Abstract
Ectromelia virus (ECTV) causes mousepox in mice, a disease very similar to smallpox in humans. ECTV and variola virus (VARV), the agent of smallpox, are closely related orthopoxviruses. Mousepox is an excellent small animal model to study the genetic and immunologic basis for resistance and susceptibility of humans to smallpox. Resistance to mousepox is dependent on a strong polarized type 1 immune response, associated with robust natural killer (NK) cell, cytotoxic T lymphocyte (CTL) and gamma interferon (IFN-γ) responses. In contrast, ECTV-susceptible mice generate a type 2 response, associated with weak NK cell, CTL and IFN-γ responses but robust IL-4 responses. Nonetheless, susceptible strains infected with mutant ECTV lacking virus-encoded IFN-γ binding protein (vIFN-γbp) (ECTV-IFN-γbpΔ) control virus replication through generation of type 1 response. Since the IL-4/IL-13/STAT-6 signaling pathways polarize type 2/T helper 2 (Th2) responses with a corresponding suppression of IFN-γ production, we investigated whether the combined absence of vIFN-γbp, and one or more host genes involved in Th2 response development, influence generation of protective immunity. Most mutant mouse strains infected with wild-type (WT) virus succumbed to disease more rapidly than WT animals. Conversely, the disease outcome was significantly improved in WT mice infected with ECTV-IFN-γbpΔ but absence of IL-4/IL-13/STAT-6 signaling pathways did not provide any added advantage. Deficiency in IL-13 or STAT-6 resulted in defective CTL responses, higher mortality rates and accelerated deaths. Deficiencies in IL-4/IL-13/STAT-6 signaling pathways significantly reduced the numbers of IFN-γ producing CD4 and CD8 T cells, indicating an absence of a switch to a Th1-like response. Factors contributing to susceptibility or resistance to mousepox are far more complex than a balance between Th1 and Th2 responses.
Introduction
Smallpox was one of the biggest human scourges when it was rife, with mortality rates of 30–40%. VARV is an orthopoxvirus (OPV) that causes smallpox, a disease that has killed more humans than all other infectious diseases combined [1–4]. As the disease was eradicated more than 30 years ago, animal models of OPV infections have been used to establish strong causal relationships and map the requirements for protection against infection in a way that is not possible in humans. ECTV is a natural pathogen of mice that causes a generalized infection known as mousepox, an excellent small animal model to study the pathogenesis of smallpox [4–7]. Both VARV and ECTV cause acute infections in their respective hosts, which either succumb to infection or clear the virus with complete recovery.
Resistance to mousepox is dependent on rapid generation of a strong polarized type 1 immune response [8]. ECTV-resistant C57BL/6 mice generate robust NK cell, CTL, and IFN-γ responses whereas in ECTV-susceptible BALB/c mice these responses are sub-optimal but high levels of IL-4 is produced. Loss of IFN-γ function in resistant mice [9,10] or over-expression of IL-4 by recombinant ECTV encoding IL-4, which abrogates IFN-γ production [11,12] results in ineffective NK cell and CTL responses and fulminant disease. The existence of virus-encoded host response modifiers that specifically target type 1 cytokines further underscores the importance of these factors in immunity to poxvirus infections [13–16]. Indeed, BALB/c mice infected with a deletion mutant virus lacking vIFN-γbp (ECTV-IFN-γbpΔ) are able to contain virus replication and overcome the infection [17]. Absence of vIFN-γbp increases IFN-γ production and as a consequence, augments cell-mediated immunity and virus control.
The cytokine milieu and cell subset-selective transcription factors play important roles in the differentiation of uncommitted naive CD4 T cells into Th1 and Th2 subsets following antigenic stimulation [18,19]. IFN-γ and IL-4 play dominant roles in the development of Th1 and Th2 subsets, respectively. IL-4 mediates its activity through the IL-4 receptor (R), which consists of a heterodimeric complex between IL-4Rα and the common γ chain [20,21]. IL-4Rα also associates with the low affinity IL-13Rα1 to form a high affinity receptor for both IL-13 and IL-4. A second IL-13 receptor, IL-13Rα2, which exists in a monomeric form, can bind to IL-13 with high affinity and modulate IL-13 responses [22,23]. Binding of IL-4 to IL-4R activates the transcription factor STAT-6, which in turn controls expression of GATA3, the master transcription regulator required for Th2 differentiation [24]. IL-13 is also capable of activating STAT-6 and shares many of the biological properties of IL-4.
A previous report pointed to a role for STAT-6 signaling and IL-4 production in contributing to susceptibility of BALB/c mice to ECTV infection [25]. STAT-6 deficient mice infected with ECTV-WT were better protected through generation of more effective NK cell and IFN-γ responses that allowed the otherwise susceptible animals to overcome infection. Curiously, IL-4 deficiency in BALB/c mice has no impact on disease outcome [8], implying that other factors like IL-13 may be sufficient to drive Th2-like responses in the absence of IL-4.
Since the generation of protective immunity and the outcome of a viral infection are influenced by both viral and host genes, we hypothesized that infection of gene knockout (GKO) BALB/c mice lacking one or more factors associated with Th2 response development with ECTV-WT or ECTV-IFN-γbpΔ would further augment the Th1 response and influence disease outcome. Contrary to our prediction, the disease was exacerbated in most groups of BALB/c mice lacking one or more components of Th2 signaling pathways compared to WT mice infected with WT virus. Conversely, the disease outcome was significantly improved in WT mice infected with the mutant virus, but absence of the IL-4/IL-13/STAT-6 signaling pathways did not provide any added advantage to the host. In fact, there were higher rates of mortality and accelerated deaths amongst mice deficient in IL-13 or STAT-6 compared with WT animals. Deficiency in IL-13 or STAT-6 dramatically reduced the antiviral CTL response. Furthermore, deficiencies in IL-4/IL-13/STAT-6 signaling pathways significantly reduced the numbers of IFN-γ producing CD4 and CD8 T cells, indicating an absence of a switch to a Th1-like response. Thus, although recovery from ECTV infection is strongly influenced, in part, by a balance between the host’s ability to produce IFN-γ and the virus’ ability to dampen its effects [17], our data indicate that factors contributing to disease susceptibility are far more complex than a balance between Th1 and Th2 responses.
Materials and Methods
Ethics Statement
This study was performed in strict accordance with the recommendations in the Australian code of practice for the care and use of animals for scientific purposes and the Australian National Health and Medical Research Council Guidelines and Policies on Animal Ethics and approved by the Animal Ethics and Experimentation Committee of the Australian National University (Protocol numbers J.IG. 62/08, J.IG. 75.09 and A2012/041).
Mice
Gene knockout (GKO) mice lacking IL-4 [26], IL-13 [27], IL-4Rα [28], STAT-6 [29] or both IL-13 and IL-4Rα were either generated on a BALB/c background or backcrossed to the BALB/cJ strain for at least 10 generations or more. Female BALB/c WT and GKO mice designated IL-4−/−, IL-13−/−, STAT-6−/−, IL-4Rα−/−, IL-13−/−/IL-4Rα−/− and were bred under specific-pathogen-free conditions at the Australian Phenomics Facility, Australian National University and used at 6–8 weeks of age. The IL-4−/− mice can respond to IL-13 whereas IL-13−/− mice have intact IL-4 responses. IL-4Rα−/− mice lack responses to both IL-4 and IL-13 but the IL-13 produced in this strain can be modulated by IL-13Rα2. IL-13−/−/IL-4Rα−/− mice have a complete absence of IL-4 and IL-13 responses. In STAT-6−/− mice, only STAT-6-independent GATA3 expression and responses can occur.
Cell lines and cell cultures
BS-C-1, an African green monkey kidney epithelial cell line, CV-1, an African green monkey kidney fibroblast cell line, P-815 (H-2d), a DBA/2 mouse-derived mastocytoma line and YAC-1 (H-2a), a Moloney murine leukemia virus-induced lymphoma line from A/Sn mouse, were obtained from American Type Culture Collection (Rockville, MD). All cells were maintained in Eagle’s Minimum Essential Medium supplemented with 10% fetal calf serum, 2mM L-glutamine, 120μg/ml penicillin and 200μg/ml streptomycin and neomycin sulfate.
Viruses and infection
WT ECTV-Moscow strain [30], designated ECTV-WT, and a vIFN-γbp deletion mutant virus, designated ECTV-IFN-γbpΔ [17] were used in this study. All ECTV stocks were propagated in BS-C-1 cells and titers determined by viral plaque assay (VPA) [31]. In all experiments, mice were inoculated subcutaneously (s.c.) with 103 PFU of virus in the flank of the left hind limb (hock) under avertin anesthesia. This dose of virus was used in order to make meaningful comparisons with previous studies [8,25].
Synthetic Peptides
ECTV-derived CD8 T cell peptide determinants [32] used in this study are shown in S1 Table. Peptides were synthesized at the Biomolecular Resource Facility, John Curtin School of Medical Research. They were purified via reverse-phase HPLC and the quality checked by mass spectroscopy. Peptides were stored in stock solutions at 10mM in 99.99% dimethyl sulfoxide (Sigma-Aldrich Inc. St. Louis, MO USA) and diluted in culture medium to the required concentration for use in the cellular assays.
NK cell and CTL assays
The standard 51Chromium release assay [8] was used to determine ex vivo anti-ECTV CTL activity, using ECTV-infected and uninfected P815 (H-2d) target cells. YAC-1 cells were used as targets for splenic NK cell cytotoxicity assays. The fold change in cytolytic activity of GKO splenocytes compared with WT splenocytes shown in the results is based on % specific lysis at a given effector: target ratio. As an example, in the data shown on CTL responses, the % specific lysis mediated by IL-13−/−/IL-4Rα−/− CTL at 75:1 effector: ratio is comparable to % specific lysis mediated by WT cells at 25:1 effector: ratio. That is, at a 3-fold lower number of splenocytes, the cytolytic activity of WT cells is comparable to that of IL-13−/−/IL-4Rα−/− cells. Similarly, the % specific lysis mediated by IL-13−/− cells at 75:1 effector: ratio is comparable to % specific lysis mediated by WT cells at 2.8:1 effector: ratio, i.e. a 27-fold lower number of WT splenocytes were required to mediate comparable levels of cytolytic activity mediated by IL-13−/− cells.
