Abstract
Underwater submergence produces autonomic changes that are observed in virtually all diving animals. This reflexly-induced response consists of apnea, a parasympathetically-induced bradycardia and a sympathetically-induced alteration of vascular resistance that maintains blood flow to the heart, brain and exercising muscles. While many of the metabolic and cardiorespiratory aspects of the diving response have been studied in marine animals, investigations of the central integrative aspects of this brainstem reflex have been relatively lacking. Because the physiology and neuroanatomy of the rat are well characterized, the rat can be used to help ascertain the central pathways of the mammalian diving response. Detailed instructions are provided on how to train rats to swim and voluntarily dive underwater through a 5 m long Plexiglas maze. Considerations regarding tank design and procedure room requirements are also given. The behavioral training is conducted in such a way as to reduce the stressfulness that could otherwise be associated with forced underwater submergence, thus minimizing activation of central stress pathways. The training procedures are not technically difficult, but they can be time-consuming. Since behavioral training of animals can only provide a model to be used with other experimental techniques, examples of how voluntarily diving rats have been used in conjunction with other physiological and neuroanatomical research techniques, and how the basic training procedures may need to be modified to accommodate these techniques, are also provided. These experiments show that voluntarily diving rats exhibit the same cardiorespiratory changes typically seen in other diving animals. The ease with which rats can be trained to voluntarily dive underwater, and the already available data from rats collected in other neurophysiological studies, makes voluntarily diving rats a good behavioral model to be used in studies investigating the central aspects of the mammalian diving response.
Keywords: Behavior, Issue 93, Rat, Rattus norvegicus, voluntary diving, diving response, diving reflex, autonomic reflex, central integration
Introduction
The diving response consists of a suite of autonomic reflexes seen in animals of all vertebrate classes 1. In response to submersion under water, this reflexly-induced response consists of apnea, bradycardia and an alteration of blood flow that maintains flow to the heart, brain and exercising muscles while limiting flow to viscera and non-exercising muscles 2. Many of the metabolic and cardiorespiratory aspects of the mammalian diving response have been well investigated 2,3, including those in humans 4,5. However, what has been relatively lacking, until recently, is investigation of the central integrative aspects of the diving response. What happens within the brainstem, and what is the neuronal step-by-step pathway, that connects afferent inputs to efferent outputs during this autonomic reflex? Answering these questions will require an appropriate animal model 6. An adage in comparative physiology, the Krogh principle 7, is that for every research question there is some animal of choice on which the problem can be most conveniently studied. A most appropriate animal for studying the central aspects of the diving response is the rat 6,8. In large part this is due to the fact that the brains of rats have been very well characterized, both anatomically and functionally, and many rat brain atlases are available 6. Additionally the rat is particularly useful in cardiorespiratory research, because the physiology of the rat is well known across all major organ systems, and the rat is well regarded as an animal model in systems biology 6. Finally, the laboratory rat is the domesticated version of the wild Rattus norvegicus, an animal that routinely swims and dives underwater 6. Based on these considerations, the rat is a good choice for studies investigating the central aspects of the mammalian diving response. In comparison, using marine animals to investigate the central aspects of the mammalian diving response would be much more difficult. This is due in large part to marine animals having comparatively large and non-uniformly sized brains, and the relative difficulty and high cost of housing these animals.
Rats have previously been used to investigate many aspects of the mammalian diving response, primarily in situations involving forced underwater submergence 9-12. However many studies in marine and aquatic animals have shown that there can be a differential response to diving based upon whether the submergence was forced or voluntary 2,13. Diving animals may show an extremely intense bradycardia during forced diving but a much less intense bradycardia during voluntary diving. The “stress” of forced submergence can significantly change the cardiovascular responses of diving in many animals 14. Small rodents such as muskrats also show a more intense diving response during forced submergence than during voluntary diving 15,16. Thus, if rats are to be used to investigate the central aspects of the mammalian diving response, investigators should be aware that a rat forcibly submerged underwater may produce a response different from that of a voluntarily diving rat.
The goal of this article is to provide detailed instructions on how to train rats to voluntarily dive underwater. These procedures are not technically difficult, but can be time-consuming. The training is conducted in such a way as to reduce the stressfulness that could otherwise be associated with forced underwater submergence. This voluntary diving technique should minimize activation of central stress pathways and thus better allow investigation of the central aspects of the diving response. By itself, training rats to voluntarily dive underwater generates no data that can be used to investigate the central aspects of the mammalian diving response. Therefore examples of how voluntarily diving rats have been used in conjunction with other physiological and neuroanatomical research techniques, and how the basic dive training may need to be modified to accommodate theses other techniques, are also provided.
Protocol
NOTE: Experimental protocols described herein conducted at Midwestern University were approved by the Midwestern University IACUC.
1. Room Requirements
Secure a procedure room that has running hot and cold running water, and a way to remove the water from the tank, typically a drain in the floor.
Use a study table on which to place the diving tank. Since rooms with floor drains are typically contoured for drainage, cut down rubber stoppers and place them under the table legs to ensure that the table top, and hence the water surface in the tank, is level.
2. Diving Tank
- Diving Tank Construction
- Construct a rectangular tank (100 x 60 x 15 cm) using 3/4 in thick Plexiglas for the bottom and 1/2 in Plexiglas for the side walls (Figure 1). Permanently attach the outside Plexiglas pieces of the tank together using cyanoacrylate cement or solvents such as trichloromethane.
- Divide the tank into 5 channels each approximately 100 cm in length, by using removable 1/2 inch thick Plexiglas cut into 85 x 15 cm pieces. Permanently place grooves in the bottom and edges of the tank into which these channel dividers can be slotted into position.
- Suspend horizontal Plexiglas pieces from the channel dividers to create a “roof” for an underwater dive “tunnel.
- In one corner of the tank place a removable raised platform, the “finish area”, for rats to haul out of the water and rest between swimming and diving trials. Rats usually groom themselves and remove water from their fur while in the finish area. Place tall sides around the edges of the finish area to insure the rats remain on the platform between training trials.
