Abstract
Background:
Early pleural tuberculosis (TB) diagnosis is particularly difficult. The aim of this study was to investigate the diagnostic accuracy of the Xpert MTB/RIF (Xpert) (Cepheid, Sunnyvale, CA) assay using pleural biopsy and pleural fluid specimens in patients with suspected pleural TB but who had a negative sputum acid-fast bacilli (AFB) smear.
Materials and Methods:
In this study, 134 sputum smear-negative suspected pleural TB patients were selected. Paired pleural fluid and pleural biopsy specimens were tested for Mycobacterium tuberculosis by standard smear-microscopy, Lowenstein-Jensen and mycobacterial growth indicator tube (MGIT) culture, and the Xpert assay. Mycobacterial culture from pleural biopsy specimens was used as a reference standard for sensitivity and specificity calculations. Detection of rifampicin resistance was compared with the MGIT method.
Results:
Of 126 evaluable patients, 55 received a diagnosis of pleural TB. The sensitivity of the Xpert assay using pleural biopsy specimens for the diagnosis of pleural TB was 85.5%, and specificity was 97.2%. The sensitivity and specificity of the Xpert assay in pleural fluid were 43.6% and 98.6%, respectively. The Xpert assay correctly identified 90.0% of phenotypic rifampicin-resistant cases and 93.9% of phenotypic rifampicin-susceptible cases.
Conclusion:
The Xpert assay on pleural biopsy specimens may provide an accurate diagnosis of pleural TB in patients who had a negative AFB smear.
Keywords: Diagnosis, pleural tuberculosis, Xpert MTB/RIF assay
INTRODUCTION
Tuberculosis (TB) is a major health problem worldwide. In 2011, approximately 8.7 million people had active TB, and 1.4 million people died from the disease.[1] Pleural TB is the second most common extrapulmonary manifestation of active Mycobacterium tuberculosis infection after lymph node TB. Diagnosis of pleural TB relies on the examination of pleural fluid and/or biopsy specimens using acid-fast microscopic examination, culture, polymerase chain reaction, evaluation of pleural fluid characteristics, and/or histopathological examination. However, these methodologies are associated with unsatisfactory sensitivity, specificity and time to diagnosis.[2,3,4,5] There is a great need for a rapid and sensitive method to diagnose pleural TB in patients who have negative findings of the examination of a sputum smear specimen.
In December 2010, the World Health Organization (WHO) endorsed the GeneXpert MTB/RIF assay (Cepheid, Sunnyvale, CA) for the rapid diagnosis of TB and multidrug-resistant TB (MDR-TB).[6] The Xpert assay is capable of detecting the M. tuberculosis while simultaneously detecting rifampicin resistance in <2 h. Several studies have assessed the Xpert assay in pulmonary TB and a meta-analysis of 16 studies gave a pooled sensitivity of 90% and a pooled specificity of 98%.[7,8,9,10,11,12,13] However, these studies have not been performed in China, a second TB-burden country in the world. More recently, evaluations of the assay have extended to a variety of nonrespiratory clinical samples from patients with extrapulmonary TB, and the specificities and sensitivities are variable.[14,15,16,17,18] Pleural fluid and/or biopsy specimens are regarded as a useful tool in the diagnosis of pleural TB, as it obtains respiratory specimens in patients with negative sputum acid-fast bacilli (AFB) smear. However, the application of the Xpert assay in pleural biopsy and pleural fluid specimens has not yet been fully evaluated.[17,18,19,20]
The aim of this study was to evaluate the accuracy of the Xpert assay using pleural biopsy and pleural fluid specimens in patients with suspected pleural TB but who had a negative sputum AFB smear.