Flow cytometry
Total and granzyme B (GzB) expressing NK cells were quantified by flow cytometry. We used anti-CD49b-PE (clone DX5) to stain NK cells and anti-CD49b (DX-5)-PE plus anti-granzyme B-APC (clone MHGB05) to stain GzB expressing NK cells. Cells were also stained with anti-CD3-FITC (clone 17A2) to exclude CD3+ NKT cells in the analysis.
Total ECTV-specific or ECTV determinant-specific IFN-γ producing CD8 T cells were enumerated using intracellular cytokine staining. The protocol for detection of ECTV-specific CD8 T cells has been described previously [17]. Briefly, 106 splenocytes were incubated with either 2x105 virus-infected P815 cells or with synthetic peptides (S1 Table) at final concentrations of 0.1μM (Kd-EVMA5265–73), 1 μM (Ld-EVM02626–34) or 10μM (Dd-EVM043140–148). After 2 hr, 0.1mg/ml brefeldin A was added and cells incubated for a further 3 hr before staining with anti-CD8α-APC (clone 53–6.7; BD Biosciences) and anti-IFN-γ- phycoerythrin (PE) (clone XMG1.2; BD Biosciences). Two hundred thousand total events were acquired on BD LSR Fortessa cell analyzer (BD Biosciences) and analyzed using FlowJo software (Tree Star Inc.). Antigen-specific IFN-γ production was calculated by subtracting the background values obtained with non-specific peptide (Herpes simplex virus-1 gB498–505) stimulation.
For enumeration of IFN-γ or IL-4 producing CD4 T cells, splenocytes from virus-infected mice were incubated at 37°C with media (unstimulated) or stimulated with Phorbol 12-Myristate 13-Acetate (PMA) plus Ionomycin (Calbiochem) (PMA: 0.1μg/ml; Ionomycin: 1μg/ml) for 5h as positive control. Following incubation, cells were stained with anti-CD4 (clone GK1.5), permeabilized for 20 min followed by intracellular staining with anti-IL-4 (clone 11B11) or anti-IFN-γ (clone XMG1.2) in staining buffer containing 0.1% saponin. Total events for cells were acquired using a BD LSR Fortessa cell analyzer and analyzed using FlowJo software (Tree Star Inc.). Antibodies were purchased from BD Biosciences, Biolegend and Invitrogen.
To estimate numbers of T-bet- or GATA-3-expressing CD4 T cells, 2 x106 spleen cells were stained without re-stimulation with anti-CD3 and anti-CD4 in a 50μl volume for 30 mins at 4°C. The cells were then washed and re-suspended in 100μl of freshly prepared fix/perm buffer (FoxP3 transcription factor staining buffer kit, e-Bioscience) and incubated for 30 mins at 4°C. The cells were then washed twice with permeablization buffer and then incubated for 40 mins at 4°C with anti-GATA3 (Clone L50–823; BD Biosciences) and anti-T-bet (Clone 4B10, Biolegend) antibodies. The cells were washed twice with permeablization buffer and resuspended in buffer just prior to analysis on BD LSR Fortessa cell analyzer.
Cell numbers in experiments were determined by multiplying the percentage of cells positive for GzmB, IFN-γ (S1, S2, S3 Figs.), IL-4 (S4 Fig.), T-bet or GATA-3 by the total number of spleen cells obtained from each mouse.
Determination of virus titers in tissues and blood
Organ tissue and blood were removed aseptically from infected mice and stored at −80°C until processed. Virus titers, expressed as log10 PFU/gram tissue or log10 PFU/100μl blood, were determined on BS-C-1 monolayers using the conventional VPA, as described previously [9].
Statistical analysis
Statistical analyses of experimental data, employing parametric and nonparametric tests as indicated in figure legends, were performed using GraphPad Prism (GraphPad Software, San Diego, USA). The Kaplan-Meier Log rank test was used to determine p values for survival proportions. Two-way ANOVA followed by Fisher’s Least Significant Difference (LSD) test was used to determine significance for viral load, NK, CD8 and CD4 T cell responses.
Results
Deficiencies in Th2 cytokine signaling exacerbate ECTV infection
Groups of WT and GKO mice were infected with ECTV-WT or ECTV-IFN-γbpΔ and monitored for 21 days to determine whether the absence or presence of vIFN-γbp during ECTV infection in BALB/c mice lacking IL-4, IL-13, IL-4Rα, STAT-6 or both IL-13 and IL-4Rα may result in better recovery rates. We compared the outcome of infection with the 2 viruses in individual mouse strains (Fig. 1), ECTV-WT infection in WT versus individual GKO mouse strains (S5 Fig.) and ECTV-IFN-γbpΔ infection of WT versus individual GKO mouse strains (S6 Fig.). The detailed statistical analysis is presented in S2 Table.
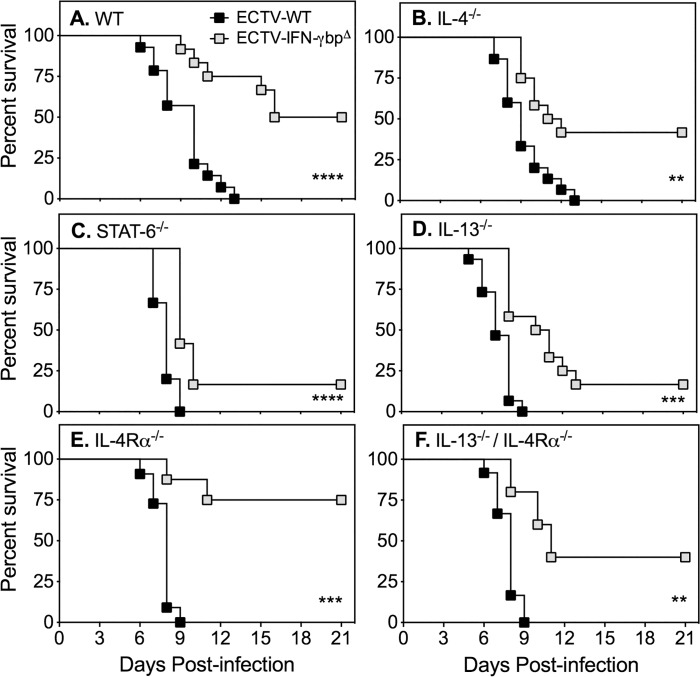
Fig 1. Response of WT and GKO BALB/c strains to infection with ECTV-WT or ECTV-IFN-γbpΔ.
Groups of female mice were infected with ECTV-WT or ECTV-IFN-γbpΔ. Mice were monitored daily for 21 days for disease signs. Data shown are combined results obtained from two separate experiments in which 5–15 mice per strain were used (S2 Table). P values for survival proportions were obtained by using Kaplan-Meier Log rank statistical test: **, p < 0.01; ***, p < 0.001; ****, p < 0.0001. The survival rates of WT mice compared to GKO mice infected with WT or mutant virus are presented in S5 and S6 Figs., respectively and the statistical analysis presented in S2 Table.
Regardless of genotype, all groups of mice infected with ECTV-WT succumbed to mousepox with 100% mortality (Fig. 1, S5 Fig., S2 Table). Notably, most GKO strains succumbed more rapidly than WT mice to ECTV-WT infection. Absence of one or more factors associated with Th2 response development did not provide any added advantage to hosts infected with WT virus. In contrast, all groups of mice infected with ECTV-IFN-γbpΔ had better rates of recovery compared with ECTV-WT infection (Fig. 1, S6 Fig., S2 Table). However, even despite the improved recovery rates compared with WT virus infection, some GKO strains infected with mutant virus succumbed to mousepox significantly more rapidly than WT mice. In particular, mice deficient in IL-13 or STAT-6 had the lowest recovery rates and succumbed to disease more rapidly than WT mice infected with ECTV-IFN-γbpΔ. These experiments established that absence of vIFN-γbp significantly assisted in the recovery of WT mice from ECTV infection but did not improve recovery of the GKO strains. They also revealed that absence of IL-13, IL-4Rα, STAT-6 or both IL-13 and IL-4Rα increased susceptibility of mice to ECTV-WT infection.
Lack of consistent correlation between increased susceptibility and viral load
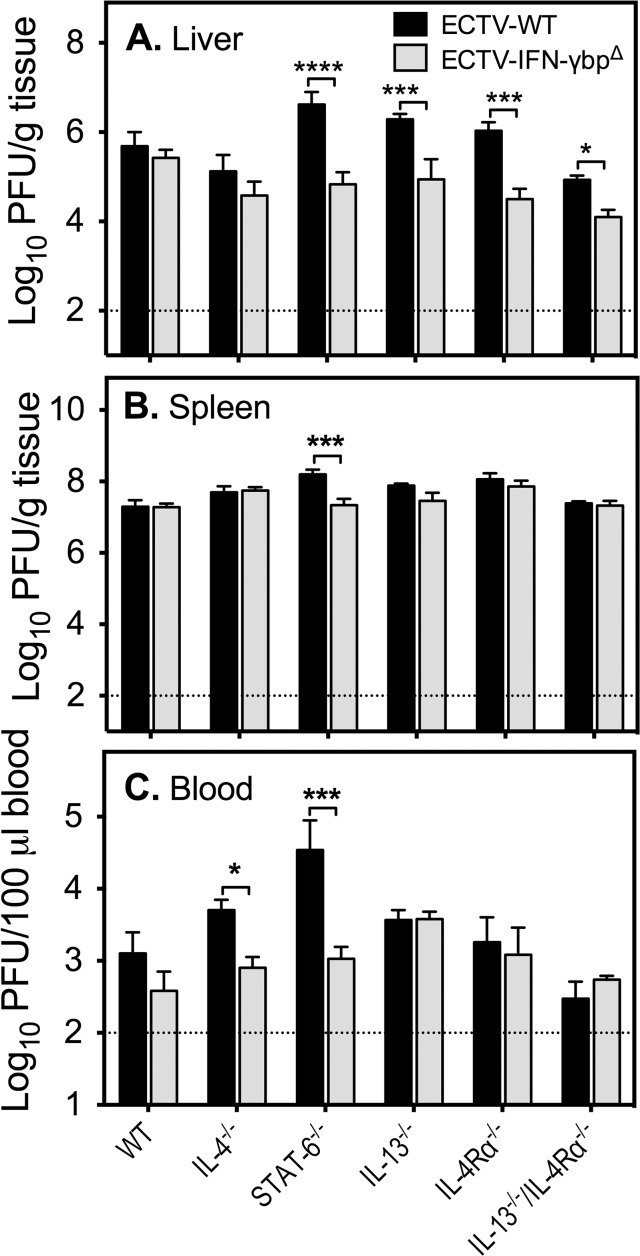
Susceptibility to ECTV is associated with uncontrolled virus replication, particularly in the liver and spleen [5–8]. We measured titers of ECTV-WT and ECTV-IFN-γbpΔ in livers, spleens and blood collected from animals at day 7 post-infection (p.i.) to determine whether the increased susceptibility of some strains was due to increased virus load. This time point was chosen as WT BALB/c mice infected with ECTV-WT begin to succumb to mousepox from day 7 p.i. as a result of high viral load [8,17].
