Abstract
Background:
The prevalence of type 2 diabetes is increasing in Malaysia, with most patients poorly controlled. Hence, this study aimed to determine nutritional and metabolic status as well as blood pressure of Malaysian patients with type 2 diabetes mellitus and identify associated risk factors for poor glycemic control.
Materials and Methods:
A total of 104 type 2 diabetic patients were recruited and completed a questionnaire covering socio-demographic status, 3-day diet records, and physical activity. Anthropometry and glycemic control parameters, lipid profile and blood pressure were also measured.
Results:
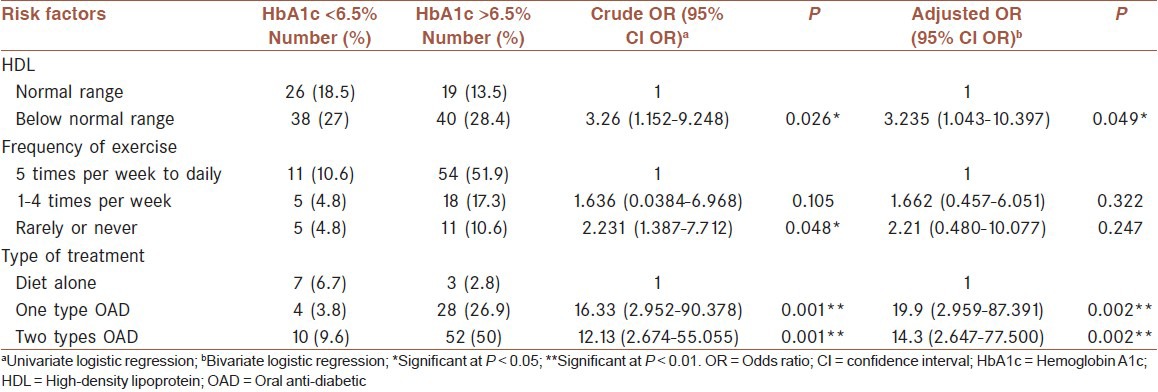
Subjects were on average 56.7±9.9 years old with a mean duration of diabetes of 6.5 ± 5.0 years. The mean hemoglobin A1c of the subjects was 7.6% ± 1.4%, with only 20.2% achieving the target goal of <6.5% with no significant differences between genders. The mean body mass index was 26.9 ± 4.7 kg/m2, with 86.5% either were overweight or obese. Only 10.6% of the subjects exercised daily. The proportions of macronutrients relative to total energy intake were consistent with the recommendations of most diabetes associations. The adjusted odds of having poor glycemic control were 3.235 (1.043-10.397) (P < 0.05) higher among those who had high density lipoprotein cholesterol levels below the normal range. Those taking one or two types of oral anti-diabetic drugs had 19.9 (2.959-87.391) (P < 0.01) and 14.3 (2.647-77.500) (P < 0.01) higher odds of poor glycemic control respectively compared to those who were being treated by diet alone.
Conclusion:
Poor glycemic control was prevalent among Malaysian diabetic patients, and this could be associated with low levels of HDL and being treated with oral anti-diabetes agents.
Keywords: Body mass index, dietary intake, glycemic control, lipid profile, nutritional status, oral hypoglycemic agents, physical activity, type 2 diabetes mellitus
INTRODUCTION
The prevalence of diabetes is increasing worldwide. Type 2 diabetes mellitus is one of the most common endocrine disorders, affecting almost 6% of the world's population.[1] It is characterized by hyperglycemia resulting from defects in insulin action, insulin secretion, or both.[2] Its prevalence in Asia ranges from 1.2% to 14.6%.[1] In Malaysia, its prevalence is above the average indicated by the International Diabetes Federation for all regions in the world. According to the Malaysian National Health Morbidity Survey 2006, it affected 14.9% of the population aged 30 years old and above in 2006,[3] increasing to 15.2% in 2010[4] and recently reported to be 22.6%.[5]
Despite aggressive diabetes support, Malaysian diabetic patients are mostly poorly controlled, with a mean hemoglobin A1c (HbA1c) of 8.7% and only 22% of patients achieving the treatment goal of <7%.[6] However, data regarding nutritional status and glycemic control and its associated risk factors in Malaysian type 2 diabetic patients remain limited and inconclusive. Better understanding of the factors associated with their improvement in diabetes control is warranted to help professionals identify determinants of diabetes management. Hence, the present study aimed to identify the current clinical and nutritional status of type 2 diabetic outpatients in a selected government teaching hospital and determine the associated risk factors for poor glycemic control.
