Abstract
Anxiety testing in zebrafish is often studied in combination with the application of pharmacological substances. In these studies, fish are routinely netted and transported between home aquaria and dosing tanks. In order to enhance the ease of compound administration, a novel method for transferring fish between tanks for drug administration was developed. Inserts that are designed for spawning were used to transfer groups of fish into the drug solution, allowing accurate dosing of all fish in the group. This increases the precision and efficiency of dosing, which becomes very important in long schedules of repeated drug administration. We implemented this procedure for use in a study examining the behavior of zebrafish in the light/dark test after administering ethanol with differing 21 day schedules. In fish exposed to daily-moderate amounts of alcohol there was a significant difference in location preference after 2 days of withdrawal when compared to the control group. However, a significant difference in location preference in a group exposed to weekly-binge administration was not observed.
This protocol can be generalized for use with all types of compounds that are water-soluble and may be used in any situation when the behavior of fish during or after long schedules of drug administration is being examined. The light/dark test is also a valuable method of assessing withdrawal-induced changes in anxiety.
Keywords: Neuroscience, Issue 93, Zebrafish, Ethanol, Behavior, Anxiety, Pharmacology, Fish, Neuroscience, Drug administration, Scototaxis
Introduction
The zebrafish (Danio rerio) is a small teleost species originating in India that is a useful model organism for behavioral1 and medical research2,3. Zebrafish are also commonly used in the testing of various pharmacological substances in order to characterize their impact on behavior. Various dosages and schedules of drug administration have been used to investigate the behavior of the zebrafish after the administration of compounds such as stimulants4, anxiolytics5 and ethanol6-8.
Our lab has investigated the effects of differing schedules of ethanol administration on zebrafish anxiety and locomotion in the well-validated light/dark assay9,20, also commonly referred to as the scototaxic assay. A new method of ethanol administration was developed to increase efficiency for repetitive, daily administration over a long period of time (21 days)6. Previously used methods were practical, however, we sought to develop a method that reduced netting, with its associated time costs, and allowed simultaneous, precisely timed administration of the drug of interest to large numbers of fish. In traditional research using ethanol, zebrafish are netted and transferred from one tank to another containing the appropriate mixture of ethanol and water10-12. While this method is widely accepted, netting zebrafish may increase the variability in the time taken to introduce and remove the fish from the drug solution. Therefore, the exact exposure to the compound of interest may vary over the course of an experiment involving repeated dosing. A method that reduces sources of error stemming from variability in transport times is thus desirable. With our method we are able to move all fish simultaneously, resulting in identical dosing time in each fish. Following ethanol exposure (described here), zebrafish can be tested in any number of behavioral assays, including those that assess anxiety. Dosing groups of fish using the new method has practical uses beyond the ability to accurately replicate and standardize dosing between subjects and across groups of fish. The advent of new software that allows for the tracking of multiple fish at once may see researchers utilize our methods to ensure replicability and accuracy in their experiments. Considering the widespread use of zebrafish as a model organism for behavioral neuroscience, this method will increase efficiency and practicality in future pharmacological studies.
In the present paradigm, a repeated dosing schedule was employed that approximately mirrors human drinking schedules. Fish were randomly assigned to one of three groups: control, daily-moderate, or weekly-binge. The dosing schedule was 21 days in duration, chosen because it significantly exceeded exposure times in previous studies7. Control fish received zero alcohol, daily-moderate fish received 0.2% alcohol once per day, and weekly-binge fish received 1.4% alcohol once per week. The light/dark task was used to assess anxiety after 2 days of withdrawal. This is a relatively simple test to administer which uses a rectangular arena in which the walls on one side are white and on the other side are dark9. Adult zebrafish robustly prefer the dark side of the arena under control conditions6,9,13. Increased anxiety is operationally defined as significantly more time spent in the dark zone, and decreased anxiety can be assumed when the fish spends relatively more time spent in the light zone. With motion-tracking software, other informative variables can also be quantified, including average velocity, immobility, meandering, and zone transitions14.
The dosing method developed in our laboratory can apply to any research in which water-soluble compounds are administered to one or more zebrafish. Many other pharmacological agents which may benefit from this methodology are currently being tested in zebrafish. Commonly tested compounds include nicotine, chlordiazepoxide, buspirone, and scopolamine, which are dissolved in a similar manner to ethanol; by mixing the appropriate amount of the chemical into water. Therefore, the general scope of this procedure is much wider and not limited to ethanol. Furthermore, after dosing with drugs for multiple days, the light/dark task is only one of many behavioral tests that could be employed. After drug administration or during withdrawal, other popular assays that can be utilized include the novel tank diving test15 and tests of social behavior such as shoaling16. The following procedure will outline an efficient method of repeatedly transferring groups of fish or individual fish into solutions containing a pharmacological compound of interest. Additionally, the process of testing anxiety with the light/dark test in groups of fish who are in withdrawal after being exposed to long schedules of alcohol administration will be described.
