Abstract
Neuropeptide Y (NPY), one of the most abundant peptides in the nervous system, exerts its effects via 5 receptor types, termed Y1, Y2, Y4, Y5 and y6. NPY’s pleiotropic functions comprise the regulation of brain activity, mood, stress coping, ingestion, digestion, metabolism, vascular and immune function. Nerve-derived NPY directly affects immune cells while NPY also acts as a paracrine and autocrine immune mediator, since immune cells themselves are capable of producing and releasing NPY. NPY is able to induce immune activation or suppression, depending on a myriad of factors such as the Y receptors activated and cell types involved.
There is an intricate relationship between psychological stress, mood disorders and the immune system. While stress represents a risk factor for the development of mood disorders, it exhibits diverse actions on the immune system as well. Conversely, inflammation is regarded as an internal stressor and is increasingly recognized to contribute to the pathogenesis of mood and metabolic disorders. Intriguingly, the cerebral NPY system has been found to protect against distinct disturbances in response to immune challenge, attenuating the sickness response and preventing the development of depression. Thus, NPY plays an important homeostatic role in balancing disturbances of physiological systems caused by peripheral immune challenge. This implication is particularly evident in the brain in which NPY counteracts the negative impact of immune challenge on mood, emotional processing and stress resilience. NPY thus acts as a unique signalling molecule in the interaction of the immune system with the brain in health and disease.
Keywords: Brain, Immune system, Neuropeptide Y
The neuropeptide Y family and its receptors (Y1, Y2, Y4, Y5)
The principal members of the neuropeptide Y (NPY) family of biologically active peptides are NPY, peptide YY (PYY) and pancreatic polypeptide (PP). Like other biologically active peptides, the NPY-related peptides are synthesized as large protein precursors which are subsequently cleaved to release the active peptides. All three members of the NPY peptide family are made of 36 amino acids. In addition, NPY, PYY and PP share the PP fold (hairpin fold) tertiary structural motif and the C-terminal tyrosine amide structure. The N-terminal amino acid of NPY and PYY is also tyrosine, which is reflected by the names of peptide YY and neuropeptide Y. The genes encoding NPY, PYY and PP have been identified and found to have very similar base sequences (Cerda-Reverter & Larhammar 2000).
The biological actions of NPY, PYY and PP are mediated by Y receptors. Of the 7 Y receptor types occurring in vertebrates, 5 types are expressed in mammals, namely Y1, Y2, Y4, Y5 and y6 (a pseudogene in humans and rats). The genes encoding the Y receptors have been characterized at the molecular and pharmacological level (Michel et al. 1998), and it is remarkable that the various Y receptors display surprisingly low sequence homologies and thus represent one of the most heterogeneous receptor families (Larhammar 1996). All Y receptors are metabotropic receptors that are coupled to pertussis toxin-sensitive Gi/o protein transduction mechanisms which decrease cAMP synthesis and protein kinase A activity (Redrobe et al. 2004a, Alexander et al. 2011). In some cells NPY can also activate pertussis toxin-insensitive Gq proteins activating protein kinase C (PKC) and extracellular signal-regulated kinase 1/2 (Herzog et al. 1992, Goldberg et al. 1998, Yang et al. 2008, Persaud & Bewick 2014).
The Y receptor types exhibit distinct affinities for the different members of the peptide family and their fragments (Cox 2007a, Alexander et al. 2011). This is of biological relevance, given that the N-terminus of NPY and PYY is readily cleaved by aminopeptidase P and dipeptidyl peptidase 4 (DP4, CD26), yielding the fragments NPY2-36, NPY3-36 and PYY3-36 which have distinct pharmacological properties. While there is not much difference in the affinities of NPY and PYY for the Y1, Y2 and Y5 receptor subtypes, NPY2-36 is a Y2 agonist, while NPY3-36 and PYY3-36 are preferential agonists at Y2 and Y5 receptors, and PP displays some selectivity for Y4 receptor (Cox 2007a, Abid et al. 2009, Alexander et al. 2011). The Y1 and Y2 receptors are the most abundant receptor types in the periphery and the central nervous system (Holzer et al. 2012).
PYY and PP are almost exclusively expressed in the digestive system, whereas NPY occurs primarily in the central and peripheral nervous system (Holzer et al. 2012). PP is synthesized by endocrine F cells of the pancreatic islets (Ekblad & Sundler 2002) and some enteroendocrine cells of the small and large intestine, these cells differing from PYY-expressing cells (Cox 2007a). Although Y4 receptors, which are preferentially targeted by PP, are expressed in the brain, PP does not seem to be produced in the central nervous system, given that the immunoreactivity which was once believed to reflect cerebral PP turned out to be NPY (Allen et al. 1983, DiMaggio et al. 1985). PYY is primarily formed by the enteroendocrine L cells which occur most abundantly in the lower gastrointestinal tract (Ekblad & Sundler 2002, McGowan & Bloom 2004, Cox 2007a, Cox 2007b, Ueno et al. 2008). Other, but minor sources of PYY are enteric neurons of the stomach (Böttcher et al. 1993) and pancreatic endocrine cells (Cox 2007b) while the expression of PYY in the brain is relatively sparse (Ekblad & Sundler 2002, Morimoto et al. 2008). Interestingly, while the major circulating form of PYY is PYY3-36, PYY1-36 seems to be predominant in the brain (Gelegen et al. 2012).
In contrast to PP and PYY, NPY is widely expressed in the body. Within the brain, NPY is one of the most abundant neuropeptides, being expressed by multiple neuronal systems from the medullary brainstem to the cerebral cortex such as the nucleus of the solitary tract, ventrolateral medulla, periaqueductal grey, locus coeruleus, paraventricular nucleus of the thalamus, hypothalamus (arcuate nucleus, paraventricular nucleus and other regions), septum, hippocampus, amygdala, basal ganglia, nucleus accumbens and cerebral cortex (Wettstein et al. 1995, Kask et al. 2002, Eaton et al. 2007). Due to the absence of specific reuptake mechanisms and their special kinetics of action, neuropeptides have long-lasting effects on target neurons (Heilig 2004). In the periphery, the major cellular sources synthesizing NPY are enteroendocrine cells of the gut (Bär et al. 2014), distinct populations of enteric neurons such as secretomotor neurons and inhibitory motoneurons (Schicho et al. 2003, Cox 2007a), primary afferent neurons (Brumovsky et al. 2007), postganglionic sympathetic neurons supplying the vascular system but also lymphoid tissues (Romano et al. 1991, Lomax et al. 2010), and immune cells (Dimitrijevic & Stanojevic 2013). Another source of NPY is platelets which, especially in rats, have been reported to release noticeable amounts of NPY into the circulation (Myers et al. 1988). The concentration of circulating NPY is in the picomolar range, and levels in the cerebrospinal fluid (CSF) are 3 fold higher compared to plasma levels (Baker et al. 2013). The half-life of NPY in plasma is uncommonly long and amounts to 40 min in humans (Ahlborg et al. 1992).
In this review, we focus on particular implications of NPY in immune regulation, on the one hand, and its involvement in brain functions underlying mood, emotional processing and stress coping, on the other hand. These implications may, at first glance, appear unrelated with each other, but there is abundant evidence for a close interaction between the immune system and the brain, which has an important bearing on psychiatric diseases including anxiety disorders and major depression (Dantzer et al. 2008, Haroon et al. 2012). As the review illustrates, the effects of NPY both in the immune and the nervous system are utterly complex. While some aspects of the peripheral immune response may be enhanced, whereas others are inhibited by NPY, the disturbances in brain function and behaviour elicited by peripheral immune challenge are uniformly counteracted by cerebral NPY in a homeostatic fashion.
NPY’s pleiotropic actions on immune cells
The first evidence that NPY could exert immunological effects was borne out by the finding that sympathetic neurons innervating lymphoid organs contain NPY (Romano et al. 1991) and NPY is co-released with noradrenaline upon stimulation (Lundberg et al. 1989). In addition to nerve-derived NPY, it was found that immune cells such as monocytes, macrophages, dendritic cells (DCs) and lymphocytes express NPY and can modulate immune cell function in a paracrine or autocrine manner (Schwarz et al. 1994). In addition to NPY, also PYY mRNA has been demonstrated in mouse macrophages (Macia et al. 2012b). Collectively, the investigation of the effects of NPY on the immune system has revealed that the peptide is implicated in a variety of inflammatory disorders such as autoimmunity, asthma, atherosclerosis and cancer (Macia et al. 2011, Dimitrijevic & Stanojevic 2013, Jaaskelainen et al. 2013).
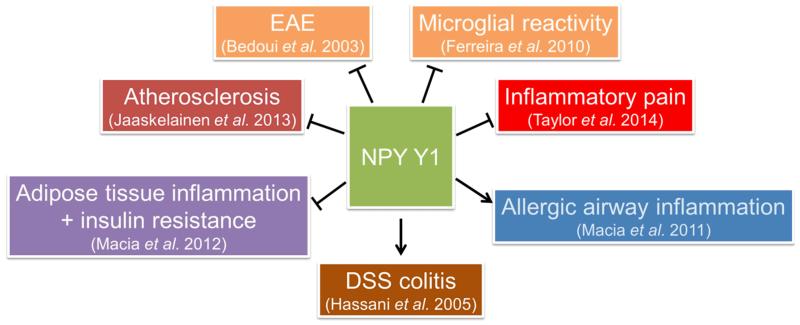
The expression of various Y receptors on immune cells enables NPY to directly influence immune function (Petitto et al. 1994). The Y1 receptor represents the most abundant receptor and has been localized to all immune cells examined thus far (Dimitrijevic & Stanojevic 2013). The effects of Y1 receptor activation on inflammation-associated disorders are summarized in Fig. 1. In contrast, reports regarding the expression of the other Y receptors are scarcer. Thus, in mice, gene expression of Y1, Y2, and Y5 receptors has been reported in macrophages, while the Y4 receptor seems not to be expressed (Singer et al. 2013). Similarly, Y1, Y2, and Y5 receptors are expressed on rat granulocytes (Mitic et al. 2011). In contrast, human neutrophils express mRNA of all 4 functional Y receptors (i.e. Y1, Y2, Y4 and Y5), and the Y4 receptor is the most abundant, while the Y2 receptor is upregulated upon neutrophil stimulation (Bedoui et al. 2008). However, despite the highest expression of the Y4 receptor, the stimulation of this receptor by PP does not elicit any changes in reactive oxygen species production or phagocytosis (Bedoui et al. 2008).
Figure 1. Involvement of the Y1 receptor in inflammation-associated disorders.
The arrow symbols denote stimulation, the tack symbols denote inhibition.
Abbreviations: DSS, dextran sulfate sodium; EAE, experimental autoimmune encephalomyelitis.
In general, it can be stated that NPY serves as a modulator of the immune system, and its distinct effects are strongly context-dependent (Tables 1 – 8). Thus, activation of different Y receptor types can mediate distinct effects of NPY while the same receptor expressed on different immune cell types can exert either pro- or anti-inflammatory actions (Wheway et al. 2005). Finally, DP4 cleaving NPY1-36 to the Y2/5 receptor agonist NPY3-36 is another regulator of NPY’s immunologic actions, being also responsible for some of the controversial effects of NPY reported in vitro and in vivo (Mitic et al. 2011).
Table 1.
Effects of NPY on adion of immune cells
| Cells | Sp | Specification | Rec | Effect | Age | Signal | Ref |
|---|---|---|---|---|---|---|---|
| Monocytes | H | U937 cell line | ↑ | (Sung et al. 1991) | |||
| Macrophages | M | Peritoneal resident cells (macrophages+lymphocytes) | ↑ | PKC | (De la Fuente et al. 1993, De la Fuente et al. 2000, De la Fuente et al. 2001a) | ||
| Macrophages | R | Peritoneal, thioglycollate-elicited | Y2 | ↑ LPS-stimulated | (Nave et al. 2004) | ||
| Macrophages | R | Peritoneal, thioglycollate-elicited | Y1 | ↓ by PYY | adult | (Stanojevic et al. 2006) | |
| Monocytes + Granulocytes | R | Blood | Y1 | ↑ LPS-stimulated | (Mitic et al. 2011) | ||
| Granulocytes | R | Air pouch, carrageenan-elicited | Y1 | ↑ | adult | (Dimitrijevic et al. 2010) | |
| Monocytes + Granulocytes | R | Air pouch, LPS-elicited | Y2+Y5 | ↓ | (Mitic et al. 2011) | ||
| Neutrophils | H | Blood | ↑ | (Sung et al. 1991) | |||
| DCs | H | Blood | ↑ | (Buttari et al. 2014) | |||
| T cells | H | Blood | Y2 | ↑ | PI3K / PKC | (Levite et al. 1998) | |
| Leukocytes | M | Peritoneal, spleen, thymus, axillary node | ↑ spleen, axillary node, thymus ↓ young: peritoneal; ↓ adult: thymus |
young | (Medina et al. 2000b) |
Abbreviations: H, human; LPS, lipopolysaccharide; M, mouse; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; PYY, peptide YY; R, rat; Rec, receptor; Sp, species.
Table 2.
Effects of NPY on cti of immune cells
| Cells | Sp | Specification | Rec | Effect / Chemoattractant | Age | Signal | Ref |
|---|---|---|---|---|---|---|---|
| Monocytes | H | Blood | ↑ fetal calf serum | (Straub et al. 2000a) | |||
| Macrophages | M | Peritoneal resident cells (macrophages+lymphocytes) | ↑ f-MLP | adult | PKC | (De la Fuente et al. 1993, De la Fuente et al. 2000, De la Fuente et al. 2001a) | |
| Macrophages | M | Peritoneal resident cells (macrophages+lymphocytes) | ↓ f-MLP | old | (De la Fuente et al. 2000, De la Fuente et al. 2001a) | ||
| Monocytes / Macrophages | M | RAW 264.7 cell line | ↓ Leishmania major | (Ahmed et al. 1998) | |||
| Macrophages + Granulocytes | H | ↓ f-MLP | (Dureus et al. 1993) | ||||
| Monocytes + Granulocytes | R | Air pouch, LPS-elicited | Y1 + Y2 | ↓ migration to air pouch | (Mitic et al. 2011) | ||
| Granulocytes | R | Air pouch, carrageenan-elicited | ↓ migration to air pouch | (Dimitrijevic et al. 2006, Dimitrijevic et al. 2010) | |||
| DCs | H | Blood | Y1 | ↑ | ERK/MAPK | (Buttari et al. 2014) | |
| Lymphocytes | M | Peritoneal resident cells (macrophages+lymphocytes) / axillary node leukocytes | ↑ f-MLP | adult | (Medina et al. 1998b) | ||
| Lymphocytes | M | Thymus | ↓ f-MLP | (Medina et al. 1998b) | |||
| Leukocytes | M | Peritoneal, spleen, thymus, axillary node | ↑ axillary node, peritoneal, f-MLP | (Medina et al. 2000b) | |||
| Leukocytes | M | Peritoneal, spleen, thymus, axillary node | ↓ thymus, f-MLP | (Medina et al. 2000b) |
Abbreviations: DCs, dendritic cells; ERK, extracellular signal regulated kinases; f-MLP, N-formyl-methionyl-leucyl-phenylalanine; H, human; LPS, lipopolysaccharide; M, mouse; MAPK, mitogen-activated protein kinases; PKC, protein kinase C; R, rat; Rec, receptor; Sp, species.
Table 3.
Effects of NPY on phagocytosis of immune cells
| Cells | Sp | Specification | Rec | Effect / Activating agent | Age | Signal | Ref |
|---|---|---|---|---|---|---|---|
| Macrophages | M | Peritoneal resident cells (macrophages+lymphocytes) | ↑ latex beads, Candida albicans | adult | PKC | (De la Fuente et al. 1993, De la Fuente et al. 2000, De la Fuente et al. 2001a) | |
| Macrophages | M | Peritoneal resident cells (macrophages+lymphocytes) | ↓ latex beads | old | (De la Fuente et al. 2000, De la Fuente et al. 2001a) | ||
| Macrophages | M | Purified peritoneal | ↓ latex beads | adult | (De la Fuente et al. 2001a) | ||
| Macrophages | M | Purified peritoneal | ↑ latex beads | old | (De la Fuente et al. 2001a) | ||
| Monocytes / Macrophages | M | RAW 264.7 cell line | ↓ Leishmania major | (Ahmed et al. 2001) | |||
| Monocytes / Macrophages | M | RAW 264.7 cell line | ↓ unopsonized E. coli + Staph. aureus | (Phan & Taylor 2013) | |||
| Monocytes / Macrophages | M | RAW 264.7 cell line | ↓ phagolysosome activation | (Phan & Taylor 2013) | |||
| Monocytes + Granulocytes | R | Blood | Y1 | ↑ zymosan | (Mitic et al. 2011) | ||
| Monocytes + Granulocytes | R | Air pouch, LPS-elicited | Y1+Y2 | ↓ zymosan | (Mitic et al. 2011) | ||
| Granulocytes | R | Air pouch, carrageenan-elicited | Y1 | ↓ zymosan | adult | (Dimitrijevic et al. 2006, Dimitrijevic et al. 2010) | |
| Neutrophils | H | Blood | ↑ E. coli (low dose) | (Bedoui et al. 2008) | |||
| Neutrophils | H | Blood | Y1+Y2 | ↓ E. coli (high dose) | (Bedoui et al. 2008) |
Abbreviations: H, human; LPS, lipopolysaccharide; M, mouse; PKC, protein kinase C; R, rat; Rec, receptor; Sp, species.
