Abstract
In utero exposure to diethylstilbestrol (DES) has been associated with increased risk of adverse health outcomes such as fertility problems and vaginal as well as breast cancer. Animal studies have linked prenatal DES exposure to lasting DNA methylation changes. We investigated genome-wide DNA methylation and in utero DES exposure in a sample of non-Hispanic white women aged 40–59 years from the Sister Study, a large United States cohort study of women with a family history of breast cancer. Using questionnaire information from women and their mothers, we selected 100 women whose mothers reported taking DES while pregnant and 100 control women whose mothers had not taken DES. DNA methylation in blood was measured at 485,577 CpG sites using the Illumina HumanMethylation450 BeadChip. Associations between CpG methylation and DES exposure status were analyzed using robust linear regression with adjustment for blood cell composition and multiple comparisons. Although four CpGs had p<105, after accounting for multiple comparisons using the false discovery rate (FDR), none reached genome-wide significance. In conclusion, adult women exposed to DES in utero had no evidence of large persistent changes in blood DNA methylation.
Introduction
The synthetic estrogen diethylstilbestrol (DES) was introduced as a drug to prevent miscarriage in the 1940’s and was widely prescribed to pregnant women. More than 2 million American women took the drug during pregnancy, exposing both themselves and their fetuses to high doses of estrogen [1]. Clinical trials during the 1950s failed to show any effect of DES on reducing the risk of miscarriage, and in 1971, DES exposure during fetal development was found to be associated with a rare form of vaginal adenocarcinoma in adolescent girls [2]. Since then, a number of other adverse health outcomes including infertility, early menopause and breast cancer, have been connected to in utero DES exposure [2–5].
Animal models show that early exposure to estrogen compounds, including DES, can change the expression levels of several genes [6–8]. Numerous animal studies have identified DES-exposure related epigenetic changes including DNA methylation and histone modifications [9–11]. While these estrogen-induced changes have mainly been observed in uterine or vaginal tissue, one study of the phytoestrogen genistein identified DNA-methylation changes in blood from 12-week old mice that had been exposed prenatally [7].
It is possible that prenatal exposure to DES induces lasting modifications to hematopoietic stem cells if the exposure occurs during early fetal development [12]. We hypothesized that exposure to DES in utero gives rise to persistent epigenetic changes (detectable in blood) and that these changes are partly responsible for the observed increased risk of infertility and cancer related health outcomes in the offspring. We conducted a study of DNA methylation patterns in whole blood from 100 women reporting that their mothers had used DES during pregnancy, and compared them to 100 women without exposure using the Illumina HumanMethylation450 BeadChip. To our knowledge this is the first study to evaluate possible effects of in utero DES exposure on genome-wide DNA methylation in humans.
Materials and Methods
Study design
All women in the study were participants of the Sister Study—a prospective cohort study of environmental and familial risk factors for breast cancer and other diseases. This cohort includes 50,884 women who were all breast cancer free at enrollment but had at least one sister diagnosed with breast cancer. All participants provided a blood sample at baseline and completed extensive questionnaires including a self-administered family history questionnaire on prenatal exposures. Detailed information about the Sister Study can be found at http://sisterstudy.niehs.nih.gov [13].
Within the Sister Study a sample of the participant’s mothers was also invited to participate in a sub study entitled the Mothers Validation Study (MVS). Sampling for the MVS was restricted to mothers under 60 who were reported to be alive at their daughters’ enrolment into the Sister Study and additionally weighted to include women whose daughters reported rare early life exposures, including DES. A total of 1802 mothers completed the MVS questionnaire which included a question about whether the she had used DES during pregnancy. The concordance between mothers and daughters reports on DES usage and exposure was addressed (S1 Table). In our sample selection we excluded 84 women with discordant mother-daughter reports on DES exposure, 163 with missing data on DES exposure, 39 women with insufficient blood samples, and 270 women because of age and ethnic restrictions (ages were limited to 40–59 years of age to capture the time period when DES was most widely prescribed and the sample was restricted to non-Hispanic whites because of possible racial/ethnic differences in methylation). Of the remaining women (n = 1246), with concordant mother-daughter DES reports, we randomly selected 100 of the 125 exposed women and 100 of the 1121 unexposed women (frequency matched by age to the exposed women) (S1 Fig.). This sample set has previously been used to study the effects of smoking on DNA methylation in blood [14].
