute kidney injury due to ischemia in humans.13 Recent studies have shown that elevated urine NGAL levels are often detectable (albeit at a lower range) in patients with CKD.14,15 Some animal model studies suggest that late upregulation of NGAL might be a useful marker for sustained renal injury after AKI and may be associated with CKD development.16 Damman et al. reported that among patients with chronic heart failure, increased urine NGAL levels are a risk factor for all-cause mortality and heart failure—related hospitalizations independent of estimated glomerular filtration rate (eGFR), albuminuria and other comorbidities.17
We investigated whether urine NGAL was an independent predictor of increased risk for cardiovascular events and/or all-cause mortality in men and women with CKD enrolled in the Chronic Renal Insufficiency Cohort (CRIC) Study.
METHODS
Study Population
The CRIC Study is a National Institutes of Health (NIH)—sponsored multi-center observational cohort which enrolled patients from seven clinical centers (consisting of 13 enrolling sites) located throughout the United States. The design of the CRIC Study and baseline characteristics of study participants have been previously described.18,19 Briefly, men and women aged 21-74 years with eGFR of 20-70 mL/min/1.73 m2 using the 4-variable MDRD (Modification of Diet in Renal Disease) Study equation were eligible for the study. Recruitment was targeted to ensure robust representation of women, individuals with diabetes mellitus and racial/ethnic minorities. Persons with polycystic kidney disease, multiple myeloma, glomerulonephritis on active immunosuppression or who had undergone kidney transplantation were excluded. A total of 3939 participants were enrolled from June 2003 through August 2008.
Laboratory and Clinical Measurements
Urine NGAL was measured at baseline6 with a two-step assay using chemiluminescent microparticle immunoassay technology on an ARCHITECT i2000SR (Abbott Laboratories, Abbott Park, IL). All urine specimens were stored long-term at −80°C and had been thawed once. Of the 3939 enrolled CRIC study participants, 229 did not have a specimen available for NGAL testing and 3386 had valid NGAL measurements. All but 12 of the urine creatinine measurements were made on a BioTek ELx808 microplate reader using a Jaffe reaction with a colorimetric endpoint and reagents from Sigma-Aldrich.
Serum creatinine assays were enzyme based for all baseline visits and performed on the Hitachi Vitros 950 AT. Serum creatinine values were calibrated to isotope-dilution mass spectrometry–traceable standards.20-22 Serum cystatin C was measured with a particle-enhanced immunonephelometric assay on the Siemens BN II System. An internal CRIC cystatin C standardization was implemented to correct for drift over time when using different calibrator lots and reagent lots.22 All lipid measurements (total and high-density lipoprotein cholesterol, triglycerides) were performed on a Hitachi 912 using an enzymatic colorimetric assay.
Demographic characteristics (such as race/ethnicity) and medication use were self-reported. Diabetes mellitus was defined as fasting plasma glucose level ≥126 mg/dL or nonfasting plasma glucose level ≥200 mg/dL or self-report of diabetes medication use. A baseline medical history questionnaire was administered to determine prevalent cardiovascular disease. Participants were asked: ‘Have you ever been diagnosed with, or has a doctor or other health professional ever told you that, you have “coronary artery disease (heart attack, angina), prior revascularization of your heart blood vessels (e.g. balloon angioplasty, coronary stenting, coronary bypass surgery)” Separate questions queried about a history of heart failure, stroke or transient ischemic attack, or peripheral artery disease (including claudication, amputation or angioplasty). Body weight and height were each measured twice and averaged for analysis and were used to calculate body mass index. Three blood pressure measurements were obtained in the sitting position after at least 5 minutes of quiet rest by trained and certified staff according to a standard protocol and averaged.23
Outcomes
We defined a priori three outcomes of interest: time to first heart failure event after enrollment, time to first ischemic atherosclerotic event after enrollment (encompassing probable or definite myocardial infarction [MI]; probable or definite ischemic stroke; or peripheral artery disease events), and time to death. We constructed separate outcomes for heart failure events and ischemic atherosclerotic events based on the known differences in their underlying pathophysiology and relationship with other vascular risk factors. Outcomes were identified through March 31, 2011. Participants who withdrew from the study or died were censored.
The CRIC Study participants were queried every six months during alternating inperson and telephone visits about whether they were hospitalized, reached end-stage renal disease, experienced a possible cardiovascular event, or underwent a selected set of diagnostic tests/procedures. International Classification of Diseases, Ninth Revision (ICD-9) discharge codes were obtained for all hospitalizations. When diagnostic or procedure codes indicative of a potential cardiovascular event were noted, medical records were retrieved for detailed review. These reviews were performed by at least two physicians for possible events of heart failure, MI, and stroke. Trained study staff reviewed medical records classified with ICD-9 codes that suggest a peripheral artery disease event.