MATERIALS AND METHODS
Subject selection
This study was the baseline assessment of a clinical trial in an outpatient clinic at Universiti Kebangsaan Malaysia Medical Centre (UKMMC) among patients with type 2 diabetes in 2009. Subjects with a confirmed diagnosis of type 2 diabetes for at least 6 months, HbA1c <12%, and those who had been treated with diet and oral anti-diabetic agents on a stable dose over the past 3 months were recruited for the study. Those who were on insulin therapy and had significant clinical cardiovascular, renal, or liver disease were excluded from the study. This study was approved by the Clinical Research and Ethics Committee of UKMMC (research project number: FF-138-2005) and all subjects gave their written consent prior to entry into the study.
Data collection
Individual interviews were conducted to collect data on socio-demographic characteristics including duration of diabetes, ethnicity, marital status, educational level, income, and employment status. Subjects were also asked to record all foods and beverages consumed in the 24-h period over 3 consecutive days including 2 weekdays and 1 weekend day. Food records were rechecked with the subjects before being analyzed for discrepancies and omissions, including food preparation, cooking method, food brand name, portion size, and ingredients, to ensure the accuracy and to improve the validity.
Measurements and analyses
All measurements were taken in the morning after fasting for at least 10 h. Subjects were weighed using a digital weighing scale (SECA; London British Indicators, UK) to the nearest 0.1 kg in light clothing without shoes. Height was measured without shoes by the height attachment on the same weighing scale (SECA; London British Indicators Ltd) to the nearest 0.1 cm. Weight and height measurements were used to calculate the body mass index (BMI). Waist circumference was also measured to the nearest 0.1 cm. Blood pressure was measured using a fully automatic blood pressure monitor (Omron M4-I; Omron Healthcare Europe BV, Hoofdorp, The Netherlands).
Fasting blood samples were drawn through the antecubital arm vein for glucose, HbA1c, insulin levels, and lipid measurements. Blood was centrifuged for 10 min at 3000 rpm and serum and plasma was stored frozen at −20°C until analysis, except that for HbA1c analysis. The blood for HbA1c was refrigerated at 4°C and analyzed within a week of collection. Fasting serum glucose, HbA1c and plasma lipid components (triglyceride, total and high-density lipoprotein [HDL]-cholesterol) were measured using a COBAS Integra(R) 800 automated analyzer (Roche Diagnostic, Basel, Switzerland) with a specific enzymatic assay. Serum low-density lipoprotein (LDL) was calculated using the Friedewald et al. equation.[7] Plasma insulin was determined using a solid-phase, two-site chemiluminescent enzyme-labeled immunometric assay (Immulite (R) 1000 Analyzer; Diagnostic Company Procedure, Deerfield IL USA). The relative insulin resistance introduced as a homeostasis model assessment (HOMA-IR) was estimated according to Matthews et al. equation.[8]
Nutrient analysis was performed using a computerized dietary analysis program (Nutritionist Pro Version 2.0; FirstData Bank, The Hearst Corp., New York). In this analysis, total carbohydrate referred to the sum of total starch, excluding fiber. The crude fiber content was listed in the Malaysian food composition table;[9]. Therefore, the total fiber content calculated from the dietary record analyses was reported as crude fiber. Assessment of under-reporting, normal reporting, and over-reporting of calorie intake by subjects was conducted based on Goldberg et al. criteria.[10]
Statistical analysis
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) software (SPSS version19. Inc., Chicago, IL, USA) and the significance level used for all tests was set at P < 0.05. Results were expressed as mean ± standard deviation. Normality was checked prior to each analysis and an equivalent nonparametric test was conducted as an alternative where appropriate. To determine associated risk factors for glycemic control, univariate and bivariate logistic regression were conducted. Poor glycemic control was considered as HbA1c >6.5%. In univariate logistic regression, each independent variable was analyzed to determine any significant association with glycemic control. Using a backward stepwise logistic regression, all factors found to be significant during the univariate logistic regression were entered together in a multivariate analysis to obtain the adjusted odds ratio (OR). The findings of the first step and final model were presented using the crude OR and adjusted OR, respectively; with a 95% confidence interval and corresponding P value.
