Abstract
BACKGROUND
Immunopathological and inflammatory processes play important roles in the initiation and development of ischemic heart disease. Hence, this study aimed to evaluate the relationship between serum levels rheumatoid factor (RF) and anti-nuclear antibodies (ANA) and severity of coronary stenotic lesions.
METHODS
Totally 140 patients with acute coronary syndrome (ACS) (n = 70) and chronic stable angina (CSA) (n = 70) that undergoing coronary angiography were enrolled in this study. ANA by the enzyme-linked immunosorbent assay (ELISA) and serum level of RF was measured by latex method. The severity of coronary stenotic lesions calculated by Gensini score. To analyze the correlations of ANA and RF to Gensini score Pearson correlation test was used. To adjust the effect of age and other confounder factors such hypertension, diabetes, hyperlipidemia and smoking multiple linear regression was used.
RESULTS
The mean serum levels of RF and ANA in CSA group were significantly higher than ACS group after adjusting for the confounder factors (P < 0.050 for ANA). Serum levels of ANA significantly correlated with severity of coronary stenotic lesions calculated by Gensini score (r = 0.40 and P < 0.050). After adjusting confounders, multiple linear regression analysis showed ANA remained independently associated with Gensini scores in ACS group (B = 0.505, P < 0.001).
CONCLUSION
Higher serum levels of ANA may be considered as independent risk factors for ACS.
Keywords: Rheumatoid Factor, Anti-Nuclear Antibodies, Acute Coronary Syndrome, Chronic Stable Angina, Gensini Score
Introduction
Atherosclerotic cardiovascular disease (CVD) is the major cause of mortality worldwide.1 Well-known CVD risk factors such as dyslipidemia, high blood pressure, diabetes, smoking, obesity, as well as genetic abnormalities, are related to only about half of the cases of coronary heart disease.2 A large amount of evidence supports the pivotal role of inflammation and immune responses in all phases of atherosclerosis, from initiation of the fatty streak to final breakout of acute coronary syndromes (ACS).3 Markers of inflammation, such as C-reactive protein, are predictive of future cardiovascular events in healthy individuals and may be useful in identifying patients with coronary artery disease who are at risk for recurrent CVD events.3,4 Several studies have documented an increased risk of atherosclerosis and myocardial infarction in patients with rheumatoid arthritis.5,6 In addition, rheumatoid arthritis is associated with a reduced life expectancy, primarily because of excessive deaths from CVD.7
Many recent published data showed that anti-nuclear antibodies (ANA) may contribute to the pathogenesis of atherosclerosis and ANA positivity is associated with the presence of coronary atherosclerosis.8 Although the association between serum levels of some inflammatory marker and ischemic heart disease (IHD) revealed, but the relationship ANA and rheumatoid factor (RF) with severity of coronary stenotic lesions have not evaluated yet.
In the present study, we aimed to find the association between RF and ANA levels with the severity of coronary stenotic lesions.
Materials and Methods
This cross-sectional study was carried out on, 140 consecutive subjects with IHD referred to the Chamran Hospital, Isfahan, Iran, between July 2013 and October 2013. Inclusion criteria are male subjects, which undergoing coronary angiography. Patients were classified into two groups according having ACS (n = 70) and chronic stable angina (CSA) (n = 70). ACS group included ST-elevation myocardial infarction, non ST-elevation myocardial infarction (NSTEMI) and unstable angina. Chronic stable angina typically manifests as a deep, poorly localized chest or arm discomfort (rarely described as pain), reproducibly precipitated by physical exertion or emotional stress, and relieved within 5-10 min by rest or sublingual nitroglycerin.9 In contrast, unstable angina is defined as angina pectoris (or equivalent type of ischemic discomfort) with at least one of three features: (1) occurring at rest (or minimal exertion) and usually lasting > 20 min (if not interrupted by the administration of a nitrate or an analgesic); (2) being severe and usually described as frank pain; or (3) occurring with a crescendo pattern (i.e., pain that awakens the patient from sleep or that is more severe, prolonged, or frequent than previously. Approximately, two-thirds of patients with unstable angina have evidence of myocardial necrosis on the basis of elevated cardiac serum markers, such as cardiac-specific troponin T or I and creatine kinase isoenzyme MB, and thus have a diagnosis of NSTEMI. The clinical diagnosis of myocardial infarction requires an integrated assessment of the history with some combination of indirect evidence of myocardial necrosis using biochemical, electrocardiographic, and imaging modalities.9
Exclusion criteria were valvular heart disease, any type of surgery, and trauma during the prior month, cardiomyopathy, liver disease, renal failure, arthritis, malignant diseases, and other inflammatory diseases and oral anticoagulant therapy. Age, smoking habits, history of hypertension and diabetes, dyslipidemia, family history of IHD s and current medications were carefully ascertained. Body mass index was calculated as weight/height2 (kg/m2). In patients with acute myocardial infarction, the serum concentration of autoantibodies was measured during 3-5 days after admission. In patients with a history of unstable angina and CSA the measurements were done at admission time. Peripheral blood (4 ml) was collected from two groups, and the serum was separated and stored at −20 °C.