In the liver, ECTV-WT replicated to significantly higher levels in STAT-6−/− mice compared to WT animals but titers were otherwise comparable across other strains (Fig. 2, S3 Table). ECTV-IFN-γbpΔ titers on the other hand were significantly lower in IL-4−/− and IL-13−/−/IL-4Rα−/− mice compared to WT mice but no other differences were evident. A comparison of ECTV-WT and ECTV-IFN-γbpΔ titers indicated that the mutant virus replicated at significantly lower levels in STAT-6−/−, IL-13−/−, IL4-Rα−/−, and IL-13−/−/IL-4Rα−/− mice (S3 Table).
Fig 2. Viral load in organs and blood of WT and GKO mice infected with ECTV-WT or ECTV-IFN-γbpΔ.
Groups of 5 mice were infected with either ECTV-WT or ECTV-IFN-γbpΔ and sacrificed at day 7 p.i. Viral load was determined by viral plaque assay and expressed as Log10 PFU virus/gram tissue. The dotted line indicates the limit of virus detection by viral plaque assay. Two-way ANOVA followed by Fisher’s LSD test for significance between (i) ECTV-WT titers in WT mice compared with all strains, (ii) ECTV-IFN-γbpΔ load in WT mice compared with all strains, and (iii) ECTV-WT compared with ECTV-IFN-γbpΔ load in individual strains (A) liver, (B) spleen, and (C) blood. The detailed statistical analysis of WT and mutant virus titers in each of the 5 GKO mouse strains is presented in S3 (Liver), S4 (Spleen) and S5 (Blood) Tables. Asterisks indicate significant differences in organ titers comparing ECTV-WT or ECTV-IFN-γbpΔ. ****, p< 0.0001; ***, 0.0001< p <0.001; **, 0.001< p <0.01; * 0.01< p <0.05.
In the spleen, the WT viral load increased significantly in STAT-6−/−, IL-13−/−, and IL-4Rα−/− mice compared with WT animals (Fig. 2, S4 Table). On the other hand, ECTV-IFN-γbpΔ titers were significantly higher in IL-4−/− and IL-4Rα−/− mice compared with WT mice. In a comparison of WT and mutant virus titers among all mouse strains, it was apparent that ECTV-IFN-γbpΔ replicated to lower levels only in the spleen of STAT-6−/− mice (S4 Table).
Finally, in the blood, ECTV-WT titers were increased in STAT-6−/− mice compared to WT mice (Fig. 2, S5 Table) but no differences in its replicative capacity were evident in the other strains. ECTV-IFN-γbpΔ replicated to higher levels in IL-13−/− mice compared to WT animals but titers were lower than that of WT virus in STAT-6−/− and IL-4−/− mice.
Taken together, with the exception of STAT-6−/− mice in which ECTV-WT titers were significantly increased in all organs compared to WT mice, there was no consistent correlation between increased susceptibility of individual mouse strains and viral load in visceral organs or blood.
The effect of vIFN-γbp on NK cell responses in GKO mice with deficiencies in Th2 cytokine signaling
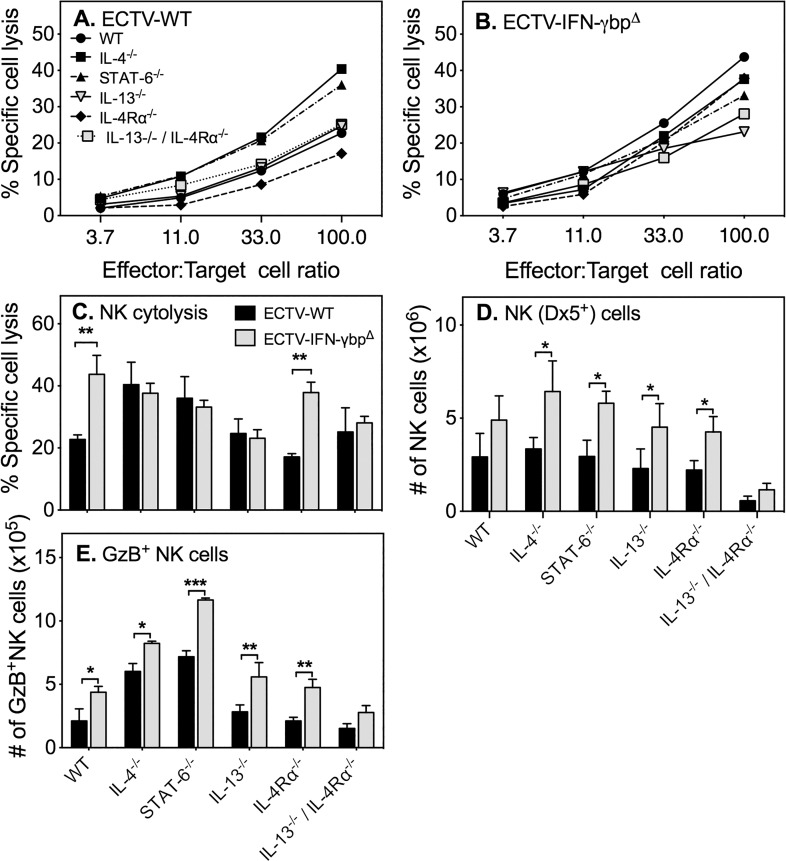
NK cells play an important role in virus control during the acute phase of ECTV infection and recovery of mice [5,17,33,34]. We measured the splenic NK cell activity at the peak of the response at day 5 p.i. in order to determine whether the absence of vIFN-γbp and/or factors associated with Th2 responses augmented these responses following infection.
ECTV-WT induced weak splenic NK cell activity in WT, IL-13−/−, IL-4Rα−/− and IL-13−/−/IL-4Rα−/− mice (Fig. 3A). The only exceptions were the IL-4−/− and STAT-6−/− strains in which the responses were about 3-fold higher than in WT animals. ECTV-IFN-γbpΔ induced a 3-fold higher NK cell cytolytic activity compared with ECTV-WT in WT and IL-4Rα−/− mice but did not affect responses in the other strains (Fig. 3B). The magnitude of NK cell responses to both mutant and WT viruses were similar in IL-4−/−, IL-13−/−, STAT-6−/− and IL-13−/−/IL-4Rα−/− mice. For the purpose of comparison, the NK cell responses to both WT and mutant mouse strains at 100: 1 effector to target cell ratio is shown in Fig. 3C.
Fig 3. NK cell responses to ECTV-WT and ECTV-IFN-γbpΔ.
Groups of 5 mice were infected with ECTV-WT or ECTV-IFN-γbpΔ, sacrificed 5 days later and the cytolytic activities of splenic NK cells measured. Data shown are % specific lysis of 51Cr-labelled YAC-1 target cells by splenic NK cells from (A) ECTV-WT-infected or (B) ECTV-IFN-γbpΔ-infected WT and GKO mouse strains. (C) Percent specific lysis of targets by splenocytes from the various strains infected with ECTV-WT or ECTV-IFN-γbpΔ at 100:1 effector-to-target ratio. (D) Numbers of GzB+ NK cells. Data shown are means SEM of results obtained from one of 3 separate experiments. Statistical significance was determined by 2-way ANOVA followed by Fisher’s LSD test for significance between groups. For panel C, (i) NK cell responses to ECTV-WT vs. ECTV-IFN-γbpΔ in WT mice (p = 0.0024) and IL-4Rα−/− mice (p < 0.0023); (ii) NK cell responses to ECTV-WT in WT mice vs IL-4−/− (p = 0.009), WT mice vs STAT-6−/− mice (**p = 0.0476); (iii) NK cell response to ECTV-IFN-γbpΔ in WT vs IL-13−/− (p = 0.0024), WT vs IL-13−/−/IL-4Rα−/− mice (*p = 0.02). For panel D, (i) GzB+ NK cell numbers in ECTV-WT vs. ECTV-IFN-γbpΔ infected strains: WT mice (p = 0.02), IL-4−/− mice (p = 0.02), STAT-6−/−(p = 0.0002), IL-13−/− (p = 0.0068) and IL-13−/−/IL-4Rα−/− mice (p = 0.008); (ii) GzB+ NK cell numbers in ECTV-WT infected WT mice vs IL-4−/− mice (p = 0.0006) and WT mice vs STAT-6−/− mice (p<0.0001); (iii) GzB+ NK cell numbers in ECTV-IFN-γbpΔ infected WT mice vs IL-4−/− mice (p = 0.0007) and WT mice vs STAT-6−/− mice (p<0.0001). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
The total numbers of DX5+ NK cells were not altered significantly in GKO strains compared with WT mice following infection with ECTV-WT, with the exception of the IL-13−/−/IL-4Rα−/− mice in which the numbers were about 5-fold lower (Fig. 3D). Numbers of NK cells increased significantly in most GKO strains following infection with the mutant virus except for WT and IL-13−/−/IL-4Rα−/− mice. We also assessed the expression of granzyme B (GzB), a serine protease found in granules of cytotoxic lymphocytes, to determine whether there was any association between the cytolytic activity of NK cells and GzB expression. Flow cytometric analysis revealed that WT virus infection increased GzB+ NK cell (CD3-DX5+) numbers in IL-4−/− and STAT-6−/− mice compared to WT mice (Fig. 3E). In contrast, mutant virus infection increased numbers of GzB+ cells in all strains compared to WT virus. Nonetheless, increases in GzB+ cell numbers did not always result in a corresponding increase in cytolytic activity (Fig. 3A-3C). As an example, infection of STAT-6−/− mice with ECTV-WT resulted in increased GzB+ cell numbers over and above those in WT mice and infection with the mutant virus further increased numbers. However, regardless of the type of virus used, the cytolytic activities of NK cells in WT and STAT-6−/− mice were comparable (Fig. 3A-3C).
