Abstract
Purpose
To demonstrate the use of a spectral-domain optical coherence tomography (SDOCT) integrated surgical microscope in glaucoma surgery.
Methods
An SDOCT system was used to interface directly with an ophthalmic surgical microscope, to allow real-time intraoperative SDOCT (iOCT) imaging during glaucoma procedures like phaco-trabeculectomy, Ahmed glaucoma valve (AGV) implantation, gonio-synechiolysis, and bleb needling. The various surgical steps during glaucoma surgeries where iOCT can be of potential help in guiding the surgeon were recorded.
Results
High-resolution, cross-sectional images of the relevant structures were achieved with the iOCT system in all procedures. The surgeon could determine the depth of the scleral dissection, the intrastomal bed, the path of the AGV tube in the eye, the release of peripheral anterior synechiae and the efficacy of needling with respect to breakage of loculations; most of these are technically ‘blind' procedures, where the outcomes are determined postoperatively. Metallic instruments cast a shadow on tissues below, thereby restricting the use of the device in its current state.
Conclusions
The iOCT system provided high quality, intraoperative, real-time imaging, which could possibly improve the safety and efficacy of the surgical procedures in glaucoma. Further studies and modifications to the iOCT are required to better understand and increase the uptake of this technology in daily practice.
Translational Relevance
The iOCT, with further advancements in its technology, could potentially provide the surgeon both quantitative and qualitative, real-time depth and tissue proximity details, thus improving the safety and accuracy of glaucoma surgery.
Keywords: glaucoma, trabeculectomy, glaucoma drainage implant, optical coherence tomography
Introduction
Optical coherence tomography (OCT) has revolutionized ophthalmology by providing the clinician an opportunity to get histology-like images of ocular structures in vivo in the clinic.1–6 A logical progression would be the integration of the OCT as an intraoperative tool to provide the surgeon a real-time, high resolution, cross-sectional, three-dimensional (3D) view of ocular tissues during surgery.
There have been previous attempts to introduce the spectral-domain (SD) OCT in to the operation theatre. The initial commercially available systems were handheld OCT devices or modified table top units, which allowed for intraoperative imaging, but required the surgeon to interrupt the surgical procedure to image. This precluded real-time feedback to the surgeon of the anatomical impact of surgical manoeuvres and increased the duration of the surgical procedure.7–10
With the recent introduction of real-time intraoperative SDOCT (iOCT) integrated on to a microscope, real-time imaging of the tissues of surgical interest and tissue-instrument interactions have become feasible.11–18 While the current focus of the published reports have been for vitreo-retinal procedures, this tool can be of potential help in glaucoma surgeries as well.11–18 In this article, we evaluate the beneficial role and pitfalls of the iOCT, while performing select glaucoma procedures like phaco-trabeculectomy, Ahmed glaucoma valve (AGV) implantation, gonio-synechiolysis, and bleb needling.
Methods
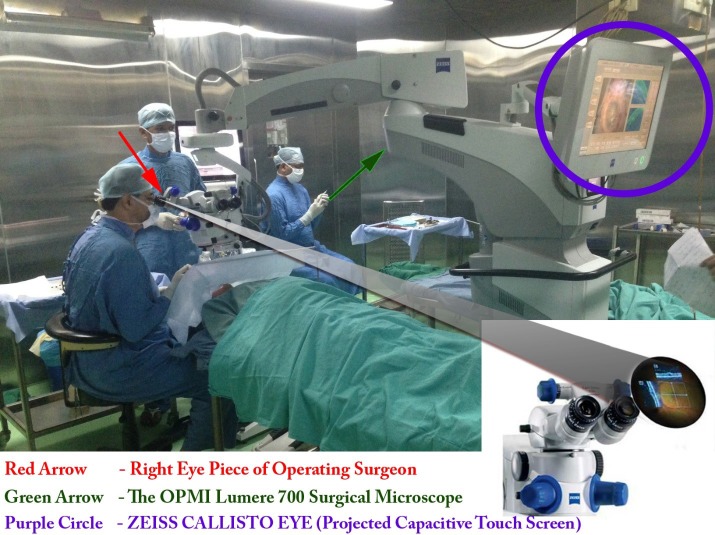
The commercially available OPMI Lumera 700 surgical microscope with an integrated RESCAN 700 SD-OCT (Carl Zeiss Meditec, Jena, Germany) was used in all surgical procedures (Fig. 1). This microscope allows real-time SDOCT visualization intraoperatively for both anterior and posterior segment surgery; no additional lens systems are required for the anterior segment. A 22″ liquid-crystal display (LCD) display screen, the Zeiss Calisto Eye, provides an intraperative surgical field on the left of the screen and a live OCT view on the right; the SDOCT images are recorded in both horizontal and vertical orientations. Written informed consent was taken from all subjects for recording the surgical procedures prior to surgery and the study adhered to the tenets of the Declaration of Helsinki.