Ethics Statement
Informed written consent was obtained from all participants prior to participation. The study was approved by the Institutional Review Boards of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health, and the Copernicus Group (http://www.cgirb.com/irb-services/).
DNA extraction, bisulfite conversion and quality control
All blood samples were collected using glass blood collection tubes with acid citrate dextrose (ACD). This was done during a home visit at baseline and the blood was stored at-20C prior to DNA extraction. Genomic DNA was extracted using automated equipment (Autopure LS, Qiagen, Valencia, CA) and quantified with Quant-iT PicoGreen dsDNA reagent (Invitrogen). One microgram (μg) of extracted DNA was bisulfite converted using the EZ DNA methylation kit (Zymo Research, Irvine, CA) according to the manufacturer’s protocol.
Illumina 450K methylation array
Methylation analysis was conducted at the NIEHS Molecular Genomics Core using the Illumina HumanMethylation450 BeadChip (Illumina, San Diego, CA). Samples were randomly assigned to eighteen beadchips with 12 spots on each using a balanced design with respect to exposure and age (<50 vs >+50). We included 6 DNA methylation controls (Zymo Research, Irvine, CA) and 10 duplicate samples, which were placed on different chips. One of the duplicates was eventually excluded due to poor methylation data quality. For the remaining 9 pairs of duplicates we calculated Pearson’s correlation coefficients for Infinium I and Infinium II probes separately (S2 Table). DNA was hybridized to the array following the manufacturer’s protocol and then scanned with an Illumina iScan (Illumina, San Diego CA). Raw intensity data were extracted using Illumina GenomeStudio software (version 2011.1).
We excluded 48,494 CpG probes based on the following criteria: 1) CpG probes with a common SNP (minor allele frequency ≥ 0.05 in Europeans based on the 1000 Genomes project data-release/20130502) located at the target CpG site; 2) CpGs with probes mapping to multiple genomic locations; 3) CpGs located on the Y chromosome.
At each CpG site on the array, methylation status was determined based on intensity measures of probes corresponding to unmethylated (U) or methylated (M) CpGs. We pre-processed the M and U intensity values separately for Infinium I and II CpG probes detailed as follows: 1) for Infinium I probes, we separately background corrected (using the Robust Multichip Average (RMA) method [15]) and quantile normalized red and green channel probes; 2) for Infinium II probes, we first corrected dye bias between U and M intensity values using the normalizeMethyLumiSet method in the R package “methylumi”, and then performed RMA background correction and quantile normalization separately for U and M intensity values; 3) The methylation level (beta value) for each CpG site was calculated as the ratio of normalized fluorescent intensities between methylated and unmethylated alleles β = M/(M+U+100).
The DNA methylation data set used in this publication can be accessed via www.sisterstudystars.org, or by contacting one of the authors.
Previously reported candidate genes affected by DES
We also specifically report on nine genes (EMB, WNT11 TGFB1, ERBB2, EGFR, LTF, EGF, FOS, and JUN) that have previously been shown to have differential gene expression in mice uterine or vaginal tissue following pre or perinatal exposure to DES [8, 11, 16–20]. We compared the mean β-value for 75 CpGs in the 5’ region (as annotated by Illumina) of these genes for women with and without DES exposure histories.
Statistical analysis
Robust linear regression was used to test the association between CpG methylation profiles (β-values) and DES exposure status. In order to adjust for multiple testing, the false discovery rate (FDR) was calculated using the q-value framework, q<0.05 was used as the cutoff for genome wide significance [21]. In the association tests we adjusted for the proportions of 6 different types of white blood cells (CD8 T cells, CD4 T cells, B cells, granulocytes, monocytes, and Natural killer cells) estimated using a method described by Houseman et al [22]. Briefly, we first utilized a publically available Illumina HumanMethylation450 dataset (GSE35069) [23] for 60 cell type specific samples to select the top 100 most informative CpGs with respect to blood cell types; based on methylation profiles of the 100 CpGs for our samples, we then estimated the composition of the 6 cell types in each sample. Additional adjustments were made for age at menarche, BMI, and parity.
Results
Participant characteristics
DES-exposed women were similar to unexposed women in regard to age, ever use of OCs, and smoking status; a greater percent of exposed women had BMI<25, menarche at 14+ years, and were nulliparous than unexposed women (Table 1).
