Abstract
Results from the present study demonstrated that transplantation of autologous bone marrow-derived mesenchymal stem cells into the lesion site in rat brain significantly ameliorated brain tissue pathological changes and brain edema, attenuated glial cell proliferation, and increased brain-derived neurotrophic factor expression. In addition, the number of cells double-labeled for 5-bromodeoxyuridine/glial fibrillary acidic protein and cells expressing nestin increased. Finally, blood vessels were newly generated, and the rats exhibited improved motor and cognitive functions. These results suggested that transplantation of autologous bone marrow-derived mesenchymal stem cells promoted brain remodeling and improved neurological functions following traumatic brain injury.
Keywords: angiogenesis, neurogenesis, neurotrophic factors, bone marrow-derived mesenchymal stem cells, traumatic brain injury, stem cell transplantation, neural regeneration
INTRODUCTION
Traumatic brain injury (TBI) results from direct impact to the head or from sudden acceleration-deceleration. The functional deficits are due to primary and secondary mechanisms[1]. The most prevalent and debilitating features in survivors of brain trauma are cognitive deficits and motor dysfunctions[2]. To date, there is no effective method for promoting functional recovery other than routine medical intervention and care[3]. Cellular therapies utilizing neural stem/progenitor cells provide promise for the treatment of brain injury[4]. However, clinical use of embryonic stem cells or fetal tissues is limited by ethical considerations and other complications. Mesenchymal stem cells represent an alternative source of stem cells for cell replacement therapies. Preclinical TBI studies tested the therapeutic efficacy of xenogenic transplantation of bone marrow-derived mesenchymal stem cells (BMSCs)[5], but results demonstrated that these cells induced rejection. Therefore, autologous transplantation of BMSCs could provide potential advantages.
BMSCs can pass through the blood-brain barrier and migrate throughout the forebrain and cerebellum, as well as differentiate into astrocytes and neurons following transplantation into the brain lesion site[6]. The present study determined the efficacy and mechanisms of autologous BMSCs transplantation for treating TBI by analyzing motor function, cognition, cell differentiation, neurotrophic factor expression, angiogenesis, and apoptosis.
RESULTS
Quantitative analysis of experimental animals
A total of 126 healthy Sprague Dawley rats were used to establish a controlled cortical impact model of TBI according to previously described methods[7]. A total of 100 rats with successfully established injury (Neurological severity score, NSS 13–17[8,9]) were randomly assigned to transplantation and control groups (n = 50, respectively) and underwent surgical transplantation of autologous BMSCs or surgical physiological saline, respectively. Finally, 100 rats were included in the final analysis.
Autologous BMSC transplantation improved brain tissue pathology in TBI rats
Histological changes were observed following hematoxylin-eosin staining. At 24 hours after injury, pathological changes, including hemorrhage, edema, vasocongestion, and inflammatory cell infiltration, occurred, although there was no significant difference between transplantation and control groups. At 28 days, the area of edema and necrosis was significantly smaller in the transplantation group, and glial cell proliferation was significantly decreased, compared with the control group (Figure 1).
Figure 1.
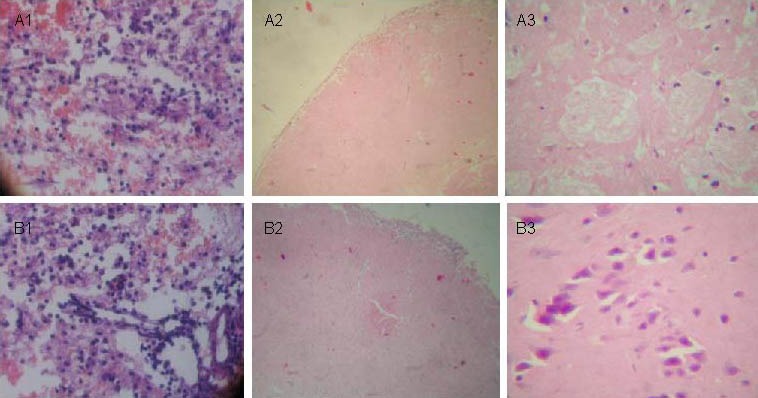
Pathological changes at different time points in brain tissues following traumatic brain injury (hematoxylin-eosin staining).