Protocol
All procedures and behavioral testing were approved by MacEwan University’s Animal Research Ethics Board under protocol number 06-11-12, which is in compliance with the Canadian Council for Animal Care’s guidelines for the care and use of experimental animals.
1. Prepare Dosing Tanks, Solutions, and Administration Schedule
Prepare an administration schedule such that the animals are dosed in the same environment and during the same time of day to avoid any confounds of time or biasing to visual stimuli.
- Obtain as many identical, 1.5 L, clear polypropylene spawning tanks as necessary for the number of group sizes. Use groups of 8 fish per tank, which allows for 2 groups of fish to be tested per day later on in the procedure (see step 3). Use one holding tank and one dosing tank per group (2x total number of groups).
- Place 400 µm spawning inserts in all holding tanks. Fill the tanks with habitat water or reverse osmosis water at the correct temperature (for zebrafish, 25-28 °C) that is consistent with the temperature fish are normally housed at. NOTE: There can be undesired chemical interactions between some drugs and the chemical components of buffered habitat water. In this situation, use reverse osmosis water buffered with minimal or no aquarium salts for drug administration, as well as for the control groups.
- Ensure that the tanks are in a neutral environment to avoid conditioning fish to external visual stimuli during dosing.
Prepare the drug solution. Mix the appropriate amount of the drug with habitat water in the spawning tanks. Prepare the 0.2% ethanol solution by combining 3 ml of a high grade ethanol (95% non-denatured ethanol) with 1,497 ml of water. Prepare the 1.4% ethanol solution by combining 21 ml of ethanol with 1,479 ml of water.
2. Netting Fish and Ethanol Administration Procedure
Carefully net fish from their habitat tanks and transfer into the appropriate holding tank containing the spawning insert. Ideally, house the fish in the spawning insert to eliminate netting altogether.
- With all fish in their respective holding tanks, gently lift the spawning insert out of the holding tank and place it into the appropriate drug solution tank (Figure 1A).
- Record the dosing time as required. Use 30 min in the ethanol solution for the procedure described here.
- If possible, have assistants help with the transfer of all experimental groups to the drug solution simultaneously to ensure precise dosing time. Alternatively, transfer one group at a time and keep track of individual groups’ dosing times (Figure 1A).
At the end of the requisite dosing period, remove the fish from the ethanol solution by carefully lifting the spawning insert out of the drug solution and placing gently back into the holding tank.
Gently net the fish in the holding tanks and place them back into their habitats until the next scheduled dosing time, or place the spawning insert back into the holding tank to eliminate netting.
As mentioned previously, if possible within the parameters of animal housing equipment, house the animals in the same tank and spawning insert that is used as the holding tank. This will eliminate netting altogether during the administration procedure.
3. Behavioral Testing
Obtain a light/dark arena 9.5 cm wide by 55 cm long and 9.5 cm deep with a white waterproof floor (Figure 1B). Affix white and black waterproof non-reflective paper to the inside walls of the arena using velcro, with half of the arena covered in white and half covered in black. Fill the arena to a depth of 5 cm with habitat water at a temperature of 25-28 °C. Maintain this temperature throughout testing.
Minimalize external visual stimuli by constructing a white three-sided enclosure for the arena to be situated in. Ensure the testing area has diffuse overhead lighting that does not cause reflections on the water surface, yet is sufficiently bright for the movement tracking software, or post-hoc manual quantification from video images.
Place the arena in the enclosure and set the recording and movement analysis parameters of the behavior tracking software. Set the trial duration to 5-15 min, depending on the research question. NOTE: Here, we used 5 min.
Transport the group of fish to be tested to the research area in the habitat tank and place them outside of the arena enclosure. Acclimate the fish for 10 min.
Gently net a fish from the appropriate group and place in the center of the light/dark arena, being sure to release the fish when it is positioned parallel to the long axis of the arena to avoid biasing the fish to the light or dark zone.
Begin recording behavior immediately after the animal is released. Watch for any software problems with tracking the fish or for fish jumping or freezing. Rotate the arena 180° after half of the subjects have been tested to prevent any confounds due to biases resulting from which end of the arena is oriented toward the open end of the enclosure.