Table 4.
Effects of NPY on ROS and NO production of immune cells
| Cells | Sp | Specification | Rec | Effect | Age | Signal | Ref |
|---|---|---|---|---|---|---|---|
| Macrophages | M | Peritoneal resident cells (macrophages + lymphocytes) | ↑ ROS (NBT reduction) | adult | PKC | (De la Fuente et al. 1993, De la Fuente et al. 2000, De la Fuente et al. 2001a) | |
| Macrophages | R | Peritoneal, thioglycollate-elicited | Y1+Y2 | ↑ ROS (PMA-stimulated) | PKC | (Dimitrijevic et al. 2005) | |
| Macrophages | R | Peritoneal, thioglycollate-elicited | Y2 | ↓ ROS (zymosan-stimulated) | (Dimitrijevic et al. 2005) | ||
| Macrophages | R | Peritoneal, thioglycollate-elicited | Y5 | ↓ ROS (PMA + zymosan-stimulated) | (Dimitrijevic et al. 2005) | ||
| Macrophages | R | Peritoneal resident cells | ↓ ROS by PYY (zymosan-stimulated) | adult | (Stanojevic et al. 2006) | ||
| Monocytes / Macrophages | M | RAW 264.7 cell line | ↓ ROS (FcR-associated) | (Phan & Taylor 2013) | |||
| Granulocytes | R | Air pouch, carrageenan-elicited | Y2+Y5 | ↓ ROS (PMA-stimulated) | (Dimitrijevic et al. 2006) | ||
| Neutrophils | H | Blood | Y5 | ↑ ROS (f-MLP-induced) | (Bedoui et al. 2008) | ||
| Macrophages | R | Peritoneal resident cells | Y1+Y2 | ↑ NO (LPS-stimulated) | young | (Dimitrijevic et al. 2008) | |
| Macrophages | R | Peritoneal resident cells | ↓ NO by PYY (LPS-stimulated) | old | (Stanojevic et al. 2006) | ||
| Granulocytes | R | Air pouch, carrageenan-elicited | Y1 | ↑ NO (LPS-stimulated) | (Dimitrijevic et al. 2006, Dimitrijevic et al. 2010) |
Abbreviations: FcR, Fc receptor; f-MLP, N-formyl-methionyl-leucyl-phenylalanine; H, human; LPS, lipopolysaccharide; M, mouse; NBT, nitroblue tetrazolium; NO, nitric oxide; PKC, protein kinase C; PMA, phorbol myristate acetate; PYY, peptide YY; R, rat; Rec, receptor; ROS, reactive oxygen species; Sp, species.
Table 5.
Effects of NPY on n production of immune cells
| Cells | Sp | Specification | Rec | Effect | Age | Signal | Ref |
|---|---|---|---|---|---|---|---|
| Macrophages | M | Peritoneal resident cells (macrophages+lymphocytes) | ↓ TNF-α (LPS-stimulated) | (De la Fuente et al. 2001a) | |||
| Macrophages | M | Peritoneal resident cells (macrophages+lymphocytes) | ↑ IL-1β (Con A-stimulated) | adult | (De la Fuente et al. 2001a) | ||
| Macrophages | M | Peritoneal resident cells (macrophages+lymphocytes) | ↓IL-1β (Con A-stimulated) | old | (De la Fuente et al. 2001a) | ||
| Macrophages | M | Peritoneal, thioglycollate-elicited | Y1 | ↓ MCP-1 + TNF (LPS- stimulated) | (Macia et al. 2012b) | ||
| Monocytes / Macrophages | M | RAW 264.7 cell line | Y1 | ↑ TGF-β1 | PI3K | (Zhou et al. 2008a) | |
| Macrophages | M | Spleen tissue slice | Y1 | ↓ IL-6 (spontaneous, α2 adrenoceptor agonist) | (Straub et al. 2000b) | ||
| Macrophages | M | Spleen tissue slice | Y1 | ↑ IL-6 (β adrenoceptor agonist) | (Straub et al. 2000b) | ||
| Macrophages | M | Peritoneal, thioglycollate-elicited | Y1 | ↑ high-mobility group box 1 secretion | young | PKC/ERK | (Zhou et al. 2013) |
| DCs | H | Blood | ↑ IL-6 + IL-10 (Th2 polarization) | (Buttari et al. 2014) | |||
| Leukocytes | M | Peritoneal resident cells (macrophages+lymphocytes) | ↓ IL-2 (Con A-stimulated) | adult | (Puerto et al. 2005) | ||
| Leukocytes | H | Blood | ↑ IL-βp, IL-6, TNF-α | (Hernanz et al. 1996) | |||
| Leukocytes | M | Peritoneal resident cells (macrophages+lymphocytes) | ↓TNF-α (LPS-stimulated) | adult | (Puerto et al. 2005) | ||
| Lymphocytes | M | ↓ IL-2 (Con A-stimulated) | adult | (Medina et al. 2000a) | |||
| T cells | M | Nonadherent splenocytes + T cell clones | ↓ IFN-α + ↑ IL-4 (Th2 polarization) | (Kawamura et al. 1998) | |||
| T cells | M | T cell lines | ↑ IL-2, IFN-γ, IL-4 | (Levite 1998) | |||
| T cells | M | Spleen | Y1 | ↓ IFN-γ (anti-CD3 Ab-stimulated) | (Bedoui et al. 2003) |
Abbreviations: Ab, antibody, Con A, concanavalin A; DCs, dendritic cells; ERK, extracellular signal regulated kinases; H, human; IFN, interferon; IL, interleukin, LPS, lipopolysaccharide; M, mouse; MCP-1, monocyte chemotactic protein-1; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; Rec, receptor; Sp, species; TGF, transforming growth factor; TNF, tumor necrosis factor.
Table 6.
Effects of NPY on microglia
| Cells | Sp | Specification | Rec | Effect | Ref |
|---|---|---|---|---|---|
| Microglia | M | N9 cell line | Y1 | ↓ motility (LPS-stimulated) | (Ferreira et al. 2012) |
| CD11b+ cells | M | Brain cortex explants | Y1 | ↓ motility (LPS-stimulated) | (Ferreira et al. 2012) |
| Microglia | M | N9 cell line | Y1 | ↓ phagocytosis (IgG-opsonized latex beads, LPS-stimulated) | (Ferreira et al. 2011) |
| Microglia | M | N9 cell line | Y1 | ↓ IL-1β, NO (LPS-stimulated) | (Ferreira et al. 2010) |
| Microglia | R | Primary, cultured from cortex | Y1 | ↓ IL-1β, TNF-α (LPS-stimulated) | (Li et al. 2014) |
Abbreviations: LPS, lipopolysaccharide; M, mouse; NO, nitric oxide; R, rat; Rec, receptor; Sp, species.
Table 7.
Effects of NPY on natural killer cells
| Cells | Sp | Specification | Effect | Age | Signal | Ref |
|---|---|---|---|---|---|---|
| NK cells | H | Blood | ↓ activity | (Nair et al. 1993) | ||
| NK cells | M | spleen, thymus, axillary node | ↓ activity (spleen) | young | (Medina et al. 1998a, De la Fuente et al. 2001b) | |
| NK cells | M | spleen, thymus, axillary node | ↑ activity (axillary node + thymus) | adult | cAMP | (Medina et al. 1998a, De la Fuente et al. 2001b) |
| NK cells | M | Peritoneal resident cells (macrophages+lymphocytes) | ↑ activity | adult | (Puerto et al. 2005) |
Abbreviations: cAMP, cyclic adenosine monophosphate; H, human; M, mouse; Sp, species.
Table 8.
Effects of NPY on lymphoproliferation
| Cells | Sp | Specification | Effect | Age | Ref |
|---|---|---|---|---|---|
| Lymphocytes | M | Peritoneal resident cells (macrophages+lymphocytes) | ↑ spontaneous lymphoproliferation | (Puerto et al. 2005) | |
| Lymphocytes | M | Peritoneal resident cells (macrophages+lymphocytes) | ↓ lymphoproliferation (Con A- or LPS-stimulated) | adult | (Puerto et al. 2005) |
| Lymphocytes | M | Spleen | ↑ spontaneous proliferation | adult | (Medina et al. 1999, Medina et al. 2000a) |
| Lymphocytes | M | Spleen, thymus, axillary node | ↓ lymphoproliferation (Con A-stimulated) | adult | (Medina et al. 1999, Medina et al. 2000a) |
Abbreviations: Con A, concanavalin A; LPS, lipopolysaccharide; M, mouse; Sp, species.
The relationship between NPY and the immune system is bidirectional. Thus, the production of NPY by immune cells can be upregulated upon immune stimulation (Singer et al. 2013). Likewise, cytokines have been reported to stimulate mouse chromaffin cells to release NPY and catecholamines, which on their own affect the immune system (Rosmaninho-Salgado et al. 2007).
NPY’s actions on monocytes, macrophages, microglia and cytokine secretion
One of the first studies examining the role of NPY on macrophages found that both NPY and PYY are able to increase adhesion (Table 1), chemotaxis (Table 2), phagocytosis of latex beads and Candida albicans (Table 3) as well as production of superoxide anions (Table 4) in resting peritoneal macrophages from male BALB/c mice (De la Fuente et al. 1993). In addition to the macrophage activating actions of NPY and PYY, a stimulation of PKC in these cells was observed (De la Fuente et al. 1993). Interestingly, in a subsequent study the effects on macrophage function were shown to change with age. Thus, while NPY increased macrophage function in adult mice, it decreased the chemotaxis and phagocytosis capacity of macrophages from old mice which under control conditions display a higher chemotaxis and phagocytosis capacity than adult mice (De la Fuente et al. 2000, De la Fuente et al. 2001a). Furthermore, NPY potentiated nitric oxide (NO) production of LPS-stimulated resident rat peritoneal macrophages solely in young rats, an effect that was mediated by Y1 and Y2 receptors (Dimitrijevic et al. 2008).
Analysis of the receptors involved revealed that in vitro NPY potentiated adhesiveness and phagocytosis of peripheral blood granulocytes and monocytes via the Y1 receptor (Mitic et al. 2011). In the same study it was found that in vivo NPY reduced inflammatory cell accumulation in LPS-induced air-pouch exudates via Y1/Y2 receptor activation, while it decreased their adhesion via Y2/Y5 receptor stimulation (Mitic et al. 2011).
NPY produced by endothelial cells has been suggested to be involved in the recruitment of leukocytes at sites of LPS-induced inflammation (Silva et al. 2003). Thus, NPY, Y1 and Y2 receptors are expressed in human umbilical venous endothelial cells, while immune challenge with lipopolysaccharide (LPS) has been reported to increase NPY, DP4 activity and to induce Y5 receptor expression, while the Y2 receptor expression is suppressed (Silva et al. 2003).
Several reports demonstrate further inhibitory actions of NPY on macrophage function. For instance, NPY inhibits spontaneous activation of human granulocytes and macrophages as well as their chemotaxis (Dureus et al. 1993). NPY, PYY, LP-NPY (a Y1/Y5 receptor agonist) and NPY13-36 (a Y2 receptor agonist) decrease peroxide production of thioglycollate-elicited rat peritoneal macrophages (Dimitrijevic et al. 2002). In addition, phagocytosis of zymosan by peritoneal macrophages is decreased through an action of the Y2 receptor (Dimitrijevic et al. 2005). PYY acting via Y1 receptors has also been demonstrated to decrease the adhesion capacity of elicited peritoneal macrophages of male rats and to suppress phagocytosis and NO production of resident macrophages (Stanojevic et al. 2006).
The partly contradicting effects of NPY on macrophage function might be due to species differences between mice and rats and to the different response modes of resident versus elicited macrophages (Stanojevic et al. 2006). The differences in phagocytosis may also depend on the activating agent or pathogen. Specifically, while NPY has been reported to increase the phagocytosis of Candida albicans (De la Fuente et al. 1993), it suppresses phagocytosis and killing of Leishmania major parasites by a murine leukaemic monocyte macrophage cell line (Raw 264.7) (Ahmed et al. 2001). Further analysis of NPY’s effects in Raw 264.7 cells disclosed a suppression of phagocytosis of unopsonized Escherichia coli and Staphylococcus aureus bioparticles (Phan & Taylor 2013). In addition, NPY decreases FcR-associated generation of reactive oxidative species and phagolysosome activation (Phan & Taylor 2013).
The effects of NPY on cytokine production (Table 5) by immune cells are likewise of a diverging nature. While NPY has been reported to increase the production of interleukin (IL)-1β, IL-6 and tumour necrosis factor-α (TNF-α) of whole blood cells from healthy subjects (Hernanz et al. 1996), it decreases TNF-α release in response to LPS as well as IL-1β and IL-2 release in response to concavalin A (Con A) in murine peritoneal macrophages (De la Fuente et al. 2001a, Puerto et al. 2005). Furthermore, NPY increases the release of the anti-inflammatory transforming growth factor-beta 1 in the Raw 264.7 cell line, a process mediated by the Y1 receptor (Zhou et al. 2008a). In addition, NPY is able to inhibit DC maturation and cytokine production in vitro (Singer et al. 2013). NPY acting via Y1 receptors likewise reduces cytokine release from thioglycollate-elicited peritoneal mouse macrophages stimulated by interferon-γ and LPS (Macia et al. 2012b). While the involvement of Y2 receptors in this effect has been excluded, macrophages of NPY/PYY double-knockout (KO) mice exhibit higher cytokine levels than those of Y1 KO mice, suggesting that Y4 or Y5 receptors could also contribute to the anti-inflammatory effect of NPY in this context (Macia et al. 2012b).
Finally, NPY affects microglia function and related neuroinflammatory processes (Malva et al. 2012) (Table 6). Murine N9 microglia cells express Y1, Y2, and Y5 receptors and the expression of NPY and the Y1 receptor by microglial cells increases after activation with LPS (Ferreira et al. 2010). Furthermore, NPY acting via the Y1 receptor inhibits the release of IL-1β and subsequent NO production in LPS-stimulated microglial cells (Ferreira et al. 2010). Subsequent studies from the same group revealed that NPY likewise inhibits LPS- and IL-1β-induced phagocytosis of opsonized latex beads (Ferreira et al. 2011) and microglial motility via the Y1 receptor (Ferreira et al. 2012). The inhibitory effects of NPY on microglial motility have been confirmed in murine cortex explants (Ferreira et al. 2012).
NPY’s effects on dendritic cells and lymphocytes
The effects of NPY on natural killer (NK) cells are age- and dose-dependent and differ in NK cells originating from different lymphoid organs (Dimitrijevic & Stanojevic 2013) (Table 7). Thus, NPY has been shown to suppress NK activity of human lymphocytes in vitro (Nair et al. 1993) and splenic NK activity in adult male rats in vivo (Saurer et al. 2006). However, the type of effect of NPY on NK cell activity has been shown to differ with age (De la Fuente et al. 2001b). Thus, NPY inhibits NK activity in spleen leukocytes of young female mice, while it stimulates NK activity in leukocytes from axillary nodes and thymus of adult and mature female mice which exhibit a decline of NK activity with age (De la Fuente et al. 2001b). Apart from age, dose is another factor modulating the type of effect of NPY on NK cell numbers. Thus it has been reported that intravenous administration of high-dose NPY increases the numbers of NK cells, lymphocytes and monocytes in the blood, while low-dose NPY decreases NK cell numbers and B cell counts (Bedoui et al. 2001). Analysis of the NPY receptors involved revealed that Y1 receptor activation decreased blood leukocyte counts, while Y5 receptor activation was associated with leukocytosis (Bedoui et al. 2002).
NPY is involved in T cell recruitment, proliferation (Table 8) and cytokine secretion. Thus it has been proven that NPY increases adhesion of human T cells to fibronectin via the Y2 receptor (Levite et al. 1998). Furthermore, NPY is able to increase spontaneous proliferation of peritoneal lymphocytes while it inhibits proliferation after stimulation with the mitogen Con A or LPS (Puerto et al. 2005, Dimitrijevic & Stanojevic 2013). These results suggest that the effects of NPY on lymphocyte proliferation depend on the activation state of these cells. Furthermore, NPY induces cytokine secretion from T cells (Levite 1998) and, interestingly, is able to induce the secretion of Th2 cytokines from Th1 T cell lines and the other way around (Levite 1998). In addition, several studies report that NPY shifts the immune balance towards a Th2 immune response (Kawamura et al. 1998, Medina et al. 2000a, Puerto et al. 2005). Pharmacological analysis attributed the Y1 receptor a role in the effect of NPY to inhibit the activation of T cells in mice (Wheway et al. 2005). The same study, however, also demonstrated that Y1 receptor signalling on DCs promotes antigen-presenting capacity and T cell priming (Wheway et al. 2005), thus providing an explanation for the modulatory role of Y1 receptor activation in lymphocyte function.