- At the opposite corner of the tank from the finish area place a chamber that only allows underwater access to the maze. This removable “start area” is only used for diving trials. NOTE: At the beginning of a diving trial a rat is lowered into the water while sitting on a platform within the start chamber; hence this platform is called the “elevator”. NOTE: Use a modular design, where the start area, finish area, and all channels dividers are removable from the tank. This is done for two reasons: 1) to facilitate filling and emptying the tank with water after removal of all channel dividers, and 2) if a rat gets disoriented, especially while underwater, the pieces can be quickly disassembled to rescue the rat from the water.
- Filling and emptying the tank with water
- Filling the Tank with water
- At the beginning of each training session, fill the tank with fresh 32 °C tap water to a depth of about 12 cm. Stir the water in the tank to minimize warm or cool pockets. NOTE: Typically it takes about 2 hr to train 12 rats. During this time water temperature decreases by about 4 °C. By the end of the training session the water cools to approximately 28 °C. Since 30 °C water is thermoneutral to rats, using this warm water, rather than room temperature water, will minimize heat loss during repetitive training.
- Emptying the Tank with water
- At the end of each training session, drain the water from the tank using a large hose, such as a 4 m long piece of 1 inch internal diameter tubing. Completely submerge the tubing in the tank, ensuring no air pockets are in the tubing. Quickly place one end of the tubing into the floor drain. The resulting siphoning effect will drain the water from the tank.
- While keeping the tank end of the tubing under the water surface to maintain the siphoning, tip the tank on edge to facilitate complete drainage of the water. NOTE: Alternatively, and rather than siphoning, install a tap near the base of the tank, and connect a hose from the tank tap to the floor drain.
3. Rat Allergen Considerations
Wear disposable gloves whenever handling and training the rats. The gloves will limit contact with the water in the tank, which invariably will contain rat urine. NOTE: The rats will become wet during the training, and the room will be filled with a “wet-rat” odor. Consequently a prophylactic regime of anti-histamines may prevent or limit the effects of rat allergies. Trainers may also need to wear disposable dust masks, or N95 masks, should they become sensitive to rat allergens.
4. Swim Training
- Daily swim training NOTE: Personal experience indicates that younger rats learn the maze better and faster, and thus starting the training with 35 g newly weaned rats is preferable to using adult rats.
- Fill tank with water. See 2.2.1.
- Insert finish area and channel dividers to create 5 swimming channels.
- During the first training session gently lower the rats by hand into the water about 3-5 cm from the finish area. NOTE: Upon their introduction to water rats will appear unsteady as they first encounter the sensation of floating. The rats will paddle about in an uncoordinated fashion while trying to locate a way to exit the water, eventually reaching the nearby finish area. Due to their innate ability to swim, in subsequent trials the rats will swim in a much more coordinated fashion toward the finish area. NOTE: Support the rats from underneath so their feet are on the investigators hands. Gently lower the rats into the water, and let them swim away from the hand, rather than dropping them into the water.
- Between trials let the rats remain in the finish area for at least 1 min. This is to allow the rats to groom and explore, so that they regard the finish area as a “safe” place to go between trials. This is in contrast to grabbing a rat that has just completed a trial and immediately positioning it back in the water to start the next trial. NOTE: Do not use external reward (i.e., food) during the training, so as to prevent activation of reward circuits in the brain.
- After the finish area wait period, gently hold the rats for 1 min before initiating the next trial. Gently dry the rats with a towel during this pre-trial hold since repetitive entry into the water will cause rats to get very wet, and drying them will prevent hypothermia.
- Repeat 4.1.3. through 4.1.5. to complete 3 to 5 trials for each rat to be trained during a daily swim training session.
- Remove all channel dividers and finish area from the tank.
- Empty the water from the tank. See 2.2.2.
- Weekly swim training schedule
- Conduct daily training sessions (see 4.1.) 5 days per week, ideally at the same time each day.
- Begin the first trial from the distance successfully negotiated on the previous day. Begin the second and third trials from an increased distance.
- With each subsequent daily training session increase the distance between where the rats are placed in the water and the finish area. For the first few training sessions increase this distance by 5-10 cm. After the rats appear more comfortable with swimming, increase the distances by 30-50 cm.
- Use larger Increases in the swimming distances while the rats are learning to swim a straight portion of a swim channel. In contrast, learning to swim the hairpin turns between channels might take 2-3 training sessions. NOTE: Often, while waiting in the finish area, rats will re-enter the water to swim, and/or submerge their head underwater while still sitting on the finish area platform.
- Repeat this daily training protocol over 3 weeks to ensure successful swimming of the entire 5 channels. This repetitious training protocol ensures successful completion of the maze, especially since the maze includes alternating left and right hairpin turns.
5. Dive Training
NOTE: After the rats have learned to successfully negotiate swimming through the maze they are ready to start dive training.
- Initial dive training (first day)
- Insert start chamber. Fill tank with water (see 2.2.1.). Ensure water level is 1 cm below the opening to the start area.
- Insert finish area and channel dividers to create 5 swimming channels.
- During the first diving session train the rats to be lowered on the elevator into the water within the start chamber. Access to the maze is from the bottom of the start chamber. During this first session the water level is low enough so the rat can easily swim from the start chamber into the maze. Allow the rat to continue swimming through the maze to the finish area.
- Between trials let the rats remain in the finish area for at least 1 min. NOTE: Do not use external reward (i.e., food) during the training, so as to prevent activation of reward circuits in the brain.
- After the finish area wait period, gently hold the rats for 1 min before initiating the next trial. Gently dry the rats with a towel during this pre-trial hold.
- Repeat 5.1.3. through 5.1.5. to complete 3 trials for each rat to be trained during this initial dive training session.
- Remove all channel dividers, start chamber and finish area from the tank.