Figure 1. .
Orientation of the surgical microscope and integrated intraoperative OCT; the OCT images are projected as a heads-up display simultaneously in the surgeon's operating field (inset) and on an LCD display screen (denoted by the circle).
The integrated OCT uses a wavelength of 840 nm and captures 27,000 A-scans per second. The device has a scan depth of 2 mm and scan length that varies from 3 to 16 mm. The axial and transverse resolutions are 5 μm and 15.5 μm, respectively. The RESCAN 700 also incorporates Z-tracking and focus control for image stabilization and image quality control. There are multiple scan protocols including ‘cube' B scans (Fig. 2a) and raster (1 or 5 line) scans (Fig. 2b). The surgeon can control the length, angle, and location of the scan either with an integrated foot pedal or with the video monitor display system.
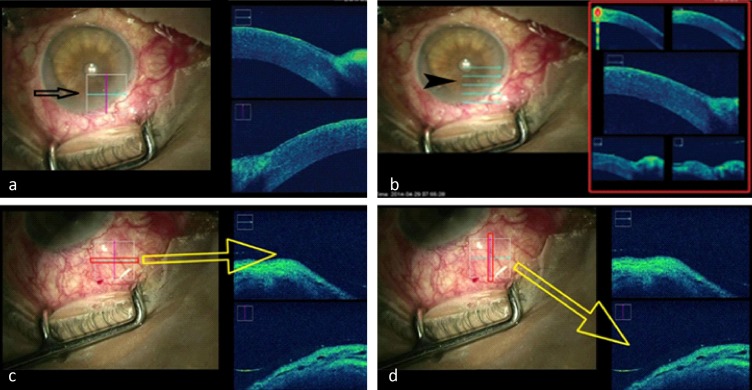
Figure 2. .
Types of scans available: left side (black arrow) shows a cube scan (a) and the right side (arrow head) shows 5-line raster scans (b); horizontal (c) and vertical (d) scans of the regions of interest and the corresponding live OCT imaging. Cross-section at the level of the blue horizontal line is shown at the top right OCT image (c) and the red vertical line is shown at the bottom right OCT image (d).
A single observer controlled and recorded all the scans with inputs from and to the surgeon so that the image quality and region of interest (ROI) was standardized. SDOCT scanning was performed through the entire length of all procedures. The output were stored in the Callisto system and later retrieved and reviewed. We used cube scans with horizontal and vertical B scans (Figs. 2c, d) that can be increased or decreased in dimensions manually, was used to image the ROI. The size of the cube was adjusted in order to achieve optimal ROI and the desirable resolution. The surgeon could provide and use live inputs due to the availability of a real time ‘heads up' display that was relayed in the surgical field of the microscope.
Of the four eyes (4 patients) that underwent surgical intervention, the first patient had medically uncontrolled primary open-angle glaucoma with a visually significant cataract; phaco-trabeculectomy was performed. The second patient underwent AGV implantation for intractable glaucoma following a failed penetrating keratoplasty. The patient that underwent phacoemulsification and gonio-synechiolysis had a history of acute angle closure with 270° peripheral anterior synechiae (PAS) and visually significant cataract. Another patient with a failing trabeculectomy bleb and raised intraocular pressure (IOP) underwent bleb needling with intraoperative injection of subconjunctival Mitomycin C.
Results
The iOCT real-time imaging was successfully done in all four cases; in the patient that underwent tube implantation, we could not record real-time surgical sections of the AGV implantation procedure due to technical difficulties where the display screen went offline during the procedure.
