Summary
Spontaneous neuronal activity is spatiotemporally structured, influencing brain computations. Nevertheless, the neuronal interactions underlying these spontaneous activity patterns, and their biological relevance, remain elusive. Here, we addressed these questions using two-photon calcium imaging of intact zebrafish larvae to monitor the neuron-to-neuron spontaneous activity fine structure in the tectum, a region involved in visual spatial detection. Spontaneous activity was organized in topographically compact assemblies, grouping functionally similar neurons rather than merely neighboring ones, reflecting the tectal retinotopic map despite being independent of retinal drive. Assemblies represent all-or-none-like sub-networks shaped by competitive dynamics, mechanisms advantageous for visual detection in noisy natural environments. Notably, assemblies were tuned to the same angular sizes and spatial positions as prey-detection performance in behavioral assays, and their spontaneous activation predicted directional tail movements. Therefore, structured spontaneous activity represents “preferred” network states, tuned to behaviorally relevant features, emerging from the circuit’s intrinsic non-linear dynamics, adapted for its functional role.
Highlights
-
•
Spontaneous functional assemblies reflect the tectal retinotopic map
-
•
Assemblies can be spontaneously engaged independently of retinal drive
-
•
Assemblies are all-or-none preferred states shaped by inhibitory competition
-
•
Assemblies are tuned to features relevant for a tectal-dependent vital behavior
The brain spontaneously produces activity patterns. Romano et al. show that this ongoing activity in a sensory brain area reflects neuronal mechanisms that assure robust circuit functioning for the extraction of behaviorally relevant sensory information.
Introduction
In order to understand how the brain interacts with the external physical world, we should first understand its intrinsic dynamics by studying activity under conditions of minimal sensory drive (Deco et al., 2013; Ringach, 2009). Indeed, in the absence of stimulation, sensory brain areas remain highly active. This ongoing spontaneous activity consumes most of the brain’s energy budget (Tomasi et al., 2013), and yet it has traditionally been considered as independent biophysical noise with no functional value for brain computations (Faisal et al., 2008; Shadlen and Newsome, 1998; Treisman, 1964).
However, several studies have emphasized the complex interaction between spontaneous activity and sensory-induced neuronal responses (Deco et al., 2011; Destexhe, 2011; Harris and Thiele, 2011; Ringach, 2009). They showed that the emergence of spontaneous activity patterns in sensory brain regions partially accounts for the across-trial variability observed in neuronal responses induced by identical sensory stimulation. Specifically, ongoing spontaneous activity can linearly sum with sensory input (Arieli et al., 1996; Azouz and Gray, 1999), inhibit and spatiotemporally confine sensory-induced responses (Petersen et al., 2003), or even non-linearly gate them (Luczak et al., 2013).
Furthermore, in recent years it was shown that spontaneous activity is spatiotemporally structured at a coarse level, according to the inter-regional brain circuitry of mice (Mohajerani et al., 2013) and humans (Fox et al., 2005; Raichle et al., 2001) and to the orientation column maps in the visual cortex of anesthetized cats (Kenet et al., 2003; Tsodyks et al., 1999). This suggests that spontaneous activity dynamically engages in coordinated network states affecting brain processing. The prevalence of spontaneous activity structure was further supported by the observation that spontaneous mean pair-wise spatiotemporal correlograms of multi-unit recordings from the ferret’s primary visual cortex were only mildly modulated by sensory inputs (Fiser et al., 2004). Moreover, the spontaneous activity structure is clearly dependent on brain state (Harris and Thiele, 2011; Luczak et al., 2009) and samples a constrained repertoire of possible neuronal responses (Luczak et al., 2009), molded by experience (Berkes et al., 2011; Han et al., 2008; Sumbre et al., 2008). Finally, the awake rodent neocortical and hippocampal neuronal networks spontaneously replay fragments of previous sensory experiences, probably contributing to higher cognitive processes like memory, learning, and priming (Carr et al., 2011; Han et al., 2008; Ringach, 2009). Despite these advances, the neuronal interactions underlying the emergence of these spontaneous patterns remain largely unexplored (apart from theoretical approaches: Deco et al., 2011, 2013; Goldberg et al., 2004), and their biological significance with respect to neuronal circuits’ behavioral roles is still unresolved.
In the present work, we addressed these two open questions by monitoring the spontaneous dynamics of the zebrafish larva optic tectum. The vertebrate optic tectum, analogous to the mammalian superior colliculus, contains functional sensory maps of the external world, and it is involved in spatial detection, attention, and the generation of commands for orienting motor behaviors (Krauzlis et al., 2013). In zebrafish, the tectum is the most complex layered brain structure and is essential for visually guided prey detection and capture (Gahtan et al., 2005). We monitored spontaneous activity by means of two-photon Ca2+ imaging of a significant fraction (∼15%) of the optic tectum with cellular resolution in intact, non-anaesthetized, non-paralyzed zebrafish larvae expressing pan-neuronally the genetically encoded calcium indicator GCaMP3 (Tian et al., 2009). This technique enabled a systematic high-resolution description of the in vivo dynamics of large neuronal circuits (Ahrens et al., 2012; Orger et al., 2008; Sumbre et al., 2008). We thus studied the structure of tectal spontaneous neuronal activity within the context of optic tectum’s functional role in vital behaviors (Gahtan et al., 2005; Niell and Smith, 2005).
We report that this spontaneous structure is organized in distinct neuronal assemblies of similarly tuned neurons, reproducing the tectum’s retinotopic-like map even in the absence of retinal drive. Furthermore, we demonstrated an interplay of non-linear cooperative and competitive neuronal tectal dynamics suited for promoting the emergence of these spontaneous patterns. Finally, the spontaneous activity structure was associated with spontaneous tail flips, and it was particularly tuned to visual features that corresponded to larva’s behavioral preferences during prey-capture assays, thus arguing for its biological relevance.
Results
Visual Functional Maps of the Tectum
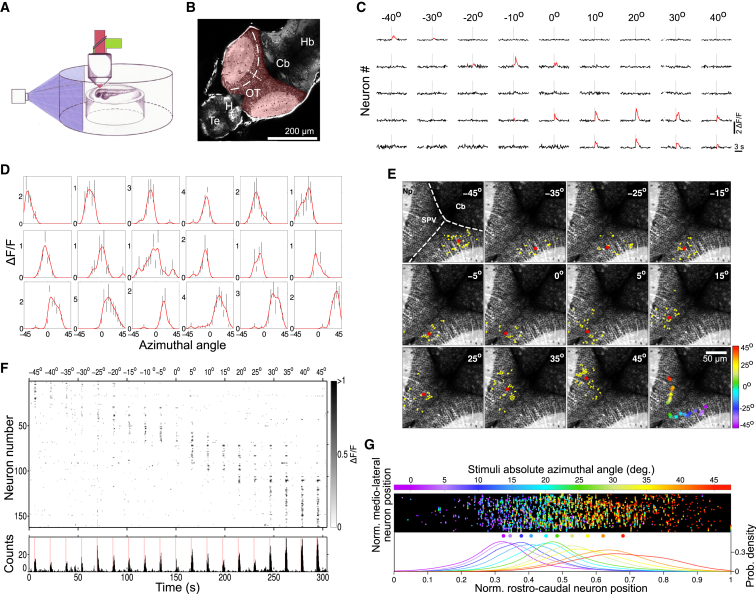
To study the optic tectum’s spontaneous activity within the frame of the tectal role in vision processing (Gahtan et al., 2005; Krauzlis et al., 2013; Niell and Smith, 2005), we first characterized the tectum’s visually induced responses in terms of stimulus spatial localization and stimulus movement direction. For this purpose, we presented to the larva light spots at different positions in the visual field and light bars moving in each of the four cardinal directions while simultaneously recording the activity of hundreds of neurons in the dorsal sensory layers of the tectum’s stratum periventriculare (SPV; 836 ± 97 neurons from both tectal hemispheres of 8 dpf larvae, 14 larvae; Figures 1A and 1B).
Figure 1.
The Zebrafish Optic Tectum Contains a Functional Retinotopic Map
(A) Agarose-embedded 8 dpf zebrafish larvae were placed on an elevated stage, within a cylindrical recording chamber, and visually stimulated using a pico-projector. Spontaneous and visually induced neuronal activities were monitored by two-photon calcium imaging.
(B) An optical section of a larva’s brain pan-neuronally expressing GCaMP3 (HuC:GCaMP3). The optic tectum is highlighted in red. The rostro-caudal and medio-lateral axes of the right tectum are schematically outlined by dashed and pointed lines, respectively. Cb, cerebellum; H, habenula; Hb, hindbrain; OT, optic tectum; Te, telencephalon.
(C) Typical single-trial neuronal ΔF/F traces induced by 1 s visual stimulation with 4° light spots. Vertical gray line: stimulus onset. Stimulus azimuthal positions are indicated at each stimulation onset (0°, negative and positive angle values represent frontal, right, and left visual field positions, respectively). Significant ΔF/F transients are highlighted in red. Breaks in the traces are discarded frames due to movement artifacts.
(D) Representative neuronal spatial tuning curves (red line). Mean ± SD of neuronal responses are shown in black.
(E) Topography examples of neuronal groups consistently activated by light spots at the azimuth angles indicated in the top right corner. Activated neurons are colored in yellow, and the neuronal groups’ centroids are depicted as red asterisks. Bottom right: the neuronal group centroids color coded according to the azimuth angle (color bar on the right). As expected from a retinotopic map, a continuous progression of centroids along the contra-lateral rostro-caudal axis is clearly observed. Top left: anatomical landmarks. Dashed lines delineate the tectal-cerebellar and inter-hemispheric tectal boundaries. Cb, cerebellum; Np, tectal neuropil; SPV, tectal somatic periventricular layer.