A recent study shows that NPY induces migration of human DCs via the Y1 receptor (Buttari et al. 2014). Furthermore, NPY is able to promote DC adhesion and a Th2 polarizing profile of the DCs (Buttari et al. 2014). These findings led the authors to conclude that NPY, on the one hand, recruits DCs to the inflammatory site but, on the other hand, exerts anti-inflammatory actions by promoting Th2 polarization (Buttari et al. 2014). Interestingly, NPY is also able to suppress experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis, by inhibiting Th1 responses via the Y1 receptor (Bedoui et al. 2003, Brod & Bauer 2012, Dimitrijevic et al. 2012).
The interaction of NPY with the immune system may also affect the skin, as exemplified by the depigmenting skin disorder vitiligo, which results from a loss of melanocytes, cells with an embryologic link to the nervous system (Kunisada et al. 2014). Autoimmunity and abnormal immune responses are implicated in the pathophysiology of the disease as vitiligo patients present with increased levels of IL-4 (Imran et al. 2012), IFN-γ (Dwivedi et al. 2013), IL-1β (Laddha et al. 2014) and other abnormalities in both humoral and cell-mediated immunity (Kemp et al. 2001). Importantly, vitiligo patients also present higher levels of NPY in plasma and skin tissue (Tu et al. 2001, Laddha et al. 2014). As NPY has been demonstrated to lead to increased release of IFN-γ and IL-4 from T cells (Levite 1998), an interaction between NPY and immune regulatory factors might also occur in vitiligo.
The neuropeptide Y (NPY) family in immune function within the gastrointestinal tract
The gut hormones PYY and PP and the enteric/sympathetic neuropeptide NPY serve multiple regulatory roles in digestion (Cox 2007a, Cox 2007b, Fujimiya & Inui 2000), gastrointestinal immune function (Wheway et al. 2007a) and gut-brain-gut communication (Holzer et al. 2012). In addition, it is emerging that these peptides play a role in the interaction between the gut microbiota and the gastrointestinal mucosa and immune system (Holzer & Farzi 2014). In order to understand these complex interdependencies it is appropriate to recall the chief functions which NPY-related peptides exert in the gastrointestinal tract.
PP is released from endocrine F cells of the pancreas following a meal and acts preferentially via Y4 and Y5 receptors to inhibit gastric emptying and reduce appetite through a vagus nerve-dependent action (Murphy & Bloom 2006, Field et al. 2010). Furthermore, PP inhibits intestinal electrolyte and water secretion (Cox 2007a) as well as intestinal motor activity and peristalsis via an action involving the enteric nervous system (Holzer et al. 1986, Fujimiya & Inui 2000).
The vagus nerve also plays a role in the postprandial release of PYY from enteroendocrine L cells in the lower gut (Fu-Cheng et al. 1997, Cox 2007b, Field et al. 2010). PYY and PYY3-36 inhibit gastrointestinal motility and secretion via an action on Y1, Y2, and Y4 receptors (Cox 2007a, Cox 2007b, Field et al. 2010, Wang et al. 2010). Through these actions, PYY slows gastric emptying and intestinal transit when fat reaches the lower gut, while the antisecretory action of PYY facilitates nutrient absorption (Van Citters & Lin 2006, Cox 2007b).
In the context of this review it is particularly worth noting that short chain fatty acids such as butyrate, which the colonic microbiota generates by fermentation of otherwise indigestible dietary fibre (Cherbut et al. 1998), stimulate L cells to release PYY via the G-protein coupled receptor Gpr41 (Samuel et al. 2008). In this way, short chain fatty acids can indirectly attenuate gastrointestinal motility as well as electrolyte and water secretion (Cox 2007b). More importantly, short chain fatty acids exert homeostatic actions on the function of the colonic mucosa and immune system (Hamer et al. 2008, Tazoe et al. 2008, Guilloteau et al. 2010, Macia et al. 2012a, Smith et al. 2013). Whether PYY plays a role in these effects of short chain fatty acids awaits to be investigated, but may be envisaged from the finding that PYY promotes mucosal cell differentiation (Hallden & Aponte 1997).
Apart from its effect to inhibit gastrointestinal motility as well as electrolyte and water secretion (Saria & Beubler 1985, Holzer et al. 1987, Holzer-Petsche et al. 1991, Cox 2007a), NPY is of distinct relevance to immune function in the gastrointestinal mucosa (Bedoui et al. 2007, Wheway et al. 2007a, Dimitrijevic & Stanojevic 2013, Chandrasekharan et al. 2013b) in which NPY-containing nerve fibres are in close contact with immune cells such as IgA-producing lymphocytes (Shibata et al. 2008).
Although the effect of NPY on immune function translates to an antiinflammatory action in many tissues, its effect in the gut needs to be labelled as proinflammatory. The effect of NPY to facilitate colonic inflammation is supported by several lines of evidence (Holzer et al. 2012). Thus, mice with a genetic deletion of NPY are to a large degree resistant to the development of dextran sulfate sodium (DSS)-induced colitis (Chandrasekharan et al. 2008, Painsipp et al. 2011). This observation is reproduced by treatment with a NPY antisense oligodeoxynucleotide (Pang et al. 2010) and by KO or antagonism of Y1 receptors (Hassani et al. 2005), which attributes Y1 receptors a key role in the proinflammatory action of NPY (Holzer et al. 2012). The inflammation-resistant phenotype of NPY and Y1 receptor KO mice results from a defect in antigen-presenting cell function, a reduction of TNF-α and IL-12 production by macrophages, and a decrease in the number of effector T cells (Wheway et al. 2005, Chandrasekharan et al. 2013a).
An implication of the NPY and Y1 receptor system in controlling colonic inflammation is corroborated by the observations that experimentally induced colitis is associated with an increase in the colonic expression of NPY (Chandrasekharan et al. 2008, Pang et al. 2010, Baticic et al. 2011), a downregulation of colonic Y1 receptors and a defective antisecretory effect of NPY (Klompus et al. 2010). The expression of NPY in sympathetic neurons of the mesenteric ganglia is also elevated in pigs with chemically induced ileitis (Pidsudko et al. 2011). In contrast, the epithelial levels of NPY and PYY are decreased in rats with DSS-induced colitis (Hirotani et al. 2008) and in mice with genetic deletion of carboxypeptidase E, an enzyme involved in the biosynthesis of many neuropeptides including NPY, in which DSS-induced colitis is exacerbated (Bär et al. 2014). These experimental data are in keeping with a decrease of colonic PYY levels in patients with inflammatory bowel disease (Tari et al. 1988, El-Salhy et al. 1997, Schmidt et al. 2005), whereas circulating levels of PYY and NPY are enhanced (Adrian et al. 1986, Straub et al. 2002). These observations suggest that the epithelial levels of NPY and PYY are exhausted by excessive release of the peptides from enteroendocrine cells and intestinal neurons, although the expression of the peptides in these cells is enhanced.
From these observations it is evident that intestinal inflammation goes along with a remodelling of the NPY system, which has an effect on mucosal permeability, immune cell function, intestinal motor activity and intestinal blood flow (Hirsch & Zukowska 2012, Chandrasekharan et al. 2013b). The impact of NPY on gut inflammation involves a heterogeneity of cellular and molecular mechanisms (Holzer et al. 2012) which include activation of antigen-presenting cells, altered regulation of T cell function, TNF-α, nuclear factor κ-light-chain-enhancer of activated B-cells (NF-κB) and oxidative stress (Wheway et al. 2005, Wheway et al. 2007a, Wheway et al. 2007b, Dimitrijevic & Stanojevic 2013, Chandrasekharan et al. 2013a, Chandrasekharan et al. 2013b). There is a close interaction between NPY and TNF-α as, on the one hand, the TNF-α system appears to be downregulated in NPY KO mice while, on the other hand, blockade of TNF-α reduces colonic NPY expression and experimental colitis (Chandrasekharan et al. 2013a). In addition, NPY enhances the expression of neuronal NO synthase which is a component of oxidative stress and subsequent inflammation (Chandrasekharan et al. 2008). The proinflammatory effect of NPY is likely to be counterregulated by the vasoconstrictor effect of the peptide (Holzer et al. 2012), since the sympathetic constriction of splanchnic resistance vessels is co-mediated by the sympathetic triad adenosine triphosphate (ATP), noradrenaline and NPY (Holzer et al. 2012). In addition, NPY is able to augment the constrictor effect of noradrenaline and ATP, this effect as well as the vasoconstrictor response to NPY being mediated by postjunctional Y1 receptors (Holzer et al. 2012).
The gut microbiota may be another cellular system that is relevant to the proinflammatory action of NPY in the digestive tract. On the one hand, the NPY/PYY system seems to have an impact on the composition and function of the gut microbiota as NPY has been found to exhibit a direct antimicrobial effect against various gut bacteria including Escherichia coli, Enterococcus faecalis, and Lactobacillus acidophilus (El Karim et al. 2008, Augustyniak et al. 2012). On the other hand, there is evidence that the gut microbiota has an influence on the NPY/PYY system. Through the generation of short chain fatty acids, the gut microbiota can directly interact with enteroendocrine cells that release PYY, besides glucagon-like peptide-1 (Cani et al. 2009, Cani & Delzenne 2011, Delzenne et al. 2011). Colonization of the mouse colon with a fermentative human microbial community increases the plasma level of PYY, an effect that is blunted by knockout of the short chain fatty acid receptor Gpr41. Gpr41 deficiency is associated with a reduced expression of PYY, an increase in intestinal transit rate and an attenuation of energy harvest (Samuel et al. 2008). Microbiota-fermented short chain fatty acids acting via Gpr41 on PYY-expressing L cells could thus be an important link in microbial-host communication and immune signalling (Holzer & Farzi 2014). The microbiota-NPY link may also have a bearing at other relays of the gut-brain axis, given that the expression of NPY in the hypothalamus of germ-free mice is higher than in conventionally raised animals (Schele et al. 2013).
NPY’s involvement in obesity-associated inflammation and inflammation-induced metabolic disturbances
An excess of nutrients leading to obesity initiates a state of low-grade inflammation which contributes to metabolic dysfunctions such as insulin resistance (Gregor & Hotamisligil 2011). In this context it is noteworthy that NPY is regarded as one of the most potent orexigenic neuropeptides, the hypothalamus and its nuclei such as the arcuate nucleus being the crucial centres in which these effects are brought about (Zhang et al. 2014). Emerging evidence indicates that Y1 receptors on cells of the hematopoietic compartment mediate a protective effect against obesity-associated inflammation, given that deletion of the Y1 receptor promotes inflammation in adipose tissue and development of insulin resistance in response to a high-fat diet (Macia et al. 2012b). Y1 receptor deletion also leads to an increase of the proinflammatory cytokine monocyte chemotactic protein 1 (Macia et al. 2012b). A further study confirms the anti-inflammatory role of NPY by showing that, in lean mice, exogenous NPY decreases adipose tissue macrophage numbers and suppresses monocytes and, in obese mice fed a high-fat diet, NPY from hematopoietic cells decreases adipose tissue macrophages (Singer et al. 2013). In contrast, the Y2 receptor has been implicated in the exaggeration of diet-induced obesity by stress, which also results in macrophage infiltration (Kuo et al. 2007).
Both NPY and PYY play important roles in metabolism and energy homeostasis (Herzog 2003). NPY promotes energy storage by promoting adipogenesis and inhibiting lipolysis (Kuo et al. 2007), and its expression is increased in the adipose tissue of obese mice and other species (Zhang et al. 2014). However, lipolysis caused by β-adrenoceptor stimulation is rather augmented by NPY, pointing to another context-dependent modulatory role of NPY (Li et al. 2012). The differential effects of NPY on lipolysis may be mediated by different second messenger systems coupled to the Y1 receptor (Li et al. 2012).
Furthermore, PYY is able to regulate function and survival of islet cells of the pancreas, which also express NPY receptors (Upchurch et al. 1994, Sam et al. 2012, Persaud & Bewick 2014). Exogenous PYY inhibits glucose-induced insulin secretion (Böttcher et al. 1993) whereas PYY KO mice display hyperinsulinaemia (Boey et al. 2006). In contrast, PYY3-36 is able to improve glucose tolerance by indirect stimulation of insulin release (Chandarana et al. 2013). In addition, PYY exerts anti-inflammatory actions in the pancreas and inhibits several transcription factors such as NF-κB (Vona-Davis & McFadden 2007). Recent studies revealed complex interactions between NPY and PYY in regulating various aspects of energy balance and glucose homeostasis by using NPY/PYY single- and double-KO mice as well as Y2 and Y4 receptor KO mice (Zhang et al. 2012). Thus, NPY/PYY double-KO mice and Y2 receptor KO mice display a reduction in spontaneous food intake (Edelsbrunner et al. 2009a, Edelsbrunner et al. 2009b), and both PYY KO and NPY/PYY KO mice show impaired hypoglycaemic responses to insulin, suggesting that the impairment of insulin action by PYY deficiency cannot be corrected by NPY deletion (Zhang et al. 2012). In line with these complex interactions of NPY and PYY, deletion of either peptide (NPY or PYY) or both peptides (NPY plus PYY) aggravates and prolongs the weight loss induced by immune challenge with Bacille Calmette-Guérin (BCG) (Painsipp et al. 2013). Importantly, the weight loss observed in the KO mice outlasted the BCG-induced decrease in food intake. Thus, alterations of food intake cannot explain the aggravated weight loss in the KO mice. Also, circulating IL-6 levels did not significantly differ between groups. These results therefore indicate that in addition to their basal effects on food intake and energy homeostasis, NPY and PYY play an important physiological role in maintaining energy homeostasis in response to infection and immune challenge (Painsipp et al. 2013).
Effects of NPY on brain function and behaviour
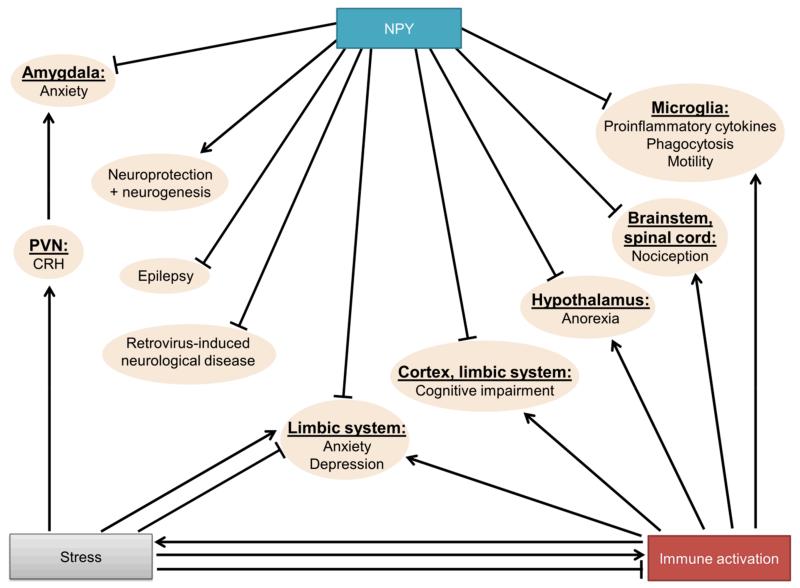
There is abundant evidence that NPY has antiepileptic effects and triggers neuroprotective pathways (Malva et al. 2012) (Fig. 3). In addition, NPY exerts mitogenic actions, inducing neuroproliferation and neurogenesis, an effect taking place in an Y1 receptor- and PKC-dependent manner (Hansel et al. 2001, Decressac et al. 2011). Furthermore, NPY is able to prevent neuronal apoptosis (Thiriet et al. 2005). Interestingly, NPY has also been reported to exert protective actions during murine retrovirus-induced neurological disease (Du et al. 2010). Specifically, NPY KO mice presented an earlier onset of histological changes together with higher virus levels in brain tissue, while the neuroinflammatory response and neuronal apoptosis were not markedly different compared to wild-type (WT) mice (Du et al. 2010). Further, NPY contributes to the control of anxiety, depression and cognition. Thus, NPY and its receptors are widely expressed in cerebral areas regulating anxiety, mood, cognition and stress resilience, such as the cortex, hippocampus, and amygdala (Heilig 2004, Morales-Medina et al. 2010, Holzer et al. 2012). In rodents, central administration of NPY reduces anxiety and depression-like behaviour (Sajdyk et al. 1999b, Stogner & Holmes 2000). In line with these pharmacological studies, central over-expression of NPY reduces anxiety-like behaviour (Thorsell et al. 2000), while NPY KO mice display an anxious and depressive-like phenotype (Bannon et al. 2000, Painsipp et al. 2011). In PYY KO mice a depression-like phenotype has been observed (Painsipp et al. 2011).