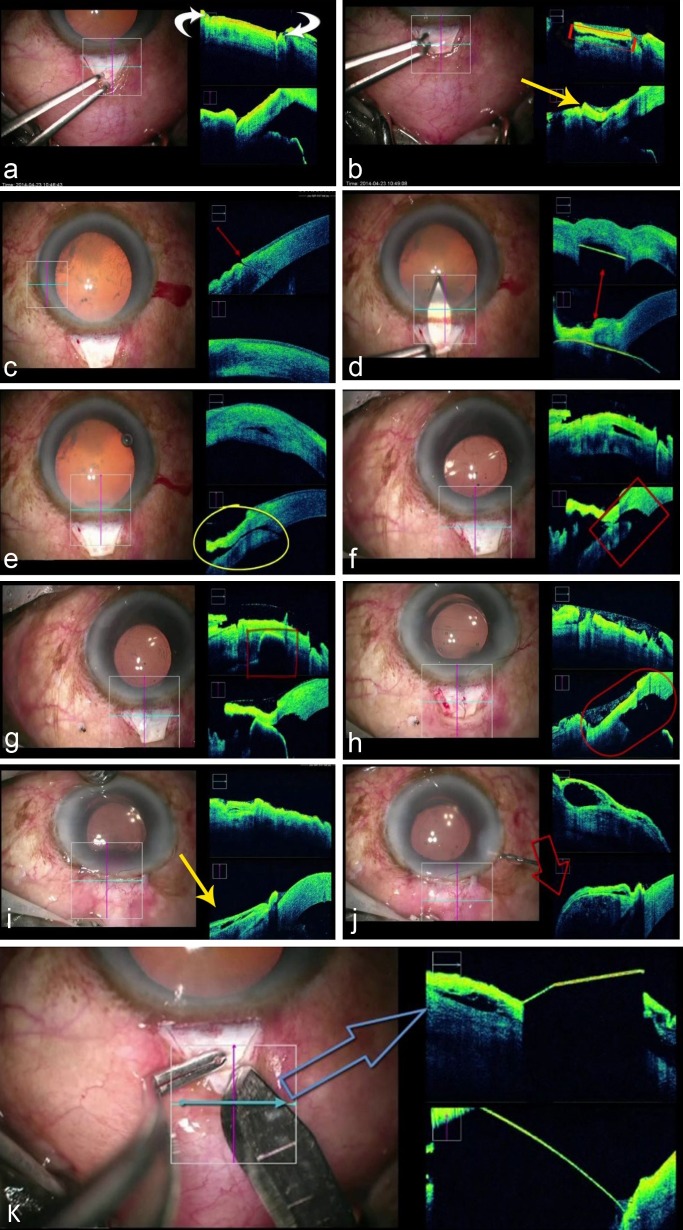
In the first patient who underwent phaco-trabeculectomy, the OCT allowed the surgeon to visualize the depth of the scleral flap dissection, side port and mainport entry, the triplanar entry wound, the trabecular stoma after deeper scleral block removal and the bleb at the end of the procedure (Fig. 3). Figure 3k demonstrates the path of the surgical instruments and the lack of visualization of structures posterior to the instruments due to impedance of the OCT light source wavelength.
Figure 3. .
Various steps of phaco-trabeculectomy and corresponding OCT scans: (a) scleral incision for the superficial scleral flap; (b) scleral flap being elevated (yellow arrow) and scleral bed after creating the flap (area in red rectangle); (c) uniplaner side port incision; (d) course of the keratome entry (hyperreflective line in both images on the OCT); (e) triplanar configuration of the main port incision; (f) inner stoma removal; (g) and (h) shows absence of deep stromal tissue–internal ostium; (i) conjuctiva before, and (j) elevated bleb after saline injection through side port and (k) demonstrating the ‘shadowing effect' caused by obstruction of light waves by metallic instruments, thus obscuring the view of underlying tissue.
The second procedure recorded was AGV implantation in an eye with refractory glaucoma in postpenetrating keratoplasty. The iOCT was of help in determining the PAS at the graft host junction. This system provided an option of viewing the intrascleral track, entry of tube in to anterior chamber, and the actual distance from cornea and iris, thus converting a blind procedure to a procedure under direct visualization (Fig. 4).