MATERIALS AND METHODS
Patient recruitment and sample collection
Participants were recruited from 4 tertiary care facilities of China from January 2011 to November 2012, the Hong Kong University Shenzhen Hospital (2000-bed), the Chest Hospital of Jiangxi Province (1100-bed), the First Affiliated Hospital of Nanchang University (3200-bed) and the Second Affiliated Hospital of Nanchang University (2100-bed). The Chest Hospital of Jiangxi Province is a specialist hospital for TB. Inclusion criteria were age >16 years and radiographic evidence of pleural effusion that led the physician to perform thoracentesis and pleural biopsy. We excluded patients with positive results of Ziehl-Neelsen (ZN) microscopic examination of a sputum specimen, because they did not require thoracentesis or biopsy for the diagnosis of pleural TB, and therefore, their inclusion might have biased the analysis of validity. We also excluded patients without at least one paired pleural fluid/pleural biopsy specimen. All subjects gave written informed consent, and the study received local and international ethical approval. Thoracentesis and pleural biopsy were performed using standard methods by the collaborating physicians, who decided the specimen volume and number. All samples that were submitted to the province reference laboratory for Mycobacteria. A total of 134 patients met the inclusion criteria and had both pleural fluid and pleural biopsy specimens were evaluated by ZN smear microscopy, culture on Lowenstein-Jensen (LJ) medium, culture on mycobacterial growth indicator tube (MGIT) 960 automated system, and the Xpert assay. Mycobacterial culture from pleural biopsy specimens was used as a gold standard for sensitivity and specificity calculations.
Sample processing
Pleural fluid samples deemed nonsterile were decontaminated using the N-acetyl-L-cysteine-sodium hydroxide (NALC-NaOH) method and concentrated by centrifugation.[21] The decontaminated pleural fluid specimen was divided into two parts: The first underwent direct ZN staining and mycobacterial culture, using an automated BACTEC MGIT 960 System (BD Biosciences, Sparks, MD) and LJ medium; and the second underwent Xpert analysis. Pleural biopsy specimens were divided into two parts: The first underwent fixation in 10% formalin, embedding in paraffin wax (for ZN testing), and hematoxylin and eosin staining; the second homogenization with a glass mortar in saline to a volume of 4 ml, and then decontaminated with standard NALC-NaOH procedure (for ZN staining, culture and Xpert analysis).
Culture using the mycobacterial growth indicator tube 960 automated system
Culture was performed according to the manufacturer's instructions for the MGIT 960 automated system. Inoculation volume was 0.5 ml per tube. The mixture was inversion mixed by hand and then inoculated and incubated at 37°C in the MGIT machine. For tubes identified as positive, a smear of a sample from the tube was prepared for examination for AFB, and further differentiation of mycobacteria was performed with molecular methods.
Culture on Lowenstein-Jensen medium
Slants were prepared from a powder base (Becton Dickinson), and 0.2 ml of each decontaminated specimen was inoculated onto each of 2 slants. Slants were incubated at 37°C in ambient CO2 and were examined visually twice weekly for 8 weeks.[22] Bacterial colonies were investigated by ZN smear and were further investigated by molecular methods.
Drug susceptibility testing
Drug susceptibility testing (DST) for rifampicin was performed with the MGIT 960 automated system with the standard critical concentration of 1 μg/ml rifampicin.
Identification of mycobacteria
For the identification of M. tuberculosis organisms and the differentiation of M. tuberculosis and nontuberculous mycobacteria from positive cultures, two commercially available DNA strip assays were used, the GenoType MTBC and CM/AS assays (Hain Lifescience GmbH, Nehren, Germany).[23]
Analysis of rifampicin-discordant strains
Bidirectional sequencing was carried out on the rifampicin resistance-determining region of the rpoB gene in all the rifampicin-discordant strains using forward (CGTTGATCAACATCCGGCCGGTG) and reverse (CCACCTTGCGGTACGGCGTT) primers and analyzed by using Chromas, version 2.33, software (Technelysium Pty. Ltd., Helensvale, Australia).[14]
Xpert procedure
The Xpert assay was performed as recently described.[14,15,16] One milliliter of decontaminated sample was diluted in 2 ml of the sample buffer included in the assay kit. The solution was vortexed for 15 s and then left to settle for 15 min, with vortexing for 15 s halfway through. A specific volume was collected using the calibrated pipette supplied with the kit and transferred to the cartridge. Cartridges were inserted into the Xpert device, and the automatically generated results were read after 90 min. Samples providing “indeterminate” Xpert results (“error,” “invalid” or “no result”), were not re-tested.