- Empty the water from the tank. See 2.2.2.
- Initial dive training (second day)
- Insert start chamber. Fill tank with water (see 2.2.1.). Ensure water level is slightly above the opening to the start area.
- Insert finish area and channel dividers to create 5 swimming channels.
- During the second dive training session the water level has been raised so that to exit the start chamber the rat has to dip its head under the edge of the start chamber to enter the maze. Consider this as the rat’s first dive. Allow the rat to then continue swimming through the maze to the finish area.
- Let the rats remain in the finish area for at least 1 min between trials. NOTE: Do not use external reward (i.e., food) during the training, so as to prevent activation of reward circuits in the brain.
- After the finish area wait period, gently hold the rats for 1 min before initiating the next trial. Gently dry the rats with a towel during this pre-trial hold.
- Repeat 5.2.3. through 5.2.5. to complete 3 trials for each rat to be trained during this dive training session.
- Remove all channel dividers, start chamber and finish area from the tank.
- Empty the water from the tank. See 2.2.2.
- Initial dive training (third day)
- Insert start chamber. Fill tank with water (see 2.2.1.), ensuring that the water level is above the opening to the start area.
- Insert finish area and channel dividers to create 5 swimming channels. Place a horizontal piece of Plexiglas immediately outside of the start area to create a 5 cm long diving tunnel.
- During the third dive training session the rat has to dip its head under the edge of the start chamber and swim 5 cm underwater to reach the open water of the swim channel. Allow the rat to then continue swimming through the maze to the finish area. NOTE: Once placed in the start area, rats initiate their own underwater submergence, and thus these are considered to be “voluntary” dives.
- Let the rats remain in the finish area for at least 1 min between trials. NOTE: Do not use external reward (i.e., food) during the training, so as to prevent activation of reward circuits in the brain.
- After the finish area wait period, gently hold the rats for 1 min before initiating the next trial. Gently dry the rats with a towel during this pre-trial hold.
- Repeat 5.3.3. through 5.3.5. to complete 3 to 5 trials for each rat to be trained during this dive training session.
- Remove all channel dividers, start chamber and finish area from the tank.
- Empty the water from the tank. See 2.2.2.
- Weekly dive training schedule
- Conduct daily training sessions (see 5.3.) 5 days per week, ideally at the same time each day.
- Begin the first trial from the distance successfully negotiated on the previous day. Begin the second and third trials with an increased diving distance.
- With each subsequent daily training session increase the length of the dive tunnel by adding additional horizontal dividers to extend the distance the rats have to swim underwater. For the first few training sessions increase this distance by 5-10 cm. After the rats appear more comfortable with diving, increase the distances by 30-50 cm. NOTE: Do not overextend the dive distance attempted during successive trials. If necessary, lift up the end of the horizontal channel cover to provide shorter dive distances. If during a dive trial a rat does not reach the end of the dive tunnel and starts to turn around underwater, quickly lift up the end of the channel cover and allow the rat to surface and continue his swim. This will enable the rat to successfully complete a longer dive distance. This positive reinforcement will keep the rat moving forward through the underwater tunnel with each dive trial.
- Use larger Increases in the diving distances while the rats are learning to dive a straight portion of a swim channel. In contrast, learning to dive around the hairpin turns between channels might take 2-3 training sessions. NOTE: Often, while waiting in the finish area, rats will re-enter the water to swim, and/or submerge their head underwater while still sitting on the finish area platform.
- Repeat this daily training protocol over 3 weeks to ensure successful diving of the entire 5 channels. This repetitious training protocol ensures successful completion of the maze, especially since the maze includes alternating left and right hairpin turns. NOTE: The full 6 week swim and dive training schedule coincides with the growth of 35 g newly weaned rats to reach a body weight of 300 g.
- After daily training sessions are complete, and the rats have been returned to their home cage, ensure rats quickly dry their fur through grooming, or if necessary, place a heating pad under their cage to keep the rats warm until their fur is dry.
6. Experimental Variations
NOTE: The basic experimental set-up and animal training have been described above. However, behavioral training only provides a model to be used with other experimental techniques to collect data of interest. Basic protocols are modified to investigate specific aspects of the diving response. Examples of these modifications, and some considerations for the collection of data using these physiological and neuroanatomical techniques, are given below.
- Implantable Telemetric Transmitters
- After completion of the training, use commercially available telemetric transmitters to transmit pulsatile arterial blood pressure from swimming and diving rats. Obtain local IACUC approval for procedures concerning surgery and post-surgical recovery. Follow the implantation procedures suggested by the company for their transmitter, and ensure complete recovery from the surgery before returning the rat into the water.
- Ensure the antenna receiving the radio signal is nearby when the rat is in the water. NOTE: Water attenuates radio signals, and so the distance the radio signal needs to travel through the water becomes a limiting factor of the tank dimensions.
- Use a hand-held antenna wand, rather than a rat-cage sized antenna, to follow the rat as it progresses through the maze. Keep the wand antenna within 30 cm of the rat to ensure that the radio signal is not lost while the rat is underwater.
- Trailing Cannulae
- Redesign the channel dividers and horizontal pieces creating the roof of the diving tunnel so that the cannula trails along behind the rat during its progression through the maze. NOTE: The modular design of the channels is of utmost importance and must also allow quick recover of the rat from the underwater maze. If a cannula snags and became dislodged from the rat, the rat could soon bleed out while underwater if the cannula is not quickly reattached.
- Use trailing arterial cannulae to record arterial blood pressure and heart rate in voluntarily diving rats. Use a 90 cm piece of PE50 as a trailing cannula. NOTE: This cannula length is long enough to connect the rat to the pressure transducer while still allowing the rat to progress through the maze, yet is short enough to minimize cannula dead space and is close enough to the pressure transducer to allow sufficient fidelity of the arterial pressure signal.
- Use trailing venous (or arterial) cannulae to inject pharmaceutical agents such as parasympathetic and sympathetic agonists and antagonists, or to inject tracers or dyes that determine the distribution of cardiac output.