(A) Control group: 24 hours (A1, × 200), 7 days (A2, × 100), and 28 days (A3, × 400) after traumatic brain injury.
(B) Transplantation group: 24 hours (B1, × 200), 7 days (B2, × 100), and 28 days (B3, × 400) after traumatic brain injury. At 28 days, edema and necrosis areas are decreased compared with the control group.
Autologous BMSC transplantation promoted glial cell proliferation in brain tissues of TBI rats
The mature glial cell marker glial fibrillary acidic protein (GFAP) was used in conjunction with 5-bromodeoxyuridine (BrdU) to identify newly generated glial cells by immunofluorescence double staining. Fluorescence microscopy revealed significantly less BrdU GFAP double-labeled cells in the control group compared with the transplantation group at 7–28 days after TBI (P < 0.05; Figure 2, Table 1).
Figure 2.
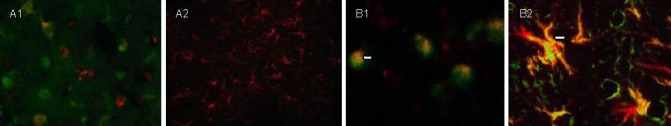
5-bromodeoxyuridine (BrdU)/glial fibrillary acidic protein (GFAP) expression in brain tissues at different time points after traumatic brain injury (immunofluorescence double staining, fluorescent microscopy).
(A) Control group (A1, × 400; A2, × 200).
(B) transplantation group (B1, B2, × 400).
Cells with green nuclei are newly generated BrdU-positive cells and red fluorescence is GFAP-positive glial cells. The number of BrdU/GFAP double-labeled cells (yellow; arrows) is less in the control group than in the transplantation group at 7 (A1, B1) and 28 days (A2, B2) after traumatic brain injury.
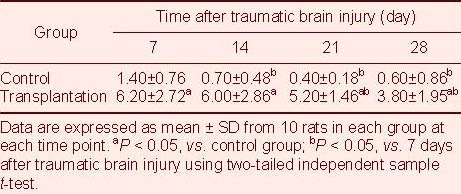
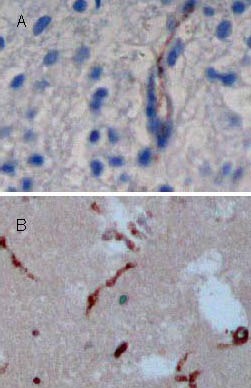
Table 1.
Number of 5-bromodeoxyuridine/glial fibrillary acidic protein double-labeled cells (cells/200 × field of view) in the lesioned brain area after traumatic brain injury
Autologous BMSC transplantation increased brain-derived neurotrophic factor (BDNF) expression in brain tissues of TBI rats
BDNF expression was analyzed using the streptavidin-peroxidase method[10]. BDNF expression was strong in the transplantation group under light microscope (Figure 3), and BDNF expression significantly increased following BMSC transplantation compared to the control group (P < 0.05; Table 2).
Figure 3.
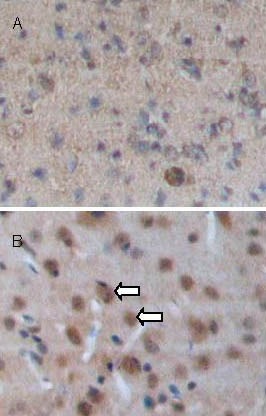
Brain-derived neurotrophic factor (BDNF) expression in brain tissues at 7 days after traumatic brain injury (streptavidin-peroxidase; × 100).
BDNF-positive cells (arrows) appear as brown staining in the cytoplasm. BDNF expression is significantly increased in the transplantation group (B) compared with the control group (A).
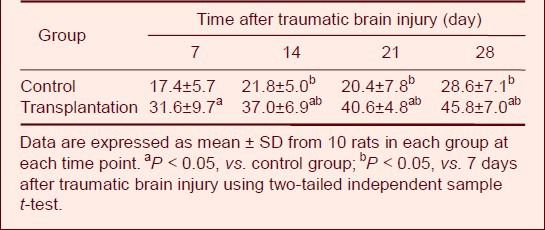
Table 2.
