Abstract
Surgery is well-established option for the treatment of Crohn’s disease that is refractory to medical therapy and for complications of the disease, including strictures, fistulas, abscesses, bleeding that cannot be controlled endoscopically, and neoplastic degeneration. For a condition like Crohn’s disease, where medical management is the rule, other indications for surgery are considered controversial, because the therapeutic effects of surgery are limited to the resolution of complications and the rate of recurrence is high, especially at sites of the surgical anastomosis. In the authors’ opinion, however, surgery should not be considered a last-resort treatment: in a variety of situations, it should be regarded as an appropriate solution for managing this disease. Based on a review of the literature and their own experience, the authors examine some of the possibilities for surgical interventions in Crohn’s disease and the roles played in these cases by diagnostic imaging modalities.
Keywords: Crohn’s disease, Surgery, Imaging for Crohn’s disease
Riassunto
La chirurgia è un’opzione terapeutica consolidata per il trattamento della malattia di Crohn refrattaria alla terapia medica e per le complicanze legate a tale patologia quali stenosi, fistole, ascessi, sanguinamento non trattabile endoscopicamente, oltre che per i casi di degenerazione neoplastica. Per condizioni come la malattia di Crohn nelle quali il trattamento medico ha un ruolo primario, altre indicazioni per la chirurgia sono controverse, poiché i suoi effetti terapeutici sono limitati alla risoluzione delle complicanze, e la frequenza di recidive è alta, soprattutto a livello dell’anastomosi. Secondo l’opinione degli autori, tuttavia, la chirurgia non deve essere considerata come ultima opzione di trattamento: in diverse situazioni dovrebbe essere ritenuta come una soluzione adeguata nel trattamento multidisciplinare della patologia. Basandosi sulla revisione della letteratura corrente e sulla propria esperienza, gli autori hanno esaminato alcune possibilità di intervento chirurgico nella malattia di Crohn ed il ruolo che svolgono in queste condizioni le tecniche di imaging.
Introduction
The clinical history of Crohn’s disease involving the gastrointestinal tract is characterized by alternating periods of subjective well-being and symptom exacerbation. The active phases of the disease are associated with clinical symptoms and histological, biological, and endoscopic evidence of inflammation. During remissions, however, the intestine is by no means lesion-free: the course of the disease is characterized by persistent subclinical inflammation, which can give rise to fibrostenotic or penetrating (fistulas, abscesses) intestinal lesions that may require surgical intervention. Aside from the management of neoplastic complications, surgery is often used to treat Crohn’s disease that is refractory to drug therapy or its complications, such as strictures, fistulas, abscesses, or bleeding that cannot be managed endoscopically. The therapeutic effects of surgery are limited exclusively to the resolution of complications because surgery is frequently followed by disease recurrence, particularly at the level of surgical anastomoses. For a disease like Crohn’s, where medical management is the rule, recourse to surgery is almost always a subject of debate. Concern that “excessive surgery” (in terms of the number of interventions as well as the amount of tissue resected) will provoke a short-bowel syndrome often leads physicians to consider surgery a last resort, and this negative perception is often transmitted to the patient. Nonetheless, the introduction in recent years of immunosuppressive and biological (anti-TNF-alpha antibodies) drugs for the treatment of Crohn’s disease has not substantially changed the incidence of surgical interventions (overall or relative to the pattern of expression at the time of surgery). The conditions that constitute indications for surgery vary widely in terms of the type of presentation (urgent or elective), the number and location of lesions within the gastrointestinal tract, and disease behavior patterns (inflammatory, stricturing, penetrating). As a result, the possible surgical solutions are equally variegated in terms of the approach (minimally invasive vs. traditional) and modality of treatment.
Accurate preoperative imaging studies capable of providing high-precision definition of the disease (lesion location, extension, presence of abscesses or fistulas) are known to play a fundamental role in surgical decision-making. The most promising role for these studies, however, is in decisions regarding the timing of surgery: identification of sclerocicatricial disease that is unresponsive to medical therapy before treatment failure is clinically evident often means that it can be managed with less complex forms of surgery.
Indications for surgery and imaging
Epidemiology
The Montreal Classification currently distinguishes several categories of Crohn’s disease based on age at onset, site of involvement, and predominant disease behavior (inflammatory, B1; stricturing, B2; and penetrating, B3) [1].
The probability of surgery in patients with Crohn’s disease and the factors correlated with it have been investigated in numerous population-based and cohort studies. A study conducted in Olmsted County, Minnesota, found that 41 % of patients whose Crohn’s disease was diagnosed between 1935 and 1975 underwent disease-related abdominal surgery at least once during follow-up (median 8.5 years after diagnosis) [2]. A recently published update on this population (1970–2004) showed that the cumulative risks of first intestinal resection were 24, 49, and 64 % 1, 10, and 30 years after diagnosis. Comparison of the findings of these two studies reveals that the likelihood of surgery has remained essentially stable over time and that roughly half of all patients will require resectional surgery within the first 10 years after diagnosis [3]. In other population-based studies, the cumulative risk of surgery 10 years after diagnosis ranged from approximately 40 to 55 % [4–6]. A report published in 2010 found that the probability of intestinal surgery in Welsh patients (1986–2003) during the first year after diagnosis was independently related to the site(s) of involvement, use of oral steroids within the first 3 months after diagnosis, and early use of thiopurines [7].
One of the current controversies regarding the degree to which new forms of treatment (immunosuppression, anti-TNFα antibodies), or their early use, has modified the natural history of the disease and the need for surgical interventions. Contrary to initial hypotheses, the introduction of thiopurine drugs has not reduced the likelihood of surgery [7, 8]. As for the biological agents, Peyrin-Biroulet et al. [9] analyzed follow-up data (median 56 months) for 296 patients with Crohn’s disease diagnosed between 2000 and 2008 and found that early use of biological therapy decreased the risk of surgery significantly, whereas the reduction was much more limited with thiopurine therapy. Long-term population-based studies have shown that small intestinal resection rates have not in effect changed since the introduction of anti-TNF-alpha therapy, and in some smaller studies, rates have actually increased in patients with fistulating disease of the small intestine [10–12]. A study conducted recently in the Netherlands found an increased incidence of urgent surgery for free perforation among patients who had been treated with anti-TNF antibodies [13]. This finding appears to be supported by data from a study in Germany of 913 patients who underwent abdominal surgery for Crohn’s over a 33-year interval (1970–2002): the period following the introduction of biological drug therapy was characterized by a significant increase in surgical procedures for peritonitis [14].
Bouguen and Peyrin-Biroulet [15] have suggested that the need for surgical intervention will decline in the future, owing not to the superior efficacy of biological therapy but to its earlier use, which can modify the natural history of the disease. The development of fibrosis (progression from B1 to B2 disease) is a biological process triggered by inflammation and possibly characterized by genetically determined increases in susceptibility [16, 17]. There is evidence to suggest, however, that this type of progression can also occur in the absence of inflammation, which means that strategies aimed at controlling inflammation might be ineffective for preventing aggravation of B2 disease or progression to more severe forms characterized by abscess and/or fistula formation (B3) [16, 18–22]. Earlier use of “aggressive” anti-inflammatory therapies, before fibrosis begins and strictures form, may promote mucosal healing, and this effect might be associated with lower rates of recurrence and, as a result, of surgical intervention [21].
In the 2009 European Crohn’s and Colitis Organization (ECCO) guidelines [23], surgery is viewed as a treatment of last resort, mainly as a result of continuing progress in the field of anti-inflammatory therapies. The ECCO acknowledges, however, that with this approach, surgery is reserved for patients with advanced disease (B3), who are at high risk for septic complications. It is interesting to note that the same guidelines point out that the risk of short-bowel syndrome is more closely correlated with complications occurring with the first operation than with the number of uncomplicated operations performed. The truth is that the stability of surgical incidence rates after the introduction of biological drug therapies has led some authors to propose earlier recourse to these drugs and others to reassess the question of the optimal timing of surgery [18].
In patients with stable short-segment involvement that is wholly or partially fibrotic component (Figs. 1, 2), early resection can produce the same mucosal healing one expects to achieve with aggressive medical therapy. However, the recurrence-free interval is likely to be longer (as demonstrated by studies of patients who have undergone surgery relatively early for acute presentations or for early onset perforation) [24, 25]. Early surgical intervention in patients with B1 disease and short-segment lesions allows one to perform simple procedures, which can be carried out with minimally invasive techniques and without the increased risk of complications (postoperative infections in particular) associated with surgical treatment of more complex disease (B3) (Fig. 3) or disease that is being managed with steroids [23, 26]. Thiopurine therapy is not believed to increase the surgical risk [26], but the risks posed by biological therapies are less well defined: there is evidence to suggest that if these drugs are not discontinued several weeks before surgery, they can negatively affect the outcome of the procedure [26].
Fig. 1.
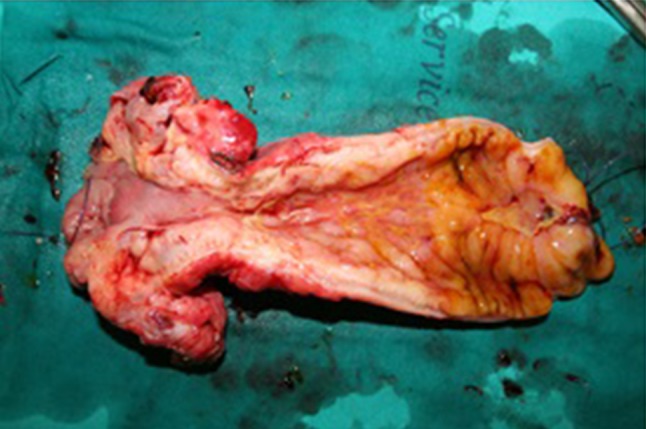
Resected segment of the ileum displaying fibrotic stricturing and inflammatory involvement with frank ulceration of the proximal resection margin
Fig. 2.
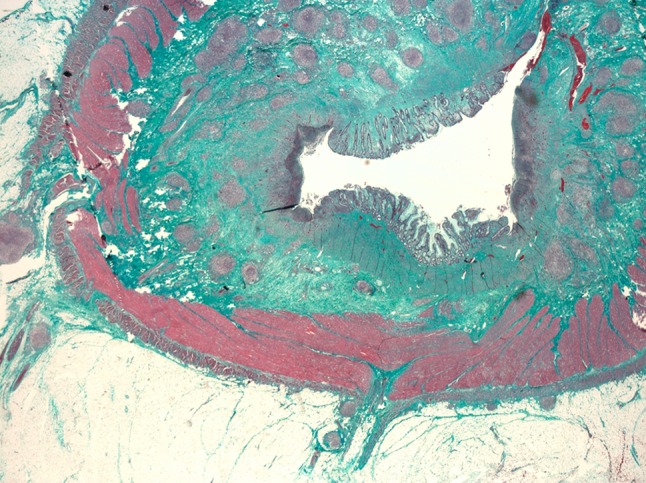
Macroscopic full-thickness specimen of the ileal wall stained with Masson’s trichrome: The submucosa is filled with collagen septa (green), which invade the tunica muscularis propria. Lymphoid aggregates are also present in the submucosal layer
Fig. 3.