Statistical analysis
The results were analyzed using the SPSS software, version 17.0 (SPSS Inc., Chicago, IL, USA). Numerical variables are reported as mean ± standard division and categorical variables as number and percentage. The categorical variables were compared using Fisher's exact tests or Pearson's Chi-square test, as appropriate. Diagnostic performance was expressed in terms of sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). All tests of significance were two-tailed; P ≤ 0.05 were considered as significant.
RESULTS
Patients
During the study period, 134 participants met the inclusion criteria and had thoracentesis performed and pleural biopsy specimens tested by Xpert assay system and conventional liquid and solid culture techniques. Patients for whom the pleural biopsy culture result was contaminated (n = 4) and patients with an invalid Xpert result (n = 4) were excluded from all analysis [Figure 1]. Of the 126 participants included in the analysis, 5 patients (4.0%) were found to be HIV positive. The mean age of the patients was 38.6 ± 13.2 years. The male-to-female ratio was 1.25. Characteristics of the enrolled 126 participants are summarized in Table 1.
Figure 1.
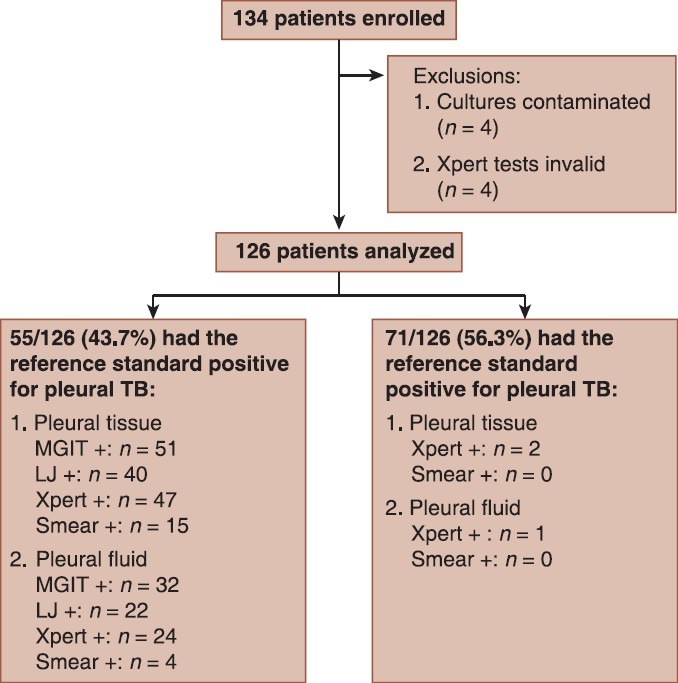
Patient enrollment flow diagram showing the number of patients enrolled and analysed: TB = Tuberculosis; LJ = Lowenstein-Jensen
Table 1.