- Withdraw blood samples from the rats using the venous (or arterial) cannulae while the rats are underwater to determine underwater catecholamine levels or blood chemistry. NOTE: Minimize the length of the trailing cannula and account for the cannula dead space during blood draws.
- Detection of Activated Brainstem Neurons
- Use immunological detection of the Fos protein to identify specific areas of the brainstem that are part of the diving response. NOTE: During repetitive initiation of a cardiorespiratory reflex, brainstem neurons that are part of that reflex circuit can become activated and produce a protein called Fos.
- Repetitively dive rats through the maze every 5 min for 2 hr for a total of 24 dives. NOTE: Other protocols that also induce neuronal Fos production can also be used. To avoid activation of brainstem neurons involved in the response to stress, the behavior that is repeated should be included as part of the behavioral training.
- Blood Corticosterone Levels during Diving
- Use corticosterone as an indicator of the level of stress rats experience during swimming and diving.
- To obtain blood for corticosterone analysis, draw 0.1 ml blood samples from the tail vein of rats 15 min after 3 voluntary swims or dives. NOTE: Preliminary experiments indicated that 15 min is sufficient time to allow for the production and release of corticosterone into the circulation and cause a peak in plasma corticosterone levels. Since corticosterone levels have a circadian rhythm, schedule all blood draws at the same time of day and corresponded with the timing of training sessions.
Representative Results
Successful completion of the described swimming and diving training procedures can decrease the stress experienced by rats when diving under water. Blood corticosterone levels indicate that repetitive daily training decreases the stressfulness associated with voluntary diving, and trained rats find diving no more stressful than being handled daily by a human (Figure 2; 17). Conversely, rats not trained in the diving protocol find voluntary diving stressful (Figure 2; 17). Additionally, both trained and untrained rats find forced diving to be the most stressful (Figure 2; 17).
The cardiovascular responses from swimming, voluntary diving and forced diving rats have been recorded using implanted telemetry devices (Figure 3; 8,17-20) and trailing cannulae (Figure 4; 21-23). Immediately upon voluntary submersion, and within a single beat, heart rate decreases by 78% and mean arterial blood pressure decreases by 25% 17. These results show that voluntarily diving rats exhibit the same cardiorespiratory changes typically seen in other diving animals. Trailing arterial cannulae have been used to inject the muscarinic antagonist atropine, which eliminates the bradycardia associated with voluntary diving (Figure 4; 21), and to determine the distribution of cardiac output 22, including cerebral blood flow 23, during voluntary diving. Trailing cannulae have also been used to show that rats ignore increasing arterial hypoxemia and hypercapnia while they are submerged 18, and that pre-existing chemoreceptor drive does not have any effect on the cardiovascular responses to voluntary diving 21.
Neurons within laminae I and II of the ventral medullary dorsal horn (MDH) express Fos during voluntary diving, and these neurons may constitute the initial brainstem afferent relay of the diving response (Figure 5; 24). Important brainstem cardiorespiratory control areas, such as the caudal pressor area (CPA), nucleus tractus solitaries (NTS), rostral ventrolateral medulla (RVLM), and peribrachial regions, all show increased Fos labeling during voluntary diving compared with swimming 25. Neurons in chemosensitive regions of the brainstem express Fos after long duration forced dives 18.
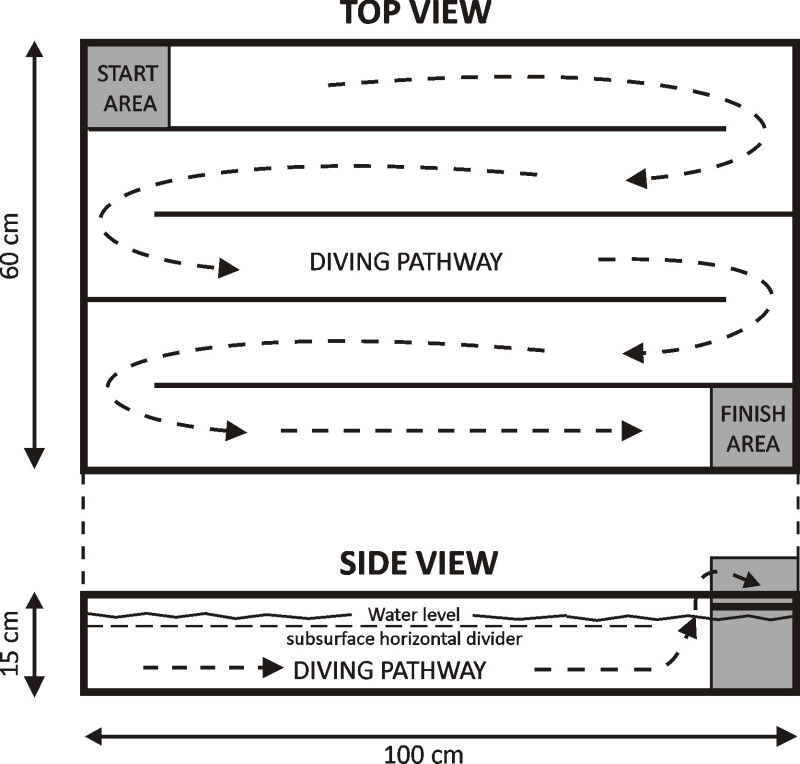 Figure 1:Schematic of Diving Tank. A Plexiglas tank (100 x 60 x 15 cm) was used to create a simple maze consisting of five 1 m long channels. The tank was filled with 30 °C tap water, and rats were initially trained to negotiate the maze by swimming on the surface of the water, from the Start Area (top left) to the Finish Area (bottom right). The rats were then trained to dive through the maze, kept underwater by horizontal Plexiglas pieces placed 2-3 cm below the water surface. [This figure has been modified from 26]