Figure 3. Homeostatic roles of NPY in the central nervous system.
The arrow symbols denote stimulation, the tack symbols denote inhibition.
Abbreviations: PVN, paraventricular nucleus of the hypothalamus.
Although not entirely consistent, the majority of clinical studies demonstrate decreased levels of NPY in plasma or CSF of patients suffering from major depression, and increased NPY levels following antidepressant treatment (Morales-Medina et al. 2010). In addition, there is evidence that a polymorphism in the NPY gene leading to a low NPY expression genotype is associated with decreased stress resilience, a risk factor for major depression, and reduced responsiveness to antidepressant treatment (Zhou et al. 2008b, Domschke et al. 2010, Mickey et al. 2011). Likewise, there is evidence for altered NPY signalling in schizophrenia (Stadlbauer et al. 2013).
The effect of NPY on mood is determined by an interaction with multiple Y receptors in the brain. Although genetic studies revealed partially controversial data, most results indicate that the Y1 receptor mediates an anxiolytic effect (Morales-Medina et al. 2010). In contrast, the Y2 receptor is involved in increasing anxiety- and depression-like behaviours (Tschenett et al. 2003, Painsipp et al. 2008a, Tasan et al. 2010), which can be related to its presynaptic localization and function as an inhibitory autoreceptor reducing NPY release (Caberlotto et al. 2000).
Despite the limited distribution of the Y4 receptor in the brain, KO of this receptor is associated with reduced anxiety- and depression-related behaviours (Painsipp et al. 2008b, Tasan et al. 2009). In contrast, the involvement of the Y5 receptor in mood and stress resilience is rather inconclusive as judged from the available findings (Kormos & Gaszner 2013).
NPY has also been proposed to affect memory processing in rodents. Thus, central administration of NPY leads to an improvement of memory in rodent amnesia models induced by either anisomycin or scopolamine (Flood et al. 1987). While NPY KO mice do not show any gross abnormalities in learning tests (Bannon et al. 2000), Y2 deficient mice have been reported to display cognitive deficits (Redrobe et al. 2004b, Greco & Carli 2006, Painsipp et al. 2008b). Interestingly, a recent study provided evidence for altered Y2 receptor signalling in symptoms associated with schizophrenia, as administration of PYY3-36 to male mice induces behavioural abnormalities relevant to schizophrenia (Stadlbauer et al. 2013).
Involvement of NPY in behavioural disturbances caused by peripheral immune challenge
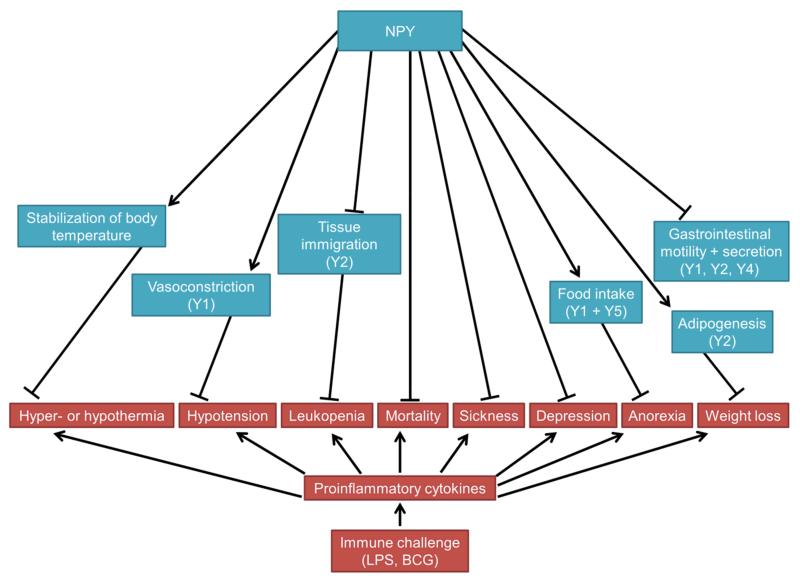
Immune challenge has been demonstrated to disturb homeostasis of many physiological systems and to contribute to the development of mood disorders such as major depression (Dantzer et al. 2008, Haroon et al. 2012) and other disturbances of brain function (Figs. 2 + 3). Cytokine-induced depression can be modelled in rodents by administration of proinflammatory cytokines or bacterial components such as LPS (Dantzer et al. 2008, Farzi et al. 2014). In this context, proinflammatory cytokines cause acute sickness behaviour by exciting afferent neurons (e.g. in the vagus nerve) and by inducing the expression of cytokines within the brain (Dantzer et al. 2008). The acute sickness response (sedation, social withdrawal, anorexia) is followed by signs of depression-like behaviour which has been demonstrated to be mediated by activation of the enzyme indoleamine-2,3-dioxygenase by proinflammatory cytokines, leading to the production of kynurenine (O’Connor et al. 2009). Interestingly, peripheral immune challenge has an impact on several brain nuclei that express NPY and various Y receptors, and there is evidence that the NPY system protects against various disturbances caused by immune activation (Holzer et al. 2012) (Figs. 2 and 3).
Figure 2. Homeostatic roles of NPY in disturbances of physiological systems caused by peripheral immune challenge.
Major receptors involved are bracketed. The arrow symbols denote stimulation, the tack symbols denote inhibition.
Abbreviations: BCG, Bacille Calmette-Guérin; LPS, lipopolysaccharide.
Specifically, NPY increases the survival of rats from septic shock induced by high doses of LPS (Hauser et al. 1993). This beneficial effect might to some extent be explained by NPY’s counterbalancing effects on the cardiovascular system. Thus, in contrast to other vasoconstrictors, the pressor response to NPY is not reduced by LPS and NPY is able to attenuate LPS induced-hypotension (Hauser et al. 1993, Felies et al. 2004, Kuncova et al. 2011). Furthermore, NPY is able to partially reverse the suppressed responsiveness to other vasoconstrictors in response to LPS (Hauser et al. 1995). A further mechanism whereby NPY attenuates effects of immune challenge is its ability to stabilize body temperature in endotoxaemic rats (Felies et al. 2004).
Pain is another hallmark of inflammation and NPY’s antinociceptive effects also extend to the inhibition of inflammatory pain (Taylor et al. 2014). Specifically, NPY is up regulated in the spinal cord during inflammation and exerts anti-hyperalgesic actions via the Y1 receptor by inhibiting the release of substance P (Taylor et al. 2014).
Finally, the beneficial effects of NPY against disturbances caused by immune activation might be mediated directly by its effects on the immune system (Nave et al. 2004). Thus, NPY is able to attenuate LPS-induced leukopenia and to stabilize granulocyte and T-lymphocyte numbers (Nave et al. 2004). Furthermore, NPY increases adhesion of monocytes to the endothelium via Y2 receptor stimulation (Nave et al. 2004) (Fig. 2). The beneficial effect of Y2 receptor activation in response to LPS is further related to an attenuation of TNF-α levels (Stadler et al. 2011).
In view of the involvement of NPY in the control of appetite and food intake it is not totally unexpected that NPY is able to attenuate cytokine-induced anorexia (Fig. 3), one aspect of the sickness response to immune activation. NPY concentrations have been reported to be increased, decreased or unchanged in hypothalamic nuclei in response to immune stimulation (McCarthy et al. 1995, Gayle et al. 1997, Gayle et al. 1998, Ilyin et al. 1998, Gayle et al. 1999, Kim et al. 2007, Carlson et al. 2009, Iwasa et al. 2010). Central administration of NPY has repeatedly been reported to block or reverse anorexia induced by immune activation (Sonti et al. 1996, McMahon et al. 1999, Kim et al. 2007).
NPY has also been implicated in other manifestations of the sickness response to immune activation such as immobility/sedation and impaired mood (Fig. 3). Y2 and Y4 receptor KO mice are particularly susceptible to the acute action of LPS to attenuate locomotion (Painsipp et al. 2008a, Painsipp et al. 2010). Intriguingly, decreased locomotion in Y4 KO mice is still evident 4 weeks after treatment with LPS (Painsipp et al. 2010). In addition, Y2 and Y4 KO mice display depression-like behaviour 4 weeks after administration of LPS, while such an effect is absent in WT mice (Painsipp et al. 2010). From these results it has been concluded that NPY acting via Y2 and Y4 receptors attenuates the development of long-term sickness- and depression-like behaviour induced by immune challenge (Painsipp et al. 2010).
Immune activation and neuroinflammation can also lead to cognitive impairment and there is evidence for the involvement of immune activation in neurodegenerative diseases such as Alzheimer’s disease (Czerniawski & Guzowski 2014, Lueg et al. 2014). While reports regarding the effects of NPY on cognitive impairment induced by peripheral immune activation are lacking, there is evidence for beneficial effects of NPY in the pathophysiology of Alzheimer’s disease (Malva et al. 2012).
Calorie restriction has been demonstrated to suppress LPS-induced cytokine release, microglial activation, fever, and sickness behaviour (MacDonald et al. 2014). In addition, calorie restriction is able to upregulate the expression of NPY in the arcuate nucleus (McShane et al. 1999). Therefore, a recent study examined the relationship between calorie restriction, LPS-stimulated microglial activation and NPY (Radler et al. 2014). A strong correlation was demonstrated between NPY and body temperature on the one side, and body temperature and microglial morphology, on the other side (Radler et al. 2014). In contrast, the authors did not find an association between NPY and microglial morphology. These results led the authors to conclude that NPY may indirectly attenuate LPS-induced microglial activation through its effects on body temperature (Radler et al. 2014).
As NPY protects from behavioural sequelae of stress, exerts neuroprotective actions and counteracts disturbances caused by immune activation via several mechanisms, it is very likely that its effects on brain function converge with its effects on the immune system in order to have a beneficial influence on mood and behaviour. However, further systematic studies are required to untangle to which extent the immunomodulating and stress-buffering actions of the NPY family contribute to the attenuation of behavioural disturbances caused by peripheral immune challenge.
NPY, stress and stress resilience
A large body of evidence has identified NPY as a crucial factor counteracting stress-induced disturbances of homeostasis (Heilig 2004). During stress exposure, NPY strongly interacts with corticotrophin-releasing hormone (CRH), a principal regulator of the hypothalamic-pituitary-adrenal (HPA) axis (Heilig et al. 1994, Sajdyk et al. 2004). A fibre tract between the hypothalamic arcuate nucleus, a major source of NPY, and the hypothalamic paraventricular nucleus (PVN), a major source of CRH, allows a crosstalk between these two neuropeptide systems (Broberger et al. 1999). In face of a stressful challenge, parvocellular neurons of the PVN respond rapidly with the transcription of CRH (Imaki et al. 1996). CRH promotes the production of adrenocorticotropic hormone, which in turn regulates adrenal glucocorticoid release.
Like CRH, NPY is also regulated by stress exposure. Depending on type and duration of stress as well as brain region examined, stress modifies the expression of NPY to counteract the biological actions of CRH (Thorsell et al. 1998, Thorsell et al. 1999, de Lange et al. 2008, McGuire et al. 2011). Moreover, NPY alters the stress response in a gender-dependent manner, given that HPA axis reactivity to stress differs between male and female mice in a NPY-related fashion (Forbes et al. 2012). Thus, stress-induced corticosterone levels are higher in male NPY KO mice than in male WT mice, an effect absent in female mice. Furthermore, stress-induced reduction of food intake is observed in female NPY KO, but not in male NPY KO mice (Forbes et al. 2012).
An important interaction between NPY and CRH with special relevance for emotional regulation takes place in the basolateral amygdala, which expresses receptors for both neuropeptides (Van Pett et al. 2000, Kask et al. 2002). Intraamygdalar CRH application induces anxiety, while NPY is anxiolytic (Sajdyk et al. 1999b, Sajdyk et al. 1999a). A direct interaction is suggested by the finding that intraamygdalar pretreatment with NPY blocks the anxiogenic action of CRH receptor stimulation (Sajdyk et al. 2006) (Fig. 3).
The behavioural effects of NPY and its ability to revert the effects of CRH raise the question whether NPY has a role in stress resilience, i.e. the ability to cope with stress. Indeed, hippocampal NPY overexpression protects from the anxiety-producing effects of stress (Thorsell et al. 2000), and repeated administration of NPY into the basolateral amygdala generates resilient mice that are protected from the negative consequences of stress (Sajdyk et al. 2008). A study by Cohen et al. (Cohen et al. 2012) describes a correlation between behavioural disruption of animals after predator scent stress and brain NPY levels. Animals whose behaviour is extremely disrupted by stress have the lowest brain NPY levels.
Furthermore, treatment of animals with NPY shortly after stress exposure significantly reduces the prevalence of behavioural maladaptation (Cohen et al. 2012). In line with these observations, recent evidence suggests a therapeutic potential of intranasal NPY to reverse stress-induced neuropsychiatric disorders. Intranasal NPY infusion, which avoids potential peripheral side effects, attenuates stress-induced anxiety and depression-like behaviour when applied before or 1 week after prolonged stress exposure (Serova et al. 2013, Serova et al. 2014). The anti-stress and anxiolytic properties of NPY are very likely related to the peptide’s action within the amygdala (Fig. 3): NPY acting on Y1 receptors reduces excitability of amygdalar neurons by decreasing NMDA-mediated excitatory currents and increasing GABA-mediated inhibitory currents via 2 divergent signal-transduction pathways (Molosh et al. 2013).
NPY is further considered a resilience factor as individuals with a low NPY-expressing haplotype show disturbed emotionality and diminished stress resilience (Zhou et al. 2008b, Mickey et al. 2011). Accumulating evidence also suggests that NPY is a protective factor preventing post-traumatic stress disorder (PTSD) development (Sah & Geracioti 2013). Thus, veterans suffering from PTSD have lower plasma and cerebrospinal NPY levels than healthy control subjects (Rasmusson et al. 2000, Sah et al. 2009). Moreover, combat veterans without PTSD have higher cerebrospinal NPY levels than combat veterans with PTSD (Sah et al. 2014). However, other reports suggest that the exposure to trauma, but not the associated PTSD, is responsible for the decrease in NPY levels (Morgan et al. 2003, Yehuda et al. 2006). A recent study of the relationship between NPY and PTSD after severe motor vehicle accidents found no difference in NPY levels between the study groups (Nishi et al. 2014). It appears that accident-related PTSD has different biological features than combat-related PTSD.
Involvement of NPY in the interaction of stress with the immune system
Stress, mood disorders and the immune system are in an intricate relationship. Thus, psychosocial stress has been reported to activate inflammatory responses via the sympathetic nervous system (Haroon et al. 2012). Activation of the HPA axis in response to stress and the subsequent release of glucocorticoids exert, on the one side, immunosuppressive effects but under certain conditions can also stimulate the immune system, in particular within the brain (Bellavance & Rivest 2014). For instance, NK cell activity is decreased in depressed patients, while the plasma levels of NPY are increased, attesting to an inverse correlation between the two factors (Irwin et al. 1991). On the other hand, patients with major depression present an exaggerated immune response in stressful situations (Pace et al. 2006), and psychological stress and depression are often associated with an aggravation of intestinal inflammation (Mittermaier et al. 2004, Ghia et al. 2009). Vice versa, an experimental study has shown that iodoacetamide-evoked gastritis induces an increase of anxiety in female mice (Painsipp et al. 2007), while DSS-induced colitis induces anxiety- and depression-related behaviour in a gender-dependent manner (Painsipp et al. 2011). The effects of colitis on behaviour seem to be modified by KO of NPY or PYY (Painsipp et al. 2011). Thus, genetic depletion of PYY prevents the anxiogenic effects of colitis in male mice, while in female mice NPY or PYY KO blocks depression-like behaviour induced by colitis (Painsipp et al. 2011). NPY and PYY thus seem to have distinct effects on the impact of intestinal inflammation on mood and behaviour.
While stress is generally believed to be harmful, certain types and intensities of stress such as predictable chronic mild stress can exert beneficial effects on behaviour (Parihar et al. 2011). In line with this contention, a repeated chronic stressor (water avoidance stress) has been demonstrated to attenuate colitis-induced behavioural disturbances in male mice (Hassan et al. 2014). Intriguingly, the behavioural resilience was however associated with an increase of hypothalamic NPY and a rise of circulating corticosterone, which points to a possible involvement of hypothalamic NPY in the protective effect of repeated stress against colitis-induced behavioural disturbances (Hassan et al. 2014). In contrast, colitis severity, levels of circulating IL-6 and IL-18, and cerebral cyclooxygenase-2 mRNA expression were not modulated by the combination of WAS and DSS. Further studies are warranted to investigate whether hypothalamic NPY induces resilience by affecting immune-related parameters not yet investigated or by interacting with the HPA axis, which also acts on the immune system.