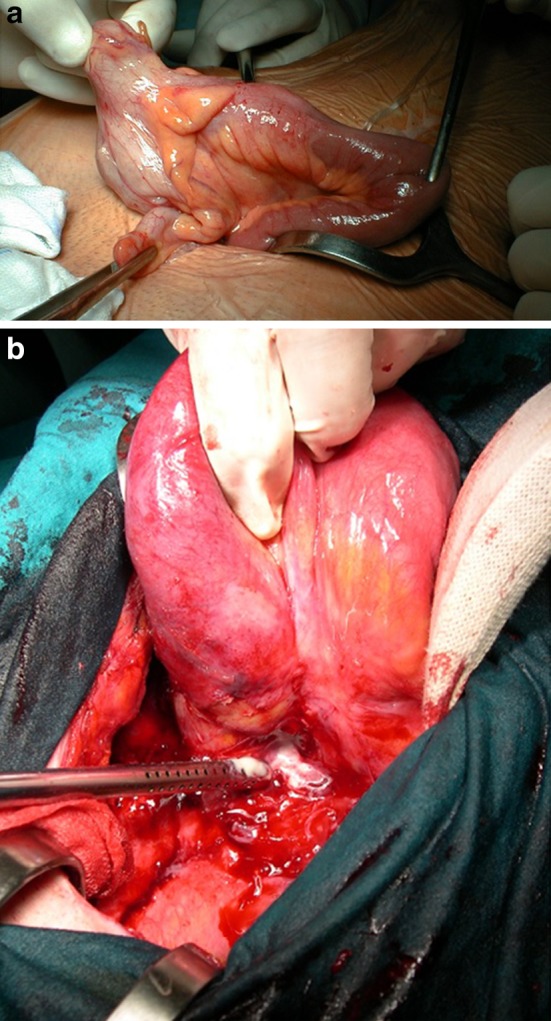
Video-assisted ileocecal resection for stricturing Crohn’s disease. a Complex procedure for fistulizing Crohn’s disease performed via laparotomy. b An abscess is clearly seen at the base of the mesentery
Clinical aspects
The classical indications for surgery are complications requiring urgent treatment (perforation, occlusion, hemorrhage), penetrating (B3) disease in which immunosuppressive or biological therapy cannot be started until abscesses or fistulas have been resolved surgically (in rare cases, with emergency surgery but more commonly urgent or even elective procedures), and cases that are or have become unresponsive to medical therapy.
Urgent presentations account for a non-negligible proportion of the indications for surgery. However, Crohn’s disease-related intestinal occlusion rarely requires emergency surgery. In 2000, Miller et al. [27] reported that Crohn’s disease was the third most common cause of small intestinal occlusion in western countries (4–7 % of all cases). In 2008 Cosnes [28] estimated that patients with Crohn’s disease have a 40 % probability of developing intestinal stenosis during the first 400 months after the diagnosis. As for abscess formation (which is not always associated with acute presentation and does not always require urgent surgical intervention), its prevalence in carious case series ranges from approximately 10 to 28 %, with a cumulative incidence of 9 % 10 years after diagnosis and 25 % after 25 years [29–31]. (The figures from many of these studies also include postoperative abscesses). Free perforation into the peritoneal cavity is much rarer, with a prevalence of 1.5 %: it frequently represents the first manifestation of the disease (30–60 %). In any case, it tends to occur early. Eighty percent of all cases occur during the first 4 years after diagnosis, and the event is not necessarily correlated with the aggressiveness of the disease [32–35]. Other less common acute complications of Crohn’s disease (especially that involving the colon) include toxic megacolon, which is present in 4.4–6.4 % of patients with colon involvement and is also an early event [36–38], and massive hemorrhage, with an incidence of around 1 %. In 15–20 % of patients with large intestinal Crohn’s disease, hemorrhage is the presenting symptom, and in 20–40 % of all cases it requires surgery [39, 40]. In many cases, an acute event leads to the diagnosis, but it is difficult to determine the true incidence because most of the published studies are retrospective analyses of cases managed in tertiary referral centers. Intraoperative diagnosis of Crohn’s disease during a scheduled appendectomy continues to be the most common scenario [41].
In the presence of complications requiring emergency or urgent treatment, the clinical presentation guides the diagnostic work-up, as it does in other conditions: endoscopy for hemorrhagic forms and ultrasound/computed tomography for occlusions, free perforations, and abscesses. Ultrasound displays high sensitivity (81 %) and specificity (93 %) in the identification of intra-abdominal abscesses [42]. Because of the risk of sepsis, immunosuppressive therapy is contraindicated in patients with abscesses. Their detection is thus an early, unequivocal indication for urgent surgery, which is often performed in two stages with the construction of a diverting stoma. In stable patients, however, ultrasound- or CT-guided percutaneous drainage of the abscess, accompanied by antibiotic therapy, can sometimes be a valid alternative to surgery. If necessary, drainage can be followed by one-stage surgery (resection and anastomosis performed in the same procedure). This approach is especially useful when the abscess develops against a background of active disease [26, 43].
Elective surgery is generally performed when the patient’s gastroenterologist sees a progressive decline in the response to medical therapy (manifested by worsening of symptoms, obstructive in most cases, although frank occlusion rarely occurs) or when the patient becomes steroid-dependent. It may also be requested when the disease produces a state of malnutrition, which is particularly important in pediatric patients because it can cause delays in growth and development [44].
Imaging
Endoscopy is the gold standard method for diagnosing and monitoring the evolution of upper GI involvement (proximal to the ligament of Treitz) and disease of the colon. However, since a single stricture of the large intestine is an indication for surgery, complementary radiological studies of the colon (barium enema, virtual colonoscopy, sonographic studies of the intestinal loops) are useful in these cases to characterize the stricture and the segments proximal to it.
Most surgically relevant lesions are found in the small intestine, which is beyond the reach of traditional endoscopy. New techniques, such as capsule endoscopy, push enteroscopy, and double-balloon endoscopy, can be useful in the diagnostic phase, although they also have limitations (low specificity in the case of capsule endoscopy, difficulties in incomplete evaluation of the small intestine in the case of push enteroscopy). These techniques are not generally used for follow-up, which relies mainly on radiological examinations.
For many years, the technique most widely used was the small-bowel enema (enteroclysis). It provided sufficiently precise information on the number and length of ileal strictures and above all their location within the gut. It has several serious drawbacks, however: it is difficult to perform and the results are not easy to interpret; it is poorly tolerated by patients; it involves considerable radiation exposure (an important consideration since patients with Crohn’s disease are often young), and it is of limited value in defining the inflammatory component of the strictures. (Inflammatory involvement of the mesentery/mesocolon will be reflected by areas in the abdominal cavity that appear “empty” but actually contain markedly thickened mesenteric tissue that has displaced the contrast medium-filled intestinal loops). For these reasons, the small-bowel enema has been widely abandoned in favor of other techniques [ultrasound, computed tomographic (CT) enterography, magnetic resonance (MR) tomography]. They can often provide a more precise picture of the inflammatory component, based on the degree of vascularization observed with the aid of intravenous contrast media. All of these methods are associated with moderately high rates of false-negative findings in the presence of short-segment jejunal involvement. In addition, although they provide more accurate information on the location of the lesions within the abdomen, they are less useful for localizing lesions along the course of the small intestine (aside from those involving the most proximal and distal segments). This importance of this information is often under-rated by radiologists and gastroenterologists, but it is of enormous importance to the surgeon.
Ultrasound
A meta-analysis published by Italian researchers in 2005 looked at the role of ultrasound in the diagnosis of Crohn’s disease. The accuracy of the examination was characterized by variability between facilities and between operators, with sensitivity values ranging from 75 to 94 % and specificities of 67–100 % [45]. Similar sensitivity (85 %) emerged from a more recent systematic review by Panes et al. [46].
An interesting study by Gasche et al. [47] evaluated the ability of ultrasound to identify intra-abdominal strictures in Crohn’s disease patients scheduled for surgery. Using intra-operative findings as the reference standard, the authors found that ultrasound correctly identified all 22 of the patients with strictures and correctly excluded the presence of these lesions in 10 of 11 cases (sensitivity 100 %, specificity 91 %). The high diagnostic accuracy of ultrasound in this setting has been confirmed by Maconi et al. [48], who reported a sensitivity of 74 % and specificity of 93 % for ultrasound detection of strictures, although, as they pointed out, the accuracy varied depending on the lesion’s location (ileum vs. colon).
The same studies also evaluated ultrasound’s role in the detection of intra-abdominal fistulas and abscesses. Gasche et al. [47] found that the examination correctly identified the presence of fistulas in 20/23 patients and correctly excluded such lesions in 9/10 (sensitivity 87 %, specificity 90 %). Similar performance was observed in the identification of intra-abdominal abscesses, which were correctly detected in 9/9 patients and correctly excluded in 22/24 (sensitivity 100 %, specificity 92 %).
These data show that, despite certain limitations related mainly to its operator-dependence and to the location of the lesions themselves (particularly those of the rectum, segments of the intestine other than the ileum, and the stomach), ultrasound is an indispensable tool for the diagnosis and characterization of Crohn’s disease and its complications.
Computed tomography
In the diagnosis and assessment of Crohn’s disease, equally important roles are played by abdominal CT and the more focused examination known as CT enterography. The latter differs from normal abdominal CT in that it allows multiplanar imaging and involves the administration of oral as well as intravenous contrast media.
An important meta-analysis by Horsthuis et al. [49] found that CT has a sensitivity of 84 % and specificity of 95 % in diagnosing Crohn’s disease and identifying the segments of the intestine affected by the disease. The systematic review conducted by Panes et al. [46] looked at the performance of CT enterography in identifying areas of stenosis in five studies [50–54] and found an overall sensitivity and specificity of 89 and 99 %, respectively. This method was also assessed in an interesting study by Chiorean et al. [55]: compared with intraoperative findings, CT enterography exhibited 92 % sensitivity with a specificity of only 39 %. The relative nonspecificity of the examination was probably related to the fact that data in this retrospective nature were collected from surgeons’ reports. Moreover, in all probability, the fact that the operative specimens were “opened” for pathologic assessment erroneously reduced the number of reference diagnoses of stricture. In the same study [55], CT enterography displayed 77 % sensitivity and 86 % specificity in the detection of fistulas and somewhat greater accuracy in the identification of intra-abdominal abscesses (sensitivity 86 %, specificity 87 %). The recent systematic review by Panes et al. [46] looked at the results of several studies and found that CT enterography had an overall sensitivity of 70 % and specificity of 97 % in the detection of fistulas. The sensitivity for abscess detection (based on review of five studies) was 84–100 % and the specificity was 95–100 %.
Computed tomographic enterography is thus a good technique for identifying intra-abdominal complications, also when surgery is being planned. Indeed, the ECCO [23] recommends it for assessing the disease and diagnosing complications of this type. It has its drawbacks, however: it requires patient preparation, it is not available in all areas, and it exposes the patient to a considerable dose of radiation. The latter feature renders CT enterography unsuitable for repeated use and limits is role in monitoring the disease.
Magnetic resonance
Magnetic resonance enterography (MRE), a more recently developed technique, is an excellent tool for evaluating abdominal complications. Like CT enterography, MRE involves the use of an oral contrast medium to distend the intestinal loops. Unlike CTE, it also requires the administration of drugs like butylbromide that reduce intestinal peristalsis, a source of motion artifacts that can diminish image quality [56].