Figure 1:Schematic of Diving Tank. A Plexiglas tank (100 x 60 x 15 cm) was used to create a simple maze consisting of five 1 m long channels. The tank was filled with 30 °C tap water, and rats were initially trained to negotiate the maze by swimming on the surface of the water, from the Start Area (top left) to the Finish Area (bottom right). The rats were then trained to dive through the maze, kept underwater by horizontal Plexiglas pieces placed 2-3 cm below the water surface. [This figure has been modified from 26]
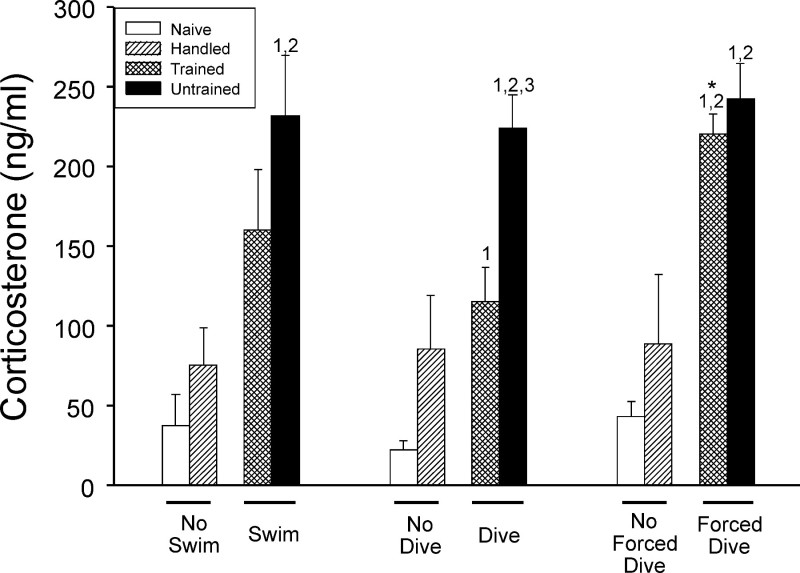 Figure 2:Corticosterone measurements. Blood draws from rat tail veins were used to measure corticosterone concentrations (mean ± SE) from rats left in their cages (Naïve), rats handled for 10 min/day (Handled), rats trained to swim and dive (Trained), and rats that received no swim or dive training (Untrained). Corticosterone was measured after trained rats had completed their swim training (Left set of bars), after trained rats had completed their voluntary dive training (Center set of bars), and after trained rats had completed their forced dive training (Right set of bars). 1 indicates value is significantly greater than Naïve; 2 indicates value is significantly greater than Handled; 3 indicates value is significantly greater than Trained; * indicates that in Trained rats value during forced dive is significantly greater than during voluntary dive. [This figure has been modified from 17]
Figure 2:Corticosterone measurements. Blood draws from rat tail veins were used to measure corticosterone concentrations (mean ± SE) from rats left in their cages (Naïve), rats handled for 10 min/day (Handled), rats trained to swim and dive (Trained), and rats that received no swim or dive training (Untrained). Corticosterone was measured after trained rats had completed their swim training (Left set of bars), after trained rats had completed their voluntary dive training (Center set of bars), and after trained rats had completed their forced dive training (Right set of bars). 1 indicates value is significantly greater than Naïve; 2 indicates value is significantly greater than Handled; 3 indicates value is significantly greater than Trained; * indicates that in Trained rats value during forced dive is significantly greater than during voluntary dive. [This figure has been modified from 17]
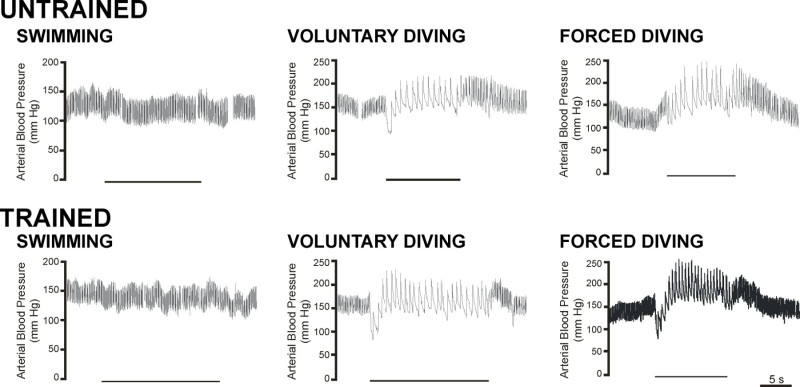 Figure 3:Arterial blood pressure traces from telemetric transmitters. Raw data traces showing pulsatile arterial blood pressure during swimming (left column), voluntary diving (middle column), and forced diving (right column) from rats trained to swim and dive through the maze (bottom row) and from rats that had not had the training procedure (top row). Diving underwater (both voluntary and forced submergence) produced an immediate bradycardia and slower onset increase in arterial pressure, whereas swimming on the surface of the water caused no such cardiovascular changes. Bars under traces indicate periods of submergence. Breaks in trace indicate periods when the telemetric signal was lost. [This figure has been modified from 17]
Figure 3:Arterial blood pressure traces from telemetric transmitters. Raw data traces showing pulsatile arterial blood pressure during swimming (left column), voluntary diving (middle column), and forced diving (right column) from rats trained to swim and dive through the maze (bottom row) and from rats that had not had the training procedure (top row). Diving underwater (both voluntary and forced submergence) produced an immediate bradycardia and slower onset increase in arterial pressure, whereas swimming on the surface of the water caused no such cardiovascular changes. Bars under traces indicate periods of submergence. Breaks in trace indicate periods when the telemetric signal was lost. [This figure has been modified from 17]
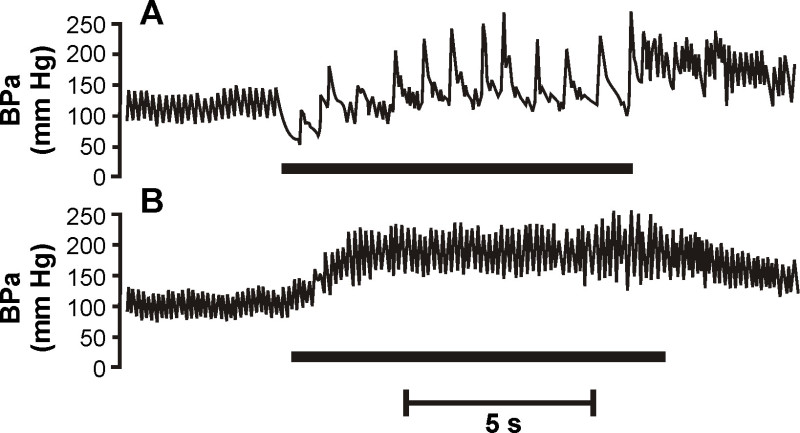 Figure 4:Atropine eliminates diving bradycardia. Original recordings of pulsatile arterial blood pressure of voluntarily diving rats (A) before and (B) after atropine pre-treatment. Traces were obtained using a trailing arterial cannula. Before atropine pre-treatment, arterial pressure decreased slightly upon submersion, but then increased to greater than pre-dive for the remainder of the dive. Heart rate was determined from adjacent pulse pressure intervals. Upon submersion there was an immediate and substantial bradycardia that was sustained for the duration of the dive. After parasympathetic blockade by atropine pre-treatment the bradycardia was eliminated. There was also an increase in arterial pressure during the dive. The bar under the trace indicates the period of submergence. [McCulloch, unpublished]