Conclusion
The NPY family of peptides and its receptors exert diverse effects in the context of neuroimmune interactions. The effects of NPY on immune cells are complex and comprise modulation of leukocyte trafficking, regulation of macrophage function, modulation of DCs and T cell priming as well as attenuation of neuroinflammation. It is also emerging that members of the NPY peptide family impact the microbiota gut-brain axis and exhibit pro-inflammatory activities in the intestine.
Within the brain, the effects of NPY can in general be considered as protective and homeostatic as NPY can counteract the detrimental influence of immune challenge on mood, emotional processing, cognition, pain sensitivity and ingestion (Fig. 3). As psychological stress can also activate the immune system, its adverse impact on brain function resembles that of immune challenge in several aspects. NPY is likewise able to buffer many detrimental effects of psychological stress. Another disease associated with an inflammatory state is obesity in which NPY has also been shown to have a modulatory influence. Finally, the NPY system plays a major role in metabolism and has been demonstrated to maintain energy homeostasis in response to infection and immune challenge.
Since NPY is not only expressed in immune cells and cerebral neurons but also in primary afferent as well as sympathetic neurons, it would seem that this peptide participates in the reciprocal interaction between the brain and immune system.
Taken all findings together, NPY emerges to play a unique homeostatic role in immune-brain interaction, which leads us to hypothesize that, on the one hand, aberrant expression and /or function of NPY and its receptors may be a pathophysiological factor in inflammation- and stress-associated disorders and, on the other hand, Y receptors may be relevant pharmacological targets in these disorders.
The discovery of the pleiotropic effects of NPY in these systems calls for an in-depth investigation of its physiological and pathological implications in neuroimmune communication. While this review highlights some of the pathological disturbances of the NPY system, there is a need for systematic studies – both experimental and clinical – to define the Y receptors that could be targeted by selective ligands to prevent and treat a multitude of the adverse effects which immune challenge and stress have on the brain.
Acknowledgements
Work in the authors’ laboratory was supported by the Austrian Science Fund (FWF grants P23097-B18 and P25912-B23).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Abid K, Rochat B, Lassahn PG, Stocklin R, Michalet S, Brakch N, Aubert JF, Vatansever B, Tella P, De Meester I, Grouzmann E. Kinetic study of neuropeptide Y (NPY) proteolysis in blood and identification of NPY3-35: a new peptide generated by plasma kallikrein. J Biol Chem. 2009;284:24715–24724. doi: 10.1074/jbc.M109.035253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian TE, Savage AP, Bacarese-Hamilton AJ, Wolfe K, Besterman HS, Bloom SR. Peptide YY abnormalities in gastrointestinal diseases. Gastroenterology. 1986;90:379–384. doi: 10.1016/0016-5085(86)90936-4. [DOI] [PubMed] [Google Scholar]
- Ahlborg G, Weitzberg E, Sollevi A, Lundberg JM. Splanchnic and renal vasoconstrictor and metabolic responses to neuropeptide Y in resting and exercising man. Acta Physiol Scand. 1992;145:139–149. doi: 10.1111/j.1748-1716.1992.tb09349.x. [DOI] [PubMed] [Google Scholar]
- Ahmed AA, Wahbi A, Nordlind K, Kharazmi A, Sundqvist KG, Mutt V, Liden S. In vitro Leishmania major promastigote-induced macrophage migration is modulated by sensory and autonomic neuropeptides. Scand J Immunol. 1998;48:79–85. doi: 10.1046/j.1365-3083.1998.00380.x. [DOI] [PubMed] [Google Scholar]
- Ahmed AA, Wahbi AH, Nordlin K. Neuropeptides modulate a murine monocyte/macrophage cell line capacity for phagocytosis and killing of Leishmania major parasites. Immunopharmacol Immunotoxicol. 2001;23:397–409. doi: 10.1081/iph-100107339. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl 1):S1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen YS, Adrian TE, Allen JM, Tatemoto K, Crow TJ, Bloom SR, Polak JM. Neuropeptide Y distribution in the rat brain. Science. 1983;221:877–879. doi: 10.1126/science.6136091. [DOI] [PubMed] [Google Scholar]
- Augustyniak D, Jankowski A, Mackiewicz P, Skowyra A, Gutowicz J, Drulis-Kawa Z. Innate immune properties of selected human neuropeptides against Moraxella catarrhalis and nontypeable Haemophilus influenzae. BMC Immunol. 2012;13:24. doi: 10.1186/1471-2172-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DG, Bertram TM, Patel PM, Barkauskas DA, Clopton P, Patel S, Geracioti TD, Jr, Haji U, O’Connor DT, Nievergelt CM, Hauger RL. Characterization of cerebrospinal fluid (CSF) and plasma NPY levels in normal volunteers over a 24-h timeframe. Psychoneuroendocrinology. 2013;38:2378–2382. doi: 10.1016/j.psyneuen.2013.04.020. [DOI] [PubMed] [Google Scholar]
- Bannon AW, Seda J, Carmouche M, Francis JM, Norman MH, Karbon B, McCaleb ML. Behavioral characterization of neuropeptide Y knockout mice. Brain Res. 2000;868:79–87. doi: 10.1016/s0006-8993(00)02285-x. [DOI] [PubMed] [Google Scholar]
- Bär F, Foh B, Pagel R, Schroder T, Schlichting H, Hirose M, Lemcke S, Klinger A, Konig P, Karsten CM, Buning J, Lehnert H, Fellermann K, Ibrahim SM, Sina C. Carboxypeptidase E Modulates Intestinal Immune Homeostasis and Protects against Experimental Colitis in Mice. PLoS One. 2014;9:e102347. doi: 10.1371/journal.pone.0102347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baticic L, Detel D, Kucic N, Buljevic S, Pugel EP, Varljen J. Neuroimmunomodulative properties of dipeptidyl peptidase IV/CD26 in a TNBS-induced model of colitis in mice. J Cell Biochem. 2011;112:3322–3333. doi: 10.1002/jcb.23261. [DOI] [PubMed] [Google Scholar]
- Bedoui S, Kromer A, Gebhardt T, Jacobs R, Raber K, Dimitrijevic M, Heine J, von Horsten S. Neuropeptide Y receptor-specifically modulates human neutrophil function. J Neuroimmunol. 2008;195:88–95. doi: 10.1016/j.jneuroim.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Bedoui S, Kuhlmann S, Nave H, Drube J, Pabst R, von Horsten S. Differential effects of neuropeptide Y (NPY) on leukocyte subsets in the blood: mobilization of B-1-like B-lymphocytes and activated monocytes. J Neuroimmunol. 2001;117:125–132. doi: 10.1016/s0165-5728(01)00328-9. [DOI] [PubMed] [Google Scholar]
- Bedoui S, Lechner S, Gebhardt T, Nave H, Beck-Sickinger AG, Straub RH, Pabst R, von Horsten S. NPY modulates epinephrine-induced leukocytosis via Y-1 and Y-5 receptor activation in vivo: sympathetic co-transmission during leukocyte mobilization. J Neuroimmunol. 2002;132:25–33. doi: 10.1016/s0165-5728(02)00278-3. [DOI] [PubMed] [Google Scholar]
- Bedoui S, Miyake S, Lin Y, Miyamoto K, Oki S, Kawamura N, Beck-Sickinger A, von Horsten S, Yamamura T. Neuropeptide Y (NPY) suppresses experimental autoimmune encephalomyelitis: NPY1 receptor-specific inhibition of autoreactive Th1 responses in vivo. J Immunol. 2003;171:3451–3458. doi: 10.4049/jimmunol.171.7.3451. [DOI] [PubMed] [Google Scholar]
- Bedoui S, von Horsten S, Gebhardt T. A role for neuropeptide Y (NPY) in phagocytosis: implications for innate and adaptive immunity. Peptides. 2007;28:373–376. doi: 10.1016/j.peptides.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Bellavance MA, Rivest S. The HPA - Immune Axis and the Immunomodulatory Actions of Glucocorticoids in the Brain. Front Immunol. 2014;5:136. doi: 10.3389/fimmu.2014.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boey D, Lin S, Karl T, Baldock P, Lee N, Enriquez R, Couzens M, Slack K, Dallmann R, Sainsbury A, Herzog H. Peptide YY ablation in mice leads to the development of hyperinsulinaemia and obesity. Diabetologia. 2006;49:1360–1370. doi: 10.1007/s00125-006-0237-0. [DOI] [PubMed] [Google Scholar]
- Böttcher G, Ekblad E, Ekman R, Hakanson R, Sundler F. Peptide YY: a neuropeptide in the gut. Immunocytochemical and immunochemical evidence. Neuroscience. 1993;55:281–290. doi: 10.1016/0306-4522(93)90472-r. [DOI] [PubMed] [Google Scholar]
- Broberger C, Visser TJ, Kuhar MJ, Hokfelt T. Neuropeptide Y innervation and neuropeptide-Y-Y1-receptor-expressing neurons in the paraventricular hypothalamic nucleus of the mouse. Neuroendocrinology. 1999;70:295–305. doi: 10.1159/000054490. [DOI] [PubMed] [Google Scholar]
- Brod SA, Bauer VL. Ingested (oral) neuropeptide Y inhibits EAE. J Neuroimmunol. 2012;250:44–49. doi: 10.1016/j.jneuroim.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Brumovsky P, Shi TS, Landry M, Villar MJ, Hokfelt T. Neuropeptide tyrosine and pain. Trends Pharmacol Sci. 2007;28:93–102. doi: 10.1016/j.tips.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Buttari B, Profumo E, Domenici G, Tagliani A, Ippoliti F, Bonini S, Businaro R, Elenkov I, Rigano R. Neuropeptide Y induces potent migration of human immature dendritic cells and promotes a Th2 polarization. FASEB J. 2014;28:3038–3049. doi: 10.1096/fj.13-243485. [DOI] [PubMed] [Google Scholar]
- Caberlotto L, Fuxe K, Hurd YL. Characterization of NPY mRNA-expressing cells in the human brain: co-localization with Y2 but not Y1 mRNA in the cerebral cortex, hippocampus, amygdala, and striatum. J Chem Neuroanat. 2000;20:327–337. doi: 10.1016/s0891-0618(00)00107-1. [DOI] [PubMed] [Google Scholar]
- Cani PD, Delzenne NM. The gut microbiome as therapeutic target. Pharmacol Ther. 2011;130:202–212. doi: 10.1016/j.pharmthera.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Cani PD, Lecourt E, Dewulf EM, Sohet FM, Pachikian BD, Naslain D, De Backer F, Neyrinck AM, Delzenne NM. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr. 2009;90:1236–1243. doi: 10.3945/ajcn.2009.28095. [DOI] [PubMed] [Google Scholar]
- Carlson DE, Le W, Chiu WC, Hoffman GE. Messenger RNA for neuropeptide Y in the arcuate nucleus increases in parallel with plasma adrenocorticotropin during sepsis in the rat. Neurosci Lett. 2009;452:146–150. doi: 10.1016/j.neulet.2009.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerda-Reverter JM, Larhammar D. Neuropeptide Y family of peptides: structure, anatomical expression, function, and molecular evolution. Biochem Cell Biol. 2000;78:371–392. [PubMed] [Google Scholar]
- Chandarana K, Gelegen C, Irvine EE, Choudhury AI, Amouyal C, Andreelli F, Withers DJ, Batterham RL. Peripheral activation of the Y2-receptor promotes secretion of GLP-1 and improves glucose tolerance. Mol Metab. 2013;2:142–152. doi: 10.1016/j.molmet.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekharan B, Bala V, Kolachala VL, Vijay-Kumar M, Jones D, Gewirtz AT, Sitaraman SV, Srinivasan S. Targeted deletion of neuropeptide Y (NPY) modulates experimental colitis. PLoS One. 2008;3:e3304. doi: 10.1371/journal.pone.0003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekharan B, Jeppsson S, Pienkowski S, Belsham DD, Sitaraman SV, Merlin D, Kokkotou E, Nusrat A, Tansey MG, Srinivasan S. Tumor necrosis factor-neuropeptide Y cross talk regulates inflammation, epithelial barrier functions, and colonic motility. Inflamm Bowel Dis. 2013a;19:2535–2546. doi: 10.1097/01.MIB.0000437042.59208.9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekharan B, Nezami BG, Srinivasan S. Emerging neuropeptide targets in inflammation: NPY and VIP. Am J Physiol Gastrointest Liver Physiol. 2013b;304:G949–57. doi: 10.1152/ajpgi.00493.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbut C, Ferrier L, Roze C, Anini Y, Blottiere H, Lecannu G, Galmiche JP. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am J Physiol. 1998;275:G1415–22. doi: 10.1152/ajpgi.1998.275.6.G1415. [DOI] [PubMed] [Google Scholar]
- Cohen H, Liu T, Kozlovsky N, Kaplan Z, Zohar J, Mathe AA. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology. 2012;37:350–363. doi: 10.1038/npp.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox HM. Neuropeptide Y receptors; antisecretory control of intestinal epithelial function. Auton Neurosci. 2007a;133:76–85. doi: 10.1016/j.autneu.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Cox HM. Peptide YY: a neuroendocrine neighbor of note. Peptides. 2007b;28:345–351. doi: 10.1016/j.peptides.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Czerniawski J, Guzowski JF. Acute neuroinflammation impairs context discrimination memory and disrupts pattern separation processes in hippocampus. J Neurosci. 2014;34:12470–12480. doi: 10.1523/JNEUROSCI.0542-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Fuente M, Bernaez I, Del Rio M, Hernanz A. Stimulation of murine peritoneal macrophage functions by neuropeptide Y and peptide YY. Involvement of protein kinase C. Immunology. 1993;80:259–265. [PMC free article] [PubMed] [Google Scholar]
- De la Fuente M, Del Rio M, Medina S. Changes with aging in the modulation by neuropeptide Y of murine peritoneal macrophage functions. J Neuroimmunol. 2001a;116:156–167. doi: 10.1016/s0165-5728(01)00297-1. [DOI] [PubMed] [Google Scholar]
- De la Fuente M, Del Rio M, Victor VM, Medina S. Neuropeptide Y effects on murine natural killer activity: changes with ageing and cAMP involvement. Regul Pept. 2001b;101:73–79. doi: 10.1016/s0167-0115(01)00262-2. [DOI] [PubMed] [Google Scholar]
- De la Fuente M, Medina S, Del Rio M, Ferrandez MD, Hernanz A. Effect of aging on the modulation of macrophage functions by neuropeptides. Life Sci. 2000;67:2125–2135. doi: 10.1016/s0024-3205(00)00799-2. [DOI] [PubMed] [Google Scholar]
- de Lange RP, Wiegant VM, Stam R. Altered neuropeptide Y and neurokinin messenger RNA expression and receptor binding in stress-sensitised rats. Brain Res. 2008;1212:35–47. doi: 10.1016/j.brainres.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Decressac M, Wright B, David B, Tyers P, Jaber M, Barker RA, Gaillard A. Exogenous neuropeptide Y promotes in vivo hippocampal neurogenesis. Hippocampus. 2011;21:233–238. doi: 10.1002/hipo.20765. [DOI] [PubMed] [Google Scholar]
- Delzenne NM, Neyrinck AM, Backhed F, Cani PD. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol. 2011;7:639–646. doi: 10.1038/nrendo.2011.126. [DOI] [PubMed] [Google Scholar]