Demographic and clinical characteristics of the study participants
Comparison of Xpert assay with culture method results for detection of mycobacterium tuberculosis
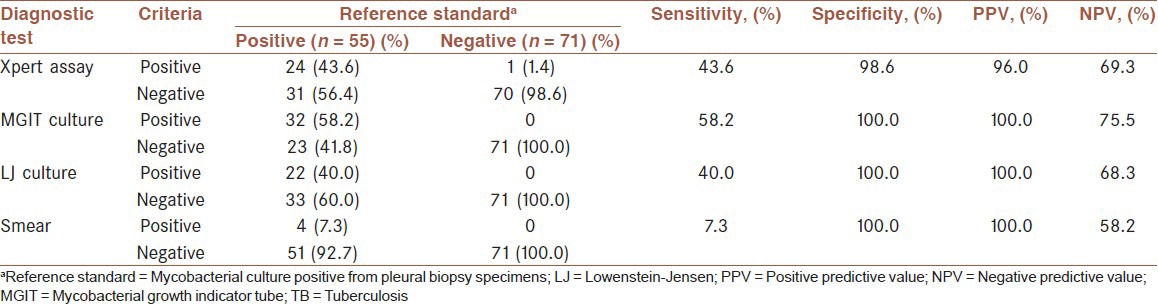
Fifty-five (43.7%) of 126 participants met the diagnostic standard of pleural TB, defined as pleural biopsy specimens that had positive culture results. All AFB from cultures in this study were demonstrated to be M. tuberculosis by DNA strip assay. For pleural biopsy specimens, Xpert assay was positive in 49 patients (38.9%), LJ culture in 40 patients (31.7%), MGIT culture in 51 patients (40.5%) and microscopy in 15 patients (11.9%). The overall sensitivity, specificity, PPV and NPV of Xpert assay using biopsy specimens for pleural TB diagnosis were 85.5%, 97.2%, 95.9% and 89.6%, respectively [Table 2]. For pleural fluid specimens, Xpert assay was positive in 25 patients (19.8%), LJ culture in 22 patients (17.5%), MGIT culture in 32 patients (25.4%) and microscopy in 4 patients (3.2%). The accuracy of the Xpert assay on pleural fluid for the diagnosis of pleural TB using pleural biopsy M. tuberculosis culture as the reference standard was determined [Table 3]. The overall sensitivity, specificity, PPV and NPV of Xpert assay using pleural fluid specimens for pleural TB diagnosis were 43.6%, 98.6%, 96.0% and 69.3%, respectively. The sensitivity of Xpert assay on pleural fluid specimens was lower to that obtained with pleural biopsy specimens (P < 0.01). Comparison of Xpert assay with smear-microscopy revealed that the sensitivity of Xpert was significantly higher than that of the smear-microscopy on pleural biopsy specimens (85.5% vs. 27.3%; P < 0.01), and on pleural fluid specimens (43.6% vs. 7.3%; P < 0.01). None of the isolated nontuberculous mycobacteria was found to be positive in the Xpert assay. Cultures had an average time to positivity (TTP) of 21 days with liquid culture and an average TTP of 5 weeks with solid media.
Table 2.
Sensitivity and specificity of conventional tests and Xpert assay using pleural biopsy for the diagnosis of pleural TB
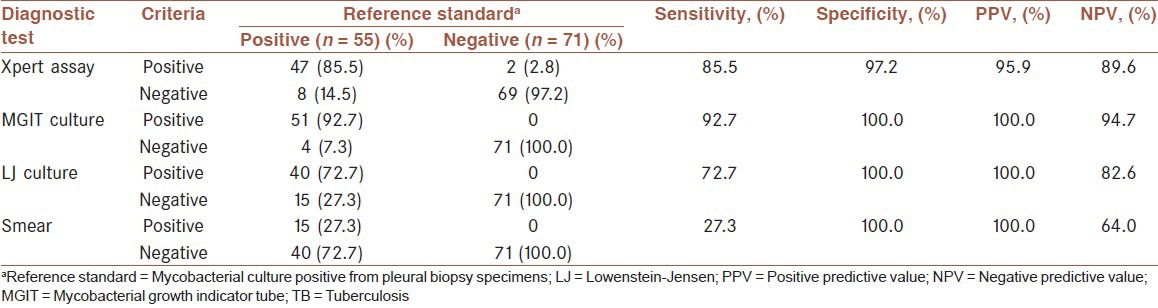
Table 3.