The sensitivity and specificity of MRE in the diagnosis of Crohn’s disease and its main intra-abdominal complications have also been analyzed in systematic reviews and meta-analyses. Panes et al. [46] reviewed data from four studies (including the meta-analysis by Horstius et al. [49], which compared MRE with endoscopy for the diagnosis of Crohn’s disease. They found an overall sensitivity of 78 % and an overall specificity of 85 %. As in ultrasonography, one of the main parameters considered diagnostic in MRE was gut wall thickness (with a normal cut-off of 4 mm). In addition, however, increased gut wall enhancement and the presence of edema also displayed diagnostic value in MRE.
Several studies also assessed the accuracy of MRE in identifying intra-abdominal complications, such as strictures. Pariente et al. [57] reviewed data from several studies that assessed the performance of MRE against ileoscopy or capsule endoscopy findings [58–60] and reported an overall sensitivity and specificity of 88 and 95 %, respectively. Similar figures emerged regarding the ability of MRE to identify fistulas (sensitivity 88 %, specificity 93 %) [57]. Somewhat different results were reported by Panes et al. [46], who reviewed data from five different studies and found overall sensitivity and specificity figures of 76 and 96 % for fistula detection. Because of its sensitivity and specificity and the fact that it does not involve exposure to radiation, the Second European Evidence-based Consensus statement lists MRE as the method of choice for identifying the transmural complications of Crohn’s disease [23]. The value of magnetic resonance in the detection of intra-abdominal abscesses has been confirmed by both of the systematic reviews published on this issue: that conducted by Panes et al., which reported sensitivity of 86 % and specificity of 93 % [46], and that of Pariente et al., who found sensitivities ranging ranged from 86 to 100 % and specificities of 93–100 % [57].
These studies highlight certain advantages that MRE offers for the identification of intra-abdominal complications, such as the absence of radiation exposure and a more limited operator-dependency as compared with ultrasound. Nonetheless, MRE also has its limitations, which are mainly related to the high cost of the examination and the limited availability of the equipment needed to perform it.
All three of the imaging techniques considered above are fundamental tools for identifying indications for surgery in patients with Crohn’s disease, and they can also provide useful information for planning the intervention (approach to be used, techniques and strategies that can reduce the risk of complications). For example, when retroperitoneal inflammation is present (or simply suspected), preoperative or intraoperative ureteral stenting is usually advisable [61]. Each of these techniques has obvious limitations and advantages, and none displays any obvious superiority over the others (except possibly CT, whose use of ionizing radiation is a major drawback). All three techniques can thus be of value in the workup of complicated Crohn’s disease that may require surgery.
Surgical techniques
The location, number, and type of lesions have important implications in terms of the surgical approach and strategies to be used. Although considerable progress has been made in the accuracy of preoperative imaging studies, the actual clinical and anatomic features of the case may necessitate an unexpected change of plans (e.g., use of open surgery instead of laparoscopy, resection instead of strictureplasty) [61].
Gastroduodenal Crohn’s disease
Crohn’s disease rarely produces lesions proximal to the ligament of Treitz (2–4 %) [62], and when it does, they rarely require surgical treatment. In the presence of strictures (generally duodenal) that are refractory to therapy, including endoscopic dilatation, there are several surgical solutions. The one associated with the lowest risk (and therefore the most widely used) involves the creation of a by-pass (usually with a gastroenteric anastomosis although gastroduodenal and duodenojejunal anastomoses are also possible), with or without vagotomy [63].
For short-segment strictures involving the first, second, and third segments of the duodenum, the Heineke–Mikulicz strictureplasty is also used. Finney strictureplasty can be used for strictures involving the fourth portion. In these cases (as when atypical strictureplasties involving anastomosis between duodenum and isolated jejunal loops are performed), the loop of Treitz may have to be completely freed so that it can be fully rotated (Fig. 4). Solutions of this type cannot be used in the presence of abscesses or fistulas, and they are more technically complex than by-passes, but they offer a major advantage in that they allow subsequent endoscopic and histological assessment. Elimination of strictures is also believed to allow mucosal healing, which can have positive effects on lesions involving the sphincter of Oddi (when present) [61, 64–67].
Fig. 4.
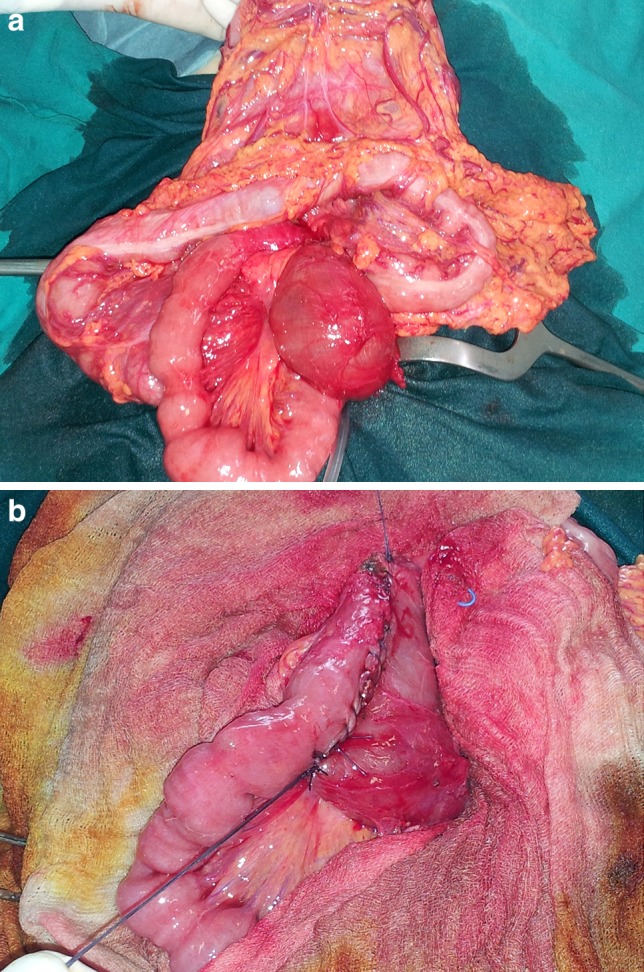
a Stenosis just below the ligament of Treitz, which is freed and partially de-rotated; appreciable dilatation of the gastroduodenal tract. b Elimination of the stenosis with Finney strictureplasty
Jejunoileal and ileocolic Crohn’s disease
Interventions involving the small intestine account for most of the surgery performed for abdominal Crohn’s disease: up to 10 % are for disease that is exclusively jejunoileal and 40–50 % are performed for disease of the terminal ileum (often with colon involvement as well) [62, 68, 69]. The situations in which surgery is indicated vary widely from short-segment primary lesions requiring relatively simple operations to recurrent abscesses and/or fistulas with severe mesenteric inflammation and disease patterns that vary (B1, B2, or B3) from one segment to another, major surgical challenges in which the normally simple process of debridement of the intestinal loops of the intestine can take hours. In complex cases like these, resolving the various problems often requires an eclectic approach and the use of diverse surgical techniques in a single operation.
Surgical treatment of ileal or ileocecal Crohn’s disease, which is, as we have seen, the most common form of the disease, has evolved over the years: from the concept of “curative” resection with margins that were free of macroscopic disease, the trend has been towards increasingly more limited resections. Given the unavoidable risk of postoperative recurrence, the aim has been to reduce the chances of short bowel syndrome caused by repeated interventions (a complication seen in up to 13.6 % of the cases reported in the 1980s) [70, 71]. This trend has been supported by the fundamental research conducted by Fazio et al. [72] in 1996, which demonstrated that the incidence of recurrence is not related to the amount of tissue resected or to the presence/absence of inflammatory disease at one or both of the resection margins. This finding has since been confirmed in a number of other studies, and it is now widely agreed that the resection should be as conservative as possible [73].
The techniques known as strictureplasties are based on the idea that in patients with stricturing disease (even if it includes an inflammatory component), elimination of the stricture without removing the inflamed tissue leads to resolution of the symptoms. Originally described by Indian authors as means for treating tubercular strictures of the gastrointestinal tract [74], strictureplasty was introduced as a means for treating ileal Crohn’s disease in the early 1980s. It was first used by Lee and Papaioannu [75] and later by Alexander-Williams and Haynes [76] as a conservative approach for the management of those relatively common cases in which a long segment of the small intestine presents by multiple short-segment strictures separated zones with no macroscopic disease. Strictureplasties are now considered a valid solution for the intestinal strictures caused by Crohn’s disease, with results that are quite similar to those offered by resection, as demonstrated by the results of a meta-analysis reported by Tichansky et al. [77].
The most widely used strictureplasty techniques are the ones described by Heinecke–Mikulicz for treatment of strictures less than 10 cm in length (although this cut-off may be overestimated) and the Finney technique, which is used for strictures up to 20 cm long. In both cases, the procedure involves a linear antimesenteric incision, which, in the Mickulicz procedure, is closed transversely. In the Finney, the intestine is first folded into a U and then closed with a side-to-side anastomosis. Another technique, the Michelassi strictureplasty, is indicated for long strictures (more than 30 cm). It is used mainly for recurrent stenosis and cases characterized by alternating long and short strictures. In these cases, the diseased segment is attached to an unaffected segment (in the colon, if necessary) with a long side-to-side anastomosis [78].
More recently, atypical strictureplasty techniques have been introduced (long anastomosis, iso- or antiperistaltic, between affected segments) (Fig. 4). The trend toward conservative management has led some surgeons, in Italy and elsewhere, to extend the indications for these atypical techniques to all forms of involvement without abscesses or fistulas (including ileocecal lesions), since strictureplasty tends to be followed by regression of active disease, even at the site of intervention [79–81] (Fig. 5). In adopting this approach, it is important to recall that patients with Crohn’s disease are up to 60 times more likely than members of the general population to develop adenocarcinoma of the small intestine [82].
Fig. 5.