- DiMaggio DA, Chronwall BM, Buchanan K, O’Donohue TL. Pancreatic polypeptide immunoreactivity in rat brain is actually neuropeptide Y. Neuroscience. 1985;15:1149–1157. doi: 10.1016/0306-4522(85)90259-3. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic M, Mitic K, Kustrimovic N, Vujic V, Stanojevic S. NPY suppressed development of experimental autoimmune encephalomyelitis in Dark Agouti rats by disrupting costimulatory molecule interactions. J Neuroimmunol. 2012;245:23–31. doi: 10.1016/j.jneuroim.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic M, Stanojevic S. The intriguing mission of neuropeptide Y in the immune system. Amino Acids. 2013;45:41–53. doi: 10.1007/s00726-011-1185-7. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic M, Stanojevic S, Micic S, Vujic V, Kovacevic-Jovanovic V, Mitic K, von Horsten S, Kosec D. Neuropeptide Y (NPY) modulates oxidative burst and nitric oxide production in carrageenan-elicited granulocytes from rat air pouch. Peptides. 2006;27:3208–3215. doi: 10.1016/j.peptides.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic M, Stanojevic S, Mitic K, Kustrimovic N, Vujic V, Miletic T, Kovacevic-Jovanovic V. Modulation of granulocyte functions by peptide YY in the rat: age-related differences in Y receptors expression and plasma dipeptidyl peptidase 4 activity. Regul Pept. 2010;159:100–109. doi: 10.1016/j.regpep.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic M, Stanojevic S, Mitic K, Kustrimovic N, Vujic V, Miletic T, Kovacevic-Jovanovic V. The anti-inflammatory effect of neuropeptide Y (NPY) in rats is dependent on dipeptidyl peptidase 4 (DP4) activity and age. Peptides. 2008;29:2179–2187. doi: 10.1016/j.peptides.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic M, Stanojevic S, Vujic V, Beck-Sickinger A, von Horsten S. Neuropeptide Y and its receptor subtypes specifically modulate rat peritoneal macrophage functions in vitro: counter regulation through Y1 and Y2/5 receptors. Regul Pept. 2005;124:163–172. doi: 10.1016/j.regpep.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic M, Stanojevic S, Vujic V, Kovacevic-Jovanovic V, Beck-Sickinger A, Demuth H, von Horsten S. Effect of neuropeptide Y on inflammatory paw edema in the rat: involvement of peripheral NPY Y1 and Y5 receptors and interaction with dipeptidyl-peptidase IV (CD26) J Neuroimmunol. 2002;129:35–42. doi: 10.1016/s0165-5728(02)00173-x. [DOI] [PubMed] [Google Scholar]
- Domschke K, Dannlowski U, Hohoff C, Ohrmann P, Bauer J, Kugel H, Zwanzger P, Heindel W, Deckert J, Arolt V, Suslow T, Baune BT. Neuropeptide Y (NPY) gene: Impact on emotional processing and treatment response in anxious depression. Eur Neuropsychopharmacol. 2010;20:301–309. doi: 10.1016/j.euroneuro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Du M, Butchi NB, Woods T, Morgan TW, Peterson KE. Neuropeptide Y has a protective role during murine retrovirus-induced neurological disease. J Virol. 2010;84:11076–11088. doi: 10.1128/JVI.01022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dureus P, Louis D, Grant AV, Bilfinger TV, Stefano GB. Neuropeptide Y inhibits human and invertebrate immunocyte chemotaxis, chemokinesis, and spontaneous activation. Cell Mol Neurobiol. 1993;13:541–546. doi: 10.1007/BF00711462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi M, Laddha NC, Shah K, Shah BJ, Begum R. Involvement of interferon-gamma genetic variants and intercellular adhesion molecule-1 in onset and progression of generalized vitiligo. J Interferon Cytokine Res. 2013;33:646–659. doi: 10.1089/jir.2012.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton K, Sallee FR, Sah R. Relevance of neuropeptide Y (NPY) in psychiatry. Curr Top Med Chem. 2007;7:1645–1659. doi: 10.2174/156802607782341037. [DOI] [PubMed] [Google Scholar]
- Edelsbrunner ME, Herzog H, Holzer P. Evidence from knockout mice that peptide YY and neuropeptide Y enforce murine locomotion, exploration and ingestive behaviour in a circadian cycle- and gender-dependent manner. Behav Brain Res. 2009a;203:97–107. doi: 10.1016/j.bbr.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelsbrunner ME, Painsipp E, Herzog H, Holzer P. Evidence from knockout mice for distinct implications of neuropeptide-Y Y2 and Y4 receptors in the circadian control of locomotion, exploration, water and food intake. Neuropeptides. 2009b;43:491–497. doi: 10.1016/j.npep.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblad E, Sundler F. Distribution of pancreatic polypeptide and peptide YY. Peptides. 2002;23:251–261. doi: 10.1016/s0196-9781(01)00601-5. [DOI] [PubMed] [Google Scholar]
- El Karim IA, Linden GJ, Orr DF, Lundy FT. Antimicrobial activity of neuropeptides against a range of micro-organisms from skin, oral, respiratory and gastrointestinal tract sites. J Neuroimmunol. 2008;200:11–16. doi: 10.1016/j.jneuroim.2008.05.014. [DOI] [PubMed] [Google Scholar]
- El-Salhy M, Danielsson A, Stenling R, Grimelius L. Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242:413–419. doi: 10.1046/j.1365-2796.1997.00237.x. [DOI] [PubMed] [Google Scholar]
- Farzi A, Reichmann F, Meinitzer A, Mayerhofer R, Jain P, Hassan AM, Frohlich EE, Wagner K, Painsipp E, Rinner B, Holzer P. Synergistic effects of NOD1 or NOD2 and TLR4 activation on mouse sickness behavior in relation to immune and brain activity markers. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felies M, von Horsten S, Pabst R, Nave H. Neuropeptide Y stabilizes body temperature and prevents hypotension in endotoxaemic rats. J Physiol. 2004;561:245–252. doi: 10.1113/jphysiol.2004.073635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira R, Santos T, Cortes L, Cochaud S, Agasse F, Silva AP, Xapelli S, Malva JO. Neuropeptide Y inhibits interleukin-1 beta-induced microglia motility. J Neurochem. 2012;120:93–105. doi: 10.1111/j.1471-4159.2011.07541.x. [DOI] [PubMed] [Google Scholar]
- Ferreira R, Santos T, Viegas M, Cortes L, Bernardino L, Vieira OV, Malva JO. Neuropeptide Y inhibits interleukin-1beta-induced phagocytosis by microglial cells. J Neuroinflammation. 2011;8 doi: 10.1186/1742-2094-8-169. 169-2094-8-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira R, Xapelli S, Santos T, Silva AP, Cristovao A, Cortes L, Malva JO. Neuropeptide Y modulation of interleukin-1{beta} (IL-1{beta})-induced nitric oxide production in microglia. J Biol Chem. 2010;285:41921–41934. doi: 10.1074/jbc.M110.164020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field BC, Chaudhri OB, Bloom SR. Bowels control brain: gut hormones and obesity. Nat Rev Endocrinol. 2010;6:444–453. doi: 10.1038/nrendo.2010.93. [DOI] [PubMed] [Google Scholar]
- Flood JF, Hernandez EN, Morley JE. Modulation of memory processing by neuropeptide Y. Brain Res. 1987;421:280–290. doi: 10.1016/0006-8993(87)91297-2. [DOI] [PubMed] [Google Scholar]
- Forbes S, Herzog H, Cox HM. A role for neuropeptide Y in the gender-specific gastrointestinal, corticosterone and feeding responses to stress. Br J Pharmacol. 2012;166:2307–2316. doi: 10.1111/j.1476-5381.2012.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu-Cheng X, Anini Y, Chariot J, Castex N, Galmiche JP, Roze C. Mechanisms of peptide YY release induced by an intraduodenal meal in rats: neural regulation by proximal gut. Pflugers Arch. 1997;433:571–579. doi: 10.1007/s004240050316. [DOI] [PubMed] [Google Scholar]
- Fujimiya M, Inui A. Peptidergic regulation of gastrointestinal motility in rodents. Peptides. 2000;21:1565–1582. doi: 10.1016/s0196-9781(00)00313-2. [DOI] [PubMed] [Google Scholar]
- Gayle D, Ilyin SE, Flynn MC, Plata-Salaman CR. Lipopolysaccharide (LPS)- and muramyl dipeptide (MDP)-induced anorexia during refeeding following acute fasting: characterization of brain cytokine and neuropeptide systems mRNAs. Brain Res. 1998;795:77–86. doi: 10.1016/s0006-8993(98)00280-7. [DOI] [PubMed] [Google Scholar]
- Gayle D, Ilyin SE, Plata-Salaman CR. Feeding status and bacterial LPS-induced cytokine and neuropeptide gene expression in hypothalamus. Am J Physiol. 1999;277:R1188–95. doi: 10.1152/ajpregu.1999.277.4.R1188. [DOI] [PubMed] [Google Scholar]
- Gayle D, Ilyin SE, Plata-Salaman CR. Central nervous system IL-1 beta system and neuropeptide Y mRNAs during IL-1 beta-induced anorexia in rats. Brain Res Bull. 1997;44:311–317. doi: 10.1016/s0361-9230(97)00159-7. [DOI] [PubMed] [Google Scholar]
- Gelegen C, Chandarana K, Choudhury AI, Al-Qassab H, Evans IM, Irvine EE, Hyde CB, Claret M, Andreelli F, Sloan SE, Leiter AB, Withers DJ, Batterham RL. Regulation of hindbrain Pyy expression by acute food deprivation, prolonged caloric restriction, and weight loss surgery in mice. Am J Physiol Endocrinol Metab. 2012;303:E659–68. doi: 10.1152/ajpendo.00033.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghia JE, Blennerhassett P, Deng Y, Verdu EF, Khan WI, Collins SM. Reactivation of inflammatory bowel disease in a mouse model of depression. Gastroenterology. 2009;136:2280–2288.e1-4. doi: 10.1053/j.gastro.2009.02.069. [DOI] [PubMed] [Google Scholar]
- Goldberg Y, Taimor G, Piper HM, Schluter KD. Intracellular signaling leads to the hypertrophic effect of neuropeptide Y. Am J Physiol. 1998;275:C1207–15. doi: 10.1152/ajpcell.1998.275.5.C1207. [DOI] [PubMed] [Google Scholar]
- Greco B, Carli M. Reduced attention and increased impulsivity in mice lacking NPY Y2 receptors: relation to anxiolytic-like phenotype. Behav Brain Res. 2006;169:325–334. doi: 10.1016/j.bbr.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Guilloteau P, Martin L, Eeckhaut V, Ducatelle R, Zabielski R, Van Immerseel F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr Res Rev. 2010;23:366–384. doi: 10.1017/S0954422410000247. [DOI] [PubMed] [Google Scholar]
- Hallden G, Aponte GW. Evidence for a role of the gut hormone PYY in the regulation of intestinal fatty acid-binding protein transcripts in differentiated subpopulations of intestinal epithelial cell hybrids. J Biol Chem. 1997;272:12591–12600. doi: 10.1074/jbc.272.19.12591. [DOI] [PubMed] [Google Scholar]
- Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- Hansel DE, Eipper BA, Ronnett GV. Neuropeptide Y functions as a neuroproliferative factor. Nature. 2001;410:940–944. doi: 10.1038/35073601. [DOI] [PubMed] [Google Scholar]
- Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AM, Jain P, Reichmann F, Mayerhofer R, Farzi A, Schuligoi R, Holzer P. Repeated predictable stress causes resilience against colitis-induced behavioral changes in mice. Front Behav Neurosci. 2014;8:386. doi: 10.3389/fnbeh.2014.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani H, Lucas G, Rozell B, Ernfors P. Attenuation of acute experimental colitis by preventing NPY Y1 receptor signaling. Am J Physiol Gastrointest Liver Physiol. 2005;288:G550–6. doi: 10.1152/ajpgi.00182.2004. [DOI] [PubMed] [Google Scholar]
- Hauser GJ, Dayao EK, Zukowska-Grojec Z. Effect of neuropeptide Y on endotoxin-induced suppression of the response to various agonists in conscious rats. Life Sci. 1995;57:235–244. doi: 10.1016/0024-3205(95)00266-9. [DOI] [PubMed] [Google Scholar]
- Hauser GJ, Myers AK, Dayao EK, Zukowska-Grojec Z. Neuropeptide Y infusion improves hemodynamics and survival in rat endotoxic shock. Am J Physiol. 1993;265:H1416–23. doi: 10.1152/ajpheart.1993.265.4.H1416. [DOI] [PubMed] [Google Scholar]
- Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF, Ekman R, Britton KT. Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends Neurosci. 1994;17:80–85. doi: 10.1016/0166-2236(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Hernanz A, Tato E, De la Fuente M, de Miguel E, Arnalich F. Differential effects of gastrin-releasing peptide, neuropeptide Y, somatostatin and vasoactive intestinal peptide on interleukin-1 beta, interleukin-6 and tumor necrosis factor-alpha production by whole blood cells from healthy young and old subjects. J Neuroimmunol. 1996;71:25–30. doi: 10.1016/s0165-5728(96)00118-x. [DOI] [PubMed] [Google Scholar]
- Herzog H. Neuropeptide Y and energy homeostasis: insights from Y receptor knockout models. Eur J Pharmacol. 2003;480:21–29. doi: 10.1016/j.ejphar.2003.08.089. [DOI] [PubMed] [Google Scholar]
- Herzog H, Hort YJ, Ball HJ, Hayes G, Shine J, Selbie LA. Cloned human neuropeptide Y receptor couples to two different second messenger systems. Proc Natl Acad Sci U S A. 1992;89:5794–5798. doi: 10.1073/pnas.89.13.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotani Y, Mikajiri K, Ikeda K, Myotoku M, Kurokawa N. Changes of the peptide YY levels in the intestinal tissue of rats with experimental colitis following oral administration of mesalazine and prednisolone. Yakugaku Zasshi. 2008;128:1347–1353. doi: 10.1248/yakushi.128.1347. [DOI] [PubMed] [Google Scholar]
- Hirsch D, Zukowska Z. NPY and stress 30 years later: the peripheral view. Cell Mol Neurobiol. 2012;32:645–659. doi: 10.1007/s10571-011-9793-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P, Bartho L, Lippe IT, Petritsch W, Leb G. Effect of pancreatic polypeptide on the motility of the guinea-pig small intestine in vitro. Regul Pept. 1986;16:305–314. doi: 10.1016/0167-0115(86)90030-3. [DOI] [PubMed] [Google Scholar]
- Holzer P, Farzi A. Neuropeptides and the microbiota-gut-brain axis. Adv Exp Med Biol. 2014;817:195–219. doi: 10.1007/978-1-4939-0897-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P, Lippe IT, Bartho L, Saria A. Neuropeptide Y inhibits excitatory enteric neurons supplying the circular muscle of the guinea pig small intestine. Gastroenterology. 1987;92:1944–1950. doi: 10.1016/0016-5085(87)90628-7. [DOI] [PubMed] [Google Scholar]
- Holzer P, Reichmann F, Farzi A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides. 2012;46:261–274. doi: 10.1016/j.npep.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer-Petsche U, Petritsch W, Hinterleitner T, Eherer A, Sperk G, Krejs GJ. Effect of neuropeptide Y on jejunal water and ion transport in humans. Gastroenterology. 1991;101:325–330. doi: 10.1016/0016-5085(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Ilyin SE, Gayle D, Flynn MC, Plata-Salaman CR. Interleukin-1beta system (ligand, receptor type I, receptor accessory protein and receptor antagonist), TNF-alpha, TGF-beta1 and neuropeptide Y mRNAs in specific brain regions during bacterial LPS-induced anorexia. Brain Res Bull. 1998;45:507–515. doi: 10.1016/s0361-9230(97)00437-1. [DOI] [PubMed] [Google Scholar]
- Imaki T, Shibasaki T, Chikada N, Harada S, Naruse M, Demura H. Different expression of immediate-early genes in the rat paraventricular nucleus induced by stress: relation to corticotropin-releasing factor gene transcription. Endocr J. 1996;43:629–638. doi: 10.1507/endocrj.43.629. [DOI] [PubMed] [Google Scholar]
- Imran M, Laddha NC, Dwivedi M, Mansuri MS, Singh J, Rani R, Gokhale RS, Sharma VK, Marfatia YS, Begum R. Interleukin-4 genetic variants correlate with its transcript and protein levels in patients with vitiligo. Br J Dermatol. 2012;167:314–323. doi: 10.1111/j.1365-2133.2012.11000.x. [DOI] [PubMed] [Google Scholar]
- Irwin M, Brown M, Patterson T, Hauger R, Mascovich A, Grant I. Neuropeptide Y and natural killer cell activity: findings in depression and Alzheimer caregiver stress. FASEB J. 1991;5:3100–3107. doi: 10.1096/fasebj.5.15.1743441. [DOI] [PubMed] [Google Scholar]
- Iwasa T, Matsuzaki T, Kinouchi R, Fujisawa S, Murakami M, Kiyokawa M, Kuwahara A, Yasui T, Irahara M. Neonatal LPS injection alters the body weight regulation systems of rats under non-stress and immune stress conditions. Int J Dev Neurosci. 2010;28:119–124. doi: 10.1016/j.ijdevneu.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Jaaskelainen AE, Seppala S, Kakko T, Jaakkola U, Kallio J. Systemic treatment with neuropeptide Y receptor Y1-antagonist enhances atherosclerosis and stimulates IL-12 expression in ApoE deficient mice. Neuropeptides. 2013;47:67–73. doi: 10.1016/j.npep.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Kask A, Harro J, von Horsten S, Redrobe JP, Dumont Y, Quirion R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci Biobehav Rev. 2002;26:259–283. doi: 10.1016/s0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- Kawamura N, Tamura H, Obana S, Wenner M, Ishikawa T, Nakata A, Yamamoto H. Differential effects of neuropeptides on cytokine production by mouse helper T cell subsets. Neuroimmunomodulation. 1998;5:9–15. doi: 10.1159/000026321. [DOI] [PubMed] [Google Scholar]
- Kemp EH, Waterman EA, Weetman AP. Immunological pathomechanisms in vitiligo. Expert Rev Mol Med. 2001;3:1–22. doi: 10.1017/S1462399401003362. [DOI] [PubMed] [Google Scholar]