Comparison of the number of brain-derived neurotrophic factor-positive cells (cells/200 × field of view) after traumatic brain injury
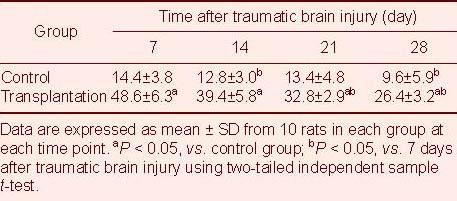
Nestin expression in brain tissues of TBI rats following autologous BMSC transplantation
Nestin expression was analyzed by immunohistochemistry, as previously described[10]. The presence of the immature cellular marker nestin was clearly detectable in the transplantation group (Figure 4). Statistical analyses showed that at 7–28 days after TBI, the number of nestin-positive cells significantly increased in brain tissues of the transplantation group compared with the control group (P < 0.05; Table 3).
Figure 4.
Nestin-positive cells in brain tissues at 14 days after traumatic brain injury (streptavidin-peroxidase; × 200).
Nestin-positive cells are visible as cells with brown staining (arrows). Nestin expression is significantly increased in brain tissues of the transplantation group (B) compared with the control group (A).
Table 3.
Comparison of the number of nestin-positive cells (cells/200 × field of view) after traumatic brain injury

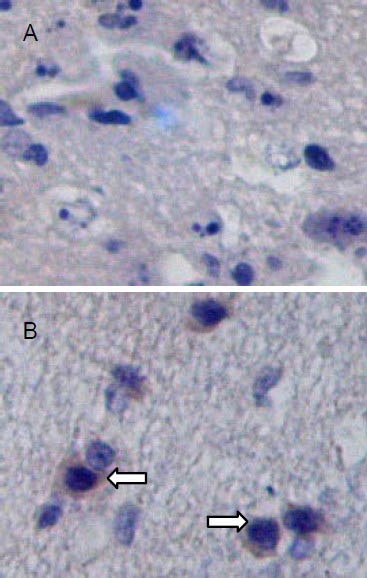
Autologous BMSC transplantation increased factor VIII expression in brain tissues of TBI rats
At 7, 14, 21, and 28 days after TBI, VIII expression was observed in brain tissues of both groups. BMSC transplantation significantly increased vascular density and vessel-to-tissue ratio in brain tissue compared with the control group (P < 0.05; Figure 5, Table 4).
Figure 5.
Factor VIII antigen expression in brain tissues at 14 days after traumatic brain injury (streptavidin-peroxidase; ×100).
Coronal sections are stained with antibodies specific to factor VIII. Positive cells appear as dark brown-stained nuclei (arrows). Factor VIII expression is significantly increased in brain tissues of the transplantation group (B) compared with the control group (A).
Table 4.
Comparison of the number of factor VIII-positive cells (cells/200 × field of view) after traumatic brain injury
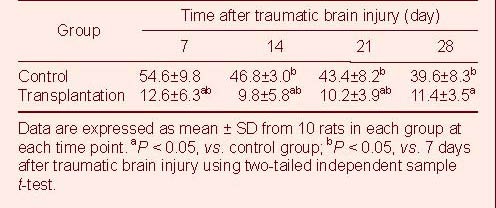
Autologous BMSC transplantation inhibited cell apoptosis in brain tissues of TBI rats
Cell apoptosis was detected by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)[11]. Several apoptotic cells were observed in both groups (Figure 6). Statistical analyses revealed significantly less TUNEL-positive cells in the transplantation group compared with the control group (P < 0.05; Table 5), suggesting that BMSC transplantation protected against cell apoptosis.
Figure 6.
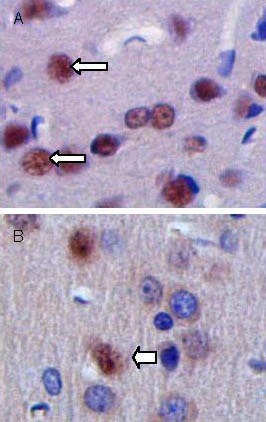
Cellular apoptosis in brain tissues at 28 days after traumatic brain injury (terminal deoxynucleotidyl transferase dUTP nick-end labeling, × 200).
Arrows show apoptotic bodies (brown). The number of apoptotic cells is increased in the control group (A) com-pared with the transplantation group (B).
Table 5.