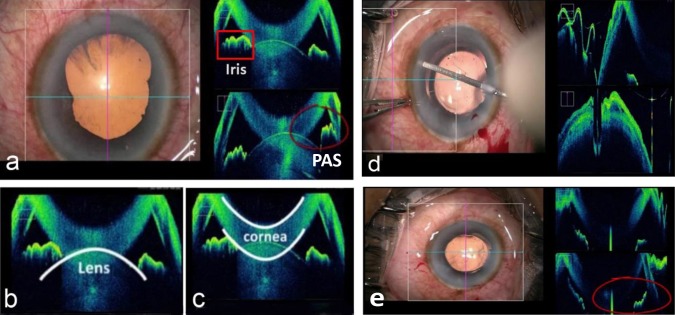
Figure 4. .
Preoperative color photograph of the patient with intractable glaucoma following failed corneal graft (a, b). iOCT image shows PAS at graft host junction (c) and cut section of Ahmed valve tube (d); the outer point of entry of the tube insertion wound is seen (e) along with the plane of insertion of the tube through the intra scleral tunnel (f); the red oval circle shows the well positioned tube in the anterior chamber (g).
In the patient that underwent gonio-synechiolysis along with phacoemulsification, a blunt spatula was used to perform gonio-synechiolysis under iOCT guidance. Figure 5a shows the presence of PAS prior to the procedure while, Figure 5e shows that the iris having fallen back after the PAS has been released.
Figure 5. .
Goniosynechiolysis under iOCT guidance: OCT image shows presence of peripheral anterior synechiae (red oval) (a); (b) and (c) OCT images to landmark the lens and cornea; (d) shows the blunt spatula while performing goniosynechiolysis and (e) shows the iris that has fallen back after PAS has been released (red oval).
In the eye where bleb needling with intraoperative subconjunctival MMC was performed to revive a failing bleb with episcleral fibrosis, the iOCT demonstrated the extent of adhesions and loculations inside the bleb at the beginning of the procedure (Fig. 6a), the breakage of these adhesions and formation of a large single hyporeflective cavity inside the bleb that was evident at the end of procedure (Fig. 6b).
Figure 6. .
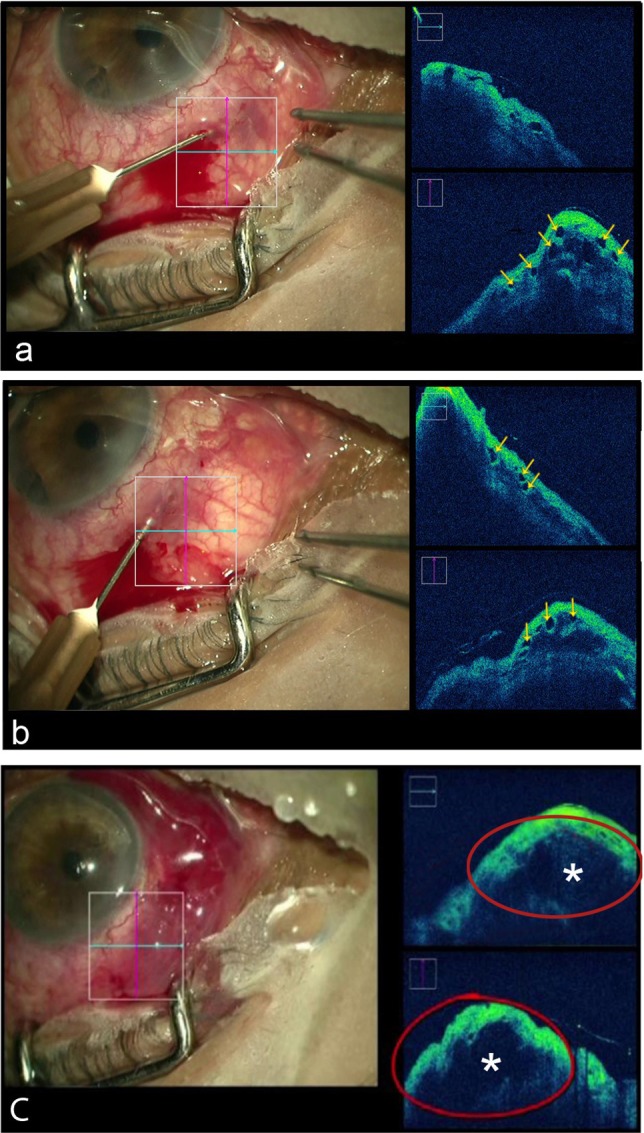
Use of iOCT in bleb needling under direct view: (a, b) yellow arrows show areas of intrableb loculations suggestive of fibrosed tissue before procedure; (c) the asterisks shows a single hyporeflective cavity inside the bleb at the end of the procedure.