RESULTS
A total of 104 subjects (40 male (38%)) were recruited into this study. Their mean age was 56.7 ± 9.94 years old and the mean duration of diabetes was 6.5 ± 5.0 years [Table 1]. Only 8.6% of the subjects worked as professionals and the rest were semi-professionals, workers, pensioners, housewives, or unemployed. The majority of the subjects had a monthly household income of <1000 RM while 20.2%, 8.6%, and 12.5% had an income of 1001-3000 RM, 3001-5000 RM, and above 5000 RM respectively. With respect to diabetes treatment, the majority of subjects (90%) were treated with oral anti-diabetic agents either on single (29%) or dual (61%) drugs whereas the rest were treated by diet alone. Hyperlipidemia was the most common pharmacologically treated co-morbid condition, with 76% of the subjects using lipid-lowering drugs (statins and/or fibrate). More than half of the subjects (59%) were taking anti-hypertensive drugs, which included B-blockers, angiotensin converting enzyme inhibitors, anti-diuretics and/or calcium antagonists.
Table 1.
Glycaemic control, anthropometric and metabolic parameters of the subjects
Most of the subjects were non-smokers, but 7 (6.7%) subjects were still actively smoking. A small proportion of the subjects (10%) reported moderate alcohol consumption. In terms of self-reported exercise activities, only a small percentage of the subjects (10.6%) exercised daily while the majority of subjects (60%) rarely or never exercised at all. Thirty-seven percent of those who reported doing exercise spent around 15-30 min exercising during each session three to four times a week.
Glycemic control and metabolic parameters
The average fasting blood glucose and HbA1c of the subjects were higher than the treatment goals. Only 28% and 20% of the subjects had fasting glycaemia and HbA1c at optimum levels [Figure 1]. More than half of the subjects (62%) were either overweight or obese [Figure 2] with the majority manifesting abdominal obesity [Figure 1]. On average, systolic blood pressure and LDL-cholesterol were higher than the recommended levels. There were no statistically significant differences between men and women in terms of glycemic and metabolic status. However, women had a significantly higher mean BMI and lower mean waist circumference compared to men (P < 0.05) [Table 1].
Figure 1.
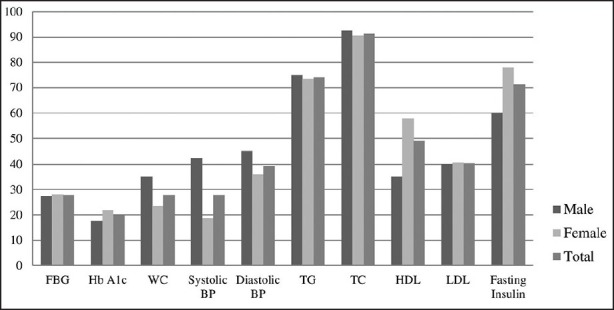
Percentage of subjects with the optimal level of glycemic control, waist circumference, lipid profile, blood pressure, and insulin level
Figure 2.
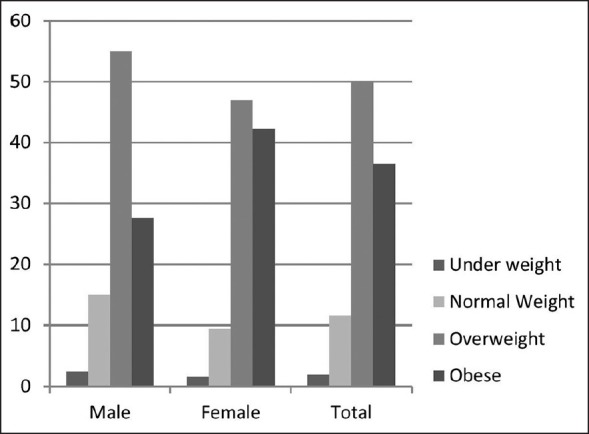
Percentage of the subjects in different body mass index categories
Dietary intake
While intake of energy, carbohydrate, protein, and fat was significantly higher among men, the percentage of calories derived from carbohydrate, protein, and fat was comparable between men and women [Table 2]. Nevertheless, about 55.8% of the subjects were under-reporting their energy intake with none of them over-reporting their energy intake. Cholesterol and sodium intake were significantly higher among male than female diabetics (P < 0.05) while calcium, Vitamin C, and fiber intake were below recommended levels suggested for Malaysian diabetic patients.