Current smoking habits were self-reported. The body mass index, seated systolic blood pressure (SBP)/diastolic blood pressure (DBP) in the upper arm, and the plasma glucose, serum lipids levels were measured after an overnight fast. The glucose and lipids (total cholesterol, high-density lipoprotein cholesterol and triglycerides) were measured enzymatically. Participants who were not taking lipid-lowering medications were classified as having dyslipidemia if their low-density lipoprotein (LDL) cholesterol concentration exceeded of 160 mg/dl. All participants treated with lipid-lowering drugs were classified as having dyslipidemia. Hypertension was defined as SBP ≥ 140 mmHg or DBP ≥ 90 mmHg and currently took antihypertensive medications. Diabetes was defined as fasting plasma glucose ≥ 126 mg/dl and currently taking anti-diabetic treatment.9,10
Serum RF levels were measured by latex method. The serum levels of RF were quantitated using standard samples with known concentrations of the factor expressed as IU/ml, provided by the manufacturer. The normal range of RF has been reported to be < 20 IU/ml. The serum levels of ANA were also measured using a commercial the enzyme-linked immunosorbent assay (ELISA) kits. The serum levels of ANA were quantitated using standard samples with known concentrations of antibodies provided by the manufacturers. The serum levels of ANA was expressed as a unit with a normal range of < 1.2 U based on kits indicator.
Gensini score was calculated for each patient according to coronary angiography results. The score was computed by assigning a severity score to each coronary stenosis according to the degree of luminal narrowing and its geographic importance. Reduction in the lumen diameter, and the roentgenographic appearance of concentric lesions and eccentric plaques were evaluated (reductions of 25, 50, 75, 90, 99 percent, and complete occlusion, were given Gensini scores of 1, 2, 4, 8, 16, and 32, respectively). Each principal vascular segment was assigned a multiplier in accordance with the functional significance of the myocardial area supplied by that segment: the left main coronary artery × 5; the proximal segment of left anterior descending (LAD) coronary artery × 2.5; the proximal segment of the circumflex artery × 2.5; the mid-segment of the LAD × 1.5; the right coronary artery, the distal segment of the LAD, the postero-lateral artery, and the obtuse marginal artery ×1; and others × 0.5 (112).
Results were presented as mean ± standard deviation for quantitative variables (median and Interquartile range were reported for abnormal variables) were summarized by absolute frequencies and percentages for categorical variables. Logarithm transformed was used for abnormal variables. Continuous variables were compared using t independents sample t-test for normal variables and Mann-Whitney test for abnormal variables. Categorical variables were, on the other hand, compared using chi-square test or Fisher’s exact test when more than 20% of cells with expected count of < 5 were observed. To analyze the correlations of ANA and RF to Gensini score, we used Pearson correlation test. Regression test was used to determined relationship between ANA, RF and severity of coronary stenosis. Multiple linear regression used to control the effect of age and other confounder factors such hypertension, diabetes, hyperlipidemia, and smoking.
P < 0.050 was considered as statistically significant. For the statistical analysis, statistical software SPSS for Windows (version 20.0, SPSS Inc., Chicago, IL, USA) was used.
