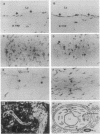
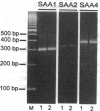
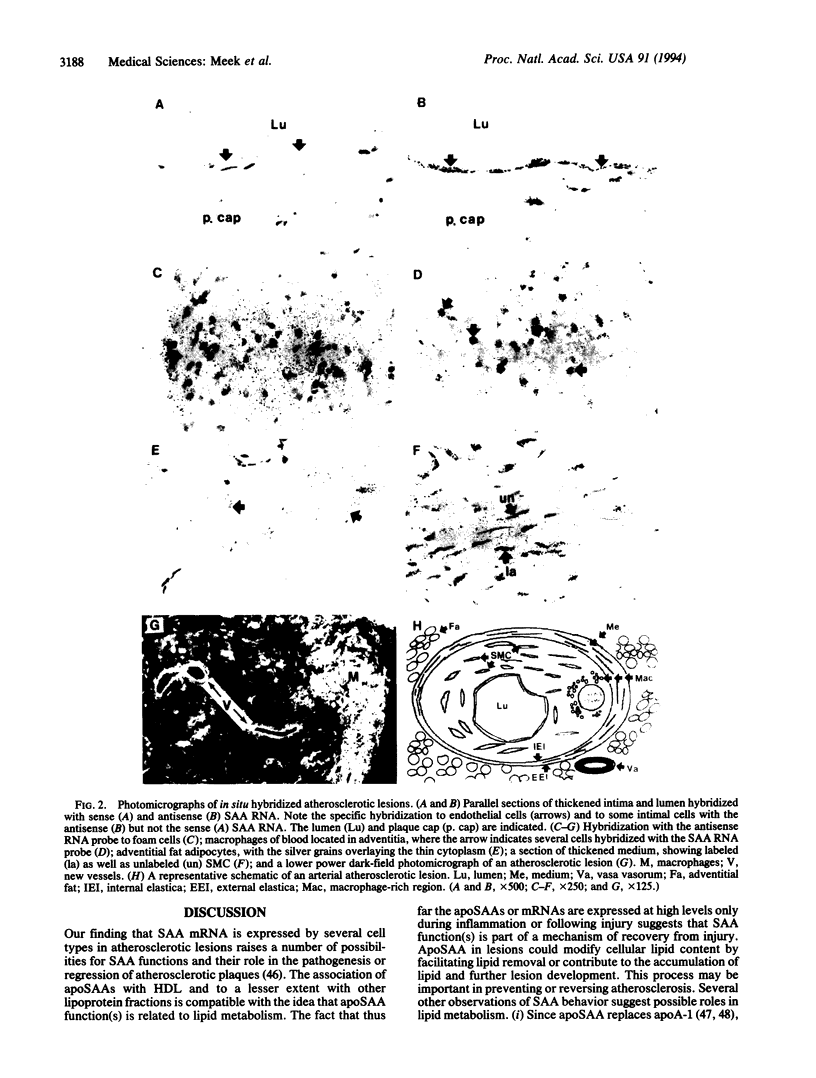
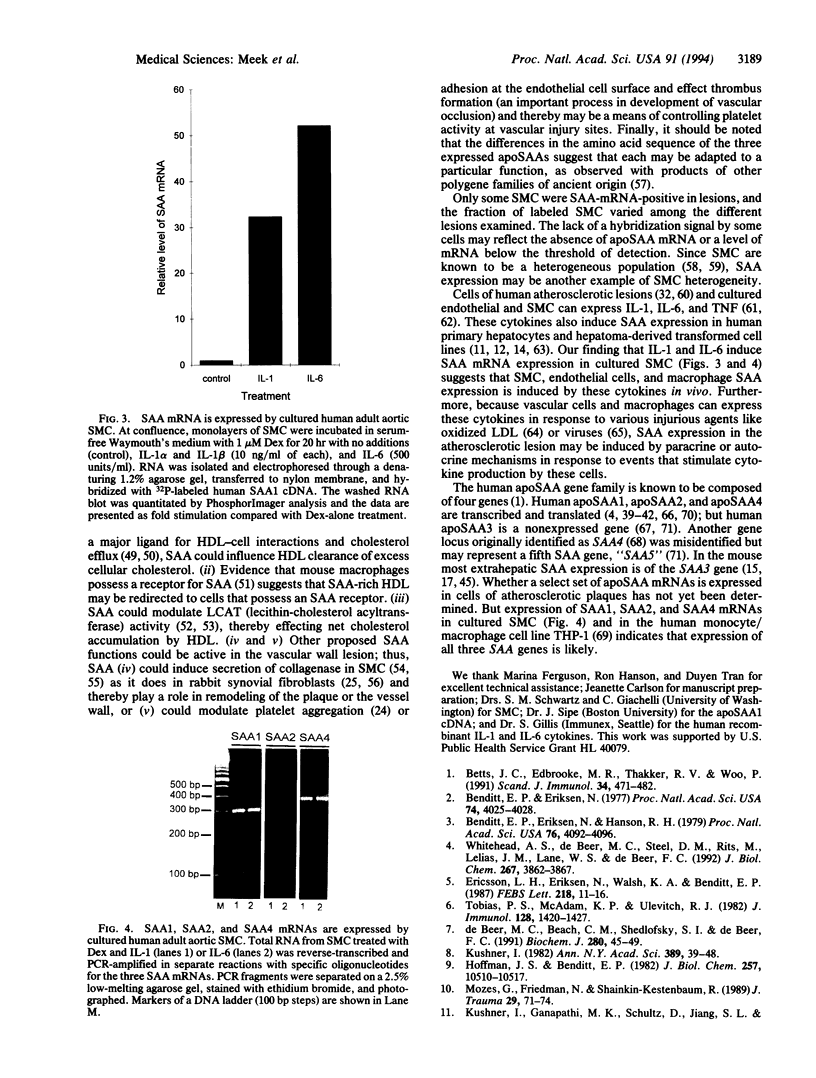
Abstract
Altered lipoprotein metabolism and vascular injury are considered to be major parts of the pathogenesis of atherosclerotic lesions. Serum amyloid A (SAA) is a family of acute-phase reactants found residing mainly on high density lipoproteins (HDL) in the circulation. Several functions for the SAAs have been proposed that could be important in atherosclerosis. These include involvement in cholesterol metabolism, participation in detoxification, depression of immune responses, and interference with platelet functions. Like other acute-phase reactants, the liver is a major site of SAA synthesis. However, studies in the mouse have revealed that several cell types including macrophages express SAA. Furthermore, we recently found that SAA mRNA expression can be induced in the human monocyte/macrophage cell line, THP-1. In the present study, human atherosclerotic lesions of coronary and carotid arteries were examined for expression of SAA mRNA by in situ hybridization. Surprisingly, SAA mRNA was found in most endothelial cells and some smooth muscle cells as well as macrophage-derived "foam cells," adventitial macrophages, and adipocytes. In addition, cultured smooth muscle cells expressed SAA1, SAA2, and SAA4 mRNAs when treated with interleukin 1 or 6 (IL-1 or IL-6) in the presence of dexamethasone. These findings give further credence to the notion that the SAAs are involved in lipid metabolism or transport at sites of injury and in atherosclerosis or may play a role in defending against viruses or other injurious agents such as oxidized lipids. Furthermore, expression of SAAs by endothelial cells is compatible with the evidence that SAA modulates platelet aggregation and function and possibly adhesion at the endothelial cell surface.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldo-Benson M. A., Benson M. D. SAA suppression of immune response in vitro: evidence for an effect on T cell-macrophage interaction. J Immunol. 1982 Jun;128(6):2390–2392. [PubMed] [Google Scholar]
- Amento E. P., Ehsani N., Palmer H., Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb. 1991 Sep-Oct;11(5):1223–1230. doi: 10.1161/01.atv.11.5.1223. [DOI] [PubMed] [Google Scholar]
- Au Y. P., Montgomery K. F., Clowes A. W. Heparin inhibits collagenase gene expression mediated by phorbol ester-responsive element in primate arterial smooth muscle cells. Circ Res. 1992 May;70(5):1062–1069. doi: 10.1161/01.res.70.5.1062. [DOI] [PubMed] [Google Scholar]
- Baumberger C., Ulevitch R. J., Dayer J. M. Modulation of endotoxic activity of lipopolysaccharide by high-density lipoprotein. Pathobiology. 1991;59(6):378–383. doi: 10.1159/000163681. [DOI] [PubMed] [Google Scholar]