Heart failure events were determined based on clinical symptoms, radiographic evidence of pulmonary congestion, physical examination of the heart and lungs, central venous hemodynamic monitoring data, and echocardiographic imaging among hospitalized patients based on the Framingham and ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) criteria.24,25 Diagnosis of probable or definite MI were based on symptoms consistent with acute ischemia, cardiac biomarker levels, and electrocardiograms as recommended by a consensus statement on the universal definition of MI.26 Two neurologists reviewed all hospitalizations suggestive of stroke. Outcomes included both probable and definite ischemic stroke. The latter was determined based on autopsy findings or sudden onset of neurologic symptoms supported with CT or MRI demonstration of an infarction in a territory where an injury or infarction would be expected to create those symptoms. The former was defined as sudden or rapid onset of one major or two minor neurologic signs or symptoms lasting for more than 24 hours or until the patient died with no evidence of hemorrhage or infarction on CT or MRI performed within 24 hours of the onset of symptoms.27 (Hemorrhagic strokes were not included in the composite outcome of ischemic atherosclerotic event.) Ascertainment of peripheral artery disease was based on nurse-abstracted hospital records indicating that amputation, bypass procedure, angioplasty or surgical/vascular procedure for abdominal aortic aneurysm or non-coronary arteries took place. Ascertainment of death was supplemented by cross-linkage with the Social Security Death Master File.
Analysis
Patient characteristics at baseline were described overall and by quintiles of urine NGAL concentration using mean ± standard deviation for quantitative variables and frequencies and percentages for categorical variables.
Kaplan-Meier analysis was used to illustrate the survival probability for each outcome measure by quintile of baseline urine NGAL concentration. Multivariable time-to-event analysis was conducted using Cox proportional hazards models. We adjusted for demographic characteristics (age, sex, race/ethnicity); conventional measures of kidney function including eGFR (estimated using an internally derived CRIC Study equation based on age, sex, race, standardized serum creatinine and cystatin C measurements22) and 24-hour urine albuminuria; established cardiovascular disease risk factors including diabetes mellitus, smoking status (never, former, current), prior history of MI, history of coronary revascularization, history of heart failure, history of ischemic stroke, history of peripheral artery disease, systolic and diastolic blood pressure, body mass index, LDL cholesterol, HDL cholesterol; and use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, use of aldosterone receptor antagonists, use of statin and use of antiplatelet agents (clopidogrel, aspirin). All comorbidities were determined at baseline concurrent with the primary biomarker measurement. We also adjusted for clinical center in all analyses (as a covariate) and verified that there was no violation of the proportional hazards assumption.
We did not a priori plan any subgroup analyses or exploration of interactions. We performed sensitivity analyses where estimation of glomerular filtration rate was estimated with the MDRD Study equation,21 where we limited the study population to those without self-reported history of cardiovascular disease (heart failure, MI, stroke and peripheral artery disease) at baseline and when we normalized urine NGAL concentration to urine creatinine concentration.
The study was approved by the institutional review committees of each participating CRIC site and all study participants provided written informed consent.
RESULTS
Baseline clinical and demographic characteristics of the participants we studied (N = 3386) overall and by urine NGAL concentration quintiles (≤6.9, >6.9-≤12.9, >12.9-≤22.6, >22.6-≤49.5, >49.5 ng/mL) are shown in Table 1. Mean age overall was 58.2 years; approximately one-half of the participants were women, non-Hispanic black/Hispanic or had diabetes mellitus. The distribution of urine NGAL levels was highly skewed (median, 17.2 [interquartile range, 8.1-39.2] ng/mL); the highest 5% of values were between 178.9 and 3069.6 ng/mL. Participants with higher urine NGAL concentrations were more likely to have lower eGFR and higher levels of albuminuria. They were also more likely to be women, non-Hispanic black or Hispanic, have diabetes mellitus and higher blood pressure (Table 1).
Table 1. Baseline clinical and demoqraphic characteristics of study participants overall and bv NGAL quintiles.