(F) Raster plot of ΔF/F responses presented in (E). Grayscale, ΔF/F amplitude. Top labels indicate the light-spot azimuth angle. For visualization purposes, we show trials rearranged according to azimuth angle (during experiments, they were presented in random order). Only responsive neurons are shown. Bottom: histogram of the neuronal Ca2+ transients. Red bars mark light-spot stimulation periods.
(G) Normalized topography of neuronal groups induced by 4° light-spot stimulation experiments (n = 9). Each point in the upper panel represents the normalized position of a visually activated neuron, whose color indicates the absolute azimuth angle of the stimulating light spot (color bar on top). Bottom: probability density distributions of the normalized rostro-caudal neuron positions according to the stimulus position. Distributions are color coded according to the top color bar. Dots represent the medians. The smooth progression of the latter reflects a robust tectal retinotopic map conserved across larvae.
In zebrafish, tectal neurons receive massive organized inputs from retinal ganglion cells (RGCs), exclusively from the contralateral eye, that synapse on the tectal neuropil creating a functional retinotopic tectal map of the contralateral visual field (Burrill and Easter, 1994; Niell and Smith, 2005). The contralateral dorsal and ventral visual hemifields are represented in the dorso-ventral tectal axis, whereas the nasal and temporal hemifields are mapped in the rostro-caudal tectal axis. Light spots presented at different positions in the visual field evoked activations (Ca2+-induced significant relative change in fluorescence, ΔF/F; Supplemental Experimental Procedures; Figures 1C and S1) of distinct neuronal groups (neuronal populations that consistently responded to a given stimulus across trials). According to these visual responses, we estimated the neuronal spatial tuning curves (their receptive field along the azimuthal position; Figure 1D). Central positions of the visual field activated rostral neuronal groups, and lateral positions activated more caudal ones, in agreement with previous studies (Niell and Smith, 2005) (Figures 1E and 1F). To enable comparisons across experiments, we summarized the tectal activation profiles across larvae by representing neuronal topographical positions in anatomically normalized tectal coordinates. Within a given optical plane, the tectal neuroanatomy possesses two functionally relevant axes: the rostro-caudal and the medio-lateral (see traces in Figure 1B). We therefore projected and normalized the Cartesian neuronal position coordinates on these axes (Supplemental Experimental Procedures). We observed that the contralateral visual field azimuth was represented along the tectal rostro-caudal axis (Figure 1G), revealing a tectal functional retinotopic map robustly conserved across different larvae.
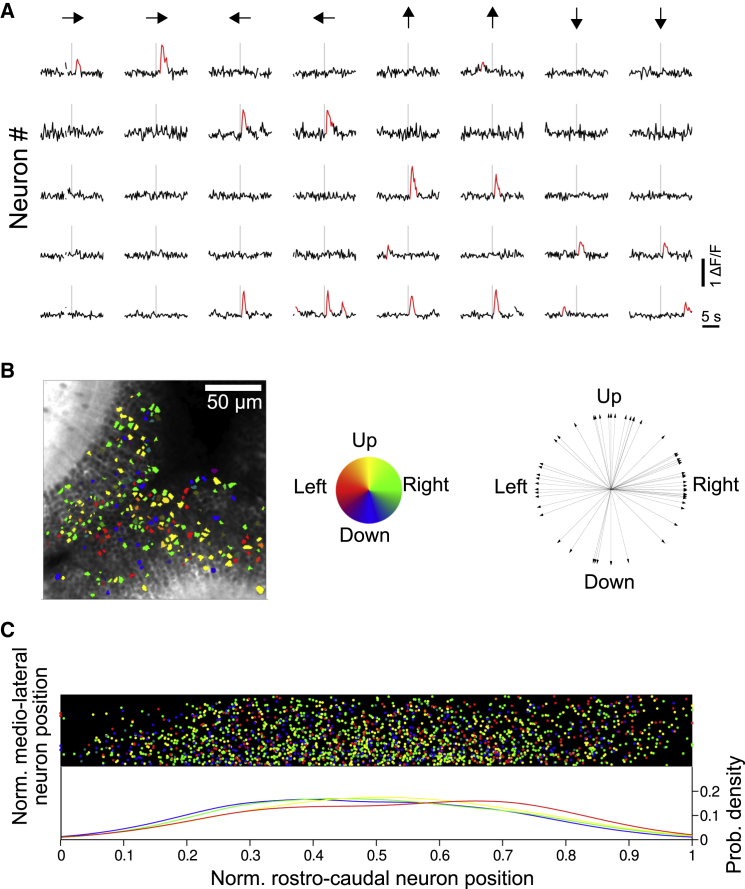
Direction-selective neurons in the larval zebrafish SPV tectal layer have already been reported (Gebhardt et al., 2013), but their precise topographic arrangement is still unknown. In contrast to the functional topographic arrangement of neurons with spatial tuning (Figure 1G), direction-selective neurons (Figure 2A) were scattered across the tectal network without showing any clear topographic organization (Figures 2B and 2C).
Figure 2.
Direction-Selective Tectal Neurons Are Topographically Scattered
(A) Typical single-trial neuronal ΔF/F responses induced by moving light bars. Vertical gray line marks stimulus onset. Arrows indicate bar moving direction. Significant ΔF/F transients are highlighted in red.
(B) Left: example of the topography of direction-selective neurons. Neurons are colored according to their direction selectivity (color wheel). Right: neuronal direction preferences of this example.
(C) Normalized topography of neuronal groups induced by direction-selectivity experiments (n = 14). Each point in the upper panel represents the normalized position of a direction-selective neuron whose color indicates its direction selectivity (same color code as that in B). Bottom: color-coded probability density distributions of the normalized rostro-caudal position of direction-selective neurons. The similarity between the distributions indicates the lack of direction-selective topographic arrangement in the tectum.
Having systematically assessed neuronal tunings for visual object localization and movement direction, we then studied tectal spontaneous network activity within the framework of these functional roles.
Spontaneous Activation of Visual Representations
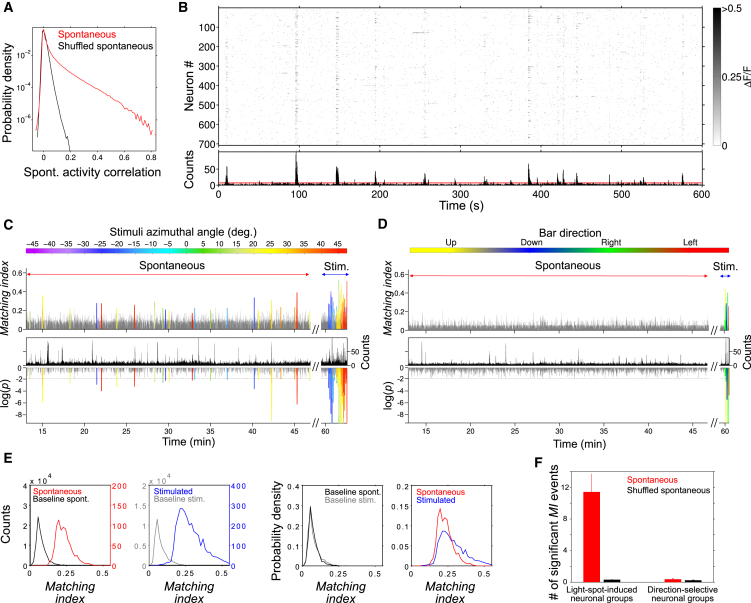
To study spontaneous activity in the tectum’s sensory visual layers, we analyzed the dynamics of neuronal populations for periods of 1 hr, in the absence of sensory stimulation. Neuronal populations showed sparse basal activity, with low average neuronal activation frequencies (0.016 ± 0.015 Hz), with less than ∼1% of the imaged population simultaneously active. Nevertheless, we observed significant pair-wise temporal correlations (Figure 3A) and frequent episodes of transient synchronous activations across neuronal subsets consisting of 2%–10% of the total population (Figure 3B), revealing concerted spontaneous neuronal dynamics.
Figure 3.
Visual Spatial Representations Spontaneously Emerge in the Absence of Sensory Stimulation
(A) Probability density distribution of pair-wise spontaneous neuronal activity correlations (red, Pearson linear coefficient, r) observed across experiments (n = 23). Only significant ΔF/F transients were considered. In black is the same distribution after neuronal interactivation interval shuffling (IAI) that destroys temporal coordination across neurons. 16% of neuronal pairs showed correlations that could not be explained by the latter distribution (r = 0.07 ± 0.06, p < 0.05).
(B) Example of a raster plot of spontaneous Ca2+ transients, revealing episodes of synchronous activations. Grayscale: ΔF/F amplitude. Bottom: histogram of the Ca2+ transients. The red line marks the threshold for significant population events.
(C and D) The degree of match between spontaneous network activations and the visually induced neuronal groups quantified by the matching index (MI), ranging between 0 and 1, corresponding to null and full match, respectively. (C) Top: example of MI dynamics for matches to neuronal groups induced by 4° light spots during stimulated and spontaneous activity periods (blue and red two-headed arrows, respectively). Significant MI peaks are colored according to the angular position they represent (the stimulus position that induced the activation of the neuronal group that best matched the spontaneous activation pattern; top color bar). Non-significant matches are shown in gray. For visualization purposes, we show trials with stimulation angles in increasing order. Bottom: corresponding population histogram of the neuronal Ca2+ transients (top, black) and the base 10 logarithm of the MI p values (bottom). MI peaks with p value < 0.01 (99% confidence; dashed gray line; see Supplemental Experimental Procedures) are considered significant and colored as in top panel. (D) Same as (C), but for direction-selective neuronal groups. Notice the absence of significant spontaneous activations.