Quantification of terminal deoxynucleotidyl transferase dUTP nick-end labeling-positive cells (cells/400 × field of view) after traumatic brain injury
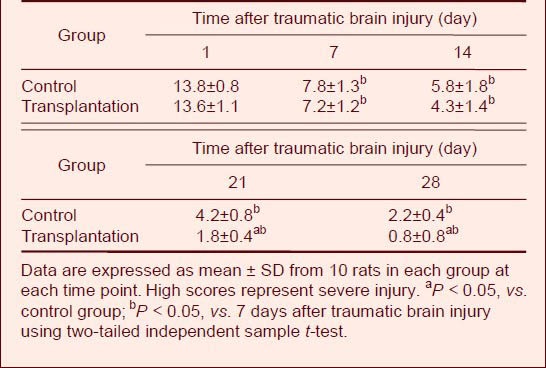
Autologous BMSC transplantation improved motor functions in TBI rats
Motor function was measured using the NSS, which is sensitive for unilateral cortical injury, because it reflects multiple asymmetries, including postural, sensory, forelimb, and hindlimb[8,9,12,13]. There was no significant difference in NSS scores between transplantation and control groups at 14 days after TBI (P > 0.05). However, at days 21 and 28, scores were significantly lower (i.e., improved) in the transplantation group compared with the control group (P < 0.05; Table 6).
Table 6.
Comparison of Neurological Severity Scores after traumatic brain injury
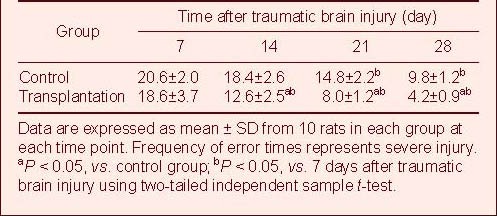
Autologous BMSC transplantation improved cognitive function in TBI rats
The Y-maze was used to measure learning and memory in the rats[14]. During the initial 7 days, the transplantation group showed no significant improvement in the test. However, performance significantly improved in the transplantation group by 14 days post-transplantation compared with the control group, and the positive effect persisted for up to 28 days (P < 0.05; Table 7).
Table 7.
Comparison of error times in the Y-electric maze test after traumatic brain injury
DISCUSSION
The present study analyzed functional outcomes after TBI in rats following surgical transplantation of autologous BMSCs, which previous studies have shown to improve functional outcomes in experimental studies of traumatic brain injury. A previous preclinical study[15] analyzed the therapeutic efficacy of xenogenic BMSC transplantation, but this method requires immunosuppressive treatment. The present study demonstrated that surgical transplantation of autologous BMSCs improved sensorimotor function after TBI. To the best of our knowledge, this is the first study to demonstrate that surgical transplantation of autologous BMSCs reduces deficits in spatial learning and sensorimotor functions after TBI. NSS results and the times of error reactions in the Y-electric maze test demonstrated that BMSC transplantation promoted recovery of sensorimotor functional deficits. Surgical transplantation of autologous BMSCs also resulted in significant treatment effects on NSS at later time points compared with the control group.
In the present study, following TBI, BMSCs induced endogenous cellular proliferation and enhanced neural plasticity. Morphological changes in brain tissue at different time points after TBI demonstrated that BMSC transplantation attenuated neuronal and nerve fiber necrosis, relieved degree of pathological reaction, protected injured neurons, prevented glial cell proliferation, and increased nerve fiber regeneration. BMSCs have been previously shown to significantly decrease glial scar formation and promote glial-axonal remodeling[16]. This could be a mechanism by which BMSCs inhibit progressive chronic processes of cell death or initiate repair of injured cerebral tissue.