| Characteristic | All (N=) | NGAL≤ 6.9 | NGAL
>6.9- ≤12.9 |
NGAL
>12.9- ≤22.6 |
NGAL >22.6
- ≤49.5 |
NGAL >49.5 | P for trend |
|---|---|---|---|---|---|---|---|
| No. | 3386 | 681 | 678 | 673 | 677 | 677 | |
| Urine NGAL (ng/ml) | 17.2 [8.1-39.2] | 4.3 [3.3-5.5] | 9.8 [8.1-11.2] | 17.2 [14.8-19.5] | 32.2 [26.7-39.2] | 104.3 [69.4-178.9] | |
| Age (y) | 58.2 ±11.0 | 58.7 ±10.3 | 59.4 ±10.0 | 59.5 ±10.9 | 57.5 ±11.3 | 56.1 ±11.9 | <0.001 |
| Female sex | 1592 (47.0%) | 103 (15.1%) | 258 (38.1%) | 381 (56.6%) | 444 (65.6%) | 406 (60.0%) | <0.001 |
| Race/ethnicity | <0.001 | ||||||
| Non-Hispanic White | 1410 (41.6%) | 396 (58.1%) | 331 (48.8%) | 263 (39.1%) | 240 (35.5%) | 180 (26.6%) | |
| Non-Hispanic Black | 1440 (42.5%) | 206 (30.2%) | 277 (40.9%) | 314 (46.7%) | 332 (49.0%) | 311 (45.9%) | |
| Hispanic | 404 (11.9%) | 40 (5.9%) | 43 (6.3%) | 76 (11.3%) | 83 (12.3%) | 162 (23.9%) | |
| Presence of diabetes mellitus | 1634 (48.3%) | 303 (44.5%) | 270 (39.8%) | 304 (45.2%) | 357 (52.7%) | 400(59.1%) | <0.001 |
| Presence of hypertension | 2915 (86.1%) | 567 (83.3%) | 570 (84.1%) | 585 (86.9%) | 599 (88.5%) | 594 (87.7%) | 0.02 |
| Current Smoker | 456 (13.5%) | 61 (9.0%) | 81 (11.9%) | 93 (13.8%) | 105(15.5%) | 116(17.1%) | <0.001 |
| History of | |||||||
| Cardiovascular Disease | 1116 (33.0%) | 233 (34.2%) | 201 (29.6%) | 228 (33.9%) | 212(31.3%) | 242 (35.7%) | 0.1 |
| Ml/prior revascularization | 740 (21.9%) | 178 (26.1%) | 137 (20.2%) | 157 (23.3%) | 131 (19.4%) | 137 (20.2%) | 0.01 |
| Heart Failure | 326 (9.6%) | 67 (9.8%) | 62 (9.1%) | 65 (9.7%) | 57 (8.4%) | 75(11.1%) | 0.6 |
| Stroke | 329 (9.7%) | 52 (7.6%) | 58 (8.6%) | 69 (10.3%) | 73 (10.8%) | 77(11.4%) | 0.1 |
| PAD | 227 (6.7%) | 36 (5.3%) | 38 (5.6%) | 46 (6.8%) | 37 (5.5%) | 70 (10.3%) | <0.001 |
| 24-h albuminuria (mg/24 h) | 69 [11- 606] | 27 [7 -147] | 33 [8 – 241] | 61 [9 – 459] | 124 [15 – 960] | 646 [50 – 2785] | <0.001 |
| eGFR (ml/min/1.73m2) | 44.4 (16.9) | 52.1 (16.0) | 49.0 (16.0) | 44.1 (15.9) | 41.3 (16.7) | 35.2(14.7) | <0.001 |
| Urine creatinine (mg/dl) | 71.5 (37.2) | 70.6 (33.2) | 77.8 (40.3) | 72.6 (39.5) | 72.1 (37.5) | 64.3 (33.8) | <0.001 |
| Cystatin C (mg/L) | 1.5 (0.6) | 1.3(0.4) | 1.4 (0.4) | 1.5 (0.5) | 1.6 (0.6) | 1.9 (0.6) | <0.001 |
| Systolic BP (mmHg) | 128.5(22.1) | 122.5 (18.2) | 125.6(19.5) | 127.3(21.7) | 130.8(23.0) | 136.5 (25.1) | <0.001 |
