Abstract
The immunosuppressant drug FK506 acts by binding to receptor proteins, FK506-binding proteins (FKBPs), which in turn can bind to and regulate a Ca(2+)-dependent phosphatase, calcineurin, and a Ca2+ release channel, the ryanodine receptor. Based on our findings in regeneration models that levels of FKBPs during neural regeneration parallel those of growth-associated protein GAP43, a calcineurin substrate that regulates neurite extension, we examined effects of FK506 in PC12 rat pheochromocytoma cells and in rat sensory ganglia. FK506 enhances neurite outgrowth in both systems by increasing sensitivity to nerve growth factor. Blockade of FK506 actions in sensory ganglia by rapamycin, an FK506 antagonist, establishes that these effects involve FKBPs. Rapamycin itself stimulates neurite outgrowth in PC12 cells. These drug effects are detected at subnanomolar concentrations, suggesting therapeutic application in diseases involving neural degeneration.
Full text
PDF
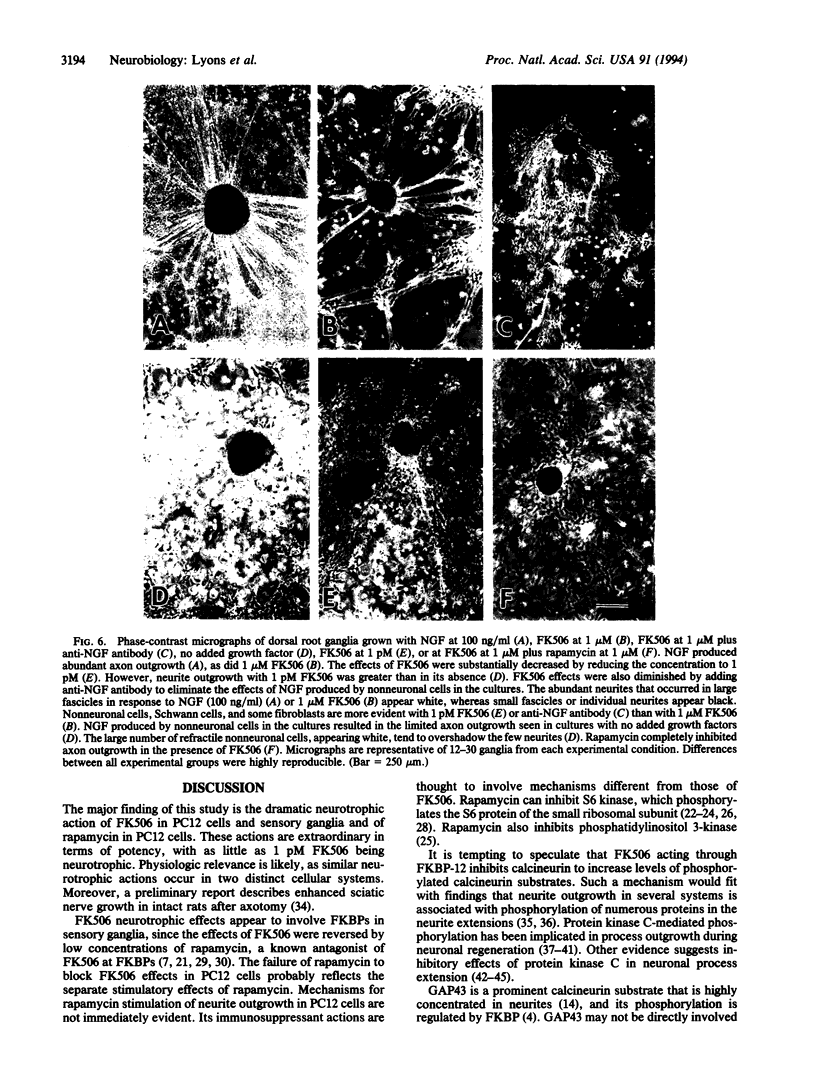

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baetge E. E., Hammang J. P. Neurite outgrowth in PC12 cells deficient in GAP-43. Neuron. 1991 Jan;6(1):21–30. doi: 10.1016/0896-6273(91)90118-j. [DOI] [PubMed] [Google Scholar]
- Bierer B. E., Mattila P. S., Standaert R. F., Herzenberg L. A., Burakoff S. J., Crabtree G., Schreiber S. L. Two distinct signal transmission pathways in T lymphocytes are inhibited by complexes formed between an immunophilin and either FK506 or rapamycin. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9231–9235. doi: 10.1073/pnas.87.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixby J. L. Protein kinase C is involved in laminin stimulation of neurite outgrowth. Neuron. 1989 Sep;3(3):287–297. doi: 10.1016/0896-6273(89)90253-5. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Ferris C. D., Snyder S. H. Nitric oxide synthase regulatory sites. Phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and calcium/calmodulin protein kinase; identification of flavin and calmodulin binding sites. J Biol Chem. 1992 Jun 5;267(16):10976–10981. [PubMed] [Google Scholar]
- Calvo V., Crews C. M., Vik T. A., Bierer B. E. Interleukin 2 stimulation of p70 S6 kinase activity is inhibited by the immunosuppressant rapamycin. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7571–7575. doi: 10.1073/pnas.89.16.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J., Kuo C. J., Crabtree G. R., Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992 Jun 26;69(7):1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Steiner J. P., Dawson V. L., Dinerman J. L., Uhl G. R., Snyder S. H. Immunosuppressant FK506 enhances phosphorylation of nitric oxide synthase and protects against glutamate neurotoxicity. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9808–9812. doi: 10.1073/pnas.90.21.9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., Bartley D. A., Uhl G. R., Snyder S. H. Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures. J Neurosci. 1993 Jun;13(6):2651–2661. doi: 10.1523/JNEUROSCI.13-06-02651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., London E. D., Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFranco A. L. Signal transduction. Immunosuppressants at work. Nature. 1991 Aug 29;352(6338):754–755. doi: 10.1038/352754a0. [DOI] [PubMed] [Google Scholar]
- Dumont F. J., Melino M. R., Staruch M. J., Koprak S. L., Fischer P. A., Sigal N. H. The immunosuppressive macrolides FK-506 and rapamycin act as reciprocal antagonists in murine T cells. J Immunol. 1990 Feb 15;144(4):1418–1424. [PubMed] [Google Scholar]
- Dumont F. J., Staruch M. J., Koprak S. L., Melino M. R., Sigal N. H. Distinct mechanisms of suppression of murine T cell activation by the related macrolides FK-506 and rapamycin. J Immunol. 1990 Jan 1;144(1):251–258. [PubMed] [Google Scholar]
- Ferrari S., Pearson R. B., Siegmann M., Kozma S. C., Thomas G. The immunosuppressant rapamycin induces inactivation of p70s6k through dephosphorylation of a novel set of sites. J Biol Chem. 1993 Aug 5;268(22):16091–16094. [PubMed] [Google Scholar]
- Fruman D. A., Klee C. B., Bierer B. E., Burakoff S. J. Calcineurin phosphatase activity in T lymphocytes is inhibited by FK 506 and cyclosporin A. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3686–3690. doi: 10.1073/pnas.89.9.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
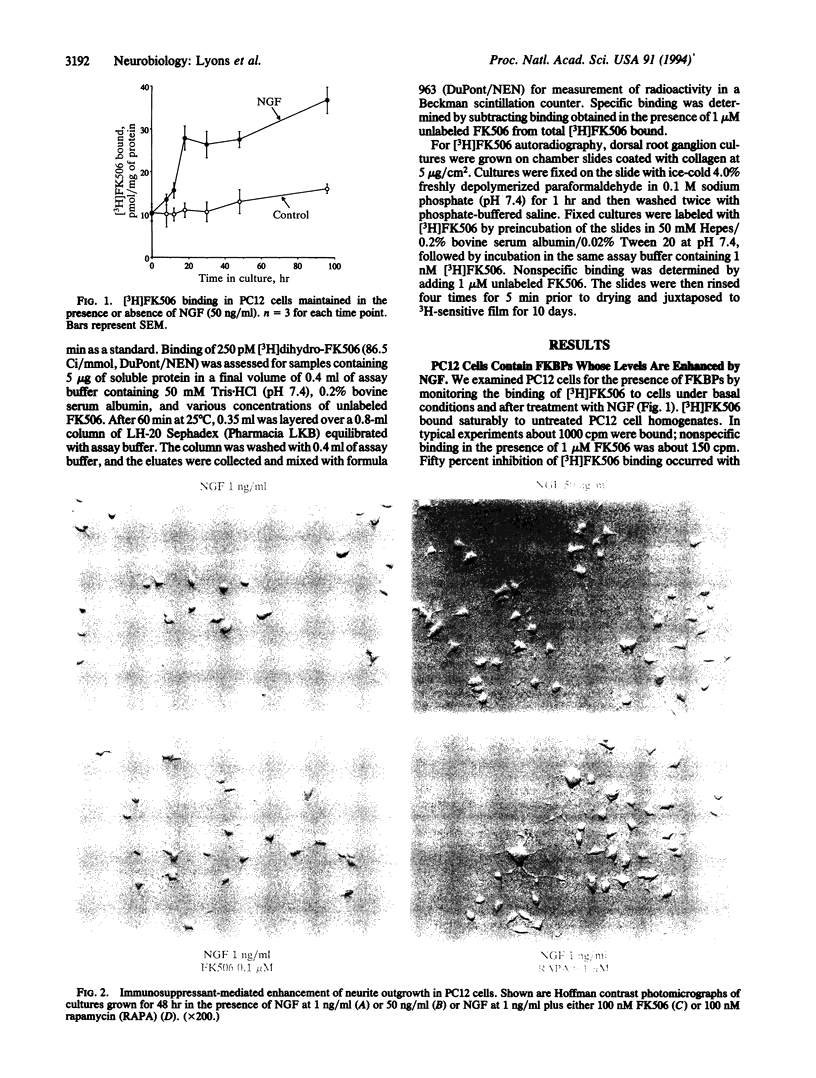
- Fujita K., Lazarovici P., Guroff G. Regulation of the differentiation of PC12 pheochromocytoma cells. Environ Health Perspect. 1989 Mar;80:127–142. doi: 10.1289/ehp.8980127. [DOI] [PMC free article] [PubMed] [Google Scholar]
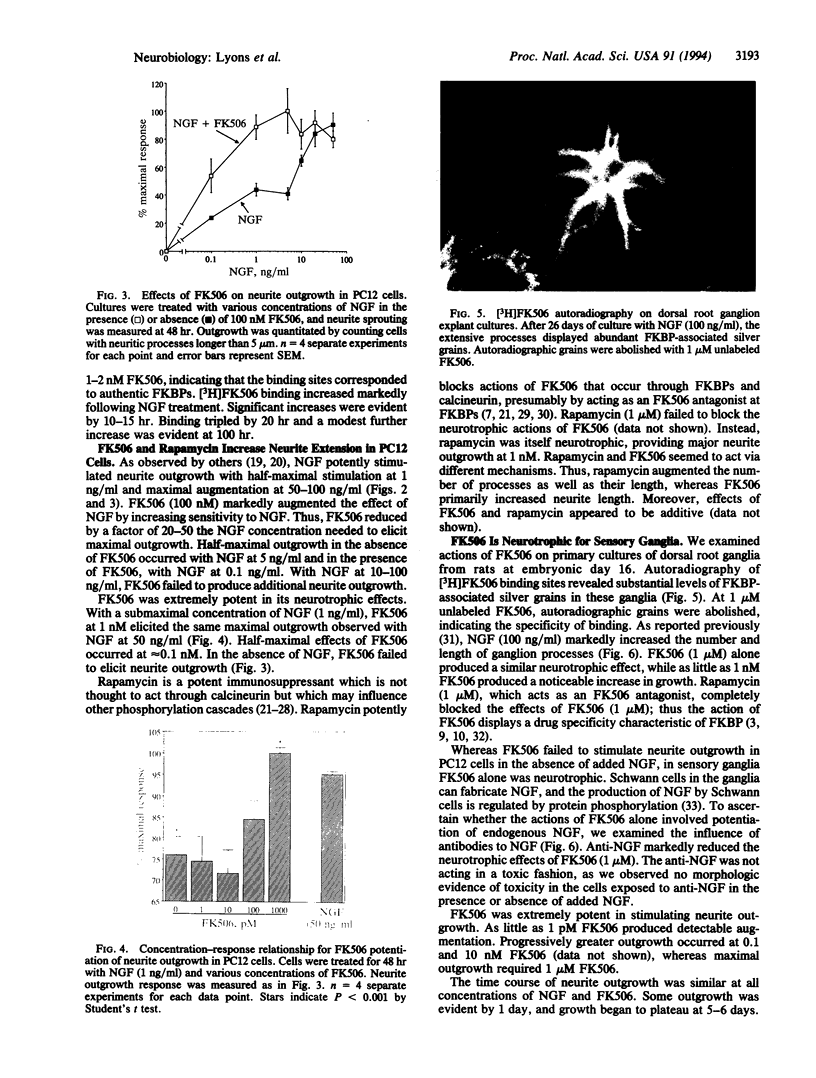
- Galat A., Lane W. S., Standaert R. F., Schreiber S. L. A rapamycin-selective 25-kDa immunophilin. Biochemistry. 1992 Mar 3;31(8):2427–2434. doi: 10.1021/bi00123a031. [DOI] [PubMed] [Google Scholar]
- Girard P. R., Kuo J. F. Protein kinase C and its 80-kilodalton substrate protein in neuroblastoma cell neurite outgrowth. J Neurochem. 1990 Jan;54(1):300–306. doi: 10.1111/j.1471-4159.1990.tb13315.x. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall F. L., Fernyhough P., Ishii D. N., Vulliet P. R. Suppression of nerve growth factor-directed neurite outgrowth in PC12 cells by sphingosine, an inhibitor of protein kinase C. J Biol Chem. 1988 Mar 25;263(9):4460–4466. [PubMed] [Google Scholar]