Varied CD8 T cell responses in mice lacking Th2 cytokine signaling is differentially regulated by vIFN-γbp
CD8 T cells are an important source of IFN-γ production in mousepox beginning at day 6 p.i. The levels of IFN-γ produced by these cells closely parallel the magnitude of their cytolytic activity [8] and both responses peak between days 7–8 p.i. We assessed whether deletion of vIFN-γbp augmented IFN-γ production and CTL activity in CD8 T cells in WT and GKO mice at day 7 p.i.
Absence of IL-4Rα signaling did not affect CTL responses since WT and IL-4Rα−/− mice infected with ECTV-WT generated similar levels of killing (Fig. 4A). The responses in IL-4−/− and IL-13−/−/IL-4Rα−/− mice were about 3-fold lower than WT mice. However, the most striking differences were evident in STAT-6−/− and IL-13−/− mice in which the responses were about 9-fold and 27-fold lower, respectively, compared to responses in WT mice. The significant reduction in CTL response in IL-13−/− mice was partially reversed when IL-4Rα was also absent. The data indicated that IL-4, STAT-6 and IL-13, which are normally associated with development of Th2 responses, play important and non-redundant roles in the generation of optimal antiviral CTL responses.
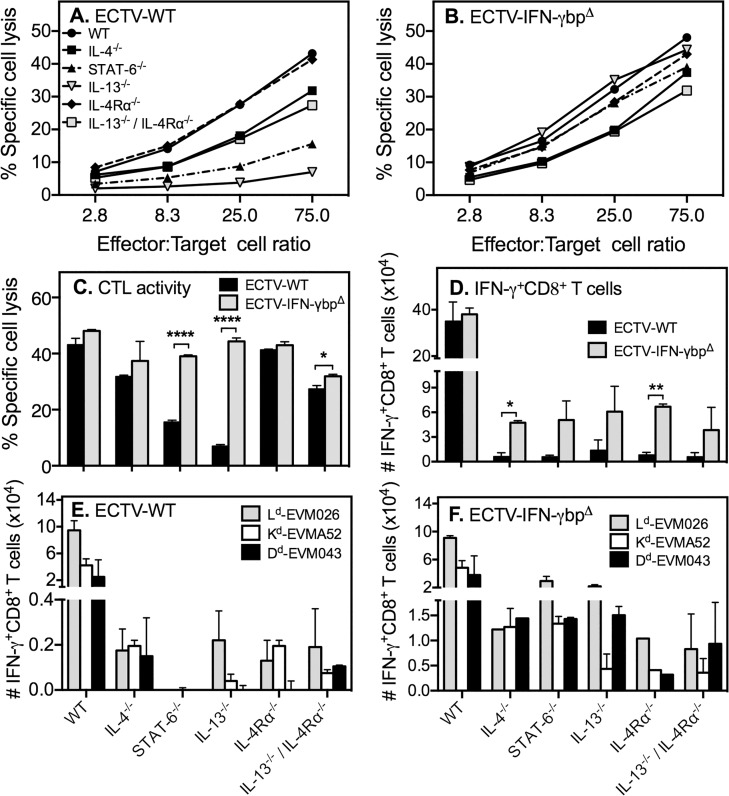
Fig 4. CD8 T cell responses to ECTV-WT and ECTV-IFN-γbpΔ.
Mice (n = 5–10/group) were infected with ECTV-WT or ECTV-IFN-γbp and sacrificed 7 days later to measure splenic CTL activity. Shown is % specific lysis of 51Cr-labelled, ECTV-infected P815 target cells by splenocytes from (A) ECTV-WT- or (B) ECTV-IFN-γbpΔ-infected WT and GKO mice. (C) Percent specific lysis of targets by splenocytes at 75:1 effector-to-target ratio. Two-way ANOVA followed by Fisher’s LSD test for significance was used for statistical analysis: (i) CTL response to ECTV-WT vs. ECTV-IFN-γbpΔ in IL-13−/− (p < 0.0001) and STAT-6−/− (p < 0.0001) (ii) CTL response to ECTV-WT in WT vs IL-4−/− (p = 0.0083), vs STAT-6−/− (p = 0.0003), vs IL-13−/− (p = 0.0001), vs IL-13−/−/IL-4Rα−/− (p = 0.0037) mice; (iii) CTL response to ECTV-IFN-γbp in WT vs STAT-6−/− (p = 0.0002); vs IL-13−/− (p = 0.0478); vs IL-4Rα−/− (p = 0.0174) and IL-13−/−/IL-4Rα−/− (p = 0.000046) mice. ****, p<0.0001. (D—F) Spleen cells from mice infected mice were re-stimulated for 5 h with ECTV-infected P815 cells or H-2d 8 T cell peptide determinants prior to intracellular IFN-γ staining followed by flow cytometry. Data shown are means of absolute numbers of ECTV-specific IFN-γ+ 8 T cells in spleens of ECTV-WT- vs. ECTV-IFN-γbpΔ-infected mice (D) and determinant-specific IFN-γ+ 8 T cells following infection with ECTV-WT (E) or ECTV-IFN-γbp (F) viruses. Two-way ANOVA followed by Fisher’s LSD test for significance was used for statistical analysis. For panels D, E and F, IFN-γ+ 8 T cell numbers in WT mice were significantly higher (p<0.0001) than numbers in all GKO strains. Data shown for D-F are representative of one of three independent experiments with similar results. *, p< 0.05; **, p < 0.01; ***, p < 0.0001.
The vIFN-γbp had minimal impact on CTL activity in WT, IL-4−/− and IL-4Rα−/− mice as these strains generated comparable responses to WT or mutant virus infection (Fig. 4A-4C). The most dramatic effects of infection with ECTV-IFN-γbpΔ in the GKO mouse strains were evident in STAT-6−/− and IL-13−/− mice. These groups had significantly diminished CTL responses to WT virus infection (Fig. 4A and 4C). However, mutant virus infection in both mouse strains induced robust CTL activities, which were almost as high as the magnitude in WT mice (Fig. 4B and 4C). Thus, vIFN-γbp had a greater deleterious effect on CD8 T responses in STAT-6−/− and IL-13−/− mice compared with the other GKO strains.
ECTV-WT induced significantly higher (p<0.0001) numbers of virus-specific IFN-γ-producing CD8 T cells in WT mice compared to all GKO strains, in which numbers were reduced by 20–40-fold (Fig. 4D). While ECTV-IFN-γbpΔ induced comparable numbers of IFN-γ-producing CD8 T cells as WT virus in WT mice, absence of vIFN-γbp only marginally increased numbers of IFN-γ+ CD8 cells in each GKO strain (Fig. 4D).
The MHC class I Ld-restricted response generally dominates at day 7 p.i. in BALB/c mice. Numbers of Ld-, Kd- and Dd-restricted determinant-specific IFN-γ+ cells were significantly higher (p<0.0001) in WT mice infected with ECTV-WT compared to all GKO strains (Fig. 4E). The reduction in virus-specific IFN-γ-producing CD8 T cell numbers seen in GKO strains (Fig. 4D) was clearly reflected in the determinant-specific responses, which were nonetheless varied (Fig. 4E). Curiously, there was a complete lack of reactivity to the three H-2d-restricted viral determinants in the STAT-6−/− strain. Although we do not have an explanation for this finding, it nonetheless suggested the possibility that the response might have been directed against other H-2d determinants.
In WT mice, ECTV-IFN-γbpΔ infection generated determinant-specific IFN-γ+ CD8 T cells (Fig. 4F) that were comparable in numbers to ECTV-WT infection. Mutant virus infection increased numbers of determinant-specific IFN-γ+ CD8 T cells in all GKO strains (Fig. 4F), over and above those induced by ECTV-WT (Fig. 4E). In particular, the biggest increases were seen in STAT-6−/− mice and IL-13−/− mice (Fig. 4F). The mechanism(s) though which absence of vIFN-γbp at least partially reconstituted the determinant-specific IFN-γ+ CD8 T cell responses in STAT-6−/− mice or increased both the total (Fig. 4D) and determinant-specific responses (Fig. 4F) in the other GKO strains is currently unknown but merits investigation.
Effect of vIFN-γbp on IFN-γ or IL-4 production by CD4 T cells deficient in Th2 cytokine signaling
The preceding data established that deficiencies in specific Th2 cytokine signaling significantly reduced the numbers of IFN-γ+ CD8 T cells and that vIFN-γbp partially modulated the response. It was therefore of interest to determine whether the combined deficiencies in Th2 cytokines and vIFN-γbp promoted Th1 responses in CD4 T cells.