- Benditt E. P., Barrett T., McDougall J. K. Viruses in the etiology of atherosclerosis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6386–6389. doi: 10.1073/pnas.80.20.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Barrett T., McDougall J. K. Viruses in the etiology of atherosclerosis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6386–6389. doi: 10.1073/pnas.80.20.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N. Amyloid protein SAA is associated with high density lipoprotein from human serum. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4025–4028. doi: 10.1073/pnas.74.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N., Hanson R. H. Amyloid protein SAA is an apoprotein of mouse plasma high density lipoprotein. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4092–4096. doi: 10.1073/pnas.76.8.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Meek R. L. Expression of the third member of the serum amyloid A gene family in mouse adipocytes. J Exp Med. 1989 May 1;169(5):1841–1846. doi: 10.1084/jem.169.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P. Origins of human atherosclerotic plaques. The role of altered gene expression. Arch Pathol Lab Med. 1988 Oct;112(10):997–1001. [PubMed] [Google Scholar]
- Benson M. D., Aldo-Benson M. Effect of purified protein SAA on immune response in vitro: mechanisms of suppression. J Immunol. 1979 May;122(5):2077–2082. [PubMed] [Google Scholar]
- Betts J. C., Edbrooke M. R., Thakker R. V., Woo P. The human acute-phase serum amyloid A gene family: structure, evolution and expression in hepatoma cells. Scand J Immunol. 1991 Oct;34(4):471–482. doi: 10.1111/j.1365-3083.1991.tb01570.x. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff C. E., Mitchell T. I., Karmilowicz M. J., Kluve-Beckerman B., Benson M. D. Autocrine induction of collagenase by serum amyloid A-like and beta 2-microglobulin-like proteins. Science. 1989 Feb 3;243(4891):655–657. doi: 10.1126/science.2536953. [DOI] [PubMed] [Google Scholar]
- Brown B. G., Zhao X. Q., Sacco D. E., Albers J. J. Lipid lowering and plaque regression. New insights into prevention of plaque disruption and clinical events in coronary disease. Circulation. 1993 Jun;87(6):1781–1791. doi: 10.1161/01.cir.87.6.1781. [DOI] [PubMed] [Google Scholar]
- Chung B. H., Anatharamaiah G. M., Brouillette C. G., Nishida T., Segrest J. P. Studies of synthetic peptide analogs of the amphipathic helix. Correlation of structure with function. J Biol Chem. 1985 Aug 25;260(18):10256–10262. [PubMed] [Google Scholar]
- Coetzee G. A., Strachan A. F., van der Westhuyzen D. R., Hoppe H. C., Jeenah M. S., de Beer F. C. Serum amyloid A-containing human high density lipoprotein 3. Density, size, and apolipoprotein composition. J Biol Chem. 1986 Jul 25;261(21):9644–9651. [PubMed] [Google Scholar]
- Ericsson L. H., Eriksen N., Walsh K. A., Benditt E. P. Primary structure of duck amyloid protein A. The form deposited in tissues may be identical to its serum precursor. FEBS Lett. 1987 Jun 22;218(1):11–16. doi: 10.1016/0014-5793(87)81008-6. [DOI] [PubMed] [Google Scholar]
- Ganapathi M. K., May L. T., Schultz D., Brabenec A., Weinstein J., Sehgal P. B., Kushner I. Role of interleukin-6 in regulating synthesis of C-reactive protein and serum amyloid A in human hepatoma cell lines. Biochem Biophys Res Commun. 1988 Nov 30;157(1):271–277. doi: 10.1016/s0006-291x(88)80043-3. [DOI] [PubMed] [Google Scholar]