(E) Left to right, count histograms of significant spontaneous (red) and stimulated (blue) MIs and the corresponding non-significant MI baselines (black and gray), and probability density distributions comparing these baselines and the significant MIs. Note the comparable magnitudes of spontaneous and stimulated significant MI events.
(F) Average number of significant MI events of visually evoked neuronal groups during spontaneous activities, per experiment, per neuronal group (red), for light-spot-induced (left) and direction-selective (right) neuronal groups. The number of significant events for these neuronal groups during IAI-shuffled spontaneous activities is shown in black.
We then asked if the patterns of these episodic spontaneous activations resembled, with single-neuron precision, those induced by visual stimulation (Figures 1 and 2). For this purpose, we calculated a matching index (MI; Supplemental Experimental Procedures) expressing the moment-to-moment degree of neuronal overlap between two given activation patterns (Hilgetag et al., 2002), in this case between spontaneous network dynamics (the spontaneous activation patterns) and the visually induced neuronal responses (i.e., neuronal groups). Significant MIs of light-spot-induced neuronal groups emerged transiently during the spontaneous activity (Figure 3C; 1.46 ± 1.79 episodes per min, or 0.23 ± 0.25 episodes per min for a given neuronal group) with MI levels comparable to those induced by light-spot stimulation (Figure 3E; mean MI of 0.23 ± 0.05 for spontaneous assemblies and 0.29 ± 0.09 for stimulations). To estimate the significance of these matches, we shuffled the neurons’ spontaneous spike times. This disruption of the temporal neuronal dynamics completely suppressed the emergence of significant MIs, thus arguing for the significance of the observed spontaneous patterns that matched the visually induced responses (Figure 3F). Moreover, we failed to observe significant spontaneous MIs of direction-selective neuronal groups (Figures 3D and 3F). These results suggest that certain spontaneous tectal activity patterns matched, with significant cellular precision, transient network states that corresponded to spatially localized visual representations.
The Spatiotemporal Structure of Spontaneous Activity
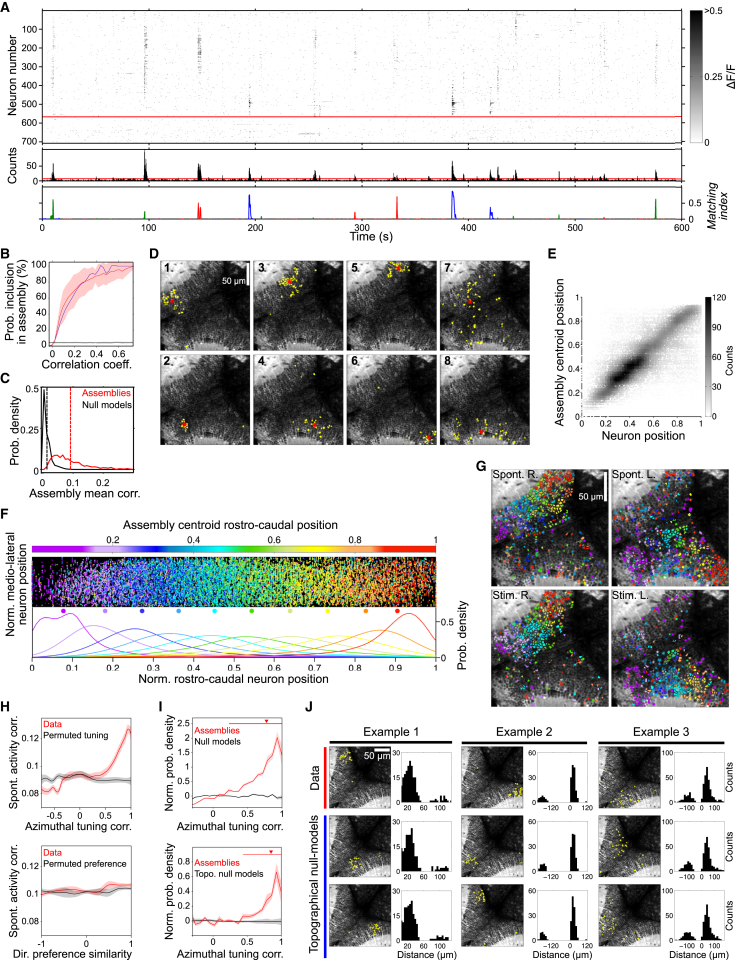
Both the emergence of spontaneous activation patterns resembling visual representations and the presence of significant spontaneous neuronal pair-wise correlations (Figure 3) motivated us to look for dynamical neuronal assemblies, i.e., groups of neurons showing significantly correlated spontaneous activities. For this purpose, we combined and adapted previously proposed dimensionality reduction and clustering methods (Lopes-dos-Santos et al., 2011; Peyrache et al., 2010; see Supplemental Experimental Procedures for details; Figures S2A–S2C). This combined technique markedly outperformed other widely used algorithms such as k-means or hierarchical clustering, as measured by cluster validation tests (Figures S2D–S2I).
Using this PCA-promax technique, we identified distinct assemblies composed, in most cases, of neighboring neurons (Figures 4A–4D). During the 1-hr experiments, we observed the emergence of 47 ± 19 spontaneous unique assemblies with a rate of 2.4 ± 0.9 per min and comprising 25 ± 9 neurons per assembly (average ± SD; Figure 4A). The spontaneous assemblies consisted of significantly temporally correlated neurons (Figure 4C), representing 80% ± 19% of the significant neuronal pair-wise correlations observed in the tectal population (Figure 4B). We therefore concluded that these assemblies accounted for a large fraction of the correlational structure of the entire imaged neuronal population.
Figure 4.
Spontaneous Emergence of a Retinotopic-like Map from the Dynamics of the Ongoing Neuronal Activities
(A) Same raster plot and histogram as in Figure 3B, with neurons sorted according to the assemblies obtained with PCA/promax, allowing the visualization of distinct neuronal assemblies. Neurons not assigned to any assembly lie below the red line. Bottom: example of the MI dynamics for three representative spontaneous assemblies (red, green, and blue). Note that each MI peak of the same color corresponds to the activation of roughly the same group of neurons.
(B) Probability of inclusion of a neuronal pair in a spontaneous assembly, as a function of their pair-wise spontaneous activity correlation coefficient, averaged across experiments (red), and for the experiment presented in (A) (blue). The same calculation for shuffled sets of surrogate assemblies (null models) is presented in black. Error bars, SD across experiments.
(C) Probability density distribution of the mean pair-wise spontaneous activity correlation coefficient of neurons within each of the spontaneous assemblies (red). The same distribution for null models is presented in black. Dashed lines: the distributions’ means (0.09 ± 0.06 for spontaneous assemblies and 0.01 ± 0.01 for null models; means and SD p < 10−5).
(D) Topographies of eight examples of spontaneous neuronal assemblies. Assembly neurons are labeled in yellow. Red asterisks: assembly centroids. 1–6: typical compact assemblies. 7–8: sparse assemblies.
(E) Density plot of the normalized rostro-caudal position of each neuron against the normalized rostro-caudal centroid position of its spontaneous assemblies. Grayscale: data count.
(F) Normalized topography of spontaneous assemblies across larvae (n = 23). Top: each point represents the normalized position of a neuron participating in a spontaneous assembly whose color indicates the normalized rostro-caudal centroid positions of its assemblies (color bar on top). Bottom: probability density distributions of the normalized rostro-caudal position of neurons for assemblies within the same range of colors (for ten bins of the color bar). Colored dots: the medians for each distribution curve.
(G) Spontaneously emerging retinotopic-like map in one typical experiment. Spont., all the spontaneous assemblies whose centroids lay on the right (R.) and left (L.) tecti. Neurons belonging to assemblies with similar topographic centroids were similarly colored. The colors represent the average normalized rostro-caudal centroid position of their assemblies. The transparency represents the SD across centroids. Stim. L. and R.: the same representation for the light-spot-induced responses. Note the resemblance between Spont. and Stim. panels, showing a graded change in colors along the rostro-caudal axes.
(H) Top: local regression of significant pair-wise spontaneous neuronal activity correlation coefficients against the pair-wise neuronal spatial tuning curve correlation coefficients (red). The same regression after randomly permuting across neurons the neuronal tuning curves (black). Bottom: same as top, but regressing against neuronal directional preference similarity (red; pair-wise dot product of direction-preference vectors) and permuting neuronal directional preferences (black).
(I) Top: probability density distribution of the pooled pair-wise correlation coefficients of azimuthal tuning curves of neurons that are grouped by a spontaneous assembly (red). This distribution was normalized by dividing by the distribution resulting from null models. For comparison, we show in black the average normalized distribution for single null model runs. Note that spontaneous assemblies show a bias toward grouping neurons with highly similar tuning curves (significant bias range and median are indicated by top red line and arrowhead). Bottom: same as top, but after normalization with topographical null models. Lines and confidence interval in (H) and (I): average and jackknife procedure across experiments.
(J) Two examples of topographical null models (blue) of three spontaneous assemblies (red). Black bar histograms: the corresponding inter-neuron distance distributions (positive and negative distances correspond to intra- and inter-hemisphere neuronal pairs, respectively).