Following transplantation into the lesion site, the number of nestin-positive cells significantly increased, and some GFAP-positive cells were observed. BrdU/GFAP double-positive cells were detected in the lesion site. These results suggested that BMSCs promoted remodeling of injured cerebral tissue. BMSC fate is regulated by interactions with the microenvironment, neighboring cells, soluble trophic factors, and mechanical stimuli. BMSCs in the brain have been shown to secrete various growth factors that promote functional outcome after injury, thereby increasing endogenous levels of these factors[17,18,19]. BMSCs also induce intrinsic parenchymal cells to produce these growth factors[15]. Recent studies have suggested that growth factors such as fibroblast growth factor-2, VEGF, and BDNF promote neurogenesis[20,21,22]. However, short duration and limited expression of endogenous growth factors provide challenges for overall and lasting treatment of damaged neurons. Indeed, transplantation of gene-modified hMSC (BDNF-hypersecreting hMSC) into a middle cerebral artery occlusion model results in better outcome[23]. In the present study, expression of the neurotrophic factor BDNF increased in the brain lesion following BMSC transplantation, suggesting that BMSCs produced BDNF and induced intrinsic parenchymal cells to produce BDNF. BMSCs induce expression of cytokines and trophic factors within parenchymal cells that enhance angiogenesis, neurogenesis, and synaptogenesis[24] in the lesion zone, and the majority of surviving BMSCs were detected in the lesion zone in the TBI + BMSC group compared with the control group.
BMSCs “home” to damaged tissue, and previous studies have shown that regenerated neuronal- and astrocyte-like cells are of bone marrow origin[16,23]. Studies have shown that intravenous administration of marrow stromal cells results in accumulation of these cells within the ischemic rat brain, and a small percentage of these cells express neuronal markers[25]. Neural stem cells are concentrated within the subventricular zone, but migration to the zone of injury is often limited by distance. However, bone marrow cells can be delivered via direct surgical injection to the region of interest. In the present study, a significantly increased number of nestin-positive cells was detected in brain tissue of the BMSC transplantation group compared with control group, suggesting that bone marrow–derived cells expressed the stem cell marker nestin.
Brain regeneration following cerebral injury requires the generation of new neurons and glial cells, as well as the generation of new blood vessels. It remains unknown which specific bone marrow–derived populations are involved in neovascularization or neuronal regeneration. However, it is likely that these could be separate cell populations. Bone marrow contains at least two populations of progenitor cells: hematopoietic and marrow stromal cells[25,26]. Marrow stromal cells have a wide differentiation potential and differentiate into cells that express neuronal markers in vitro and in vivo[25]. In contrast, hematopoietic stem cells are the putative progenitor cells for the endothelium. BMSCs mobilize in response to injury, differentiate into endothelial cells, and decrease endothelial apoptosis. Results from the present study showed that BMSCs transplanted to the lesion site contributed to neovascularization. In addition, factor VIII expression significantly increased in brain tissue of the BMSC transplantation group compared to the control group. BMSCs treatment significantly increased vascular density in brain tissue compared with the control group, suggesting that BMSCs increased angiogenesis and reduced injury in TBI rats.
Studies have suggested a strong correlation between neovascularization and neurogenesis in the brain. In the adult hippocampus, newly dividing cells are found in dense clusters associated with the vasculature, suggesting that neurogenesis occurs in an angiogenic niche[27]. These findings have clinical importance and provide novel therapeutic strategies for enhancing regeneration and recovery after TBI.
Bone marrow-derived progenitor cells differentiated into endothelial cells and nestin-expressing cells, which suggested that surgical transplantation of autologous bone marrow stem cells could provide effective treatments for TBI. However, further studies are needed to accurately quantify angiogenesis and neurogenesis following BMSC transplantation in the TBI-injured brain. Repair of brain injury is a major therapeutic challenge, but recent progress in cellular transplantation, drugs, gene therapy, and molecular therapies has increased optimism for future cures. The combination of strategies could provide sufficient structural and functional recovery.
In conclusion, autologous BMSC transplantation provided therapeutic benefits and promoted remodeling of injured cerebral tissue. This method could provide strategies for repairing brain injury and improving neurological function after TBI.
MATERIALS AND METHODS
Design
A randomized, controlled animal experiment.
Time and setting
The present study was performed at the Experimental Center of Henan Provincial People's Hospital, China, from December 2009 to March 2011.
Materials
A total of 126 healthy, male, Sprague Dawley rats, aged 18–22 months and weighing 250–300 g, were provided by the Laboratory Animal Center of Tongji Medical College in Central China, University of Sciences and Technology. All experimental procedures were performed in accordance with the Guidance Suggestions For the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[28].