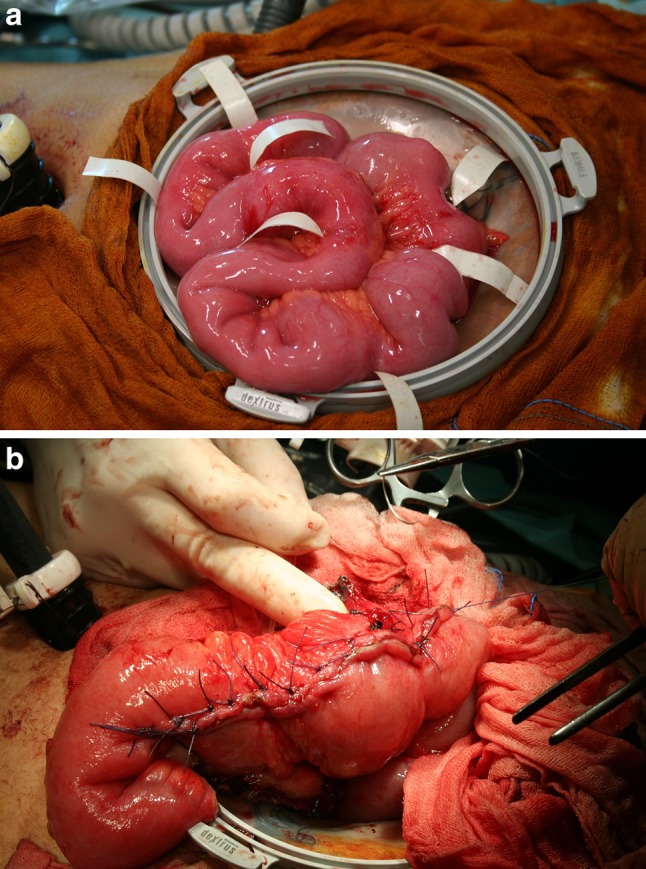
Multiple areas of ileal involvement (a). Treatment with atypical (modified Michelassi) strictureplasty. Video-assisted intervention with the Hand Port (b)
In a meta-analysis published by Canavan [83] Crohn’s disease was associated with a relative risk of small intestinal cancer of 33.2 (15, 9, 60, 9), and this risk has not decreased over the past 30 years. Similar results emerged from a meta-analysis conducted by other English investigators and published in 2007, where the relative risk was 28.4 (95 % CI 14.46–55.66) compared with the normal population [84]. It is currently impossible to identify subjects who are at risk for neoplastic transformation (which can also be associated with other malignancies, such as lymphomas). For one thing, in patients with exclusively ileal disease, the true onset of disease may be much earlier than the diagnosis. Initial-stage Crohn’s disease cannot be diagnosed with the imaging modalities currently being used for this purpose (i.e., ultrasound, CT, CT enterography, MR enterography), and in many cases the diagnosis is made unexpectedly on the basis of histological examination of resected tissue. Double-balloon enteroscopic biopsy of suspicious lesions could increase preoperative diagnosis of early-stage disease, but as noted above, routine use this approach is simply not possible right now. In light of these considerations, along with sporadic reports of adenocarcinoma arising at the site of strictureplasties [85, 86], the trend toward wider use of strictureplasty should be regarded with caution, especially in elderly patients or those with long-standing disease. In addition, as suggested by Michelassi himself, any lesion at the site of a strictureplasty that is even vaguely suspicious should be biopsied, and given the non-negligible frequency of carcinoids involving the appendix, appendectomies should always be performed with ileocecal strictureplasties (Fig. 6).
Fig. 6.
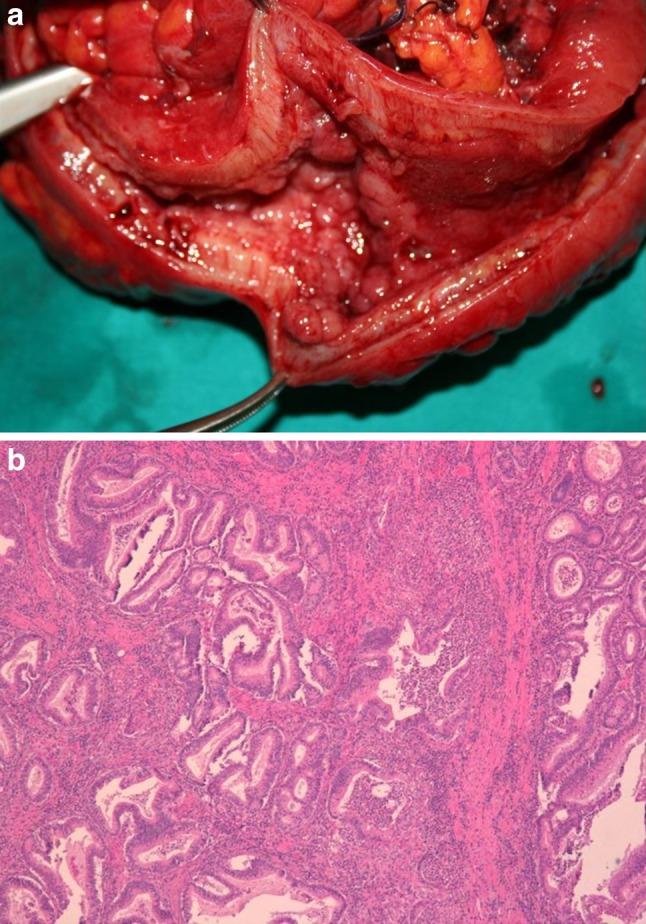
a Ileocolic specimen resected for stricturing/penetrating Crohn’s disease. b Histological examination: focal adenocarcinoma (T2N0)
In the rare cases in which small intestinal malignancies are diagnosed preoperatively (usually tumors located in areas of the gut that can be reached with traditional endoscopy), oncologically radical segmental resections should be performed whenever possible. Nonetheless, the prognosis in these cases is still worse than it is in colorectal forms of the disease, and it is even worse when cancers develop in segments of the intestine where fecal transit has been eliminated by surgical by-pass. The latter technique has in fact been abandoned (at least as a permanent solution) because it is associated with a high incidence of cancers that are already in the advanced stages when symptoms develop [87–89].
Resection is still the technique of choice for ileocecal lesions, and it is the only solution for patients with penetrating-abscess forming disease and active fistulas. As noted above, preoperative ultrasound- or CT-guided abscess drainage can sometimes eliminate the need for surgery or at least render it less urgent.
The objective of the preoperative workup should be to provide the most precise information possible on the number, extension, and characteristics of the lesions, including the presence and predominant nature (fibrotic or inflammatory) of strictures as well as the extramural extension of the inflammatory changes. Ultrasound is limited by its operator-dependency, but it is also widely available, and in addition to characterizing strictures (with or without the aid of acoustic contrast media proposed by some), it can also reveal extramural complications of Crohn’s disease, although its accuracy in this setting is inferior to that of CT [42, 45, 90, 91].
In all probability, ultrasound will be used more and more frequently (within the limits of its operator-dependency), but for the moment, CT and MRI remain the gold-standard methods for assessment of the small intestine. With these imaging modalities, disease extension and activity status can be reliably defined on the basis of wall thickening, increases in wall density after the administration of contrast media, and increased visibility of the vasa recta adjacent to the involved loop (“comb sign”). The severity and activity status of the disease can be defined on the basis of the degree of wall thickening and the presence of submucosal edema and gut wall ulceration, while the characteristics of the wall thickening (homogenous or stratified) on MRI can be used to differentiate active disease from fibrosclerotic forms. CT and MRI display similarly high accuracy in identifying extramural complications [26, 92–95].
Currently, CT is more widely used, in part because it is more widely available and in part because it is probably preferable for use in urgent situations. However, MRI is superior for distinguishing between inflammatory and sclerosing disease (which, as we have seen, is an important factor when early surgery is being considered), and the fact that it entails no radiation exposure is also an advantage. For these reasons, MRI is likely to play an increasing role in the future [96].
Ileal and ileocolic resections are potentially simple procedures that can sometimes be performed with minimally invasive techniques, but in patients with recurrent penetrating, fistulizing disease, these operations can be highly complex. Fistulas can involve other segments of the GI tract or other organs/systems. The most common forms are enterovesical, enterovaginal, and enterocutaneous. Fistulectomy and closure of the orifice is sufficient on the nondigestive side of the fistula. When the fistula involves different segments of the gastrointestinal tract, the presence of disease in the segment with secondary involvement (in many cases the sigmoid colon or duodenum). This is fundamental for deciding whether the orifice can be closed with sutures or whether minimal resection is required [26].
The type of anastomosis that should be used to restore continuity after an ileal or ileocolic resection has been widely debated. A meta-analysis published in 2007 showed the stapled side-to-side closures offer greater anastomotic safety [97], but they can also cause blind pouches that may be more susceptible to early recurrence of inflammatory disease. To eliminate this risk, the side-to-side technique known as the functional end-to-end (FEEA) anastomosis was developed. It can be sutured manually or mechanically and is currently regarded as the method of choice in the ECCO and ACOI Guidelines [23, 26]. A recent review of 44 RCTs by Van Loo et al. [98] found no differences between hand-sewn and stapled anastomoses in terms of short-term results or the incidence of recurrence. The fundamental requisites for a successful anastomosis are the absence of traction and good vascularization of the stumps, and the T-T (manual) anastomosis offers excellent results. It is easy to construct, especially when the stumps are of similar caliber, and it probably holds up better than a FEEA if endoscopic dilatation has to be performed for recurrent short-segment strictures.
Colorectal Crohn’s disease
Crohn’s disease is frequently confined exclusively to the colon (20–30 % of cases) [62]. The diagnosis is usually made endoscopically, and the presence of stenosis that prevents passage of the scope may be an indication for surgery. In these cases, radiological studies (barium enema, CT colonography) can be used to assess the condition of the gut upstream from the obstruction, but this type of disease is rarely associated with penetrating lesions like those seen in the ileum.
The most widely accepted approach for isolated colon lesions involves limited resection of the involved segment, but treatment becomes more complex when there are multiple areas of involvement in the colon (with or without ileal disease). In these cases, many authors advise subtotal colectomy with an ileorectal anastomosis, although the presence of multiple, widely separated areas of involvement might also justify the use of double resection [26]. However, Crohn’s disease of the colon is known to be associated with an increased risk of post-resectional recurrence: the percentage of patients who will require additional colon surgery within 5.5 years may be as high as 62 % [99–101]. For this reason, in the absence of jejunoileal disease, multiple sites of involvement in the colon may be an indication for total colectomy (or proctocolectomy when there is rectal involvement) with construction of a permanent ileostomy [99].
Rectal involvement is often associated with strictures or fibrosis, which requires proctectomy and permanent ostomy construction. If the patient also has active, untreatable, perianal disease, surgery is associated with a high risk of septic complications, and many surgeons, therefore, advise a two-stage approach involving colostomy or ileostomy followed by proctectomy several months later, when the exclusion of fecal transit has resolved the perianal sepsis [61].
In addition to providing updated imaging data, one of the aims of the preoperative workup should be the identification of a suitable site for ostomy, even when there is only a limited possibility that one will be needed. When possible, this should be done with the aid of an experienced stoma therapist and the site should always be marked. During resective surgery (ileal, ileocolic, colic), protective colostomies and especially protective ileostomies often have to be constructed to reduce the risk anastomotic failure related to the presence of abscesses, current therapy with steroids (and in some authors’ opinions also with more recently developed agents like the anti-TNF alpha antibodies), multiple resections, and malnutritional states [102].
Although the risk of colon cancer is increased in patients with Crohn’s disease, the increase is less marked than it is in ulcerative colitis [103]. Major advances are being made in the field of diagnostic endoscopy, including the development of image-magnification and high-definition imaging technologies, so if the patient has been followed regularly and appropriately, unexpected postoperative diagnoses of cancers are rare. Resections of the colon (where the imperative regarding minimal resection is less absolute) must be performed in accordance with the criteria of oncologic radicality, even in cases in which the malignancy of the lesion is uncertain (presence of high-grade dysplasia on biopsy of a lesion that could not be removed endoscopically) [26].
However, colon involvement in Crohn’s disease can also take the form of pancolitis. The clinical picture in these cases is characterized by inflammatory disease that is poorly controlled with medication, similar to that of ulcerative colitis. In fact, the differential diagnosis may be difficult based on endoscopic findings alone, unless there are also extracolic lesions that point to Crohn’s disease [104, 105]. The clinical course of this type of disease also resembles that of ulcerative colitis: surgery may be required for an acute evolution that results in toxic megacolon [106], but in most cases surgery is performed because the disease cannot be managed medically. Because of the high rate of recurrence, a preoperative diagnosis of Crohn’s disease was for many years considered a contraindication to proctocolectomy with construction of an ileoanal pouch, which is used in patients with ulcerative colitis. In case series in which Crohn’s disease was diagnosed after single-stage surgery for a preoperative diagnosis of ulcerative colitis, 10-year pouch failure rates were as high as 45 % [107–109].