Discussion
This article demonstrates the feasibility of real-time iOCT imaging in glaucoma surgery. The evolution of intraoperative OCT started with preclinical animal studies predominantly for use in vitreoretinal surgeries. The role of the intraoperative real-time iOCT in vitreoretinal surgeries is now well established.11–18
Ehlers et al.13 demonstrated the feasibility of real-time iOCT for anterior and posterior segment surgery with a microscope integrated iOCT system with heads-up display surgeon feedback; both real-time and static imaging were obtained. The iOCT (RESCAN 700) also has a heads-up display system; a similar system with is the Cole Eye Institute microscope integrated iOCT prototype (Cleveland, Ohio, USA).13,18,19 Ehlers et al.1 first reported the successful integration of a microscope mounted OCT (MMOCT) into a surgical microscope, where the same platform was used to record surgeries in both animal and human volunteers.
Handheld SDOCT (Envisu; Bioptigen, Inc.) has been previously used to obtain high-resolution intraoperative images.20 One of the major limitations of the handheld devices is the lack of stability of a fixed platform and the need for an experienced technician to acquire good quality images; motion artefacts during acquisition can result in poor image quality. Another concern is that since the device has to be stabilized by using a support bar, intraoperative sterility might be compromised.8 Further limitations include the lack of image registration, thereby impeding repeatability and that the images are cross-sectional and not real-time in vivo. This means that acquisition of scans require considerable skill and time, which can be potentially disruptive to the surgeon and decrease the uptake of intraoperative imaging by surgeons.7-10
The potential applications in glaucoma surgeries have been summarized in our paper. In trabeculectomy, the iOCT could be useful in training residents and fellows with regard to the depth of the scleral flap and to possibly determine completeness and extent of the stromal ostium. The depth and thickness of the superficial scleral flap could be critical in achieving a functioning bleb; this importance is more accentuated in nonpenetrating glaucoma surgeries wherein prevention of ‘penetration' is vital for a successful procedure. The iOCT could also potentially help the surgeon in determining if the iris is plugging the ostium at the end of the surgery. During glaucoma drainage device implantation, the iOCT could possibly play a role in determining proper intrascleral entry and placement of tube in the anterior chamber/pars plana. The nearly blind procedure of creating an intrascleral passage for the AGV tube and precise positioning of the tube in the anterior chamber are crucial in the success of valve placement. While performing goniosynechiolysis, the iOCT could allow the surgeon to actually visualize the real-time posterior displacement of the iris after goniosynechiolysis with improvements in technology in the future. While performing goniosynechiolysis, the iOCT could allow the surgeon to perform the procedure without the use of an intraoperative gonioscopy lens. It could also help in visualization of the extent of the PAS, assessment of adequacy of synechiolysis, and judging the extent of angle opened at the end of the procedure, if the hardware and the software are further advanced to improve localization and image quality. The inverted image of the cornea (Fig. 5) is a well-known entity noted when using Fourier domain OCT; imaging on the OCT involves a series of Fourier transforms to resolve the depth information in the image; this transform of the interference spectrum results in a “real” and “imaginary” image (similar to the concept of complex numbers). It is this imaginary image that appears as the inverted image in the OCT; the observer has to simply ignore the inverted image.
One of the areas where the iOCT could be useful as a definite role is the direct visualization of the anatomical success of bleb needling; the breaking of adhesions and the opening of the scleral bed under the conjunctiva is currently at best a blind procedure with the formation of a diffuse bleb at the end of the surgery, the only indicator.
To the best of our knowledge, this is the first report of the use of the iOCT in glaucoma surgeries. The iOCT might not find a role in routine glaucoma procedures, neither might it replace the practice of using goniolens for angle procedures. However, it could provide an alternative method to deal with angle imaging in hazy corneas as in failed keratoplasty or in cases of congenital glaucoma with opaque corneas. Similarly in microincision glaucoma surgeries, the iOCT might help in placement of stents like the iStent and Express implants in the appropriate anatomical space.
One of the applications of the iOCT in the future could be as a teaching tool for residents and fellows; the ability to record and document the surgery would be an added advantage. This technology might also reduce the steep learning curve of technically challenging surgeries (like nonpenetrating glaucoma surgeries and drainage device implantation).