Table 1. Baseline characteristics of DES-exposed and unexposed women selected from the Sister Study.
| Characteristics | Exposed (n = 100)% | Unexposed (n = 100%) | P d |
|---|---|---|---|
| 40–49 yr | 42 | 42 | |
| 50–59 yr | 58 | 58 | 1.00 |
| Body Mass Index | |||
| <20 | 7 | 7 | |
| 20–24.9 | 49 | 38 | |
| 25–29.9 | 26 | 25 | |
| ≥30 | 18 | 30 | 0.22 |
| Age at Menarche | |||
| <12 yr | 15 | 25 | |
| 12–13 yr | 51 | 49 | |
| ≥14 yr | 34 | 26 | 0.17 |
| Age at Menopause a | |||
| <45 yr | 11 | 8 | |
| 45–55yr | 29 | 31 | |
| ≥55 yr | 2 | 1 | 0.66 |
| Menopause Status | |||
| Postmenopausal | 45 | 45 | |
| Premenopausal | 55 | 55 | 1.00 |
| Oral Contraceptive Use | |||
| Ever | 90 | 92 | |
| Never | 10 | 8 | 0.62 |
| HRT Use b | |||
| Current | 8 | 13 | |
| Ever | 15 | 12 | |
| Never | 22 | 19 | 0.42 |
| Parity | |||
| 0 | 40 | 21 | |
| 1 | 13 | 16 | |
| 2 | 29 | 38 | |
| ≥3 | 18 | 25 | 0.03 |
| Smoking Status c | |||
| Current | 8 | 4 | |
| Former | 35 | 34 | |
| Never | 54 | 58 | 0.60 |
| Mothers Education at Age 13 c | |||
| <HS, HS/GED | 55 | 60 | |
| Some College | 12 | 10 | |
| Associate/Tech | 12 | 9 | |
| Bach degree | 16 | 17 | |
| Grad degree | 4 | 2 | 0.82 |
| Family Income During Childhood | |||
| Well Off | 9 | 9 | |
| Middle Income | 75 | 69 | |
| Low Income | 13 | 17 | |
| Poor | 3 | 5 | 0.73 |
| Maternal Smoking c | |||
| Definitely | 34 | 32 | |
| Probably | 5 | 6 | |
| Probably not | 4 | 2 | |
| Definitely not | 56 | 59 | 0.83 |
| Birth Weight c | |||
| <2500 g | 13 | 6 | |
| 2500-<4000g | 70 | 71 | |
| 4000+ g | 7 | 9 | 0.25 |
| Gestational Age c | |||
| Not Early, 2+Weeks | 67 | 68 | |
| Early, 2–4 Weeks | 10 | 5 | |
| Early, 1+ months | 6 | 4 | 0.40 |
aAge at menopause was ascertained among the 45 exposed and 45 unexposed women who were postmenopausal
bHRT was ascertained among postmenopausal women only
cColumn % does not add to 100% due to missing data
dBased on Chi-Square test
Epigenome wide study
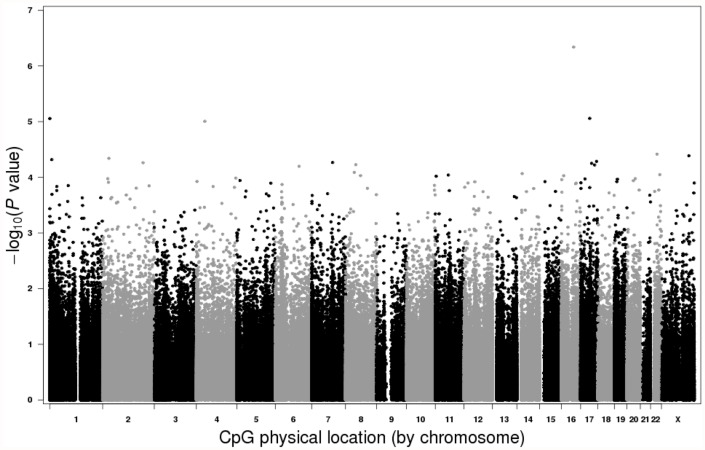
In our primary model, adjusted for multiple testing and blood cell composition, four CpGs in two different genes (KIFC3, and DCAKD) had nominal p values < 10-5, and an additional 18 CpGs in 18 different genes had nominal p values < 10-4 (Fig. 1). None of these 22 CpGs achieved genome-wide significance after considering multiple comparisons (q<0.05). Additional adjustments for age at menarche, BMI and parity did not affect the results.