As early as 1991, Hyman et al. [110] reported good pouch function in a moderately high percentage of cases, and this outcome was associated (albeit nonsignificantly) with the absence before surgery of clinical signs suggestive of Crohn’s disease. These findings, which were confirmed in a recent review [111], suggest that single-stage pouch procedures in un tempo may not always be unsuccessful [110, 111] and that ileal pouch-anal anastomosis might be attempted in certain patients with preoperative diagnoses of Crohn’s disease of the colon, provided, of course, that they have no perianal or small intestinal involvement and that they are willing and prepared to undergo complex surgery with a moderate-to-high risk of failure [112]. Several fairly complex salvage procedures have been described to treat disease recurrence or complications at the site of the ileoanal pouch, and anti-TNF-alphas antibody therapy can also produce appreciable results in these cases [113–115].
Laparoscopy and Crohn’s disease
Like all abdominal surgery, laparoscopic procedures are being used more and more frequently to manage Crohn’s disease. A review published by Lesperance in 2009 found that minimally invasive techniques were used in only 6 % of the 50,000 interventions performed in the United States in 2000-2004 for Crohn’s disease, and this figure showed little or no change over the 5-year period examined by the authors. However, these data were extracted from hospital discharge forms examined at the national level, and they contrast with those from surgical referral centers, where the rate of laparoscopic procedures is much higher [116].
Laparoscopic intervention can be difficult, not only in cases that are obviously complex (extensive, recurrent B3 disease). Problems may also arise in situations that seem relatively simple. Use of laparoscopy allows (and sometimes facilitates) full-length visualization of the small intestine and the identification of disease involvement at this level, including lesions that were missed on preoperative imaging studies. (The latter studies are associated with non-negligible rates of false-negative findings, especially in the presence of short-segment jejunal involvement.). However, laparotomy is often required for reliable identification of strictures and above all for the conservative treatment (strictureplasty). In addition, if the ileal mesentery is inflamed (and this can be easily documented with various preoperative imaging modalities), it will be especially thick and fragile. Under these circumstances, the instruments normally used to obtain hemostasis during laparoscopic procedures (ultrasound and/or radiofrequency scalpels) may be hard to use, and proper extracorporeal resection and anastomosis will have to be performed through a short laparotomy (Fig. 7).
Fig. 7.
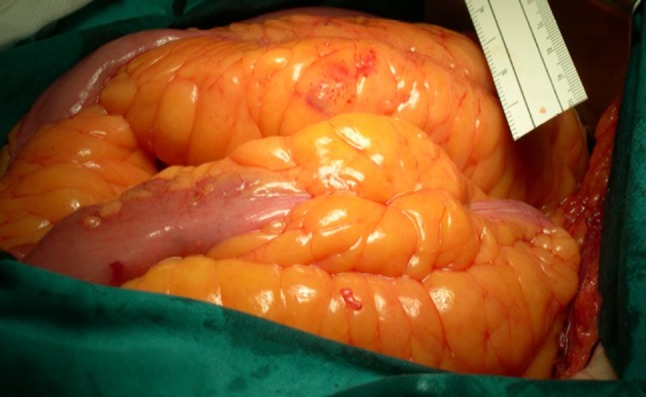
Ileal involvement with massive inflammatory thickening of the mesentery and appreciable “fat wrapping”
A review of the various case series indicates that “pure laparoscopy” is rarely used in real-life clinical practice: in all cases, a mini-laparotomy has to be performed to remove the resected segment, and the incision has to be fairly long since the disease tends to produce inflammatory masses of considerable size. Laparotomy-assisted techniques are much more widely used, i.e., those in which the lesion is isolated laparoscopically and the resection and anastomosis (or strictureplasty) are done extracorporeally (at least in part) via appropriately placed incisions that are much smaller than those required for open surgery. In hand-assisted laparoscopic surgery (HALS), the surgeon can actually insert his/her hand into the abdominal cavity without loss of the pneumoperitoneum. Its advantages include a more rapid learning curve and the possibility to tactilely explore the entire small intestine during the laparoscopic procedure (Figs. 3, 8) [117, 118].
Fig. 8.
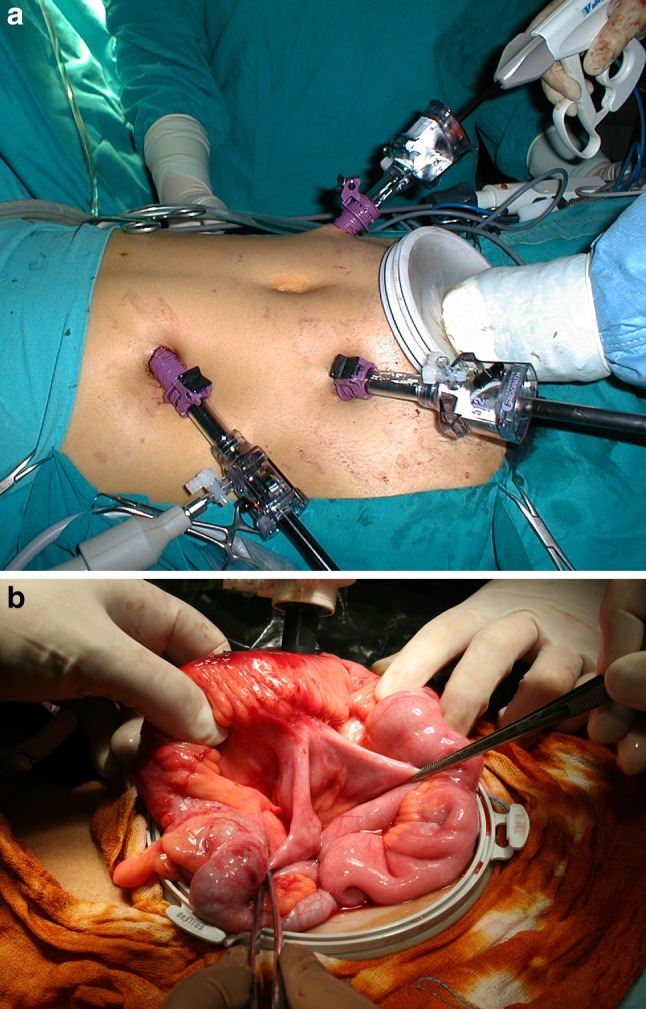
Hand-assisted laparoscopy performed with the aid of a Hand Port inserted in a hypogastric Pfannestiel laparotomy incision measuring 7 cm (a). The Hand Port is also used for video-assisted isolation of the stricture and the enterocolic fistula (b)
In all of the case series analyzed thus far (most of which come from surgical referral centers for chronic IBD), the results obtained with laparoscopy are reportedly better. It is important to recall, however, that the meta-analysis conducted by Van Loo found that use of laparoscopy had no impact on the incidence of recurrence. On the other hand, laparo-assisted approaches may reduce the risk of postoperative adhesions, thereby simplifying any subsequent surgical procedures, and importance of this advantage should not be underrated [98, 119].
Moreover, there do not appear to be any contraindications to an early discharge for patients who undergo minimally invasive (even laparo-assisted) procedures for short-segment involvement, but this does not apply to single-incision laparoscopic surgery (SILS), given the relatively low number of cases that have been analyzed thus far.
Conclusions
Surgery continues to play fundamental roles in the treatment of Crohn’s disease, considering the high percentage of patients who undergo surgery. Surgical interventions should be regarded not as adverse events caused by unsuccessful medical therapy, but as therapeutic tools whose use should be timed to ensure the lowest risk possible to the patient, as one of the many weapons available to the multidisciplinary team whose treatment strategies are based on an integrated assessment of the patient. Experiences with the management of perianal Crohn’s disease have shown that that medical therapy often produces the best results after surgical intervention, and the same might ultimately prove to be true for other areas of involvement. Prompt surgical intervention for fibrotic stricturing disease, as soon as it becomes clear that it is not responding to medical therapy, means that medical therapies can still be used for early-stage treatment or even prevention of recurrences.
Accurate imaging (endoscopic and above all radiological) is an essential component of multidiscliplinary management of Crohn’s disease. Today’s radiologists are accustomed to working with gastroenterologists in the diagnosis and follow-up phases and with surgeons in the preoperative evaluation (for urgent and elective surgery), but as the ability of diagnostic imaging modalities to characterize Crohn’s disease continues to improve, radiologists’ collaboration with gastroenterologists and surgeons will be a fundamental part of decisions regarding the timing of surgical intervention.
Conflict of interest
Fiorenzo Botti, Flavio Caprioli, Diego Pettinari, Alberto Carrara, Andrea Magarotto, Ettore Contessini Avesani declare that they have no conflict of interest.