Sensitivity and specificity of conventional tests and Xpert assay using pleural fluid for the diagnosis of pleural TB
Performance of the Xpert assay for the detection of rifampicin resistance
Results of culture to determine drug susceptibility were available for 43 of 55 M. tuberculosis culture-positive patients, with 23.3% (10/43) of cases resistant to rifampicin. The sensitivity and specificity of the Xpert test compared to phenotypic DST were found to be 90.0% (9/10) for correctly determining rifampicin resistance and 93.9% (31/33) for correctly determining rifampicin susceptibility. However, there were 3 patients whose phenotypic DST results for rifampicin were in discordance with the Xpert result. Two of these samples were rifampicin sensitive by phenotypic DST but rifampicin resistant by Xpert, and 1 sample was rifampicin resistant by phenotypic DST and rifampicin sensitive by Xpert. For these cases, phenotypic DST was repeated first. Furthermore, this discrepancy in results was resolved by bidirectional sequencing. Of the 2 phenotypically proven rifampicin-sensitive strains, 1 was found to have a wild-type sequence, whereas the other one sequencing showed a rpoB mutation in codon 531 (TCG to TTG). The remaining sample showed the presence of a mutation at codon 526 (CAC to TAC) upon sequencing, resulting in a histidine-to-tyrosine exchange. Considering the phenotypic DST and sequencing results together, the sensitivity using Xpert was found to be 90.9% (10/11), and the specificity was found to be 96.9% (31/32).
DISCUSSION
Despite a growing number of studies showing promising results of Xpert for rapid detection of M. tuberculosis respiratory specimens, there are relatively few data on the use of Xpert to evaluate nonrespiratory specimens, especially those obtained from pleural fluid and pleural biopsy, and of these data, there have been variable results with regard to the diagnostic performance of Xpert.[14,15,16,17,18,19,20] In the present study, we used the Xpert assay for direct M. tuberculosis detection in pleural fluid and pleural biopsy specimens in a high TB-endemic country. To the best of our knowledge, this study is the largest study to date evaluating the performance of Xpert using pleural biopsy for the diagnosis of pleural TB.
This study demonstrates that Xpert assay performs well in biopsy specimens for rapid and accurate diagnosis of pleural TB. However, sensitivity of detection in pleural fluid specimens (42.9%) was lower than that in the pleural biopsy specimens (85.5%). The sensitivity of the Xpert assay for detecting pleural TB using pleural fluid sample was slightly higher than previously reported,[17,18,19,20] whereas the specificity was similar. Of the 56 pleural TB patients, 32 (57.1%) were Xpert false-negative on pleural fluid specimens. Compared with the pleural TB patients with accurate results, those with false-negative Xpert results had less extensive caseous necrosis (data not shown). Pleural TB is a paucibacillary form of the disease, as indicated in a previous study,[10,15] the low detection ability in some sterile specimens, such as pleural fluid, could be explained by the low bacterial load contained in these samples. Techniques based on nucleic acid amplification have recently been considered for pleural TB diagnosis, in order to improve sensitivity and specificity.[2,24] The lower efficiency obtained in these pleural fluid specimens with Xpert (though better than the sensitivity achieved by other methods) highlights the assay's limited detection ability in very-low-yield samples.
Furthermore, not only M. tuberculosis detection but also rapidly determining the patient's MDR status is of prime importance in bringing to an end the spread of MDR-TB and decreasing mortality. Conventional DST results take at least 2 months from the time when the culture is inoculated. One of the important strengths of the Xpert assay is its ability to rapid detect the presence of resistance to rifampicin. When analyzing the performance of the rifampicin component of the Xpert assay, culture-determined rifampicin resistance was used as the gold standard. The sensitivity and specificity of the Xpert assay to detect rifampicin resistance were similar to that previously reported studies.[13] Sequencing of the three discrepant samples resolved 1 of them, resulting in an increased specificity of 97%. Recent studies have highlighted a problem with false-positive results of tests for rifampicin resistance,[25,26] and corrective measures have been instituted in the recent G4 version of the test, including revisions to the diagnostic platform software and redesign of the oligonucleotide probes.[27] WHO recommends further confirmatory tests following detection of rifampicin-resistant M. tuberculosis strains.[28]
Accurate quantification of the M. tuberculosis load in patient samples may allow for the evaluation of the patient's infectiousness, the evaluation of the disease severity and the monitoring of treatment. One limitation of this technique is that, in detecting M. tuberculosis DNA, they cannot distinguish between viable and nonviable microorganisms. However, higher Xpert assay loads were associated with decreased MGIT culture TTP, consistent with previous data indicating that the Xpert assay's semiquantitative results could be used to estimate the M. tuberculosis load.[10] One limitation of this study is that the cost-effectiveness and turnaround time of the Xpert assay were not evaluated.
