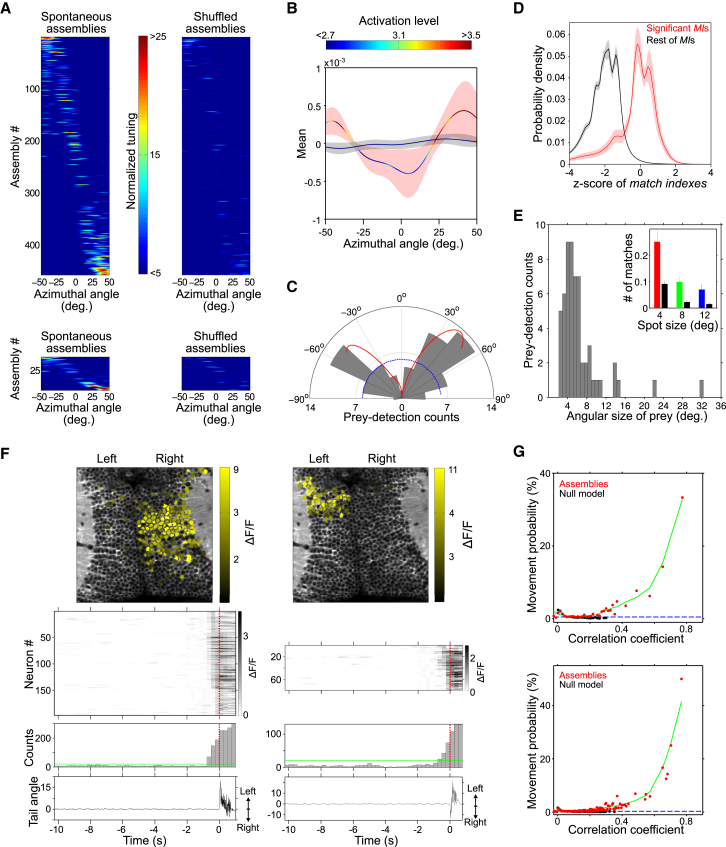
Figure 7.
Behavioral Correlate of the Tectum’s Spontaneous Neuronal Assemblies
(A) Normalized azimuthal tuning of all spontaneous assemblies from all experiments (top left, n = 9) and for a typical experiment (bottom left). Same analysis for the null model runs (right). Color scale bar, normalized azimuthal tuning.
(B) Average across experiments of the mean normalized azimuthal tuning of spontaneous assemblies (curve with red confidence interval) and the null model runs (curve with black confidence interval). Confidence intervals, SD. Curves are color-coded according to the average activation levels for each azimuth angle (top color bar). Note the clear over-representation of lateral positions in both spatial tuning and activation.
(C) Polar plot of the relative angle between the larva and the paramecia at the detection instant preceding a prey-capture behavior (gray bars, n = 60). Negative angles: right visual field. Blue dashed line indicates homogeneous angular performance. Red curve: mean normalized azimuthal tuning of spontaneous assemblies as in (B), positioned and rescaled to have the same mean and range as the gray bars to allow comparison. Note the similar trend between the behavioral and spontaneous activity preferences.
(D) Probability density distributions of the significant (red) and non-significant (black) MIs between spontaneous assembly patterns and the 4°-light-spot average response patterns, Z scored with respect to the MIs across the corresponding stimulation trials. Confidence intervals: jackknife procedure. 89% of the significant MIs between stimulated groups and spontaneous assemblies lay between Z score values of −2 and 2, suggesting match levels comparable to the ones obtained across trials of the concerned light-spot stimulations.
(E) Histogram of the angular size of the prey (paramecia) on the larva’s retina at the moment of detection. Note the peak around ∼4°–5°. Inset: the number of spontaneous assemblies that significantly matched (as measured by MI) neuronal groups induced by light spots of 4° (red), 8° (green), and 12° (blue) per stimulated neuronal group, per experiment. The number of significant matches to spontaneous assemblies’ topographical null models are shown in black. Note the better match for 4° light spots with respect to the larger light spots.
(F) Two examples of spontaneous activation of a topographically compact tectal assembly before a spontaneous tail movement. Bottom to top: tail angle trace (zero, a straight tail; positive, left bends; negative, right bends), histogram of the spontaneously active tectal neurons (green line, threshold for significant population events; red dotted line, tail-flip onset), raster plot of spontaneous activations of tectal neurons active before the movement’s onset (red dotted line, tail-flip onset), and topography of the spontaneously activated assembly. Time is relative to tail-flip onset. Note that neurons activated immediately before movement onset are located in the tectal hemisphere contralateral to the initial tail movement direction.
(G) Probability of a tail movement onset as a function of the correlation between all the assembly patterns and the spontaneous tectal network activity for the imaging frame preceding movement onset (red dots, raw data; green curve, regression fit). Black dots and curve: same calculation for randomized assemblies (null models). Dashed blue line: average probability of a movement onset at any given imaging frame. Top, typical experiment; bottom, pooled experiments (n = 5).