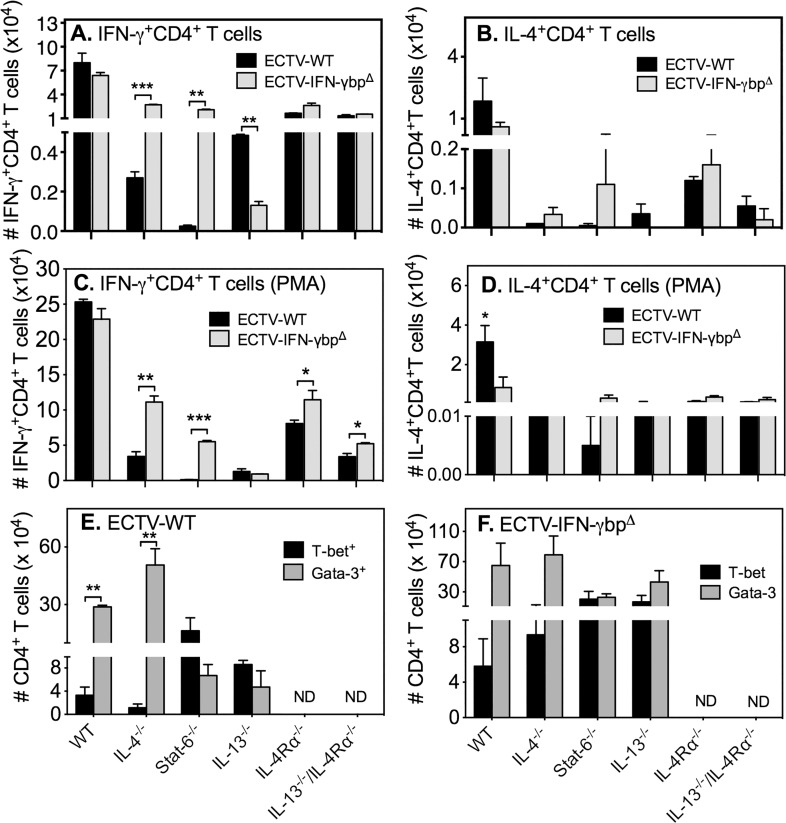
Intriguingly, WT mice infected with ECTV-WT or ECTV-IFN-γbpΔ generated the highest numbers of IFN-γ+ CD4 T cells whereas the numbers in all GKO strains were significantly (p<0.0001) reduced (Fig. 5A). Of note, STAT-6−/− mice infected with WT virus had the lowest number of IFN-γ producing CD4 T cells but it was the only strain in which mutant virus infection increased numbers significantly by nearly 90-fold. Similarly, in the case of IL-4 production, WT mice infected with WT or mutant virus generated the largest number of IL-4+ CD4 T cells but numbers were significantly lower (p<0.01) in all GKO strains of mice (Fig. 5B). Data shown in Fig. 5A and 5B are using non-stimulated cells. CD4 T cells from IL-4−/− mice did not produce IL-4 as expected but IL-4+ CD4 T cell numbers were also minimal in STAT-6−/− and IL-13−/− mice. In vitro stimulation of splenocytes with PMA + ionomycin increased the numbers of IFN-γ (Fig. 5C) and IL-4 (Fig. 5D) producing CD4 T cells in all groups but the response in WT cells was still much higher than in GKO cells.
Fig 5. Numbers of CD4 T cells expressing IFN-γ, IL-4, T-bet or GATA-3.
Mice (n = 5–10/group) were infected with ECTV-WT or ECTV-IFN-γbpΔ, sacrificed on day 7 p.i. and splenocytes used for intracellular staining for IFN-γ, IL-4, T-bet or GATA-3 without stimulation (A and B) or with PMA + ionomycin stimulation (C and D). Shown are means of absolute numbers of IFN-γ+CD4 T cells with no stimulation (A) or PMA + ionomycin stimulation (C), IL-4+CD4 T cells with no stimulation (B) or PMA + ionomycin stimulation (D). Also shown are means of absolute numbers of T-bet+ and GATA-3+ CD4 T cells following ECTV-WT (E) or ECTV-IFN-γbpΔ (F) infection. Two-way ANOVA followed by Fisher’s LSD test for significance was used. For panel A, numbers of IFN-γ+CD4 T cells in WT mice for both viruses were significantly higher (p<0.0001) compared to numbers in all GKO strains. In IL-4−/− and STAT-6−/− mice, IFN-γ+CD4 T cell numbers generated by ECTV-IFN-γbpΔ infection were significantly higher (p = 0.0005 and p = 0.0013, respectively) compared to WT virus infection. In IL-13−/− mice, IFN-γ+CD4 T cell numbers generated by WT virus infection were significantly higher (p = 0.0034) compared to ECTV-IFN-γbpΔ infection. For panel B, numbers of IL-4+CD4 T cells in WT mice for both viruses were significantly higher (p<0.01) compared to numbers in all GKO strains. No other significant differences were found. For panel C, numbers of IFN-γ+CD4 T cells in WT mice for both viruses were significantly higher (p<0.0001 in ECTV-WT and p < 0.001 in ECTV-IFN-γbpΔ) compared to numbers in all GKO strains. In IL-4−/−, STAT-6−/− IL-4Rα−/− and IL-13−/− / IL-4Rα−/− mice, IFN-γ+CD4 T cell numbers generated by ECTV-IFN-γbpΔ infection were significantly higher compared to WT virus infection. For panel D, numbers of IL-4+CD4 T cells in WT mice for ECTV-WT virus was significantly higher (p<0.05) compared to numbers in all GKO strains. In WT mice, IL-4+CD4 T cell numbers generated by WT virus infection were significantly higher (p<0.05) compared to ECTV-IFN-γbpΔ infection. For panel E, T-bet+ CD4 T cell numbers were significantly increased (p<0.01) in STAT-6−/− and IL-13−/− mice compared to WT animals. For panel F, no significant differences were found between strains. Data shown for panels A and B are from one of two independent experiments with similar results. Data shown for panels C-F are from one experiment. *, p < 0.05; **, p < 0.01; ***, p < 0.0001.
We interrogated CD4 T cells by flow cytometry to detect expression of intracellular T-bet and GATA-3, the signature Th1 and Th2 transcription factors, respectively. In WT mice infected with ECTV-WT, CD4 T cells were predominantly GATA-3+ (Fig. 5E). IL-4 deficiency further increased GATA-3+ CD4 T cell numbers whereas STAT-6 or IL-13 deficiency increased T-bet+ cell numbers over and above those in WT mice. On the other hand, ECTV-IFN-γbpΔ infection did not significantly change T-bet+ or GATA-3+ cell numbers in GKO strains compared to those in WT mice. However, in comparing numbers induced by ECTV-WT infection (Fig. 5C), both T-bet+ and GATA-3+ cell numbers were increased in all strains following infection with ECTV-IFN-γbpΔ (Fig. 5D). Increases in T-bet+ cell numbers did not necessarily translate to increased numbers of IFN-γ+ CD4 T cells (Fig. 5A and 5C) and likewise, increases in GATA-3+ cells did not result in increases in IL-4+ CD4 T cells (Fig. 5, panels B and D). On the contrary, deficiency in specific Th2 cytokines, transcription factor or cytokine receptor resulted in reduced numbers of IFN-γ+ and IL-4+ CD4 T cells. vIFN-γbp modulated the IFN-γ response only in STAT-6−/− mice but that did not assist in the recovery of this strain from mutant virus infection. Deficiency in Th2 cytokine signaling did not bias CD4 T cells towards a Th1 response.
Discussion
A type 1/Th1-like cytokine response coupled with robust cell-mediated immunity is critical for recovery from mousepox whereas a type 2/Th2-like response associated with an absent or weak IFN-γ response and cell-mediated immunity is associated with susceptibility [8]. Deficiency in IFN-γ [9,10] or its suppression through over-expression of IL-4 by recombinant ECTV in resistant mice results in generation of poor cell-mediated immunity, overcomes resistance to mousepox and results in fulminant disease [11,12]. While it may be speculated that type 1-like responses were important for recovery of humans from smallpox, there is circumstantial evidence that Th2-biased responses can significantly exacerbate the disease and result in increased mortality.
In non-vaccinated humans, about 90% of cases were of the ordinary-type smallpox with a case fatality rate of about 30–40% [35]. A more severe form of the disease, hemorrhagic-type smallpox, characterized by mucosal and skin hemorrhage, was mainly seen in pregnant women. Early hemorrhagic-type smallpox was associated with almost 100% mortality regardless of vaccination status and pregnant women with smallpox had 3–4 times higher case fatality rates than non-pregnant women and men of the same age groups [35,36]. Although the reasons for higher case fatality rates were not known at the time when smallpox was endemic, it is now recognized that an immune bias towards a Th2-like response associated with pregnancy [37–39] may have been responsible. That Th2-biased response can exacerbate smallpox even in vaccinated individuals is significant. In this regard, the demonstration that infection of previously vaccinated mice with recombinant ECTV encoding IL-4 can completely overcome protective immunity and genetic resistance [11,12] helped establish that over exuberant Th2-biased responses increase susceptibility to OPV infections. This raises the question of whether defective or sub-optimal Th2 responses allow for the generation of more effective antiviral immunity. Our results indicate they do not.
Deficiencies in IL-4, STAT-6, IL-13 and/or IL-4Rα signaling on immune response generation were varied and further modulated by vIFN-γbp but 3 broad categories of outcomes were evident. In the first, the IL-4−/− mice had comparable recovery rates to WT mice regardless of whether they were infected with WT or mutant virus. In the second, the IL-4Rα−/− and IL-13−/−/IL-4Rα−/− mice exhibited increased susceptibility to WT virus infection but the absence of vIFN-γbp during infection mitigated the increased susceptibility. Finally, mice deficient in IL-13 or STAT-6 were significantly more susceptible than WT animals in response to WT or mutant virus infection. Susceptibility to ECTV is attributed to uncontrolled generalized virus replication in visceral organs, including in the liver [6,7], spleen and blood [40]. However, in the current study, viral load in organs and blood 7 days after infection did not consistently correlate with increased susceptibility, with the exception of STAT-6−/− mice. This outcome is surprising and might have been a consequence of the extent to which deficiencies in IL-4, IL-13 and/or IL-4Rα, combined with immunomodulation by vIFN-γbp impacted on inflammation, the immune response and virus control. Viral load in the liver and spleen generally increase exponentially over time if not effectively controlled by the immune response. However, the blood viral load is usually only increased following primary, secondary and subsequent occurrence of viremia following release of virions from the visceral organs. It is possible that kinetics of virus replication in the different GKO mouse strains was different and measurement of viral load at a single time-point might not have revealed the extent to which the viruses replicated and spread. However, the fact that most GKO mice succumbed to mousepox at or after 6 days post-infection suggests that measurement of viral titers at day 7 should have indicated if uncontrolled virus replication was the cause of death. Another finding of note is that deficiency in IL-13 or IL-4Rα resulted in comparable viral titers in liver, spleen and blood but titers were lower in the liver and blood in IL-13−/−/IL-4Rα−/− mice. While the reason for this is unclear, it might be explained, at least in part by the CTL responses generated in the absence of IL-13 or both IL-13 and IL-4Rα (see below).