We quantified the assemblies’ features by the implementation of several normalized dimensionless indexes that enabled us to unbiasedly compare across experiments. To infer the significance of the measured properties of the assemblies, we controlled against sets of shuffled surrogate assemblies (null models). For this purpose, we first shuffled neuronal identities by permuting the indexing of the neuronal population. Then, we built the null models by grouping neurons according to the original assemblies, but using the permuted neuronal indexing. This was repeated for 1,000 rounds, creating a null model set of mock random clusters with randomized topographies, conserving the rest of the statistics in the data (firing rates, pair-wise correlations, etc.). This allowed us to test for the significance of the original assemblies’ features, with respect to the shuffled clusters.
Using this approach, we observed that spontaneous assemblies were mostly constrained to either tectal hemisphere (71% of them were significantly unilateral, laterality index > 0.74, p < 0.05, where 0 corresponds to an assembly whose neurons are equally distributed across hemispheres, and 1 indicates a completely unilateral arrangement). Moreover, 51% showed significant topographical compactness (Figures 4D and S7B; compactness index > 0.46, p < 0.05, ranging between 0 and 1, quantifying the spatial tightness of the assembly; see Figures S5A and S5C for compactness index examples and calculation). The centroids of spontaneous assemblies were arranged in a continuous progression along the rostro-caudal tectal axis, both in pooled (Figure 4F) and individual experiments (top panels in Figure 4G), and the normalized rostro-caudal neuron positions linearly predicted the normalized rostro-caudal centroid positions of the assemblies to which they belong (Figure 4E; r = 0.7, p < 10−5, explaining 49% of the variance), thus demonstrating that neighboring neurons tend to have spontaneously correlated activity. In contrast, we did not find such arrangement across opposite hemispheres (Figures S3A and S3B), suggesting no topographic organization according to the inter-hemispheric spontaneous correlations. These results imply that spontaneous assemblies tend to be formed by neighboring neurons of the same tectal hemisphere, often resulting in topographically compact neuronal clusters.
Interestingly, light-spot-induced and spontaneous assemblies within the same hemisphere displayed comparable topographical arrangements (compare top and bottom panels in Figure 4G and Figure S3C; light-spot-induced assemblies were defined through the previously used PCA-promax procedure).
We found that neuronal pairs with similar spatial tuning curves tended to show higher spontaneous correlations, while this was not true for direction preference similarity (Figure 4H). Accordingly, spontaneous assemblies had a significant bias to regroup neurons with similar spatial tuning curves, when compared to the shuffled null models (top panel in Figure 4I; p < 10−5 for tuning curve correlation coefficients > 0.25, not explained by the null model). We then asked if this result could be explained solely by a combination of a retinotopic map and the usually observed tendency for higher spontaneous correlations between nearby neurons (Kerr et al., 2007; Smith and Kohn, 2008). To test this possibility, we built an additional set of null models with the additional constraint of conserving the relative pair-wise physical distances between neurons found in the original assemblies (topographical null models; Figure 4J). This method enabled us to destroy the functional specificity of the assemblies while keeping intact their topographic compactness and the tectum’s retinotopic map. Remarkably, despite the preservation of the distribution of pair-wise distances, the original assemblies still had a significant bias toward neurons with similar tunings, when compared to these null models (bottom panel in Figure 4I). Nor could topographical null models of light-spot-induced neuronal groups completely explain the transient spontaneous emergence of spatially localized visual representations (Figure S3D). Therefore, spontaneous assemblies do not simply derive from the coarse law of increase in pair-wise spontaneous correlations with decrease in inter-neuronal distance. On the contrary, there is a clear specificity in the composition of spontaneous neuronal assemblies such that they grouped functionally related neurons rather than exclusively neighboring ones. In agreement with these results, spontaneous correlations were related to the neuronal signal correlations (the pair-wise correlations of the mean neuronal responses to an ensemble of stimuli). But notably, noise correlations (the pair-wise correlations of trial-to-trial variability in neuronal responses) were also significantly associated with the spontaneous correlations (Figure S4H). This suggests that the fluctuations of neuronal responses across trials are related to the neuronal interactions represented in the spontaneous tectal assemblies. On the other hand, spontaneous assemblies were not significantly composed of similar direction-selective neurons, and therefore the assemblies were not directionally tuned, when compared to null models (p > 0.05; Figures S3E–S3G). These results suggest that the fine local topographic structure of the tectal functional retinotopic map, but not the directional map, can be reconstructed from the dynamics of the tectal spontaneous activity.
Importantly, the similarity between population neuronal dynamics during spontaneous and visually induced activity was also supported by other methods completely independent of PCA and promax. First, multidimensional scaling (MDS), allowed for a qualitative validation of the global structure of spontaneous and induced neuronal population dynamics (Figures S4A–S4E). We then estimated that spontaneous neuronal assemblies explained the clusters of neurons with correlated light-spot-induced responses with only ∼10% error (Figures S4F and S4G; Supplemental Experimental Procedures).
Therefore, tectal spontaneous neuronal activity is organized in dynamic assemblies mostly resembling neuronal response patterns induced by spatially localized visual stimuli. This suggests that structured tectal spontaneous activity is tightly associated with the functional role of the tectum in visual spatial detection.
Spontaneous Assemblies Do Not Reflect Retinal Inputs
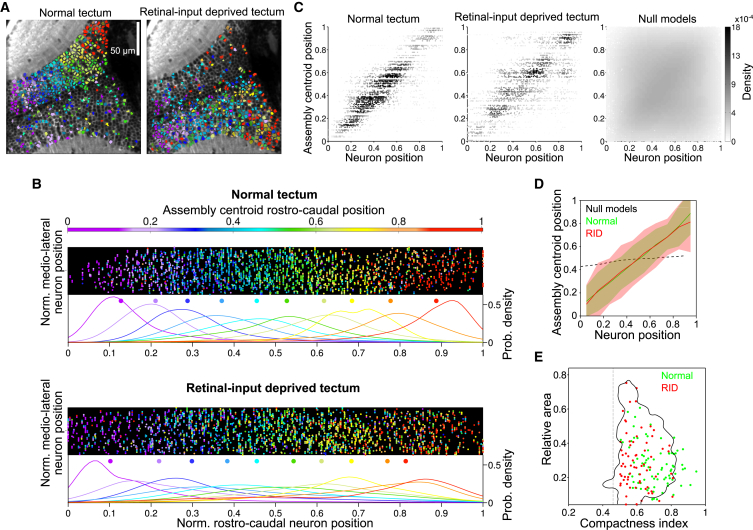
To test whether the topographical organization of tectal spontaneous assemblies resulted from structured inputs arriving from the retina (the vast majority of the tectal afferents; Burrill and Easter, 1994), we performed unilateral retinal ablations or eye enucleations 1–3 days before the experiments. Since the retina projects only to the contralateral tectum, we could perform the experiment and its control in the same larva. Both retinal-input-deprived (RID) tecti and their control tecti displayed spontaneous assemblies in a continuous topographical arrangement along the rostro-caudal tectal axis, indicating that we could reconstruct the functional retinotopic map from the spontaneous activity, even in the absence of retinal inputs (Figures 5A–5D; the neuron’s position linearly predicted the assembly’s centroid position for both RID and control tecti, r = 0.6 and r = 0.74, respectively). Compared to normal tecti, neurons in the deprived tecti were spontaneously active at similar levels (control ΔF/F: 0.58 ± 0.2; deprived ΔF/F: 0.59 ± 0.19; p = 0.055), yet at slightly lower frequencies (control: 0.03 ± 0.04 Hz; deprived: 0.029 ± 0.03 Hz; p = 0.005) and mildly longer durations (control: 0.51 ± 0.21 s; deprived: 0.49 ± 0.24 s; p < 10−5) (Figures S6A–S6C). Assemblies within the deprived tecti spread over similar areas (control: 0.28 ± 0.16; deprived: 0.31 ± 0.19; p = 0.67) but were less compact with respect to those found in the normal tecti (control: 0.61 ± 0.18; deprived: 0.5 ± 0.14; p < 10−5) (Figures S6D and S6E). This difference in compactness could be explained by shuffling ∼25% of the neuronal composition of normal tecti assemblies (Figure 5E; p < 0.01). Therefore, this indicates that spontaneous assemblies of the optic tectum (the major retino-recipient brain region in zebrafish) are not the simple outcome of correlated feed-forward inputs arriving from the retina.
Figure 5.
Spontaneous Assemblies Are Not Driven by Retino-Tectal Inputs
(A) Example of a spontaneously emerging retinotopic-like map 24 hr after retinal-input deprivation (RID) of the left tectum (right eye enucleation at 7 dpf). Spontaneous assemblies whose centroids lay on the normal and RID tectum are shown on the left and right panels, respectively. Data are represented as in Figure 4G.
(B) Normalized topography of spontaneous assemblies for normal (top) and RID (bottom) tecti (n = 5). Data are represented as in Figure 4F.
(C) Density plot of the normalized rostro-caudal position of each neuron against the normalized rostro-caudal centroid position of its spontaneous assemblies for both the normal (left) and RID tecti (middle) and for assemblies’ null models (right). Grayscale: data density.
(D) Regression of the data shown in (C) for the normal (green) and RID (red) tecti (mean and 95% confidence interval). The dashed black line shows the regression result for null models.
(E) Relative area as a function of compactness index (see Supplemental Experimental Procedures) for spontaneous assemblies in the normal (green) and RID (red) tecti. Only significantly compact assemblies are shown (significance threshold indicated by dashed line). The black solid line outlines the region occupied by 99% of the normal tecti spontaneous assemblies with random 25% rearrangement of their neuronal composition. Spontaneous assemblies in RID tecti are not significantly different from this rearranged assembly population.