Results
The mean of age was 56.37 ± 10.75 and 60.0 ± 10.53 in CSA and ACS group, respectively. Table 1 shows baseline characteristics in study population. Pharmacological treatment by Aspirin and statins are more prevalent among CSA group (P < 0.001). Then the Gensini scoring system was introduced to evaluate the severity and extent of coronary stenotic lesions. The mean serum levels of RF, ANA and Gensini score in study groups are demonstrated in table 2. The median and isolated thoracic aortitis serum levels of RF in CSA group 3.0 (1.0-5.0) IU/ml were significantly higher than ACS group 3 (1.0-8.0) IU/ml, P = 0.589). Pearson correlation test for RF, ANA and Gensini score showed ANA is positively correlated with Gensini score (r = 0.247 and P = 0.004), but no significant correlation has seen between RF and Gensini score (r = −0.001 and P = 0.994) (data not shown). After adjusting for confounders such as age, hypertension, diabetes, hyperlipidemia and smoking multiple linear stepwise regression analysis showed ANA remained independently associated with Gensini scores in ACS group (B = 0.471, P < 0.001) so could be consider as a predictor for coronary artery disease severity (Table 3).
Table 1.
Baseline characteristics in study population
| Variable | ACS | CSA | P |
|---|---|---|---|
| Age (mean ± SD) | 56.37 ± 10.75 | 60.0 ± 10.53 | 0.046 |
| Body mass index (mean ± SD) | 25.16 ± 3.92 | 25.60 ± 3.80 | 0.494 |
| Hypertension [n (%)] | 26 (37.7) | 28 (40.0) | 0.779 |
| Hyperlipidemia [n (%)] | 14 (20.0) | 28 (40.0) | 0.010 |
| Diabetes mellitus [n (%)] | 23 (32.9) | 23 (32.9) | > 0.999 |
| Smoking [n (%)] | 33 (47.1) | 30 (42.9) | 0.610 |
| FH [n (%)] | 32 (45.7) | 27 (38.6) | 0.392 |
| Statin use [n (%)] | 12 (17.1) | 34 (48.6) | < 0.001 |
| Aspirin use [n (%)] | 35 (50.0) | 55 (78.6) | < 0.001 |
Chi-square, t test; SD: Standard deviation; ACS: Acute coronary syndrome
CSA: Chronic stable angina; FH: Familial hypercholesterolemia
Table 2.
Rheumatoid factor (RF) and Gensini score in study population
| Variable | ACS | CSA | P |
|---|---|---|---|
| RF (IU/ml)* | 3.00 (1.0-5.0) | 3.0 (1.0-8.0) | 0.589 |
| ANA (IU/ml)* | 0.28 (0.2-0.4) | 0.4 (0.3-0.6) | 0.001 |
| Gensini | 25.70 (13.0-42.5) | 26.0 (15.7-58.0) | 0.209 |
Median (interquartile range);
Mann-Whitney test; ACS: Acute coronary syndrome; CSA: Chronic stable angina RF: Rheumatoid factor; ANA: Anti-nuclear antibodies
Table 3.
Relationship between rheumatoid factor (RF) and anti-nuclear antibodies (ANA) with Gensini score (log transformed) in study population
| Study groups | Crude |
Adjusted model |
||||||
|---|---|---|---|---|---|---|---|---|
| RF |
ANA |
RF |
ANA |
|||||
| Standard beta | P | Standard beta | P | Standard beta | P | Standard beta | P | |
| ACS | -0.073 | 0.55 | 0.400 | 0.001 | -0.102 | 0.880 | 0.505 | < 0.001 |
| CSA | -0.020 | 0.87 | 0.037 | 0.767 | -0.034 | 0.797 | 0.013 | 0.922 |
Adjusted model: For controlling the effect of age and other confounder factors such hypertension, diabetes, hyperlipidemia and smoking we used multiple linear regression; RF: Rheumatoid factor; ANA: Anti-nuclear antibodies; ACS: Acute coronary syndrome; CSA: Chronic stable angina
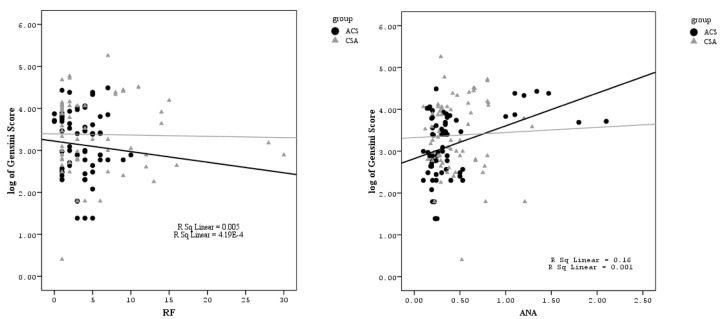
Using multivariate regression analysis for all collected variables, we demonstrated that Gensini scores were significantly associated with ANA (Figure 1).