- Handschumacher R. E., Harding M. W., Rice J., Drugge R. J., Speicher D. W. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984 Nov 2;226(4674):544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Hagino A. Blockage of nerve growth factor action in PC12h cells by staurosporine, a potent protein kinase inhibitor. J Neurochem. 1989 Dec;53(6):1675–1685. doi: 10.1111/j.1471-4159.1989.tb09230.x. [DOI] [PubMed] [Google Scholar]
- Hsu L. The effects of 12-O-tetradecanoylphorbol-13-acetate (TPA) on axonal elongation and fasciculation. Anat Embryol (Berl) 1989;179(5):511–518. doi: 10.1007/BF00319595. [DOI] [PubMed] [Google Scholar]
- Jayaraman T., Brillantes A. M., Timerman A. P., Fleischer S., Erdjument-Bromage H., Tempst P., Marks A. R. FK506 binding protein associated with the calcium release channel (ryanodine receptor). J Biol Chem. 1992 May 15;267(14):9474–9477. [PubMed] [Google Scholar]
- Jin Y. J., Albers M. W., Lane W. S., Bierer B. E., Schreiber S. L., Burakoff S. J. Molecular cloning of a membrane-associated human FK506- and rapamycin-binding protein, FKBP-13. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6677–6681. doi: 10.1073/pnas.88.15.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y. J., Burakoff S. J., Bierer B. E. Molecular cloning of a 25-kDa high affinity rapamycin binding protein, FKBP25. J Biol Chem. 1992 Jun 5;267(16):10942–10945. [PubMed] [Google Scholar]
- Jin Y. J., Burakoff S. J. The 25-kDa FK506-binding protein is localized in the nucleus and associates with casein kinase II and nucleolin. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7769–7773. doi: 10.1073/pnas.90.16.7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz J., Henriquez R., Schneider U., Deuter-Reinhard M., Movva N. R., Hall M. N. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell. 1993 May 7;73(3):585–596. doi: 10.1016/0092-8674(93)90144-f. [DOI] [PubMed] [Google Scholar]
- Kuo C. J., Chung J., Fiorentino D. F., Flanagan W. M., Blenis J., Crabtree G. R. Rapamycin selectively inhibits interleukin-2 activation of p70 S6 kinase. Nature. 1992 Jul 2;358(6381):70–73. doi: 10.1038/358070a0. [DOI] [PubMed] [Google Scholar]
- Levi A., Biocca S., Cattaneo A., Calissano P. The mode of action of nerve growth factor in PC12 cells. Mol Neurobiol. 1988 Fall;2(3):201–226. doi: 10.1007/BF02935346. [DOI] [PubMed] [Google Scholar]