As with every new technology, the iOCT has got limitations. The majority of the limitations of this tool are surgeon and machine related. The restricted scanning area and the need to move the scanning zone to target the instrument tip requires a rather cumbersome coordination between the surgeon and assistant/technician manning the scanner; refocussing to the ROI is constantly required to achieve good quality images.1 Distortion of images during eye or microscope movements also causes motion artefacts. There is a learning curve for focusing and aligning the ROI and the inconvenience of simultaneously looking at both the surgical field and the OCT image simultaneously. The synchronization between the two areas under observation has a definite learning curve. The other major limitation is the shadowing effect caused by the metallic instruments currently used, that renders the underlying tissues invisible (Fig. 5).13
The integration of real-time in vivo OCT imaging with the operating microscope is still in its infancy. Instruments that would allow the wavelength of the OCT light to pass through need to be developed to overcome the shadowing effect. Improvements in the resolution of the integrated OCT and possible color coding to determine tissue thickness could be other potential areas that need to be addressed.
An area where real-time intraoperative OCT guidance could possibly improve performance during surgery is during tube implantation via a scleral tunnel (essentially a blind procedure), determining the depth of the scleral flap during nonpenetrating glaucoma procedures and visualization of the Schlemm's canal during canalostomy. The use of the device would also increase if real-time quantitative measures are available along with desired depth proximity alarms.
Advancements to make the iOCT more interactive to include surgeon feedback regarding the depth of the incision and integrating lasers to this effect using built-in software could pave the way for a controlled ‘robotic' approach to ophthalmic surgery.
The still images and live real-time imaging demonstrated by the SDOCT provides guidance to the surgeon in two dimensions, and it would be interesting to see if 3D imaging can further enhance the safety and efficacy of the procedure. Given the logistics and technical issues associated with creation of true 3D images, it would be rather too early to consider bringing 3D into ophthalmic surgery. Nonetheless, the authors believe that if such imaging technology is made available, then it is likely to not only increase the safety of surgical procedures but also reduce the learning curve for the beginners.
We believe that future studies should determine if any significant differences (if any) of ease of surgery and quality of desired outcome after surgery (e.g., depth achieved in nonpenetrating procedures or intraoperative visualization in viscocanalostomy) exist by including a control group (a non-SDOCT guided group); this would increase the validity of incorporating the technology intraoperatively. Studies comparing longitudinal outcomes, intra-and postoperative results of SDOCT guided versus nonguided surgery would possibly increase the uptake of OCT guided procedures.
Further studies and modifications to the iOCT are required to better understand and increase the uptake of this technology in daily practice. The prohibitive costs could potentially truncate the role of this promising technology as a teaching tool and be a major hurdle in the device uptake, as was the case when the OCT was introduced initially as an imaging tool in ophthalmology.
Acknowledgments
The authors thank Abdul Muthalib for audio-visual editing.
Disclosure: R.S. Kumar, None; M.U. Jariwala, None; Sathidevi A. V, None; J.P. Venugopal, None; N.K. Puttaiah, None; D.R.A. S, None; R. Balu, None; R. Shetty, None
References
- 1.Ehlers JP, Tao YK, Farsiu S, et al. Visualization of real-time intraoperative manoeuvres with a microscope-mounted spectral domain optical coherence tomography system. Retina. 2013;33:232–236. doi: 10.1097/IAE.0b013e31826e86f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen TC, Zeng A, Sun W, et al. Spectral domain optical coherence tomography and glaucoma. Int Ophthalmol Clin. 2008;48:29–45. doi: 10.1097/IIO.0b013e318187e801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen TC, Cense B, Pierce MC, et al. Spectral domain optical coherence tomography: ultrahigh-speed, ultra high resolution ophthalmic imaging. Arch Ophthalmol. 2005;123:1715–1720. doi: 10.1001/archopht.123.12.1715. [DOI] [PubMed] [Google Scholar]