Figure 1.
Linear regression analysis to demonstrate relationship between anti-nuclear antibodies and (log transformed) Gensini scores ACS: Acute coronary syndrome; CSA: Chronic stable angina; ANA: Anti-nuclear antibodies
Discussion
Our results appear to show ANA could be considered as a strong predictor for coronary artery disease. Our results showed an association between RF in male patients with a history of CSA. Moreover, we found that as this study was conducted in a relatively small population, and the correlation was not very strong. However, if the results are confirmed in large studies,7,8,11,12 the present findings may provide new insights into the physiological roles of RF and ANA in atherosclerosis.
Previous study which carried out by Chang et al. showed RF is present in up to 15% of elderly subjects and may arise through polyclonal B cell activation due to infectious organisms or by antigen-driven proliferation of B cells associated with autoimmune diseases, including rheumatoid arthritis.13 He demonstrated as RF is strongly associated with rheumatoid arthritis so it is associated with increased cardiovascular morbidity and mortality, the increased risk in their study population is unlikely to be due to active rheumatoid arthritis or its treatment.14 Other studies have reported that higher level of RF is an independent risk factor for CAD in men from the general population.15 Our result is the same as that reported by Edwards et al.16 They investigated whether the presence of RF was associated with an increased risk of coronary artery disease among a population of elderly men and women in the Hertfordshire Cohort Study. They found that RF was associated with an increased likelihood of coronary artery disease in men (odds ratio = 3.1, 95% confidence interval 1.7-5.4, P < 0.001), but not in women. RF appears to cause direct tissue damage in RA as a component of immune complexes via activating the complement system.17 It may cause damage to the endothelium in a similar manner in IHD. The presence of immunoglobulins and complement components has been demonstrated in atherosclerotic plaques providing evidence for immune complex reactions.18 Indeed autoantibody productions are conditions strongly associated with RA. A few autoantibodies including anti-oxidized (ox LDL), RF, anti-cyclic citrullinated peptides, ANA, anticardiolipin antibodies are considered autoimmune factors associated with atherosclerosis in autoimmune disease, as well as in the general populations.19 In the conventional view, the antigen-antibody reaction is prone to enhance inflammation and results in exacerbation of atherosclerosis.
According to previous studies the RF and ANA are likely to play an important role in atherogenesis, but our result showed that these factors cannot be used as precipitating factor of ACS. However, these factors are not acute inflammatory factors and present in chronic inflammatory disease. Indeed the initiation of the atherosclerotic process can occur early in life,20 and these auto-antibodies may play role in the initiation of the atherosclerotic process. In the some study association between ANA and IHD was unclear.21
Limitation
The present study is limited by its cross-sectional nature and small size population, so we could not evaluate outcome measures. This study strength using Gensini score to evaluate the extent and severity of coronary stenotic lesions.
Conclusion
RF and ANA is likely to play an important role in atherogenesis, but our result showed that Higher serum levels of ANA in patients with IHD may be considered as independent risk factors for ACS.
Acknowledgments
This study was financially supported by a grant (No. 392050) as a residency thesis from Isfahan University of Medical Sciences, Isfahan, Iran.