After the trial has ended, gently net and remove the fish from the arena to a holding tank or habitat tank.
4. Analysis
Examine time spent in light versus dark zones. For each group and each fish, obtain the relative time spent in the light and dark zones and analyze using a one sample t-test (or Wilcoxon signed rank test for nonparametric data; difference from (half of the total trial time) 150 sec) to determine if groups significantly prefer one area over the other.
To compare preferences, calculate a preference index by subtracting the time spent in the light zone from the time spent in the dark zone and compare differences between groups. t-tests can be used to compare two groups. Compare multiple groups with a one-way analysis of variance utilizing Tukey’s HSD post hoc test where necessary (or Kruskal-Wallis test with Dunn’s multiple comparison post hoc test for nonparametric data).
Compare velocity, number of zone transitions, meandering, and immobility across groups. Use one-way analysis of variance utilizing Tukey’s HSD post hoc test where necessary (or Kruskal-Wallis test with Dunn’s multiple comparison post hoc test for nonparametric data).
Representative Results
To maintain accuracy and control in pharmacological studies with zebrafish it is important to time the duration of ethanol administration consistently and accurately as described above. Our procedure can increase the ease and throughput of the dosing procedure. The administration of ethanol on either a weekly-binge or daily-moderate schedule resulted in altered anxiety levels, measured with the light/dark test, compared to controls. When tested two days after the last dosing, zebrafish in the control group (who received no alcohol, but were still moved to dosing tanks) displayed the expected pattern of behavior, with control fish spending significantly more time on the dark side of the arena (Figure 2A) similar to naive zebrafish in other studies6,9,13. Fish in the weekly binge group showed no preference for either the light or dark zones in the task when tested two days after their last ethanol administration (Figure 2B). Interestingly, fish in the daily-moderate group significantly preferred the light side of the arena (Figure 2C); in contrast to the control group. The preference index indicated a significant difference between control and daily-moderate groups (Figure 2D). There were no significant differences in swimming speed, or immobility across groups (Table 1), and therefore, this effect was not due to a motor deficit in the fish.
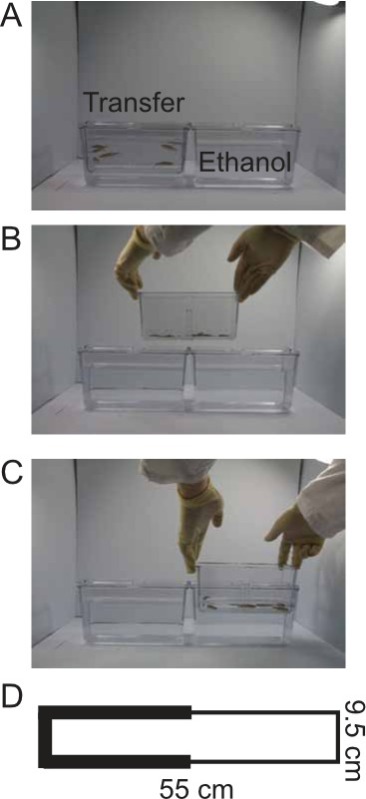 Figure 1: Transfer Procedure and the Light/Dark arena. These photos illustrate the transfer procedure. (A) First, the fish are situated in the transfer tank (left). Dosing tank containing ethanol is pictured (right). (B-C) Moving the fish from the transfer tank to the ethanol solution requires the researcher to lift the spawning insert out of the transfer tank and into the ethanol solution tank. (D) The light/dark arena as illustrated measured 9.5 cm wide by 9.5 cm deep and 55 cm long. White flooring is used, along with black walls on half of the arena (left) and white walls on the other half, creating a light and dark zone.
Figure 1: Transfer Procedure and the Light/Dark arena. These photos illustrate the transfer procedure. (A) First, the fish are situated in the transfer tank (left). Dosing tank containing ethanol is pictured (right). (B-C) Moving the fish from the transfer tank to the ethanol solution requires the researcher to lift the spawning insert out of the transfer tank and into the ethanol solution tank. (D) The light/dark arena as illustrated measured 9.5 cm wide by 9.5 cm deep and 55 cm long. White flooring is used, along with black walls on half of the arena (left) and white walls on the other half, creating a light and dark zone.