Spontaneous Assemblies Represent Preferred Network States
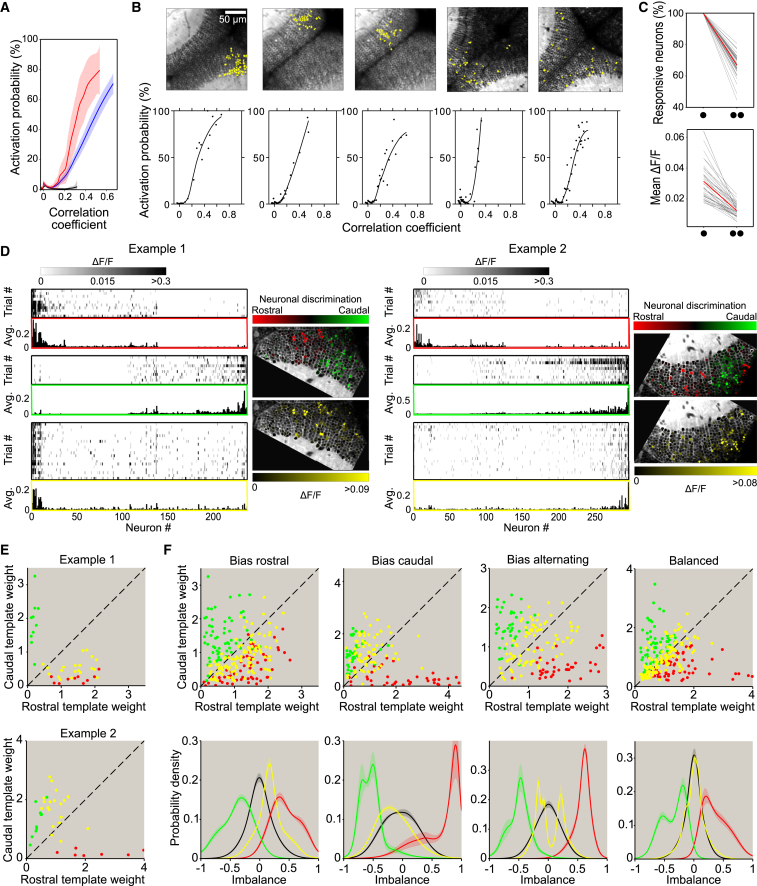
To study the dynamic principles underlying the emergence of spontaneous assemblies, we analyzed how the spontaneous coordinated activations of the neurons within an assembly affected the activity of the assembly’s neurons. For this purpose, we calculated the activation probability of neurons (i.e., the fraction of imaging frames in which the neuron was active) within a given assembly, as a function of the correlation between the instantaneous activity pattern of the tectal population and the topography of the assembly being tested (the latter correlation indicated how well the spontaneous activity at any given moment matched the spontaneous assembly being studied). The obtained activation/correlation profiles showed strong activation probabilities (“facilitations”) that sharply increased as the spontaneous activity pattern gradually resembled the topography of the tested assembly. This implies that the spontaneous neuronal assembly patterns represent network states that are “preferred” by the assembly neurons, since these neurons are markedly more prone to be activated when the spontaneous network activation better resembles the pattern of the assembly they belong to (up to an ∼40-fold increase compared to the null model; Figures 6A, 6B, and S7A). The upper-third most-steep “facilitations” showed step-like activation profiles capable of producing an all-or-nothing-like effect, while the other two-thirds showed more gradual, rather linear, “facilitations.” This result suggests that the dynamics of assembly neurons is capable of abruptly switching from sparse weak to fully correlated activations.
Figure 6.
The Optic Tectum Shows Non-Linear Cooperative and Competitive Dynamics
(A) Activation probability of spontaneous assembly neurons as a function of the correlation between the assembly pattern and the spontaneous tectal network activity. Average activation probability “facilitation” profiles for the upper-third most-steep curves (red, non-linear step-like group) and for the rest of the curves (blue, linear group). As a control, the curves for null models are presented in black. Curves, regression fits across assemblies; confidence interval, SEs.
(B) Top: topography examples of spontaneous assemblies with step-like non-linear dynamics (assembly neurons in yellow). Bottom: corresponding raw data (dots) and regression fits (black curves) of the activation probabilities of the assembly’s neurons. Note the all-or-none-like nature of these curves, suggesting highly recurrent facilitatory cooperative dynamics.
(C) Percentage of responsive neurons (top) and the population average of the mean ΔF/F responses across trials (bottom; only responsive neurons) of individual-light-spot and simultaneous-light-spot stimulation, schematically indicated by a single and a pair of black dots, respectively. Gray lines, single experiments; red lines, average. In top panel, percentages are relative to the combined total of neurons responsive to the two light spots presented individually.
(D) Two examples of single-trial (rasters) and average (bar plots) tectal response patterns to individual light spots (top and middle panels; 10 trials; red and green for most rostral and most caudal light spots, respectively) and simultaneous stimulation with both light spots (bottom panel, 20 trials, yellow). Grayscale: ΔF/F amplitude in rasters. Only responsive neurons are shown. Respective color-coded topographies of neurons that preferentially responded to either the most rostral or most caudal light spot in individual light-spot stimulation (top) and of the average ΔF/F tectal response pattern to simultaneous-light-spot stimulation (bottom). Note how responses to simultaneous stimulation are dominated by the most rostral or the most caudal light-spot representation in examples 1 and 2, respectively.
(E) Visualization of the linear decomposition analysis of the two examples shown in (D). Decomposition weights of single-trial responses to individual (red dots, rostral; green dots, caudal) and simultaneous (yellow dots) light spots with respect to the two average single-spot templates (bar plots in D). Dashed line: equal (balanced) weights. Note how yellow dots overlap with red (top) and green (bottom) dots.
(F) Left to right, visualization of the decomposition analysis of pooled experiments according to four categories: bias competition for rostral neuronal group, bias competition for the caudal group, alternated bias across trials, and balanced responses (no bias of a given group). Top: decomposition weights, color code as in (E). Bottom: distributions of the corresponding imbalance indexes. As a control, we show the expected distribution for a balanced linear response summation scenario estimated from all the possible pair-wise sums of the corresponding individual-light-spot trial responses (black line).
Interestingly, assemblies showing step-like activations were more topographically compact (Figure S7B; compactness index, step-like group: 0.61 ± 0.18; linear group: 0.5 ± 0.17; p < 10−5), and their spatial receptive fields were better tuned than the linear ones (Figure S7C; average assembly normalized tuning peak of 10.1 ± 6.0 for step-like assemblies and 7.7 ± 5.0 for linear assemblies, p = 10−5). Thus, this suggested that spontaneous assemblies with all-or-nothing-like activations carried more information about visual spatial representations than assemblies with linear ones.
These results imply that tectal spontaneous assemblies are stable “preferred,” coherently tuned network states, entrained by a non-linear synergistic interaction between assembly neurons.
Tectal Assemblies Show Biased Competitive Dynamics
To investigate the principles underlying the delineation of the compact topography of the neuronal assemblies, we tested whether the tectal assemblies are subjected to inter-assembly inhibitory competition. To this end, we simultaneously presented to one of the larva’s eyes two 4° light spots (angular separation: Δ10°–Δ30°) while monitoring neuronal activations of the contralateral tectum. We observed that simultaneous-light-spot stimuli induced neuronal responses of lower magnitude (in terms of induced-responses amplitude and number of responsive neurons) than those induced by each of the two light spots presented individually (Figure 6C), suggesting that neuronal responses are affected by inhibitory interactions during the simultaneous stimulations. The lower-magnitude responses to the simultaneous-two-light-spot stimuli could be the result of a uniform inhibition across the whole tectal network. Alternatively, it may represent a specific competition between the neuronal groups induced by each light spot. Our results showed that simultaneous-light-spot trial responses did not represent a simple superposition of both individual-light-spot neuronal groups, but rather were biased toward one of these groups (Figures 6D and S7D). This suggests that the simultaneous presentation of two light spots did not induce a global uniform tectal inhibition, but instead represents competitive inhibitory interactions between the neuronal groups induced by each of the individual light spots.
To quantify this competition, we assessed the extent of the contribution of each of the two individual-light-spot neuronal representations to the explanation of the simultaneous-light-spot trial responses. To this end, we decomposed every trial response induced by a simultaneous light spot with respect to a weighted linear summation of two individual-light-spot-induced template patterns. These latter templates were estimated as the across-trial average responses to each individual light spot when presented separately (e.g., red and green bar plots in Figure 6D; see Supplemental Experimental Procedures). Thus, to analyze the relative contributions of each individual-light-spot-induced neuronal group, for each trial of simultaneous stimulation we computed an imbalance index, defined as the difference between both template weights divided by their sum. This index ranges between −1 and 1, where 0 indicates exact balanced linear summation of the individual-light-spot average responses (no inhibitory interaction between the two neuronal groups), while 1 and −1, respectively, indicate that only the most rostral or the most caudal template was enough to explain the simultaneous-light-spot trial response (winner-takes-all interactions).
We observed competitive interactions between the neuronal groups that were not compatible with a balanced linear summation. In 70% of the experiments (14 out of 20), simultaneous-stimulation response trials were characterized by a neuronal group being more strongly activated (i.e., having a greater decomposition weight) than the other (examples in Figures 6E and S7E). To further characterize this phenomenon, we classified experiments in a data-driven manner (Figure S7F): (1) responses were dominated by the most rostral neuronal groups (30%); (2) dominated by the most caudal neuronal groups (20%); (3) alternating domination of either the rostral or caudal neuronal groups (20%); (4) balanced linear summation (20%) (Figure 6F).