- Gordon D., Reidy M. A., Benditt E. P., Schwartz S. M. Cell proliferation in human coronary arteries. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4600–4604. doi: 10.1073/pnas.87.12.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Guyton J. R., Klemp K. F. Early extracellular and cellular lipid deposits in aorta of cholesterol-fed rabbits. Am J Pathol. 1992 Oct;141(4):925–936. [PMC free article] [PubMed] [Google Scholar]
- Hoffman J. S., Benditt E. P. Changes in high density lipoprotein content following endotoxin administration in the mouse. Formation of serum amyloid protein-rich subfractions. J Biol Chem. 1982 Sep 10;257(17):10510–10517. [PubMed] [Google Scholar]
- Hoffman J. S., Benditt E. P. Changes in high density lipoprotein content following endotoxin administration in the mouse. Formation of serum amyloid protein-rich subfractions. J Biol Chem. 1982 Sep 10;257(17):10510–10517. [PubMed] [Google Scholar]
- Hoffman J. S., Benditt E. P. Plasma clearance kinetics of the amyloid-related high density lipoprotein apoprotein, serum amyloid protein (apoSAA), in the mouse. Evidence for rapid apoSAA clearance. J Clin Invest. 1983 Apr;71(4):926–934. doi: 10.1172/JCI110847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisilevsky R., Subrahmanyan L. Serum amyloid A changes high density lipoprotein's cellular affinity. A clue to serum amyloid A's principal function. Lab Invest. 1992 Jun;66(6):778–785. [PubMed] [Google Scholar]
- Kisilevsky R., Subrahmanyan L. Serum amyloid A changes high density lipoprotein's cellular affinity. A clue to serum amyloid A's principal function. Lab Invest. 1992 Jun;66(6):778–785. [PubMed] [Google Scholar]
- Kluve-Beckerman B., Drumm M. L., Benson M. D. Nonexpression of the human serum amyloid A three (SAA3) gene. DNA Cell Biol. 1991 Nov;10(9):651–661. doi: 10.1089/dna.1991.10.651. [DOI] [PubMed] [Google Scholar]
- Kluve-Beckerman B., Dwulet F. E., Benson M. D. Human serum amyloid A. Three hepatic mRNAs and the corresponding proteins in one person. J Clin Invest. 1988 Nov;82(5):1670–1675. doi: 10.1172/JCI113779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluve-Beckerman B., Long G. L., Benson M. D. DNA sequence evidence for polymorphic forms of human serum amyloid A (SAA). Biochem Genet. 1986 Dec;24(11-12):795–803. doi: 10.1007/BF00554519. [DOI] [PubMed] [Google Scholar]
- Ku G., Thomas C. E., Akeson A. L., Jackson R. L. Induction of interleukin 1 beta expression from human peripheral blood monocyte-derived macrophages by 9-hydroxyoctadecadienoic acid. J Biol Chem. 1992 Jul 15;267(20):14183–14188. [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Auger K. R., Robbins A. H., Birinyi L. K., Dinarello C. A. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am J Pathol. 1986 Aug;124(2):179–185. [PMC free article] [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Birinyi L. K., Auger K. R., Dinarello C. A. Inducible interleukin-1 gene expression in human vascular smooth muscle cells. J Clin Invest. 