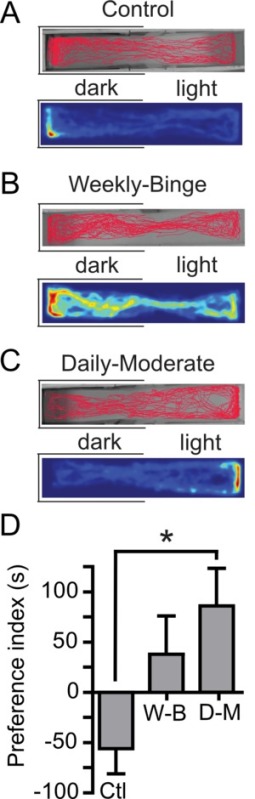 Figure 2: Representative results and trackplots of ethanol dosing procedure on three groups of zebrafish after 2 days withdrawal. (A) A representative trackplot of a single control zebrafish movement path throughout the 5 min light/dark trial. Below is the same zebrafish trackplot represented as a heatmap, which is a colored representation of zebrafish movement throughout the trial, based on the time the fish spent in the location represented by each pixel. (B) A representative trackplot of a single zebrafish from the weekly-binge group throughout the 5 min light/dark task. Below is the heatmap from the same zebrafish. (C) A representative trackplot of a single daily-moderate zebrafish movement throughout the 5 min light/dark trial. Below is the heatmap from the same zebrafish. (D) The preference index was calculated for all groups by subtracting time spent in the light zone from time spent in the dark zone. Negative numbers indicate preference for the dark zone. Positive numbers indicate preference for the light zone. Results indicate a significant difference in preference between control and daily-moderate groups at 2 days withdrawal *p <0.05 (one-way ANOVA). Note there is also a significant preference for dark in the control group, p <0.05 (one sample t-test, difference from 0), and a significant preference for light in the daily-moderate group, p <0.05 (one sample t-test, difference from 0). Please click here to view a larger version of this figure.
Figure 2: Representative results and trackplots of ethanol dosing procedure on three groups of zebrafish after 2 days withdrawal. (A) A representative trackplot of a single control zebrafish movement path throughout the 5 min light/dark trial. Below is the same zebrafish trackplot represented as a heatmap, which is a colored representation of zebrafish movement throughout the trial, based on the time the fish spent in the location represented by each pixel. (B) A representative trackplot of a single zebrafish from the weekly-binge group throughout the 5 min light/dark task. Below is the heatmap from the same zebrafish. (C) A representative trackplot of a single daily-moderate zebrafish movement throughout the 5 min light/dark trial. Below is the heatmap from the same zebrafish. (D) The preference index was calculated for all groups by subtracting time spent in the light zone from time spent in the dark zone. Negative numbers indicate preference for the dark zone. Positive numbers indicate preference for the light zone. Results indicate a significant difference in preference between control and daily-moderate groups at 2 days withdrawal *p <0.05 (one-way ANOVA). Note there is also a significant preference for dark in the control group, p <0.05 (one sample t-test, difference from 0), and a significant preference for light in the daily-moderate group, p <0.05 (one sample t-test, difference from 0). Please click here to view a larger version of this figure.
| Velocity | Immobility | |
| (cm/sec) | (sec) | |
| 2-days Wd | 2-days Wd | |
| Control (n = 13) | 9.1 ± 0.6 | 1.9 ± 0.6 |
| Binge (n = 14) | 9.8 ± 0.5 | 0.8 ± 0.2 |
| Chronic (n = 15) | 10.3 ± 0.5 | 2.5 ± 1.0 |
Table 1: Average velocity and immobility during the light/dark test. The average swim velocity (cm/sec) and immobility (sec) of a representative group of zebrafish after 2 days of withdrawal (mean ± S.E.M). Here, no significant differences in either velocity or immobility were found. Used with Permission from Holcombe et al., (2013).
Discussion
Previous studies involving drug administration in zebrafish have simply relied on netting fish to transport them from their home tank into the drug solution12,16. Netting is not always consistent and often takes longer than expected due to the escape response of the zebrafish, which has significant individual variability. Traditional transfer methods, while useful, can be improved upon by decreasing the amount of total time the fish spend outside of water, as well as decreasing the amount of variability in transfer time between animals. In addition to improving practicality, this method gives researchers the ability to dose large groups of fish simultaneously in a solution for a precise amount of time. Previous methods require either dosing a single fish, or attempting to net whole groups of fish simultaneously. The former is slow and decreases throughput, whereas the latter is an awkward and difficult task. Furthermore, motion-tracking systems that can assess the behavior of multiple fish at once can benefit from the precision of this method. Fish could be transferred directly from the spawning insert into the shoaling arena, which would ensure near simultaneous exposure to the arena for all fish.