Table 2.
Comparisons of daily dietary intake between men and women
Factors associated with glycemic control
The type of treatment, HDL level, and frequency of exercise per week each made a significant contribution to glycemic control [Table 3]. The crude ORs showed that levels of HDL, frequency of exercise, and type of treatment contributed significantly to poor glycemic control. The adjusted OR showed that subjects who had below normal levels of HDL were 3.235 (1.043-10.397) times more at risk of poor glycemic control. In addition, the odds of having poor glycemic control among those who were treated with one and two types of OAD agents were 19.9 (2.959-87.391) and 14.3 (2.647-77.500), respectively, compared with those being under treatment with diet alone.
Table 3.
Crude OR and AOR of poor good glycemic control
DISCUSSION
This study described the general characteristics of and factors associated with poor glycemic control among a sample of Malaysian type 2 diabetics from a selected outpatient clinic of the University Hospital. Subjects in this study were on average in their 50s. Data obtained from the National Health Morbidity survey II showed that the highest prevalence of known diabetes was among those aged 60-64 years (26.1%).[3]
Only 20.2% and 27.9% of these patients achieved the target for HbA1c and fasting blood glucose according to recommended levels for Malaysian diabetic patients; these rates were similar to those for other diabetic patients from another Malaysian government university hospital. Such levels of HbA1c are a concern as tight glycemic control is important to prevent or minimize the development of diabetes-related complications in patients with type 2 diabetes.[11] Subjects in this study also suffered other diabetes-related comorbidities such as hyperlipidemia, hypertension, and obesity. Overall, the evidence shows that the conventional dietary and medical approaches fail to achieve favorable glycemic control in Malaysian diabetic patients, and there is a tangible need for complementary approaches to improving glycemic control.[12]
The mean BMI of the subjects in this study was 26 kg/m2, indicating that on average, subjects were overweight. The prevalence of overweight and obesity in the study subjects was 86.5%, similar to the prevalence in diabetic patients in Kelantan.[11] However, the prevalence of overweight and obese in this study was more than double compared to what reported in the national diabetes study conducted in Kelantan 10 years ago.[13] This discrepancy could be due to the passage of time. However, the mean BMI of the Asian subjects in this study was lower compared to that of patients from other ethnic backgrounds. For example in a study in the United States, the mean BMI of African-American and Native Hawaiian was 27.9 and 28.4, respectively.[14] These data confirm that the risk of type 2 diabetes starts at a lower BMI for Asians than other ethnicities.[15] Indeed, most subjects had a waist circumference above the normal range, reflecting central obesity. Studies showed that Asian populations, especially South Asians, are more prone to abdominal obesity which leads to increased IR compared with their Western counterparts.[16]
Only a small percentage exercised daily, while 59.6% of the subjects rarely or never exercised. This percentage is one-fold higher than that reported by Nelson et al.,[17] who found that 31% of adults with type 2 diabetes in the US did not exercise regularly. Similar data from diabetic patients in Kelantan also showed that the majority of diabetic patients do not perform physical activity regularly.[11] Indeed, for those who reported regular exercise (36.5%), the time spent exercising (15-30 min for each session 3-4 times a week) was far below the recommendation for diabetic patients[18,19] of 150 min of moderate intensity exercise per week.