| Diastolic BP (mmHg) | 71.5 (12.7) | 70.5(11.9) | 71.1 (11.9) | 70.7 (12.6) | 71.9 (13.0) | 73.3 (13.9) | <0.001 |
| Body Mass Index (kg/m2) | 32.2(7.9) | 31.2 (6.7) | 31.6(6.9) | 32.3(7.8) | 33.5(8.8) | 32.5 (9.0) | <0.001 |
| Cholesterol | |||||||
| Total (mg/dl) | 183.4(45.5) | 176.9 (42.4) | 179.6(41.1) | 184.0(43.2) | 185.0(44.8) | 191.5 (53.9) | <0.001 |
| LDL (mg/dl) | 102.4(35.5) | 99.1 (33.9) | 99.0 (32.1) | 103.7(34.6) | 104.7(36.5) | 105.5 (39.5) | <0.001 |
| HDL (mg/dl) | 47.5 (15.4) | 45.7(14.6) | 47.1 (14.7) | 49.6 (16.7) | 47.9 (15.6) | 47.4 (15.1) | 0.02 |
| Use of | |||||||
| Statins | 1851 (55.0%) | 380 (56.1%) | 384 (57.1%) | 357 (53.3%) | 357 (53.0%) | 373 (55.7%) | 0.5 |
| ACEi or ARB | 2284 (67.9%) | 499 (73.7%) | 468 (69.5%) | 451 (67.3%) | 437 (64.9%) | 429 (64.0%) | <0.001 |
| β-blockers | 1643 (48.9%) | 315 (46.5%) | 307 (45.6%) | 335 (50.0%) | 335 (49.8%) | 351 (52.4%) | 0.08 |
| Calcium channel blockers | 1364 (40.6%) | 243 (35.9%) | 270 (40.1%) | 270 (40.3%) | 287 (42.6%) | 294 (43.9%) | 0.03 |
| Antiplatelet agents | 1543 (45.6%) | 350 (51.4%) | 319 (47.1%) | 300 (44.6%) | 292 (43.1%) | 282 (41.7%) | 0.003 |
| Aldosterone antagonists | 132(3.9%) | 29 (4.3%) | 33 (4.9%) | 30 (4.5%) | 20 (3.0%) | 20 (3.0%) | 0.2 |
Note: Values for categorical variables are given as frequency (percentage); values for continuous variables are given as mean ± standard deviation or median [interquartile range], Quintiles of NGAL concentration expressed as ng/mL. Conversion factors for units: creatinine in mg/dL to μmol/L, ×88.4; cholesterol in mg/dL to mmol/L, ×0.02586;
ACEi, angiotensin-converting enzyme inhibitor; BP, blood pressure; eGFR, estimated glomerular filtration rate ; gelatinase-associated lipocalin (NGAL); HDL, hiqh-density lipoprotein; LDL, low-density lipoprotein; Ml, myocardial infarction; PAD, peripheral artery disease
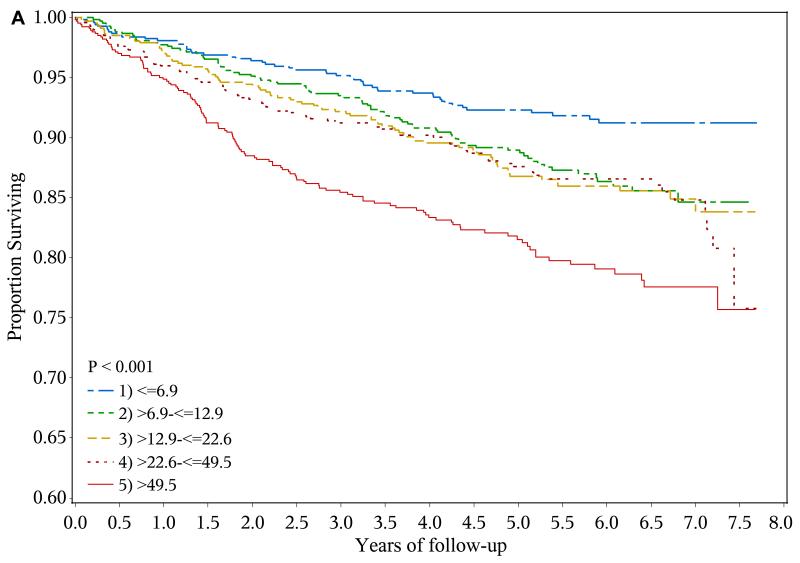
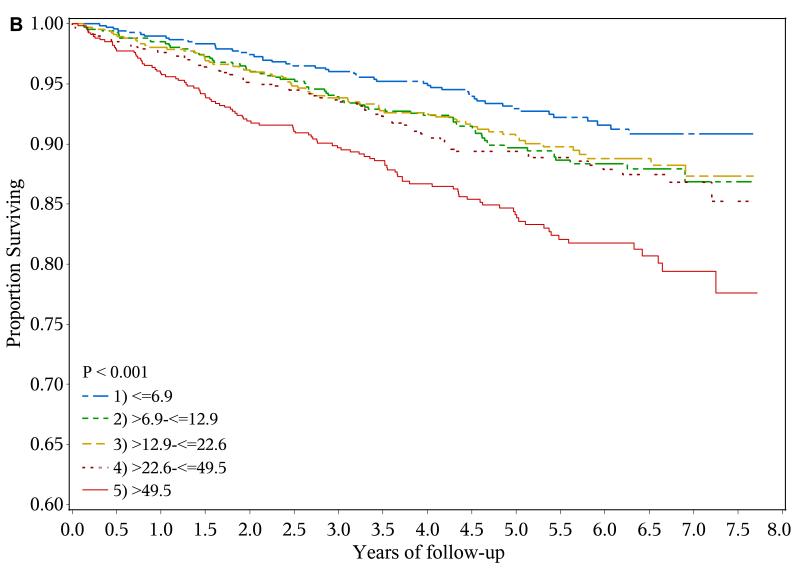
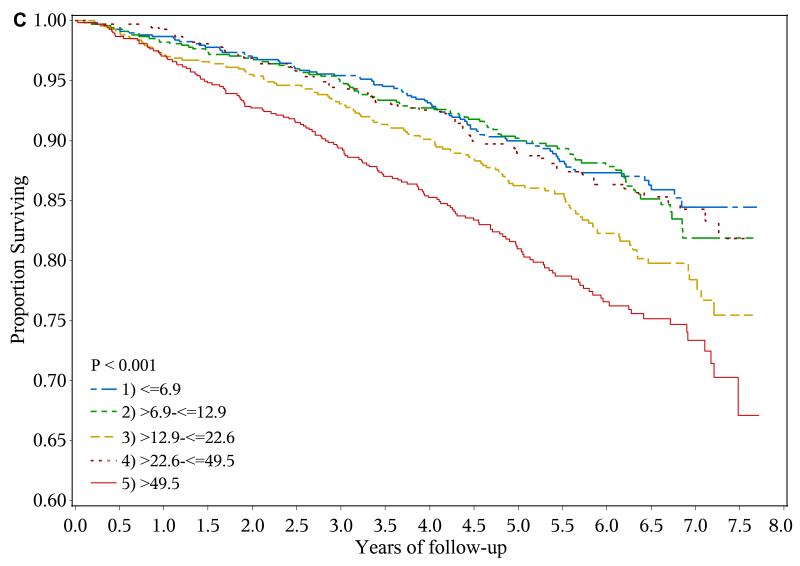
A total of 428 heart failure events (event rate, 2.61 per 100 person-years), 361 ischemic atherosclerotic events (event rate, 2.18 per 100 person-years) and 522 deaths (event rate, 2.87 per 100 years-years) were observed during follow-up. The crude event rate for each outcome was higher with higher levels of urine NGAL (Table 2 and Figures 1a-1c).