These results demonstrate that the tectal functional assemblies are subjected to competition in 70% of the cases such that, when simultaneously engaged, the resulting activation pattern is biased toward one of the assemblies. We thus suggest that the spontaneous functional assemblies of the tectum are topographically delineated by this inter-assembly biased competitive dynamics.
Behavioral Correlate of the Tectum’s Spontaneous Neuronal Assemblies
Since we found that spontaneous assemblies are related to the optic tectum’s role in spatial vision, we thus further studied whether the assemblies’ population spatial tuning reflected parameters pertinent to visually guided tectum-dependent behaviors. For this purpose, we compared the functional tuning (i.e., spatial and size tuning) of spontaneous assemblies with the larva’s detection performance during prey-capture behavior.
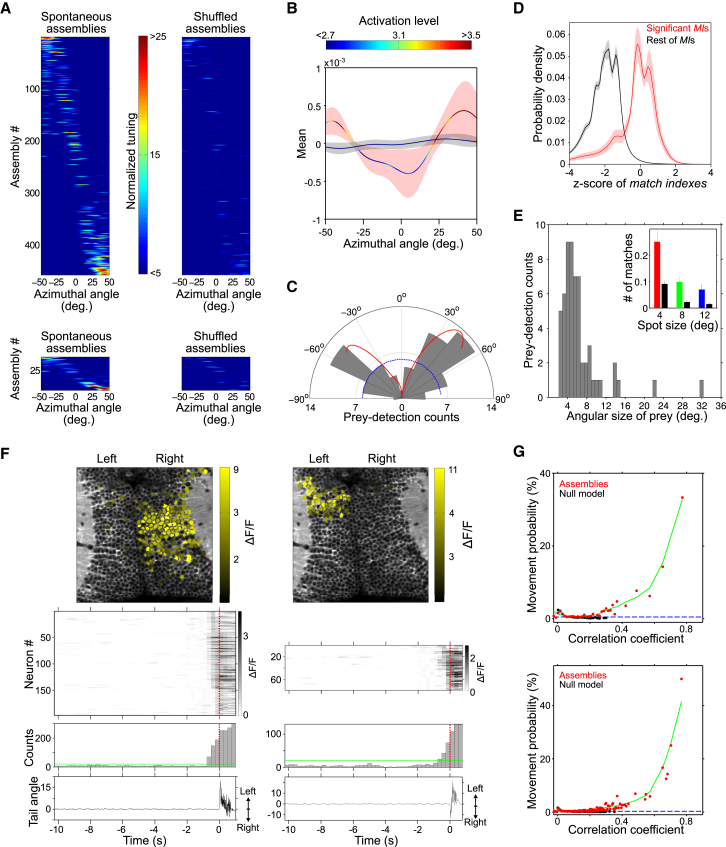
We first studied how the larva’s visual field is represented by the tectal spontaneous assemblies. For this, we estimated the receptive fields of the assemblies (the normalized sum of the spatial tuning curves of all the neurons within an assembly). To correct for uneven tectal representations of the visual field, we further divided the spontaneous assemblies’ receptive fields by the average receptive field of shuffled null models, resulting in normalized assembly receptive fields independent of any potential sampling bias in the data (i.e., if a particular visual field position is represented by a considerably larger neuronal population than other positions). After this correction, we observed that spontaneous assemblies representing lateral position of the visual field had significantly narrower spatial tuning curves and showed significantly higher activation levels (AL, the overall integral of the MI) than those representing rostral positions (Figures 7A and 7B).
Figure 7.
Behavioral Correlate of the Tectum’s Spontaneous Neuronal Assemblies
(A) Normalized azimuthal tuning of all spontaneous assemblies from all experiments (top left, n = 9) and for a typical experiment (bottom left). Same analysis for the null model runs (right). Color scale bar, normalized azimuthal tuning.
(B) Average across experiments of the mean normalized azimuthal tuning of spontaneous assemblies (curve with red confidence interval) and the null model runs (curve with black confidence interval). Confidence intervals, SD. Curves are color-coded according to the average activation levels for each azimuth angle (top color bar). Note the clear over-representation of lateral positions in both spatial tuning and activation.
(C) Polar plot of the relative angle between the larva and the paramecia at the detection instant preceding a prey-capture behavior (gray bars, n = 60). Negative angles: right visual field. Blue dashed line indicates homogeneous angular performance. Red curve: mean normalized azimuthal tuning of spontaneous assemblies as in (B), positioned and rescaled to have the same mean and range as the gray bars to allow comparison. Note the similar trend between the behavioral and spontaneous activity preferences.
(D) Probability density distributions of the significant (red) and non-significant (black) MIs between spontaneous assembly patterns and the 4°-light-spot average response patterns, Z scored with respect to the MIs across the corresponding stimulation trials. Confidence intervals: jackknife procedure. 89% of the significant MIs between stimulated groups and spontaneous assemblies lay between Z score values of −2 and 2, suggesting match levels comparable to the ones obtained across trials of the concerned light-spot stimulations.
(E) Histogram of the angular size of the prey (paramecia) on the larva’s retina at the moment of detection. Note the peak around ∼4°–5°. Inset: the number of spontaneous assemblies that significantly matched (as measured by MI) neuronal groups induced by light spots of 4° (red), 8° (green), and 12° (blue) per stimulated neuronal group, per experiment. The number of significant matches to spontaneous assemblies’ topographical null models are shown in black. Note the better match for 4° light spots with respect to the larger light spots.
(F) Two examples of spontaneous activation of a topographically compact tectal assembly before a spontaneous tail movement. Bottom to top: tail angle trace (zero, a straight tail; positive, left bends; negative, right bends), histogram of the spontaneously active tectal neurons (green line, threshold for significant population events; red dotted line, tail-flip onset), raster plot of spontaneous activations of tectal neurons active before the movement’s onset (red dotted line, tail-flip onset), and topography of the spontaneously activated assembly. Time is relative to tail-flip onset. Note that neurons activated immediately before movement onset are located in the tectal hemisphere contralateral to the initial tail movement direction.
(G) Probability of a tail movement onset as a function of the correlation between all the assembly patterns and the spontaneous tectal network activity for the imaging frame preceding movement onset (red dots, raw data; green curve, regression fit). Black dots and curve: same calculation for randomized assemblies (null models). Dashed blue line: average probability of a movement onset at any given imaging frame. Top, typical experiment; bottom, pooled experiments (n = 5).
To assess this spontaneous lateral over-representation of the visual field with respect to the larva’s behavior, we monitored larva behavior during prey-capture assays to evaluate their visual detection capabilities. Zebrafish larva’s hunting behavior is mostly driven by vision and dependent on the tectum (Gahtan et al., 2005). It begins by detection of the prey followed by a sequence of discrete locomotor maneuvers, some of which are uniquely performed during hunting (Bianco et al., 2011; Gahtan et al., 2005; McElligott and O’malley, 2005). We filmed the behavior of zebrafish larvae (n = 60) in the presence of low-density paramecia using a high-speed video camera (200 Hz). We defined the instant of prey detection as the video frame preceding the onset of a prey-capture behavior. For all confirmed prey-capture behaviors, we measured the zebrafish-paramecium distances and relative angles at the moment of paramecium detection. The distribution of these relative angles showed a strong bias toward lateral positions of the visual field (Figure 7C). Remarkably, this larva’s tendency to better detect paramecia at lateral angles matched the spontaneous assemblies’ over-representation of the lateral visual field (Figure 7C).
We then asked if there was also a parallel between the angular tuning of spontaneous assemblies and the angular size of paramecia on the larva’s retina at the moment of detection. From visually induced responses, we observed that light spots of 4° induced the activation of more compact and spatially constrained assemblies than those of 8° and 12° (Figure S5B; compactness indexes for 4°: 0.57 ± 0.18, 8°: 0.42 ± 0.15, 12°: 0.43 ± 0.17; 4° versus 8°: p < 10−5, 4° versus 12°: p < 10−5). Moreover, spontaneous assemblies better and more often matched neuronal groups activated by light spots of 4° compared to those induced by 8° and 12° light spots, as measured by activation density maps (Figure S5D; correlation between density maps of stimulated neuronal groups and their best-matching spontaneous assembly: 4°: 0.80 ± 0.13, 8°: 0.65 ± 0.18, 12°: 0.62 ± 0.19; 4° versus 8°: p < 10−5, 4° versus 12°: p < 10−5). This was also true when we quantified this similarity by the neuron-to-neuron topographical overlap between the visually induced neuronal groups and the spontaneous assemblies’ patterns (inset in Figure 7E; as quantified by the matching index for the topographic overlap between these patterns, number of significant spontaneous MIs per stimulated neuronal group, per experiment, 4°: 0.25 ± 0.04, 8°: 0.09 ± 0.02; 12°: 0.07 ± 0.02; 4° versus 8°: p = 0.003, 4° versus 12°: p = 10−5). Importantly, the significant pattern matches between spontaneous assemblies and neuronal groups induced by 4° light spots were comparable to those representing the inter-trial variability of the induced neuronal responses (Figure 7D). This suggests that the spontaneous assemblies were indistinguishable from the single-trial response patterns induced by 4° light spots. Remarkably, when we determined the angular size of paramecia on the larva’s retina, at the moment of paramecia detection (Bianco et al., 2011; McElligott and O’malley, 2005), we observed that larvae most frequently initiated a prey-capture behavior when paramecia were at a distance corresponding to ∼4°–5° on the larva’s retina (Figure 7E).