1986 Dec;78(6):1432–1438. doi: 10.1172/JCI112732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke R. P., Bock V., Valet G., Rothe G. Inhibition of the oxidative burst response of N-formyl peptide-stimulated neutrophils by serum amyloid-A protein. Biochem Biophys Res Commun. 1991 May 15;176(3):1100–1105. doi: 10.1016/0006-291x(91)90397-p. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Giachelli C. M., Reidy M. A., Schwartz S. M. Rat carotid neointimal smooth muscle cells reexpress a developmentally regulated mRNA phenotype during repair of arterial injury. Circ Res. 1992 Oct;71(4):759–768. doi: 10.1161/01.res.71.4.759. [DOI] [PubMed] [Google Scholar]
- McKnight G. L., Reasoner J., Gilbert T., Sundquist K. O., Hokland B., McKernan P. A., Champagne J., Johnson C. J., Bailey M. C., Holly R. Cloning and expression of a cellular high density lipoprotein-binding protein that is up-regulated by cholesterol loading of cells. J Biol Chem. 1992 Jun 15;267(17):12131–12141. [PubMed] [Google Scholar]
- Meek R. L., Benditt E. P. Amyloid A gene family expression in different mouse tissues. J Exp Med. 1986 Dec 1;164(6):2006–2017. doi: 10.1084/jem.164.6.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek R. L., Benditt E. P. Rat tissues express serum amyloid A protein-related mRNAs. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1890–1894. doi: 10.1073/pnas.86.6.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek R. L., Eriksen N., Benditt E. P. Murine serum amyloid A3 is a high density apolipoprotein and is secreted by macrophages. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7949–7952. doi: 10.1073/pnas.89.17.7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell T. I., Coon C. I., Brinckerhoff C. E. Serum amyloid A (SAA3) produced by rabbit synovial fibroblasts treated with phorbol esters or interleukin 1 induces synthesis of collagenase and is neutralized with specific antiserum. J Clin Invest. 1991 Apr;87(4):1177–1185. doi: 10.1172/JCI115116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozes G., Friedman N., Shainkin-Kestenbaum R. Serum amyloid A: an extremely sensitive marker for intensity of tissue damage in trauma patients and indicator of acute response in various diseases. J Trauma. 1989 Jan;29(1):71–74. [PubMed] [Google Scholar]
- O'Brien K. D., Gordon D., Deeb S., Ferguson M., Chait A. Lipoprotein lipase is synthesized by macrophage-derived foam cells in human coronary atherosclerotic plaques. J Clin Invest. 1992 May;89(5):1544–1550. doi: 10.1172/JCI115747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram J. F., Johnson C. J., Brown T. A. Interaction of high density lipoprotein with its receptor on cultured fibroblasts and macrophages. Evidence for reversible binding at the cell surface without internalization. J Biol Chem. 1987 Feb 15;262(5):2405–2410. [PubMed] [Google Scholar]
- Pech M. A., Myara I., Vedie B., Moatti N. LDL modifiées et athérosclérose. Nature des modifications. Propriétés physicochimiques et biologiques. Ann Biol Clin (Paris) 1992;50(4):213–227. [PubMed] [Google Scholar]