Absence of IL-4, STAT-6, IL-13 or IL-4Rα signaling clearly had a significant impact on both CD4 and CD8 T cell responses, which were modulated to some extent by vIFN-γbp. Contrary to our prediction, the numbers of IFN-γ+ CD4 and CD8 T cells as well as IL-4+ CD4 T cells were decreased in all GKO strains compared to WT mice. Absence of vIFN-γbp during infection had minimal effects on CD4 T cell responses with the exception of STAT-6−/− mice in which mutant virus infection significantly increased IFN-γ+ CD4 T cell numbers. The most striking reductions in the anti-ECTV CTL responses were seen with WT virus infected STAT-6−/− and IL-13−/− mice, which were almost completely reversed when these strains were infected with ECTV-IFN-γbpΔ. Deficiency in the IL-4Rα alone did not have any impact on the CTL response but in the IL-13−/− mice, the significant reduction in CTL activity was partially reversed when IL-4Rα was also absent. This is an interesting finding and although the mechanisms involved are not known, it suggests that in the absence of IL-13, IL-4 signaling through the IL-Rα was far more detrimental to induction of antiviral CTL responses than in the complete absence of IL-4 and IL-13 responses in IL-13−/−/IL-4Rα−/− mice. We speculate that IL-13 might modulate the IL-4 response in induction of ECTV-specific CTL responses. Another important finding in the STAT-6−/− mice is the complete lack of reactivity of CD8 T cells to any of the H-2d determinants that were tested. One explanation for the complete lack of reactivity to the three H-2d-restricted viral determinants in the STAT-6−/− strain is that the response might have been directed against other determinants. It is also possible that results could be influenced by subtle changes in reactivity of cells maturing in a STAT-6-deficient environment. Such changes in immune responses may be due to altered peptide expression that might be negligible or difficult to identify or quantify, but should nonetheless be considered for investigations in our future studies. It is curious why Th2-response linked genes influence the reactivity of CD8 T responses but may be related, at least in part, to potential defects in antigen presentation by dendritic cells as discussed below.
Although IFN-γ impacts on the quality and magnitude of the anti-ECTV CTL response [8], it was evident that the augmented CTL activity in IL-13−/− and STAT-6−/− mice was not related to the marginally increased numbers of virus-specific IFN-γ+ CD8 cells, which occurred in all GKO strains. It is also possible that factors other than CD8 T cell-derived IFN-γ influenced the cytolytic activity of CTL in IL-13−/− and STAT-6−/− mice. As CD4 T cells are important for generation of optimal CTL responses to ECTV infection [41], we considered the possibility that IFN-γ-producing CD4 T cells may have impacted on the magnitude of CTL responses. While this might have been the case in STAT-6−/− animals (Fig. 5A) in which numbers of IFN-γ+ CD4 cells substantially increased following infection with the mutant virus, it did not hold true for the IL-13−/− mice. There is therefore little or no evidence for any consistent correlation between the numbers of IFN-γ+ CD4 T cells and the magnitude of the CD8 T cell-mediated CTL responses in individual mouse strains.
STAT-6-dependent IL-4 production has been purported to be associated with susceptibility of BALB/c mice to ECTV infection [25] but IL-4 deficiency in BALB/c mice has no impact on disease outcome [8]. Since IL-4−/− mice can respond to IL-13, we hypothesized that the cytokine might compensate for absence of IL-4 to drive Th2-like responses. Our data established that this was not the case and that the responses to, and outcome of infection with, ECTV in IL-4−/−, IL-13−/− and STAT-6−/−mice are very different. First, IL-13−/− and STAT-6−/− mice were far more susceptible to WT or mutant virus infection than IL-4−/− mice. Second, the CTL response to WT virus was marginally reduced in IL-4−/− mice compared to WT animals, but the effect was far more significant in IL-13−/− and STAT-6−/− mice. This is an important and novel finding in that factors normally associated with Th2 responses play critical and non-redundant roles in the generation of optimal antiviral CD8 CTL responses. The mechanism(s) through which IL-13 or STAT-6 impact on CTL responses generation during ECTV infection is currently unknown but merit a detailed investigation.
Naïve CD8+ T cells in IL-4−/−, IL-13−/− and STAT6−/− mice express lower levels of IL-4Rα making them less responsive to IL-4 and IL-13 during T cell priming [42]. It is therefore possible that in IL-13−/− mice, IL-4 signaling alone through activation of STAT-6 may promote sufficient down-regulation of CD8 co-receptor densities on naïve CD8+ T cells following cognate antigen encounter during ECTV infection, and lessen the anti-viral capacity of effector CD8+ T cells by reducing avidity and polyfunctionality [43–45]. In relation to the role of STAT-6 in CTL responses, it has been reported that the activation and function of CTL may be impaired in STAT-6−/− mice during infection with the intracellular parasite Toxoplasma gondii, likely as a consequence of reduced levels of CD86 expression on DC [46]. The demonstration that activation of CD8 T cells in STAT-6−/− mice was restored after transfer of splenic adherent cells or bone-marrow derived DC from WT mice whereas transfer of naïve WT CD8 T cells into STAT-6−/− mice were not activated suggested a defect in antigen presentation [46]. It is known that in vitro, IL-13, like IL-4, in combination with GM-CSF can induce generation of functional DC [47], suggesting that IL-13 may be important in differentiation of DC via STAT-6 signaling. We speculate that IL-4R/IL-13R-associated STAT-6 signaling might be important in CD8 T cell activation and function during ECTV infection, possibly through maturation of antigen presenting cells, such as dendritic cells (DC). Apart from promoting the expression of several cell surface molecules responsible for antigen presentation; including MHC II, CD80, and CD86, IL-4/STAT-6 signaling regulates T-cell proliferation by decreasing the expression of p27Kip1, a known cyclin-dependent kinase inhibitor. In CD8 cells, STAT6 is required for Tc2 differentiation as the production of IL-4 and IL-5 is completely lost with STAT6 deficiency (reviewed in [48]). Taken together, STAT6 is required for IL-4-stimulated T-cell functions.
That STAT-6 deficiency further exacerbates susceptibility of BALB/c mice to ECTV infection is in stark contrast to a previous report in which STAT-6−/− mice were found to be more resistant than WT BALB/c mice due to augmented NK cell and IFN-γ responses [25]. In the current study, apart from a 2–3-fold increase in the cytolytic activity of NK cells in STAT-6−/− mice, all other responses were either far worse or no different to WT mice. We believe that there may be a simple explanation for this discrepancy. In the present study we used STAT-6−/− mice that were backcrossed to the BALB/c background for at least 10 generations [29]. In the previous study, STAT-6−/− mice generated on a 129 background [49] and backcrossed to the BALB/c background for only 6 generations were used. Given that several of the 129 sub-strains are resistant to mousepox [10], we believe that the 129/Sv background genes may have influenced the outcome of infection in that study. It is likely that STAT-6 deficiency in ECTV-resistant C57BL/6 or 129 mouse strains might further augment Th1 responses but this is not the case in the ECTV-susceptible BALB/c mice. Consistent with this proposition, we have found that C57BL/6 mice deficient in IL-4 generate more robust CTL and IFN-γ responses which correlated with more efficient virus control compared to WT littermate controls (data not shown). Furthermore, when the STAT-6−/− mice used in the previous study [25,49] were backcrossed to the BALB/c background 10–12 times, they were found to be more susceptible to ECTV-WT infection than WT littermates (data not shown). Clearly, STAT-6 deficiency does not increase the resistance of BALB/c mice to ECTV infection. On the contrary, STAT-6 deficiency further increased susceptibility of the BALB/c strain to ECTV.
Our finding that deficiencies in IL-4/IL-13/STAT-6 signaling pathways significantly reduced the numbers of IFN-γ producing CD4 and CD8 T cells indicates an absence of a switch to a Th1-like response and might be unique to this viral model. For example, Th1/Th2 cytokine production by IL-13−/− cells is varied depending on in vitro or in vivo stimulation conditions and the type of cells involved [27]. IL-13 deficient splenocytes or CD4 T cells stimulated with mitogens produce reduced levels of IL-4 but similar levels of IFN-γ compared to WT cells. Under Th1/Th2 culture conditions, IL-13−/− CD4 T cells produce dramatically reduced levels of IL-4 but only a 2-fold higher amount of IFN-γ. In contrast, mast cells from IL-13−/− and WT mice stimulated with PMA and ionomycin produce comparable levels of IL-4. In vivo, challenge of IL-13−/− mice with schistosome eggs reduced IL-4 levels only by about 3-fold whereas challenge with the parasite N. brasiliensis had no impact on Th2 response generation or IL-4 production by lymph node cells or purified CD4 T cells.
It is conceivable that regulation of Th2 response generation in vivo during infection with a virus is far more complex than how it is understood to occur in in vitro culture conditions. Various stimuli, cell types and signalling pathways are involved in orchestrating Th2 responses of which there are also variants [19,50–52]. Indeed, Th2 differentiation has been shown to occur in mice that are deficient in IL-4, IL-13, IL-4Rα or STAT-6 and cytokines like thymic stromal lymphopoietin, IL-25 and IL-33 can induce Th2 responses [19,27,51,52]. There is consensus, however, that GATA3 is required for this process, even if expressed at low levels [53–55]. In the current study, we found that numbers of GATA3+ CD4 T cells were highest in both WT and IL-4−/− mice infected with WT virus but numbers were reduced in the GKO strains. These strains also had the highest number of T-bet+ CD4 T cells. T-bet can negatively regulate GATA3 [56] and in STAT-6−/− mice, T-bet+ CD4 T cell numbers were increased with a corresponding decrease in GATA-3+ CD4 T cells. These changes, however, did not translate to an increase in IFN-γ+ CD4 T cells. Overall, there was little correlation between GATA-3 and IL-4 expression or T-bet and IFN-γ expression in CD4 T cells regardless of the type of infecting virus. We believe that the use of transgenic CD4 and CD8 T cells, specific for a model antigen, and deficient in IL-4, IL-13, IL-4Rα or STAT-6 might help better understand the molecular mechanisms involved in driving Th2 responses in BALB/c mice infected with ECTV. The adoptive transfer of such transgenic cells into recipient mice combined with the use of recombinant ECTV encoding the model antigen for infection is an approach that we propose to undertake.
Conclusion
We have established that deficiency in specific factors associated with Th2 response development and/or the absence of vIFN-γ-bp during infection of BALB/c mice with ECTV does not bias development of a Th1 response. IL-4, STAT-6 and IL-13, which are normally associated with development of Th2 responses, play important and non-redundant roles in the generation of optimal antiviral CTL responses. Our data also established that the absence of IL-13 and STAT-6 further increased the susceptibility of BALB/c mice to ECTV infection. We conclude that host resistance or susceptibility to mousepox is far more complex and goes beyond the presence or absence of select genetic factors that control Th differentiation.