- Kim YW, Kim KH, Ahn DK, Kim HS, Kim JY, Lee DC, Park SY. Time-course changes of hormones and cytokines by lipopolysaccharide and its relation with anorexia. J Physiol Sci. 2007;57:159–165. doi: 10.2170/physiolsci.RP003407. [DOI] [PubMed] [Google Scholar]
- Klompus M, Ho W, Sharkey KA, McKay DM. Antisecretory effects of neuropeptide Y in the mouse colon are region-specific and are lost in DSS-induced colitis. Regul Pept. 2010;165:138–145. doi: 10.1016/j.regpep.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Kormos V, Gaszner B. Role of neuropeptides in anxiety, stress, and depression: from animals to humans. Neuropeptides. 2013;47:401–419. doi: 10.1016/j.npep.2013.10.014. [DOI] [PubMed] [Google Scholar]
- Kuncova J, Sykora R, Chvojka J, Sviglerova J, Stengl M, Krouzecky A, Nalos L, Matejovic M. Plasma and tissue levels of neuropeptide y in experimental septic shock: relation to hemodynamics, inflammation, oxidative stress, and hemofiltration. Artif Organs. 2011;35:625–633. doi: 10.1111/j.1525-1594.2010.01154.x. [DOI] [PubMed] [Google Scholar]
- Kunisada T, Tezulka K, Aoki H, Motohashi T. The stemness of neural crest cells and their derivatives. Birth Defects Res C Embryo Today. 2014;102:251–262. doi: 10.1002/bdrc.21079. [DOI] [PubMed] [Google Scholar]
- Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, Lee EW, Burnett MS, Fricke ST, Kvetnansky R, Herzog H, Zukowska Z. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med. 2007;13:803–811. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- Laddha NC, Dwivedi M, Mansuri MS, Singh M, Patel HH, Agarwal N, Shah AM, Begum R. Association of neuropeptide Y (NPY), interleukin-1B (IL1B) genetic variants and correlation of IL1B transcript levels with vitiligo susceptibility. PLoS One. 2014;9:e107020. doi: 10.1371/journal.pone.0107020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larhammar D. Structural diversity of receptors for neuropeptide Y, peptide YY and pancreatic polypeptide. Regul Pept. 1996;65:165–174. doi: 10.1016/0167-0115(96)00110-3. [DOI] [PubMed] [Google Scholar]
- Levite M. Neuropeptides, by direct interaction with T cells, induce cytokine secretion and break the commitment to a distinct T helper phenotype. Proc Natl Acad Sci U S A. 1998;95:12544–12549. doi: 10.1073/pnas.95.21.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levite M, Cahalon L, Hershkoviz R, Steinman L, Lider O. Neuropeptides, via specific receptors, regulate T cell adhesion to fibronectin. J Immunol. 1998;160:993–1000. [PubMed] [Google Scholar]
- Li Q, Dong C, Li W, Bu W, Wu J, Zhao W. Neuropeptide Y protects cerebral cortical neurons by regulating microglial immune function. Neural Regen Res. 2014;9:959–967. doi: 10.4103/1673-5374.133140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Guan H, Yang K. Neuropeptide Y potentiates beta-adrenergic stimulation of lipolysis in 3T3-L1 adipocytes. Regul Pept. 2012;178:16–20. doi: 10.1016/j.regpep.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Lomax AE, Sharkey KA, Furness JB. The participation of the sympathetic innervation of the gastrointestinal tract in disease states. Neurogastroenterol Motil. 2010;22:7–18. doi: 10.1111/j.1365-2982.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Lueg G, Gross CC, Lohmann H, Johnen A, Kemmling A, Deppe M, Groger J, Minnerup J, Wiendl H, Meuth SG, Duning T. Clinical relevance of specific T-cell activation in the blood and cerebrospinal fluid of patients with mild Alzheimer’s disease. Neurobiol Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Rudehill A, Sollevi A, Fried G, Wallin G. Co-release of neuropeptide Y and noradrenaline from pig spleen in vivo: importance of subcellular storage, nerve impulse frequency and pattern, feedback regulation and resupply by axonal transport. Neuroscience. 1989;28:475–486. doi: 10.1016/0306-4522(89)90193-0. [DOI] [PubMed] [Google Scholar]
- MacDonald L, Hazi A, Paolini AG, Kent S. Calorie restriction dose-dependently abates lipopolysaccharide-induced fever, sickness behavior, and circulating interleukin-6 while increasing corticosterone. Brain Behav Immun. 2014;40:18–26. doi: 10.1016/j.bbi.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Macia L, Rao PT, Wheway J, Sierro F, Mackay F, Herzog H. Y1 signalling has a critical role in allergic airway inflammation. Immunol Cell Biol. 2011;89:882–888. doi: 10.1038/icb.2011.6. [DOI] [PubMed] [Google Scholar]
- Macia L, Thorburn AN, Binge LC, Marino E, Rogers KE, Maslowski KM, Vieira AT, Kranich J, Mackay CR. Microbial influences on epithelial integrity and immune function as a basis for inflammatory diseases. Immunol Rev. 2012a;245:164–176. doi: 10.1111/j.1600-065X.2011.01080.x. [DOI] [PubMed] [Google Scholar]
- Macia L, Yulyaningsih E, Pangon L, Nguyen AD, Lin S, Shi YC, Zhang L, Bijker M, Grey S, Mackay F, Herzog H, Sainsbury A. Neuropeptide Y1 receptor in immune cells regulates inflammation and insulin resistance associated with diet-induced obesity. Diabetes. 2012b;61:3228–3238. doi: 10.2337/db12-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malva JO, Xapelli S, Baptista S, Valero J, Agasse F, Ferreira R, Silva AP. Multifaces of neuropeptide Y in the brain--neuroprotection, neurogenesis and neuroinflammation. Neuropeptides. 2012;46:299–308. doi: 10.1016/j.npep.2012.09.001. [DOI] [PubMed] [Google Scholar]
- McCarthy HD, Dryden S, Williams G. Interleukin-1 beta-induced anorexia and pyrexia in rat: relationship to hypothalamic neuropeptide Y. Am J Physiol. 1995;269:E852–7. doi: 10.1152/ajpendo.1995.269.5.E852. [DOI] [PubMed] [Google Scholar]
- McGowan BM, Bloom SR. Peptide YY and appetite control. Curr Opin Pharmacol. 2004;4:583–588. doi: 10.1016/j.coph.2004.06.007. [DOI] [PubMed] [Google Scholar]
- McGuire JL, Larke LE, Sallee FR, Herman JP, Sah R. Differential Regulation of Neuropeptide Y in the Amygdala and Prefrontal Cortex during Recovery from Chronic Variable Stress. Front Behav Neurosci. 2011;5:54. doi: 10.3389/fnbeh.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon CD, Buxton DF, Elsasser TH, Gunter DR, Sanders LG, Steele BP, Sartin JL. Neuropeptide Y restores appetite and alters concentrations of GH after central administration to endotoxic sheep. J Endocrinol. 1999;161:333–339. doi: 10.1677/joe.0.1610333. [DOI] [PubMed] [Google Scholar]
- McShane TM, Wilson ME, Wise PM. Effects of lifelong moderate caloric restriction on levels of neuropeptide Y, proopiomelanocortin, and galanin mRNA. J Gerontol A Biol Sci Med Sci. 1999;54:B14–21. doi: 10.1093/gerona/54.1.b14. [DOI] [PubMed] [Google Scholar]
- Medina S, Del Rio M, Ferrandez MD, Hernanz A, De la Fuente M. Changes with age in the modulation of natural killer activity of murine leukocytes by gastrin-releasing peptide, neuropeptide Y and sulfated cholecystokinin octapeptide. Neuropeptides. 1998a;32:549–555. doi: 10.1016/s0143-4179(98)90084-1. [DOI] [PubMed] [Google Scholar]
- Medina S, Del Rio M, Hernanz A, De la Fuente M. Age-related changes in the neuropeptide Y effects on murine lymphoproliferation and interleukin-2 production. Peptides. 2000a;21:1403–1409. doi: 10.1016/s0196-9781(00)00284-9. [DOI] [PubMed] [Google Scholar]
- Medina S, Del Rio M, Hernanz A, De la Fuente M. The NPY effects on murine leukocyte adherence and chemotaxis change with age. Adherent cell implication. Regul Pept. 2000b;95:35–45. doi: 10.1016/s0167-0115(00)00134-8. [DOI] [PubMed] [Google Scholar]
- Medina S, Del Rio M, Manuel Victor V, Hernanz A, De la Fuente M. Changes with ageing in the modulation of murine lymphocyte chemotaxis by CCK-8S, GRP and NPY. Mech Ageing Dev. 1998b;102:249–261. doi: 10.1016/s0047-6374(98)00014-1. [DOI] [PubMed] [Google Scholar]
- Medina S, Rio MD, Cuadra BD, Guayerbas N, Fuente MD. Age-related changes in the modulatory action of gastrin-releasing peptide, neuropeptide Y and sulfated cholecystokinin octapeptide in the proliferation of murine lymphocytes. Neuropeptides. 1999;33:173–179. doi: 10.1054/npep.1999.0020. [DOI] [PubMed] [Google Scholar]
- Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- Mickey BJ, Zhou Z, Heitzeg MM, Heinz E, Hodgkinson CA, Hsu DT, Langenecker SA, Love TM, Pecina M, Shafir T, Stohler CS, Goldman D, Zubieta JK. Emotion processing, major depression, and functional genetic variation of neuropeptide Y. Arch Gen Psychiatry. 2011;68:158–166. doi: 10.1001/archgenpsychiatry.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitic K, Stanojevic S, Kustrimovic N, Vujic V, Dimitrijevic M. Neuropeptide Y modulates functions of inflammatory cells in the rat: distinct role for Y1, Y2 and Y5 receptors. Peptides. 2011;32:1626–1633. doi: 10.1016/j.peptides.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Mittermaier C, Dejaco C, Waldhoer T, Oefferlbauer-Ernst A, Miehsler W, Beier M, Tillinger W, Gangl A, Moser G. Impact of depressive mood on relapse in patients with inflammatory bowel disease: a prospective 18-month follow-up study. Psychosom Med. 2004;66:79–84. doi: 10.1097/01.psy.0000106907.24881.f2. [DOI] [PubMed] [Google Scholar]
- Molosh AI, Sajdyk TJ, Truitt WA, Zhu W, Oxford GS, Shekhar A. NPY Y1 receptors differentially modulate GABAA and NMDA receptors via divergent signal-transduction pathways to reduce excitability of amygdala neurons. Neuropsychopharmacology. 2013;38:1352–1364. doi: 10.1038/npp.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Medina JC, Dumont Y, Quirion R. A possible role of neuropeptide Y in depression and stress. Brain Res. 2010;1314:194–205. doi: 10.1016/j.brainres.2009.09.077. [DOI] [PubMed] [Google Scholar]
- Morgan CA, 3rd, Rasmusson AM, Winters B, Hauger RL, Morgan J, Hazlett G, Southwick S. Trauma exposure rather than posttraumatic stress disorder is associated with reduced baseline plasma neuropeptide-Y levels. Biol Psychiatry. 2003;54:1087–1091. doi: 10.1016/s0006-3223(03)00433-5. [DOI] [PubMed] [Google Scholar]
- Morimoto R, Satoh F, Murakami O, Totsune K, Saruta M, Suzuki T, Sasano H, Ito S, Takahashi K. Expression of peptide YY in human brain and pituitary tissues. Nutrition. 2008;24:878–884. doi: 10.1016/j.nut.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854–859. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- Myers AK, Farhat MY, Vaz CA, Keiser HR, Zukowska-Grojec Z. Release of immunoreactive-neuropeptide by rat platelets. Biochem Biophys Res Commun. 1988;155:118–122. doi: 10.1016/s0006-291x(88)81057-x. [DOI] [PubMed] [Google Scholar]
- Nair MP, Schwartz SA, Wu K, Kronfol Z. Effect of neuropeptide Y on natural killer activity of normal human lymphocytes. Brain Behav Immun. 1993;7:70–78. doi: 10.1006/brbi.1993.1007. [DOI] [PubMed] [Google Scholar]
- Nave H, Bedoui S, Moenter F, Steffens J, Felies M, Gebhardt T, Straub RH, Pabst R, Dimitrijevic M, Stanojevic S, von Horsten S. Reduced tissue immigration of monocytes by neuropeptide Y during endotoxemia is associated with Y2 receptor activation. J Neuroimmunol. 2004;155:1–12. doi: 10.1016/j.jneuroim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Nishi D, Hashimoto K, Noguchi H, Matsuoka Y. Serum neuropeptide Y in accident survivors with depression or posttraumatic stress disorder. Neurosci Res. 2014 doi: 10.1016/j.neures.2014.03.009. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Painsipp E, Herzog H, Holzer P. Evidence from knockout mice that neuropeptide-Y Y2 and Y4 receptor signalling prevents long-term depression-like behaviour caused by immune challenge. J Psychopharmacol. 2010;24:1551–1560. doi: 10.1177/0269881109348171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painsipp E, Herzog H, Holzer P. Implication of neuropeptide-Y Y2 receptors in the effects of immune stress on emotional, locomotor and social behavior of mice. Neuropharmacology. 2008a;55:117–126. doi: 10.1016/j.neuropharm.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painsipp E, Herzog H, Sperk G, Holzer P. Sex-dependent control of murine emotional-affective behaviour in health and colitis by peptide YY and neuropeptide Y. Br J Pharmacol. 2011;163:1302–1314. doi: 10.1111/j.1476-5381.2011.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painsipp E, Kofer MJ, Farzi A, Dischinger US, Sinner F, Herzog H, Holzer P. Neuropeptide Y and peptide YY protect from weight loss caused by Bacille Calmette-Guerin in mice. Br J Pharmacol. 2013;170:1014–1026. doi: 10.1111/bph.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painsipp E, Wultsch T, Edelsbrunner ME, Tasan RO, Singewald N, Herzog H, Holzer P. Reduced anxiety-like and depression-related behavior in neuropeptide Y Y4 receptor knockout mice. Genes Brain Behav. 2008b;7:532–542. doi: 10.1111/j.1601-183X.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painsipp E, Wultsch T, Shahbazian A, Edelsbrunner M, Kreissl MC, Schirbel A, Bock E, Pabst MA, Thoeringer CK, Huber HP, Holzer P. Experimental gastritis in mice enhances anxiety in a gender-related manner. Neuroscience. 2007;150:522–536. doi: 10.1016/j.neuroscience.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang XH, Li TK, Xie Q, He FQ, Cui de J, Chen YQ, Huang XL, Gan HT. Amelioration of dextran sulfate sodium-induced colitis by neuropeptide Y antisense oligodeoxynucleotide. Int J Colorectal Dis. 2010;25:1047–1053. doi: 10.1007/s00384-010-0964-z. [DOI] [PubMed] [Google Scholar]
- Parihar VK, Hattiangady B, Kuruba R, Shuai B, Shetty AK. Predictable chronic mild stress improves mood, hippocampal neurogenesis and memory. Mol Psychiatry. 2011;16:171–183. doi: 10.1038/mp.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud SJ, Bewick GA. Peptide YY: more than just an appetite regulator. Diabetologia. 2014;57:1762–1769. doi: 10.1007/s00125-014-3292-y. [DOI] [PubMed] [Google Scholar]
- Petitto JM, Huang Z, McCarthy DB. Molecular cloning of NPY-Y1 receptor cDNA from rat splenic lymphocytes: evidence of low levels of mRNA expression and [125I]NPY binding sites. J Neuroimmunol. 1994;54:81–86. doi: 10.1016/0165-5728(94)90234-8. [DOI] [PubMed] [Google Scholar]
- Phan TA, Taylor AW. The neuropeptides alpha-MSH and NPY modulate phagocytosis and phagolysosome activation in RAW 264.7 cells. J Neuroimmunol. 2013;260:9–16. doi: 10.1016/j.jneuroim.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidsudko Z, Wasowicz K, Kaleczyc J, Klimczuk M, Bossowska A, Majewski M, Adriaensen D, Timmermans JP. The influence of ileitis on the neurochemistry of the caudal mesenteric ganglion in the pig. Neurogastroenterol Motil. 2011;23:e213–22. doi: 10.1111/j.1365-2982.2011.01694.x. [DOI] [PubMed] [Google Scholar]
- Puerto M, Guayerbas N, Alvarez P, De la Fuente M. Modulation of neuropeptide Y and norepinephrine on several leucocyte functions in adult, old and very old mice. J Neuroimmunol. 2005;165:33–40. doi: 10.1016/j.jneuroim.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Radler ME, Wright BJ, Walker FR, Hale MW, Kent S. Calorie restriction increases lipopolysaccharide-induced neuropeptide Y immunolabeling and reduces microglial cell area in the arcuate hypothalamic nucleus. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Hauger RL, Morgan CA, Bremner JD, Charney DS, Southwick SM. Low baseline and yohimbine-stimulated plasma neuropeptide Y (NPY) levels in combat-related PTSD. Biol Psychiatry. 2000;47:526–539. doi: 10.1016/s0006-3223(99)00185-7. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Carvajal C, Kask A, Dumont Y, Quirion R. Neuropeptide Y and Its Receptor Subtypes in the Central Nervous System: Emphasis on Their Role in Animal Models of Psychiatric Disorders. Handb. Exp. Pharmacol. 2004a:101–136. [Google Scholar]