- Perez-Reyes N., Halbert C. L., Smith P. P., Benditt E. P., McDougall J. K. Immortalization of primary human smooth muscle cells. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1224–1228. doi: 10.1073/pnas.89.4.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyart C., Wajcman H., Kister J. Molecular adaptation of hemoglobin function in mammals. Respir Physiol. 1992 Oct;90(1):3–17. doi: 10.1016/0034-5687(92)90130-o. [DOI] [PubMed] [Google Scholar]
- Raynes J. G., Eagling S., McAdam K. P. Acute-phase protein synthesis in human hepatoma cells: differential regulation of serum amyloid A (SAA) and haptoglobin by interleukin-1 and interleukin-6. Clin Exp Immunol. 1991 Mar;83(3):488–491. doi: 10.1111/j.1365-2249.1991.tb05666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynes J. G., McAdam K. P. Serum amyloid A isoforms in inflammation. Scand J Immunol. 1991 Jun;33(6):657–666. doi: 10.1111/j.1365-3083.1991.tb02538.x. [DOI] [PubMed] [Google Scholar]
- Sack G. H., Jr, Talbot C. C., Jr The human serum amyloid A locus SAA4 is a pseudogene. Biochem Biophys Res Commun. 1992 Mar 16;183(2):362–366. doi: 10.1016/0006-291x(92)90489-8. [DOI] [PubMed] [Google Scholar]
- Salomon R. N., Underwood R., Doyle M. V., Wang A., Libby P. Increased apolipoprotein E and c-fms gene expression without elevated interleukin 1 or 6 mRNA levels indicates selective activation of macrophage functions in advanced human atheroma. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2814–2818. doi: 10.1073/pnas.89.7.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaguri Y., Yanagi H., Fukuda S., Nakano R., Morimatsu M. [Expression of oncogenes by smooth muscle cells in atherosclerotic lesions]. Rinsho Byori. 1990 Jan;38(1):19–25. [PubMed] [Google Scholar]
- Schwartz S. M., Heimark R. L., Majesky M. W. Developmental mechanisms underlying pathology of arteries. Physiol Rev. 1990 Oct;70(4):1177–1209. doi: 10.1152/physrev.1990.70.4.1177. [DOI] [PubMed] [Google Scholar]
- Sellar G. C., Whitehead A. S. Localization of four human serum amyloid A (SAA) protein superfamily genes to chromosome 11p: characterization of a fifth SAA-related gene sequence. Genomics. 1993 Jun;16(3):774–776. doi: 10.1006/geno.1993.1265. [DOI] [PubMed] [Google Scholar]
- Shor A., Kuo C. C., Patton D. L. Detection of Chlamydia pneumoniae in coronary arterial fatty streaks and atheromatous plaques. S Afr Med J. 1992 Sep;82(3):158–161. [PubMed] [Google Scholar]
- Sipe J. D., Colten H. R., Goldberger G., Edge M. D., Tack B. F., Cohen A. S., Whitehead A. S. Human serum amyloid A (SAA): biosynthesis and postsynthetic processing of preSAA and structural variants defined by complementary DNA. Biochemistry. 1985 Jun 4;24(12):2931–2936. doi: 10.1021/bi00333a018. [DOI] [PubMed] [Google Scholar]
- Stary H. C. The sequence of cell and matrix changes in atherosclerotic lesions of coronary arteries in the first forty years of life. Eur Heart J. 1990 Aug;11 (Suppl E):3–19. doi: 10.1093/eurheartj/11.suppl_e.3. [DOI] [PubMed] [Google Scholar]