Supporting Information
Data are from one of the three separate experiments showing intracellular IFN-γ expression (Y-axis) by splenic 8 T cells (X-axis) from WT and GKO mice. The numbers in the upper right quadrants in individual panels indicate percentages of IFN-γ-producing 8 T cells after stimulation with (from left) the irrelevant control (HSV-1 gB) peptide (first column), whole virus (ECTV) (second column), Ld-EVM026 (third column), Kd-EVMA52 (forth column) or Dd-EVM043 peptides (fifth column). Absolute numbers of IFN-γ+ 8 T were obtained by multiplying the percentage of cells with the total number of splenocytes from each mouse for each strain.
(TIF)
Data are from one of the three separate experiments showing intracellular IFN-γ expression (Y-axis) by splenic 8 T cells (X-axis) from WT and GKO mice. The numbers in the upper right quadrants in individual panels indicate percentages of IFN-γ-producing 8 T cells after stimulation with (from left) the irrelevant control (HSV-1 gB) peptide (first column), whole virus (ECTV) (second column), Ld-EVM026 (third column), Kd-EVMA52 (fourth column) or Dd-EVM043 peptides (fifth column). Absolute numbers of IFN-γ+ 8 T were obtained by multiplying the percentage of cells with the total number of splenocytes from each mouse for each strain.
(TIF)
Data are from one of three separate experiments showing intracellular IFN-γ expression (Y-axis) in unstimulated splenic CD4 T cells (X-axis) from naïve (first column), ECTV-WT- (second column) or ECTV-IFN-γbpΔ- (third column) infected WT and GKO mice. The numbers in the upper right quadrants in individual panels indicate percentages of IFN-γ-producing CD4 T cells.
(TIF)
Data are from one of three separate experiments showing intracellular IL-4 expression (Y-axis) in unstimulated splenic CD4 T cells (X-axis) from naïve (first column), ECTV-WT- (second column) or ECTV-IFN-γbpΔ- (right column) infected WT and GKO mice. The numbers in the upper right quadrants in individual panels indicate percentages of IL-4-producing CD4 T cells.
(TIF)
Data in this figure is the same as in Fig. 1 but presented to compare survival curves of each GKO strain with wild type BALB/c mice. P values were obtained by using Kaplan-Meier Log rank statistical test: *, p < 0.05.
(TIF)
Data in this figure is the same as in Fig. 1 but presented to compare survival curves of each GKO strain with wild type BALB/c mice. P values were obtained by using Kaplan-Meier Log rank statistical test: *, p < 0.05.
(TIF)
a EVM represents nomenclature for ECTV-specific 8 T cell determinants.
(DOCX)
a ECTV-WT vs. ECTV-IFN-γbpΔ P value using Logrank (Mantel-Cox) test. For extremely significant (****) P < 0.0001; extremely significant (***) 0.0001< P <0.001; very significant (**) 0.001< P <0.01; significant (*) 0.01< P <0.05; not significant (ns) P ≥ 0.05. b Number in brackets is the median survival time in days. c Number of animals in group. d BALB/c.WT vs. GKO strain P value using Logrank (Mantel-Cox) test. e undefined
(DOCX)
a To evaluate significant differences between groups, viral titers were log transformed and 2-way ANOVA performed followed by Fisher’s LSD test. For extremely significant (****) P < 0.0001; extremely significant (***) 0.0001< P <0.001; very significant (**) 0.001< P <0.01; significant (*) 0.01< P <0.05; not significant (ns) P ≥ 0.05. b ECTV-WT vs. ECTV-IFN-γbpΔ. c BALB/c.WT vs. GKO strain.
(DOCX)
a To evaluate significant differences between groups, viral titers were log transformed and 2-way ANOVA performed followed by Fisher’s LSD test. For extremely significant (****) P < 0.0001; extremely significant (***) 0.0001< P <0.001; very significant (**) 0.001< P <0.01; significant (*) 0.01< P <0.05; not significant (ns) P ≥ 0.05. b ECTV-WT vs. ECTV-IFN-γbpΔ. c BALB/c.WT vs. GKO.
(DOCX)
a To evaluate significant differences between groups, viral titres were log transformed and 2-way ANOVA performed followed by Fisher’s LSD test. For extremely significant (****) P < 0.0001; extremely significant (***) 0.0001< P <0.001; very significant (**) 0.001< P <0.01; significant (*) 0.01< P <0.05; not significant (ns) P ≥ 0.05. b ECTV-WT vs. ECTV-IFN-γbpΔ. c BALB/c.WT vs. GKO strain.
(DOCX)
Acknowledgments
We thank the Australian National University Animal Breeding and Research Facility and the John Curtin School of Medical Research Microscopy and Flow Cytometry Research Facility.
Data Availability
All data are included within the paper.
Funding Statement
This work was funded by the National Health and Medical Research Council of Australia (https://www.nhmrc.gov.au/) grant numbers APP1007980 and 471426. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Oldstone M (1998) Viruses, plagues and history New York: Oxford University Press. [Google Scholar]
- 2. Miller J, Engelberg S, Broad W (2001) Germs: The ultimate weapon London: Simon and Schuster. [Google Scholar]
- 3. McFadden G (2005) Poxvirus tropism. Nat Rev Microbiol 3: 201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stanford MM, McFadden G, Karupiah G, Chaudhri G (2007) Immunopathogenesis of poxvirus infections: forecasting the impending storm. Immunol Cell Biol 85: 93–102. [DOI] [PubMed] [Google Scholar]
- 5. Buller RM, Palumbo GJ (1991) Poxvirus pathogenesis. Microbiol Rev 55: 80–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Esteban DJ, Buller RM (2005) Ectromelia virus: the causative agent of mousepox. J Gen Virol 86: 2645–2659. [DOI] [PubMed] [Google Scholar]
- 7. Fenner F (1949) Mouse-pox; infectious ectromelia of mice; a review. J Immunol 63: 341–373. [PubMed] [Google Scholar]
- 8. Chaudhri G, Panchanathan V, Buller RM, van den Eertwegh AJ, Claassen E, et al. (2004) Polarized type 1 cytokine response and cell-mediated immunity determine genetic resistance to mousepox. Proc Natl Acad Sci U S A 101: 9057–9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karupiah G, Fredrickson TN, Holmes KL, Khairallah LH, Buller RM (1993) Importance of interferons in recovery from mousepox. J Virol 67: 4214–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Panchanathan V, Chaudhri G, Karupiah G (2005) Interferon function is not required for recovery from a secondary poxvirus infection. Proc Natl Acad Sci U S A 102: 12921–12926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen N, Bellone CJ, Schriewer J, Owens G, Fredrickson T, et al. (2011) Poxvirus interleukin-4 expression overcomes inherent resistance and vaccine-induced immunity: pathogenesis, prophylaxis, and antiviral therapy. Virology 409: 328–337. 10.1016/j.virol.2010.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jackson RJ, Ramsay AJ, Christensen CD, Beaton S, Hall DF, et al. (2001) Expression of mouse interleukin-4 by a recombinant ectromelia virus suppresses cytolytic lymphocyte responses and overcomes genetic resistance to mousepox. J Virol 75: 1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alcami A, Smith GL (1995) Cytokine receptors encoded by poxviruses: a lesson in cytokine biology. Immunol Today 16: 474–478. [DOI] [PubMed] [Google Scholar]
- 14. Alcami A, Smith GL (1996) Receptors for gamma-interferon encoded by poxviruses: implications for the unknown origin of vaccinia virus. Trends Microbiol 4: 321–326. [DOI] [PubMed] [Google Scholar]
- 15. McFadden G, Graham K, Ellison K, Barry M, Macen J, et al. (1995) Interruption of cytokine networks by poxviruses: lessons from myxoma virus. J Leukoc Biol 57: 731–738. [DOI] [PubMed] [Google Scholar]
- 16. Seet BT, Johnston JB, Brunetti CR, Barrett JW, Everett H, et al. (2003) Poxviruses and immune evasion. Annu Rev Immunol 21: 377–423. [DOI] [PubMed] [Google Scholar]
- 17. Sakala IG, Chaudhri G, Buller RM, Nuara AA, Bai H, et al. (2007) Poxvirus-encoded gamma interferon binding protein dampens the host immune response to infection. J Virol 81: 3346–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamane H, Paul WE (2013) Early signaling events that underlie fate decisions of naive CD4(+) T cells toward distinct T-helper cell subsets. Immunol Rev 252: 12–23. 10.1111/imr.12032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu J, Yamane H, Paul WE (2010) Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 28: 445–489. 10.1146/annurev-immunol-030409-101212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chatila TA (2004) Interleukin-4 receptor signaling pathways in asthma pathogenesis. Trends Mol Med 10: 493–499. [DOI] [PubMed] [Google Scholar]
- 21. Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE (1999) The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol 17: 701–738. [DOI] [PubMed] [Google Scholar]
- 22. Chiaramonte MG, Mentink-Kane M, Jacobson BA, Cheever AW, Whitters MJ, et al. (2003) Regulation and function of the interleukin 13 receptor alpha 2 during a T helper cell type 2-dominant immune response. J Exp Med 197: 687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wood N, Whitters MJ, Jacobson BA, Witek J, Sypek JP, et al. (2003) Enhanced interleukin (IL)-13 responses in mice lacking IL-13 receptor alpha 2. J Exp Med 197: 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ansel KM, Djuretic I, Tanasa B, Rao A (2006) Regulation of Th2 differentiation and Il4 locus accessibility. Annu Rev Immunol 24: 607–656. [DOI] [PubMed] [Google Scholar]
- 25. Mahalingam S, Karupiah G, Takeda K, Akira S, Matthaei KI, et al. (2001) Enhanced resistance in STAT6-deficient mice to infection with ectromelia virus. Proc Natl Acad Sci U S A 98: 6812–6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Noben-Trauth N, Kropf P, Muller I (1996) Susceptibility to Leishmania major infection in interleukin-4-deficient mice. Science 271: 987–990. [DOI] [PubMed] [Google Scholar]
- 27. McKenzie GJ, Emson CL, Bell SE, Anderson S, Fallon P, et al. (1998) Impaired development of Th2 cells in IL-13-deficient mice. Immunity 9: 423–432. [DOI] [PubMed] [Google Scholar]