Utilizing this new method produced significant results when implemented on three groups of zebrafish over a 21 day period. Control fish received no alcohol, daily-moderate fish received a small amount of alcohol once per day, and weekly-binge fish received a large dose of alcohol once per week over the 21 day period. In the light/dark task, the dosing procedure did not change the normal preference of the zebrafish for the dark zone under control conditions. However, after 2 days of ethanol withdrawal, zebrafish in the daily-moderate group preferred the light zone of the arena, a reversal of preference. Statistical analysis confirms a significant change in zone preference. It is possible that the dosing procedure resulted in ethanol seeking behavior due to a conditioned place preference (CPP), with the fish seeking ethanol (an appetitive stimulus), and preferring the white zone due to its relative similarity to the bright dosing area. Recent research reveals that CPP can occur in zebrafish and in areas where zebrafish are dosed with ethanol17,18. Weekly binge-fish showed no preference 2 days post ethanol, and though evidence about neural mechanisms accounting for this finding is lacking, it is possible that infrequent but large doses of alcohol may damage the brain, possibly leaving fish unable to discriminate between zones.
Another advantage that this dosing procedure confers on researchers is its generalizability. As described, the procedure produces clear and useful results in zebrafish. However, the procedure is not limited solely to zebrafish. The constraints on the procedure are related to the size and limits of the dosing tanks, animals and testing apparatus, as well as ability to analyze data. With the use of motion tracking software, recent studies have investigated many additional variables throughout a trial. White avoidance habituation, squares crossed, locomotion habituation, latency to the white zone, erratic swimming, thigmotaxis (time spent near the arena walls), and risk assessment can also be quantified14. It is conceivable to use this procedure in other model organisms, such as goldfish, which have been used previously to investigate the role of tolerance and withdrawal19. Researchers hoping to investigate the effects of other pharmacological agents or environmental toxins, the use of other behavioral assays, or other marine or freshwater animals, could also utilize this basic methodology.
Using this protocol is simple and effective. However, if results are not as expected, consider troubleshooting the motion-tracking system to ensure proper data collection. Tracking should be smooth and accurate across the trials. The tracking system should be recording the movement of the body-center of the fish at all times. Any jumping or erratic movement of the tracking dot due to visual artifacts, such as light reflections on water ripples, or improper tracking settings may confound results. Some discrepancies in results may arise from small differences in arena design or dosing environments. To be sure of replication, the arena should be as described, particularly with respect to using a white floor to maximize tracking efficiency in zebrafish. With other fish that are lighter in color, a dark floor should be used to aid in motion tracking. Similarly, dosing the fish in an environment that is free from any visual stimuli that could possibly bias the fish is extremely important. Any distinct color, pattern or hue that is perceivable to the fish could confound results. A neutral, light colored background similar to that in the housing system is recommended.
While it is feasible to modify this method as necessary for other species of fish, results may vary in terms of innate light/dark preference20-21. It is also important to note even the procedure described here still causes some stress. Although netting is significantly reduced, the procedure still involves a very short period of time where the fish must be out of water, possibly inducing a stress response. Ramsay and colleagues22 found that netted zebrafish had at least double the levels of cortisol in their body compared to those that were not netted. Since cortisol is a hormone linked to stress11 this may alter the behavioral response that is measured. While our method reduces netting, it does not eliminate it. However, it gives researchers the opportunity to eliminate the incidence of netting if they desire by housing zebrafish in the spawning inserts. Future studies should investigate whether the use of the spawning insert for transfer of fish can decrease the stress response relative to netting. Additionally, it is crucially important to maintain precise dosing times and transfer times between groups. Changes in dosing times or large differences in time spent out of water could alter results. To reduce possible circadian effects, it is also critical to dose the fish during similar times of the day. In the current experiment fish were dosed and tested between 10 AM and 2 PM. Accurate and reliable replication of this procedure relies on repeating the protocol with precision.
Disclosures
The authors acknowledge Joshua Gallup for use of his photography equipment used for Figure 1. This work was supported by a Natural Sciences and Engineering Research Council (NSERC) Canada Discovery grant (to T.J.H.).
Acknowledgments
The authors have nothing to disclose.