- Liu J., Albers M. W., Wandless T. J., Luan S., Alberg D. G., Belshaw P. J., Cohen P., MacKintosh C., Klee C. B., Schreiber S. L. Inhibition of T cell signaling by immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity. Biochemistry. 1992 Apr 28;31(16):3896–3901. doi: 10.1021/bi00131a002. [DOI] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Liu Y. C., Storm D. R. Dephosphorylation of neuromodulin by calcineurin. J Biol Chem. 1989 Aug 5;264(22):12800–12804. [PubMed] [Google Scholar]
- Matsuoka I., Meyer M., Thoenen H. Cell-type-specific regulation of nerve growth factor (NGF) synthesis in non-neuronal cells: comparison of Schwann cells with other cell types. J Neurosci. 1991 Oct;11(10):3165–3177. doi: 10.1523/JNEUROSCI.11-10-03165.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M. P., Guthrie P. B., Kater S. B. Intracellular messengers in the generation and degeneration of hippocampal neuroarchitecture. J Neurosci Res. 1988 Oct-Dec;21(2-4):447–464. doi: 10.1002/jnr.490210236. [DOI] [PubMed] [Google Scholar]
- McKeon F. When worlds collide: immunosuppressants meet protein phosphatases. Cell. 1991 Sep 6;66(5):823–826. doi: 10.1016/0092-8674(91)90426-y. [DOI] [PubMed] [Google Scholar]
- Mehta S., Hsu L., Jeng A. Y., Chen K. Y. Neurite outgrowth and protein phosphorylation in chick embryonic sensory ganglia induced by a brief exposure to 12-O-tetradecanoylphorbol 13-acetate. J Neurochem. 1993 Mar;60(3):972–981. doi: 10.1111/j.1471-4159.1993.tb03244.x. [DOI] [PubMed] [Google Scholar]
- Meiri K. F., Bickerstaff L. E., Schwob J. E. Monoclonal antibodies show that kinase C phosphorylation of GAP-43 during axonogenesis is both spatially and temporally restricted in vivo. J Cell Biol. 1991 Mar;112(5):991–1005. doi: 10.1083/jcb.112.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. S., Gross J. L., Moskal J. R. Inhibition of protein kinase C activity promotes the neurotrophic action of epidermal and basic fibroblast growth factors. Brain Res. 1988 Nov 8;473(1):141–146. doi: 10.1016/0006-8993(88)90325-3. [DOI] [PubMed] [Google Scholar]
- Phelps C. H., Gage F. H., Growdon J. H., Hefti F., Harbaugh R., Johnston M. V., Khachaturian Z. S., Mobley W. C., Price D. L., Raskind M. Potential use of nerve growth factor to treat Alzheimer's disease. Neurobiol Aging. 1989 Mar-Apr;10(2):205–207. doi: 10.1016/0197-4580(89)90032-8. [DOI] [PubMed] [Google Scholar]
- Price D. J., Grove J. R., Calvo V., Avruch J., Bierer B. E. Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science. 1992 Aug 14;257(5072):973–977. doi: 10.1126/science.1380182. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991 Jan 18;251(4991):283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L., Crabtree G. R. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992 Apr;13(4):136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Skene J. H. Axonal growth-associated proteins. Annu Rev Neurosci. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- Snipes G. J., Costello B., McGuire C. B., Mayes B. N., Bock S. S., Norden J. J., Freeman J. A. Regulation of specific neuronal and nonneuronal proteins during development and following injury in the rat central nervous system. Prog Brain Res. 1987;71:155–175. doi: 10.1016/s0079-6123(08)61821-x. [DOI] [PubMed] [Google Scholar]
- Steiner J. P., Dawson T. M., Fotuhi M., Glatt C. E., Snowman A. M., Cohen N., Snyder S. H. High brain densities of the immunophilin FKBP colocalized with calcineurin. Nature. 1992 Aug 13;358(6387):584–587. doi: 10.1038/358584a0. [DOI] [PubMed] [Google Scholar]
- Swanson S. K., Born T., Zydowsky L. D., Cho H., Chang H. Y., Walsh C. T., Rusnak F. Cyclosporin-mediated inhibition of bovine calcineurin by cyclophilins A and B. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3741–3745. doi: 10.1073/pnas.89.9.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P. K., Albers M. W., Chang H., Faber L. E., Schreiber S. L. Association of a 59-kilodalton immunophilin with the glucocorticoid receptor complex. Science. 1992 May 29;256(5061):1315–1318. doi: 10.1126/science.1376003. [DOI] [PubMed] [Google Scholar]
- Tetzlaff W., Zwiers H., Lederis K., Cassar L., Bisby M. A. Axonal transport and localization of B-50/GAP-43-like immunoreactivity in regenerating sciatic and facial nerves of the rat. J Neurosci. 1989 Apr;9(4):1303–1313. doi: 10.1523/JNEUROSCI.09-04-01303.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H., Barde Y. A. Physiology of nerve growth factor. Physiol Rev. 1980 Oct;60(4):1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- Timerman A. P., Ogunbumni E., Freund E., Wiederrecht G., Marks A. R., Fleischer S. The calcium release channel of sarcoplasmic reticulum is modulated by FK-506-binding protein. Dissociation and reconstitution of FKBP-12 to the calcium release channel of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1993 Nov 5;268(31):22992–22999. [PubMed] [Google Scholar]
- Yankner B. A., Benowitz L. I., Villa-Komaroff L., Neve R. L. Transfection of PC12 cells with the human GAP-43 gene: effects on neurite outgrowth and regeneration. Brain Res Mol Brain Res. 1990 Jan;7(1):39–44. doi: 10.1016/0169-328x(90)90071-k. [DOI] [PubMed] [Google Scholar]
- Yem A. W., Tomasselli A. G., Heinrikson R. L., Zurcher-Neely H., Ruff V. A., Johnson R. A., Deibel M. R., Jr The Hsp56 component of steroid receptor complexes binds to immobilized FK506 and shows homology to FKBP-12 and FKBP-13. J Biol Chem. 1992 Feb 15;267(5):2868–2871. [PubMed] [Google Scholar]