- 28. Noben-Trauth N, Shultz LD, Brombacher F, Urban JF Jr., Gu H, et al. (1997) An interleukin 4 (IL-4)-independent pathway for CD4+ T cell IL-4 production is revealed in IL-4 receptor-deficient mice. Proc Natl Acad Sci U S A 94: 10838–10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaplan MH, Schindler U, Smiley ST, Grusby MJ (1996) Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity 4: 313–319. [DOI] [PubMed] [Google Scholar]
- 30. Chen W, Drillien R, Spehner D, Buller RM (1992) Restricted replication of ectromelia virus in cell culture correlates with mutations in virus-encoded host range gene. Virology 187: 433–442. [DOI] [PubMed] [Google Scholar]
- 31. Scalzo AA, Farrell HE, Karupiah G (2000) Techniques for studying murine natural killer cells in defense against viral infection. Methods Mol Biol 121: 163–177. [DOI] [PubMed] [Google Scholar]
- 32. Tscharke DC, Woo WP, Sakala IG, Sidney J, Sette A, et al. (2006) Poxvirus CD8+ T-cell determinants and cross-reactivity in BALB/c mice. J Virol 80: 6318–6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parker AK, Parker S, Yokoyama WM, Corbett JA, Buller RM (2007) Induction of natural killer cell responses by ectromelia virus controls infection. J Virol 81: 4070–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fang M, Orr MT, Spee P, Egebjerg T, Lanier LL, et al. (2011) CD94 is essential for NK cell-mediated resistance to a lethal viral disease. Immunity 34: 579–589. 10.1016/j.immuni.2011.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fenner F HD, Arita I, Jezek Z, Ladnyi ID (1988) Smallpox and its eradication. Geneva: World Health Organization. [Google Scholar]
- 36. AR R (1972) Smallpox Bombay: The Kothari Book Depot. [Google Scholar]
- 37. Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG (1993) Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol 151: 4562–4573. [PubMed] [Google Scholar]
- 38. Tranchot-Diallo J, Gras G, Parnet-Mathieu F, Benveniste O, Marce D, et al. (1997) Modulations of cytokine expression in pregnant women. Am J Reprod Immunol 37: 215–226. [DOI] [PubMed] [Google Scholar]
- 39. Trowsdale J, Betz AG (2006) Mother's little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol 7: 241–246. [DOI] [PubMed] [Google Scholar]
- 40. Chaudhri G, Panchanathan V, Bluethmann H, Karupiah G (2006) Obligatory requirement for antibody in recovery from a primary poxvirus infection. J Virol 80: 6339–6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Karupiah G, Buller RM, Van Rooijen N, Duarte CJ, Chen J (1996) Different roles for CD4+ and CD8+ T lymphocytes and macrophage subsets in the control of a generalized virus infection. J Virol 70: 8301–8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wijesundara DK, Tscharke DC, Jackson RJ, Ranasinghe C (2013) Reduced interleukin-4 receptor alpha expression on CD8+ T cells correlates with higher quality anti-viral immunity. PLoS One 8: e55788 10.1371/journal.pone.0055788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kienzle N, Olver S, Buttigieg K, Groves P, Janas ML, et al. (2005) Progressive differentiation and commitment of CD8+ T cells to a poorly cytolytic CD8low phenotype in the presence of IL-4. J Immunol 174: 2021–2029. [DOI] [PubMed] [Google Scholar]
- 44. Apte SH, Baz A, Groves P, Kelso A, Kienzle N (2008) Interferon-gamma and interleukin-4 reciprocally regulate CD8 expression in CD8+ T cells. Proc Natl Acad Sci U S A 105: 17475–17480. 10.1073/pnas.0809549105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wijesundara DK, Jackson RJ, Tscharke DC, Ranasinghe C (2013) IL-4 and IL-13 mediated down-regulation of CD8 expression levels can dampen anti-viral CD8(+) T cell avidity following HIV-1 recombinant pox viral vaccination. Vaccine 31: 4548–4555. 10.1016/j.vaccine.2013.07.062 [DOI] [PubMed] [Google Scholar]
- 46. Jin D, Takamoto M, Hu T, Taki S, Sugane K (2009) STAT6 signalling is important in CD8 T-cell activation and defence against Toxoplasma gondii infection in the brain. Immunology 127: 187–195. 10.1111/j.1365-2567.2008.02935.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Alters SE, Gadea JR, Holm B, Lebkowski J, Philip R (1999) IL-13 can substitute for IL-4 in the generation of dendritic cells for the induction of cytotoxic T lymphocytes and gene therapy. J Immunother 22: 229–236. [DOI] [PubMed] [Google Scholar]
- 48. Goenka S, Kaplan MH (2011) Transcriptional regulation by STAT6. Immunol Res 50: 87–96. 10.1007/s12026-011-8205-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, et al. (1996) Essential role of Stat6 in IL-4 signalling. Nature 380: 627–630. [DOI] [PubMed] [Google Scholar]
- 50. Paul WE (2010) What determines Th2 differentiation, in vitro and in vivo? Immunol Cell Biol 88: 236–239. 10.1038/icb.2010.2 [DOI] [PubMed] [Google Scholar]
- 51. Paul WE, Zhu J (2010) How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol 10: 225–235. 10.1038/nri2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pulendran B, Artis D (2012) New paradigms in type 2 immunity. Science 337: 431–435. 10.1126/science.1221064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pai SY, Truitt ML, Ho IC (2004) GATA-3 deficiency abrogates the development and maintenance of T helper type 2 cells. Proc Natl Acad Sci U S A 101: 1993–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zheng W, Flavell RA (1997) The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89: 587–596. [DOI] [PubMed] [Google Scholar]
- 55. Zhu J, Min B, Hu-Li J, Watson CJ, Grinberg A, et al. (2004) Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol 5: 1157–1165. [DOI] [PubMed] [Google Scholar]
- 56. Usui T, Preiss JC, Kanno Y, Yao ZJ, Bream JH, et al. (2006) T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J Exp Med 203: 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data are from one of the three separate experiments showing intracellular IFN-γ expression (Y-axis) by splenic 8 T cells (X-axis) from WT and GKO mice. The numbers in the upper right quadrants in individual panels indicate percentages of IFN-γ-producing 8 T cells after stimulation with (from left) the irrelevant control (HSV-1 gB) peptide (first column), whole virus (ECTV) (second column), Ld-EVM026 (third column), Kd-EVMA52 (forth column) or Dd-EVM043 peptides (fifth column). Absolute numbers of IFN-γ+ 8 T were obtained by multiplying the percentage of cells with the total number of splenocytes from each mouse for each strain.
(TIF)
Data are from one of the three separate experiments showing intracellular IFN-γ expression (Y-axis) by splenic 8 T cells (X-axis) from WT and GKO mice. The numbers in the upper right quadrants in individual panels indicate percentages of IFN-γ-producing 8 T cells after stimulation with (from left) the irrelevant control (HSV-1 gB) peptide (first column), whole virus (ECTV) (second column), Ld-EVM026 (third column), Kd-EVMA52 (fourth column) or Dd-EVM043 peptides (fifth column). Absolute numbers of IFN-γ+ 8 T were obtained by multiplying the percentage of cells with the total number of splenocytes from each mouse for each strain.
(TIF)
Data are from one of three separate experiments showing intracellular IFN-γ expression (Y-axis) in unstimulated splenic CD4 T cells (X-axis) from naïve (first column), ECTV-WT- (second column) or ECTV-IFN-γbpΔ- (third column) infected WT and GKO mice. The numbers in the upper right quadrants in individual panels indicate percentages of IFN-γ-producing CD4 T cells.
(TIF)
Data are from one of three separate experiments showing intracellular IL-4 expression (Y-axis) in unstimulated splenic CD4 T cells (X-axis) from naïve (first column), ECTV-WT- (second column) or ECTV-IFN-γbpΔ- (right column) infected WT and GKO mice. The numbers in the upper right quadrants in individual panels indicate percentages of IL-4-producing CD4 T cells.
(TIF)
Data in this figure is the same as in Fig. 1 but presented to compare survival curves of each GKO strain with wild type BALB/c mice. P values were obtained by using Kaplan-Meier Log rank statistical test: *, p < 0.05.
(TIF)
Data in this figure is the same as in Fig. 1 but presented to compare survival curves of each GKO strain with wild type BALB/c mice. P values were obtained by using Kaplan-Meier Log rank statistical test: *, p < 0.05.
(TIF)
a EVM represents nomenclature for ECTV-specific 8 T cell determinants.
(DOCX)
a ECTV-WT vs. ECTV-IFN-γbpΔ P value using Logrank (Mantel-Cox) test. For extremely significant (****) P < 0.0001; extremely significant (***) 0.0001< P <0.001; very significant (**) 0.001< P <0.01; significant (*) 0.01< P <0.05; not significant (ns) P ≥ 0.05. b Number in brackets is the median survival time in days. c Number of animals in group. d BALB/c.WT vs. GKO strain P value using Logrank (Mantel-Cox) test. e undefined
(DOCX)
a To evaluate significant differences between groups, viral titers were log transformed and 2-way ANOVA performed followed by Fisher’s LSD test. For extremely significant (****) P < 0.0001; extremely significant (***) 0.0001< P <0.001; very significant (**) 0.001< P <0.01; significant (*) 0.01< P <0.05; not significant (ns) P ≥ 0.05. b ECTV-WT vs. ECTV-IFN-γbpΔ. c BALB/c.WT vs. GKO strain.
(DOCX)
a To evaluate significant differences between groups, viral titers were log transformed and 2-way ANOVA performed followed by Fisher’s LSD test. For extremely significant (****) P < 0.0001; extremely significant (***) 0.0001< P <0.001; very significant (**) 0.001< P <0.01; significant (*) 0.01< P <0.05; not significant (ns) P ≥ 0.05. b ECTV-WT vs. ECTV-IFN-γbpΔ. c BALB/c.WT vs. GKO.
(DOCX)
a To evaluate significant differences between groups, viral titres were log transformed and 2-way ANOVA performed followed by Fisher’s LSD test. For extremely significant (****) P < 0.0001; extremely significant (***) 0.0001< P <0.001; very significant (**) 0.001< P <0.01; significant (*) 0.01< P <0.05; not significant (ns) P ≥ 0.05. b ECTV-WT vs. ECTV-IFN-γbpΔ. c BALB/c.WT vs. GKO strain.
(DOCX)
Data Availability Statement
All data are included within the paper.