Finally, we asked whether the spontaneous activation of a tectal assembly can be related to the generation of a behavioral output. For this purpose, we monitored spontaneous tectal activities in head-restrained larvae so we could also monitor the spontaneous tail motor behaviors. Under this condition, larvae produced brief isolated episodes of tail movements (< 300 ms; 40 ± 14 episodes per hr). Remarkably, we observed that 11% of these spontaneous movement episodes (22 out of 199, 4.4 episodes per hr; n = 5) were immediately preceded by the spontaneous activation of a topographically compact tectal assembly (Figures 7F and S7G; compactness index = 0.74 ± 0.1). As a control, we used tectal spontaneous activations to predict the onset of tail movements of other larvae. Using this method, we found that only 0.2% of the movement episodes were preceded by compact tectal assemblies. Given the frequency of spontaneous assembly activations and tail movements, the probability of predicting 11% of the episodes by chance is in the order of 10−20. Notably, when the activation of a spontaneous assembly preceded a tail flip with kinematics that indicated a turning movement (14 out of 22), in 93% of the cases the assembly corresponded to the contralateral tectal hemisphere (Figures 7F and S7G; 13 out of 14, 7 to each side; p = 9 × 10−4). We then calculated the probability of a tail movement onset during the imaging frame i as a function of the correlation between the tectal activity pattern at frame i-1 and the patterns of all the spontaneous neuronal assemblies found. The latter correlation indicates how well the pattern of tectal activity preceding a tail flip matches the topographies of a spontaneous neuronal assembly. We observed a sharp step-like increase of this probability as the spontaneous tectal activity gradually matched the topography of a spontaneous assembly (Figure 7G).
In summary, these results indicate a behavioral correlate of spontaneous tectal assemblies and that these assemblies are particularly tuned to relevant visual information, paralleling behavioral performance observed in a vital tectum-dependent behavior (prey-tracking).
Discussion
Contemporary theories of sensory perception regard the brain not as a stimulus-driven processor, but rather as an active system with rich intrinsic dynamics that interacts with environmental sensory information and the organism’s internal states (Engel et al., 2001; Fiser et al., 2004; Harris, 2005; Ringach, 2009). Structured spontaneous brain activity in sensory brain areas is seen as a reflection of this phenomenon, and thus it should reveal features about the neuronal circuits’ functional role.
We report, at the single-neuron level, that tectal spontaneous activity is significantly correlated (Figures 3A and 3B) and spatiotemporally organized in topographically compact assemblies of neurons with matching receptive fields (Figure 4). Although it might be expected that this result could reflect the combination of functional topography and the well-known tendency of nearby neurons to show higher spontaneous correlations (Kerr et al., 2007; Smith and Kohn, 2008), we further demonstrated that these assemblies do not simply assemble neighboring neurons, but showed significant specificity for functionally similar neurons (Figure 4I), resulting in coherently tuned subnetworks. Hence, these spontaneously engaging tectal assemblies are true functional neuronal assemblies.
We further assessed the neuronal interactions underlying the emergence of structured spontaneous activity. Remarkably, tectal assemblies are not necessarily driven by common retinal inputs (Figure 5) and might exclusively rely on tectal recurrent connectivity (Deco et al., 2011; Ringach, 2009), although we cannot exclude the involvement of other brain sources. We observed all-or-nothing-like synergistic facilitation between assembly neurons, suited for their efficient and robust coordinated recruitment (Figures 6A and 6B). Moreover, we found evidence for lateral competitive dynamics between visually evoked neuronal groups (Figures 6C–6E), suggesting that biased reciprocal inhibitions between neighboring assemblies may shape their compact topography. Therefore, we expect that under weak external drive, these tectal dynamic principles will spontaneously generate structured activation patterns out of noise. This scenario is analogous to the heuristic optimization algorithm simulated annealing, where random small fluctuations drive stochastic exploration of an “energy landscape” (the tectum’s dynamic repertoire), revealing stable system states (the neuronal assemblies). These states would represent robust neuronal circuit modes that may be dynamically recruited during sensory-induced brain responses (Destexhe, 2011; MacLean et al., 2005; Ringach, 2009), avoiding less reliable intermediate states (Marre et al., 2009). This points to spontaneous assemblies as an unavoidable consequence of the optic tectum’s functional role.
In the same line, spontaneous assemblies matched visual representations corresponding to angular sizes (4°) found to initiate a prey-capture behavior (Bianco et al., 2011; McElligott and O’malley, 2005) (Figures 7D and 7E). This could reflect the tectum’s functional strategy for reducing the high dimensionality of the external world by the formation of tectal functional sub-networks, whose visual resolving capability is suited to extract information of vital importance to the organism. Indeed, the tectum is involved in behaviors that require the visual detection of small objects by means of an identified neuronal circuit capable of filtering large visual stimuli, making it most responsive to 4° stimuli (Del Bene et al., 2010). The filtered visual inputs would favor the tectal representation for angular sizes of 4° through experience-dependent plasticity, explaining their preponderance in spontaneous activity. In the same line, the population of spontaneous tectal assemblies showed an over-representation for lateral regions of the visual field (Figure 7B), which matched the observed behavioral tendency in visual detection performance (Figure 7C). These observations suggest that the structure of the tectal spontaneous activity showed features also observed at the behavioral level, stressing its biological significance. Furthermore, we showed that the activation of a spontaneous tectal assembly significantly increased the probability of a subsequent spontaneous tail movement (Figure 7G), demonstrating a behavioral correlate of patterned spontaneous activity in a sensory brain region. Spontaneous behaviors were preceded by compact tectal assemblies in only 11% of the cases. We thus interpret these events as a reflection of the tectal functional connectivity adapted for robust visual detection of biologically relevant features and the generation of orienting motor commands.
Neuronal clusters showing spontaneously correlated activity do not necessarily imply neuronal interconnectivity, but could reflect functional processing modules (Harris, 2005). Our results indicate that tectal spontaneous spatiotemporal activity patterns represent robust “preferred” network states compatible with tectal functional principles, adapted for robust visual detection, associated in some cases with orienting motor behaviors. This suggests that structured tectal spontaneous activity emerges from neuronal mechanisms suited for the tectum’s role in vital behaviors such as prey tracking in low-contrast, crowded, cluttered natural environments.
Alternatively to this scenario, the compact neuronal assemblies could represent active top-down cognitive process (Engel et al., 2001; Ringach, 2009) (e.g., spatial attention or expectations), while the sparse ones may reflect global network modulations induced by the cholinergic or serotonergic systems.
Experimental Procedures
Animals
Experiments were performed in 8 days post-fertilization (dpf) larvae of a transgenic Huc:GCaMP3 Nacre line. All experiments were approved by Le Comité d’Éthique pour l’Expérimentation Animale Charles Darwin (Ce5/2009/027).
In Vivo Calcium Imaging
Larvae were restrained in 1.8% low-melting agarose and placed within a cylindrical chamber filled with E3 embryo medium (Figure 1A). Calcium dynamics were monitored using a custom-built two-photon microscope with a 25× NA1.05 objective and a Ti:sapphire laser tuned at 920 nm. Scanning was performed at 3.91 Hz on ∼200 μm × 200 μm optic tectum planes at different depths. When simultaneously monitoring behavior, we filmed tail movements of head-restrained larva illuminated with an 820 nm LED, using a mini microscope connected to a high-speed camera (200 Hz) with a FF01-842 Semrock short-pass filter. For synchronization of video recordings, visual stimuli, and two-photon imaging, we used a I/O TTL board.
Visual Stimulation
Visual stimuli covered a field of view of ∼90° × 90°. Light-bar stimuli were 4° wide and moved in the four cardinal directions at 45°/s. Light spots lasted for 1 s. They were presented at 19 different positions, from −45° to 45° in steps of 5°. We performed four trials for each stimulation, and the inter-trial time interval was 7 s. Stimulation order was randomized.
Analysis of Neuronal Visual Responses
For each stimulation, we averaged across trials the neuronal responses within a 2 s time window after the trial onsets and only considered neurons that responded in at least 50% of the trials. Neuronal spatial tuning curves were estimated by the normalized sum of 10°-wide Gaussian curves centered at the 4°-light-spot azimuthal positions. Neuronal direction selectivity was the difference of the average neuronal responses to bars moving in opposite cardinal directions, divided by their sum. The direction selectivity vector of each neuron was the vectorial sum of their up-down and left-right selectivities.
Detection of Neuronal Assemblies
The method and its benchmarking are explained in detail in the Supplemental Experimental Procedures. Briefly, we first reduced the dimensionality of the neuronal network activity using principal component analysis (PCA) by only keeping principal components (PCs) with significant eigenvalues (Peyrache et al., 2010). To determine the neuronal composition of the assemblies, we worked on a space of obliquely rotated components (Lopes-dos-Santos et al., 2011), which tended to group the PC loadings along non-perpendicular rotated PCs (rPCs). Neurons were assigned to a given assembly defined by a rPC if their loadings on that rPC exceeded a data-driven threshold value.
Matching Index
The MI quantifies the proportion of neuronal activations that are common to two given network activation patterns, with respect to the total number of activations present in both patterns. It is defined as
where both Pati and Patj are binary network activation patterns (binary vectors representing the imaged neuronal population, with ones indicating active neurons and zeros for the silent ones).