Figure 4:Atropine eliminates diving bradycardia. Original recordings of pulsatile arterial blood pressure of voluntarily diving rats (A) before and (B) after atropine pre-treatment. Traces were obtained using a trailing arterial cannula. Before atropine pre-treatment, arterial pressure decreased slightly upon submersion, but then increased to greater than pre-dive for the remainder of the dive. Heart rate was determined from adjacent pulse pressure intervals. Upon submersion there was an immediate and substantial bradycardia that was sustained for the duration of the dive. After parasympathetic blockade by atropine pre-treatment the bradycardia was eliminated. There was also an increase in arterial pressure during the dive. The bar under the trace indicates the period of submergence. [McCulloch, unpublished]
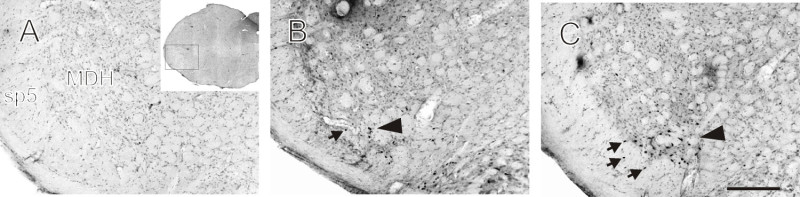 Figure 5: Fos labeling within the MDH. Photomicrographs of the trigeminal medullary dorsal horn (MDH) and the spinal trigeminal tract (sp5) in rats trained to dive underwater. (A) In a control rat that did not repetitively dive there is no Fos labeling. (B) In a swimming rat there is very little Fos label in the MDH (large arrowhead) or paratrigeminal nucleus (small arrow) within sp5. (C) In a diving rat there is more Fos labeling ventrally in both the MDH (large arrowhead) and paratrigeminal nucleus (small arrows) compared to the swimming and control rat. Insert in panel (A) indicates the rostral-caudal location of panels A-C. Scale bar in panel C is 100 μm. [This figure has been modified from 24]
Figure 5: Fos labeling within the MDH. Photomicrographs of the trigeminal medullary dorsal horn (MDH) and the spinal trigeminal tract (sp5) in rats trained to dive underwater. (A) In a control rat that did not repetitively dive there is no Fos labeling. (B) In a swimming rat there is very little Fos label in the MDH (large arrowhead) or paratrigeminal nucleus (small arrow) within sp5. (C) In a diving rat there is more Fos labeling ventrally in both the MDH (large arrowhead) and paratrigeminal nucleus (small arrows) compared to the swimming and control rat. Insert in panel (A) indicates the rostral-caudal location of panels A-C. Scale bar in panel C is 100 μm. [This figure has been modified from 24]
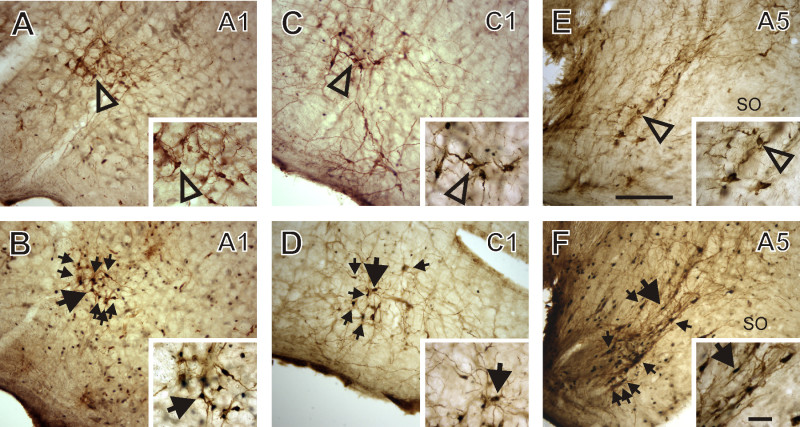 Figure 6: Activated catecholaminergic neurons from diving rats. Photomicrographs show the medullas of a non-diving control rat (A, C, and E) and a voluntarily diving rat (B, D, and F). The brain tissue was immunohistologically processed for both Fos and tyrosine hydroxylase (TH), producing brown TH somas and black Fos nuclei. Open arrowheads identify single-labeled TH-positive neurons, while solid arrows identify Fos+TH double-labeled neurons. A1 neurons are identified in A and B. C1 neurons are identified in C and D. A5 neurons are identified in E and F. More Fos and TH double-labeled are seen in the A1, C1, and A5 regions of the diving rat than in the non-diving control rat. Calibration bar in E is for panels A-F, and is 250 μm. Calibration bar in inset in F is for all insets, and is 50 μm. [This figure has been modified from 26]
Figure 6: Activated catecholaminergic neurons from diving rats. Photomicrographs show the medullas of a non-diving control rat (A, C, and E) and a voluntarily diving rat (B, D, and F). The brain tissue was immunohistologically processed for both Fos and tyrosine hydroxylase (TH), producing brown TH somas and black Fos nuclei. Open arrowheads identify single-labeled TH-positive neurons, while solid arrows identify Fos+TH double-labeled neurons. A1 neurons are identified in A and B. C1 neurons are identified in C and D. A5 neurons are identified in E and F. More Fos and TH double-labeled are seen in the A1, C1, and A5 regions of the diving rat than in the non-diving control rat. Calibration bar in E is for panels A-F, and is 250 μm. Calibration bar in inset in F is for all insets, and is 50 μm. [This figure has been modified from 26]
Discussion
Rats in their feral form can and do exploit semi-aquatic environments, and will often dive underwater while foraging for food 6. Thus it is not too surprising that rats can be very easily trained to voluntarily dive underwater. The described training procedures may last up to 6 weeks, which will bring newly weaned rats to a body size used in most adult rat brain atlases (~300 g). Thus the brains from these trained animals will be more readily comparable to the anatomical structures identified in these atlases.