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104(22):2746–53. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 2.Buttar HS, Li T, Ravi N. Prevention of cardiovascular diseases: Role of exercise, dietary interventions, obesity and smoking cessation. Exp Clin Cardiol. 2005;10(4):229–49. [PMC free article] [PubMed] [Google Scholar]
- 3.Getz GS, Vanderlaan PA, Reardon CA. The immune system and murine atherosclerosis. Curr Drug Targets. 2007;8(12):1297–306. doi: 10.2174/138945007783220669. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 5.Liuzzo G, Biasucci LM, Gallimore JR, Grillo RL, Rebuzzi AG, Pepys MB, et al. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med. 1994;331(7):417–24. doi: 10.1056/NEJM199408183310701. [DOI] [PubMed] [Google Scholar]
- 6.Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107(9):1303–7. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 7.Park YB, Ahn CW, Choi HK, Lee SH, In BH, Lee HC, et al. Atherosclerosis in rheumatoid arthritis: morphologic evidence obtained by carotid ultrasound. Arthritis Rheum. 2002;46(7):1714–9. doi: 10.1002/art.10359. [DOI] [PubMed] [Google Scholar]
- 8.Liang KP, Kremers HM, Crowson CS, Snyder MR, Therneau TM, Roger VL, et al. Autoantibodies and the risk of cardiovascular events. J Rheumatol. 2009;36(11):2462–9. doi: 10.3899/jrheum.090188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaziano JM. Global burden of cardiovascular disease. In: Bonow RO, Mann DL, Zipes DP, Libby P. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 9th. Philadelphia, PA: Elsevier Health Sciences; 2011. p. 935. [Google Scholar]
- 10.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51(3):606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 12.Jafarzadeh A, Poorgholami M, Nemati M, Rezayati MT. High serum levels of rheumatoid factor and anti-phosphatidylserine antibody in patients with ischemic heart disease. Iran J Immunol. 2011;8(1):34–44. [PubMed] [Google Scholar]
- 13.Chang MH, Ma T, Liou Y, Tien L, Wu EH, Lai CH, et al. Rheumatoid Factor is a Strong Risk Factor for Coronary Artery Disease in Men with Metabolic Syndrome. Acta Cardiol Sin. 2010;26:8993. [Google Scholar]
- 14.Abou-Raya S, Abou-Raya A, Naim A, Abuelkheir H. Rheumatoid arthritis, periodontal disease and coronary artery disease. Clin Rheumatol. 2008;27(4):421–7. doi: 10.1007/s10067-007-0714-y. [DOI] [PubMed] [Google Scholar]
- 15.Avalos I, Chung CP, Oeser A, Gebretsadik T, Shintani A, Kurnik D, et al. Increased augmentation index in rheumatoid arthritis and its relationship to coronary artery atherosclerosis. J Rheumatol. 2007;34(12):2388–94. [PubMed] [Google Scholar]
- 16.Edwards CJ, Syddall H, Goswami R, Goswami P, Dennison EM, Arden NK, et al. The autoantibody rheumatoid factor may be an independent risk factor for ischaemic heart disease in men. Heart. 2007;93(10):1263–7. doi: 10.1136/hrt.2006.097816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, Gabriel SE. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005;52(2):402–11. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 18.Tighe H, Carson DA. Rheumatoid factor. In: Ruddy S, Harris ED, Sledge CB. Kelley's Textbook of Rheumatology. 6th. Philadelphia, PA: W.B. Saunders Company; 2001. pp. 151-60.–60. [Google Scholar]
- 19.Vossenaar ER, Robinson WH. Citrullination and autoimmune disease: 8th Bertine Koperberg meeting. Ann Rheum Dis. 2005;64(10):1513–5. doi: 10.1136/ard.2005.045716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuzcu EM, Kapadia SR, Tutar E, Ziada KM, Hobbs RE, McCarthy PM, et al. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation. 2001;103(22):2705–10. doi: 10.1161/01.cir.103.22.2705. [DOI] [PubMed] [Google Scholar]
- 21.Holmqvist ME, Wedren S, Jacobsson LT, Klareskog L, Nyberg F, Rantapaa-Dahlqvist S, et al. No increased occurrence of ischemic heart disease prior to the onset of rheumatoid arthritis: results from two Swedish population-based rheumatoid arthritis cohorts. Arthritis Rheum. 2009;60(10):2861–9. doi: 10.1002/art.24855. [DOI] [PubMed] [Google Scholar]