Overall, Xpert may be a potentially useful additional tool using biopsy specimens for rapid diagnosis of pleural TB in patients with AFB smear-negative sputum results, and allowing these vulnerable individuals to get onto TB treatment earlier. Further studies should be done to evaluate the clinical impact of the Xpert assay, including evaluation of the outcomes and effect on clinical practice decisions, management outcome, and development of new diagnostic algorithms; the cost-effectiveness and feasibility of implementing the assay.
AUTHOR'S CONTRIBUTIONS
JD, ZH and XL designed and carried out the research, collected the data, interviewed the patients, coordinated the study, participated in all of the research, and prepared the manuscript. QL provided assistance in designing and conducting the research, collected the data, and participated in manuscript preparation. GX and JL provided assistance in laboratory tests. XX performed the statistical analyses. WL corrected the English manuscript and revised further statistical data. All authors have read and approved the content of the manuscript.
ACKNOWLEDGMENTS
We thank Dr. Li for his kind technical assistance. This study was financially supported by National Natural Science Foundation of China (Research No. 81170006).
Footnotes
Source of Support: Nil
Conflict of Interest: No conflict of interests.
REFERENCES
- 1.Geneva, Switzerland: World Health Organization; 2012. World Health Organization. Global Tuberculosis Report 2012. WHO/HTM/TB/2012.6. [Google Scholar]
- 2.Trajman A, Pai M, Dheda K, van Zyl Smit R, Zwerling AA, Joshi R, et al. Novel tests for diagnosing tuberculous pleural effusion: What works and what does not? Eur Respir J. 2008;31:1098–106. doi: 10.1183/09031936.00147507. [DOI] [PubMed] [Google Scholar]
- 3.Porcel JM. Tuberculous pleural effusion. Lung. 2009;187:263–70. doi: 10.1007/s00408-009-9165-3. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Q, Chen YQ, Qin SM, Tao XN, Xin JB, Shi HZ. Diagnostic accuracy of T-cell interferon-γ release assays in tuberculous pleurisy: A meta-analysis. Respirology. 2011;16:473–80. doi: 10.1111/j.1440-1843.2011.01941.x. [DOI] [PubMed] [Google Scholar]
- 5.Sahn SA, Huggins JT, San José ME, Álvarez-Dobaño JM, Valdés L. Can tuberculous pleural effusions be diagnosed by pleural fluid analysis alone? Int J Tuberc Lung Dis. 2013;17:787–93. doi: 10.5588/ijtld.12.0892. [DOI] [PubMed] [Google Scholar]
- 6.Geneva, Switzerland: World Health Organization; 2010. World Health Organization. Roadmap for Rolling Out Xpert MTB/RIF for Rapid Diagnosis of TB and MDR-TB. [Google Scholar]
- 7.Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K, et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol. 2010;48:229–37. doi: 10.1128/JCM.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–15. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller MB, Popowitch EB, Backlund MG, Ager EP. Performance of Xpert MTB/RIF RUO assay and IS6110 real-time PCR for Mycobacterium tuberculosis detection in clinical samples. J Clin Microbiol. 2011;49:3458–62. doi: 10.1128/JCM.05212-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Grady J, Bates M, Chilukutu L, Mzyece J, Cheelo B, Chilufya M, et al. Evaluation of the Xpert MTB/RIF assay at a tertiary care referral hospital in a setting where tuberculosis and HIV infection are highly endemic. Clin Infect Dis. 2012;55:1171–8. doi: 10.1093/cid/cis631. [DOI] [PubMed] [Google Scholar]