Methods
Establishment of TBI model
A controlled, cortical impact model of TBI was established according to Feeney's method[29]. The rats were fasted for 16 hours prior to surgery. During the experiment, the rats were anesthetized by intraperitoneal injection of 100 g/L chloral hydrate (0.35 g/kg) and placed in a prone position. After the skin was sterilized, a median incision was made on the scalp, and the periosteum was stripped to expose the right parietal bone. A 5-mm diameter hole was drilled 1.5 mm posterior to the frontoparietal suture and 2.5 mm lateral to the median line, leaving the dura mater intact. A 20-g counterweight was vertically dropped from a 30-cm long copper tube to strike dura mater and induce right parietal lobe TBI. Gentamicin (4 × 107 IU/L; 5 drops) was administered to the cut, bone wax was used to seal the hole, and the scalp was sutured. At 24 hours after establishment of the TBI model, neurological functions were measured by NSS[8,9]. A total of 100 rats with scores between 13–17 were used for analysis.
BMSC preparation and transplantation
All animals received an intraperitoneal injection of BrdU, twice daily for one week (50 mg/kg; Sigma, St. Louis, MO, USA) and 7 days prior to model establishment to label dividing cells. At 24 hours after model establishment, the rats were intraperitoneally anesthetized with 350 mg/kg body weight chloral hydrate. A vertical incision was made along the anterior margin of the tibia to expose the proximal part of the facies medialis tibiae. A 2-mL heparin-infiltrated syringe was inserted into the tibia, and 0.4 mL bone marrow was harvested. The bone marrow was mixed with 5 mL phosphate buffered saline (PBS) and mechanically dispersed. The single cell suspension was transferred to an aseptic centrifuge tube and centrifuged. The supernatant was discarded, and cells were resuspended in an equal volume of D-Hank's solution. The cells were then centrifuged and the mononuclear cell layer was gently triturated into a single-cell suspension. Mononuclear cell suspensions were quantified following trypan blue staining (0.2%) and dilutions were adjusted to 1 × 106/100 µL. BMSCs exhibited typical spindle-shape morphology, and cell type was confirmed by flow cytometry. BMSCs (1 × 106) were suspended in 100 µL saline and were delivered via direct surgical injection to the brain lesion site[30]. In the control group, equal amounts of physiological saline were injected.
Neurological functional evaluation
Neurological function was assessed using NSS[8,9], which is composed of motor (muscle status, abnormal movement), sensory (visual, tactile, and proprioceptive), reflex, and beam walking tests. A greater NSS score represents more severe injury. Evaluation began prior to TBI and was performed once daily after TBI.
The Y-electric maze test was used to assess processing speed[14]. Briefly, rats were placed in the maze, and tasks were recorded in the following manner: after electric shock, the rat escaped from the starting zone to the safety zone within 10 seconds, i.e., correct reaction. If the rat did not reach the safety zone within 10 seconds, an error reaction was recorded. Error reaction times were recorded once daily, and all measurements were performed by observers blinded to individual treatment.
Preparation of brain tissue samples
Ten rats were sacrificed at 24 hours and 7, 14, 21, and 28 days after model establishment. The rats were sacrificed by anesthesia overdose with an intraperitoneal injection of 300 mg/kg chloral hydrate. Intracardiac perfusion was then performed with 100 mL cold saline. Brain tissues from the lesion zone of each group were harvested for preparation of paraffin sections, which were used for histological analysis and immunohistochemical staining[13].
Observation of pathological changes using hematoxylin-eosin staining
Hematoxylin-eosin staining was employed to measure morphological changes. The paraffin sections were subjected to de-waxing and hydration, followed by Harris hematoxylin staining for 5 minutes. The sections were then differentiated in 0.6% hydrochloric acid alcohol for 30 seconds, counterstained in acidified eosin alcohol for 1–2 minutes, dehydrated, cleared, and the lesion and boundary zones were observed by light microscope (Olympus, Tokyo, Japan).