Despite the high prevalence of overweight and obesity in this population, only 23% of the subjects were consuming more energy than they actually required, which may be due to under-reporting in 55.8% of subjects. This study found that the percentage of total calories consumed as carbohydrate, protein, and fat was 55.7, 16.5, and 27.5 respectively, which is consistent with a previous study by Moy and Suriah.[20] These proportions did not exceed the levels recommended for Malaysian diabetic patients.[21] The sodium intake of male subjects exceeded recommended levels while the intake of calcium, fiber, and Vitamin C was below the range recommended for Malaysian diabetic patients.[21]
In the agreement with the present study, other studies showed that poor compliance to diet and physical activity among Malaysian diabetic patients has always been a matter of concern.[22,23,24] Efforts should be made to enhance diabetic patients’ awareness regarding the notorious impact of a healthy lifestyle on glycemic control and preventing diabetes complications.
In this study, type of treatment, lack of physical activity and low levels of HDL were potential risk factors for poor glycemic control. The association between increasing physical activity levels and improvements in glycemic control has been demonstrated by several studies.[18,25,26,27] The odds of having poor glycemic control in patients who rarely did exercise or were less physically active were significant. However, they were no more significant in the adjusted ORs. One possible explanation is that exercise modulates glycemic control by increasing HDL. The association between exercise and HDL level was previously reported by several studies.[28,29] Indeed, patients who were taking two types of OAD also had higher odds of having poor glycemic control compared to those treated with diet alone or one type of OAD. Although an association between higher number of medications and an unfavorable glycemic outcome has previously been reported,[30] the causality link is questionable, that is, doctors will prescribe more medications to those who are poorly controlled. The subject's compliance to medication can also contribute to this association, but was not taken into account in this study. Worth mentioning that only few numbers of the subjects in this study was under treatment with diet alone. This fact weakened the results derived from this part.
Despite previous reports of the association between dietary fiber and glycemic control,[31,32] this study found no evidence of such an association. One explanation is that since only a few subjects met the requirements for fiber intake, the statistical analysis was unable to show any significant association. Overall low fiber intake in diabetic patients could be a matter of concern, and medical nutrition therapy should place more emphasis on increasing fiber intake.
With regards factors associated with poor glycemic control, other studies have highlighted other associated risk factors, including a longer duration of diabetes,[30,33,34] age,[30] LDL, HDL, and blood pressure.[35] However, this study found no evidence of any association between the mentioned variables and glycemic control.
Small sample size should be mentioned as one of the limitations of this study. Indeed, this study was limited to a sample from a selected teaching hospital, and thus data from other centers are required to determine whether the finding in this study can be generalized to the diabetes care setting. Furthermore, the population in this study was that of diabetic outpatients excluding those who were under insulin therapy. Under-reporting by the subjects and not measuring their compliance to medications are other limitations of this study. Despite these inherent limitations, data from this study can be used as a baseline for further research in this area. Indeed, the present study is one of the few studies to assess in detail the diet of Malaysian type 2 diabetic patients.
CONCLUSION
Subjects of this study were mostly overweight and obese with having abdominal obesity. They had low intake of fiber; calcium and Vitamin C compared to recommended levels for type 2 diabetic patients. Besides, hypertension and dyslipidemia were also prevalent among subjects of this study. Poor glycemic control was prevalent among diabetic patients and can be contributed to a lack of appropriate levels of physical activity, low levels of HDL, and a higher number of medications. Future research should further evaluate the contribution of factors affecting glycemic control in a larger population.
AUTHOR'S CONTRIBUTIONS
MYBN and KNA contributed in the conception or design of the work, SF and MYBN contributed on analysis, or interpretation of data for the work. SF and MYBN contributed in drafting the work and revising it critically for important intellectual content. MYBN has approved final version to be published. MYBN and KNA have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGEMENTS
We would like to thank all hospital staffs for their support in the data collection and all the patients who took part in this study.
Footnotes
Source of Support: This study was granted by “University Kebangsaan Malaysia”.
Conflict of Interest: None declared.