- 4.Sharma R, Sharma A, Arora T, et al. Application of anterior segment optical coherence tomography in glaucoma. Surv Ophthalmol. 2014;59:311–327. doi: 10.1016/j.survophthal.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Lee L. Clinical applications and new developments of optical coherence tomography: an evidence-based review. Clin Exp Optom. 2007;90:317–335. doi: 10.1111/j.1444-0938.2007.00151.x. [DOI] [PubMed] [Google Scholar]
- 6.Matonti F, Hoffart L, Alessi G, et al. Spectral-domain optical coherence tomography in anterior segment imaging: the 3rd dimension. J Fr Ophtalmol. 2009;32:727–734. doi: 10.1016/j.jfo.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Dayani PN, Maldonado R, Farsiu S, et al. Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery. Retina. 2009;29:1457–1468. doi: 10.1097/IAE.0b013e3181b266bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chavala SH, Farsiu S, Maldonado R, et al. Insights into advanced retinopathy of prematurity using handheld spectral domain optical coherence tomography imaging. Ophthalmology. 2009;116:2448–2456. doi: 10.1016/j.ophtha.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maldonado RS, Izatt JA, Sarin N, et al. Optimizing hand-held spectral domain optical coherence tomography imaging for neonates, infants, and children. Invest Ophthalmol Vis Sci. 2010;51:2678–2685. doi: 10.1167/iovs.09-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malhotra C, Shetty R, Kumar RS, et al. In vivo imaging of riboflavin penetration during collagen cross-linking with hand-held spectral domain optical coherence tomography. J Refract Surg. 2012;28:776–780. doi: 10.3928/1081597X-20121011-05. [DOI] [PubMed] [Google Scholar]
- 11.Ehlers JP, Tao YK, Farsiu S, et al. Integration of a spectral domain optical coherence tomography system into a surgical microscope for intraoperative imaging. Invest Ophthalmol Vis Sci. 2011;52:3153–3159. doi: 10.1167/iovs.10-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binder S, Falkner-Radler CI, Hauger C, et al. Feasibility of intrasurgical spectral-domain optical coherence tomography. Retina. 2011;31:1332–1336. doi: 10.1097/IAE.0b013e3182019c18. [DOI] [PubMed] [Google Scholar]
- 13.Ehlers JP, Kaiser PK, Srivastava SK. Intraoperative optical coherence tomography using the RESCAN 700: preliminary results from the DISCOVER study. Br J Ophthalmol. 2014;98:1329–1332. doi: 10.1136/bjophthalmol-2014-305294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehlers JP, Ohr MP, Kaiser PK, et al. Novel micro-architectural dynamics in rhegmatogenous retinal detachments identified with intraoperative optical coherence tomography. Retina. 2013;33:1428–1434. doi: 10.1097/IAE.0b013e31828396b7. [DOI] [PubMed] [Google Scholar]
- 15.Ehlers JP, Tam T, Kaiser PK, et al. Utility of intraoperative optical coherence tomography during vitrectomy surgery for vitreo-macular traction syndrome. Retina. 2014;34:1341–1346. doi: 10.1097/IAE.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehlers JP, Xu D, Kaiser PK, et al. Intrasurgical dynamics of macular hole surgery: an assessment of surgery-induced ultra-structural alterations with intraoperative optical coherence tomography. Retina. 2014;34:213–221. doi: 10.1097/IAE.0b013e318297daf3. [DOI] [PubMed] [Google Scholar]
- 17.Ehlers JP, Kernstine K, Farsiu S, et al. Analysis of pars plana vitrectomy for optic pit-related maculopathy with intraoperative optical coherence tomography: a possible connection with the vitreous cavity. Arch Ophthalmol. 2011;129:1483–1486. doi: 10.1001/archophthalmol.2011.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehlers JP, Tao YK, Srivastava SK. The value of intraoperative optical coherence tomography imaging in vitreo-retinal surgery. Curr Opin Ophthalmol. 2014;25:221–227. doi: 10.1097/ICU.0000000000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehlers JP, Kaiser PK, Singh RP, et al. Application of intraoperative OCT to ophthalmic surgery: PIONEER 18-month iOCT vitreoretinal results. Am J Ophthalmol. 2014;33:887–892. [Google Scholar]
- 20.Hahn P, Migacz J, O'Donnell R, et al. Preclinical evaluation and intraoperative human retinal imaging with a high-resolution microscope-integrated spectral domain optical coherence tomography device. Retina. 2013;33:1328–1337. doi: 10.1097/IAE.0b013e3182831293. [DOI] [PMC free article] [PubMed] [Google Scholar]