Fig 1. Manhattan plot.
Log10 transformed DES association p-values for individual CpGs are plotted in relation to their chromosome location. No CpGs reached genome wide significance.
Candidate gene comparisons
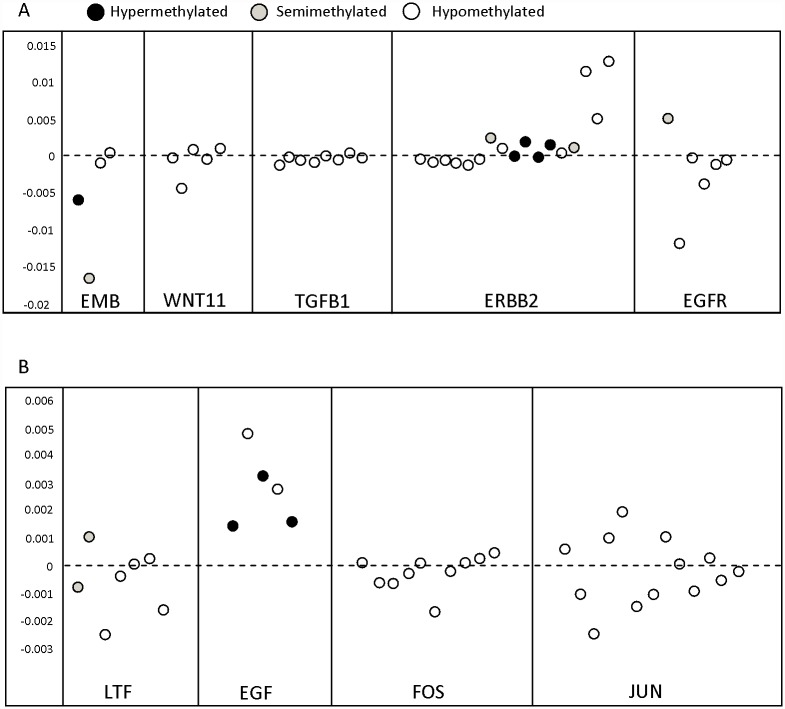
We examined DNA methylation values at 75 CpG sites located in the 5’ regions of nine previously reported genes. At these sites the average differences in DNA methylation between exposed and unexposed women were < 1%. None of the 75 CpG sites remained significant after adjusting for multiple comparisons (Fig. 2 and S3 Table).
Fig 2. Distribution of CpGs in the 5’end of 9 genes associated with differential methylation in animal models exposed to DES.
All CpGs are ordered from 5’ to 3’. Y axis shows the difference in mean beta values of exposed and unexposed women. Circle color depicts methylation status of individual CpGs (Black = Hyper-methylated [≥70%], Grey = Semi-methylated [<70%>30%], White = Hypo-methylated [≤30%]). All sites were selected based on annotations from the Illumina manifest. A) Genes that are up regulated in mice exposed to DES. B) Genes that are down regulated in mice exposed to DES.
Discussion
Epidemiologic studies have shown that women exposed to the synthetic estrogen DES in utero have increased risk of breast and vaginal cancer, preterm delivery, infertility, early menarche and other health outcomes[2, 4, 5, 24], and some of these risks, specifically concerning breast and vaginal cancer, persist as women grow older [3]. Although we hypothesized that persistent DNA methylation changes might be detectable in blood from women exposed to DES in utero, we found no evidence of this in our sample of non-Hispanic white women aged 40–59 years.
Several studies have found differences in gene expression and/or DNA methylation in adult mice or rats exposed to DES during development or early life [8, 17, 25]. Hyper or hypo-methylation of the 5’ promoter regions has previously been shown to control gene expression [26] which led us to focus on CpGs in this region of the gene. However, for 75 CpGs in the 5’ regions of the nine genes identified in rodent studies, we found no significant differences between exposed and unexposed women.