REFERENCES
- 1.Adeghate E, Schattner P, Dunn E. An update on the etiology and epidemiology of diabetes mellitus. Ann N Y Acad Sci. 2006;1084:1–29. doi: 10.1196/annals.1372.029. [DOI] [PubMed] [Google Scholar]
- 2.Das SK, Elbein SC. The genetic basis of type 2 diabetes. Cellscience. 2006;2:100–31. doi: 10.1901/jaba.2006.2-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Letchuman GR, Wan Nazaimoon WM, Wan Mohamad WB, Chandran LR, Tee GH, Jamaiyah H, et al. Prevalence of diabetes in the Malaysian National Health Morbidity Survey III 2006. Med J Malaysia. 2010;65:180–6. [PubMed] [Google Scholar]
- 4.Rampal S, Rampal L, Rahmat R, Zain AM, Yap YG, Mohamed M, et al. Variation in the prevalence, awareness, and control of diabetes in a multiethnic population: A nationwide population study in Malaysia. Asia Pac J Public Health. 2010;22:194–202. doi: 10.1177/1010539509334816. [DOI] [PubMed] [Google Scholar]
- 5.Wan Nazaimoon WM, Md Isa SH, Wan Mohamad WB, Khir AS, Kamaruddin NA, Kamarul IM, et al. Prevalence of diabetes in Malaysia and usefulness of HbA1c as a diagnostic criterion. Diabet Med. 2013;30:825–8. doi: 10.1111/dme.12161. [DOI] [PubMed] [Google Scholar]
- 6.Mafauzy M, Hussein Z, Chan SP. The status of diabetes control in Malaysia: Results of DiabCare 2008. Med J Malaysia. 2011;66:175–81. [PubMed] [Google Scholar]
- 7.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 8.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 9.Tee ES, Nasir IA, Khatijah I, editors. Kuala Lumpur: Malaysian Food Composition Database Programme, Institute for Medical Research; 1997. Nutrient Composition of Malaysian Foods. [Google Scholar]
- 10.Goldberg GR, Black AE, Jebb SA, Cole TJ, Murgatroyd PR, Coward WA, et al. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur J Clin Nutr. 1991;45:569–81. [PubMed] [Google Scholar]
- 11.Abougalambou SS, Mohamed M, Sulaiman SA, Abougalambou AS, Hassali MA. Current clinical status and complications among type 2 diabetic patients in Universiti Sains Malaysia hospital. Int J Diabetes Mellit. 2010;2:184–8. [Google Scholar]
- 12.Firouzi S, Barakatun-Nisak MY, Ismail A, Majid HA, Nor Azmi K. Role of probiotics in modulating glucose homeostasis: Evidence from animal and human studies. Int J Food Sci Nutr. 2013;64:780–6. doi: 10.3109/09637486.2013.775227. [DOI] [PubMed] [Google Scholar]
- 13.Mafauzy M, Mokhtar N, Mohamad WB, Musalmah M. Diabetes mellitus and associated cardiovascular risk factors in north-east Malaysia. Asia Pac J Public Health. 1999;11:16–9. doi: 10.1177/101053959901100104. [DOI] [PubMed] [Google Scholar]
- 14.Maskarinec G, Grandinetti A, Matsuura G, Sharma S, Mau M, Henderson BE, et al. Diabetes prevalence and body mass index differ by ethnicity: The Multiethnic Cohort. Ethn Dis. 2009;19:49–55. [PMC free article] [PubMed] [Google Scholar]
- 15.Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, et al. Diabetes in Asia: Epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–40. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 16.Mohan V. Age, body mass index and type 2 diabetes-associations modified by ethnicity. Diabetologia. 2003;46:1063–70. doi: 10.1007/s00125-003-1158-9. [DOI] [PubMed] [Google Scholar]
- 17.Nelson KM, Reiber G, Boyko EJ, NHANES III. Diet and exercise among adults with type 2 diabetes: Findings from the third national health and nutrition examination survey (NHANES III) Diabetes Care. 2002;25:1722–8. doi: 10.2337/diacare.25.10.1722. [DOI] [PubMed] [Google Scholar]
- 18.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1433–8. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- 19.Albright A, Franz M, Hornsby G, Kriska A, Marrero D, Ullrich I, et al. American College of Sports Medicine position stand. Exercise and type 2 diabetes. Med Sci Sports Exerc. 2000;32:1345–60. doi: 10.1097/00005768-200007000-00024. [DOI] [PubMed] [Google Scholar]