Table 2. Number of events, event rate, and adjusted HR by quintiles of baseline urine NGAL concentration.
| NGAL≤6.9 | NGAL>6.9 -≤12.9 | NGAL>12.9 - ≤22.6 | NGAL>22.6 - ≤49.51 | NGAL>49.5 | ||
|---|---|---|---|---|---|---|
| Heart Failure Events | ||||||
| No. of events | 53 | 81 | 84 | 87 | 123 | |
| Total no. of years of follow-up | 3525.8 | 3377.0 | 3257.2 | 3282.2 | 2940.7 | |
| Event rates (per 100 person-y) | 1.50 | 2.40 | 2.58 | 2.65 | 4.18 | |
| Adjusted* HR (95%CI) | Reference | 1.45 (1.01-2.08) | 1.09 (0.75-1.59) | 1.01 (0.68-1.49) | 1.05 (0.69-1.59) | |
| Adjusted* HR (95%CI) | 0.995 (0.984-1.006) per 0.1-unit increase in log urine NGAL | |||||
| Ischemic Atherosclerotic Events# | ||||||
| No. of events | 50 | 69 | 64 | 73 | 105 | |
| Total no. of years of follow-up | 3527.9 | 3392.3 | 3298.0 | 3332.5 | 3033.0 | |
| Event rates (per 100 person-y) | 1.42 | 2.03 | 1.94 | 2.19 | 3.46 | |
| Adjusted* HR (95%CI) | Reference | 1.54 (1.06-2.23) | 1.26 (0.85-1.87) | 1.52 (1.02-2.27) | 1.83 (1.20-2.81) | |
| Adjusted* HR (95%CI) | 1.012 (1.001-1.023) per 0.1-unit increase in log urine NGAL | |||||
| All-Cause Mortality | ||||||
| No. of events | 84 | 86 | 115 | 88 | 149 | |
| Total no. of years of follow-up | 3779.5 | 3701.7 | 3591.7 | 3692.7 | 3448.1 | |
| Event rates (per 100 person-y) | 2.22 | 2.32 | 3.20 | 2.38 | 4.32 | |
| Adjusted* HR (95%CI) | Reference | 1.02 (0.74-1.39) | 1.17 (0.86-1.58) | 0.81 (0.58-1.14) | 1.15 (0.81-1.62 ) | |
| Adjusted* HR (95%CI) | 1.005 (0.996-1.014) per 0.1-unit increase in log urine NGAL | |||||
Note: Quintiles of NGAL concentration expressed as ng/mL.
CI, confidence interval; gelatinase-associated lipocalin (NGAL); HR, hazard ratio;
Composite of myocardial infarction, peripheral artery disease, ischemic stroke
Adjusted for age, sex, race/ethnicity, estimated glomerular filtration rate , 24-hour urine albumin, diabetes mellitus, smoking status, prior history of myocardial infarction, history of coronary revascularization, history of heart failure, history of ischemic stroke, history of peripheral artery disease, blood pressure, body mass index, LDL cholesterol, HDL cholesterol, and use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, aldosterone receptor antagonists, statins and antiplatelet agents
Figure 1.
Risk of adverse outcomes and levels of urine neutrophil gelatinase-associated lipocalin (NGAL)
a. Kaplan-Meier curves showing risk of heart failure event by baseline urine NGAL quintiles (ng/ml)
b. Kaplan-Meier curves showing risk of ischemic atherosclerotic event by baseline urine NGAL quintiles (ng/ml)
c. Kaplan-Meier curves showing risk of death by baseline urine NGAL quintiles (ng/ml)
In unadjusted analysis, compared with their counterparts in the lowest quintile (≤ 6.9 ng/ml), participants in the highest quintile of urine NGAL (> 49.5 ng/ml) had unadjusted hazard ratios (HRs) for experiencing a heart failure event, ischemic atherosclerotic event or death of 2.71 (95% confidence interval [CI], 1.96-3.74), 2.43 (95% CI, 1.74-3.41) and 1.96 (95% CI, 1.50-2.56), respectively. However, after adjustment for eGFR, 24-hour urine albumin, demographic characteristics, traditional cardiovascular disease risk factors and medication use, higher urine NGAL level was independently associated with only ischemic atherosclerotic events (adjusted HR for highest vs. lowest quintile, 1.83; 95% CI, 1.20-2.81)(Table 2).
In sensitivity analyses, results were similar when the MDRD Study equation was used to estimate glomerular filtration rate with adjusted HR for ischemic atherosclerotic events of 1.78 (95% CI, 1.16-2.74) when comparing highest vs. lowest quintile. Higher levels of urine NGAL also remained significantly associated with higher risks of future ischemic atherosclerotic events in participants who did not report a history of cardiovascular disease at study enrollment, or when urine NGAL concentration was normalized to urine creatinine (Tables S1-S3).