- Steel D. M., Sellar G. C., Uhlar C. M., Simon S., DeBeer F. C., Whitehead A. S. A constitutively expressed serum amyloid A protein gene (SAA4) is closely linked to, and shares structural similarities with, an acute-phase serum amyloid A protein gene (SAA2). Genomics. 1993 May;16(2):447–454. doi: 10.1006/geno.1993.1209. [DOI] [PubMed] [Google Scholar]
- Steinkasserer A., Weiss E. H., Schwaeble W., Linke R. P. Heterogeneity of human serum amyloid A protein. Five different variants from one individual demonstrated by cDNA sequence analysis. Biochem J. 1990 May 15;268(1):187–193. doi: 10.1042/bj2680187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz A., Hocke G., Saïle R., Puchois P., Fruchart J. C. Influence of serum amyloid A on cholesterol esterification in human plasma. Biochim Biophys Acta. 1989 Nov 28;1006(2):173–178. doi: 10.1016/0005-2760(89)90192-6. [DOI] [PubMed] [Google Scholar]
- Tobias P. S., McAdam K. P., Ulevitch R. J. Interactions of bacterial lipopolysaccharide with acute-phase rabbit serum and isolation of two forms of rabbit serum amyloid A. J Immunol. 1982 Mar;128(3):1420–1427. [PubMed] [Google Scholar]
- Uhlar C. M., Burgess C. J., Sharp P. M., Whitehead A. S. Evolution of the serum amyloid A (SAA) protein superfamily. Genomics. 1994 Jan 15;19(2):228–235. doi: 10.1006/geno.1994.1052. [DOI] [PubMed] [Google Scholar]
- Urieli-Shoval S., Meek R. L., Hanson R. H., Ferguson M., Gordon D., Benditt E. P. Preservation of RNA for in situ hybridization: Carnoy's versus formaldehyde fixation. J Histochem Cytochem. 1992 Dec;40(12):1879–1885. doi: 10.1177/40.12.1280665. [DOI] [PubMed] [Google Scholar]
- Watson G., Coade S., Woo P. Analysis of the genomic and derived protein structure of a novel human serum amyloid A gene, SAA4. Scand J Immunol. 1992 Nov;36(5):703–712. doi: 10.1111/j.1365-3083.1992.tb03131.x. [DOI] [PubMed] [Google Scholar]
- Webb C. F., Tucker P. W., Dowton S. B. Expression and sequence analyses of serum amyloid A in the Syrian hamster. Biochemistry. 1989 May 30;28(11):4785–4790. doi: 10.1021/bi00437a040. [DOI] [PubMed] [Google Scholar]
- Whitehead A. S., de Beer M. C., Steel D. M., Rits M., Lelias J. M., Lane W. S., de Beer F. C. Identification of novel members of the serum amyloid A protein superfamily as constitutive apolipoproteins of high density lipoprotein. J Biol Chem. 1992 Feb 25;267(6):3862–3867. [PubMed] [Google Scholar]
- Yap S. H., Moshage H. J., Hazenberg B. P., Roelofs H. M., Bijzet J., Limburg P. C., Aarden L. A., van Rijswijk M. H. Tumor necrosis factor (TNF) inhibits interleukin (IL)-1 and/or IL-6 stimulated synthesis of C-reactive protein (CRP) and serum amyloid A (SAA) in primary cultures of human hepatocytes. Biochim Biophys Acta. 1991 Feb 19;1091(3):405–408. doi: 10.1016/0167-4889(91)90207-e. [DOI] [PubMed] [Google Scholar]
- Zimlichman S., Danon A., Nathan I., Mozes G., Shainkin-Kestenbaum R. Serum amyloid A, an acute phase protein, inhibits platelet activation. J Lab Clin Med. 1990 Aug;116(2):180–186. [PubMed] [Google Scholar]
- de Beer M. C., Beach C. M., Shedlofsky S. I., de Beer F. C. Identification of a novel serum amyloid A protein in BALB/c mice. Biochem J. 1991 Nov 15;280(Pt 1):45–49. doi: 10.1042/bj2800045. [DOI] [PMC free article] [PubMed] [Google Scholar]