- 20.Moy FM, Suriah A. Anthropometry and dietary intake of type 2 diabetes patients attending an outpatient clinic. Malays J Nutr. 2002;8:63–73. [PubMed] [Google Scholar]
- 21.Kuala Lumpur, Malaysia: Malaysian Dietetic Association; 2005. Malaysian Dietetic Association. Medical Nutrition Therapy Guidelines for Type 2 Diabetes. [Google Scholar]
- 22.Mafauzy M. Diabetes control and complications in public hospitals in Malaysia. Med J Malaysia. 2006;61:477–83. [PubMed] [Google Scholar]
- 23.Mafauzy M. Diabetes mellitus in Malaysia. Med J Malaysia. 2006;61:397–8. [PubMed] [Google Scholar]
- 24.Mafauzy M. Diabetes control and complications in private primary healthcare in Malaysia. Med J Malaysia. 2005;60:212–7. [PubMed] [Google Scholar]
- 25.Kavouras SA, Panagiotakos DB, Pitsavos C, Chrysohoou C, Anastasiou CA, Lentzas Y, et al. Physical activity, obesity status, and glycemic control: The ATTICA study. Med Sci Sports Exerc. 2007;39:606–11. doi: 10.1249/mss.0b013e31803084eb. [DOI] [PubMed] [Google Scholar]
- 26.Toledo FG, Menshikova EV, Ritov VB, Azuma K, Radikova Z, DeLany J, et al. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes. 2007;56:2142–7. doi: 10.2337/db07-0141. [DOI] [PubMed] [Google Scholar]
- 27.Aylin K, Arzu D, Sabri S, Handan TE, Ridvan A. The effect of combined resistance and home-based walking exercise in type 2 diabetes patients. Int J Diabetes Dev Ctries. 2009;29:159–65. doi: 10.4103/0973-3930.57347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiens B, Jörgensen I, Lewis S, Jensen G, Lithell H, Vessby B, et al. Increased plasma HDL-cholesterol and apo A-1 in sedentary middle-aged men after physical conditioning. Eur J Clin Invest. 1980;10:203–9. doi: 10.1111/j.1365-2362.1980.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 29.Kodama S, Tanaka S, Saito K, Shu M, Sone Y, Onitake F, et al. Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: A meta-analysis. Arch Intern Med. 2007;167:999–1008. doi: 10.1001/archinte.167.10.999. [DOI] [PubMed] [Google Scholar]
- 30.Juarez DT, Sentell T, Tokumaru S, Goo R, Davis JW, Mau MM. Factors associated with poor glycemic control or wide glycemic variability among diabetes patients in Hawaii, 2006-2009. Prev Chronic Dis. 2012;9:120065. doi: 10.5888/pcd9.120065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM, Brinkley LJ. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med. 2000;342:1392–8. doi: 10.1056/NEJM200005113421903. [DOI] [PubMed] [Google Scholar]
- 32.McIntosh M, Miller C. A diet containing food rich in soluble and insoluble fiber improves glycemic control and reduces hyperlipidemia among patients with type 2 diabetes mellitus. Nutr Rev. 2001;59:52–5. doi: 10.1111/j.1753-4887.2001.tb06976.x. [DOI] [PubMed] [Google Scholar]
- 33.Khattab M, Khader YS, Al-Khawaldeh A, Ajlouni K. Factors associated with poor glycemic control among patients with type 2 diabetes. J Diabetes Complications. 2010;24:84–9. doi: 10.1016/j.jdiacomp.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Benoit SR, Fleming R, Philis-Tsimikas A, Ji M. Predictors of glycemic control among patients with Type 2 diabetes: A longitudinal study. BMC Public Health. 2005;5:36. doi: 10.1186/1471-2458-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabanayagam C, Shankar A, Saw SM, Tai ES, Lim SC, Lee JJ, et al. Prevalence of diabetes mellitus, glycemic control, and associated factors in a Malay population in Singapore. Asia Pac J Public Health. 2009;21:385–98. doi: 10.1177/1010539509343958. [DOI] [PubMed] [Google Scholar]