Human and animal studies
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sedlack RE, Whisnant J, Elveback LR, Kurland LT. Incidence of Crohn’s disease in Olmsted County, Minnesota, 1935–1975. Am J Epidemiol. 1980;112:759–763. doi: 10.1093/oxfordjournals.aje.a113048. [DOI] [PubMed] [Google Scholar]
- 3.Peyrin-Biroulet L, Harmsen WS, Tremaine WJ, Zinsmeister AR, Sandborn WJ, Loftus EV., Jr Surgery in a population-based cohort of Crohn’s disease from Olmsted County, Minnesota (1970–2004) Am J Gastroenterol. 2012;107:1693–1701. doi: 10.1038/ajg.2012.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agrez MV, Valente RM, Pierce W, Melton LJ, 3rd, van Heerden JA, Beart RW., Jr Surgical history of Crohn’s disease in a well-defined population. Mayo Clin Proc. 1982;57:747–752. [PubMed] [Google Scholar]
- 5.Binder V, Hendriksen C, Kreiner S. Prognosis in Crohn’s disease-based on results from aregional patient group from the county of Copenhagen. Gut. 1985;26:146–150. doi: 10.1136/gut.26.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solberg IC, Vatn MH, Hoie O, Stray N, Sauar J, Jahnsen J, Moum B, Lygren I. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5:1430–1438. doi: 10.1016/j.cgh.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Ramadas AV, Gunesh S, Thomas GA, Williams GT, Hawthorne AB. Natural history of Crohn’s disease in a population-based cohort from Cardiff (1986–2003): a study of changes in medical treatment and surgical resection rates. Gut. 2010;59:1200–1206. doi: 10.1136/gut.2009.202101. [DOI] [PubMed] [Google Scholar]
- 8.Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP. Impact of the increasing use of immunosuppressants in Crohn’s disease on the need for intestinal surgery. Gut. 2005;54:237–241. doi: 10.1136/gut.2004.045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peyrin-Biroulet L, Oussalah A, Williet N, Pillot C, Bresler L, Bigard MA. Impact of azathioprine and tumour necrosis factor antagonists on the need for surgery in newly diagnosed Crohn’s disease. Gut. 2011;60:930–936. doi: 10.1136/gut.2010.227884. [DOI] [PubMed] [Google Scholar]
- 10.Lazarev M, Ullman T, Schraut WH, Kip KE, Saul M, Regueiro M. Small bowel resection rates in Crohn’s disease and the indication for surgery over time: experience from a large tertiary care center. Inflamm Bowel Dis. 2010;16:830–835. doi: 10.1002/ibd.21118. [DOI] [PubMed] [Google Scholar]
- 11.Bewtra M, Su C, Lewis JD. Trends in hospitalization rates for inflammatory bowel disease in the United States. Clin Gastroenterol Hepatol. 2007;5:597–601. doi: 10.1016/j.cgh.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen GC, Tuskey A, Dassopoulos T, Harris ML, Brant SR. Rising hospitalization rates for inflammatory bowel disease in the United States between 1998 and 2004. Inflamm Bowel Dis. 2007;13:1529–1535. doi: 10.1002/ibd.20250. [DOI] [PubMed] [Google Scholar]
- 13.Eshuis EJ, Griffioen GH, Stokkers PC, Ubbink DT, Bemelman WA. Anti tumour necrosis factor as risk factor for free perforations in Crohn’s disease? A case–control study. Colorectal Dis. 2012;14:578–584. doi: 10.1111/j.1463-1318.2011.02764.x. [DOI] [PubMed] [Google Scholar]
- 14.Siassi M, Weiger A, Hohenberger W, Kessler H. Changes in surgical therapy for Crohn’s disease over 33 years: a prospective longitudinal study. Int J Colorectal Dis. 2007;22:319–324. doi: 10.1007/s00384-006-0150-5. [DOI] [PubMed] [Google Scholar]
- 15.Bouguen G, Peyrin-Biroulet L. Surgery for adult Crohn’s disease: what is the actual risk? Gut. 2011;60:1178–1181. doi: 10.1136/gut.2010.234617. [DOI] [PubMed] [Google Scholar]
- 16.Ippoliti A, Devlin S, Mei L, Yang H, Papadakis KA, Vasiliauskas EA, McGovern DP, Abreu MT, Melmed G, Shaye O, Enayati P, Chen G, Choi J, Taylor K, Landers CJ, Rotter JI, Targan SR. Combination of innate and adaptive immune alterations increased the likelihood of fibrostenosis in Crohn’s disease. Inflamm Bowel Dis. 2010;16:1279–1285. doi: 10.1002/ibd.21196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV., Jr Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology. 2010;139:1147–1155. doi: 10.1053/j.gastro.2010.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latella G, Caprilli R, Travis S. In favour of early surgery in Crohn’s disease: a hypothesis to be tested. J Crohns Colitis. 2011;5:1–4. doi: 10.1016/j.crohns.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777–782. doi: 10.1136/gut.49.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papi C, Festa V, Fagnani C, Stazi A, Antonelli G, Moretti A, Koch M, Capurso L. Evolution of clinical behaviour in Crohn’s disease: predictive factors of penetrating complications. Dig Liver Dis. 2005;37:247–253. doi: 10.1016/j.dld.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Van Assche G, Vermeire S, Rutgeerts P. The potential for disease modification in Crohn’s disease. Nat Rev Gastroenterol Hepatol. 2010;7:79–85. doi: 10.1038/nrgastro.2009.220. [DOI] [PubMed] [Google Scholar]
- 22.Eshuis EJ, Stokkers PC, Bemelman WA. Decision-making in ileocecal Crohn’s disease management: surgery versus pharmacotherapy. Expert Rev Gastroenterol Hepatol. 2010;4:181–189. doi: 10.1586/egh.10.3. [DOI] [PubMed] [Google Scholar]
- 23.Dignass A, Van Assche G, Lindsay JO, Lemann M, Soderholm J, Colombel JF, Danese S, D’Hoore A, Gassull M, Gomollon F, Hommes DW, Michetti P, O’Morain C, Oresland T, Windsor A, Stange EF, Travis SP. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: current management. J Crohns Colitis. 2010;4:28–62. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Cullen G, O’Toole A, Keegan D, Sheahan K, Hyland JM, O’Donoghue DP. Long-term clinical results of ileocecal resection for Crohn’s disease. Inflamm Bowel Dis. 2007;13:1369–1373. doi: 10.1002/ibd.20220. [DOI] [PubMed] [Google Scholar]
- 25.Latella G, Cocco A, Angelucci E, Viscido A, Bacci S, Necozione S, Caprilli R. Clinical course of Crohn’s disease first diagnosed at surgery for acute abdomen. Dig Liver Dis. 2009;41:269–276. doi: 10.1016/j.dld.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Infantino A, Aratari A, Botti F, Carrara A, Casciani E, Catarci M, Cerro P, Contessini-Avesani E, Coscia M, Di Mitri R, Fazio V, Ficari F, Gallese N, Gentilini L, Gualdi G, Grassi GB, Laureti S, Mancini S, Masselli G, Nudo F, Nudo R, Papi C, Podda M, Poggioli G, Pronestì M, Speciale A, Solina G, Tonelli F, S. T, Ugolini F (2011) Linee guida acoi m di Crohn chirurgica (in collaborazione con siccr)—ACOI website
- 27.Miller G, Boman J, Shrier I, Gordon PH. Etiology of small bowel obstruction. Am J Surg. 2000;180:33–36. doi: 10.1016/s0002-9610(00)00407-4. [DOI] [PubMed] [Google Scholar]
- 28.Cosnes J. Crohn’s disease phenotype, prognosis, and long-term complications: what to expect? Acta Gastroenterol Belg. 2008;71:303–307. [PubMed] [Google Scholar]
- 29.Greenstein AJ, Sachar DB, Greenstein RJ, Janowitz HD, Aufses AH., Jr Intraabdominal abscess in Crohn’s (ileo) colitis. Am J Surg. 1982;143:727–730. doi: 10.1016/0002-9610(82)90046-0. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi A, Matsui T, Sakurai T, Ueki T, Nakabayashi S, Yao T, Futami K, Arima S, Ono H. The clinical characteristics and outcome of intraabdominal abscess in Crohn’s disease. J Gastroenterol. 2004;39:441–448. doi: 10.1007/s00535-003-1317-2. [DOI] [PubMed] [Google Scholar]
- 31.Feagins LA, Holubar SD, Kane SV, Spechler SJ. Current strategies in the management of intra-abdominal abscesses in Crohn’s disease. Clin Gastroenterol Hepatol. 2011;9:842–850. doi: 10.1016/j.cgh.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 32.Greenstein AJ, Mann D, Sachar DB, Aufses AH., Jr Free perforation in Crohn’s disease: I. A survey of 99 cases. Am J Gastroenterol. 1985;80:682–689. [PubMed] [Google Scholar]
- 33.Greenstein AJ, Sachar DB, Mann D, Lachman P, Heimann T, Aufses AH., Jr Spontaneous free perforation and perforated abscess in 30 patients with Crohn’s disease. Ann Surg. 1987;205:72–76. doi: 10.1097/00000658-198701000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinberg DM, Cooke WT, Alexander-Williams J. Free perforation in Crohn’s disease. Gut. 1973;14:187–190. doi: 10.1136/gut.14.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freeman HJ. Spontaneous free perforation of the small intestine in Crohn’s disease. Can J Gastroenterol. 2002;16:23–27. doi: 10.1155/2002/284958. [DOI] [PubMed] [Google Scholar]
- 36.Latella G, Vernia P, Viscido A, Frieri G, Cadau G, Cocco A, Cossu A, Tomei E, Caprilli R. Gi distension in severe ulcerative colitis. Am J Gastroenterol. 2002;97:1169–1175. doi: 10.1111/j.1572-0241.2002.05691.x. [DOI] [PubMed] [Google Scholar]
- 37.Grieco MB, Bordan DL, Geiss AC, Beil AR., Jr Toxic megacolon complicating Crohn’s colitis. Ann Surg. 1980;191:75–80. doi: 10.1097/00000658-198001000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Witte J, Shivananda S, Lennard-Jones JE, Beltrami M, Politi P, Bonanomi A, Tsianos EV, Mouzas I, Schulz TB, Monteiro E, Clofent J, Odes S, Limonard CB, Stockbrugger RW, Russel MG. Disease outcome in inflammatory bowel disease: mortality, morbidity and therapeutic management of a 796-person inception cohort in the European collaborative study on inflammatory bowel disease (EC-IBD) Scand J Gastroenterol. 2000;35:1272–1277. doi: 10.1080/003655200453610. [DOI] [PubMed] [Google Scholar]
- 39.Belaiche J, Louis E, D’Haens G, Cabooter M, Naegels S, De Vos M, Fontaine F, Schurmans P, Baert F, De Reuck M, Fiasse R, Holvoet J, Schmit A, Van Outryve M (1999) Acute lower gastrointestinal bleeding in Crohn’s disease: characteristics of a unique series of 34 patients. Belgian IBD Research Group. Am J Gastroenterol 94:2177–2181 [DOI] [PubMed]
- 40.Pardi DS, Loftus EV, Jr, Tremaine WJ, Sandborn WJ, Alexander GL, Balm RK, Gostout CJ. Acute major gastrointestinal hemorrhage in inflammatory bowel disease. Gastrointest Endosc. 1999;49:153–157. doi: 10.1016/s0016-5107(99)70479-7. [DOI] [PubMed] [Google Scholar]