- 11.Menzies NA, Cohen T, Lin HH, Murray M, Salomon JA. Population health impact and cost-effectiveness of tuberculosis diagnosis with Xpert MTB/RIF: A dynamic simulation and economic evaluation. PLoS Med. 2012;9:e1001347. doi: 10.1371/journal.pmed.1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawn SD, Mwaba P, Bates M, Piatek A, Alexander H, Marais BJ, et al. Advances in tuberculosis diagnostics: The Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis. 2013;13:349–61. doi: 10.1016/S1473-3099(13)70008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang K, Lu W, Wang J, Zhang K, Jia S, Li F, et al. Rapid and effective diagnosis of tuberculosis and rifampicin resistance with Xpert MTB/RIF assay: A meta-analysis. J Infect. 2012;64:580–8. doi: 10.1016/j.jinf.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Hillemann D, Rüsch-Gerdes S, Boehme C, Richter E. Rapid molecular detection of extrapulmonary tuberculosis by the automated GeneXpert MTB/RIF system. J Clin Microbiol. 2011;49:1202–5. doi: 10.1128/JCM.02268-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vadwai V, Boehme C, Nabeta P, Shetty A, Alland D, Rodrigues C. Xpert MTB/RIF: A new pillar in diagnosis of extrapulmonary tuberculosis? J Clin Microbiol. 2011;49:2540–5. doi: 10.1128/JCM.02319-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Causse M, Ruiz P, Gutiérrez-Aroca JB, Casal M. Comparison of two molecular methods for rapid diagnosis of extrapulmonary tuberculosis. J Clin Microbiol. 2011;49:3065–7. doi: 10.1128/JCM.00491-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moure R, Martín R, Alcaide F. Effectiveness of an integrated real-time PCR method for detection of the Mycobacterium tuberculosis complex in smear-negative extrapulmonary samples in an area of low tuberculosis prevalence. J Clin Microbiol. 2012;50:513–5. doi: 10.1128/JCM.06467-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tortoli E, Russo C, Piersimoni C, Mazzola E, Dal Monte P, Pascarella M, et al. Clinical validation of Xpert MTB/RIF for the diagnosis of extrapulmonary tuberculosis. Eur Respir J. 2012;40:442–7. doi: 10.1183/09031936.00176311. [DOI] [PubMed] [Google Scholar]
- 19.Friedrich SO, von Groote-Bidlingmaier F, Diacon AH. Xpert MTB/RIF assay for diagnosis of pleural tuberculosis. J Clin Microbiol. 2011;49:4341–2. doi: 10.1128/JCM.05454-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porcel JM, Palma R, Valdés L, Bielsa S, San-José E, Esquerda A. Xpert® MTB/RIF in pleural fluid for the diagnosis of tuberculosis. Int J Tuberc Lung Dis. 2013;17:1217–9. doi: 10.5588/ijtld.13.0178. [DOI] [PubMed] [Google Scholar]
- 21.Kent PT, Kubica GP. Atlanta, GA: Centers for Disease Control; 1985. Public Health Mycobacteriology: A Guide for the Level III Laboratory. [Google Scholar]
- 22.Weyer K. Geneva, Switzerland: World Health Organization; 1998. Laboratory Services in Tuberculosis Control. Part III: culture. [Google Scholar]
- 23.Richter E, Rüsch-Gerdes S, Hillemann D. Evaluation of the GenoType Mycobacterium assay for identification of mycobacterial species from cultures. J Clin Microbiol. 2006;44:1769–75. doi: 10.1128/JCM.44.5.1769-1775.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pai M, Flores LL, Hubbard A, Riley LW, Colford JM., Jr Nucleic acid amplification tests in the diagnosis of tuberculous pleuritis: A systematic review and meta-analysis. BMC Infect Dis. 2004;4:6. doi: 10.1186/1471-2334-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: A multicentre implementation study. Lancet. 2011;377:1495–505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawn SD, Brooks SV, Kranzer K, Nicol MP, Whitelaw A, Vogt M, et al. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: A prospective study. PLoS Med. 2011;8:e1001067. doi: 10.1371/journal.pmed.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geneva: Foundation for Innovative New Diagnostics; 2011. FIND. Performance of Xpert MTB/RIF Version G4 Assay. [Google Scholar]
- 28.Geneva, Switzerland: World Health Organization; 2011. World Health Organization. Rapid Implementation of the Xpert MTB/RIF Diagnostic Test: Technical and Operational “How-to”; Practical Considerations. [Google Scholar]