BrdU/GFAP immunofluorescence
BrdU and GFAP immunofluorescence was performed on the brain sections to identify transplanted BMSCs and specific cell types[7]. The paraffin-embedded sections were de-paraffinized in Histoclear and rehydrated in a 100–70% ethanol gradient, followed by distilled water. Endogenous peroxidase was inhibited by incubating the sections in 0.3% hydrogen peroxide at room temperature for 20 minutes. The sections were then incubated with primary rabbit BrdU polyclonal antibody BrdU (Sigma; 1: 80) at 4°C overnight. After three PBS washes, the sections were incubated in fluoresceine isothiocyanate-conjugated goat anti-rabbit IgG (Roche, Indianapolis, IN, USA) (1: 30) in PBS at 37°C for 2 hours. After three PBS wash steps, the sections were incubated in goat anti-mouse GFAP (Roche; 1: 100) at 4°C overnight. Finally, sections were washed three times with PBS and incubated in Cy3-conjugated mouse anti-goat IgG (Roche; 1: 30) in PBS at 37°C for 2 hours. Cover slips were washed and mounted with 50% glycerin in PBS before imaging (fluorescence microscope, Leica, Solms, Germany). Negative control sections underwent identical preparations for immunohistochemical staining. BrdU-GFAP positive cells were quantified in five random areas in the lesion boundary zone at 200 × magnification under fluorescence microscope.
BDNF, nestin, and factor VIII immunohistochemistry
BDNF, nestin, and factor VIII expressions were detected using the streptavidin-peroxidase method[10] with primary antibodies: rabbit anti-BDNF (Sigma), nestin (Roche), and factor VIII (Roche). The staining was performed strictly according to the instruction of the staining kit. The sections were then incubated with biotinylated goat anti-rabbit IgG secondary antibodies diluted 1: 100 in PBS for 20 minutes at 37°C. The sections were then washed in PBS and incubated for 30 minutes at 37°C in streptavidin peroxidase. The sections were observed by light microscope following diaminobenzidine coloration.
Detection of cell apoptosis using TUNEL staining
Cell apoptosis was analyzed using a DeadEnd Colorimetric TUNEL system (Roche)[11]. Apoptotic cells in injured brain tissues were detected using the In Situ Apoptosis Detection Kit (Roche) according to manufacturer instructions. TUNEL-positive cells were quantified in five random areas from five different sections at 400 × magnification under a light microscope. The number of TUNEL-positive cells was represented by cells/400 × field of view.
Statistical analysis
Data were expressed as mean ± SD and analyzed with SPSS for Windows (version 12.0; SPSS, Chicago, IL, USA) for two-tailed independent sample t-test. P < 0.05 was considered statistically significant.
Acknowledgments:
We thank Dr. Zhiqi Hu, Nanfang Hospital for help in discussions. We also thank Xuekun Wang and Zhiying Li, Henan Provincial People's Hospital for their help.
Footnotes
Conflict of interest: None declared.
Funding: This work was financially supported by the Science and Technology Tackle Program of Henan Province, No. 0424420054.
Ethical approval: The project received full ethical approval by Animal Ethics Committee of Henan Provincial People's Hospital, China.
(Edited by He YS, Qu F/Su LL/Wang L)
REFERENCES
- [1].Cernak I, Noble-Haeusslein LJ. Traumatic brain injury: an overview of pathobiology with emphasis on military populations. J Cereb Blood Flow Metab. 2010;30(2):255–266. doi: 10.1038/jcbfm.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].De Guise E, Feyz M, LeBlanc J, et al. Overview of traumatic brain injury patients at a tertiary trauma centre. Can J Neurol Sci. 2005;32(2):186–193. doi: 10.1017/s0317167100003954. [DOI] [PubMed] [Google Scholar]
- [3].Narayan RK, Michel ME, Ansell B, et al. Clinical trials in head injury. J Neurotrauma. 2002;19(5):503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tsyb AF, Roshal LM, Konoplyannikov AG, et al. Evaluation of psychoemotional status of rats after brain injury and systemic transplantation of mesenchymal stem cells. Bull Exp Biol Med. 2007;143(4):539–542. doi: 10.1007/s10517-007-0174-z. [DOI] [PubMed] [Google Scholar]
- [5].Lu D, Li Y, Wang L, et al. Intraarterial administration of marrow stromal cells in a rat model of traumatic brain injury. J Neurotrauma. 2001;18:813–819. doi: 10.1089/089771501316919175. [DOI] [PubMed] [Google Scholar]