Author Contributions
S.A.R. carried out the imaging experiments, analyzed the data, and built the setup and software. T.P. generated the elav3:GCaMP3 transgenic line, participated in discussions about the data, and performed some eye enucleations. V.P.-S., A.J., and G.S. built the setup. M.H. carried out the behavioral assays. V.P.-S. and A.J. developed software. S.A.R. and G.S. conceived the experiments and wrote the manuscript.
Acknowledgments
We thank M.-m. Poo, B. Barbour, Y. Frégnac, G. de Polavieja, and Y. Loewenstein for comments on the manuscript and E. Schenidman, J. Boulanger-Weill, and M.S. Murmu for helpful discussions. This work was supported by EraSysBio+ Zebrain, ERC stg 243106, ANR-10-LABX-54 MEMO LIFE, ANR-11-IDEX-0001-02 PSL∗ Research University, and Avenir grant INSERM.
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information
References
- Ahrens M.B., Li J.M., Orger M.B., Robson D.N., Schier A.F., Engert F., Portugues R. Brain-wide neuronal dynamics during motor adaptation in zebrafish. Nature. 2012;485:471–477. doi: 10.1038/nature11057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arieli A., Sterkin A., Grinvald A., Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- Azouz R., Gray C.M. Cellular mechanisms contributing to response variability of cortical neurons in vivo. J. Neurosci. 1999;19:2209–2223. doi: 10.1523/JNEUROSCI.19-06-02209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkes P., Orbán G., Lengyel M., Fiser J. Spontaneous cortical activity reveals hallmarks of an optimal internal model of the environment. Science. 2011;331:83–87. doi: 10.1126/science.1195870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco I.H., Kampff A.R., Engert F. Prey capture behavior evoked by simple visual stimuli in larval zebrafish. Front. Syst. Neurosci. 2011;5:101. doi: 10.3389/fnsys.2011.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrill J.D., Easter S.S., Jr. Development of the retinofugal projections in the embryonic and larval zebrafish (Brachydanio rerio) J. Comp. Neurol. 1994;346:583–600. doi: 10.1002/cne.903460410. [DOI] [PubMed] [Google Scholar]
- Carr M.F., Jadhav S.P., Frank L.M. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat. Neurosci. 2011;14:147–153. doi: 10.1038/nn.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G., Jirsa V.K., McIntosh A.R. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat. Rev. Neurosci. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Deco G., Jirsa V.K., McIntosh A.R. Resting brains never rest: computational insights into potential cognitive architectures. Trends Neurosci. 2013;36:268–274. doi: 10.1016/j.tins.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Del Bene F., Wyart C., Robles E., Tran A., Looger L., Scott E.K., Isacoff E.Y., Baier H. Filtering of visual information in the tectum by an identified neural circuit. Science. 2010;330:669–673. doi: 10.1126/science.1192949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A. Intracellular and computational evidence for a dominant role of internal network activity in cortical computations. Curr. Opin. Neurobiol. 2011;21:717–725. doi: 10.1016/j.conb.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Engel A.K., Fries P., Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat. Rev. Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Faisal A.A., Selen L.P.J., Wolpert D.M. Noise in the nervous system. Nat. Rev. Neurosci. 2008;9:292–303. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiser J., Chiu C., Weliky M. Small modulation of ongoing cortical dynamics by sensory input during natural vision. Nature. 2004;431:573–578. doi: 10.1038/nature02907. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahtan E., Tanger P., Baier H. Visual prey capture in larval zebrafish is controlled by identified reticulospinal neurons downstream of the tectum. J. Neurosci. 2005;25:9294–9303. doi: 10.1523/JNEUROSCI.2678-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt C., Baier H., Del Bene F. Direction selectivity in the visual system of the zebrafish larva. Front Neural Circuits. 2013;7:111. doi: 10.3389/fncir.2013.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J.A., Rokni U., Sompolinsky H. Patterns of ongoing activity and the functional architecture of the primary visual cortex. Neuron. 2004;42:489–500. doi: 10.1016/s0896-6273(04)00197-7. [DOI] [PubMed] [Google Scholar]
- Han F., Caporale N., Dan Y. Reverberation of recent visual experience in spontaneous cortical waves. Neuron. 2008;60:321–327. doi: 10.1016/j.neuron.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K.D. Neural signatures of cell assembly organization. Nat. Rev. Neurosci. 2005;6:399–407. doi: 10.1038/nrn1669. [DOI] [PubMed] [Google Scholar]
- Harris K.D., Thiele A. Cortical state and attention. Nat. Rev. Neurosci. 2011;12:509–523. doi: 10.1038/nrn3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgetag C., Kötter R., Stephan K., Sporns O. Computational methods for the analysis of brain connectivity. In: Ascoli G., editor. Computational Neuroanatomy–Principles and Methods. Humana Press; Totowa: 2002. pp. 295–335. [Google Scholar]
- Kenet T., Bibitchkov D., Tsodyks M., Grinvald A., Arieli A. Spontaneously emerging cortical representations of visual attributes. Nature. 2003;425:954–956. doi: 10.1038/nature02078. [DOI] [PubMed] [Google Scholar]
- Kerr J.N.D., de Kock C.P.J., Greenberg D.S., Bruno R.M., Sakmann B., Helmchen F. Spatial organization of neuronal population responses in layer 2/3 of rat barrel cortex. J. Neurosci. 2007;27:13316–13328. doi: 10.1523/JNEUROSCI.2210-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzlis R.J., Lovejoy L.P., Zénon A. Superior colliculus and visual spatial attention. Annu. Rev. Neurosci. 2013;36:165–182. doi: 10.1146/annurev-neuro-062012-170249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes-dos-Santos V., Conde-Ocazionez S., Nicolelis M.A.L., Ribeiro S.T., Tort A.B.L. Neuronal assembly detection and cell membership specification by principal component analysis. PLoS ONE. 2011;6:e20996. doi: 10.1371/journal.pone.0020996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak A., Barthó P., Harris K.D. Spontaneous events outline the realm of possible sensory responses in neocortical populations. Neuron. 2009;62:413–425. doi: 10.1016/j.neuron.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak A., Bartho P., Harris K.D. Gating of sensory input by spontaneous cortical activity. J. Neurosci. 2013;33:1684–1695. doi: 10.1523/JNEUROSCI.2928-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean J.N., Watson B.O., Aaron G.B., Yuste R. Internal dynamics determine the cortical response to thalamic stimulation. Neuron. 2005;48:811–823. doi: 10.1016/j.neuron.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Marre O., Yger P., Davison A.P., Frégnac Y. Reliable recall of spontaneous activity patterns in cortical networks. J. Neurosci. 2009;29:14596–14606. doi: 10.1523/JNEUROSCI.0753-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott M.B., O’malley D.M. Prey tracking by larval zebrafish: axial kinematics and visual control. Brain Behav. Evol. 2005;66:177–196. doi: 10.1159/000087158. [DOI] [PubMed] [Google Scholar]
- Mohajerani M.H., Chan A.W., Mohsenvand M., LeDue J., Liu R., McVea D.A., Boyd J.D., Wang Y.T., Reimers M., Murphy T.H. Spontaneous cortical activity alternates between motifs defined by regional axonal projections. Nat. Neurosci. 2013;16:1426–1435. doi: 10.1038/nn.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell C.M., Smith S.J. Functional imaging reveals rapid development of visual response properties in the zebrafish tectum. Neuron. 2005;45:941–951. doi: 10.1016/j.neuron.2005.01.047. [DOI] [PubMed] [Google Scholar]
- Orger M.B., Kampff A.R., Severi K.E., Bollmann J.H., Engert F. Control of visually guided behavior by distinct populations of spinal projection neurons. Nat. Neurosci. 2008;11:327–333. doi: 10.1038/nn2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C.C.H., Hahn T.T.G., Mehta M., Grinvald A., Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc. Natl. Acad. Sci. USA. 2003;100:13638–13643. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrache A., Benchenane K., Khamassi M., Wiener S.I., Battaglia F.P. Principal component analysis of ensemble recordings reveals cell assemblies at high temporal resolution. J. Comput. Neurosci. 2010;29:309–325. doi: 10.1007/s10827-009-0154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringach D.L. Spontaneous and driven cortical activity: implications for computation. Curr. Opin. Neurobiol. 2009;19:439–444. doi: 10.1016/j.conb.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen M.N., Newsome W.T. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J. Neurosci. 1998;18:3870–3896. doi: 10.1523/JNEUROSCI.18-10-03870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.A., Kohn A. Spatial and temporal scales of neuronal correlation in primary visual cortex. J. Neurosci. 2008;28:12591–12603. doi: 10.1523/JNEUROSCI.2929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumbre G., Muto A., Baier H., Poo M.M. Entrained rhythmic activities of neuronal ensembles as perceptual memory of time interval. Nature. 2008;456:102–106. doi: 10.1038/nature07351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., Hires S.A., Mao T., Huber D., Chiappe M.E., Chalasani S.H., Petreanu L., Akerboom J., McKinney S.A., Schreiter E.R. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Wang G.J., Volkow N.D. Energetic cost of brain functional connectivity. Proc. Natl. Acad. Sci. USA. 2013;110:13642–13647. doi: 10.1073/pnas.1303346110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman M. Noise and Weber’s law: the discrimination of brightness and other dimensions. Psychol. Rev. 1964;71:314–330. doi: 10.1037/h0042445. [DOI] [PubMed] [Google Scholar]
- Tsodyks M., Kenet T., Grinvald A., Arieli A. Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science. 1999;286:1943–1946. doi: 10.1126/science.286.5446.1943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.