DISCUSSION
In a large, diverse cohort of adults with CKD, we found that urine NGAL was independently associated with development of ischemic atherosclerotic events but not heart failure events or all-cause mortality.
While CKD (as defined by reduced glomerular filtration rate and/or proteinuria) has been shown to be a strong and independent predictor of cardiovascular disease events and death in many studies,2-4,28 much less is known about whether other measures of kidney function and/or structural integrity are differentially associated with specific types of cardiovascular disease events and all-cause mortality. Our finding that after controlling for traditional cardiovascular risk factors including eGFR and albuminuria, higher urine NGAL levels were associated with a higher adjusted rate of ischemic atherosclerotic events (but not other cardiovascular disease events and death) is novel.
There are several potential explanations for why urine NGAL may be independently associated with future ischemic atherosclerotic events in individuals with CKD. First, as NGAL expression is upregulated by ischemia,12 it is possible that higher urine NGAL levels reflect heavier burden of vascular disease in the kidney and, by extrapolation, other vascular beds. In a prior cross-sectional study of 120 untreated, non-diabetic patients with primary hypertension, higher urine NGAL levels were independently associated with 4-fold higher risk of left ventricular hypertrophy, even after adjusting for age, gender and blood pressure values.29 The authors hypothesized that urine NGAL may reflect subclinical inflammation and endothelial damage.
Second, urine NGAL, as a marker of tubular injury, may identify dimensions of kidney function that are not fully captured by eGFR or albuminuria. Although not all the pathophysiological links between CKD and cardiovascular disease are completely elucidated, some sequelae of CKD—such as elevated levels of fibroblast growth factor 2330—may be directly cardiotoxic.31 A recent study reported that the association between higher levels of fibroblast growth factor 23 and adverse outcomes is largely limited to the subset of patients with low fractional excretion of phosphorus, perhaps a sign of renal tubular dysfunction.32 Data are now accumulating that markers of renal tubular dysfunction and injury might supply information about risk of adverse outcomes beyond eGFR and albuminuria. For example, among 565 elderly community-dwelling men, higher levels of urine kidney injury molecule 1—another biomarker of tubular injury— was a risk factor for developing heart failure.33
Although we believe it is less likely, we cannot exclude the possibility that our results are because urine NGAL levels reflect filtered load of plasma NGAL, which can originate from neutrophils. A number of studies have linked plasma NGAL levels to inflammation, atherosclerosis and cardiovascular disease.14,34 Some studies suggest that the Abbott ARCHITECT platform detects mostly the monomeric form of NGAL,35 the predominant form secreted by distal tubule epithelial cells.36 In contrast, the dimeric form is the predominant form secreted by neutrophils.37,38 However, there remains considerable uncertainty in the field.39
Our observation that urine NGAL concentration was not independently associated with mortality is consistent with the few studies that have examined this association in other settings (e.g. community-dwelling men and HIV-infected women40,41). This and our findings regarding lack of association with heart failure events as well as persistent association with ischemic atherosclerotic events need to be replicated in future studies.
Strengths of our work include the cohort study design, large sample size and number of clinical outcomes, the detailed and careful clinical characterization of study participants, the rigorous outcome adjudication process, and the diversity of the study population (concerning both race-ethnicity as well as the presence/absence of diabetes and other comorbid conditions). Since CRIC Study enrollees volunteered to attend elective research study visits, we do not expect them to have rapid serum creatinine fluctuations from decreased kidney perfusion which would confound estimation of glomerular filtration rate.
Our finding should be viewed in the context of the following limitations. By design, some underlying CKD causes (eg, polycystic kidney disease) were not represented and patients aged 75 years and older were not enrolled. The NGAL measurements were done with urine samples that were not processed immediately after voiding under conditions fully controlled by the research team. Suboptimal handling could have led to random error from protein degradation or other processes, which would bias our results towards the null. Dipstick urinalysis results were not available to assess for the presence of urinary tract infection, and the presence of urinary neutrophils would lead to higher urine NGAL concentrations. Concurrent serum or plasma NGAL measurements were not available. Finally, urine NGAL concentration was ascertained only at study entry, and we were unable to examine changes in NGAL over time and subsequent outcomes.
In summary, we found that among adults with CKD, elevated urine NGAL was associated with an increased incidence of ischemic atherosclerotic events (independent of eGFR and albuminuria and other several other potential confounders), but not heart failure events or all-cause mortality. We believe that our finding deserves validation (potentially in cohorts enrolling more elderly patients and encompassing more diverse etiologies of kidney disease) and may have the potential to further our understanding of the connections between kidney and cardiovascular disease.
Supplementary Material
Acknowledgements
The CRIC Study Investigators comprise Lawrence J. Appel, MD, MPH, Harold I. Feldman, MD, MSCE, Alan S. Go, MD, Jiang He, MD, PhD, John W. Kusek, PhD, James P. Lash, MD, Akinlolu Ojo, MD, PhD, Mahboob Rahman, MD, and Raymond R. Townsend, MD.