After being placed in the start area most rats will begin their underwater swim within 20 sec. However, occasionally a rat will take up to 5 min or more before initiating its voluntary dive. While it may be tempting at this stage to force the rats into the underwater tunnel, this generally should be avoided to prevent the rats from associating the water with a negative experience. Rodents can be stubborn and may initially refuse to dive during the training sessions, but once they realize the only way to exit the water is by completing the dive through the maze, they usually initiate their dives soon after being placed in the start area.
A critical aspect of the repetitious and methodical training procedures is that they are conducted in such a way to reduce the stress experienced by the rats. Letting rats explore their environment, especially while in the finishing area between trials, seems to further reduce stress. Rats will often re-enter the water to swim, and/or submerge their head underwater while still sitting on the finish area platform. This suggests that rats are not inherently water aversive. Additionally, while it is not uncommon for rats to produce fecal pellets during swimming or diving, or when waiting in the finish area, daily training results in fewer fecal pellets 17. In general the less stressed the rats are during the training, the fewer pellets they will produce. Any fecal pellets that are produced are removed from the water or finish area as soon as possible to keep the water relatively clean.
Rats may occasionally get bloody noses while swimming and diving, which may be a result of insufflation of water into the nasal passages. The appearance of blood may be due to osmotic stresses within the nasal mucosa. While in the finish area between trials the rats will groom themselves. As a consequence blood from the nose can get redistributed over the rats head and snout, giving the rats a slight reddish tinge, especially around the eyes, during the course of a training session. Also, an occasional rat may find diving stressful and/or have negative diving experiences (i.e., by turning around and getting lost while diving underwater (see note after 5.4.3. on how to prevent this from occurring)). In these rats porphyrin may appear in the corners of their eyes, signaling a stress response.
The size of the diving tank will to some degree determine the room requirements. The described tank is designed to have rats swim 2-3 cm under the surface of the water through a 5 m long Plexiglas maze to give an underwater swimming duration of 10-15 sec 17,19,24,26,27. Should an experiment be designed to measure responses from a longer duration dive, or from a deeper dive underwater, the tank may need to be re-designed. The room requirements could then also change to fit the dimensions of a re-designed diving tank. If there is no floor drain available in the procedure room, the water from the tank can be collected in a large container, such as a 60 gal garbage can, which can then be emptied elsewhere in a convenient manner.
The Fos technique can be utilized with other neuronal detection methods to further identify and characterize neurons that are part of the brainstem circuitry of the diving response. For instance, Fos detection in conjunction with tyrosine hydroxylase staining has identified catecholaminergic neurons in the A1, C1, A2, A5 and sub-coeruleus areas (Figure 6; 26), and globosa neurons within the lateral A7 area 26,27, that are activated during voluntary diving. Also, Fos detection in conjunction with the retrograde tracer Cholera toxin has identified the cell bodies of cardiac vagal motorneurons within the external formation of the nucleus ambiguus that are activated during voluntary diving 20.
Investigating the central nervous integration of the cardiorespiratory responses to diving is important for a number of reasons 6,8,28. The diving response enables animals, including humans, to remain submerged underwater without breathing for extended periods of time. The diving response represents a functional reorganization of brainstem homeostatic control, and demonstrates one of the most powerful patterns of autonomic reflexes observed in animals. The diving response may also be important clinically in humans as part of the trigemino-cardiac reflex, nasopharyngeal reflex, and/or sudden infant death syndrome. Finally, an understanding of the neuronal circuitry that exists within the brainstem of rats will help determine how cortical afferent signals can modify basic brainstem autonomic reflexes. All of these considerations make study of the central aspects of the mammalian diving response inherently worthwhile and interesting. Using the described procedures to train rats to voluntarily dive underwater will allow better investigation of the central aspects of the mammalian diving response than will the use of forced dived animals. This is because the training procedures as described 1) reduce activation of CNS stress circuitry, and 2) do not activate CNS reward circuitry because external rewards are not used.
Disclosures
The author is a consultant for Stoelting Company, and provided the overall design and specifications of the McCulloch Dive Tank Maze to them for commercial purposes.
Acknowledgments
Research supported by funding from the Midwestern University Office of Research and Sponsored Programs. Thanks also to the Midwestern University Animal Facility and Erik Warren.