References
- Spence R, Gerlach G, Lawrence C, Smith C. The behaviour and ecology of the zebrafish, Danio rerio. Biological Reviews. 2008;83(1):13–34. doi: 10.1111/j.1469-185X.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- Langheinrich U. Zebrafish: A new model on the pharmaceutical catwalk. BioEssays. 2003;25(9):904–912. doi: 10.1002/bies.10326. [DOI] [PubMed] [Google Scholar]
- Santoriello C, Zon LI. Hooked! Modeling human disease in zebrafish. Journal of Clinical Investigation. 2012;122(7):2337–2343. doi: 10.1172/JCI60434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N, Greene K, Dydinski A, Gerlai R. Effects of nicotine and alcohol on zebrafish ( Danio rerio) shoaling. Behavioural brain research. 2012. [DOI] [PubMed]
- Bencan Z, Sledge D, Levin ED. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacology Biochemistry and Behavior. 2009;94(1):75–80. doi: 10.1016/j.pbb.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcombe A, Howorko A, Powell RA, Schalomon M, Hamilton TJ. Reversed Scototaxis during Withdrawal after Daily-Moderate, but Not Weekly-Binge Administration of Ethanol in Zebrafish. PLoS ONE. 2013;8(5) doi: 10.1371/journal.pone.0063319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur P, Guo S. Differences of acute versus chronic ethanol exposure on anxiety-like behavioral responses in zebrafish. Behavioural Brain Research. 2011;219(2):234–239. doi: 10.1016/j.bbr.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugos C, Rabin R. Ethanol effects on three strains of zebrafish: model system for genetic investigations. Pharmacology Biochemistry and Behavior. 2003. [DOI] [PubMed]
- Maximino C, et al. Scototaxis as anxiety-like behavior in fish. Nature Protocols. 2010;5(2):209–216. doi: 10.1038/nprot.2009.225. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Lee V, Blaser R. Effects of acute and chronic ethanol exposure on the behavior of adult zebrafish (Danio rerio) Pharmacology Biochemistry and Behavior. 2006;85(4):752–761. doi: 10.1016/j.pbb.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan RJ, et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behavioural brain research. 2009;205(1):38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer DL, et al. Effects of anxiolytics in zebrafish: Similarities and differences between benzodiazepines, buspirone and ethanol. Pharmacology Biochemistry and Behavior. 2011;99(3):480–486. doi: 10.1016/j.pbb.2011.04.021. [DOI] [PubMed] [Google Scholar]
- Serra MC, Mattioli R. Natural preference of zebrafish (Danio rerio) for a dark environment. Braz J Med Biol Res. 1999;32(12):1551–1553. doi: 10.1590/s0100-879x1999001200016. [DOI] [PubMed] [Google Scholar]
- Maximino C, et al. Behavioral and neurochemical changes in the zebrafish leopard strain. Genes Brain Behav. 2013;12(5):576–582. doi: 10.1111/gbb.12047. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiology & Behavior. 2007;90(1):54–58. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Chatterjee D, Pereira T, Sawashima T, Krishnannair R. Acute and chronic alcohol dose: population differences in behavior and neurochemistry of zebrafish. Genes, Brain and Behavior. 2009;8(6):586–599. doi: 10.1111/j.1601-183X.2009.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur P, Berberoglu MA, Guo S. Preference for ethanol in zebrafish following a single exposure. Behavioural Brain Research. 2011;217(1):128–133. doi: 10.1016/j.bbr.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renninger SL, et al. Investigating the genetics of visual processing, function and behaviour in zebrafish. Neurogenetics. 2011;12:97–116. doi: 10.1007/s10048-011-0273-x. [DOI] [PubMed] [Google Scholar]
- Crawshaw LI, et al. Tolerance and withdrawal in goldfish exposed to ethanol. Physiology & Behaviour. 2006;87(3):460–468. doi: 10.1016/j.physbeh.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Hamilton TJ, Holcombe A. Tresguerres, M.CO2-induced ocean acidification increases anxiety in rockfish via alteration of GABAA receptor functioning. Proceedings of the Royal Society B. 2014. [DOI] [PMC free article] [PubMed]
- Ramsay JM, Feist GW, Varga ZM, Westerfield M, Kent ML, Schreck CB. Whole-body cortisol response of zebrafish to acute net handling stress. Aquaculture. 2009;297(1-4):157–164. doi: 10.1016/j.aquaculture.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton TJ, Paz-Yepes J, Morrison RA, Palenik B, Tresguerres M. Exposure to bloom-like concentrations of two marine Synechococcus cyanobacteria (strains CC9311 and CC9902) differentially alters fish behaviour. Conservation Physiology. 2014;2(1) doi: 10.1093/conphys/cou020. [DOI] [PMC free article] [PubMed] [Google Scholar]