Strengths of this study include the availability of both mothers and daughters reports on DES exposure, and genome-wide coverage of CpG methylation sites (representing ~ 2% of CpGs in the genome). Limitations include lack of detailed information on dose, route of exposure, timing, or duration of DES use. Additionally we had limited power to detect small effect sizes (defined by the absolute methylation difference between two groups, divided by the standard deviation). With a sample size of 200 (1:1 case/control ratio) and assuming a p-value threshold of 10-8, our study had 80% power to detect CpG sites with effect sizes of at least 0.67. The average effect size of our top 4 CpG sites was 0.5, which would require a study sample size of 354 to obtain 80% power.
In this study we used DNA from blood rather than from a known target tissue such as vagina, cervix or endometrium. Histologic changes in target tissues, including vaginal epithelium, are known to correlate with dose of DES exposure and with increased risk of adverse outcomes such as vaginal carcinoma [3, 27]. Although it may be possible to compare methylation in target tissues using archival formalin-fixed paraffin-embedded (FFPE) samples, it is difficult to find adequate FFPE samples from DES-exposed women. In addition, methylation analysis of degraded and cross-linked FFPE samples present substantial technical challenges. In conclusion, although we cannot rule out the possibility of effects at younger ages or in other tissues, our study finds no evidence of large persistent effects of in utero DES exposure on blood DNA methylation.
Supporting Information
The 100 exposed women were randomly selected from the 125 eligible exposed women. The 100 unexposed women were selected based on age frequency matching to the exposed women.
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank Kevin Gerrish and Laura Wharey at the NIEH microarray core for processing and running the Illumina 450K bead arrays.
Data Availability
Ethical considerations prevent public sharing of data. Data requests may be sent to the corresponding author, or pursued through the study website: https://www.sisterstudystars.org.
Funding Statement
This study was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
References
- 1. Heinonen OP (1973) Diethylstilbestrol in pregnancy. Frequency of exposure and usage patterns. Cancer 31: 573–577. [DOI] [PubMed] [Google Scholar]
- 2. Herbst AL, Ulfelder H, Poskanzer DC (1971) Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med 284: 878–881. [DOI] [PubMed] [Google Scholar]
- 3. Hoover RN, Hyer M, Pfeiffer RM, Adam E, Bond B, Cheville AL, et al. (2011) Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med 365: 1304–1314. 10.1056/NEJMoa1013961 [DOI] [PubMed] [Google Scholar]
- 4. Palmer JR, Hatch EE, Rosenberg CL, Hartge P, Kaufman RH, Titus-Ernstoff L, et al. (2002) Risk of breast cancer in women exposed to diethylstilbestrol in utero: prelimiinary results (United States). Cancer Causes Control 13: 753–758. [DOI] [PubMed] [Google Scholar]
- 5. Palmer JR, Wise LA, Hatch EE, Troisi R, Titus-Ernstoff L, Strohsnitter W, et al. (2006) Prenatal diethylstilbestrol exposure and risk of breast cancer. Cancer Epidemiol Biomarkers Prev 15: 1509–1514. [DOI] [PubMed] [Google Scholar]
- 6. Block K, Kardana A, Igarashi P, Taylor HS (2000) In utero diethylstilbestrol (DES) exposure alters Hox gene expression in the developing mullerian system. FASEB J 14: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 7. Vanhees K, Coort S, Ruijters EJ, Godschalk RW, van Schooten FJ, Barjesteh van Waalwijk van Doorn-Khosrovani S. (2011) Epigenetics: prenatal exposure to genistein leaves a permanent signature on the hematopoietic lineage. FASEB J 25: 797–807. 10.1096/fj.10-172155 [DOI] [PubMed] [Google Scholar]
- 8. Newbold RR, Jefferson WN, Grissom SF, Padilla-Banks E, Snyder RJ, Lobenhofer EK (2007) Developmental exposure to diethylstilbestrol alters uterine gene expression that may be associated with uterine neoplasia later in life. Mol Carcinog 46: 783–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bromer JG, Wu J, Zhou Y, Taylor HS (2009) Hypermethylation of homeobox A10 by in utero diethylstilbestrol exposure: an epigenetic mechanism for altered developmental programming. Endocrinology 150: 3376–3382. 10.1210/en.2009-0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li S, Hursting SD, Davis BJ, McLachlan JA, Barrett JC (2003) Environmental exposure, DNA methylation, and gene regulation: lessons from diethylstilbesterol-induced cancers. Ann N Y Acad Sci 983: 161–169. [DOI] [PubMed] [Google Scholar]