References
- Butler PJ, Jones DR. In: Advances in Comparative Physiology and Biochemistry. Lowenstein O, editor. Vol. 8. Academic Press; 1982. pp. 179–364. [DOI] [PubMed] [Google Scholar]
- Butler PJ, Jones DR. Physiology of diving birds and mammals) Physiol. Rev. 1997;77:837–899. doi: 10.1152/physrev.1997.77.3.837. [DOI] [PubMed] [Google Scholar]
- Butler PJ. Metabolic regulation in diving birds and mammals. Resp. Physiol. Neurobiol. 2004;141:297–315. doi: 10.1016/j.resp.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Foster GE, Sheel AW. The human diving response, its function, and its control. Scand. J. Med. Sci. Sports. 2005;15:3–12. doi: 10.1111/j.1600-0838.2005.00440.x. [DOI] [PubMed] [Google Scholar]
- Lindholm P, Lundgren CEG. The physiology and pathophysiology of human breath-hold diving. J. Appl. Physiol. 2009;106:284–292. doi: 10.1152/japplphysiol.90991.2008. [DOI] [PubMed] [Google Scholar]
- McCulloch PF. Animal models for investigating the central control of the mammalian diving response. Front. Physiol. 2012;3:1–16. doi: 10.3389/fphys.2012.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A. The progress of physiology. Am. J. Physiol. 1929;90:243–251. [Google Scholar]
- Panneton WM, Gan Q, Juric R. The rat: a laboratory model for studies of the diving response. J. Appl. Physiol. 2010;108:811–820. doi: 10.1152/japplphysiol.00600.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC. Autonomic nervous control of cardiovascular responses during diving in the rat. Am. J. Physiol. 1974;227:601–605. doi: 10.1152/ajplegacy.1974.227.3.601. [DOI] [PubMed] [Google Scholar]
- Lin YC, Baker DG. Cardiac output and its distribution during diving in the rat. Am. J. Physiol. 1975;228:733–737. doi: 10.1152/ajplegacy.1975.228.3.733. [DOI] [PubMed] [Google Scholar]
- Huang TF, Peng YI. Role of the chemoreceptors in diving bradycardia in the rat. Jap. J. Physiol. 1976;26:395–401. doi: 10.2170/jjphysiol.26.395. [DOI] [PubMed] [Google Scholar]
- Fahlman A, Bostrom BL, Dillon KH, Jones DR. The genetic component of the forced diving bradycardia response in mammals. Front. Physiol. 2011;2:1–7. doi: 10.3389/fphys.2011.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blix AS, Folkow B. In: Handbook of Physiology. Shepher JT, Abboud FM, editors. American Physiological Society; 1984. pp. 917–944. [Google Scholar]
- Kooyman GL. Vol. 200. Springer-Verlag; 1989. Diverse Divers. [Google Scholar]
- MacArthur RA, Karpan CM. Heart rates of muskrats diving under simulated field conditions: persistence of the bradycardia response and factors modifying its expression. Can. J. Zool. 1989;67:1783–1792. [Google Scholar]
- McCulloch PF, Jones DR. Cortical influences on diving bradycardia in muskrats (Ondatra zibethicus) Physiol. Zool. 1990;63:1098–1117. [Google Scholar]
- McCulloch PF, Dinovo KM, Connolly TM. The cardiovascular and endocrine responses to voluntary and forced diving in trained and untrained rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:224–234. doi: 10.1152/ajpregu.00592.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneton WM, Gan Q, Dahms TE. Cardiorespiratory and neural consequences of rats brought past their aerobic dive limit. J. Appl. Physiol. 2010;109:1256–1269. doi: 10.1152/japplphysiol.00110.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotiyanonta JS, DiNovo KM, McCulloch PF. Bilateral sectioning of the anterior ethmoidal nerves does not eliminate the diving response in voluntarily diving rats. Physiol. Reports. 2013;1 doi: 10.1002/phy2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneton WM, Anch AM, Panneton WM, Gan Q. Parasympathetic preganglionic cardiac motoneurons labeled after voluntary diving. Front. Physiol. 2014;5:1–10. doi: 10.3389/fphys.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch PF, Ollenberger GP, Bekar LK, West NH. Trigeminal and chemoreceptor contributions to bradycardia during voluntary dives in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1997;273:814–822. doi: 10.1152/ajpregu.1997.273.2.R814. [DOI] [PubMed] [Google Scholar]
- Ollenberger GP, Matte G, Wilkinson AA, West NH. Relative distribution of blood flow in rats during surface and submerged swimming. Comp. Biochem. Physiol. A. 1998;119:271–277. doi: 10.1016/s1095-6433(97)00427-3. [DOI] [PubMed] [Google Scholar]
- Ollenberger GP, West NH. Distribution of regional cerebral blood flow in voluntarily diving rats. J. Exp. Biol. 1998;201:549–558. doi: 10.1242/jeb.201.4.549. [DOI] [PubMed] [Google Scholar]
- McCulloch PF. Activation of the trigeminal medullary dorsal horn during voluntary diving in rats. Brain Res. 2005;1051:194–198. doi: 10.1016/j.brainres.2005.05.059. [DOI] [PubMed] [Google Scholar]
- Panneton WM, et al. Activation of brainstem neurons by underwater diving in the rat. Front. Physiol. 2012;3:1–13. doi: 10.3389/fphys.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch PF, Panneton WM. Activation of brainstem catecholaminergic neurons during voluntary diving in rats. Brain Res. 2003;984:42–53. doi: 10.1016/s0006-8993(03)03051-8. [DOI] [PubMed] [Google Scholar]
- McCulloch PF. Globosa neurons: a distinct subgroup of noradrenergic neurons in the caudal pons of rats. Brain Res. 2003;964:164–167. doi: 10.1016/s0006-8993(02)04156-2. [DOI] [PubMed] [Google Scholar]
- Panneton WM. The mammalian diving response: an enigmatic reflex to preserve life. Physiology. 2013;28:284–297. doi: 10.1152/physiol.00020.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


