Figure 2.
P1aABD Associates Preferentially with the N-ter Lobe of CaM
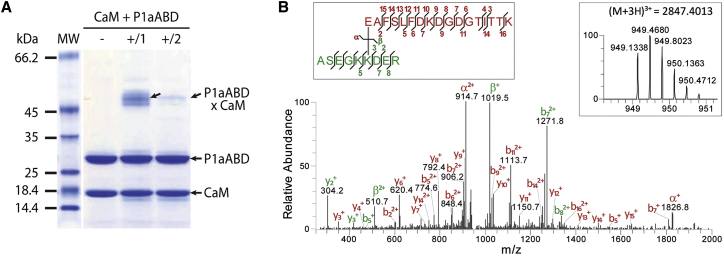
(A) SDS-PAGE analysis of the P1aABD/CaM complex crosslinked by EDC and sulfo-NHS. Proteins were incubated in the binding buffer either in the absence of crosslinker (-), or subjected to EDC and sulfo-NHS in a one-step (+/1) or two-step (+/2) reaction (for details see Supplemental Materials and Methods). Complexes of crosslinked P1aABD and CaM are readily visible as a higher-molecular weight band (arrow).
(B) MS/MS spectrum identifying a pair of crosslinked peptides after in-gel proteolysis of the complex band obtained by two-step crosslinking of CaM and P1aABD. Fragment ions are annotated for the α-peptide (red) and the β-peptide (green). Insets show mapping of the fragment ions onto the crosslinked peptide sequences (left) and the corresponding high-resolution MS spectrum displaying the isotopic distribution of the crosslinking product (right).
see also Table S1.