- 11. Li S, Washburn KA, Moore R, Uno T, Teng C, Newbold RR, et al. (1997) Developmental exposure to diethylstilbestrol elicits demethylation of estrogen-responsive lactoferrin gene in mouse uterus. Cancer Res 57: 4356–4359. [PubMed] [Google Scholar]
- 12. Smith Z, Chan M, Mikkelsen T, Gu H, Gnirke A, Regev A, et al. (2012) A unique regulatory phase of DNA methylation in the early mammalian embryo. Nature 484: 339–344. 10.1038/nature10960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(2014) The Sister Study Cohort Description. NIEHS (National Institute of Environmental Health Sciences)
- 14. Harlid S, Xu Z, Panduri V, Sandler DP, Taylor JA (2014) CpG sites associated with cigarette smoking: analysis of epigenome-wide data from the sister study. Environ Health Perspect 122: 673–678. 10.1289/ehp.1307480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4: 249–264. [DOI] [PubMed] [Google Scholar]
- 16. Kamiya K, Sato T, Nishimura N, Goto Y, Kano K, Iguchi T, et al. (1996) Expression of estrogen receptor and proto-oncogene messenger ribonucleic acids in reproductive tracts of neonatally diethylstilbestrol-exposed female mice with or without post-puberal estrogen administration. Exp Clin Endocrinol Diabetes 104: 111–122. [DOI] [PubMed] [Google Scholar]
- 17. Miyagawa S, Suzuki A, Katsu Y, Kobayashi M, Goto M, Handa H, et al. (2004) Persistent gene expression in mouse vagina exposed neonatally to diethylstilbestrol. J Mol Endocrinol 32: 663–677. [DOI] [PubMed] [Google Scholar]
- 18. Nakamura T, Miyagawa S, Katsu Y, Watanabe H, Mizutani T, Sato T, et al. (2012) Wnt family genes and their modulation in the ovary-independent and persistent vaginal epithelial cell proliferation and keratinization induced by neonatal diethylstilbestrol exposure in mice. Toxicology 296: 13–19. 10.1016/j.tox.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 19. Nelson KG, Sakai Y, Eitzman B, Steed T, McLachlan J (1994) Exposure to diethylstilbestrol during a critical developmental period of the mouse reproductive tract leads to persistent induction of two estrogen-regulated genes. Cell Growth Differ 5: 595–606. [PubMed] [Google Scholar]
- 20. Yamashita S, Takayanagi A, Shimizu N (2001) Effects of neonatal diethylstilbestrol on c-fos and c-jun protooncogene expression in the mouse uterus. Histol Histopathol 16: 131–140. [DOI] [PubMed] [Google Scholar]
- 21. Storey JD, Tibshirani R (2003) Statistical significance for genomewide studies. Proc Natl Acad Sci U S A 100: 9440–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, et al. (2012) DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics 13: 86 10.1186/1471-2105-13-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reinius LE, Acevedo N, Joerink M, Pershagen G, Dahlen SE, Greco D, et al. (2012) Differential DNA methylation in purified human blood cells: implications for cell lineage and studies on disease susceptibility. PloS One 7: e41361 10.1371/journal.pone.0041361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. D’Aloisio AA, DeRoo LA, Baird DD, Weinberg CR, Sandler DP (2013) Prenatal and infant exposures and age at menarche. Epidemiology 4: 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bredfeldt TG, Greathouse KL, Safe SH, Hung MC, Bedford MT, Walker CL. (2010) Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT. Mol Endocrinol 24: 993–1006. 10.1210/me.2009-0438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Suzuki MM, Bird A (2008) DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet 9: 465–476. 10.1038/nrg2341 [DOI] [PubMed] [Google Scholar]
- 27. O’Brien PC, Noller KL, Robboy SJ, Barnes AB, Kaufman RH, Tilley BC, et al. (1979) Vaginal epithelial changes in young women enrolled in the National Cooperative Diethylstilbestrol Adenosis (DESAD) project. Obstet Gynecol 53: 300–308. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The 100 exposed women were randomly selected from the 125 eligible exposed women. The 100 unexposed women were selected based on age frequency matching to the exposed women.
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
Ethical considerations prevent public sharing of data. Data requests may be sent to the corresponding author, or pursued through the study website: https://www.sisterstudystars.org.