We thank Theodore Mifflin at the University of Pennsylvania Translational Core Central Laboratory for technical assistance with the urine NGAL assays.
Support: Funding for the CRIC Study was obtained under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (grants U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by the following: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/National Center for Advancing Translational Sciences (NCATS) UL1TR000003, Johns Hopkins University grant UL1 TR-000424, University of Maryland General Clinical Research Center grant M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, grant UL1TR000439 from the NCATS component of the NIH and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research grant UL1TR000433, University of Illinois at Chicago Clinical and Translational Science Award UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology grant P30GM103337, and Kaiser Permanente NIH/National Center for Research Resources UCSF-CTSI grant UL1 RR-024131. Drs Liu, Go, Feldman and Hsu are also supported by grant U01DK85649 as members of the Chronic Kidney Disease Biomarker Consortium.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: Dr Liu has received donation of biomarker reagents/measurements for research studies from Abbott and CMIC Holdings Co Ltd; Dr Hsu has received donation of biomarker reagents/measurements for research studies from Abbott. The other authors declare that they have no other relevant financial interests.
Contributions: research idea and study design: KDL, WY, ASG, AHA, HIF, MJF, JH, RRK, JWK, ERM, SS, MRW, C-yH; data acquisition: ASG, AHA, HIF, JH, RRK, SRM, ERM, SS, C-yH; data analysis/interpretation: KDL, WY, ASG, AHA, HIF, MJF, JH, RRK, JWK, SRM, ERM, SER, MRW, C-yH; statistical analysis: WY, AHA, KT, C-yH; supervision or mentorship: C-yH; study oversight, management and funding: JWK. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. C-yH takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted, and that any discrepancies from the study as planned have been explained.
Supplementary Material
Table S1: Adjusted HR by quintiles of baseline NGAL after excluding patients with history of CVD.
Table S2: Adjusted HR by quintiles of baseline urine NGAL/creatinine ratio.
Table S3: Ischemic atherosclerotic events separated into MI, peripheral artery disease, and ischemic stroke.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
REFERENCES
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007 Nov 7;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004 Sep 23;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010 Feb 3;303(5):423–429. doi: 10.1001/jama.2010.39. 2010. [DOI] [PubMed] [Google Scholar]
- 4.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010 Jun 12;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Israni AK, Kasiske BL. Laboratory assessment of kidney disease: glomerular filtration rate, urinalysis, and proteinuria. In: Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu ASL, Brenner BM, editors. Brenner and Rector’s The Kidney. 9th ed. Elsevier Saunders; Philadelphia: 2012. pp. 868–869. [Google Scholar]
- 6.Liu KD, Yang W, Anderson AH, et al. Urine neutrophil gelatinase-associated lipocalin levels do not improve risk prediction of progressive chronic kidney disease. Kidney Int. 2013 May;83(5):909–914. doi: 10.1038/ki.2012.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolignano D, Donato V, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am. J. Kidney Dis. 2008 Sep;52(3):595–605. doi: 10.1053/j.ajkd.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Fassett RG, Venuthurupalli SK, Gobe GC, Coombes JS, Cooper MA, Hoy WE. Biomarkers in chronic kidney disease: a review. Kidney Int. 2011 Oct;80(8):806–821. doi: 10.1038/ki.2011.198. [DOI] [PubMed] [Google Scholar]
- 9.Peralta CA, Katz R, Bonventre JV, et al. Associations of urinary levels of kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) with kidney function decline in the Multi-Ethnic Study of Atherosclerosis (MESA) Am. J. Kidney Dis. 2012 Dec;60(6):904–911. doi: 10.1053/j.ajkd.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhavsar NA, Kottgen A, Coresh J, Astor BC. Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM-1) as predictors of incident CKD stage 3: the Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Kidney Dis. 2012 Aug;60(2):233–240. doi: 10.1053/j.ajkd.2012.02.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith ER, Lee D, Cai MM, et al. Urinary neutrophil gelatinase-associated lipocalin may aid prediction of renal decline in patients with non-proteinuric Stages 3 and 4 chronic kidney disease (CKD) Nephrol. Dial. Transplant. 2013 Jun;28(6):1569–1579. doi: 10.1093/ndt/gfs586. [DOI] [PubMed] [Google Scholar]
- 12.Paragas N, Qiu A, Zhang Q, et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat. Med. 2011 Feb;17(2):216–222. doi: 10.1038/nm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 14.Bolignano D, Coppolino G, Lacquaniti A, Buemi M. From kidney to cardiovascular diseases: NGAL as a biomarker beyond the confines of nephrology. Eur. J. Clin. Invest. 2010 Mar;40(3):273–276. doi: 10.1111/j.1365-2362.2010.02258.x. [DOI] [PubMed] [Google Scholar]
- 15.Cruz DN, Gaiao S, Maisel A, Ronco C, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of cardiovascular disease: a systematic review. Clin. Chem. Lab. Med. 2012 Sep 1;50(9):1533–1545. doi: 10.1515/cclm-2012-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko GJ, Grigoryev DN, Linfert D, et al. Transcriptional analysis of kidneys during repair from AKI reveals possible roles for NGAL and KIM-1 as biomarkers of AKI-to-CKD transition. Am J Physiol Renal Physiol. 2010 Jun;298(6):F1472–1483. doi: 10.1152/ajprenal.00619.2009. [DOI] [PubMed] [Google Scholar]