- 41.Sands BE. From symptom to diagnosis: clinical distinctions among various forms of intestinal inflammation. Gastroenterology. 2004;126:1518–1532. doi: 10.1053/j.gastro.2004.02.072. [DOI] [PubMed] [Google Scholar]
- 42.Maconi G, Sampietro GM, Parente F, Pompili G, Russo A, Cristaldi M, Arborio G, Ardizzone S, Matacena G, Taschieri AM, Bianchi Porro G. Contrast radiology, computed tomography and ultrasonography in detecting internal fistulas and intra-abdominal abscesses in Crohn’s disease: a prospective comparative study. Am J Gastroenterol. 2003;98:1545–1555. doi: 10.1111/j.1572-0241.2003.07521.x. [DOI] [PubMed] [Google Scholar]
- 43.Gutierrez A, Lee H, Sands BE. Outcome of surgical versus percutaneous drainage of abdominal and pelvic abscesses in Crohn’s disease. Am J Gastroenterol. 2006;101:2283–2289. doi: 10.1111/j.1572-0241.2006.00757.x. [DOI] [PubMed] [Google Scholar]
- 44.Kelts DG, Grand RJ, Shen G, Watkins JB, Werlin SL, Boehme C. Nutritional basis of growth failure in children and adolescents with Crohn’s disease. Gastroenterology. 1979;76:720–727. [PubMed] [Google Scholar]
- 45.Fraquelli M, Colli A, Casazza G, Paggi S, Colucci A, Massironi S, Duca P, Conte D. Role of US in detection of Crohn disease: meta-analysis. Radiology. 2005;236:95–101. doi: 10.1148/radiol.2361040799. [DOI] [PubMed] [Google Scholar]
- 46.Panes J, Bouzas R, Chaparro M, Garcia-Sanchez V, Gisbert JP, Martinez de Guerenu B, Mendoza JL, Paredes JM, Quiroga S, Ripolles T, Rimola J. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther. 2011;34:125–145. doi: 10.1111/j.1365-2036.2011.04710.x. [DOI] [PubMed] [Google Scholar]
- 47.Gasche C, Moser G, Turetschek K, Schober E, Moeschl P, Oberhuber G. Transabdominal bowel sonography for the detection of intestinal complications in Crohn’s disease. Gut. 1999;44:112–117. doi: 10.1136/gut.44.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maconi G, Bollani S, Bianchi Porro G. Ultrasonographic detection of intestinal complications in Crohn’s disease. Dig Dis Sci. 1996;41:1643–1648. doi: 10.1007/BF02087914. [DOI] [PubMed] [Google Scholar]
- 49.Horsthuis K, Stokkers PC, Stoker J. Detection of inflammatory bowel disease: diagnostic performance of cross-sectional imaging modalities. Abdom Imaging. 2008;33:407–416. doi: 10.1007/s00261-007-9276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fiorino G, Bonifacio C, Peyrin-Biroulet L, Minuti F, Repici A, Spinelli A, Fries W, Balzarini L, Montorsi M, Malesci A, Danese S. Prospective comparison of computed tomography enterography and magnetic resonance enterography for assessment of disease activity and complications in ileocolonic Crohn’s disease. Inflamm Bowel Dis. 2011;17:1073–1080. doi: 10.1002/ibd.21533. [DOI] [PubMed] [Google Scholar]
- 51.Hassan C, Cerro P, Zullo A, Spina C, Morini S. Computed tomography enteroclysis in comparison with ileoscopy in patients with Crohn’s disease. Int J Colorectal Dis. 2003;18:121–125. doi: 10.1007/s00384-002-0455-y. [DOI] [PubMed] [Google Scholar]
- 52.Solem CA, Loftus EV, Jr, Fletcher JG, Baron TH, Gostout CJ, Petersen BT, Tremaine WJ, Egan LJ, Faubion WA, Schroeder KW, Pardi DS, Hanson KA, Jewell DA, Barlow JM, Fidler JL, Huprich JE, Johnson CD, Harmsen WS, Zinsmeister AR, Sandborn WJ. Small-bowel imaging in Crohn’s disease: a prospective, blinded, 4-way comparison trial. Gastrointest Endosc. 2008;68:255–266. doi: 10.1016/j.gie.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 53.Turetschek K, Schober E, Wunderbaldinger P, Bernhard C, Schima W, Puespoek A, Vogelsang H, Moeschl P, Mostbeck G. Findings at helical CT-enteroclysis in symptomatic patients with Crohn disease: correlation with endoscopic and surgical findings. J Comput Assist Tomogr. 2002;26:488–492. doi: 10.1097/00004728-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 54.Voderholzer WA, Beinhoelzl J, Rogalla P, Murrer S, Schachschal G, Lochs H, Ortner MA. Small bowel involvement in Crohn’s disease: a prospective comparison of wireless capsule endoscopy and computed tomography enteroclysis. Gut. 2005;54:369–373. doi: 10.1136/gut.2004.040055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiorean MV, Sandrasegaran K, Saxena R, Maglinte DD, Nakeeb A, Johnson CS. Correlation of CT enteroclysis with surgical pathology in Crohn’s disease. Am J Gastroenterol. 2007;102:2541–2550. doi: 10.1111/j.1572-0241.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- 56.Maccioni F, Viscido A, Broglia L, Marrollo M, Masciangelo R, Caprilli R, Rossi P. Evaluation of Crohn disease activity with magnetic resonance imaging. Abdom Imaging. 2000;25:219–228. doi: 10.1007/s002610000004. [DOI] [PubMed] [Google Scholar]
- 57.Pariente B, Peyrin-Biroulet L, Cohen L, Zagdanski AM, Colombel JF. Gastroenterology review and perspective: the role of cross-sectional imaging in evaluating bowel damage in Crohn disease. AJR Am J Roentgenol. 2011;197:42–49. doi: 10.2214/AJR.11.6632. [DOI] [PubMed] [Google Scholar]
- 58.Florie J, Horsthuis K, Hommes DW, Nio CY, Reitsma JB, van Deventer SJ, Stoker J. Magnetic resonance imaging compared with ileocolonoscopy in evaluating disease severity in Crohn’s disease. Clin Gastroenterol Hepatol. 2005;3:1221–1228. doi: 10.1016/s1542-3565(05)00853-0. [DOI] [PubMed] [Google Scholar]
- 59.Maccioni F, Bruni A, Viscido A, Colaiacomo MC, Cocco A, Montesani C, Caprilli R, Marini M. MR imaging in patients with Crohn disease: value of t2- versus t1-weighted gadolinium-enhanced MR sequences with use of an oral superparamagnetic contrast agent. Radiology. 2006;238:517–530. doi: 10.1148/radiol.2381040244. [DOI] [PubMed] [Google Scholar]
- 60.Martin DR, Danrad R, Herrmann K, Semelka RC, Hussain SM. Magnetic resonance imaging of the gastrointestinal tract. Top Magn Reson Imaging. 2005;16:77–98. doi: 10.1097/01.rmr.0000179461.55234.7d. [DOI] [PubMed] [Google Scholar]
- 61.Fichera A, Michelassi F. Surgical treatment of Crohn’s disease. J Gastrointest Surg. 2007;11:791–803. doi: 10.1007/s11605-006-0068-9. [DOI] [PubMed] [Google Scholar]
- 62.Michelassi F, Balestracci T, Chappell R, Block GE (1991) Primary and recurrent Crohn’s disease. Experience with 1379 patients. Ann Surg 214:230–238 (discussion 238–240) [DOI] [PMC free article] [PubMed]
- 63.Reynolds HL Jr., Stellato TA (2001) Crohn’s disease of the foregut. Surg Clin North Am. 81:117–135, viii [DOI] [PubMed]
- 64.Yamamoto T, Bain IM, Connolly AB, Allan RN, Keighley MR. Outcome of strictureplasty for duodenal Crohn’s disease. Br J Surg. 1999;86:259–262. doi: 10.1046/j.1365-2168.1999.01022.x. [DOI] [PubMed] [Google Scholar]
- 65.Worsey MJ, Hull T, Ryland L, Fazio V. Strictureplasty is an effective option in the operative management of duodenal Crohn’s disease. Dis Colon Rectum. 1999;42:596–600. doi: 10.1007/BF02234132. [DOI] [PubMed] [Google Scholar]
- 66.Racz JM, Davies W. Severe stricturing Crohn’s disease of the duodenum: a case report and review of surgical options. Int J Surg Case Rep. 2012;3:242–245. doi: 10.1016/j.ijscr.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alemanno G, Sturiale A, Bellucci F, Giudici F, Tonelli F. Resolving sphincter of oddi incontinence for primary duodenal Crohn’s disease with strictureplasty. Int J Surg Case Rep. 2013;4:149–152. doi: 10.1016/j.ijscr.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farmer RG, Hawk WA, Turnbull RB., Jr Clinical patterns in Crohn’s disease: a statistical study of 615 cases. Gastroenterology. 1975;68:627–635. [PubMed] [Google Scholar]
- 69.Tan WC, Allan RN. Diffuse jejunoileitis of Crohn’s disease. Gut. 1993;34:1374–1378. doi: 10.1136/gut.34.10.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hellers G. Crohn’s disease in Stockholm County 1955–1974. A study of epidemiology, results of surgical treatment and long-term prognosis. Acta Chir Scand Suppl. 1979;490:1–84. [PubMed] [Google Scholar]
- 71.Cooke WT, Mallas E, Prior P, Allan RN. Crohn’s disease: course, treatment and long term prognosis. Q J Med. 1980;49:363–384. [PubMed] [Google Scholar]
- 72.Fazio VW, Marchetti F, Church M, Goldblum JR, Lavery C, Hull TL, Milsom JW, Strong SA, Oakley JR, Secic M (1996) Effect of resection margins on the recurrence of Crohn’s disease in the small bowel. A randomized controlled trial. Ann Surg 224:563–571 (discussion 571–563) [DOI] [PMC free article] [PubMed]
- 73.Botti F, Carrara A, Antonelli B, Quadri F, Maino M, Cesana B, Contessini-Avesani E. the minimal bowel resection in Crohn’s disease: analysis of prognostic factors on the surgical recurrence. Ann Ital Chir. 2003;74:627–633. [PubMed] [Google Scholar]
- 74.Katariya RN, Sood S, Rao PG, Rao PL. Stricture-plasty for tubercular strictures of the gastro-intestinal tract. Br J Surg. 1977;64:496–498. doi: 10.1002/bjs.1800640713. [DOI] [PubMed] [Google Scholar]
- 75.Lee EC, Papaioannou N. Minimal surgery for chronic obstruction in patients with extensive or universal Crohn’s disease. Ann R Coll Surg Engl. 1982;64:229–233. [PMC free article] [PubMed] [Google Scholar]
- 76.Alexander-Williams J, Haynes IG. Conservative operations for Crohn’s disease of the small bowel. World J Surg. 1985;9:945–951. doi: 10.1007/BF01655400. [DOI] [PubMed] [Google Scholar]
- 77.Tichansky D, Cagir B, Yoo E, Marcus SM, Fry RD. Strictureplasty for Crohn’s disease: meta-analysis. Dis Colon Rectum. 2000;43:911–919. doi: 10.1007/BF02237350. [DOI] [PubMed] [Google Scholar]
- 78.Michelassi F. Side-to-side isoperistaltic strictureplasty for multiple Crohn’s strictures. Dis Colon Rectum. 1996;39:345–349. doi: 10.1007/BF02049480. [DOI] [PubMed] [Google Scholar]
- 79.Cristaldi M, Sampietro GM, Danelli PG, Bollani S, Bianchi Porro G, Taschieri AM. Long-term results and multivariate analysis of prognostic factors in 138 consecutive patients operated on for Crohn’s disease using “bowel-sparing” techniques. Am J Surg. 2000;179:266–270. doi: 10.1016/s0002-9610(00)00334-2. [DOI] [PubMed] [Google Scholar]
- 80.Tonelli F, Fazi M, Di Martino C. Ileocecal strictureplasty for Crohn’s disease: long-term results and comparison with ileocecal resection. World J Surg. 2010;34:2860–2866. doi: 10.1007/s00268-010-0708-9. [DOI] [PubMed] [Google Scholar]