- [6].Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96(19):10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Boulton RA, Hodgson HJ. Assessing cell proliferation: a methodological review. Clin Sci (Lond) 1995;88(2):119–130. doi: 10.1042/cs0880119. [DOI] [PubMed] [Google Scholar]
- [8].Beni-Adani L, Gozes I, Cohen Y, et al. A peptide derived from activity-dependent neuroprotective protein (ADNP) ameliorates injury response in closed head injury in mice. J Pharmacol Exp Ther. 2001;296:57–63. [PubMed] [Google Scholar]
- [9].Moussaieff A, Shein NA, Tsenter J, et al. Incensole acetate: a novel neuroprotective agent isolated from Boswellia carterii. J Cereb Blood Flow Metab. 2008;28(7):1341–1352. doi: 10.1038/jcbfm.2008.28. [DOI] [PubMed] [Google Scholar]
- [10].Hsi ED. A practical approach for evaluating new antibodies in the clinical immunohistochemistry laboratory. Arch Pathol Lab Med. 2001;125(2):289–294. doi: 10.5858/2001-125-0289-APAFEN. [DOI] [PubMed] [Google Scholar]
- [11].Bu X, Khankaldyyan V, Gonzales-Gomez I, et al. Species-specific urokinase receptor ligands reduce glioma growth and increase survival primarily by an antiangiogenesis mechanism. Lab Invest. 2004;84(6):667–678. doi: 10.1038/labinvest.3700089. [DOI] [PubMed] [Google Scholar]
- [12].Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- [13].Zhang SX, Bu XY, Liu M, et al. Temporal muscle sticking combined with autologous bone marrow stem cell mobilization for the treatment of ischemic cerebrovascular diseases in rats. Zhonghua Linchuang Yishi Zazhi: Dianzi Ban. 2009;3:771–779. [Google Scholar]
- [14].Wang YC. The rational use of Y-electric maze in the test of the learning and memory abilities of the rat. Zhongguo Xingwei Yixue Kexue. 2005;14:69–70. [Google Scholar]
- [15].Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma. 2004;21:33–39. doi: 10.1089/089771504772695922. [DOI] [PubMed] [Google Scholar]
- [16].Li Y, Chen J, Zhang CL, et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49(3):407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- [17].Whetton AD, Graham GJ. Homing and mobilization in the stem cell niche. Trends Cell Biol. 1999;9(6):233–238. doi: 10.1016/s0962-8924(99)01559-7. [DOI] [PubMed] [Google Scholar]
- [18].Askari AT, Unzek S, Popovic ZB, et al. Effect of stromal-cell- derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362(9385):697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- [19].Mahmood A, Lu D, Chopp M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery. 2004;55(5):1185–1193. doi: 10.1227/01.neu.0000141042.14476.3c. [DOI] [PubMed] [Google Scholar]
- [20].Yoshimura S, Teramoto T, Whalen MJ, et al. FGF-2 regulates neurogenesis and degeneration in the dentate gyrus after traumatic brain injury in mice. J Clin Invest. 2003;112(8):1202–1210. doi: 10.1172/JCI16618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jin K, Zhu Y, Sun Y, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99(18):11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82(6):1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- [23].Nomura T, Honmou O, Harada K, et al. I.V. infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience. 2005;136(1):161–169. doi: 10.1016/j.neuroscience.2005.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang T, Xu Z, Jiang W, et al. Cell-to-cell contact induces mesenchymal stem cell to differentiate into cardiomyocyte and smooth muscle cell. Int J Cardiol. 2006;109(1):74–81. doi: 10.1016/j.ijcard.2005.05.072. [DOI] [PubMed] [Google Scholar]
- [25].Bianco P, Riminucci M, Gronthos S, et al. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19(3):180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- [26].Baum CM, Weissman IL, Tsukamoto AS, et al. Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci U S A. 1992;89(7):2804–2808. doi: 10.1073/pnas.89.7.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425(4):479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [28].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions For the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [29].Feeney DM, Boyeson MG, Linn RT, et al. Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res. 1981;211(1):67–77. doi: 10.1016/0006-8993(81)90067-6. [DOI] [PubMed] [Google Scholar]
- [30].Zhang F, Bu XY, Zhang SX, et al. Autologous mesenchymal stem cells transplanting for ischemic brain injury. Zhonghua Shiyong Zhenduan yu Zhiliao Zazhi. 2009;23:240–243. [Google Scholar]