- 17.Damman K, Masson S, Hillege HL, et al. Clinical outcome of renal tubular damage in chronic heart failure. Eur. Heart J. 2011 Nov;32(21):2705–2712. doi: 10.1093/eurheartj/ehr190. [DOI] [PubMed] [Google Scholar]
- 18.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J. Am. Soc. Nephrol. 2003 Jul;14(7 Suppl 2):S148–153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 19.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clinical journal of the American Society of Nephrology: CJASN. 2009 Aug;4(8):1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joffe M, Hsu CY, Feldman HI, et al. Variability of creatinine measurements in clinical laboratories: results from the CRIC study. Am. J. Nephrol. 2010;31(5):426–434. doi: 10.1159/000296250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin. Chem. 2007 Apr;53(4):766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 22.Anderson AH, Yang W, Hsu CY, et al. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2012 Aug;60(2):250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Townsend RR, Anderson AH, Chen J, et al. Metabolic syndrome, components, and cardiovascular disease prevalence in chronic kidney disease: findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Nephrol. 2011;33(6):477–484. doi: 10.1159/000327618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N. Engl. J. Med. 1971 Dec 23;285(26):1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 25.Einhorn PT, Davis BR, Massie BM, et al. The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Heart Failure Validation Study: diagnosis and prognosis. Am. Heart J. 2007 Jan;153(1):42–53. doi: 10.1016/j.ahj.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007 Nov 27;116(22):2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 27.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999 Apr;30(4):736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 28.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003 Oct 28;108(17):2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 29.Leoncini G, Mussap M, Viazzi F, et al. Combined use of urinary neutrophil gelatinase-associated lipocalin (uNGAL) and albumin as markers of early cardiac damage in primary hypertension. Clin. Chim. Acta. 2011 Oct 9;412(21-22):1951–1956. doi: 10.1016/j.cca.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 30.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011 Jun 15;305(23):2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J. Clin. Invest. 2011 Nov;121(11):4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dominguez JR, Shlipak MG, Whooley MA, Ix JH. Fractional excretion of phosphorus modifies the association between fibroblast growth factor-23 and outcomes. J. Am. Soc. Nephrol. 2013 Mar;24(4):647–654. doi: 10.1681/ASN.2012090894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlsson AC, Larsson A, Helmersson-Karlqvist J, et al. Urinary kidney injury molecule 1 and incidence of heart failure in elderly men. Eur J Heart Fail. 2013 Apr;15(4):441–446. doi: 10.1093/eurjhf/hfs187. [DOI] [PubMed] [Google Scholar]
- 34.Daniels LB, Barrett-Connor E, Clopton P, Laughlin GA, Ix JH, Maisel AS. Plasma neutrophil gelatinase-associated lipocalin is independently associated with cardiovascular disease and mortality in community-dwelling older adults: The Rancho Bernardo Study. J. Am. Coll. Cardiol. 2012 Mar 20;59(12):1101–1109. doi: 10.1016/j.jacc.2011.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nickolas TL, Forster CS, Sise ME, et al. NGAL (Lcn2) monomer is associated with tubulointerstitial damage in chronic kidney disease. Kidney Int. 2012 Sep;82(6):718–722. doi: 10.1038/ki.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nickolas TL, Schmidt-Ott KM, Canetta P, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J. Am. Coll. Cardiol. 2012 Jan 17;59(3):246–255. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai L, Rubin J, Han W, Venge P, Xu S. The origin of multiple molecular forms in urine of HNL/NGAL. Clinical journal of the American Society of Nephrology: CJASN. 2010 Dec;5(12):2229–2235. doi: 10.2215/CJN.00980110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martensson J, Xu S, Bell M, Martling CR, Venge P. Immunoassays distinguishing between HNL/NGAL released in urine from kidney epithelial cells and neutrophils. Clin. Chim. Acta. 2012 Oct 9;413(19-20):1661–1667. doi: 10.1016/j.cca.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Glassford NJ, Schneider AG, Xu SY, et al. The nature and discriminatory value of urinary neutrophil gelatinase-associated lipocalin in critically ill patients at risk of acute kidney injury. Intensive Care Med. Oct 2013;39(10):1714–1724. doi: 10.1007/s00134-013-3040-7. [DOI] [PubMed] [Google Scholar]
- 40.Helmersson-Karlqvist J, Larsson A, Carlsson AC, et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is associated with mortality in a community-based cohort of older Swedish men. Atherosclerosis. 2013 Apr;227(2):408–413. doi: 10.1016/j.atherosclerosis.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Peralta CA, Scherzer R, Grunfeld C, et al. Urinary biomarkers of kidney injury are associated with all-cause mortality in the Women’s Interagency HIV Study (WIHS) HIV Med. 2014 May;15(5):291–300. doi: 10.1111/hiv.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.