- 81.Michelassi F, Hurst RD, Melis M, Rubin M, Cohen R, Gasparitis A, Hanauer SB, Hart J. Side- to-side isoperistaltic strictureplasty in extensive Crohn’s disease: a prospective longitudinal study. Ann Surg. 2000;232:401–408. doi: 10.1097/00000658-200009000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dossett LA, White LM, Welch DC, Herline AJ, Muldoon RL, Schwartz DA, Wise PE. Small bowel adenocarcinoma complicating Crohn’s disease: case series and review of the literature. Am Surg. 2007;73:1181–1187. [PubMed] [Google Scholar]
- 83.Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment Pharmacol Ther. 2006;23:1097–1104. doi: 10.1111/j.1365-2036.2006.02854.x. [DOI] [PubMed] [Google Scholar]
- 84.von Roon AC, Reese G, Teare J, Constantinides V, Darzi AW, Tekkis PP. The risk of cancer in patients with Crohn’s disease. Dis Colon Rectum. 2007;50:839–855. doi: 10.1007/s10350-006-0848-z. [DOI] [PubMed] [Google Scholar]
- 85.Marchetti F, Fazio VW, Ozuner G. Adenocarcinoma arising from a strictureplasty site in Crohn’s disease. report of a case. Dis Colon Rectum. 1996;39:1315–1321. doi: 10.1007/BF02055130. [DOI] [PubMed] [Google Scholar]
- 86.Jaskowiak NT, Michelassi F. Adenocarcinoma at a strictureplasty site in Crohn’s disease: report of a case. Dis Colon Rectum. 2001;44:284–287. doi: 10.1007/BF02234306. [DOI] [PubMed] [Google Scholar]
- 87.Michelassi F, Testa G, Pomidor WJ, Lashner BA, Block GE. Adenocarcinoma complicating Crohn’s disease. Dis Colon Rectum. 1993;36:654–661. doi: 10.1007/BF02238592. [DOI] [PubMed] [Google Scholar]
- 88.Ribeiro MB, Greenstein AJ, Heimann TM, Yamazaki Y, Aufses AH., Jr Adenocarcinoma of the small intestine in Crohn’s disease. Surg Gynecol Obstet. 1991;173:343–349. [PubMed] [Google Scholar]
- 89.Greenstein AJ, Sachar D, Pucillo A, Kreel I, Geller S, Janowitz HD, Aufses A., Jr Cancer in Crohn’s disease after diversionary surgery. A report of seven carcinomas occurring in excluded bowel. Am J Surg. 1978;135:86–90. doi: 10.1016/0002-9610(78)90015-6. [DOI] [PubMed] [Google Scholar]
- 90.Martinez MJ, Ripolles T, Paredes JM, Blanc E, Marti-Bonmati L. Assessment of the extension and the inflammatory activity in Crohn’s disease: comparison of ultrasound and MRI. Abdom Imaging. 2009;34:141–148. doi: 10.1007/s00261-008-9365-y. [DOI] [PubMed] [Google Scholar]
- 91.Pallotta N, Tomei E, Viscido A, Calabrese E, Marcheggiano A, Caprilli R, Corazziari E. Small intestine contrast ultrasonography: an alternative to radiology in the assessment of small bowel disease. Inflamm Bowel Dis. 2005;11:146–153. doi: 10.1097/00054725-200502000-00008. [DOI] [PubMed] [Google Scholar]
- 92.Koh DM, Miao Y, Chinn RJ, Amin Z, Zeegen R, Westaby D, Healy JC. MR imaging evaluation of the activity of Crohn’s disease. AJR Am J Roentgenol. 2001;177:1325–1332. doi: 10.2214/ajr.177.6.1771325. [DOI] [PubMed] [Google Scholar]
- 93.Wold PB, Fletcher JG, Johnson CD, Sandborn WJ. Assessment of small bowel Crohn disease: noninvasive peroral CT enterography compared with other imaging methods and endoscopy—feasibility study. Radiology. 2003;229:275–281. doi: 10.1148/radiol.2291020877. [DOI] [PubMed] [Google Scholar]
- 94.Horsthuis K, Bipat S, Bennink RJ, Stoker J. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology. 2008;247:64–79. doi: 10.1148/radiol.2471070611. [DOI] [PubMed] [Google Scholar]
- 95.Schmidt S, Lepori D, Meuwly JY, Duvoisin B, Meuli R, Michetti P, Felley C, Schnyder P, van Melle G, Denys A. Prospective comparison of MR enteroclysis with multidetector spiral-CT enteroclysis: interobserver agreement and sensitivity by means of “sign-by-sign” correlation. Eur Radiol. 2003;13:1303–1311. doi: 10.1007/s00330-002-1710-x. [DOI] [PubMed] [Google Scholar]
- 96.Desmond AN, O’Regan K, Curran C, McWilliams S, Fitzgerald T, Maher MM, Shanahan F. Crohn’s disease: factors associated with exposure to high levels of diagnostic radiation. Gut. 2008;57:1524–1529. doi: 10.1136/gut.2008.151415. [DOI] [PubMed] [Google Scholar]
- 97.Simillis C, Purkayastha S, Yamamoto T, Strong SA, Darzi AW, Tekkis PP. A meta-analysis comparing conventional end-to-end anastomosis vs. other anastomotic configurations after resection in Crohn’s disease. Dis Colon Rectum. 2007;50:1674–1687. doi: 10.1007/s10350-007-9011-8. [DOI] [PubMed] [Google Scholar]
- 98.van Loo ES, Dijkstra G, Ploeg RJ, Nieuwenhuijs VB. Prevention of postoperative recurrence of Crohn’s disease. J Crohns Colitis. 2012;6:637–646. doi: 10.1016/j.crohns.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 99.Fichera A, McCormack R, Rubin MA, Hurst RD, Michelassi F. Long-term outcome of surgically treated Crohn’s colitis: a prospective study. Dis Colon Rectum. 2005;48:963–969. doi: 10.1007/s10350-004-0906-3. [DOI] [PubMed] [Google Scholar]
- 100.Longo WE, Ballantyne GH, Cahow CE. Treatment of Crohn’s colitis. Segmental or total colectomy? Arch Surg. 1988;123:588–590. doi: 10.1001/archsurg.1988.01400290070011. [DOI] [PubMed] [Google Scholar]
- 101.Prabhakar LP, Laramee C, Nelson H, Dozois RR. Avoiding a stoma: role for segmental or abdominal colectomy in Crohn’s colitis. Dis Colon Rectum. 1997;40:71–78. doi: 10.1007/BF02055685. [DOI] [PubMed] [Google Scholar]
- 102.Tzivanakis A, Singh JC, Guy RJ, Travis SP, Mortensen NJ, George BD. Influence of risk factors on the safety of ileocolic anastomosis in Crohn’s disease surgery. Dis Colon Rectum. 2012;55:558–562. doi: 10.1097/DCR.0b013e318247c433. [DOI] [PubMed] [Google Scholar]
- 103.Laukoetter MG, Mennigen R, Hannig CM, Osada N, Rijcken E, Vowinkel T, Krieglstein CF, Senninger N, Anthoni C, Bruewer M. Intestinal cancer risk in Crohn’s disease: a meta-analysis. J Gastrointest Surg. 2011;15:576–583. doi: 10.1007/s11605-010-1402-9. [DOI] [PubMed] [Google Scholar]
- 104.Pezim ME, Pemberton JH, Beart RW, Jr, Wolff BG, Dozois RR, Nivatvongs S, Devine R, Ilstrup DM. Outcome of “indeterminant” colitis following ileal pouch-anal anastomosis. Dis Colon Rectum. 1989;32:653–658. doi: 10.1007/BF02555768. [DOI] [PubMed] [Google Scholar]
- 105.Deutsch AA, McLeod RS, Cullen J, Cohen Z. Results of the pelvic-pouch procedure in patients with Crohn’s disease. Dis Colon Rectum. 1991;34:475–477. doi: 10.1007/BF02049932. [DOI] [PubMed] [Google Scholar]
- 106.Greenstein AJ, Sachar DB, Gibas A, Schrag D, Heimann T, Janowitz HD, Aufses AH., Jr Outcome of toxic dilatation in ulcerative and Crohn’s colitis. J Clin Gastroenterol. 1985;7:137–143. doi: 10.1097/00004836-198504000-00007. [DOI] [PubMed] [Google Scholar]
- 107.Edwards CM, Warren BF, Shepherd NA. Ileal pouch-anal anastomosis for Crohn’s disease. Gut. 1999;44:896. doi: 10.1136/gut.44.6.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Keighley MR, Allan RN, Sanders DS. Ileal pouch-anal anastomosis for Crohn’s disease. Gut. 1999;44:440–441. doi: 10.1136/gut.44.3.439b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sagar PM, Dozois RR, Wolff BG. Long-term results of ileal pouch-anal anastomosis in patients with Crohn’s disease. Dis Colon Rectum. 1996;39:893–898. doi: 10.1007/BF02053988. [DOI] [PubMed] [Google Scholar]
- 110.Hyman NH, Fazio VW, Tuckson WB, Lavery IC. Consequences of ileal pouch-anal anastomosis for Crohn’s colitis. Dis Colon Rectum. 1991;34:653–657. doi: 10.1007/BF02050345. [DOI] [PubMed] [Google Scholar]
- 111.Melton GB, Kiran RP, Fazio VW, He J, Shen B, Goldblum JR, Achkar JP, Lavery IC, Remzi FH. Do preoperative factors predict subsequent diagnosis of Crohn’s disease after ileal pouch-anal anastomosis for ulcerative or indeterminate colitis? Colorectal Dis. 2010;12:1026–1032. doi: 10.1111/j.1463-1318.2009.02014.x. [DOI] [PubMed] [Google Scholar]
- 112.Sagar PM, Pemberton JH. Intraoperative, postoperative and reoperative problems with ileoanal pouches. Br J Surg. 2012;99:454–468. doi: 10.1002/bjs.8697. [DOI] [PubMed] [Google Scholar]
- 113.Garrett KA, Remzi FH, Kirat HT, Fazio VW, Shen B, Kiran RP. Outcome of salvage surgery for ileal pouches referred with a diagnosis of Crohn’s disease. Dis Colon Rectum. 2009;52:1967–1974. doi: 10.1007/DCR.0b013e3181b77d1e. [DOI] [PubMed] [Google Scholar]
- 114.Ferrante M, D’Haens G, Dewit O, Baert F, Holvoet J, Geboes K, De Hertogh G, Van Assche G, Vermeire S, Rutgeerts P. Efficacy of infliximab in refractory pouchitis and Crohn’s disease-related complications of the pouch: a Belgian case series. Inflamm Bowel Dis. 2010;16:243–249. doi: 10.1002/ibd.21037. [DOI] [PubMed] [Google Scholar]
- 115.Shen B, Remzi FH, Lavery IC, Lopez R, Queener E, Shen L, Goldblum J, Fazio VW. Administration of adalimumab in the treatment of Crohn’s disease of the ileal pouch. Aliment Pharmacol Ther. 2009;29:519–526. doi: 10.1111/j.1365-2036.2008.03920.x. [DOI] [PubMed] [Google Scholar]
- 116.Lesperance K, Martin MJ, Lehmann R, Brounts L, Steele SR. National trends and outcomes for the surgical therapy of ileocolonic Crohn’s disease: a population-based analysis of laparoscopic vs open approaches. J Gastrointest Surg. 2009;13:1251–1259. doi: 10.1007/s11605-009-0853-3. [DOI] [PubMed] [Google Scholar]
- 117.Tilney HS, Constantinides VA, Heriot AG, Nicolaou M, Athanasiou T, Ziprin P, Darzi AW, Tekkis PP. Comparison of laparoscopic and open ileocecal resection for Crohn’s disease: a metaanalysis. Surg Endosc. 2006;20:1036–1044. doi: 10.1007/s00464-005-0500-3. [DOI] [PubMed] [Google Scholar]
- 118.Cima RR, Pendlimari R, Holubar SD, Pattana-Arun J, Larson DW, Dozois EJ, Wolff BG, Pemberton JH. Utility and short-term outcomes of hand-assisted laparoscopic colorectal surgery: a single-institution experience in 1103 patients. Dis Colon Rectum. 2011;54:1076–1081. doi: 10.1007/DCR.0b013e3182155904. [DOI] [PubMed] [Google Scholar]
- 119.Aytac E, Stocchi L, Remzi FH, Kiran RP. Is laparoscopic surgery for recurrent Crohn’s disease beneficial in patients with previous primary resection through midline laparotomy? A case-matched study. Surg Endosc. 2012;26:3552–3556. doi: 10.1007/s00464-012-2361-x. [DOI] [PubMed] [Google Scholar]