- Redrobe JP, Dumont Y, Herzog H, Quirion R. Characterization of neuropeptide Y, Y(2) receptor knockout mice in two animal models of learning and memory processing. J Mol Neurosci. 2004b;22:159–166. doi: 10.1385/JMN:22:3:159. [DOI] [PubMed] [Google Scholar]
- Romano TA, Felten SY, Felten DL, Olschowka JA. Neuropeptide-Y innervation of the rat spleen: another potential immunomodulatory neuropeptide. Brain Behav Immun. 1991;5:116–131. doi: 10.1016/0889-1591(91)90011-x. [DOI] [PubMed] [Google Scholar]
- Rosmaninho-Salgado J, Alvaro AR, Grouzmann E, Duarte EP, Cavadas C. Neuropeptide Y regulates catecholamine release evoked by interleukin-1beta in mouse chromaffin cells. Peptides. 2007;28:310–314. doi: 10.1016/j.peptides.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Sah R, Ekhator NN, Jefferson-Wilson L, Horn PS, Geracioti TD., Jr. Cerebrospinal fluid neuropeptide Y in combat veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology. 2014;40:277–283. doi: 10.1016/j.psyneuen.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah R, Ekhator NN, Strawn JR, Sallee FR, Baker DG, Horn PS, Geracioti TD., Jr. Low cerebrospinal fluid neuropeptide Y concentrations in posttraumatic stress disorder. Biol Psychiatry. 2009;66:705–707. doi: 10.1016/j.biopsych.2009.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah R, Geracioti TD. Neuropeptide Y and posttraumatic stress disorder. Mol Psychiatry. 2013;18:646–655. doi: 10.1038/mp.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Fitz SD, Shekhar A. The role of neuropeptide Y in the amygdala on corticotropin-releasing factor receptor-mediated behavioral stress responses in the rat. Stress. 2006;9:21–28. doi: 10.1080/10253890600557315. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Johnson PL, Leitermann RJ, Fitz SD, Dietrich A, Morin M, Gehlert DR, Urban JH, Shekhar A. Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic-adrenal-pituitary axis activity or hyperthermia. J Neurosci. 2008;28:893–903. doi: 10.1523/JNEUROSCI.0659-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res. 1999a;100:207–215. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A, Gehlert DR. Interactions between NPY and CRF in the amygdala to regulate emotionality. Neuropeptides. 2004;38:225–234. doi: 10.1016/j.npep.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Vandergriff MG, Gehlert DR. Amygdalar neuropeptide Y Y1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. Eur J Pharmacol. 1999b;368:143–147. doi: 10.1016/s0014-2999(99)00018-7. [DOI] [PubMed] [Google Scholar]
- Sam AH, Gunner DJ, King A, Persaud SJ, Brooks L, Hostomska K, Ford HE, Liu B, Ghatei MA, Bloom SR, Bewick GA. Selective ablation of peptide YY cells in adult mice reveals their role in beta cell survival. Gastroenterology. 2012;143:459–468. doi: 10.1053/j.gastro.2012.04.047. [DOI] [PubMed] [Google Scholar]
- Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saria A, Beubler E. Neuropeptide Y (NPY) and peptide YY (PYY) inhibit prostaglandin E2-induced intestinal fluid and electrolyte secretion in the rat jejunum in vivo. Eur J Pharmacol. 1985;119:47–52. doi: 10.1016/0014-2999(85)90320-6. [DOI] [PubMed] [Google Scholar]
- Saurer TB, Ijames SG, Lysle DT. Neuropeptide Y Y1 receptors mediate morphine-induced reductions of natural killer cell activity. J Neuroimmunol. 2006;177:18–26. doi: 10.1016/j.jneuroim.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Schele E, Grahnemo L, Anesten F, Hallen A, Backhed F, Jansson JO. The gut microbiota reduces leptin sensitivity and the expression of the obesity-suppressing neuropeptides proglucagon (Gcg) and brain-derived neurotrophic factor (Bdnf) in the central nervous system. Endocrinology. 2013;154:3643–3651. doi: 10.1210/en.2012-2151. [DOI] [PubMed] [Google Scholar]
- Schicho R, Schemann M, Pabst MA, Holzer P, Lippe IT. Capsaicin-sensitive extrinsic afferents are involved in acid-induced activation of distinct myenteric neurons in the rat stomach. Neurogastroenterol Motil. 2003;15:33–44. doi: 10.1046/j.1365-2982.2003.00384.x. [DOI] [PubMed] [Google Scholar]
- Schmidt PT, Ljung T, Hartmann B, Hare KJ, Holst JJ, Hellstrom PM. Tissue levels and post-prandial secretion of the intestinal growth factor, glucagon-like peptide-2, in controls and inflammatory bowel disease: comparison with peptide YY. Eur J Gastroenterol Hepatol. 2005;17:207–212. doi: 10.1097/00042737-200502000-00012. [DOI] [PubMed] [Google Scholar]
- Schwarz H, Villiger PM, von Kempis J, Lotz M. Neuropeptide Y is an inducible gene in the human immune system. J Neuroimmunol. 1994;51:53–61. doi: 10.1016/0165-5728(94)90128-7. [DOI] [PubMed] [Google Scholar]
- Serova LI, Laukova M, Alaluf LG, Pucillo L, Sabban EL. Intranasal neuropeptide Y reverses anxiety and depressive-like behavior impaired by single prolonged stress PTSD model. Eur Neuropsychopharmacol. 2014;24:142–147. doi: 10.1016/j.euroneuro.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Serova LI, Tillinger A, Alaluf LG, Laukova M, Keegan K, Sabban EL. Single intranasal neuropeptide Y infusion attenuates development of PTSD-like symptoms to traumatic stress in rats. Neuroscience. 2013;236:298–312. doi: 10.1016/j.neuroscience.2013.01.040. [DOI] [PubMed] [Google Scholar]
- Shibata M, Hisajima T, Nakano M, Goris RC, Funakoshi K. Morphological relationships between peptidergic nerve fibers and immunoglobulin A-producing lymphocytes in the mouse intestine. Brain Behav Immun. 2008;22:158–166. doi: 10.1016/j.bbi.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Silva AP, Cavadas C, Baisse-Agushi B, Spertini O, Brunner HR, Grouzmann E. NPY, NPY receptors, and DPP IV activity are modulated by LPS, TNF-alpha and IFN-gamma in HUVEC. Regul Pept. 2003;116:71–79. doi: 10.1016/s0167-0115(03)00191-5. [DOI] [PubMed] [Google Scholar]
- Singer K, Morris DL, Oatmen KE, Wang T, DelProposto J, Mergian T, Cho KW, Lumeng CN. Neuropeptide Y is produced by adipose tissue macrophages and regulates obesity-induced inflammation. PLoS One. 2013;8:e57929. doi: 10.1371/journal.pone.0057929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonti G, Ilyin SE, Plata-Salaman CR. Neuropeptide Y blocks and reverses interleukin-1 beta-induced anorexia in rats. Peptides. 1996;17:517–520. doi: 10.1016/0196-9781(96)00016-2. [DOI] [PubMed] [Google Scholar]
- Stadlbauer U, Langhans W, Meyer U. Administration of the Y2 receptor agonist PYY3-36 in mice induces multiple behavioral changes relevant to schizophrenia. Neuropsychopharmacology. 2013;38:2446–2455. doi: 10.1038/npp.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler J, Le TP, Haas P, Nave H. Distinct effects of NPY13-36, a specific NPY Y2 agonist, in a model of rodent endotoxemia on leukocyte subsets and cytokine levels. Ann Anat. 2011;193:486–493. doi: 10.1016/j.aanat.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Stanojevic S, Vujic V, Kovacevic-Jovanovic V, Mitic K, Kosec D, Horsten S, Dimitrijevic M. Age-related effect of peptide YY (PYY) on paw edema in the rat: the function of Y1 receptors on inflammatory cells. Exp Gerontol. 2006;41:793–799. doi: 10.1016/j.exger.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Stogner KA, Holmes PV. Neuropeptide-Y exerts antidepressant-like effects in the forced swim test in rats. Eur J Pharmacol. 2000;387:R9–10. doi: 10.1016/s0014-2999(99)00800-6. [DOI] [PubMed] [Google Scholar]
- Straub RH, Herfarth H, Falk W, Andus T, Scholmerich J. Uncoupling of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis in inflammatory bowel disease? J Neuroimmunol. 2002;126:116–125. doi: 10.1016/s0165-5728(02)00047-4. [DOI] [PubMed] [Google Scholar]
- Straub RH, Mayer M, Kreutz M, Leeb S, Scholmerich J, Falk W. Neurotransmitters of the sympathetic nerve terminal are powerful chemoattractants for monocytes. J Leukoc Biol. 2000a;67:553–558. doi: 10.1002/jlb.67.4.553. [DOI] [PubMed] [Google Scholar]
- Straub RH, Schaller T, Miller LE, von Horsten S, Jessop DS, Falk W, Scholmerich J. Neuropeptide Y cotransmission with norepinephrine in the sympathetic nerve-macrophage interplay. J Neurochem. 2000b;75:2464–2471. doi: 10.1046/j.1471-4159.2000.0752464.x. [DOI] [PubMed] [Google Scholar]
- Sung CP, Arleth AJ, Feuerstein GZ. Neuropeptide Y upregulates the adhesiveness of human endothelial cells for leukocytes. Circ Res. 1991;68:314–318. doi: 10.1161/01.res.68.1.314. [DOI] [PubMed] [Google Scholar]
- Tari A, Teshima H, Sumii K, Haruma K, Ohgoshi H, Yoshihara M, Kajiyama G, Miyachi Y. Peptide YY abnormalities in patients with ulcerative colitis. Jpn J Med. 1988;27:49–55. doi: 10.2169/internalmedicine1962.27.49. [DOI] [PubMed] [Google Scholar]
- Tasan RO, Lin S, Hetzenauer A, Singewald N, Herzog H, Sperk G. Increased novelty-induced motor activity and reduced depression-like behavior in neuropeptide Y (NPY)-Y4 receptor knockout mice. Neuroscience. 2009;158:1717–1730. doi: 10.1016/j.neuroscience.2008.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasan RO, Nguyen NK, Weger S, Sartori SB, Singewald N, Heilbronn R, Herzog H, Sperk G. The central and basolateral amygdala are critical sites of neuropeptide Y/Y2 receptor-mediated regulation of anxiety and depression. J Neurosci. 2010;30:6282–6290. doi: 10.1523/JNEUROSCI.0430-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BK, Fu W, Kuphal KE, Stiller CO, Winter MK, Chen W, Corder GF, Urban JH, McCarson KE, Marvizon JC. Inflammation enhances Y1 receptor signaling, neuropeptide Y-mediated inhibition of hyperalgesia, and substance P release from primary afferent neurons. Neuroscience. 2014;256:178–194. doi: 10.1016/j.neuroscience.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazoe H, Otomo Y, Kaji I, Tanaka R, Karaki SI, Kuwahara A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J Physiol Pharmacol. 2008;59(Suppl 2):251–262. [PubMed] [Google Scholar]
- Thiriet N, Deng X, Solinas M, Ladenheim B, Curtis W, Goldberg SR, Palmiter RD, Cadet JL. Neuropeptide Y protects against methamphetamine-induced neuronal apoptosis in the mouse striatum. J Neurosci. 2005;25:5273–5279. doi: 10.1523/JNEUROSCI.4893-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A, Carlsson K, Ekman R, Heilig M. Behavioral and endocrine adaptation, and up-regulation of NPY expression in rat amygdala following repeated restraint stress. Neuroreport. 1999;10:3003–3007. doi: 10.1097/00001756-199909290-00024. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Michalkiewicz M, Dumont Y, Quirion R, Caberlotto L, Rimondini R, Mathe AA, Heilig M. Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y overexpression. Proc Natl Acad Sci U S A. 2000;97:12852–12857. doi: 10.1073/pnas.220232997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A, Svensson P, Wiklund L, Sommer W, Ekman R, Heilig M. Suppressed neuropeptide Y (NPY) mRNA in rat amygdala following restraint stress. Regul Pept. 1998;75-76:247–254. doi: 10.1016/s0167-0115(98)00075-5. [DOI] [PubMed] [Google Scholar]
- Tschenett A, Singewald N, Carli M, Balducci C, Salchner P, Vezzani A, Herzog H, Sperk G. Reduced anxiety and improved stress coping ability in mice lacking NPY-Y2 receptors. Eur J Neurosci. 2003;18:143–148. doi: 10.1046/j.1460-9568.2003.02725.x. [DOI] [PubMed] [Google Scholar]
- Tu C, Zhao D, Lin X. Levels of neuropeptide-Y in the plasma and skin tissue fluids of patients with vitiligo. J Dermatol Sci. 2001;27:178–182. doi: 10.1016/s0923-1811(01)00134-7. [DOI] [PubMed] [Google Scholar]
- Ueno H, Yamaguchi H, Mizuta M, Nakazato M. The role of PYY in feeding regulation. Regul Pept. 2008;145:12–16. doi: 10.1016/j.regpep.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Upchurch BH, Aponte GW, Leiter AB. Expression of peptide YY in all four islet cell types in the developing mouse pancreas suggests a common peptide YY-producing progenitor. Development. 1994;120:245–252. doi: 10.1242/dev.120.2.245. [DOI] [PubMed] [Google Scholar]
- Van Citters GW, Lin HC. Ileal brake: neuropeptidergic control of intestinal transit. Curr Gastroenterol Rep. 2006;8:367–373. doi: 10.1007/s11894-006-0021-9. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Vona-Davis L, McFadden DW. PYY and the pancreas: inhibition of tumor growth and inflammation. Peptides. 2007;28:334–338. doi: 10.1016/j.peptides.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Wang L, Gourcerol G, Yuan PQ, Wu SV, Million M, Larauche M, Tache Y. Peripheral peptide YY inhibits propulsive colonic motor function through Y2 receptor in conscious mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G45–56. doi: 10.1152/ajpgi.00349.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein JG, Earley B, Junien JL. Central nervous system pharmacology of neuropeptide Y. Pharmacol Ther. 1995;65:397–414. doi: 10.1016/0163-7258(95)98598-k. [DOI] [PubMed] [Google Scholar]
- Wheway J, Herzog H, Mackay F. NPY and receptors in immune and inflammatory diseases. Curr Top Med Chem. 2007a;7:1743–1752. doi: 10.2174/156802607782341046. [DOI] [PubMed] [Google Scholar]
- Wheway J, Herzog H, Mackay F. The Y1 receptor for NPY: a key modulator of the adaptive immune system. Peptides. 2007b;28:453–458. doi: 10.1016/j.peptides.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Wheway J, Mackay CR, Newton RA, Sainsbury A, Boey D, Herzog H, Mackay F. A fundamental bimodal role for neuropeptide Y1 receptor in the immune system. J Exp Med. 2005;202:1527–1538. doi: 10.1084/jem.20051971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Guan H, Arany E, Hill DJ, Cao X. Neuropeptide Y is produced in visceral adipose tissue and promotes proliferation of adipocyte precursor cells via the Y1 receptor. FASEB J. 2008;22:2452–2464. doi: 10.1096/fj.07-100735. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006;59:660–663. doi: 10.1016/j.biopsych.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Zhang L, Nguyen AD, Lee IC, Yulyaningsih E, Riepler SJ, Stehrer B, Enriquez RF, Lin S, Shi YC, Baldock PA, Sainsbury A, Herzog H. NPY modulates PYY function in the regulation of energy balance and glucose homeostasis. Diabetes Obes Metab. 2012;14:727–736. doi: 10.1111/j.1463-1326.2012.01592.x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Cline MA, Gilbert ER. Hypothalamus-adipose tissue crosstalk: neuropeptide Y and the regulation of energy metabolism. Nutr Metab (Lond) 2014;11 doi: 10.1186/1743-7075-11-27. 27-7075-11-27. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JR, Xu Z, Jiang CL. Neuropeptide Y promotes TGF-beta1 production in RAW264.7 cells by activating PI3K pathway via Y1 receptor. Neurosci Bull. 2008a;24:155–159. doi: 10.1007/s12264-008-0130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JR, Zhang LD, Wei HF, Wang X, Ni HL, Yang F, Zhang T, Jiang CL. Neuropeptide Y induces secretion of high-mobility group box 1 protein in mouse macrophage via PKC/ERK dependent pathway. J Neuroimmunol. 2013;260:55–59. doi: 10.1016/j.jneuroim.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhu G, Hariri AR, Enoch MA, Scott D, Sinha R, Virkkunen M, Mash DC, Lipsky RH, Hu XZ, Hodgkinson CA, Xu K, Buzas B, Yuan Q, Shen PH, Ferrell RE, et al. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008b;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]